Review on Metal Powder Manufactured by Technologies Utilizing Centrifugal Force
Abstract
1. Introduction
2. Atomization Processes
2.1. Plasma Rotating Electrode Process (PREP)
| Type | Critical Size for Irregular Particles (μm) | Shape | Causes of Irregular Particles | Ref. |
|---|---|---|---|---|
| Ni-11Mo-8Al-3Ta-2Cr-1Re | >150 | wrinkled | Only fully melted droplets can spheroidize into spheres, driven by surface tension. Incomplete melting inevitably leads to irregular particle shapes. | [13] |
| TiAl | >150 | non-spherical | The low electrode rotation speed during the initial stage generated insufficient centrifugal force on the liquid films. | [16] |
| Ti6Al4V | >250 | flat | Semi-solid liquid films are torn away and solidify into irregular shapes before surface tension can spheroidize them. | [17] |
| 316 steels | >150 | - | Surface peeling from semi-solid films generates irregular particles; the extent of peeling and mass fraction of such powder depend on specific properties of different alloys | [15,18] |
| CoCrMo | >150 | |||
| Ni3Al-based IC21 | jagged | Centrifugal force ejected core defects with high melting point which resisted spheroidization, forming irregular particles that retained their initial morphology. | [19] |
2.2. Centrifugal Atomization (CA)
3. Disintegration Modes and Prediction of Particle Size
4. Challenges and Insights for PREP and CA
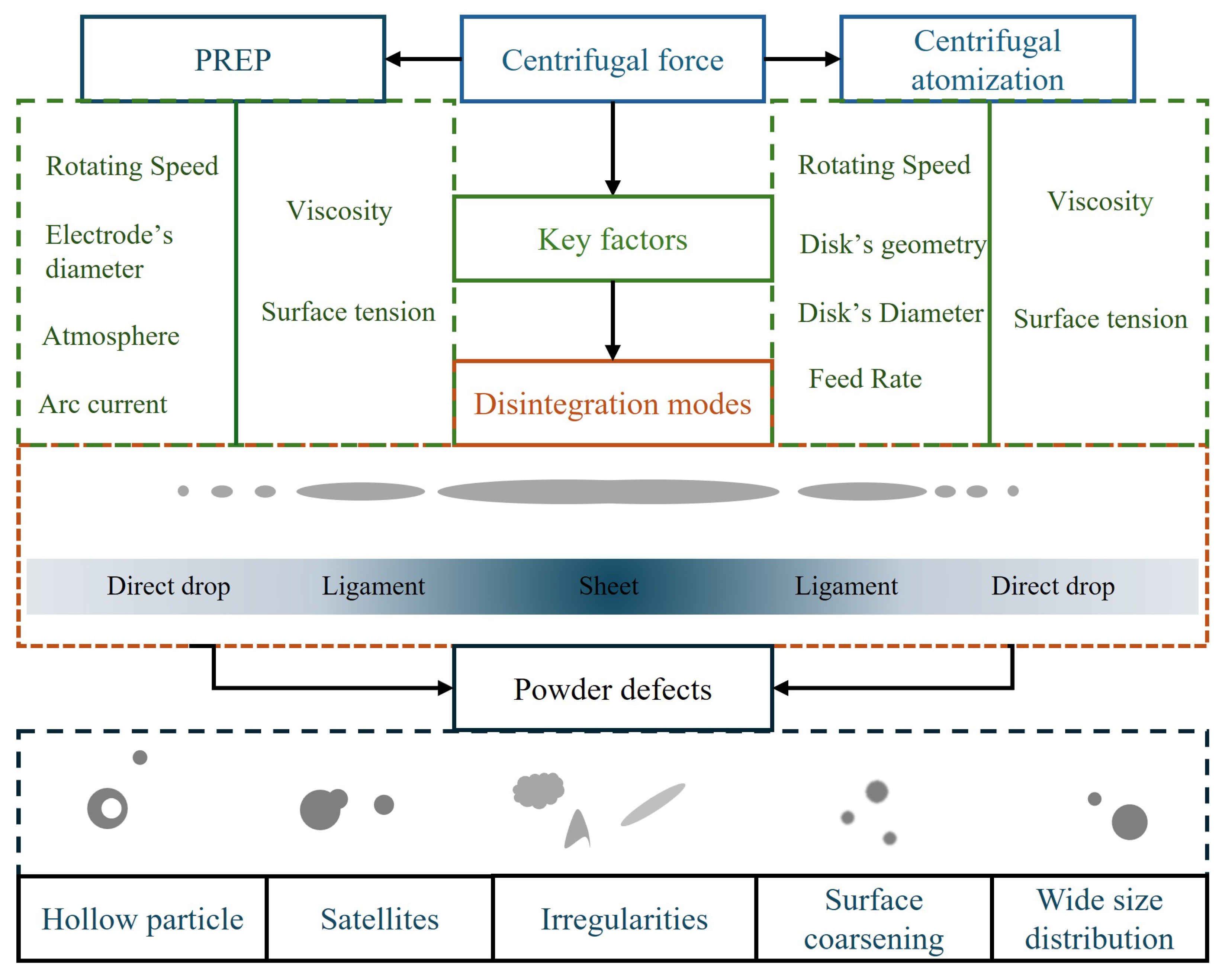
4.1. Powder Defects
4.2. Limitations for PREP and CA
5. Summary and Perspective
5.1. Scalability Challenges for Industrial Adoption
5.2. Potential Integration with Advanced AM Feedstock Requirements
5.3. Prospects for Metallic Glasses and Composites
5.4. Environmental and Energy Efficiency Considerations
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pérez-Ruiz, J.D.; de Lacalle, L.N.L.; Velilla-Díaz, W.; Mesa, J.A.; Gómez, G.; Maury, H.; Urbikain, G.; Gonzalez, H. Evaluating the feasibility of using crystalline patterns induced by PBF-LB to predict strength enhancing orientations. Mater. Des. 2025, 254, 114006. [Google Scholar] [CrossRef]
- Tekoglu, E.; Bae, J.-S.; Kim, H.-A.; Lim, K.-H.; Liu, J.; Doležal, T.D.; Kim, S.Y.; Alrizqi, M.A.; Penn, A.; Chen, W.; et al. Superior high-temperature mechanical properties and microstructural features of LPBF-printed In625-based metal matrix composites. Mater. Today 2024, 80, 297–307. [Google Scholar] [CrossRef]
- Yao, Z.; Qiu, W.; Chen, C.; Bao, X.; Luo, K.; Deng, Y.; Xue, W.; Li, X.; Hu, Q.; Guo, J.; et al. Making High Thermoelectric and Superior Mechanical Performance Nb0.88Hf0.12FeSb Half-Heusler via Additive Manufacturing. Adv. Sci. 2024, 11, 2403705. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Yadaiah, N.; Prakash, C.; Ramakrishna, S.; Dixit, S.; Gulta, L.R.; Buddhi, D. Laser powder bed fusion: A state-of-the-art review of the technology, materials, properties & defects, and numerical modelling. J. Mater. Res. Technol. 2022, 20, 2109–2172. [Google Scholar] [CrossRef]
- Sagar, V.R.; Lorin, S.; Göhl, J.; Quist, J.; Cromvik, C.; Mark, A.; Jareteg, K.; Edelvik, F.; Wärmefjord, K.; Söderberg, R.; et al. Investigating the Sensitivity of Particle Size Distribution on Part Geometry in Additive Manufacturing. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition (IMECE), Electronic Network, Porland, OR, USA, 16–19 November 2020. [Google Scholar]
- Brika, S.E.; Letenneur, M.; Dion, C.A.; Brailovski, V. Influence of particle morphology and size distribution on the powder flowability and laser powder bed fusion manufacturability of Ti-6Al-4V alloy. Addit. Manuf. 2020, 31, 100929. [Google Scholar] [CrossRef]
- Yang, D.Y.; Peng, H.X.; Fu, Y.Q.; Cao, F.Y.; Ning, Z.L.; Guo, S.; Jia, Y.D.; Liu, N.; Sun, J.F. Nucleation on Thermal History and Microstructural Evolution of Atomized Ti-48Al Nano and Micro-Powders. Nanosci. Nanotechnol. Lett. 2015, 7, 603–610. [Google Scholar] [CrossRef]
