Carbon Fixation from Industrial Flue Gas via CO2 Mineral Carbonation: Principles, Technical Advances, and Future Directions
Abstract
1. Introduction
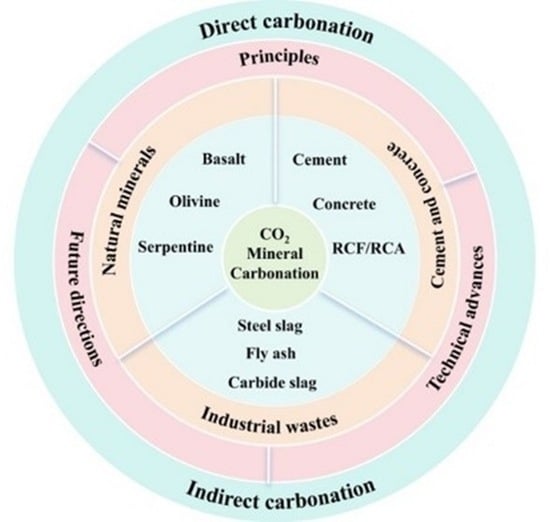
2. Principles for CO2 Mineral Carbonation
2.1. Direct Carbonation
2.2. Indirect Carbonation
2.3. Emerging Technologies and Principles
2.4. Physical–Chemical Characterization of CO2 Mineral Carbonation
3. Research Progress of CO2 Mineral Carbonation
