Mechanisms of Durability Degradation in Recycled Fine Aggregate Concrete of Varying Strengths Induced by Chloride and Sulfate Dry–Wet Cycles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Mixtures
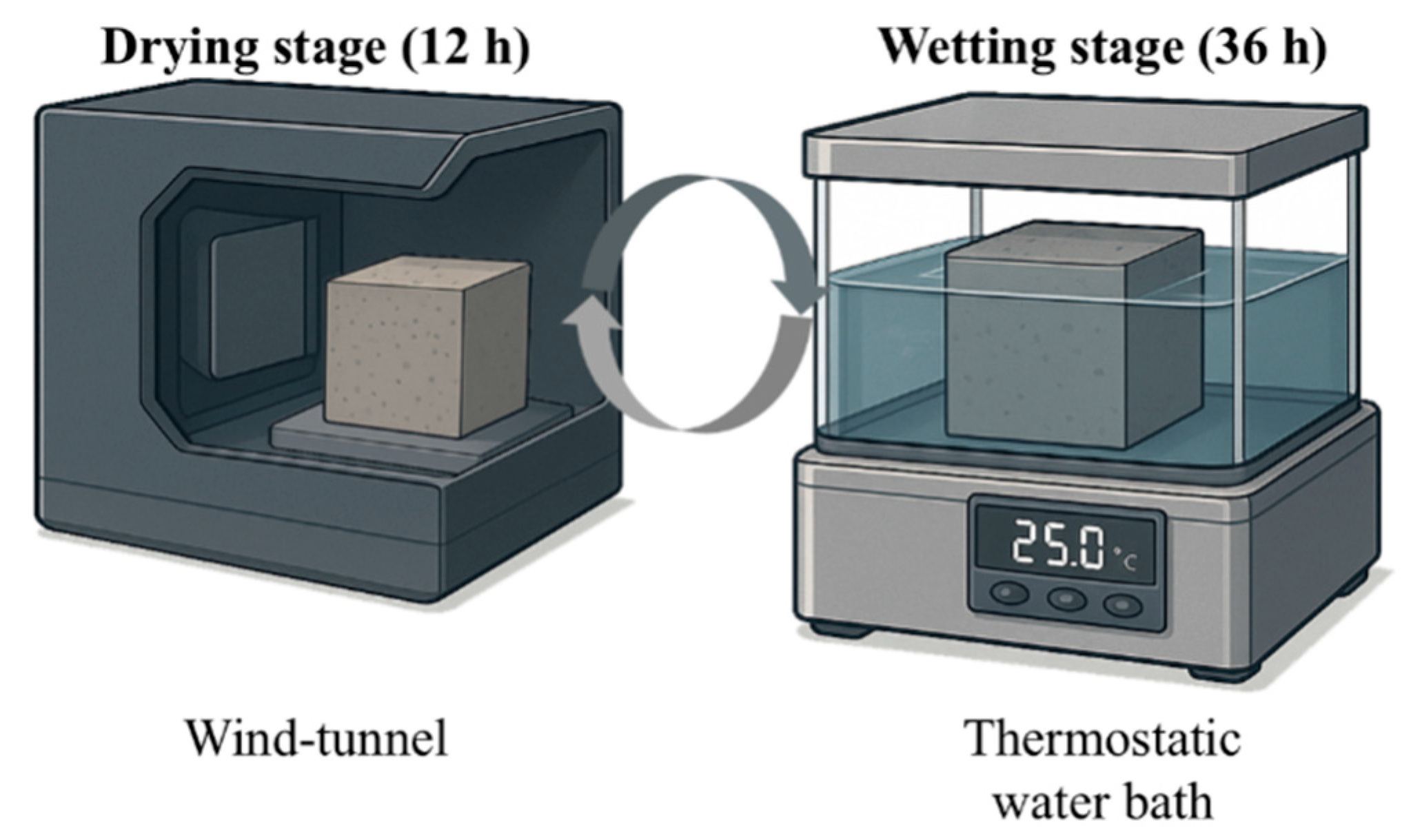
2.2. Exposures
2.3. Test Methods
3. Results
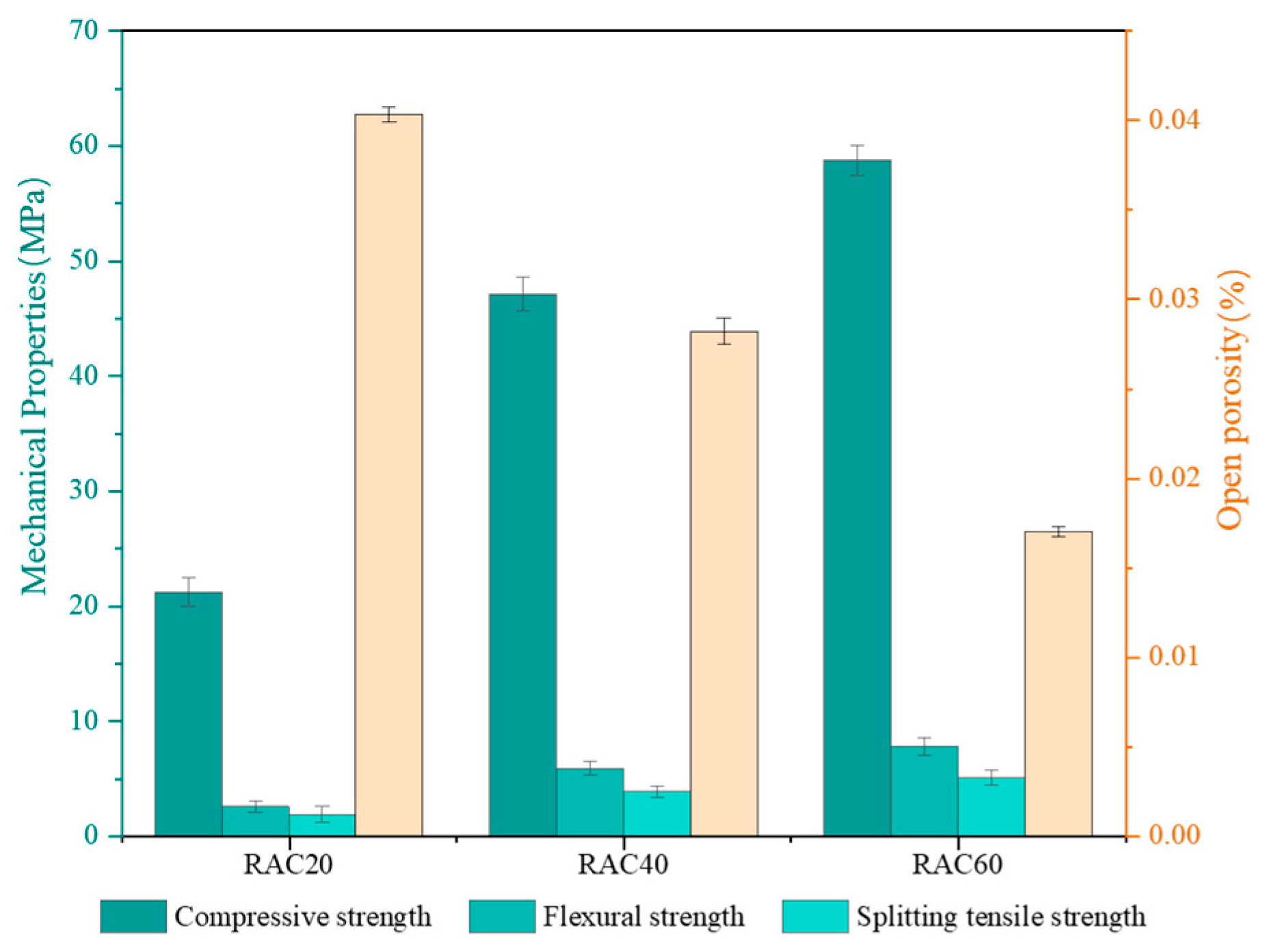