- Zhang, J.; Han, F.Z. Experimental study and parameter optimization on sustainable and efficient machining GH4169 with rotating short arc milling method. Int. J. Adv. Manuf. Technol. 2022, 119, 2023–2042. [Google Scholar] [CrossRef]
- Park, J.Y.; Park, K.B.; Kang, J.W.; Kim, H.G.; Hwang, N.M.; Park, H.K. Spheroidization behavior of water-atomized 316 stainless steel powder by inductively-coupled thermal plasma. Mater. Today Commun. 2020, 25, 101488. [Google Scholar] [CrossRef]
- Wang, P.; Gan, P.; Ren, X.N.; Yu, Z.Y. Numerical simulation on metallic droplet deformation and breakup concerning particle morphology and hollow particle formation during gas atomization. Trans. Nonferrous Met. Soc. China 2024, 34, 2074–2094. [Google Scholar] [CrossRef]
- Sanetrnik, D.; Hausnerova, B.; Ponizil, P.; Novak, M.; Monkova, K. Flow-induced defects during metal injection molding: Role of powder morphology. Phys. Fluids 2024, 36, 083334. [Google Scholar] [CrossRef]
- Li, Y.C.; Sui, Y.; Feng, Y.C.; Zhang, Y.; Li, Y.; Song, M.H.; Gong, S.L.; Xie, Y. Powder Synthesis and Characterization of Al0.5CoCrFeNi High-Entropy Alloy for Additive Manufacturing Prepared by the Plasma Rotating Electrode Process. ACS Omega 2024, 9, 18358–18365. [Google Scholar] [CrossRef]
- Han, Z.Y.; Zhang, P.X.; Lei, L.M.; Liang, S.J.; Wang, Q.X.; Lai, Y.J.; Li, J.S. Morphology and particle analysis of the Ni3Al-based spherical powders manufactured by supreme-speed plasma rotating electrode process. J. Mater. Res. Technol. 2020, 9, 13937–13944. [Google Scholar] [CrossRef]
- Hsu, T.I.; Wei, C.M.; Wu, L.D.; Li, Y.P.; Chiba, A.; Tsai, M.H. Nitinol powders generate from Plasma Rotation Electrode Process provide clean powder for biomedical devices used with suitable size, spheroid surface and pure composition. Sci. Rep. 2018, 8, 13776. [Google Scholar] [CrossRef]
- Nie, Y.; Tang, J.J.; Yang, B.B.; Lei, Q.; Yu, S.; Li, Y.P. Comparison in characteristic and atomization behavior of metallic powders produced by plasma rotating electrode process. Adv. Powder Technol. 2020, 31, 2152–2160. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, M.H.; Li, Y.; Li, Y.C.; Gong, S.L.; Zhang, B. Effect of Preparation Process on the Microstructure and Characteristics of TiAl Pre-Alloyed Powder Fabricated by Plasma Rotating Electrode Process. Crystals 2024, 14, 562. [Google Scholar] [CrossRef]
- Tang, J.J.; Nie, Y.; Lei, Q.; Li, Y.P. Characteristics and atomization behavior of Ti-6Al-4V powder produced by plasma rotating electrode process. Adv. Powder Technol. 2019, 30, 2330–2337. [Google Scholar] [CrossRef]
- Nie, Y.; Tang, J.; Teng, J.; Ye, X.; Yang, B.; Huang, J.; Yu, S.; Li, Y. Particle defects and related properties of metallic powders produced by plasma rotating electrode process. Adv. Powder Technol. 2020, 31, 2912–2920. [Google Scholar] [CrossRef]
- Wang, Q.; Zhen, Z.; Liang, S. Preparation of IC21 Spherical Powders by PREP Process. J. Phys. Conf. Ser. 2021, 012055. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, S.Y.; Tan, P.; Wang, J.; Xiang, C.S.; Tang, H.P. A comparative study of Ti-6A1-4V powders for additive manufacturing by gas atomization, plasma rotating electrode process and plasma atomization. Powder Technol. 2018, 333, 38–46. [Google Scholar] [CrossRef]
- Yang, X.B.; Tang, H.P.; Zhu, J.L.; Hei, W.W.; Yang, G.Y.; Li, X.H. The influence of lanthanum oxide on the properties of PREP tungsten powder. Rare Met. Mater. Eng. 2022, 51, 3025–3030. [Google Scholar] [CrossRef]
- Li, H.; Zhang, S.H.; Chen, Q.Y.; Du, Z.Y.; Chen, X.Y.; Chen, X.D.; Zhou, S.Y.; Mei, S.W.; Ke, L.D.; Sun, Q.L.; et al. High-Quality Spherical Silver Alloy Powder for Laser Powder Bed Fusion Using Plasma Rotating Electrode Process. Micromachines 2024, 15, 396. [Google Scholar] [CrossRef]
- Liming, Z.; Xinhua, M.; Ke, H.; Xin, L. Quantitative Analysis for the Shape Indicator of Spherical Ti-6Al-4V Powder by Image Analysis Method. RARE Met. Mater. Eng. 2020, 49, 950–955. [Google Scholar]
