Rational Design of Amino Acid-Modified Halide Perovskites for Highly Efficient and Cost-Effective Light-Emitting Diodes
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis Process of FAPbBr3 QDs
2.2. Purification
2.3. Fabricating Process of PeLED Device
3. Results
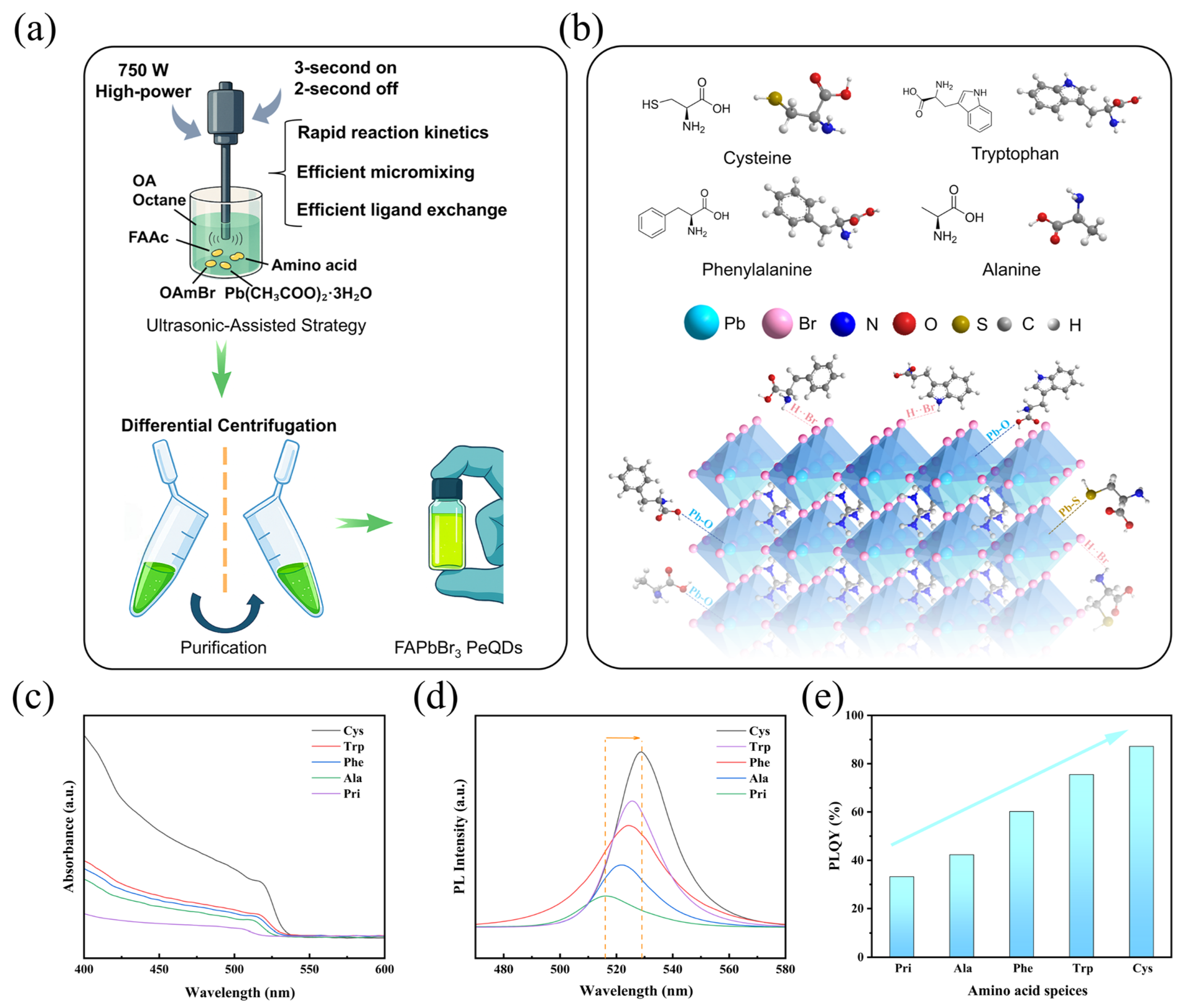
3.1. Optical Properties of Amino Acid Ligand-Doped Materials
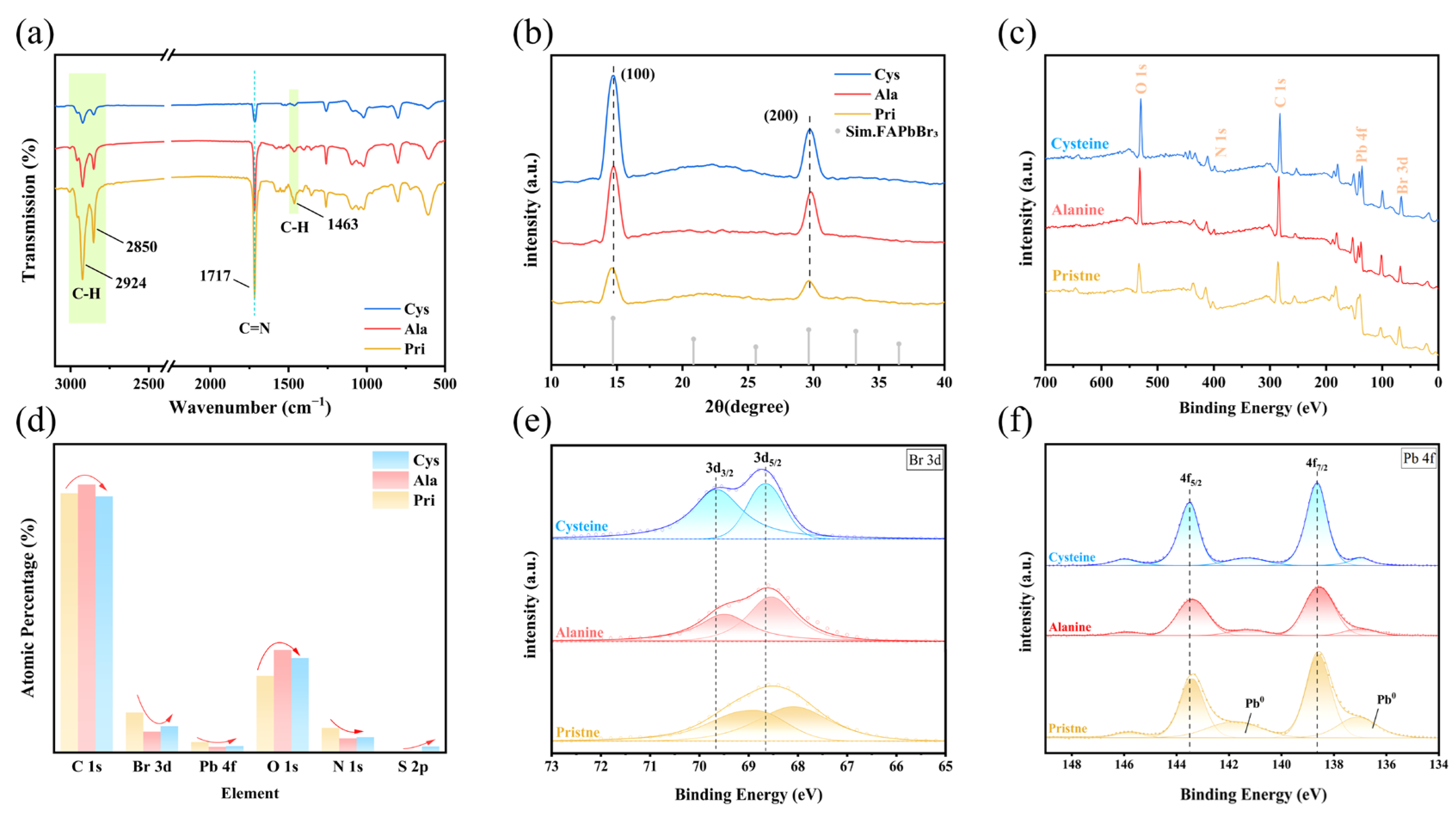
3.2. Research on the Topological Structure of Amino Acid Ligand Doping
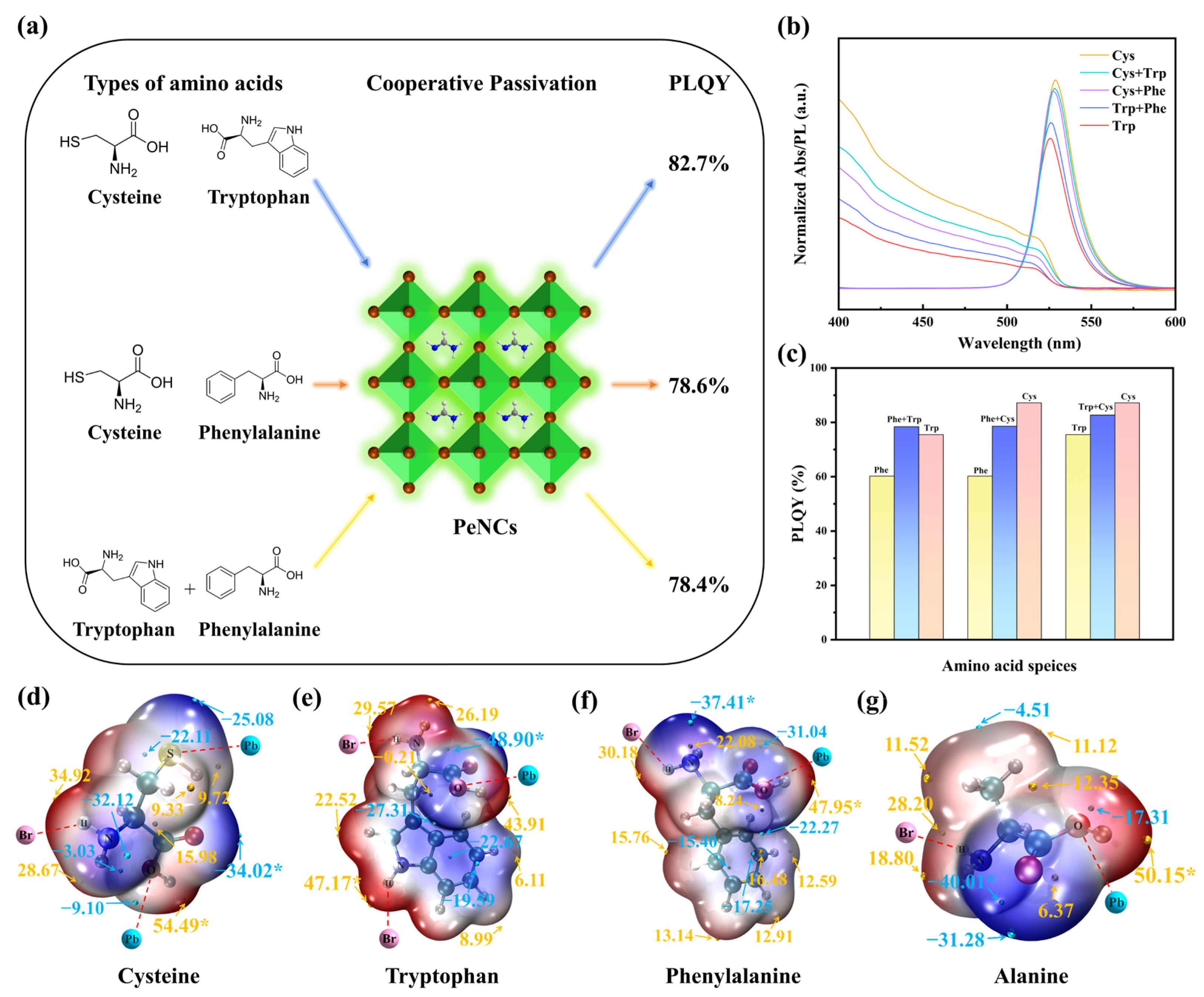
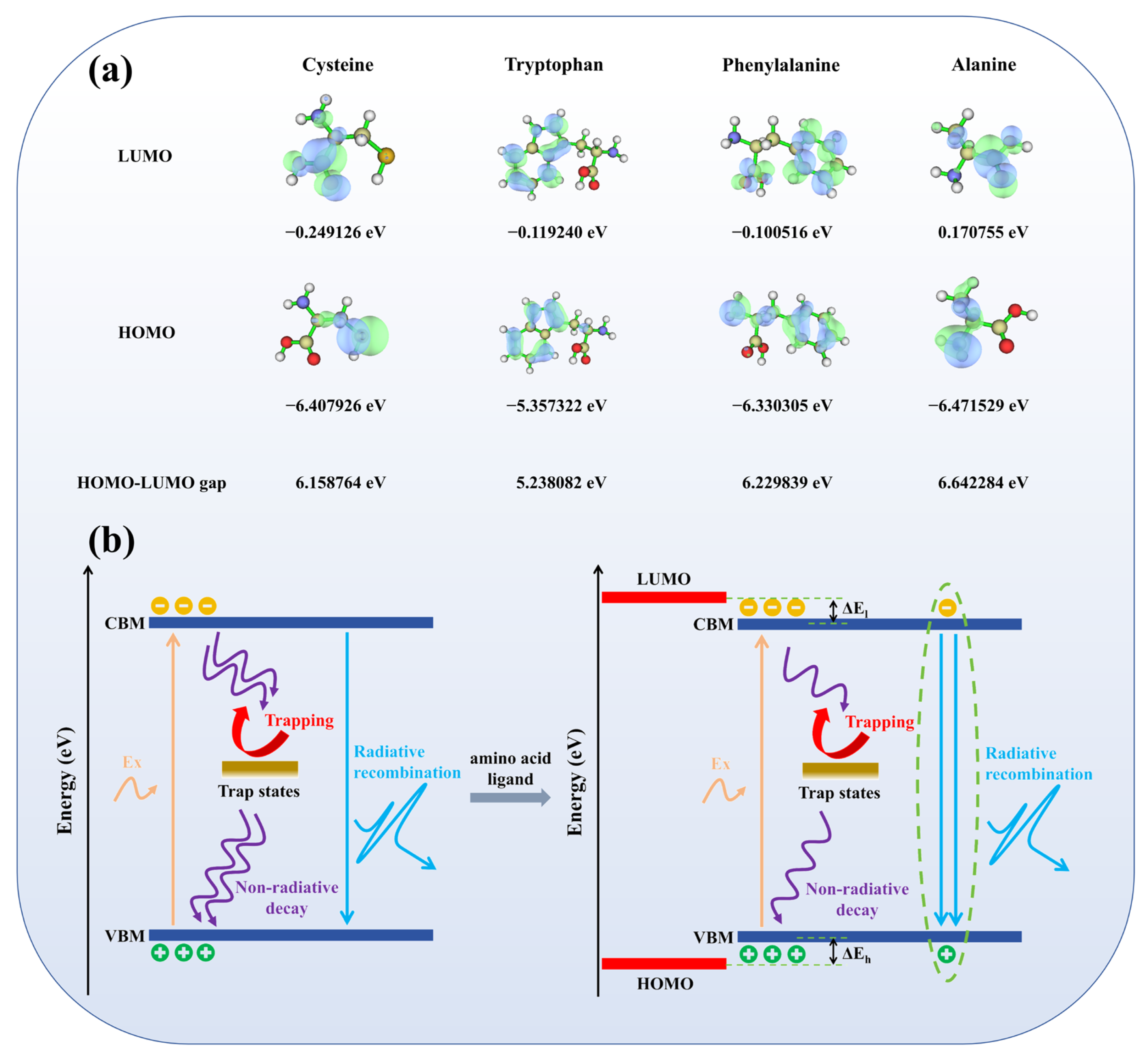
3.3. Multiligand Studies and Electronic Structure Research
3.4. Perovskite Light-Emitting Diodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ou, Q.; Bao, X.; Zhang, Y.; Shao, H.; Xing, G.; Li, X.; Shao, L.; Bao, Q. Band structure engineering in metal halide perovskite nanostructures for optoelectronic applications. Nano Mater. Sci. 2019, 1, 268–287. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, D.; Zhou, X.; Lian, L.; Shen, C.; Su, C.; Fang, S.; Liang, X.; Yuan, F.; Hou, L.; et al. In-Situ Surface Repair of FAPbBr3 Quantum Dots toward High-Performance Pure-Green Perovskite Light-Emitting Diodes. Nano Lett. 2024, 24, 12196–12203. [Google Scholar] [CrossRef]
- Shooshtari, M.; Kim, S.; Pahlavan, S.; Rivera-Sierra, G.; Través, M.J.; Serrano-Gotarredona, T.; Bisquert, J.; Linares-Barranco, B. Advancing Logic Circuits with Halide Perovskite Memristors for Next-Generation Digital Systems. SmartMat 2025, 6, e70032. [Google Scholar] [CrossRef]
- Tian, C.; Liu, D.; Dong, Y.; Wang, Y.; Yang, T.; Yang, Y.; Zhang, M.; Zhao, E.; Wu, N.; Zhang, Z.; et al. Multifunctional Organic Molecule for Defect Passivation of Perovskite for High-Performance Indoor Solar Cells. Materials 2025, 18, 179. [Google Scholar] [CrossRef]
- Bhatia, H.; Ghosh, B.; Debroye, E. Colloidal FAPbBr3 perovskite nanocrystals for light emission: What’s going on? J. Mater. Chem. C 2022, 10, 13437–13461. [Google Scholar] [CrossRef]