3.1. Initial Performance Befor Cycling
3.2. Physical–Mechanical Properties
3.2.1. Compressive Strength
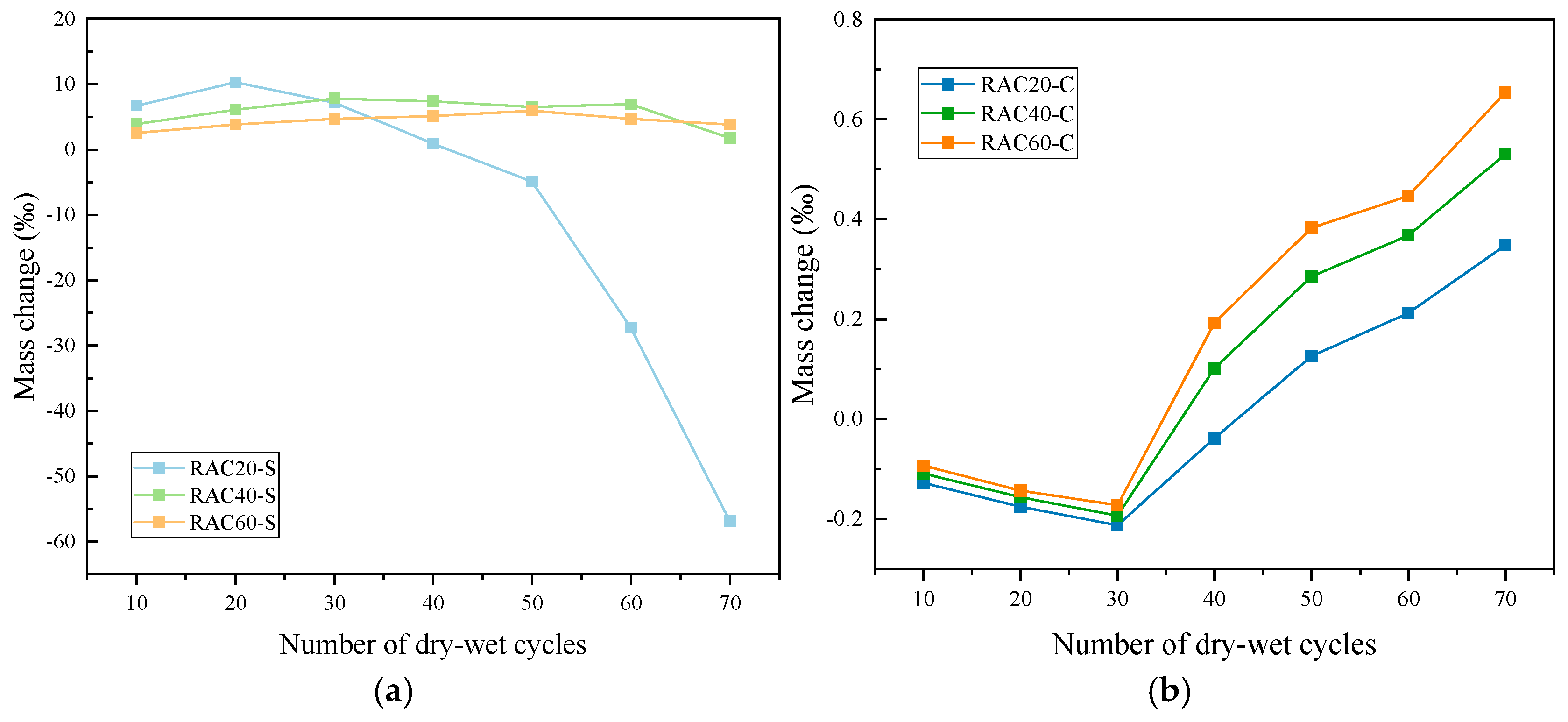
3.2.2. Mass Change
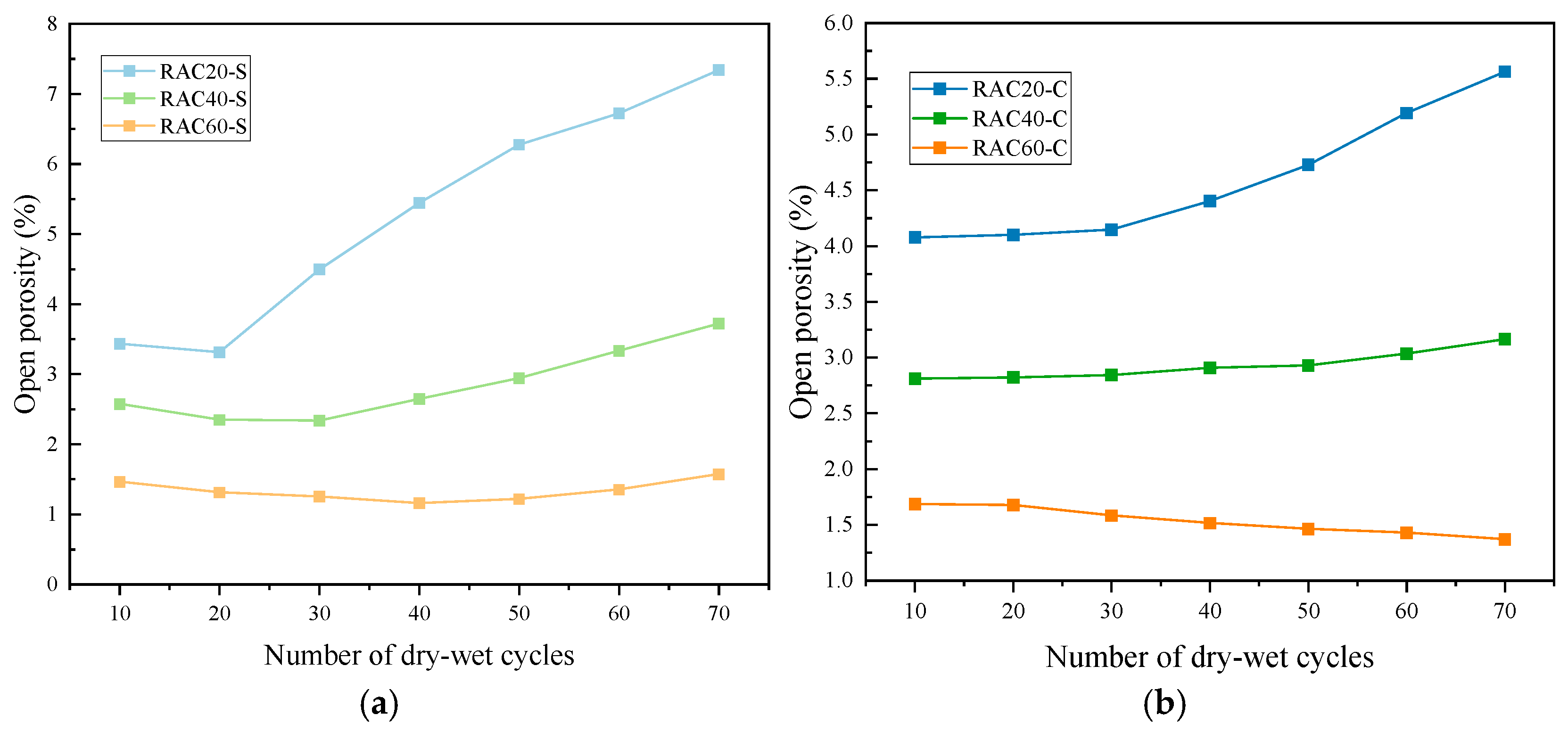
3.2.3. Open Porosity
3.3. Durability
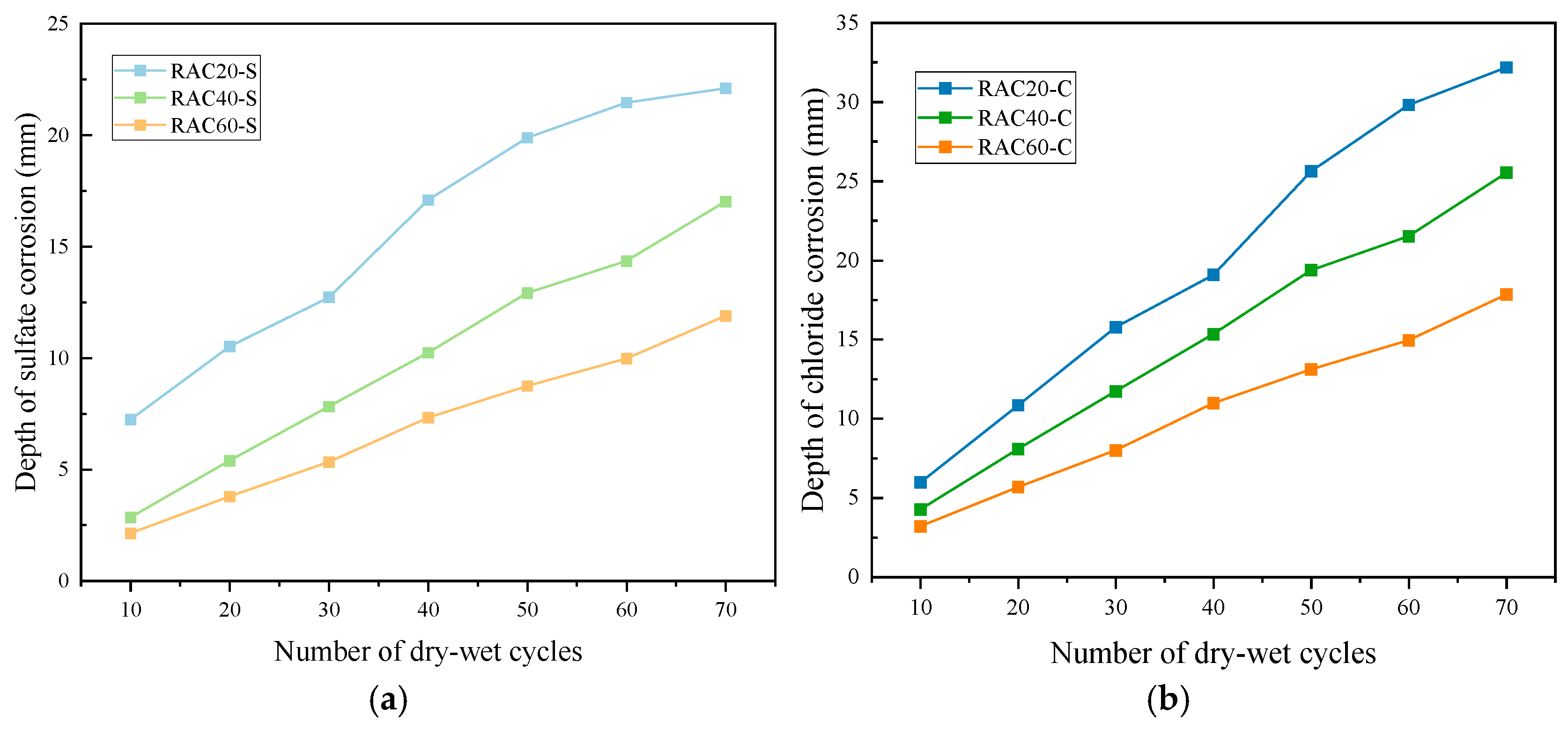
3.3.1. Erosion Depth