- Yin, J.O.; Chen, G.; Zhao, S.Y.; Ge, Y.; Li, Z.F.; Yang, P.J.; Han, W.Z.; Wang, J.; Tang, H.P.; Cao, P. Microstructural characterization and properties of Ti-28Ta at.% powders produced by plasma rotating electrode process. J. Alloys Compd. 2017, 713, 222–228. [Google Scholar] [CrossRef]
- Ruan, G.; Liu, C.; Qu, H.; Guo, C.; Li, G.; Li, X.; Zhu, Q. A comparative study on laser powder bed fusion of IN718 powders produced by gas atomization and plasma rotating electrode process. Mater. Sci. Eng. A 2022, 850, 143589. [Google Scholar] [CrossRef]
- Drokonov, D.; Zadykyan, G.; Korsmik, R. Investigation of the influence of nickel-based alloy powder EP648, obtained by plasma rotating electrode process, on powder utilization rate, structure and chemical composition, applied to direct laser deposition. J. Phys. Conf. Ser. 2018, 012058. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, M.; Li, Y.; Gong, S.; Yang, Y. Characterization of K390 Powder Prepared by Plasma Rotating Electrode Processing. J. Phys. Conf. Ser. 2025, 012019. [Google Scholar] [CrossRef]
- Jiang, J.; Li, S.; Lai, Y.; Wang, Q.; Liang, S. Preparation and characterization of a special high speed steel powder prepared by plasma rotating electrode process. Adv. Powder Technol. 2025, 36, 104886. [Google Scholar] [CrossRef]
- Cui, Y.J.; Zhao, Y.F.; Numata, H.; Bian, H.K.; Wako, K.; Yamanaka, K.; Aoyagi, K.; Zhang, C.; Chiba, A. Effects of plasma rotating electrode process parameters on the particle size distribution and microstructure of Ti-6Al-4 V alloy powder. Powder Technol. 2020, 376, 363–372. [Google Scholar] [CrossRef]
- Cui, Y.J.; Zhao, Y.F.; Numata, H.; Yamanaka, K.; Bian, H.K.; Aoyagi, K.; Chiba, A. Effects of process parameters and cooling gas on powder formation during the plasma rotating electrode process. Powder Technol. 2021, 393, 301–311. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Cui, Y.J.; Numata, H.; Bian, H.K.; Wako, K.; Yamanaka, K.; Aoyagi, K.; Chiba, A. Centrifugal granulation behavior in metallic powder fabrication by plasma rotating electrode process. Sci. Rep. 2020, 10, 18446. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, Y.; Vyacheslav, T.; Song, C.; Zhou, H.; Wang, D. Effects of particle size on processability of AlSi10Mg alloy manufactured by selective laser melting. Acta Met. Sin 2022, 59, 147–156. [Google Scholar]
- He, W.W.; Liu, Y.; Tang, H.P.; Li, Y.P.; Liu, B.; Liang, X.P.; Xi, Z.P. Microstructural characteristics and densification behavior of high-Nb TiAl powder produced by plasma rotating electrode process. Mater. Des. 2017, 132, 275–282. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.Y.; Wang, B.; Yao, C.G. Comparative study of IN600 superalloy produced by two powder metallurgy technologies: Argon Atomizing and Plasma Rotating Electrode Process. Vacuum 2018, 156, 302–309. [Google Scholar] [CrossRef]
- Fan, X.P.; Tian, Q.X.; Chu, X.; Liaw, P.K.; Tong, Y.; Chen, S.Y.; Meng, F.C. Microstructure and mechanical properties of Co 31.5 Cr 7 Fe 30 Ni 31.5 high-entropy alloy powder produced by plasma rotating electrode process and its applications in additive manufacturing. J. Mater. Res. Technol. 2024, 31, 1924–1938. [Google Scholar] [CrossRef]
- Xiang, C.S.; Wang, X.F.; Yang, J.; Li, H.T.; Fan, X.P.; Ge, Z.H. Preparation of High-Quality 316H Austenitic Stainless-Steel Powder by Electroslag Remelting and Plasma-Rotating Electrode Process. Powder Metall. Met. Ceram. 2022, 60, 653–661. [Google Scholar] [CrossRef]
- Yang, W.G.; Li, M.A.; Su, S.; Xiao, S.L.; Chen, Y.Y. Effects of carbon addition on the microstructure and mechanical property of in-situ reinforced TiAl matrix composite powders produced by plasma rotating electrode process. J. Mater. Res. Technol. 2023, 27, 5204–5218. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, S.Y.; Tan, P.; Yin, J.O.; Zhou, Q.; Ge, Y.; Li, Z.F.; Wang, J.; Tang, H.P.; Cao, P. Shape memory TiNi powders produced by plasma rotating electrode process for additive manufacturing. Trans. Nonferrous Met. Soc. China 2017, 27, 2647–2655. [Google Scholar] [CrossRef]