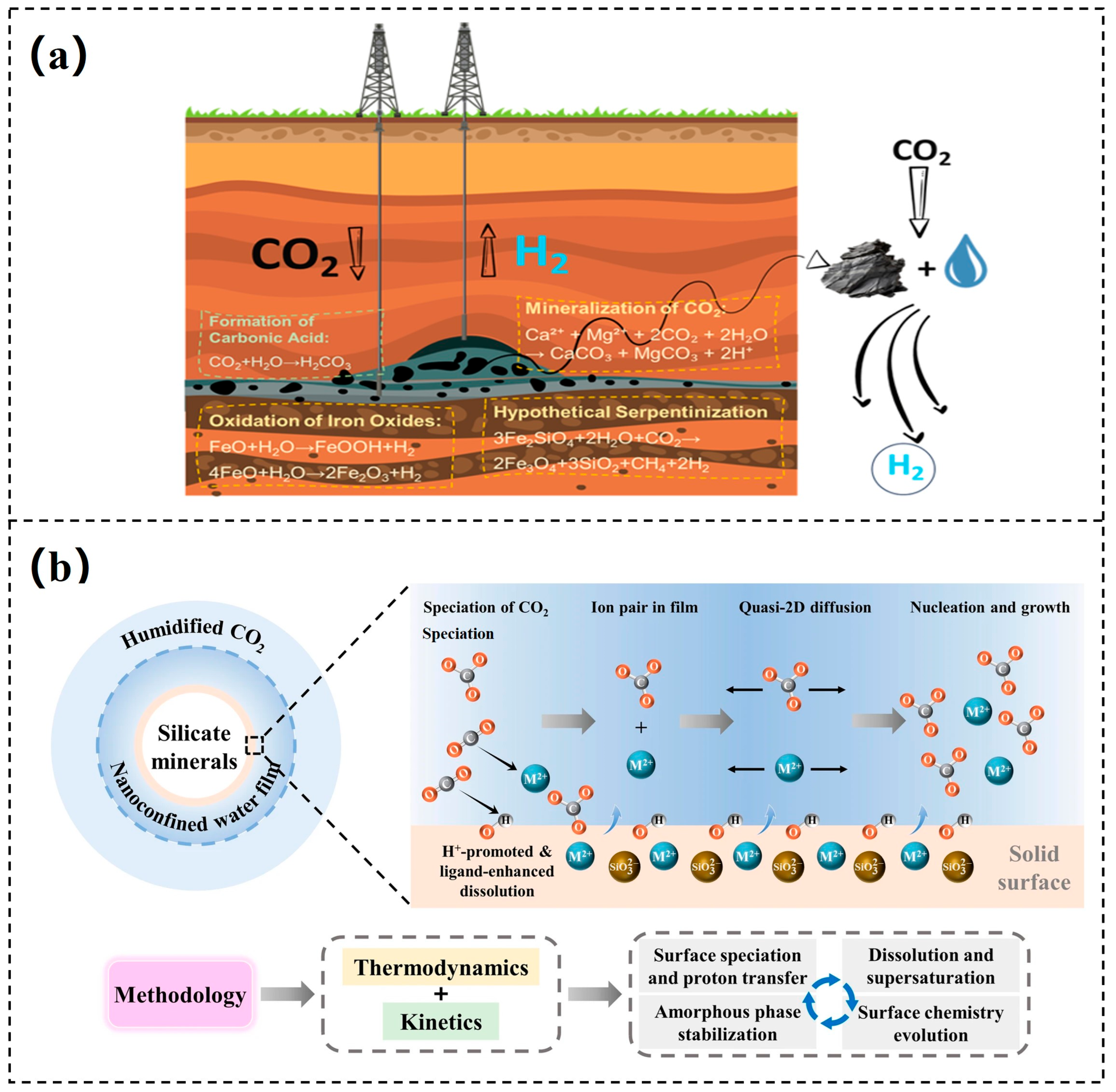
3.1. Geological Mineralization
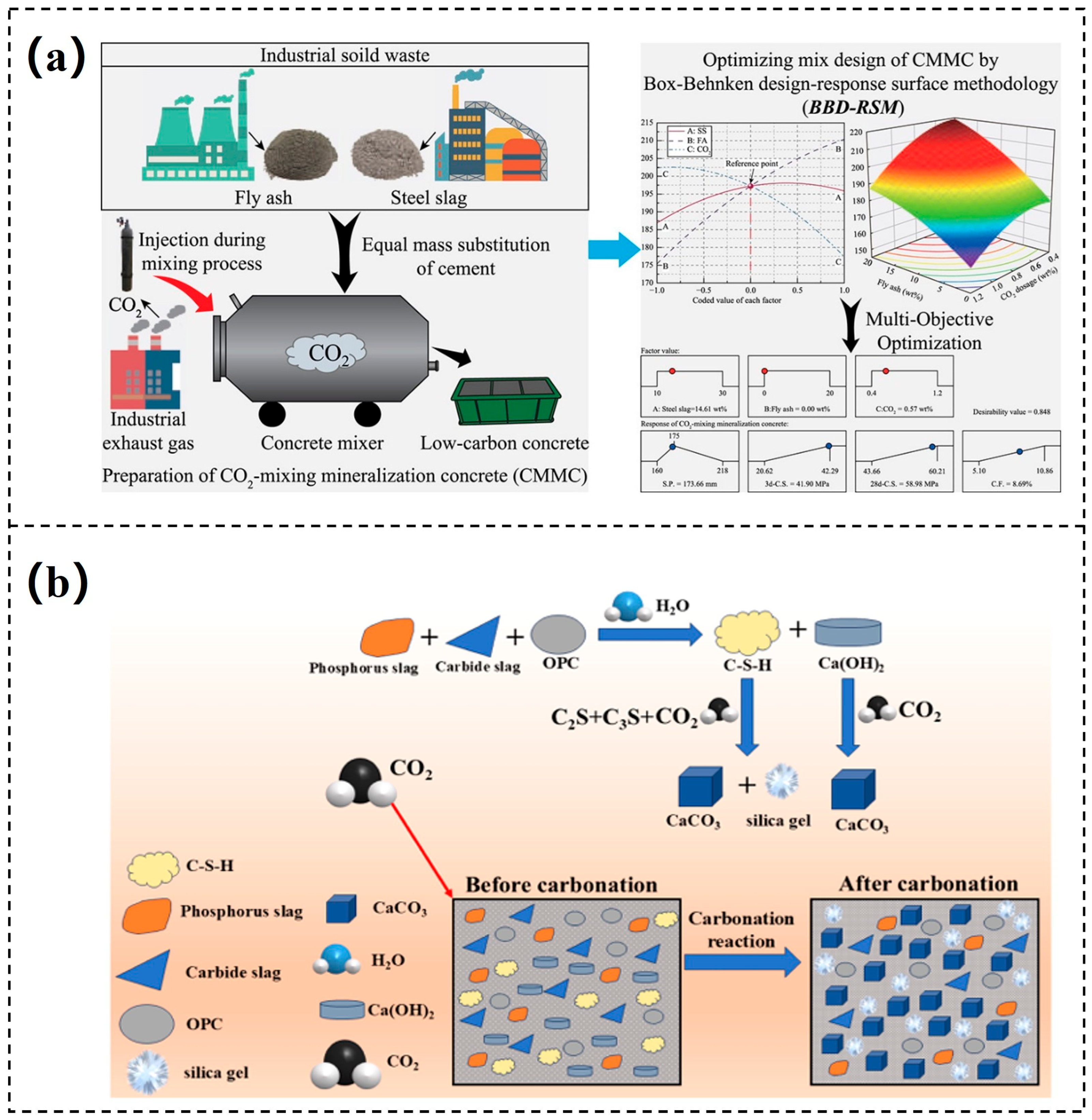
3.2. Mineralization of Cement and Concrete
3.3. Industrial Solid Waste Mineralization
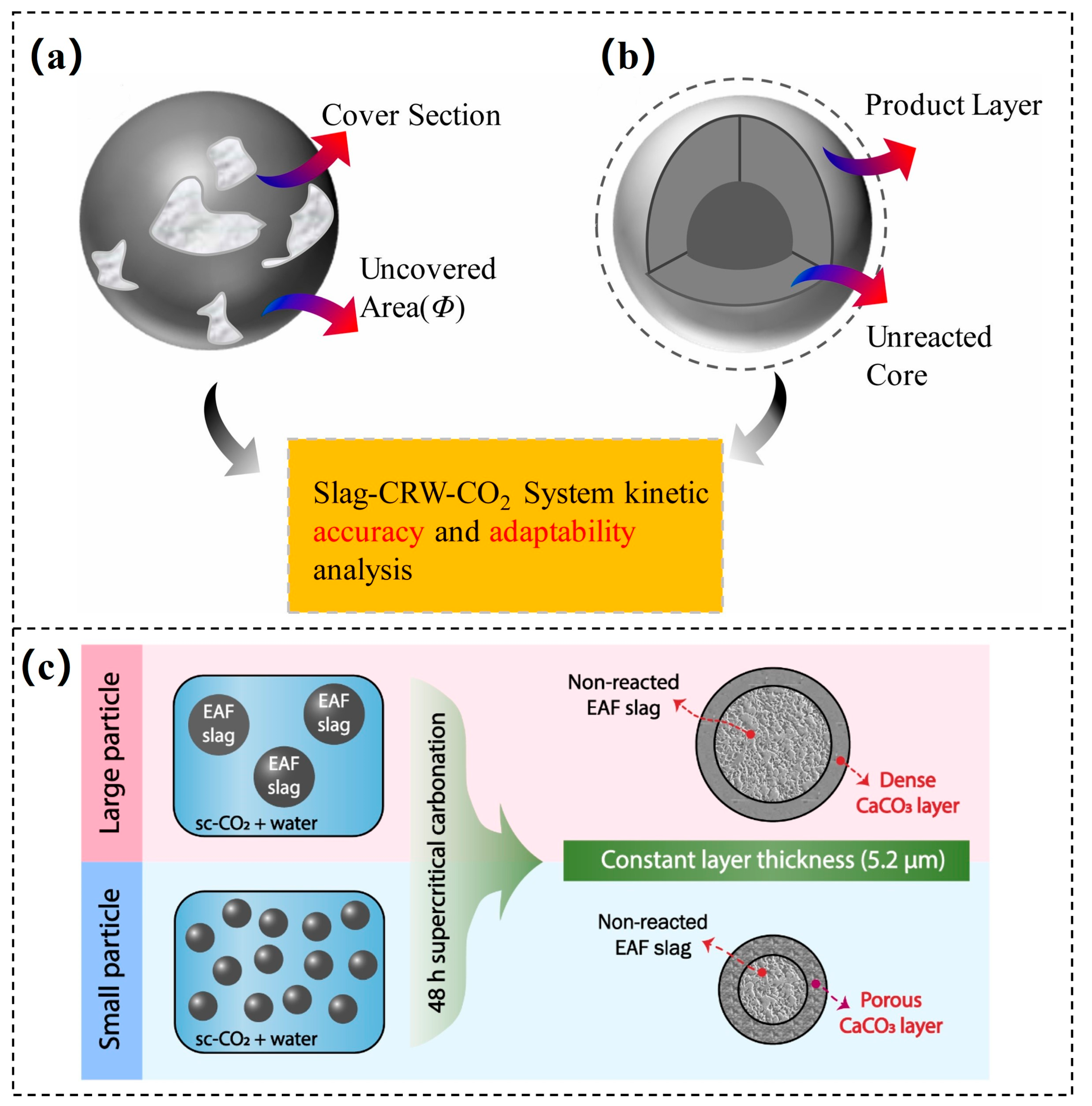
3.3.1. Steel Slag
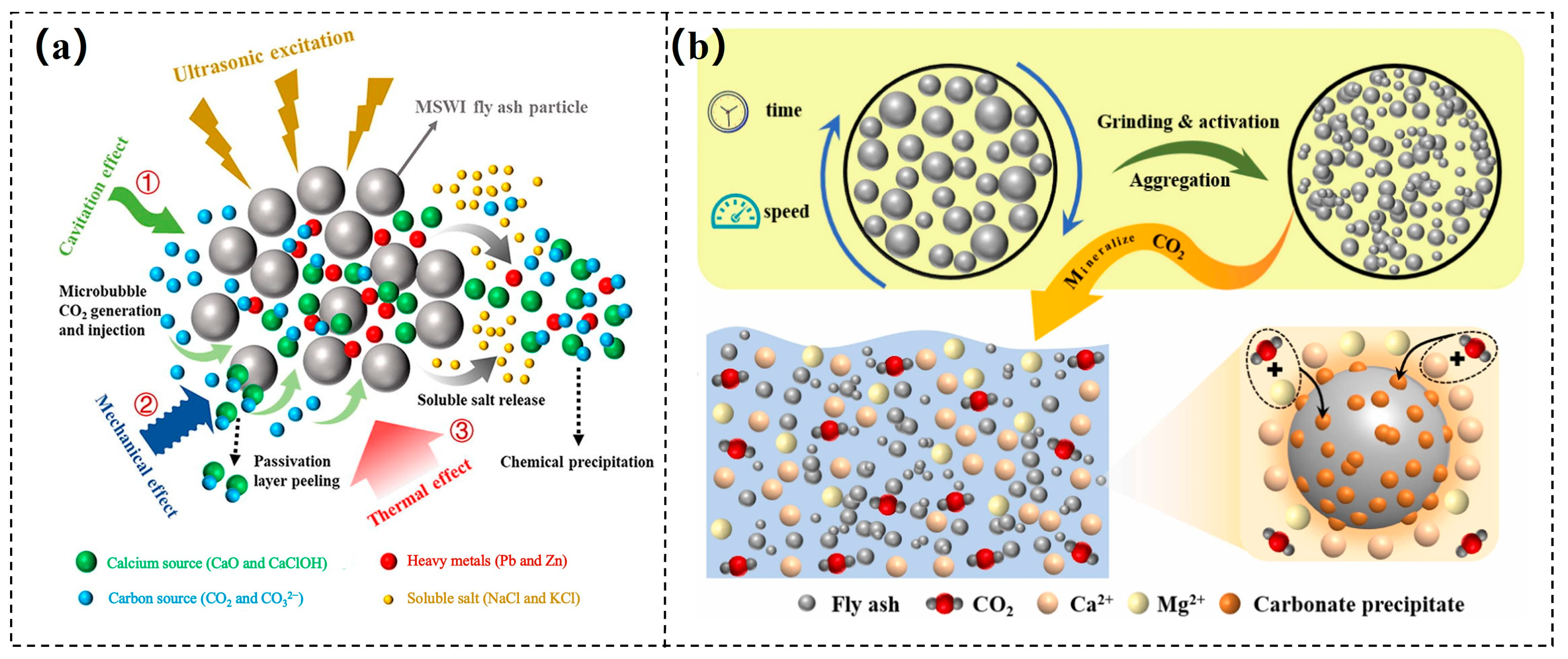
3.3.2. Fly Ash
4. Applications of CO2 Mineral Carbonation
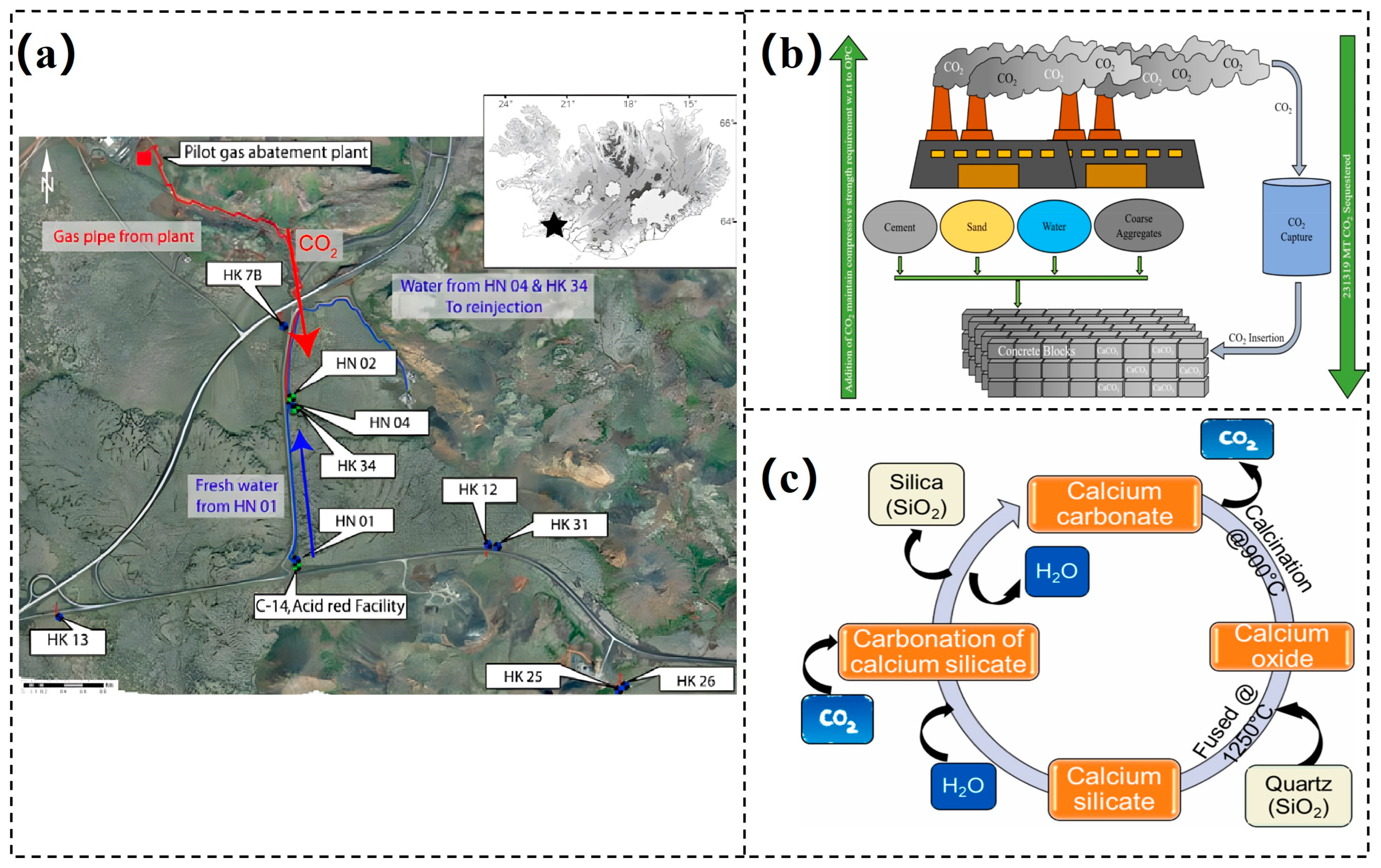
4.1. Geological CO2 Storage
4.2. Carbonation of Cement-Based Materials
4.3. Indirect Carbonation for Calcium Carbonate Production
4.4. Carbonation of Industrial Solid Waste
5. Challenges and Future Prospects
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- International Energy Agency. Global Energy Review 2025. 2025. Available online: https://www.iea.org/reports/global-energy-review-2025 (accessed on 7 September 2025).