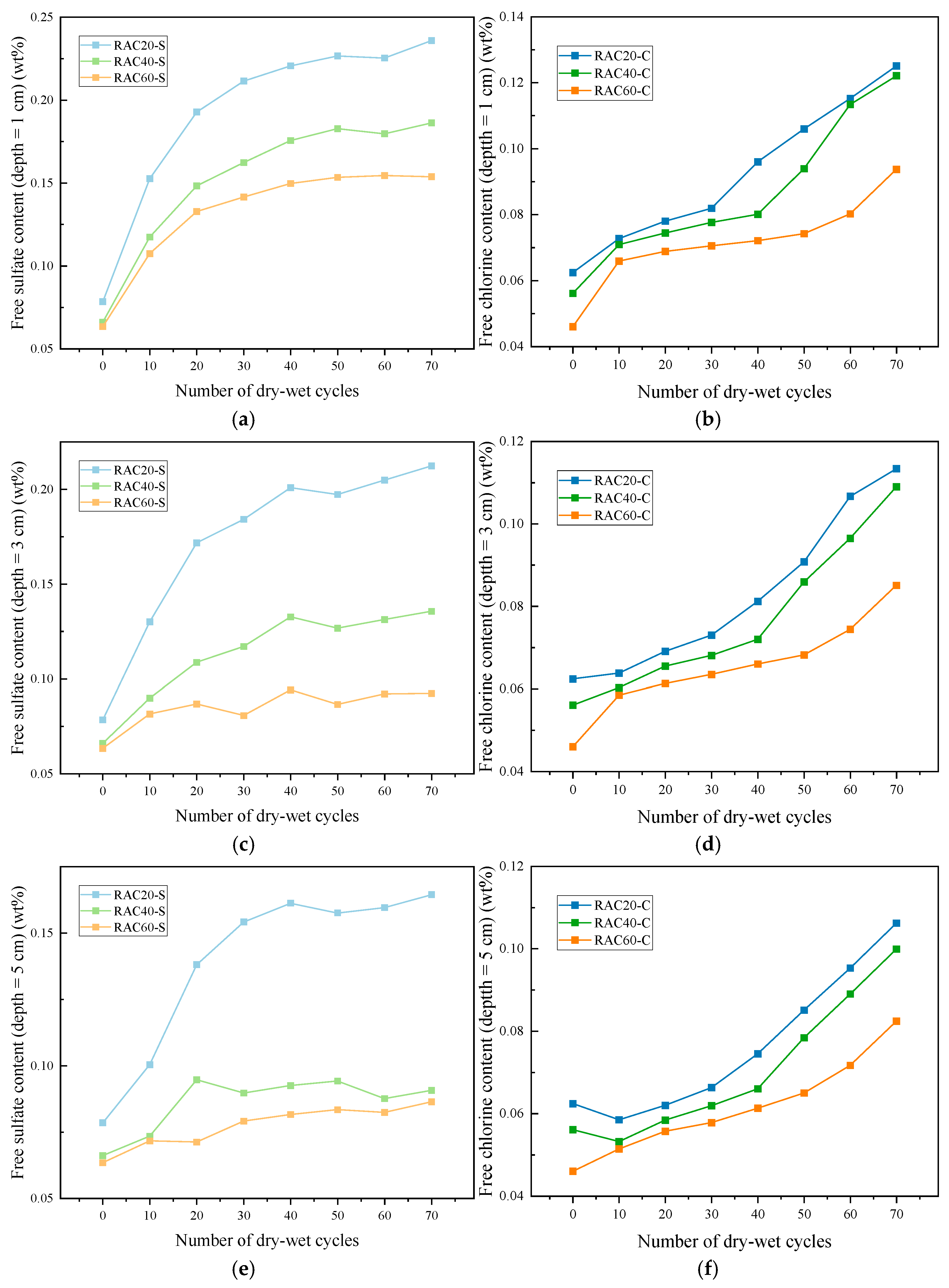
3.3.2. Erosion Content
3.4. Microscopic Properties
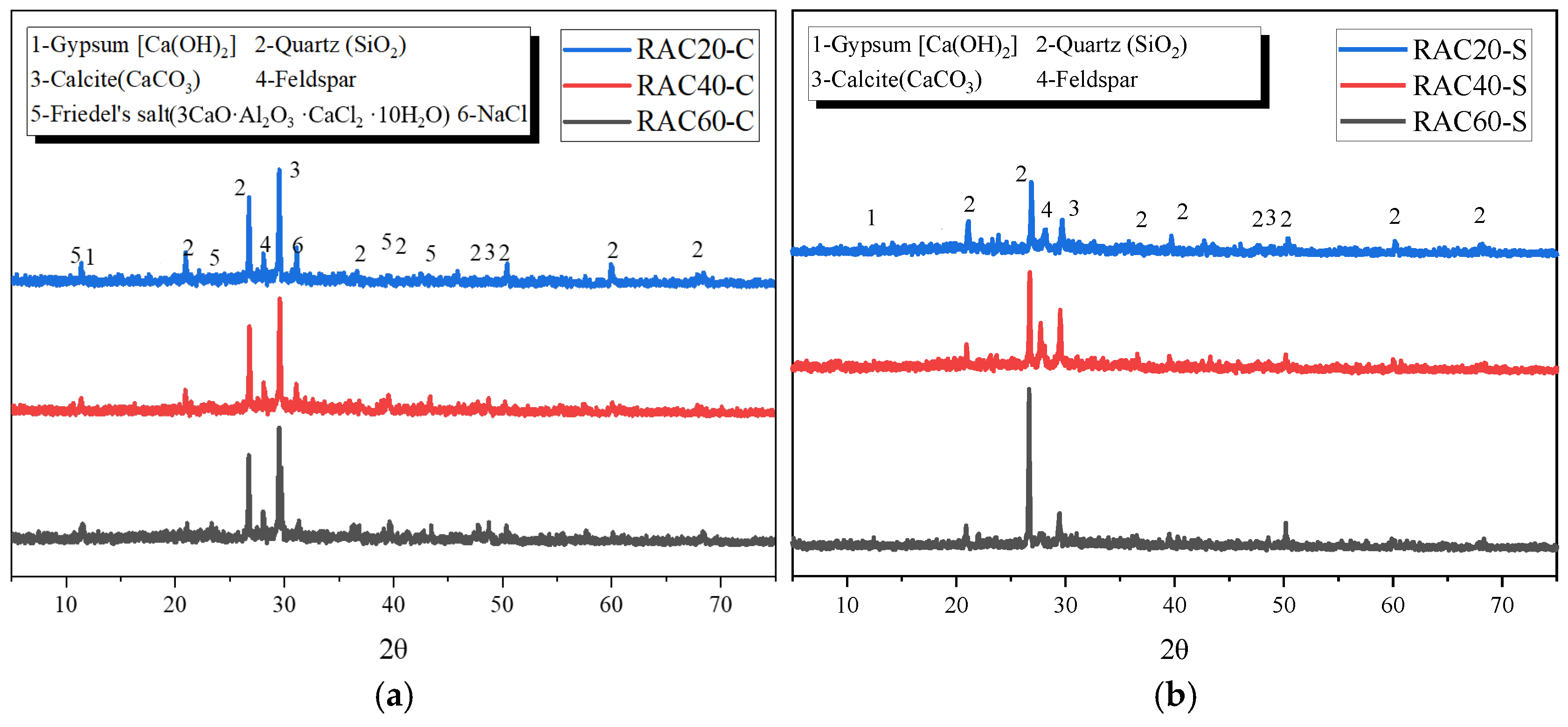
3.4.1. XRD Analysis
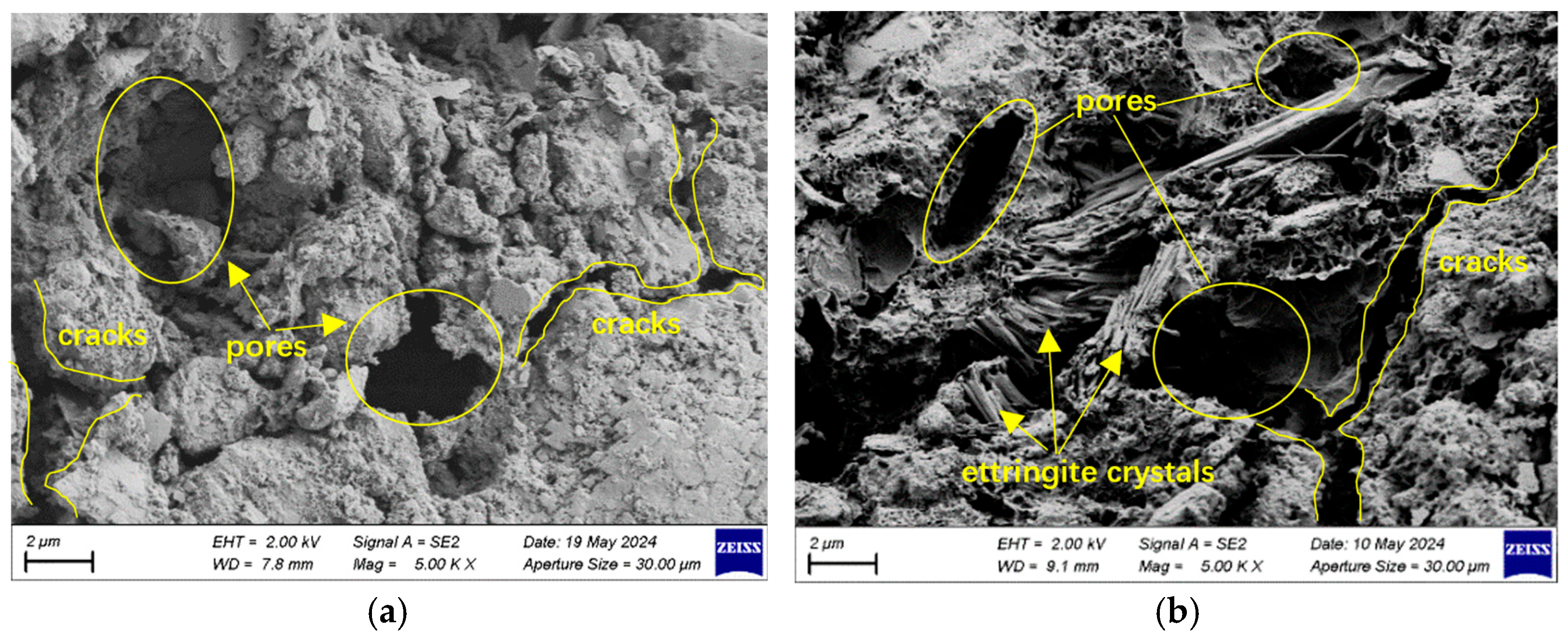
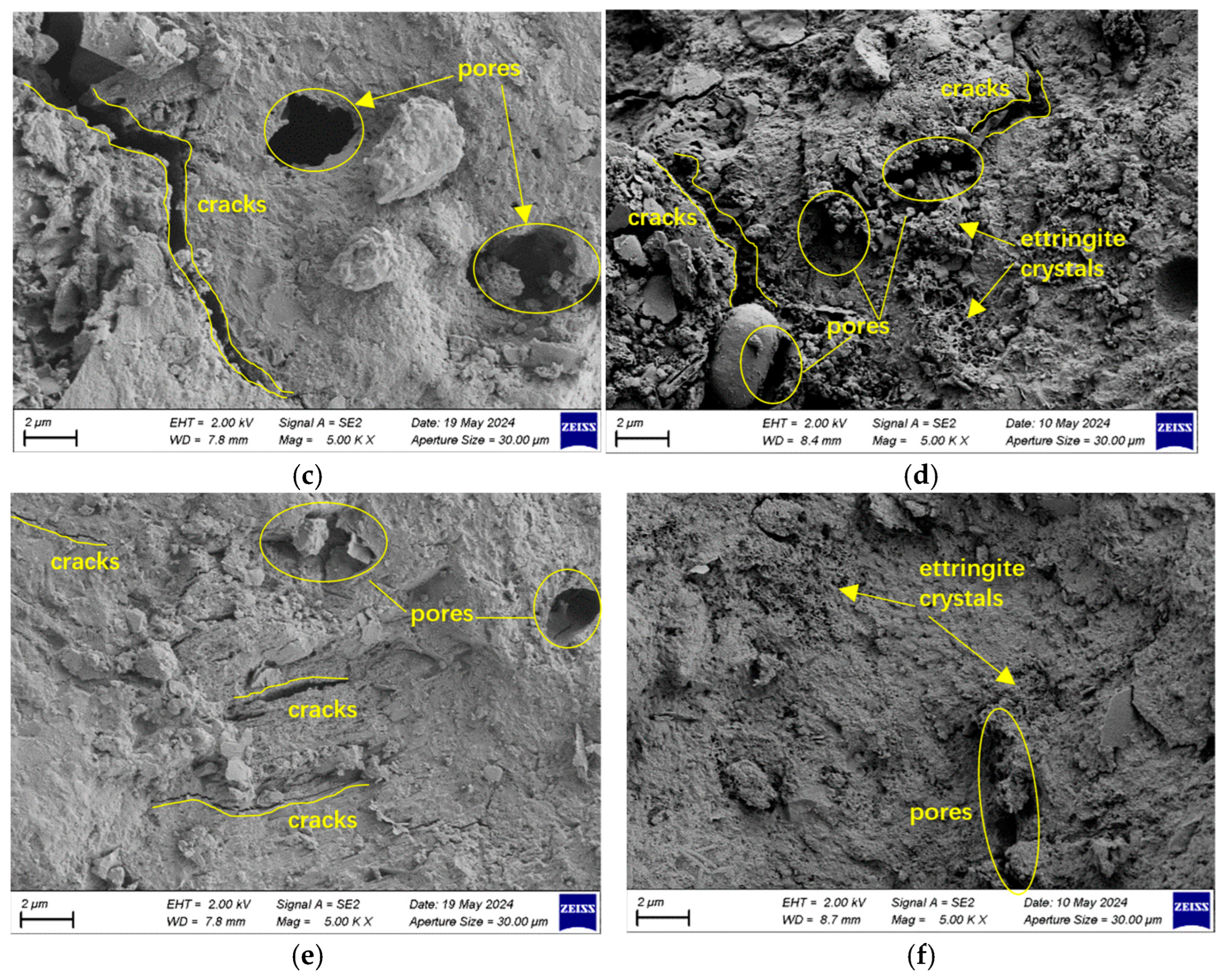
3.4.2. SEM Analysis
4. Conclusions
- (1)
- All RAC groups with three design strengths achieved their target compressive strengths, and a clear negative correlation was observed between open porosity and strength. The open porosities of RAC20, RAC40, and RAC60 were 4.03%, 2.35%, and 1.70%, respectively, indicating that the strength enhancement is closely associated with matrix densification.