- Suastiyanti, D.; Pratomo, H.; Hamdani, A.H. Designing a centrifugal atomization machine with trial manufacturing Sn powder through various rotation. J. Ceram. Process. Res. 2021, 22, 705–713. [Google Scholar] [CrossRef]
- Li, L.; Peng, L.; Zhao, W. Effects of process parameters on the spreading morphology of disc surface and aluminium powder produced by centrifugal atomisation. Powder Metall. 2023, 66, 509–518. [Google Scholar] [CrossRef]
- Salges, S.A.C.; Pijuan, J.; Hernández, R.; Riera, M.D. Effect of processing parameters on copper powder produced by novel hybrid atomisation technique. Powder Metall. 2020, 63, 142–148. [Google Scholar] [CrossRef]
- Plookphol, T.; Wisutmethangoon, S.; Gonsrang, S. Influence of process parameters on SAC305 lead-free solder powder produced by centrifugal atomization. Powder Technol. 2011, 214, 506–512. [Google Scholar] [CrossRef]
- Cegarra, S.A.; Pijuan, J.; Riera, M.D. Cooling Rate Modeling and Evaluation during Centrifugal Atomization Process. J. Manuf. Mater. Process. 2023, 7, 112. [Google Scholar] [CrossRef]
- Bruncko, M.; Erman, Z.; Kirbis, P.; Anzel, I. The Application of Centrifugal Atomization Method for Preparation of Rapidly Solidified Nd-Fe-B Flakes Used for Production of Permanent Magnets. J. Mater. Eng. Perform. 2018, 27, 5136–5140. [Google Scholar] [CrossRef]
- Tian, L.; Anderson, I.; Riedemann, T.; Russell, A. Production of fine calcium powders by centrifugal atomization with rotating quench bath. Powder Technol. 2017, 308, 84–93. [Google Scholar] [CrossRef]
- He, W.; Lv, X.; Pan, F.; Li, X.; Yan, Z. Novel preparation process of iron powders with semisteel by rotary cup atomizer. Powder Technol. 2019, 356, 1087–1096. [Google Scholar] [CrossRef]
- Wolf, S.; Riedemann, T.M.; Barclay, J.; Holladay, J.; Anderson, I.E.; Cui, J. Synthesis and magnetic performance of gadolinium powder produced with rotating disk atomization. Powder Technol. 2020, 359, 331–336. [Google Scholar] [CrossRef]
- Sungkhaphaitoon, P.; Wisutmethangoon, S.; Plookphol, T. Influence of Process Parameters on Zinc Powder Produced by Centrifugal Atomisation. Mater. Res. 2017, 20, 718–724. [Google Scholar] [CrossRef]
- Denmud, N.; Plookphol, T. Characteristics of SAC305 Lead-Free Powder Prepared by Centrifugal Atomization. Key Eng. Mater. 2018, 777, 322–326. [Google Scholar] [CrossRef]
- Zhao, Y.Y. A simplified model for velocity and temperature evolution of alloy droplets in centrifugal atomisation and spray deposition. In Pricm 5: The Fifth Pacific Rim International Conference on Advanced Materials and Processing, Pts 1–5; Zhong, Z.Y., Saka, H., Kim, T.H., Holm, E.A., Han, Y.F., Xie, X.S., Eds.; Materials Science Forum: Zurich, Switzerland, 2005; Volume 475–479, pp. 4261–4265. [Google Scholar]
- Gao, S.; Fu, A.; Xie, Z.; Liao, T.; Cao, Y.; Liu, B. Preparation and Microstructure of High-Activity Spherical TaNbTiZr Refractory High-Entropy Alloy Powders. Materials 2023, 16, 791. [Google Scholar] [CrossRef]
- Mantripragada, V.T.; Kumar, K.; Kumar, P.; Sarkar, S. Modeling of Powder Production During Centrifugal Atomization. J. Sustain. Metall. 2021, 7, 620–629. [Google Scholar] [CrossRef]
- Ravry, B.; Mathieu, A.; Allenou, J.; Sinardet, B.; Pernot, S.; Bernard, F.; Stepnik, B.; Demoisson, F. Centrifugal atomization of stainless-steel rotating rods melted by a high-power LASER beam. Adv. Powder Technol. 2022, 33, 103631. [Google Scholar] [CrossRef]
- Guo, Y.; Li, C.; Deng, N.; Sun, H.; Feng, S.; Zhang, Y.; Li, X.; Ci, E.; Li, J. Preparation of high sphericity monodisperse aluminum microspheres by pulsated orifice ejection method. Mater. Today Commun. 2022, 30, 103110. [Google Scholar] [CrossRef]