- Mao, T.; Xu, X.; Winkler, P.M.; Siri, C.; Poliukhina, E.; Silva, P.J.; Xu, N.; Hu, Y.; Al Zahabi, K.; La Polla, R.; et al. Stabilizing effect of amino acids on protein and colloidal dispersions. Nature 2025, 645, 915–921. [Google Scholar] [CrossRef]
- Yu, L.; Feng, L.; Wei, Z.; Wang, S.; Feng, Y.; Shen, Y.; Cai, J.; Wu, J.; Xiao, Y. Tunable Fluorescent Artificial Receptor Biosensor Based on Programmable Lanthanide Metal-Organic Framework for Highly Selective Neurotransmitter Detection. Adv. Funct. Mater. 2023, 33, 2300309. [Google Scholar] [CrossRef]
- Park, S.J.; Yu, C.; Shim, K.I.; Hong, G.P.; Song, S.; Park, J.H.; Hwang, S.K.; Choi, Y.J.; Han, J.W.; Kwon, M.S.; et al. Tailored self-assembled monolayer molecules for perovskite/PERC tandem solar cells with efficiencies over 30%. Energy Environ. Sci. 2025, 18, 9105–9113. [Google Scholar] [CrossRef]
- Corrie, A.M.; Touche, M.L.D.; Williams, D.R. Thermodynamic considerations in coordination. Part XIV. Formation constants for lead(II)–amino-acid complexes and their use in computing the complexing competition between lead(II) and in vivo essential metal ions, and in computer evaluation of ligands currently employed as lead(II) chelating therapeuticals. J. Chem. Soc. Dalton Trans. 1973, 23, 2561–2565. [Google Scholar] [CrossRef]
- Fiuza-Maneiro, N.; Sun, K.; López-Fernández, I.; Gómez-Graña, S.; Müller-Buschbaum, P.; Polavarapu, L. Ligand Chemistry of Inorganic Lead Halide Perovskite Nanocrystals. ACS Energy Lett. 2023, 8, 1152–1191. [Google Scholar] [CrossRef]
- Liu, L.; Najar, A.; Wang, K.; Du, M.; Liu, S. Perovskite Quantum Dots in Solar Cells. Adv. Sci. 2022, 9, 2104577. [Google Scholar] [CrossRef]
- Haydous, F.; Gardner, J.M.; Cappel, U.B. The impact of ligands on the synthesis and application of metal halide perovskite nanocrystals. J. Mater. Chem. A 2021, 9, 23419–23443. [Google Scholar] [CrossRef]
- Dai, J.; Xi, J.; Li, L.; Zhao, J.; Shi, Y.; Zhang, W.; Ran, C.; Jiao, B.; Hou, X.; Duan, X.; et al. Charge Transport Between Coupling Colloidal Perovskite Quantum Dots Assisted by Functional Conjugated Ligands. Angew. Chem. Int. Ed. 2018, 57, 5754–5758. [Google Scholar] [CrossRef]