- (2)
- Under sulfate exposure, the compressive strength of RAC20 decreased by 60% after 70 cycles, showing severe degradation. RAC40 experienced an 18.0% loss, whereas RAC60 maintained a net gain of +2.5%, demonstrating strong resistance to sulfate attack. In chloride environments, strength reductions were milder: RAC20 lost 30.7%, RAC40 lost 6.9%, and RAC60 still exhibited a +6.6% increase. These results indicate that high-strength matrices effectively resist aggressive ion penetration.
- (3)
- In sulfate environments, the mass of RAC20 initially increased but then sharply declined, with a final loss of 56.8‰, whereas RAC60 consistently retained a net gain of +3.8‰. Similar trends were observed in porosity evolution: the porosity of RAC20 more than doubled to 7.34%, while RAC60’s porosity decreased to 1.16% during the first 40 cycles and only slightly increased to 1.57% thereafter, highlighting its remarkable corrosion resistance. Under chloride exposure, all three groups showed slight increases in mass or porosity, or even densification, with high-strength concrete exhibiting the most pronounced improvement.
- (4)
- After 70 cycles, the chloride and sulfate penetration depths in RAC20 reached 24.85 mm and 32.19 mm, respectively, whereas RAC60 showed significantly lower values of 14.65 mm and 17.84 mm, corresponding to reductions of 41.0% and 44.6%. Ion content measurements revealed that low-strength concretes accumulated significantly more chloride ions and sulfates in the shallow layer (0–30 mm) than high-strength counterparts, indicating that porosity and ITZ densification govern ion diffusion rates and distribution patterns.
- (5)
- SEM and XRD analyses indicate that, under sulfate exposure, the primary degradation mechanism is the expansive formation of ettringite and gypsum, which leads to increased porosity and propagation of microcracks. In contrast, under chloride exposure, degradation is mainly dominated by crystal filling and localized chemical reactions, while the formation of calcium silicate hydrate in high-strength concrete effectively suppresses pore expansion and mitigates deterioration.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Concrete | Compressive Strength /MPa | Flexural Strength /MPa | Splitting Tensile Strength /MPa | Open Porosity /(vol%) |
|---|---|---|---|---|
| RAC20 | 20.2 (1.3) | 2.6 (0.3) | 1.9 (0.4) | 4.03 (0.07) |
| RAC40 | 47.1 (2.1) | 5.9 (0.6) | 3.9 (0.3) | 2.82 (0.14) |
| RAC60 | 62.7 (2.2) | 7.8 (0.7) | 5.1 (0.5) | 1.70 (0.06) |
| Dry–Wet Cycles | 10 | 20 | 30 | 40 | 50 | 60 | 70 |
|---|---|---|---|---|---|---|---|
| RAC20-S | 11.79 (0.9) | 14.15 (1.0) | −6.43 (0.4) | −20.30 (1.3) | −38.81 (2.8) | −50.30 (3.6) | −60.09 (4.5) |
| RAC40-S | 4.88 (0.4) | 9.34 (0.7) | 9.55 (0.6) | 3.40 (0.3) | −2.55 (0.1) | −10.40 (0.9) | −18.05 (1.1) |
| RAC60-S | 4.70 (0.3) | 7.72 (0.4) | 8.89 (0.5) | 10.74 (0.9) | 9.56 (0.4) | 6.88 (0.5) | 2.52 (0.4) |
| RAC20-C | −0.93 (0.2) | −1.40 (0.2) | −2.33 (0.1) | −7.44 (0.5) | −13.95 (1.0) | −23.26 (1.8) | −30.70(2.3) |
| RAC40-C | 0.22 (0.1) | 0.00 (0.1) | −0.43 (0.1) | −1.72 (0.1) | −2.15 (0.2) | −4.30 (0.3) | −6.88 (0.4) |
| RAC60-C | 0.34 (0.2) | 0.51 (0.1) | 2.38 (0.2) | 3.74 (0.2) | 4.75 (0.3) | 5.43 (0.4) | 6.62 (0.4) |
| Dry–Wet Cycles | 10 | 20 | 30 | 40 | 50 | 60 | 70 |
|---|---|---|---|---|---|---|---|
| RAC20-S | 6.71 (0.5) | 10.29 (0.7) | 7.16 (0.6) | 0.89 (0.1) | −4.92 (0.3) | −20.29 (1.5) | −46.82 (3.6) |
| RAC40-S | 3.90 (0.2) | 6.07 (0.4) | 7.81 (0.7) | 7.38 (0.6) | 6.51 (0.5) | 6.94 (0.5) | 1.74 (0.2) |
| RAC60-S | 2.55 (0.2) | 3.83 (0.3) | 4.68 (0.4) | 5.11 (0.4) | 5.96 (0.4) | 4.68 (0.4) | 3.83 (0.3) |
| RAC20-C | −0.13 (0.1) | −0.18 (0.1) | −0.21 (0.1) | −0.04 (0.0) | 0.13 (0.1) | 0.21 (0.1) | 0.35 (0.1) |
| RAC40-C | −0.11 (0.1) | −0.16 (0.1) | −0.19 (0.1) | 0.10 (0.1) | 0.29 (0.1) | 0.37 (0.1) | 0.53 (0.1) |
| RAC60-C | −0.09 (0.0) | −0.14 (0.1) | −0.17 (0.1) | 0.19 (0.1) | 0.38 (0.1) | 0.45 (0.1) | 0.65 (0.2) |
| Dry–Wet Cycles | 10 | 20 | 30 | 40 | 50 | 60 | 70 |
|---|---|---|---|---|---|---|---|
| RAC20-S | 3.43 (0.21) | 3.31 (0.23) | 4.49 (0.32) | 5.44 (0.44) | 6.28 (0.46) | 6.72 (0.42) | 7.34 (0.52) |
| RAC40-S | 2.57 (0.11) | 2.35 (0.16) | 2.33 (0.25) | 2.64 (0.21) | 2.94 (0.18) | 3.33 (0.24) | 3.72 (0.19) |
| RAC60-S | 1.46 (0.12) | 1.31 (0.08) | 1.25 (0.32) | 1.16 (0.08) | 1.22 (0.10) | 1.35 (0.09) | 1.57 (0.12) |
| RAC20-C | 4.08 (0.32) | 4.10 (0.28) | 4.15 (0.12) | 4.40 (0.27) | 4.73 (0.28) | 5.19 (0.42) | 5.56 (0.38) |
| RAC40-C | 2.81 (0.15) | 2.82 (0.16) | 2.84 (0.26) | 2.91 (0.11) | 2.93 (0.18) | 3.03 (0.22) | 3.16 (0.24) |
| RAC60-C | 1.68 (0.10) | 1.67 (0.11) | 1.58 (0.12) | 1.51 (0.08) | 1.46 (0.10) | 1.43 (0.12) | 1.37 (0.09) |
| Dry–Wet Cycles | 10 | 20 | 30 | 40 | 50 | 60 | 70 |
|---|---|---|---|---|---|---|---|
| RAC20-S | 7.23 (0.47) | 10.52 (1.04) | 12.72 (0.89) | 17.09 (1.30) | 19.88 (1.28) | 21.46 (1.26) | 22.10 (1.32) |
| RAC40-S | 2.84 (0.30) | 5.39 (0.73) | 7.82 (0.62) | 10.23 (0.88) | 12.92 (1.03) | 14.35 (1.31) | 17.02 (1.12) |
| RAC60-S | 2.13 (0.21) | 3.78 (0.36) | 5.33 (0.46) | 7.32 (0.81) | 8.74 (0.97) | 9.97 (1.02) | 11.89 (1.06) |
| RAC20-C | 5.97 (0.42) | 10.85 (0.95) | 15.78 (1.24) | 19.08 (1.22) | 25.64 (1.33) | 29.82 (2.04) | 32.19 (1.66) |
| RAC40-C | 4.26 (0.38) | 8.09 (0.67) | 11.73 (1.01) | 15.35 (1.12) | 19.38 (0.96) | 21.53 (1.23) | 25.53 (1.44) |
| RAC60-C | 3.20 (0.31) | 5.67 (0.46) | 8.00 (0.66) | 10.98 (0.91) | 13.11 (0.84) | 14.96 (1.05) | 17.84 (0.85) |
| Cycles | 10 | 20 | 30 | 40 | 50 | 60 | 70 | |
|---|---|---|---|---|---|---|---|---|