- Murthy, I.N.; Rao, J.B. Evaluation of the microstructure, secondary dendrite arm spacing, and mechanical properties of Al-Si alloy castings made in sand and Fe-Cr slag molds. Int. J. Miner. Metall. Mater. 2017, 24, 784–793. [Google Scholar] [CrossRef]
- Zhao, J.; Zhong, H.G.; Han, K.; Li, R.X.; Xu, Z.S.; Zhai, Q.J. Relationship between secondary dendrite arm spacing and local solidification time of 30Cr2Ni4MoV alloy at slow cooling rates. J. Iron Steel Res. Int. 2018, 25, 821–829. [Google Scholar] [CrossRef]
- Ramirez-Vidaurri, L.E.; Castro-Román, M.; Herrera-Trejo, M.; Fraga-Chavez, K.L. Secondary dendritic arm spacing and cooling rate relationship for an ASTM F75 alloy. J. Mater. Res. Technol. 2022, 19, 5049–5065. [Google Scholar] [CrossRef]
- Shaoyang, Z.; Jingou, Y.; Lei, S.; Yuan, G.; Gang, C. Ti-60Ta Powders Produced by PREP and Their Properties. RARE Met. Mater. Eng. 2017, 46, 1679–1683. [Google Scholar]
- Zuo, Z.B.; Hu, R.; Luo, X.; Wang, Q.X.; Li, C.X.; Zhu, Z.; Lan, J.; Liang, S.J.; Tang, H.K.; Zhang, K. Solidification Behavior and Microstructures Characteristics of Ti-48Al-3Nb-1.5Ta Powder Produced by Supreme-Speed Plasma Rotating Electrode Process. Acta Metall. Sin. Engl. Lett. 2023, 36, 1221–1234. [Google Scholar] [CrossRef]
- Yamanoglu, R.; Zeren, M.; German, R.M. Solidification Characteristics of Atomized AlCu4Mg1-SiC Composite Powders. J. Min. Metall. Sect. B Metall. 2012, 48, 73–79. [Google Scholar] [CrossRef]
- Xie, B.; Fan, Y.; Zhao, S. Characterization of Ti6Al4V powders produced by different methods for selective laser melting. Mater. Res. Express 2021, 8, 076510. [Google Scholar] [CrossRef]
- Karimi, P.; Keshavarz, M.K.; Sadeghi, E.; Habibnejad, M.; Vlasea, M. Interplay of process, microstructure, and mechanical performance in electron beam-powder bed fusion of Ti48Al2Nb2Cr. Addit. Manuf. 2023, 77, 103811. [Google Scholar] [CrossRef]
- Smythe, S.A.; Thomas, B.M.; Jackson, M. Recycling of Titanium Alloy Powders and Swarf through Continuous Extrusion (ConformTM) into Affordable Wire for Additive Manufacturing. Metals 2020, 10, 843. [Google Scholar] [CrossRef]
- Heaney, D.F. Powders for metal injection molding (MIM). In Handbook of Metal Injection Molding; Woodhead Publishing: Cambridge, UK, 2019; pp. 45–56. [Google Scholar]
- Prather, C.A.; Craig, C.D.; Baumann, J.M.; Morgen, M.M. Experimental and theoretical investigation of rotary atomization dynamics for control of microparticle size during spray congealing process. Powder Technol. 2023, 418, 118278. [Google Scholar] [CrossRef]
- Kumar, P.; Sarkar, S. Experimental investigation of liquid disintegration on slotted disc in centrifugal atomization process. Chem. Eng. Res. Des. 2019, 145, 76–84. [Google Scholar] [CrossRef]
- Westerlund, T.; Hoikka, T. On the modeling of mineral fiber formation. Comput. Chem. Eng. 1989, 13, 1153–1163. [Google Scholar] [CrossRef]
- Bizjan, B.; Sirok, B.; Hocevar, M.; Orbanic, A. Liquid ligament formation dynamics on a spinning wheel. Chem. Eng. Sci. 2014, 119, 187–198. [Google Scholar] [CrossRef]
- Butuzov, A.I.; Pukhovoi, I.I. Liquid-film flow regimes on a rotating surface. J. Eng. Phys. Thermophys. 1976, 31, 891. [Google Scholar] [CrossRef]
- Li, Y.; Sisoev, G.M.; Shikhmurzaev, Y.D. Spinning disk atomization: Theory of the ligament regime. Phys. Fluids 2018, 30, 092101. [Google Scholar] [CrossRef]
- Thomas, S.; Faghri, A.; Hankey, W. Experimental Analysis and Flow Visualization of a Thin Liquid Film on a Stationary and Rotating Disk. J. Fluids Eng. 1991, 113, 73–80. [Google Scholar] [CrossRef]
- Peng, H.; Wang, N.; Wang, D.; Ling, X. Experimental Study on the Critical Characteristics of Liquid Atomization by a Spinning Disk. Ind. Eng. Chem. Res. 2016, 55, 6175–6185. [Google Scholar] [CrossRef]
- Liu, J.X.; Yu, Q.B.; Qin, Q. Numerical study on film disintegration by centrifugal atomisation using rotating cup. Powder Metall. 2013, 56, 288–294. [Google Scholar] [CrossRef]