- Li, Y.; Deng, M.; Zhang, X.; Qian, L.; Xiang, C. Proton-Prompted Ligand Exchange to Achieve High-Efficiency CsPbI3 Quantum Dot Light-Emitting Diodes. Nano-Micro Lett. 2024, 16, 105. [Google Scholar] [CrossRef]
- Deng, C.; Huang, Q.; Fu, Z.; Lu, Y. Ligand Engineering of Inorganic Lead Halide Perovskite Quantum Dots Toward High and Stable Photoluminescence. Nanomaterials 2024, 14, 1201. [Google Scholar] [CrossRef]
- Lee, A.; Kim, J.; Lee, D.; Song, M.H. Efficient and Stable Perovskite Nanocrystal Light-Emitting Diodes with Sulfobetaine-Based Ligand Treatment. ACS Appl. Electron. Mater. 2023, 5, 5325–5331. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, J.; Zhao, G.; Wang, G.; Liu, P.; Li, H.; Hou, X.; Qiang, P.; Yang, Y.; Su, Q.; et al. Efficient blue CsPbBr3 perovskite nanocrystals synthesis with the assistance of zwitterionic straight chain amino acids. Colloids Surf. A Physicochem. Eng. Asp. 2023, 673, 131793. [Google Scholar] [CrossRef]
- Morad, V.; Stelmakh, A.; Svyrydenko, M.; Feld, L.G.; Boehme, S.C.; Aebli, M.; Affolter, J.; Kaul, C.J.; Schrenker, N.J.; Bals, S.; et al. Designer phospholipid capping ligands for soft metal halide nanocrystals. Nature 2024, 626, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Zhao, Q.; Wei, P.; Li, G. Surface Ligands for Perovskite Quantum Dots. ChemSusChem 2025, 18, e202401875. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Zhang, W.; Chu, Y. Hybrid Amino Acid Ligand-Regulated Excited Dynamics of Highly Luminescent Perovskite Quantum Dots for Bright White Light-Emitting Diodes. Nanomaterials 2024, 14, 1266. [Google Scholar] [CrossRef]
- Song, H.; Yang, J.; Lim, S.G.; Lee, J.; Jeong, W.H.; Choi, H.; Lee, J.H.; Kim, H.Y.; Lee, B.R.; Choi, H. On the surface passivating principle of functional thiol towards efficient and stable perovskite nanocrystal solar cells. Chem. Eng. J. 2023, 454, 140224. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, B.; Zhou, X.; Yuan, F.; Yin, C.; Wang, H.; Chen, H.; Ji, X.; Liang, X.; Shen, C.; et al. Ligand-Induced Cation–π Interactions Enable High-Efficiency, Bright, and Spectrally Stable Rec. 2020 Pure-Red Perovskite Light-Emitting Diodes. Adv. Mater. 2023, 35, 2303938. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Wang, S.; Zhang, C.; Luo, H.; Li, X.; Wang, H.; Cao, F.; Shen, P.; Zhang, J.; Yang, X. Ten-Gram-Scale Synthesis of FAPbX3 Perovskite Nanocrystals by a High-Power Room-Temperature Ultrasonic-Assisted Strategy and Their Electroluminescence. Adv. Mater. Technol. 2020, 5, 1901089. [Google Scholar] [CrossRef]
- Yang, M.; Tian, T.; Feng, W.; Wang, L.; Wu, W.-Q. Custom Molecular Design of Ligands for Perovskite Photovoltaics. Acc. Mater. Res. 2021, 2, 1141–1155. [Google Scholar] [CrossRef]
- Feng, W.; Tan, Y.; Yang, M.; Jiang, Y.; Lei, B.-X.; Wang, L.; Wu, W.-Q. Small amines bring big benefits to perovskite-based solar cells and light-emitting diodes. Chem 2022, 8, 351–383. [Google Scholar] [CrossRef]
- Cheng, H.; Ding, S.; Hao, M.; Wang, L.; Steele, J.A. Perovskite quantum dots: What’s next? Next Energy 2024, 4, 100152. [Google Scholar] [CrossRef]
- Chen, S.; Wei, J.; Pang, Q. Enhancing Photoluminescence and Stability of CsPbI3 Perovskite Quantum Dots via Cysteine Post-Processing. Crystals 2022, 13, 45. [Google Scholar] [CrossRef]
- Chen, D.; Ko, P.K.; Li, C.-H.A.; Zou, B.; Geng, P.; Guo, L.; Halpert, J.E. Amino Acid-Passivated Pure Red CsPbI3 Quantum Dot LEDs. ACS Energy Lett. 2023, 8, 410–416. [Google Scholar] [CrossRef]
- Chang, Y.; Tong, G.; Liu, L.; Li, J.; Yang, J.; Chen, Z.; Li, Z.; Zhang, S.; Zhou, R.; Jiang, Y. Synergistic ligand mediated anion exchange of CsPbI3 quantum dots for high performance white LED and anti-counterfeiting. EcoMat 2024, 6, e12439. [Google Scholar] [CrossRef]