| 10 mm | RAC20-S | 0.079 (0.008) | 0.153 (0.011) | 0.193 (0.009) | 0.212 (0.013) | 0.221 (0.007) | 0.227 (0.010) | 0.225 (0.008) |
| RAC40-S | 0.066 (0.006) | 0.117 (0.010) | 0.148 (0.008) | 0.162 (0.010) | 0.176 (0.011) | 0.183 (0.009) | 0.180 (0.009) | |
| RAC60-S | 0.063 (0.006) | 0.107 (0.008) | 0.133 (0.005) | 0.142 (0.009) | 0.150 (0.007) | 0.153 (0.006) | 0.155 (0.010) | |
| RAC20-C | 0.062 (0.005) | 0.073 (0.009) | 0.078 (0.006) | 0.082 (0.006) | 0.096 (0.009) | 0.106 (0.005) | 0.115 (0.009) | |
| RAC40-C | 0.056 (0.006) | 0.071 (0.006) | 0.074 (0.007) | 0.078 (0.007) | 0.080 (0.005) | 0.094 (0.007) | 0.113 (0.006) | |
| RAC60-C | 0.046 (0.005) | 0.066 (0.007) | 0.069 (0.008) | 0.071 (0.005) | 0.072 (0.007) | 0.074 (0.008) | 0.080 (0.005) | |
| 30 mm | RAC20-S | 0.079 (0.005) | 0.130 (0.007) | 0.172 (0.010) | 0.184 (0.007) | 0.201 (0.011) | 0.197 (0.008) | 0.205 (0.009) |
| RAC40-S | 0.066 (0.006) | 0.090 (0.006) | 0.109 (0.005) | 0.117 (0.008) | 0.133 (0.007) | 0.127 (0.011) | 0.131 (0.005) | |
| RAC60-S | 0.063 (0.005) | 0.082 (0.008) | 0.087 (0.007) | 0.081 (0.006) | 0.094 (0.008) | 0.087 (0.007) | 0.092 (0.006) | |
| RAC20-C | 0.062 (0.008) | 0.064 (0.009) | 0.069 (0.009) | 0.073 (0.005) | 0.081 (0.005) | 0.091 (0.005) | 0.107 (0.007) | |
| RAC40-C | 0.056 (0.005) | 0.060 (0.005) | 0.066 (0.005) | 0.068 (0.007) | 0.072 (0.009) | 0.086 (0.006) | 0.097 (0.009) | |
| RAC60-C | 0.046 (0.007) | 0.059 (0.007) | 0.061 (0.008) | 0.064 (0.006) | 0.066 (0.005) | 0.068 (0.008) | 0.074 (0.005) | |
| 50 mm | RAC20-S | 0.079 (0.006) | 0.100 (0.009) | 0.138 (0.008) | 0.154 (0.011) | 0.161 (0.008) | 0.158 (0.012) | 0.160 (0.010) |
| RAC40-S | 0.066 (0.005) | 0.073 (0.007) | 0.095 (0.009) | 0.090 (0.010) | 0.093 (0.009) | 0.094 (0.010) | 0.088 (0.008) | |
| RAC60-S | 0.063 (0.007) | 0.072 (0.005) | 0.071 (0.006) | 0.079 (0.007) | 0.082 (0.007) | 0.083 (0.007) | 0.082 (0.008) | |
| RAC20-C | 0.062 (0.007) | 0.059 (0.007) | 0.062 (0.007) | 0.066 (0.008) | 0.075 (0.006) | 0.085 (0.006) | 0.095 (0.005) | |
| RAC40-C | 0.056 (0.008) | 0.053 (0.006) | 0.058 (0.005) | 0.062 (0.007) | 0.066 (0.005) | 0.078 (0.008) | 0.089 (0.007) | |
| RAC60-C | 0.046 (0.005) | 0.051 (0.006) | 0.056 (0.007) | 0.058 (0.005) | 0.061 (0.006) | 0.065 (0.007) | 0.072 (0.005) |
References
- Zhong, X.; Hu, M.; Deetman, S.; Steubing, B.; Lin, H.X.; Hernandez, G.A.; Harpprecht, C.; Zhang, C.; Tukker, A.; Behrens, P. Global Greenhouse Gas Emissions from Residential and Commercial Building Materials and Mitigation Strategies to 2060. Nat. Commun. 2021, 12, 6126. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, M.; Zhao, D.; Duo, L.; Lu, C. Optimizing the Ecological Network of Resource-Based Cities to Enhance the Resilience of Regional Ecological Networks. Environ. Sci. Pollut. Res. 2024, 31, 17182–17205. [Google Scholar] [CrossRef]
- Kim, J. Construction and Demolition Waste Management in Korea: Recycled Aggregate and Its Application. Clean Technol. Environ. Policy 2021, 23, 2223–2234. [Google Scholar] [CrossRef]
- Hoang, N.H.; Ishigaki, T.; Kubota, R.; Tong, T.K.; Nguyen, T.T.; Nguyen, H.G.; Yamada, M.; Kawamoto, K. Waste Generation, Composition, and Handling in Building-Related Construction and Demolition in Hanoi, Vietnam. Waste Manag. 2020, 117, 32–41. [Google Scholar] [CrossRef]
- Lv, H.; Li, Y.; Yan, H.-B.; Wu, D.; Shi, G.; Xu, Q. Examining Construction Waste Management Policies in Mainland China for Potential Performance Improvements. Clean Technol. Environ. Policy 2021, 23, 445–462. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, C.; Cai, Z.; Zhu, P.; Liu, R.; Liu, H. Effect of Strength of Parent Concrete on Utilization of Recycled Aggregate Concrete: A Review. J. Build. Eng. 2025, 103, 112187. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Y.; Zhang, L.; Zeng, L.; Wang, X.; Dang, J. Influence of Geometric Shape, Pore Structure and Surface Modification of Recycled Fine Aggregate on the Rheology Behaviour and Strength Development of Mortar. J. Build. Eng. 2024, 91, 109604. [Google Scholar] [CrossRef]
- Bao, J.; Wang, Y.; Zhang, H.; Li, S.; Zhang, P.; Qin, L.; Song, Q. Effect of Loading-Induced Damage on Chloride Ingress Behavior of Recycled Aggregate Concrete: A Comprehensive Review. Cem. Concr. Compos. 2023, 141, 105123. [Google Scholar] [CrossRef]
- Guo, H.; Shi, C.; Guan, X.; Zhu, J.; Ding, Y.; Ling, T.-C.; Zhang, H.; Wang, Y. Durability of Recycled Aggregate Concrete–A Review. Cem. Concr. Compos. 2018, 89, 251–259. [Google Scholar] [CrossRef]
- Mao, Y.; Liu, J.; Shi, C. Autogenous Shrinkage and Drying Shrinkage of Recycled Aggregate Concrete: A Review. J. Clean. Prod. 2021, 295, 126435. [Google Scholar] [CrossRef]
- Thomas, C.; De Brito, J.; Cimentada, A.; Sainz-Aja, J.A. Macro- and Micro- Properties of Multi-Recycled Aggregate Concrete. J. Clean. Prod. 2020, 245, 118843. [Google Scholar] [CrossRef]
- Pedro, D.; de Brito, J.; Evangelista, L. Influence of the Use of Recycled Concrete Aggregates from Different Sources on Structural Concrete. Constr. Build. Mater. 2014, 71, 141–151. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, H.; Yan, X.; Yang, L.; Zhu, L.; Liu, H. Recycled Coarse Aggregate from Parent Concrete with Supplementary Cementitious Materials under Freeze-Thaw Environment: Recyclability, Environment and Economic Evaluation. J. Build. Eng. 2024, 84, 108699. [Google Scholar] [CrossRef]
- Silva, R.V.; Brito, J.D.; Neves, S.R.; Dhir, R. Prediction of Chloride Ion Penetration of Recycled Aggregate Concrete. Mater. Res. 2015, 18, 427–440. [Google Scholar] [CrossRef]
- GB/T 14684-2022; Sand for Construction. China Standards Press: Beijing, China, 2022.