- Wang, D.; Ling, X.; Peng, H. Simulation of ligament mode breakup of molten slag by spinning disk in the dry granulation process. Appl. Therm. Eng. 2015, 84, 437–447. [Google Scholar] [CrossRef]
- Hinze, J.O.; Milborn, H. Atomization of Liquids by Means of a Rotating Cup. J. Appl. Mech. 1950, 17, 145–153. [Google Scholar] [CrossRef]
- Kamiya, T. An analysis of the ligament-type disintegration of thin liquid film at the edge of rotating disk. J. Chem. Eng. Jpn. 1972, 5, 391–396. [Google Scholar] [CrossRef]
- Liu, J.; Yu, Q.; Guo, Q. Experimental investigation of liquid disintegration by rotary cups. Chem. Eng. Sci. 2012, 73, 44–50. [Google Scholar] [CrossRef]
- Zdujić, M.; Uskoković, D. Production of atomized metal and alloy powders by the rotating electrode process. Sov. Powder Met. Met. Ceram. 1990, 29, 683. [Google Scholar] [CrossRef]
- Champagne, B.; Angers, R. REP atomization mechanisms. Int. J. Powder Metall. 1984, 16, 125–128. [Google Scholar]
- Neikov, O.D. Chapter 5—Atomization and Granulation. In Handbook of Non-Ferrous Metal Powders; Neikov, O.D., Naboychenko, S.S., Murashova, I.V., Gopienko, V.G., Frishberg, I.V., Lotsko, D.V., Eds.; Elsevier: London, UK, 2009; pp. 102–142. [Google Scholar]
- Liu, Y.; Liang, S.; Han, Z.; Song, J.; Wang, Q. A novel model of calculating particle sizes in plasma rotating electrode process for superalloys. Powder Technol. 2018, 336, 406–414. [Google Scholar] [CrossRef]
- Frost, A.R. Rotary atomization in the ligament formation mode. J. Agric. Eng. Res. 1981, 26, 63–78. [Google Scholar] [CrossRef]
- Fraser, R.P.; Dombrowski, N.; Routley, J.H. The filming of liquids by spinning cups. Chem. Eng. Sci. 1963, 18, 323–337. [Google Scholar] [CrossRef]
- Wang, D.; Ling, X.; Peng, H.; Cui, Z.; Yang, X. High-temperature analogy experimental investigation on dry granulating characteristic of rotating disk for waste heat utilization of molten slag. Appl. Therm. Eng. 2017, 125, 846–855. [Google Scholar] [CrossRef]
- Wang, D.; Cui, Z.; Yu, J.; Yang, X. Particle characteristics of centrifugal atomization by rotary disk for Newtonian viscous fluid. Food Mach. 2018, 34, 75–80. [Google Scholar]
- Zhao, Y.Y. Analysis of flow development in centrifugal atomization: Part I. Film thickness of a fully spreading melt. Model. Simul. Mater. Sci. Eng. 2004, 12, 959–971. [Google Scholar] [CrossRef]
- Tan, Y.; Jia, M.; Yan, H.; Shi, J.; Shen, X.; Wu, J.; Zhu, X. Dynamic film fragmentation in a rotating disk atomizer: A comparative study of fluids with diverse viscosities. Chem. Eng. Sci. 2024, 298, 120340. [Google Scholar] [CrossRef]
- Mantripragada, V.T.; Sarkar, S. Prediction of drop size from liquid film thickness during rotary disc atomization process. Chem. Eng. Sci. 2017, 158, 227–233. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Jacobs, M.H.; Dowson, A.L. Liquid flow on a rotating disk prior to centrifugal atomization and spray deposition. Metall. Mater. Trans. B 1998, 29, 1357–1369. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Dowson, A.L.; Jacobs, M.H. Modelling of liquid flow after a hydraulic jump on a rotating disk prior to centrifugal atomization. Model. Simul. Mater. Sci. Eng. 2000, 8, 55–65. [Google Scholar] [CrossRef]
- Yanagisawa, M. Slip effect for thin liquid film on a rotating disk. J. Appl. Phys. 1987, 61, 1034–1037. [Google Scholar] [CrossRef]
- Ahmed, M.; Youssef, M.S. Influence of spinning cup and disk atomizer configurations on droplet size and velocity characteristics. Chem. Eng. Sci. 2014, 107, 149–157. [Google Scholar] [CrossRef]
- Tan, Y.; Chen, X.D.; Jia, M.J.; He, W.C.; Qin, Y.L.; Ding, B.; Wu, J.J. Evaluating rotating disk vs. cup atomizers: Atomization performance in film fragmentation mode. Chem. Eng. Sci. 2025, 306, 121251. [Google Scholar] [CrossRef]