- Deng, Z.; Zhu, B.; Davis, S.J.; Ciais, P.; Guan, D.; Gong, P.; Liu, Z. Global carbon emissions and decarbonization in 2024. Nat. Rev. Earth Environ. 2025, 6, 231–233. [Google Scholar] [CrossRef]
- Zscheischler, J.; Raymond, C.; Chen, Y.; Le Grix, N.; Libonati, R.; Rogers, C.D.W.; White, C.J.; Wolski, P. Compound weather and climate events in 2024. Nat. Rev. Earth Environ. 2025, 6, 240–242. [Google Scholar] [CrossRef]
- Newman, R.; Noy, I. The global costs of extreme weather that are attributable to climate change. Nat. Commun. 2023, 14, 6103. [Google Scholar] [CrossRef]
- Pan, S.-Y.; Chen, Y.-H.; Fan, L.-S.; Kim, H.; Gao, X.; Ling, T.-C.; Chiang, P.-C.; Pei, S.-L.; Gu, G. CO2 mineralization and utilization by alkaline solid wastes for potential carbon reduction. Nat. Sustain. 2020, 3, 399–405. [Google Scholar] [CrossRef]
- Zhou, S.; Li, L.; Ji, L.; Dai, B.; Wang, Z.; Benhelal, E.; Bolan, N.S.; Feron, P.; Yu, H. Progress in recyclable chemicals for sustainable ex-situ CO2 mineralisation. Green Energy Resour. 2024, 2, 100087. [Google Scholar] [CrossRef]
- Seifritz, W. CO2 disposal by means of silicates. Nature 1990, 345, 486. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, W.; Geng, Z.; Wang, S.; He, J.; Zhang, D.; Zhu, T. Enhanced strategies for carbon dioxide removal using natural minerals. Innov. Mater. 2025, 3, 100145. [Google Scholar] [CrossRef]
- Galina, N.R.; Arce, G.L.A.F.; Maroto-Valer, M.; Ávila, I. Experimental Study on Mineral Dissolution and Carbonation Efficiency Applied to pH-Swing Mineral Carbonation for Improved CO2 Sequestration. Energies 2023, 16, 2449. [Google Scholar] [CrossRef]
- Strefler, J.; Amann, T.; Bauer, N.; Kriegler, E.; Hartmann, J. Potential and costs of carbon dioxide removal by enhanced weathering of rocks. Environ. Res. Lett. 2018, 13, 34010. [Google Scholar] [CrossRef]
- Pan, S.-Y.; Shah, K.J.; Chen, Y.-H.; Wang, M.-H.; Chiang, P.-C. Deployment of Accelerated Carbonation Using Alkaline Solid Wastes for Carbon Mineralization and Utilization Toward a Circular Economy. ACS Sustain. Chem. Eng. 2017, 5, 6429–6437. [Google Scholar] [CrossRef]
- Rahmanihanzaki, M.; Hemmati, A. A review of mineral carbonation by alkaline solidwaste. Int. J. Greenh. Gas Control 2022, 121, 103798. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, C.; Huang, P.; Zhang, Y.; Sun, J. Modeling and response surface methodology optimization of reaction parameters for aqueous mineral carbonation by steel slag. Carbon Capture Sci. Technol. 2024, 12, 100229. [Google Scholar] [CrossRef]
- Liu, W.; Teng, L.; Rohani, S.; Qin, Z.; Zhao, B.; Xu, C.C.; Ren, S.; Liu, Q.; Liang, B. CO2 mineral carbonation using industrial solid wastes: A review of recent developments. Chem. Eng. J. 2021, 416, 129093. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, Y.; Liu, H.; Boczkaj, G.; Cao, Y.; Wang, C. Carbon dioxide sequestration by industrial wastes through mineral carbonation: Current status and perspectives. J. Clean. Prod. 2024, 434, 140258. [Google Scholar] [CrossRef]
- Gu, Q.; Wang, T.; Wu, W.; Wang, D.; Jin, B. Influence of pretreatments on accelerated dry carbonation of MSWI fly ash under medium temperatures. Chem. Eng. J. 2021, 414, 128756. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, H.; Miao, E.; Wang, Y.; Zhang, T.; Xiao, Y.; Liu, Z.; Ma, J.; Xiong, Z.; Zhao, Y.; et al. Accelerated CO2 mineralization technology using fly ash as raw material: Recent research advances. Chem. Eng. J. 2024, 488, 150676. [Google Scholar] [CrossRef]
- Wang, F.; Dreisinger, D.; Jarvis, M.; Hitchins, T.; Dyson, D. Quantifying kinetics of mineralization of carbon dioxide by olivine under moderate conditions. Chem. Eng. J. 2019, 360, 452–463. [Google Scholar] [CrossRef]
- Myers, C.A.; Nakagaki, T.; Akutsu, K. Quantification of the CO2 mineralization potential of ironmaking and steelmaking slags under direct gas-solid reactions in flue gas. Int. J. Greenh. Gas Control 2019, 87, 100–111. [Google Scholar] [CrossRef]
- Zhang, Y.; Ying, Y.; Xing, L.; Zhan, G.; Deng, Y.; Chen, Z.; Li, J. Carbon dioxide reduction through mineral carbonation by steel slag. J. Environ. Sci. 2025, 152, 664–684. [Google Scholar] [CrossRef]
- You, X.; Hu, X.; Xiao, Z.; Bairq, Z.A.S.; Chen, W.; Banthia, N.; Shi, C. Thermodynamic calculation of CaCO3 polymorphs from aqueous carbonation of Portland cement with the addition of organic additives. Cem. Concr. Compos. 2025, 164, 106279. [Google Scholar] [CrossRef]
- Chen, K.; Tian, S.; Li, J.; Lin, L.; Gao, Y.; Qin, W.; Hu, E.; Jiang, J. Water-insoluble amine-looping process induces CO2 mineral carbonation without consumption of exogenous alkaline reagents. Chem. Eng. J. 2025, 503, 158298. [Google Scholar] [CrossRef]
- Ukwattage, N.L.; Ranjith, P.G.; Yellishetty, M.; Bui, H.H.; Xu, T. A laboratory-scale study of the aqueous mineral carbonation of coal fly ash for CO2 sequestration. J. Clean. Prod. 2015, 103, 665–674. [Google Scholar] [CrossRef]
- Hosseini, T.; Selomulya, C.; Haque, N.; Zhang, L. Indirect Carbonation of Victorian Brown Coal Fly Ash for CO2 Sequestration: Multiple-Cycle Leaching-Carbonation and Magnesium Leaching Kinetic Modeling. Energy Fuels 2014, 28, 6481–6493. [Google Scholar] [CrossRef]