- GB/T 14685-2022; Pebble and Crushed Stone for Construction. China Standards Press: Beijing, China, 2022.
- GB/T 25176-2010; Recycled Fine Aggregate for Concrete and Mortar. China Standards Press: Beijing, China, 2010.
- Xuyong, C.; Jiawei, M.; Qiaoyun, W.; Jie, L.; Shukai, C.; Zhuo, L. Study of the Effect of Pretreatment on Coarse Recycled Aggregate in Self-Compacting Concrete: Rheology, Mechanical Properties, and Microstructures. J. Build. Eng. 2025, 101, 111744. [Google Scholar] [CrossRef]
- GB/T 50081-2019; Standard for Test Methods of Concrete Physical and Mechanical Properties. China Architecture and Building Press: Beijing, China, 2019.
- ASTM D1141-98; Standard Practice for Preparation of Substitute Ocean Water. American Society for Testing and Materials: West Conshohocken, PA, USA, 2021.
- Yang, S.; Qin, Y.; Kou, J.; Duan, M.; Zhang, X.; Zhou, H.; Cheng, X. Sulfate Resistance of UHPC during Dry-Wet Cycling and Energy Dissipation under Compression. J. Build. Eng. 2024, 95, 110149. [Google Scholar] [CrossRef]
- Chen, C.; Chen, Y.; He, J.; Zhu, P.; Liu, R.; Wang, X. Chloride Ion-Induced Deterioration in Concrete under Dry-Wet Cycling Using the Air-Drying Method. Constr. Build. Mater. 2024, 451, 138750. [Google Scholar] [CrossRef]
- Chen, C.; Chen, Y.; Cai, Z.; Zhang, X.; Zhu, P.; Liu, R.; Liu, H. Prediction Model for Equivalent Exposure Time in Indoor Dry-Wet Cycling Tests for Concrete in Marine Tidal Zones. Constr. Build. Mater. 2024, 451, 138778. [Google Scholar] [CrossRef]
- Chen, C.; Wang, L.; Liu, R.; Zhu, P.; Liu, H.; Wang, X.; Yu, J.; Chen, Y. Chloride Penetration of Concrete Exposed to Dry-Wet Cycle with Various Dry-Wet Ratios and Temperature. Constr. Build. Mater. 2023, 400, 132883. [Google Scholar] [CrossRef]
- GB/T 50082-2009; Standard for Test Methods of Long-Term Performance and Durability of Ordinary Concrete. China Architecture and Building Press: Beijing, China, 2009.
- ASTM C114-23; Standard Test Methods for Chemical Analysis of Hydraulic Cement. American Society for Testing and Materials: West Conshohocken, PA, USA, 2023.
- Ren, J.; Lai, Y.; Bai, R.; Qin, Y. The Damage Mechanism and Failure Prediction of Concrete under Wetting–Drying Cycles with Sodium Sulfate Solution. Constr. Build. Mater. 2020, 264, 120525. [Google Scholar] [CrossRef]
- Muhammad, F.; Harun, M.; Ahmed, A.; Kabir, N.; Khalid, H.R.; Hanif, A. Influence of Bonded Mortar on Recycled Aggregate Concrete Properties: A Review. Constr. Build. Mater. 2024, 432, 136564. [Google Scholar] [CrossRef]
- Wang, K.; Guo, J.; Wu, H.; Yang, L. Influence of Dry-Wet Ratio on Properties and Microstructure of Concrete under Sulfate Attack. Constr. Build. Mater. 2020, 263, 120635. [Google Scholar] [CrossRef]
- Dao, X.H.; Bui, P.T.; Ogawa, Y.; Kawai, K. Effect of the Strength Grade of Parent Concrete on the Performance of Recycled Aggregate Treated by Cement-Fly Ash Slurry under Prolonged Soaking Duration. Constr. Build. Mater. 2024, 411, 134528. [Google Scholar] [CrossRef]
- Wang, L.; Chen, C.; Liu, R.; Zhu, P.; Liu, H.; Jiang, H.; Yu, J. Chloride Corrosion Process of Concrete with Different Water–Binder Ratios under Variable Temperature Drying–Wetting Cycles. Materials 2024, 17, 2263. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, S.; Li, Z.; Yan, X. Experimental Investigation of the Strain Rate Effect on Crack Initiation and Crack Damage Thresholds of Hard Rock under Quasi-Static Compression. Acta Geotech. 2023, 18, 903–920. [Google Scholar] [CrossRef]
- Wang, X.; Du, G.; Cai, L.; Ren, J.; Wang, W. Effect of Crystallizer Treatment on Chloride Diffusion and Microstructure of Recycled Aggregate Concrete. Constr. Build. Mater. 2022, 321, 126273. [Google Scholar] [CrossRef]
- Wagner, M.; Decker, M.; Kunther, W.; Machner, A.; Beddoe, R.E.; Heisig, A.; Heinz, D. Gypsum Formation Mechanisms and Their Contribution to Crystallisation Pressure in Sulfate Resistant Hardened Cement Pastes during Early External Sulfate Attack at Low Sulfate Concentrations. Cem. Concr. Res. 2023, 168, 107138. [Google Scholar] [CrossRef]