- Sahoo, K.; Kumar, S. Influence of surface and edge profile of a spinning disc on its atomization characteristics in direct drop mode. Chem. Eng. Sci. 2024, 287, 119743. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, H.; Zhu, X.; Lv, Y.W.; He, X.Y.; Liao, Q. On the centrifugal granulation characteristics by rotary disk: Effect of outer edge structure. Appl. Therm. Eng. 2019, 159, 113977. [Google Scholar] [CrossRef]
- Munekata, M.; Oseto, T.; Kurishima, H.; Yshikawa, H. Effect of Disk Edge Profile on Scattering Characteristics of Liquid Droplets Splashed from Spinning Disk. Open J. Fluid Dyn. 2013, 3, 22–27. [Google Scholar] [CrossRef]
- Peng, H.; Shan, X.K.; Ling, X.; Wang, D.X.; Li, J. Ligament-type granulation of molten slag in different rotary disk configurations. Appl. Therm. Eng. 2018, 128, 1565–1578. [Google Scholar] [CrossRef]
- Wu, J.J.; Wang, H.; Zhu, X.; Liao, Q.; Li, K. Cold experiment of slag centrifugal granulation by rotary atomizer: Effect of atomizer configuration. Appl. Therm. Eng. 2017, 111, 1557–1564. [Google Scholar] [CrossRef]
- Labrecque, C.; Angers, R.; Tremblay, R.; Dube, D. Inverted disk centrifugal atomization of AZ91 magnesium alloy. Can. Metall. Q. 1997, 36, 169–175. [Google Scholar] [CrossRef]
- Wang, J.; Peng, H.; Ling, X. Ligament Mode Disintegration of Liquid Film at the Rotary Disk Rim in Waste Heat Recovery Process of Molten Slag. Energy Procedia 2014, 61, 1824–1829. [Google Scholar] [CrossRef]
- Wang, D.; Ling, X.; Peng, H.; Cui, Z.; Yang, X. Experimental Investigation of Ligament Formation Dynamics of Thin Viscous Liquid Film at Spinning Disk Edge. Ind. Eng. Chem. Res. 2016, 55, 9267–9275. [Google Scholar] [CrossRef]
- Li, H.; Deng, X. Prediction of powder particle size during centrifugal atomisation using a rotating disk. Sci. Technol. Adv. Mater. 2007, 8, 264–270. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, Q.; Ma, W.; Liu, J.; Tao, S. Experimental investigation on centrifugal granulation of molten yellow phosphorus slag. Chem. Eng. Res. Des. 2023, 197, 548–557. [Google Scholar] [CrossRef]
- Li, M.; Zhao, J.; Zhang, X.; Xu, N.; Meng, H.; Wu, Z.; Wang, S. Effects of granulator structure and cooperating mode with slag tube on the centrifugal granulation characteristics of molten slag. Appl. Therm. Eng. 2021, 193, 117026. [Google Scholar] [CrossRef]
- Shao, C.; Kang, Y.; Xing, H.W.; Liu, C.; Lin, W.L.; Sun, R.J.; Zhou, J. Experimental and simulation on the granulation process of blast furnace slag. Steel Vanadium Titan. 2024, 45, 104–114. [Google Scholar]
- Ciurans-Oset, M.; Mouzon, J.; Akhtar, F. Fabrication of spherical Ti-V-Zr-Nb-Mo-Hf-Ta-W refractory high-entropy alloy by a combination of spark plasma sintering, cryogenic grinding, and plasma centrifugal atomization. J. Alloys Compd. 2025, 1037, 182178. [Google Scholar] [CrossRef]
- Yang, W.G.; Li, M.G.; Xiao, S.L.; Chen, Y.Y. Design and investigation of strength-ductility TiAl matrix composites with a novel dual-layers couple reinforced structure. Mater. Sci. Eng. A 2024, 918, 147482. [Google Scholar] [CrossRef]
- Wen, F.; Liu, W.T.; Fu, A.; Huang, Q.L.; Wang, J.; Cao, Y.K.; Qiu, J.W.; Liu, B. Microstructure and Mechanical Properties of the Powder Metallurgy Nb-16Si-24Ti-2Al-2Cr Alloy. Materials 2024, 17, 4155. [Google Scholar] [CrossRef]
- Sungkhaphaitoon, P.; Plookphol, T.; Wisutmethangoon, S. Design and development of a centrifugal atomizer for producing zinc metal powder. Int. J. Appl. Phys. Math. 2012, 2, 77–82. [Google Scholar] [CrossRef]
- Uskokovic, D.; Uskokovic, V. Magical spherical particles produced by centrifugal atomization. Powder Technol. 2024, 444, 120017. [Google Scholar] [CrossRef]
- Strondl, A.; Lyckfeldt, O.; Brodin, H.; Ackelid, U. Characterization and Control of Powder Properties for Additive Manufacturing. JOM 2015, 67, 549–554. [Google Scholar] [CrossRef]
- Sutton, A.T.; Kriewall, C.S.; Leu, M.C.; Newkirk, J.W. Powder characterisation techniques and effects of powder characteristics on part properties in powder-bed fusion processes. Virtual Phys. Prototyp. 2017, 12, 3–29. [Google Scholar] [CrossRef]