- Gao, Y.; Tao, Y.; Li, G.; Shen, P.; Pellenq, R.J.-M.; Poon, C.S. Moisture-driven carbonation kinetics for ultrafast CO2 mineralization. Proc. Natl. Acad. Sci. USA 2025, 122, e2418239121. [Google Scholar] [CrossRef] [PubMed]
- Miao, E.; Du, Y.; Wang, H.; Xiong, Z.; Zhao, Y.; Zhang, J. Experimental study and kinetics on CO2 mineral sequestration by the direct aqueous carbonation of pepper stalk ash. Fuel 2021, 303, 121230. [Google Scholar] [CrossRef]
- Shao, X.; Qin, B.; Shi, Q.; Yang, Y.; Ma, Z.; Xu, Y.; Hao, M.; Jiang, Z.; Jiang, W. Study on the sequestration capacity of fly ash on CO2 and employing the product to prevent spontaneous combustion of coal. Fuel 2023, 334, 126378. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, L.; Cui, K.; Wang, H.; Fu, T. Carbon capture and storage technology by steel-making slags: Recent progress and future challenges. Chem. Eng. J. 2023, 455, 140552. [Google Scholar] [CrossRef]
- Chen, Q.; Ding, W.; Sun, H.; Peng, T.; Ma, G. Utilization of Phosphogypsum to Prepare High-Purity CaCO3 in the NH4Cl–NH4OH-CO2 System. ACS Sustain. Chem. Eng. 2020, 8, 11649–11657. [Google Scholar] [CrossRef]
- Hu, J.; Liu, W.; Wang, L.; Liu, Q.; Chen, F.; Yue, H.; Liang, B.; Lü, L.; Wang, Y.; Zhang, G.; et al. Indirect mineral carbonation of blast furnace slag with (NH4)2SO4 as a recyclable extractant. J. Energy Chem. 2017, 26, 927–935. [Google Scholar] [CrossRef]
- Chen, T.-L.; Jiang, W.; Shen, A.-L.; Chen, Y.-H.; Pan, S.-Y.; Chiang, P.-C. CO2 Mineralization and Utilization Using Various Calcium-Containing Wastewater and Refining Slag via a High-Gravity Carbonation Process. Ind. Eng. Chem. Res. 2020, 59, 7140–7150. [Google Scholar] [CrossRef]
- Capelo, S.; de Oliveira, R.; Stampino, I.G.; Casals-Terré, A.; Giancola, S.; Galan-Mascaros, J. A thorough assessment of mineral carbonation of steel slag and refractory waste. J. CO2 Util. 2024, 82, 102770. [Google Scholar] [CrossRef]
- Kusin, F.M.; Hasan, S.; Molahid, V.L.M.; Yusuff, F.M.; Jusop, S. Carbon dioxide sequestration of iron ore mining waste under low-reaction condition of a direct mineral carbonation process. Environ. Sci. Pollut. Res. Int. 2023, 30, 22188–22210. [Google Scholar] [CrossRef]
- Viola, V.; Catauro, M.; D’Amore, A.; Perumal, P. Assessing the carbonation potential of wood ash for CO2 sequestration. Low-Carbon Mater. Green Constr. 2024, 2, 12. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, L.; Cao, X.; Li, Q.; Gan, M.; Wang, Y. Progress, challenges, and prospects of CO2 mineral sequestration in basalt: A critical review. Appl. Energy 2025, 381, 125127. [Google Scholar] [CrossRef]
- Snæbjörnsdóttir, S.Ó.; Sigfússon, B.; Marieni, C.; Goldberg, D.; Gislason, S.R.; Oelkers, E.H. Carbon dioxide storage through mineral carbonation. Nat. Rev. Earth Environ. 2020, 1, 90–102. [Google Scholar] [CrossRef]
- Zakharova, N.V.; Goldberg, D.S.; Sullivan, E.C.; Herron, M.M.; Grau, J.A. Petrophysical and geochemical properties of Columbia River flood basalt: Implications for carbon sequestration. Geochem. Geophys. Geosyst. 2012, 13, Q11001. [Google Scholar] [CrossRef]
- Marieni, C.; Matter, J.M.; Teagle, D.A.H. Experimental study on mafic rock dissolution rates within CO2-seawater-rock systems. Geochim. Cosmochim. Acta 2020, 272, 259–275. [Google Scholar] [CrossRef]
- Al-Yaseri, A.; Desouky, M.; Aljawad, M.S.; Hassanpouryouzband, A. Evidence of hydrogen release during CO2 sequestration in basalt. Int. J. Hydrogen Energy 2025, 101, 1183–1190. [Google Scholar] [CrossRef]
- Kantzas, E.P.; Val Martin, M.; Lomas, M.R.; Eufrasio, R.M.; Renforth, P.; Lewis, A.L.; Taylor, L.L.; Mecure, J.-F.; Pollitt, H.; Vercoulen, P.V.; et al. Substantial carbon drawdown potential from enhanced rock weathering in the United Kingdom. Nat. Geosci. 2022, 15, 382–389. [Google Scholar] [CrossRef]
- Beerling, D.J.; Kantzas, E.P.; Lomas, M.R.; Taylor, L.L.; Zhang, S.; Kanzaki, Y.; Eufrasio, R.M.; Renforth, P.; Mecure, J.-F.; Pollitt, H.; et al. Transforming US agriculture for carbon removal with enhanced weathering. Nature 2025, 638, 425–434. [Google Scholar] [CrossRef]
- Chen, Y.; Kanan, M.W. Thermal Ca2+/Mg2+ exchange reactions to synthesize CO2 removal materials. Nature 2025, 638, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Liu, Q.; Bao, Q.; Wang, Z.; Xie, Y.; Michelle, T.; Zhao, W.; Xian, C. Review on in-situ CO2 mineralization sequestration: Mechanistic understanding and research frontiers. Int. J. Coal Sci. Technol. 2025, 12, 15. [Google Scholar] [CrossRef]
- Kane, S.; Olsson, J.A.; Miller, S.A. Greenhouse gas emissions of global construction material production. Environ. Res. Infrastruct. Sustain. 2025, 5, 015020. [Google Scholar] [CrossRef]
- Xue, Q.; Zhang, L.; Mei, K.; Li, X.; Newell, P.; Wang, Y.; Cheng, X.; Zheng, W. CO2-induced evolution of chemical, structural and mechanical properties of reinforced concrete: A review. Constr. Build. Mater. 2022, 353, 129069. [Google Scholar] [CrossRef]
- Tonin, N.R.; Ghosh, S.K.; Rajagopalan, B.; Hubler, M.H. Lifetime Reduction of Concrete Infrastructure due to Global Warming in the United States. Concr. Int. 2024, 46, 50–56. [Google Scholar]
- Kim, W.K.; Lee, J.; Park, J.; Moon, J. Carbon sequestration in cementitious systems through CO2-rich hydration and chemically enforced CO2 mineralization. J. CO2 Util. 2024, 84, 102834. [Google Scholar] [CrossRef]