- Vásquez-Montoya, M.; Montoya, J.F.; Ramirez, D.; Jaramillo, F. Understanding the precursor chemistry for one-step deposition of mixed cation perovskite solar cells by methylamine route. J. Energy Chem. 2021, 57, 386–391. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, K.; Qian, J.; Huang, Y.; Wang, X.; Ge, M.; Shen, W.; Huang, F.; Wang, M.; Zhang, W.; et al. Crystallization control and multisite passivation of perovskites with amino acid to boost the efficiency and stability of perovskite solar cells. J. Mater. Chem. C 2020, 8, 17482–17490. [Google Scholar] [CrossRef]
- Huang, Q.; Yin, W.; Gao, B.; Zeng, Q.; Yao, D.; Zhang, H.; Zhao, Y.; Zheng, W.; Zhang, J.; Yang, X.; et al. Enhancing crystal integrity and structural rigidity of CsPbBr3 nanoplatelets to achieve a narrow color-saturated blue emission. Light. Sci. Appl. 2024, 13, 111. [Google Scholar] [CrossRef]
- Zhang, W.; Pathak, S.; Sakai, N.; Stergiopoulos, T.; Nayak, P.K.; Noel, N.K.; Haghighirad, A.A.; Burlakov, V.M.; deQuilettes, D.W.; Sadhanala, A.; et al. Enhanced optoelectronic quality of perovskite thin films with hypophosphorous acid for planar heterojunction solar cells. Nat. Commun. 2015, 6, 10030. [Google Scholar] [CrossRef]
- Liu, Y.-P.; Lamers, N.; Zhang, Z.; Zaiats, N.; Mikkelsen, A.; Wallentin, J.; Dittmann, R.; Timm, R. Ion Migration and Redox Reactions in Axial Heterojunction Perovskite CsPb(Br1–xClx)3 Nanowire Devices Revealed by Operando Nanofocused X-ray Photoelectron Spectroscopy. ACS Nano 2024, 18, 34763–34775. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, Y.; Zhang, Y.; Han, X.; Xiao, K.; Nabahat, M.; Arbiol, J.; Llorca, J.; Ibañez, M.; Cabot, A. PbS–Pb–CuxS Composites for Thermoelectric Application. ACS Appl. Mater. Interfaces 2021, 13, 51373–51382. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chen, H.; Grater, L.; Liu, C.; Yang, Y.; Teale, S.; Maxwell, A.; Mahesh, S.; Wan, H.; Chang, Y.; et al. Anion optimization for bifunctional surface passivation in perovskite solar cells. Nat. Mater. 2023, 22, 1507–1514. [Google Scholar] [CrossRef]
- Liu, J.; He, J.; Ma, D.; He, J.; Wu, W. Chiral Molecular Environment Determining Selective Coordination of Cysteine to Perovskite Halogen Vacancies. Sol. RRL 2023, 7, 2200935. [Google Scholar] [CrossRef]