- Zhang, C.; Li, J.; Yu, M.; Lu, Y.; Liu, S. Mechanism and Performance Control Methods of Sulfate Attack on Concrete: A Review. Materials 2024, 17, 4836. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Zhang, Q. Study on the Strength and Chloride Ion Resistance of Sulfate Aluminate Cement-Based Coating Doped with Shell Powder. Constr. Build. Mater. 2025, 485, 141766. [Google Scholar] [CrossRef]
- Li, P.; Wang, X.; Peng, J.; Li, D. Chloride Penetration and Material Characterisation of Carbonated Concrete under Various Simulated Marine Environment Exposure Conditions. Constr. Build. Mater. 2024, 429, 135885. [Google Scholar] [CrossRef]
- Long, W.-J.; Zhang, X.; Feng, G.-L.; Xie, J.; Xing, F.; Dong, B.; Zhang, J.; Khayat, K.H. Investigation on Chloride Binding Capacity and Stability of Friedel’s Salt in Graphene Oxide Reinforced Cement Paste. Cem. Concr. Compos. 2022, 132, 104603. [Google Scholar] [CrossRef]
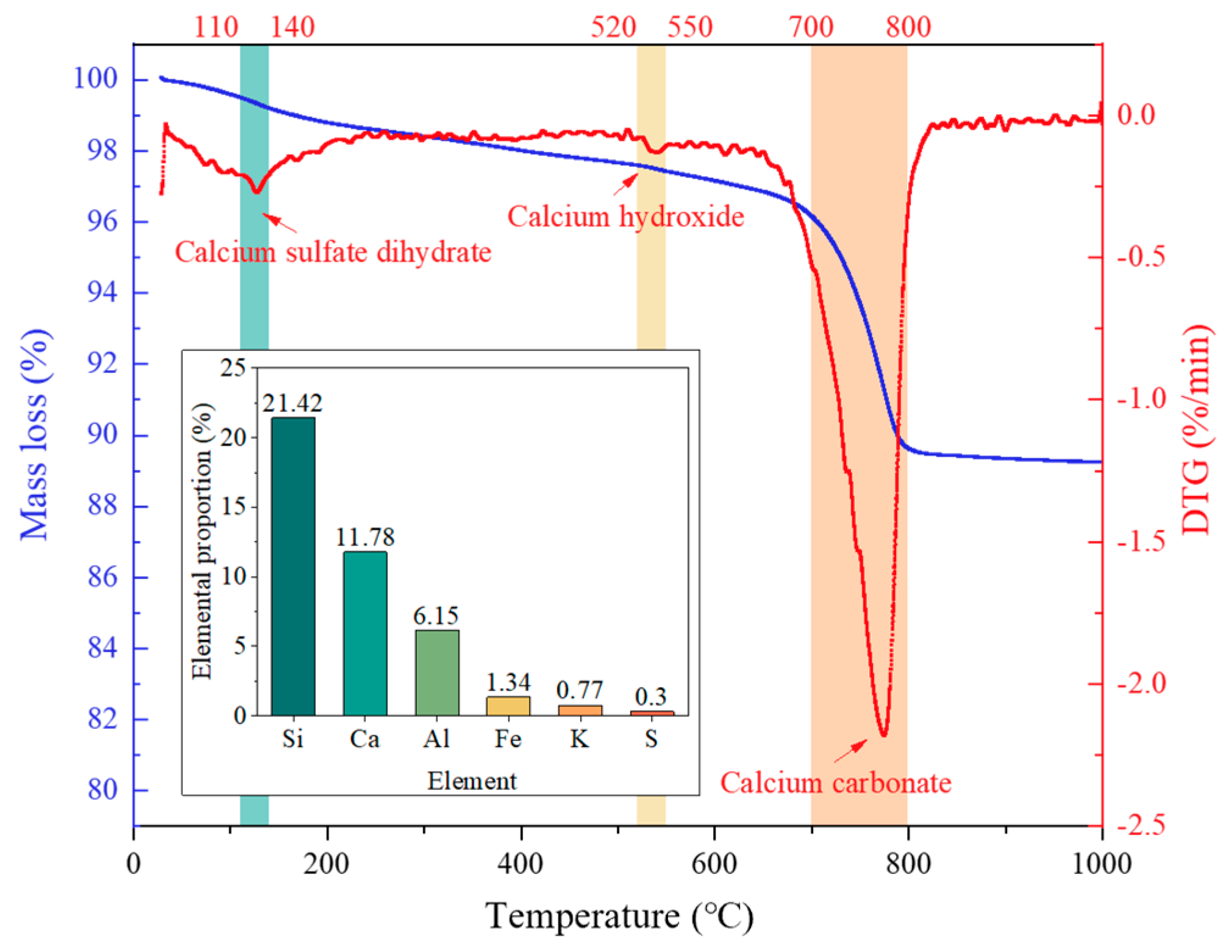


| P.O 32.5 Cement | P.O 42.5 Cement | Fly Ash | Silica Fume | ||
|---|---|---|---|---|---|
| Elemental proportion (wt%) | CaO | 60.19 | 59.22 | 3.81 | 0.85 |
| SiO2 | 19.21 | 18.55 | 52.53 | 88.24 | |
| Al2O3 | 8.55 | 9.02 | 28.33 | 0.77 | |
| Fe2O3 | 3.68 | 4.55 | 3.7 | 0.85 | |
| MgO | 1.27 | 1.36 | 1.16 | 0.94 | |
| MnO | 0.14 | 0.12 | 0.21 | <0.01 | |
| K2O | 0.71 | 0.63 | 1.70 | 1.28 | |
| TiO2 | 0.24 | 0.20 | 0.94 | <0.01 | |
| SO3 | 2.08 | 2.03 | 1.88 | <0.01 | |
| Cl | 0.04 | 0.03 | <0.01 | <0.01 | |
| Loss on ignition (wt%) | 3.20 | 2.57 | 1.83 | 1.79 | |
| Specific surface area (m2/kg) | 380 | 369 | 430 | 23,000 | |
| Apparent density (kg/m3) | 3060 | 3152 | 2524 | 2721 | |
| Bulk density (kg/m3) | 1400 | 1447 | 1120 | 270 | |
| Apparent Density (kg/m3) | Water Absorption (wt%) | Crushing Index (wt%) | |
|---|---|---|---|
| Natural coarse aggregate | 2687 | 0.4 | 4.3 |
| Recycled fine aggregate | 2394 | 8.8 | 25.6 |
| Component | RAC20 | RAC40 | RAC60 |
|---|---|---|---|
| Natural coarse aggregate | 1017 | 1081 | 1180 |
| Recycled fine aggregate | 649 | 591 | 503 |
| Cement | 421 | 429 | 370 |
| Fly ash | 0 | 50 | 114 |
| Silica fume | 0 | 25 | 85 |
| Water | 152 | 134 | 102 |
| Superplasticizer | 0.63 | 0.75 | 0.85 |
| Specimens | Specimen Purpose | |||
|---|---|---|---|---|
| RAC20 | RAC40 | RAC60 | Total | |
| 6 | 6 | 6 | 18 | Compressive strength at 7 and 28 days of curing. |
| 36 | 36 | 36 | 108 | Compressive strength, free chloride ion content, and chloride penetration depth during chloride salt dry–wet cycles. |
| 3 | 3 | 3 | 9 | Open porosity variation and mass change during chloride salt dry–wet cycles. |
| 36 | 36 | 36 | 108 | Compressive strength, free sulfate ion content, and sulfate penetration depth during sulfate dry–wet cycles. |
| 3 | 3 | 3 | 9 | Open porosity variation and mass change during sulfate dry–wet cycles. |
| 36 | 36 | 36 | 108 | Compressive strength, free chloride and sulfate ion content, and chloride and sulfate penetration depth during compound salt dry–wet cycles. |
| 3 | 3 | 3 | 9 | Open porosity variation and mass change during compound salt dry–wet cycles. |
| 4 | 4 | 4 | 16 | For backup. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Alimatu Adama, K.; Liu, R.; Chen, Y.; Zhang, X.; Liu, H. Mechanisms of Durability Degradation in Recycled Fine Aggregate Concrete of Varying Strengths Induced by Chloride and Sulfate Dry–Wet Cycles. Materials 2025, 18, 4985. https://doi.org/10.3390/ma18214985
Chen C, Alimatu Adama K, Liu R, Chen Y, Zhang X, Liu H. Mechanisms of Durability Degradation in Recycled Fine Aggregate Concrete of Varying Strengths Induced by Chloride and Sulfate Dry–Wet Cycles. Materials. 2025; 18(21):4985. https://doi.org/10.3390/ma18214985
Chicago/Turabian StyleChen, Chunhong, Kamara Alimatu Adama, Ronggui Liu, Yunchun Chen, Xiaolin Zhang, and Hui Liu. 2025. "Mechanisms of Durability Degradation in Recycled Fine Aggregate Concrete of Varying Strengths Induced by Chloride and Sulfate Dry–Wet Cycles" Materials 18, no. 21: 4985. https://doi.org/10.3390/ma18214985
APA StyleChen, C., Alimatu Adama, K., Liu, R., Chen, Y., Zhang, X., & Liu, H. (2025). Mechanisms of Durability Degradation in Recycled Fine Aggregate Concrete of Varying Strengths Induced by Chloride and Sulfate Dry–Wet Cycles. Materials, 18(21), 4985. https://doi.org/10.3390/ma18214985