- Zajac, M.; Skocek, J.; Haha, M.B.; Deja, J. CO2 Mineralization Methods in Cement and Concrete Industry. Energies 2022, 15, 3597. [Google Scholar] [CrossRef]
- Ostovari, H.; Müller, L.; Skocek, J.; Bardow, A. From Unavoidable CO2 Source to CO2 Sink? A Cement Industry Based on CO2 Mineralization. Environ. Sci. Technol. 2021, 55, 5212–5223. [Google Scholar] [CrossRef]
- Ahmed, O.; Ahmad, S.; Adekunle, S.K. Carbon dioxide sequestration in cementitious materials: A review of techniques, material performance, and environmental impact. J. CO2 Util. 2024, 83, 102812. [Google Scholar] [CrossRef]
- Fu, X.; Guerini, A.; Zampini, D.; Loria, A.F.R. Storing CO2 while strengthening concrete by carbonating its cement in suspension. Commun. Mater. 2024, 5, 109. [Google Scholar] [CrossRef]
- Liang, C.; Li, B.; Guo, M.-Z.; Hou, S.; Wang, S.; Gao, Y.; Wang, X. Effects of early-age carbonation curing on the properties of cement-based materials: A review. J. Build. Eng. 2024, 84, 108495. [Google Scholar] [CrossRef]
- Li, L.; Wu, M. An overview of utilizing CO2 for accelerated carbonation treatment in the concrete industry. J. CO2 Util. 2022, 60, 102000. [Google Scholar] [CrossRef]
- Cheng, X.; Tian, W.; Yuan, Q.; Lacey, A.; Chen, W.; Guo, J.; Cai, J. Response surface optimization of CO2-mixing mineralization concrete: Balancing mechanical properties and carbon sequestration. Constr. Build. Mater. 2025, 471, 140648. [Google Scholar] [CrossRef]
- Wang, X.; Guo, M.-Z.; Ling, T.-C. Production of a novel belite-ternesite cement with high CO2 reactivity using 100% municipal solid waste incineration (MSWI) residues. Resour. Conserv. Recycl. 2024, 209, 107785. [Google Scholar] [CrossRef]
- Li, L.; Liu, Q.; Huang, T.; Peng, W. Mineralization and utilization of CO2 in construction and demolition wastes recycling for building materials: A systematic review of recycled concrete aggregate and recycled hardened cement powder. Sep. Purif. Technol. 2022, 298, 121512. [Google Scholar] [CrossRef]
- Shi, C.; Wu, Z.; Cao, Z.; Ling, T.C.; Zheng, J. Performance of mortar prepared with recycled concrete aggregate enhanced by CO2 and pozzolan slurry. Cem. Concr. Compos. 2018, 86, 130–138. [Google Scholar] [CrossRef]
- Mehdipour, I.; Falzone, G.; La Plante, E.C.; Simonetti, D.; Neithalath, N.; Sant, G. How Microstructure and Pore Moisture Affect Strength Gain in Portlandite-Enriched Composites That Mineralize CO2. ACS Sustain. Chem. Eng. 2019, 7, 13053–13061. [Google Scholar] [CrossRef]
- Li, Y.; Fu, T.; Wang, R.; Li, Y. An assessment of microcracks in the interfacial transition zone of recycled concrete aggregates cured by CO2. Constr. Build. Mater. 2020, 236, 117543. [Google Scholar] [CrossRef]
- Liu, G.-M.; Gao, R.-C.; Liu, F.; Yue, W.-P.; Yang, Y.; Liao, Y.-P.; Liew, J.-X. Analysis of CO2 storage efficiency and performance of low carbon cement binders containing carbide slag and phosphorus slag. J. CO2 Util. 2025, 93, 103037. [Google Scholar] [CrossRef]
- Quader, M.A.; Ahmed, S.; Ghazilla, R.A.R.; Ahmed, S.; Dahari, M. A comprehensive review on energy efficient CO2 breakthrough technologies for sustainable green iron and steel manufacturing. Renew. Sustain. Energy Rev. 2015, 50, 594–614. [Google Scholar] [CrossRef]
- Gao, W.; Zhou, W.; Lyu, X.; Liu, X.; Su, H.; Li, C.; Wang, H. Comprehensive utilization of steel slag: A review. Powder Technol. 2023, 422, 118449. [Google Scholar] [CrossRef]
- Yan, D.-m.; Jiang, H.-f.; Yang, J. Research status of comprehensive utilization of iron from red mud. In Proceedings of the SPIE 12598, Eighth International Conference on Energy Materials and Electrical Engineering (ICEMEE 2022), Guangzhou, China, 22–24 July 2022. [Google Scholar] [CrossRef]
- Pan, S.-Y.; Chung, T.-C.; Ho, C.-C.; Hou, C.-J.; Chen, Y.-H.; Chiang, P.-C. CO2 Mineralization and Utilization using Steel Slag for Establishing a Waste-to-Resource Supply Chain. Sci. Rep. 2017, 7, 17227. [Google Scholar] [CrossRef] [PubMed]
- Ruiying, W.; Tao, Q.; Hongfeng, J.; Gang, D.; Canhua, L.; Shujing, Z.; Jiamao, L.; Chen, Z. Study on preparation technology and properties of calcium based CO2 absorbent from acid leaching steel slag. Green Energy Resour. 2025, 3, 100140. [Google Scholar] [CrossRef]
- Wei, C.; Dong, J.; Zhang, H.; Wang, X. Kinetics model adaptability analysis of CO2 sequestration process utilizing steelmaking slag and cold-rolling wastewater. J. Hazard. Mater. 2021, 404, 124094. [Google Scholar] [CrossRef]
- Polettini, A.; Pomi, R.; Stramazzo, A. Carbon sequestration through accelerated carbonation of BOF slag: Influence of particle size characteristics. Chem. Eng. J. 2016, 298, 26–35. [Google Scholar] [CrossRef]
- Liu, G.; Schollbach, K.; van der Laan, S.; Tang, P.; Florea, M.V.A.; Brouwers, H.J.H. Recycling and utilization of high volume converter steel slag into CO2 activated mortars—The role of slag particle size. Resour. Conserv. Recycl. 2020, 160, 104883. [Google Scholar] [CrossRef]
- Gomari, K.E.; Gomari, S.R.; Hughes, D.; Ahmed, T. Exploring the potential of steel slag waste for carbon sequestration through mineral carbonation: A comparative study of blast-furnace slag and ladle slag. J. Environ. Manag. 2024, 351, 119835. [Google Scholar] [CrossRef]