- Allen, O.J.; Kang, J.; Qian, S.; Hinsch, J.J.; Zhang, L.; Wang, Y. A theoretical review of passivation technologies in perovskite solar cells. Energy Mater. 2024, 4, 400037. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, J.; Liu, Z.; Li, A.; Miyasaka, T.; Wang, X. Zwitterion Dual-Modification Strategy for High-Quality NiOx and Perovskite Films for Solar Cells. Small 2024, 20, 2400356. [Google Scholar] [CrossRef]
- Ma, Y.; Gong, J.; Zeng, P.; Liu, M. Recent Progress in Interfacial Dipole Engineering for Perovskite Solar Cells. Nano-Micro Lett. 2023, 15, 173. [Google Scholar] [CrossRef]
- Trinh, C.T.; Minh, D.N.; Nguyen, V.L.; Ahn, K.J.; Kang, Y.; Lee, K.-G. An experimental study on the blinking suppression mechanism of organic-inorganic formamidinium lead halide perovskite quantum dots on N-Type semiconductors. APL Mater. 2020, 8, 031102. [Google Scholar] [CrossRef]
- Guo, S.; Li, Y.; Mao, Y.; Tao, W.; Bu, K.; Fu, T.; Zhao, C.; Luo, H.; Hu, Q.; Zhu, H.; et al. Reconfiguring band-edge states and charge distribution of organic semiconductor–incorporated 2D perovskites via pressure gating. Sci. Adv. 2022, 8, eadd1984. [Google Scholar] [CrossRef]
- García-Fernández, A.; Kammlander, B.; Riva, S.; Kühn, D.; Svanström, S.; Rensmo, H.; Cappel, U.B. Interface Energy Alignment between Lead Halide Perovskite Single Crystals and TIPS-Pentacene. Inorg. Chem. 2023, 62, 15412–15420. [Google Scholar] [CrossRef]
- Xiong, S.; Li, D.; Xie, J.; Wu, H.; Ma, Z.; Li, B.; Liu, X.; Fahlman, M.; Chu, J.; Pan, C.; et al. Disentangling perovskite surface work functions and electron extraction energy offsets to drive high photovoltaic efficiency. Sci. Bull. 2025, 70, 1968–1975. [Google Scholar] [CrossRef]
- Mei, X.; Ren, B.; Qiu, J.; Sun, Z.; Zhang, X. Complementary Dual-Ligands Resurfacing CsPbI3 Perovskite Quantum Dots for High-Performance Solar Cells. Small 2025, 21, 2504748. [Google Scholar] [CrossRef]