- Kim, J.; Azimi, G. The CO2 sequestration by supercritical carbonation of electric arc furnace slag. J. CO2 Util. 2021, 52, 101667. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, D.; Gu, L.; Yuan, H.; Zhang, X. Enhanced carbonation of steel slag blocks using various chemical additives. J. Build. Eng. 2025, 105, 112518. [Google Scholar] [CrossRef]
- Zeng, B.; Zhuang, X.; Han, W.; Jia, S.; Zhang, Z.; Mo, L.; Kishi, T. Resource efficiency and sustainability of carbonated steel slag -cement with sodium salicylate. Constr. Build. Mater. 2025, 464, 140107. [Google Scholar] [CrossRef]
- Liu, L.; Fan, X.; Gan, M.; Wei, J.; Gao, Z.; Sun, Z.; Ji, Z.; Wu, Y.; Li, J. Microwave-enhanced selective leaching calcium from steelmaking slag to fix CO2 and produce high value-added CaCO3. Sep. Purif. Technol. 2024, 330, 125395. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Zhao, Z.; Hou, X.; Zhang, H.; Wen, C.; Wang, X.; Li, Y.; Wang, W. Study of a coal fly ash-based integrated CO2 capture-mineralization material: Preparation method, modification mechanism, and CO2 mineralization property. Sci. Total Environ. 2024, 955, 177201. [Google Scholar] [CrossRef]
- Ćwik, A.; Casanova, I.; Rausis, K.; Koukouzas, N.; Zarębska, K. Carbonation of high-calcium fly ashes and its potential for carbon dioxide removal in coal fired power plants. J. Clean. Prod. 2018, 202, 1026–1034. [Google Scholar] [CrossRef]
- Dananjayan, R.R.T.; Kandasamy, P.; Andimuthu, R. Direct mineral carbonation of coal fly ash for CO2 sequestration. J. Clean. Prod. 2016, 112, 4173–4182. [Google Scholar] [CrossRef]
- Shao, X.; Qin, B.; Shi, Q.; Yang, Y.; Ma, Z.; Li, Y.; Jiang, Z.; Jiang, W. The impacts of CO2 mineralization reaction on the physicochemical characteristics of fly ash: A study under different reaction conditions of the water-to-solid ratio and the pressure of CO2. Energy 2024, 287, 129676. [Google Scholar] [CrossRef]
- Chen, J.; Fu, C.; Mao, T.; Shen, Y.; Li, M.; Lin, X.; Li, X.; Yan, J. Study on the accelerated carbonation of MSWI fly ash under ultrasonic excitation: CO2 capture, heavy metals solidification, mechanism and geochemical modelling. Chem. Eng. J. 2022, 450, 138418. [Google Scholar] [CrossRef]
- Jiang, Z.; Qin, B.; Shi, Q.; Ma, Z.; Shao, X.; Xu, Y.; Hao, M.; Yang, Y. Effect of ball milling activation on CO2 mineralization performance in fly ash and fire resistance capabilities of mineralized product. J. Environ. Chem. Eng. 2024, 12, 113954. [Google Scholar] [CrossRef]
- Pan, S.-Y.; Hung, C.-H.; Chan, Y.-W.; Kim, H.; Li, P.; Chiang, P.-C. Integrated CO2 Fixation, Waste Stabilization, and Product Utilization via High-Gravity Carbonation Process Exemplified by Circular Fluidized Bed Fly Ash. ACS Sustain. Chem. Eng. 2016, 4, 3045–3052. [Google Scholar] [CrossRef]
- Mehdizadeh, H.; Cheng, X.; Mo, K.H.; Ling, T.-C. Upcycling of waste hydrated cement paste containing high-volume supplementary cementitious materials via CO2 pre-treatment. J. Build. Eng. 2022, 52, 104396. [Google Scholar] [CrossRef]
- Sarkar, M.; Maiti, M.; Mandal, S.; Xu, S. Enhancing concrete resilience and sustainability through fly ash-assisted microbial biomineralization for self-healing: From waste to greening construction materials. Chem. Eng. J. 2024, 481, 148148. [Google Scholar] [CrossRef]
- McGrail, B.P.; Schaef, H.T.; Spane, F.A.; Cliff, J.B.; Qafoku, O.; Horner, J.A.; Thompson, C.J.; Owen, A.T.; Sullivan, C.E. Field Validation of Supercritical CO2 Reactivity with Basalts. Environ. Sci. Technol. Lett. 2017, 4, 6–10. [Google Scholar] [CrossRef]
- Matter, J.M.; Broecker, W.S.; Gislason, S.R.; Gunnlaugsson, E.; Oelkers, E.H.; Stute, M.; Sigurdardóttir, H.; Stefansson, A.; Alfreðsson, H.A.; Aradóttir, E.S.; et al. The CarbFix Pilot Project–Storing carbon dioxide in basalt. Energy Procedia 2011, 4, 5579–5585. [Google Scholar] [CrossRef]
- von Strandmann, P.A.E.P.; Burton, K.W.; Snæbjörnsdóttir, S.O.; Sigfússon, B.; Aradóttir, E.S.; Gunnarsson, I.; Alfredsson, H.A.; Mesfin, K.G.; Oelkers, E.H.; Gislason, S.R. Rapid CO2 mineralisation into calcite at the CarbFix storage site quantified using calcium isotopes. Nat. Commun. 2019, 10, 1983. [Google Scholar] [CrossRef] [PubMed]
- Sigfusson, B.; Gislason, S.R.; Matter, J.M.; Stute, M.; Gunnlaugsson, E.; Gunnarsson, I.; Aradottir, E.S.; Sigurdardottir, H.; Mesfin, K.; Alfredsson, H.A.; et al. Solving the carbon-dioxide buoyancy challenge: The design and field testing of a dissolved CO2 injection system. Int. J. Greenh. Gas Control 2015, 37, 213–219. [Google Scholar] [CrossRef]
- Matter, J.M.; Stute, M.; Snæbjörnsdottir, S.Ó.; Oelkers, E.H.; Gislason, S.R.; Aradottir, E.S.; Sigfusson, B.; Gunnarsson, I.; Sigurdardottir, H.; Gunnlaugsson, E.; et al. Rapid carbon mineralization for permanent disposal of anthropogenic carbon dioxide emissions. Science 2016, 352, 1312–1314. [Google Scholar] [CrossRef] [PubMed]
- Snæbjörnsdóttir, S.Ó.; Oelkers, E.H.; Mesfin, K.; Aradóttir, E.S.; Dideriksen, K.; Gunnarsson, I.; Gunnlaugsson, E.; Matter, J.M.; Stute, M.; Gislason, S.R. The chemistry and saturation states of subsurface fluids during the in situ mineralisation of CO2 and H2S at the CarbFix site in SW-Iceland. Int. J. Greenh. Gas Control 2017, 58, 87–102. [Google Scholar] [CrossRef]
- Trias, R.; Ménez, B.; le Campion, P.; Zivanovic, Y.; Lecourt, L.; Lecoeuvre, A.; Schmitt-Kopplin, P.; Uhl, J.; Gislason, S.R.; Alfreðsson, H.A.; et al. High reactivity of deep biota under anthropogenic CO2 injection into basalt. Nat. Commun. 2017, 8, 1063. [Google Scholar] [CrossRef]
- McGrail, B.P.; Spane, F.A.; Amonette, J.E.; Thompson, C.R.; Brown, C.F. Injection and Monitoring at the Wallula Basalt Pilot Project. Energy Procedia 2014, 63, 2939–2948. [Google Scholar] [CrossRef]
- McGrail, B.P.; Schaef, H.T.; Spane, F.A.; Horner, J.A.; Owen, A.T.; Cliff, J.B.; Qafoku, O.; Thompson, C.J.; Sullivan, E.C. Wallula Basalt Pilot Demonstration Project: Post-injection Results and Conclusions. Energy Procedia 2017, 114, 5783–5790. [Google Scholar] [CrossRef]
- Supriya; Chaudhury, R.; Sharma, U.; Thapliyal, P.C.; Singh, L.P. Low-CO2 emission strategies to achieve net zero target in cement sector. J. Clean. Prod. 2023, 417, 137466. [Google Scholar] [CrossRef]
- Monkman, S.; MacDonald, M.; Hooton, R.D.; Sandberg, P. Properties and durability of concrete produced using TON as an accelerating admixture. Cem. Concr. Compos. 2016, 74, 218–224. [Google Scholar] [CrossRef]
- Monkman, S.; MacDonald, M. On carbon dioxide utilization as a means to improve the sustainability of ready-mixed concrete. J. Clean. Prod. 2017, 167, 365–375. [Google Scholar] [CrossRef]
- Monkman, S.; MacDonald, M. Carbon dioxide upcycling into industrially produced concrete blocks. Constr. Build. Mater. 2016, 124, 127–132. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Li, Y.; Tian, J.; You, X.; Mao, Y.; Hu, X.; Shi, C. Carbonated concrete brick capturing carbon dioxide from cement kiln exhaust gas. Case Stud. Constr. Mater. 2022, 17, e01474. [Google Scholar] [CrossRef]
- Lu, B.; Drissi, S.; Liu, J.; Hu, X.; Song, B.; Shi, C. Effect of temperature on CO2 curing, compressive strength and microstructure of cement paste. Cem. Concr. Res. 2022, 157, 106827. [Google Scholar] [CrossRef]
- Bettenhausen, C. US Steel probes carbon capture. CEN Glob. Enterp. 2023, 101, 13. [Google Scholar] [CrossRef]
- Yang, B.; Shao, C.; Hu, X.; Ngata, M.R.; Aminu, M.D. Advances in Carbon Dioxide Storage Projects: Assessment and Perspectives. Energy Fuels 2023, 37, 1757–1776. [Google Scholar] [CrossRef]
- Hanifa, M.; Agarwal, R.; Sharma, U.; Thapliyal, P.C.; Singh, L.P. A review on CO2 capture and sequestration in the construction industry: Emerging approaches and commercialised technologies. J. CO2 Util. 2023, 67, 102292. [Google Scholar] [CrossRef]
- Sahu, S.; Meininger, R.C. Sustainability and Durability of Solidia Cement Concrete: Based on alternative CO2 functional cement chemistry. Concr. Int. 2020, 42, 29–34. Available online: https://www.proquest.com/trade-journals/sustainability-durability-solidia-cement-concrete/docview/2431226621/se-2 (accessed on 6 September 2025).
- Liao, M.; Yao, Y. Applications of artificial intelligence-based modeling for bioenergy systems: A review. GCB Bioenergy 2021, 13, 774–802. [Google Scholar] [CrossRef]
- Xu, W.; Wang, Y.; Zhang, D.; Yang, Z.; Yuan, Z.; Lin, Y.; Yan, H.; Zhou, X.; Yang, C. Transparent AI-assisted chemical engineering process: Machine learning modeling and multi-objective optimization for integrating process data and molecular-level reaction mechanisms. J. Clean. Prod. 2024, 448, 141412. [Google Scholar] [CrossRef]
- Hosseinionari, H.; Seethaler, R. The integration of Model Predictive Control and deep Reinforcement Learning for efficient thermal control in thermoforming processes. J. Manuf. Process. 2024, 115, 82–93. [Google Scholar] [CrossRef]
- Arroyo, J.; Manna, C.; Spiessens, F.; Helsen, L. Reinforced model predictive control (RL-MPC) for building energy management. Appl. Energy 2022, 309, 118346. [Google Scholar] [CrossRef]
- Xie, H.; Yue, H.; Zhu, J.; Liang, B.; Li, C.; Wang, Y.; Xie, L.; Zhou, X. Scientific and Engineering Progress in CO2 Mineralization Using Industrial Waste and Natural Minerals. Engineering 2015, 1, 150–157. [Google Scholar] [CrossRef]
- Wang, T.; Yi, Z.; Song, J.; Zhao, C.; Guo, R.; Gao, X. An industrial demonstration study on CO2 mineralization curing for concrete. iScience 2022, 25, 104261. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Wang, X.; Ma, Z.; Cheng, Y.; Xie, B.; Liu, M.; Li, J.; Sun, C.; Wang, W. Carbon Fixation from Industrial Flue Gas via CO2 Mineral Carbonation: Principles, Technical Advances, and Future Directions. Materials 2025, 18, 4935. https://doi.org/10.3390/ma18214935
Sun Y, Wang X, Ma Z, Cheng Y, Xie B, Liu M, Li J, Sun C, Wang W. Carbon Fixation from Industrial Flue Gas via CO2 Mineral Carbonation: Principles, Technical Advances, and Future Directions. Materials. 2025; 18(21):4935. https://doi.org/10.3390/ma18214935
Chicago/Turabian StyleSun, Yanli, Xujiang Wang, Zhipeng Ma, Yanmei Cheng, Bingbing Xie, Mengning Liu, Jingwei Li, Chenggong Sun, and Wenlong Wang. 2025. "Carbon Fixation from Industrial Flue Gas via CO2 Mineral Carbonation: Principles, Technical Advances, and Future Directions" Materials 18, no. 21: 4935. https://doi.org/10.3390/ma18214935
APA StyleSun, Y., Wang, X., Ma, Z., Cheng, Y., Xie, B., Liu, M., Li, J., Sun, C., & Wang, W. (2025). Carbon Fixation from Industrial Flue Gas via CO2 Mineral Carbonation: Principles, Technical Advances, and Future Directions. Materials, 18(21), 4935. https://doi.org/10.3390/ma18214935