- Shi, J.; Li, F.; Jin, Y.; Liu, C.; Cohen-Kleinstein, B.; Yuan, S.; Li, Y.; Wang, Z.; Yuan, J.; Ma, W. In Situ Ligand Bonding Management of CsPbI3 Perovskite Quantum Dots Enables High-Performance Photovoltaics and Red Light-Emitting Diodes. Angew. Chem. Int. Ed. 2020, 59, 22230–22237. [Google Scholar] [CrossRef]
- Han, S.; Jeong, W.H.; Seo, G.; Choi, S.; Lee, D.G.; Chae, W.; Ahn, H.; Lee, T.K.; Choi, H.; Choi, J.; et al. Synergistic Hybrid-Ligand Passivation of Perovskite Quantum Dots: Suppressing Reduced-Dimensionality and Enhancing Optoelectronic Performance. Adv. Mater. 2025, 37, 2410128. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Meng, Y.; Yang, Z.; Ye, Y.; Lin, Q.; Meng, Z.; Hong, H.; Ye, S.; Cheng, Z.; Lan, Q.; et al. Efficient CsPbBr3 Perovskite Light-Emitting Diodes via Novel Multi-Step Ligand Exchange Strategy Based on Zwitterionic Molecules. ACS Appl. Mater. Interfaces 2024, 16, 10389–10397. [Google Scholar] [CrossRef]
- Bae, J.; Lee, S.; Ahn, J.; Kim, J.H.; Wajahat, M.; Chang, W.S.; Yoon, S.-Y.; Kim, J.T.; Seol, S.K.; Pyo, J. 3D-Printed Quantum Dot Nanopixels. ACS Nano 2020, 14, 10993–11001. [Google Scholar] [CrossRef]
- Deng, Y.; Lin, X.; Fang, W.; Di, D.; Wang, L.; Friend, R.H.; Peng, X.; Jin, Y. Deciphering exciton-generation processes in quantum-dot electroluminescence. Nat. Commun. 2020, 11, 2309. [Google Scholar] [CrossRef] [PubMed]
- Yassitepe, E.; Yang, Z.; Voznyy, O.; Kim, Y.; Walters, G.; Castañeda, J.A.; Kanjanaboos, P.; Yuan, M.; Gong, X.; Fan, F.; et al. Amine-Free Synthesis of Cesium Lead Halide Perovskite Quantum Dots for Efficient Light-Emitting Diodes. Adv. Funct. Mater. 2016, 26, 8757–8763. [Google Scholar] [CrossRef]
- Sun, C.; Jiang, Y.; Cui, M.; Qiao, L.; Wei, J.; Huang, Y.; Zhang, L.; He, T.; Li, S.; Hsu, H.-Y.; et al. High-performance large-area quasi-2D perovskite light-emitting diodes. Nat. Commun. 2021, 12, 2207. [Google Scholar] [CrossRef] [PubMed]
- Dyrvik, E.G.; Warby, J.H.; McCarthy, M.M.; Ramadan, A.J.; Zaininger, K.-A.; Lauritzen, A.E.; Mahesh, S.; Taylor, R.A.; Snaith, H.J. Reducing Nonradiative Losses in Perovskite LEDs through Atomic Layer Deposition of Al2O3 on the Hole-Injection Contact. ACS Nano 2023, 17, 3289–3300. [Google Scholar] [CrossRef] [PubMed]
- Elimelech, O.; Oded, M.; Harries, D.; Banin, U. Spontaneous Patterning of Binary Ligand Mixtures on CdSe Nanocrystals: From Random to Janus Packing. ACS Nano 2023, 17, 5852–5860. [Google Scholar] [CrossRef]
- Jing, Y.; Low, A.K.Y.; Liu, Y.; Feng, M.; Lim, J.W.M.; Loh, S.M.; Rehman, Q.; Blundel, S.A.; Mathews, N.; Hippalgaonkar, K.; et al. Stable and Highly Emissive Infrared Yb-Doped Perovskite Quantum Cutters Engineered by Machine Learning. Adv. Mater. 2024, 36, 2405973. [Google Scholar] [CrossRef]
- Jin, H.; Yeong Park, G.; Kyong Kim, M.; Cha, J.; Seok Ham, D.; Kim, M. Eco-friendly Solvent-Processible and highly luminescent perovskite nanocrystals with polymer zwitterions for Air-Stable optoelectronics. Chem. Eng. J. 2023, 459, 141531. [Google Scholar] [CrossRef]
- Kirsch, C.; Naujoks, T.; Haizmann, P.; Frech, P.; Peisert, H.; Chassé, T.; Brütting, W.; Scheele, M. Zwitterionic Carbazole Ligands Enhance the Stability and Performance of Perovskite Nanocrystals in Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2023, 15, 32744–32752. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Qiu, M. Rational Design of Amino Acid-Modified Halide Perovskites for Highly Efficient and Cost-Effective Light-Emitting Diodes. Materials 2025, 18, 4982. https://doi.org/10.3390/ma18214982
Chen H, Qiu M. Rational Design of Amino Acid-Modified Halide Perovskites for Highly Efficient and Cost-Effective Light-Emitting Diodes. Materials. 2025; 18(21):4982. https://doi.org/10.3390/ma18214982
Chicago/Turabian StyleChen, Hongyu, and Mingxia Qiu. 2025. "Rational Design of Amino Acid-Modified Halide Perovskites for Highly Efficient and Cost-Effective Light-Emitting Diodes" Materials 18, no. 21: 4982. https://doi.org/10.3390/ma18214982
APA StyleChen, H., & Qiu, M. (2025). Rational Design of Amino Acid-Modified Halide Perovskites for Highly Efficient and Cost-Effective Light-Emitting Diodes. Materials, 18(21), 4982. https://doi.org/10.3390/ma18214982