- Zhong, C.; Chen, J.; Linnenbrink, S.; Gasser, A.; Sui, S.; Poprawe, R. A comparative study of Inconel 718 formed by High Deposition Rate Laser Metal Deposition with GA powder and PREP powder. Mater. Des. 2016, 107, 386–392. [Google Scholar] [CrossRef]
- Guo, R.-P.; Xu, L.; Zong, B.Y.-P.; Yang, R. Characterization of Prealloyed Ti-6Al-4V Powders from EIGA and PREP Process and Mechanical Properties of HIPed Powder Compacts. Acta Metall. Sin. Engl. Lett. 2017, 30, 735–744. [Google Scholar] [CrossRef]
- Cegarra, S.A.; Maicas, H.; Pijuan, J. Centrifugal Atomization and Characterization of Fe-Si-B Amorphous Alloys. Materials 2025, 18, 510. [Google Scholar] [CrossRef]
- Ravry, B.; Mathieu, A.; Bernard, F.; Demoisson, F. Atomization regime observation and characterization of an atomized pure zinc rod during centrifugal atomization using a LASER beam. Powder Technol. 2023, 415, 118139. [Google Scholar] [CrossRef]
- Long, L.; Lei, P.; Wei, Z. Study of liquid spreading and particle size distribution during the preparation of aluminum alloy powder by rotary disc atomization. J. Exp. Fluid Mech. 2023, 38, 1–10. [Google Scholar] [CrossRef]
- Angers, R.; Tremblay, R.; Dube, D. Formation of irregular particles during centrifugal atomization of AZ91 alloy. Mater. Lett. 1997, 33, 13–18. [Google Scholar] [CrossRef]
- Angers, R.; Tremblay, R.; Desrosiers, L.; Dube, D. Rotating disk coatings for centrifugal atomization of aluminium and magnesium alloys. Can. Metall. Q. 1996, 35, 291–297. [Google Scholar] [CrossRef]
- Neikov, O.D.; Gopienko, V.G. Chapter 18—Production of Titanium and Titanium Alloy Powders. In Handbook of Non-Ferrous Metal Powders, 2nd ed.; Neikov, O.D., Naboychenko, S.S., Yefimov, N.A., Eds.; Elsevier: London, UK, 2019; pp. 549–570. [Google Scholar]
- Yamanoglu, R.; German, R.M.; Karagoz, S.; Bradbury, W.L.; Zeren, M.; Li, W.; Olevsky, E.A. Microstructural investigation of as cast and PREP atomised Ti-6Al-4V alloy. Powder Metall. 2011, 54, 604–607. [Google Scholar] [CrossRef]
- Xie, J.W.; Zhao, Y.Y.; Dunkley, J.J. Effects of processing conditions on powder particle size and morphology in centrifugal atomisation of tin. Powder Metall. 2004, 47, 168–172. [Google Scholar] [CrossRef]
- Ho, K.H.; Zhao, Y. Modelling thermal development of liquid metal flow on rotating disc in centrifugal atomisation. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2004, 365, 336–340. [Google Scholar] [CrossRef]
- Gonsrang, S.; Plookphol, T.; Wisutmethangoon, S. The Effect of Rotating Atomizer Geometry on the SAC305 Lead-Free Solder Powder Production. Available online: https://www.researchgate.net/publication/267768898_THE_EFFECT_OF_ROTATING_ATOMIZER_GEOMETRY_ON_THE_SAC305_LEAD-FREE_SOLDER_POWDER_PRODUCTION (accessed on 15 October 2025).
- Yu, S.; Zhao, Y.G.; Zhao, G.Y.; Liu, Q.; Yao, B.C.; Liu, H. Review on preparation technology and properties of spherical powders. Int. J. Adv. Manuf. Technol. 2024, 132, 1053–1069. [Google Scholar] [CrossRef]
- Yim, S.; Bian, H.; Aoyagi, K.; Yamanaka, K.; Chiba, A. Effect of powder morphology on flowability and spreading behavior in powder bed fusion additive manufacturing process: A particle-scale modeling study. Addit. Manuf. 2023, 72, 103612. [Google Scholar] [CrossRef]
- Luo, Y.W.; Liu, F.; Tan, L.M.; Ma, X.; Zhang, Y.; Zeng, Q.; Wang, Y.; Zhang, D.J.; Sun, J.; Huang, L. Effect of powder characteristic on microstructure and mechanical property of additive manufacturing nickel-based superalloy IN718. J. Mater. Res. Technol. 2025, 37, 1705–1715. [Google Scholar] [CrossRef]
- Baskoro, A.S.; Fauzian, A.; Basalamah, H.; Kiswanto, G.; Winarto, W. Improving weld penetration by employing of magnetic poles’ configurations to an autogenous tungsten inert gas (TIG) welding. Int. J. Adv. Manuf. Technol. 2018, 99, 1603–1613. [Google Scholar] [CrossRef]
- Dong, W.; Meng, Y.; Xu, F.M.; Han, Y.; Wang, Y.Y.; Wang, X.M.; Zhao, Y. Preparation of Sn-Pb Spherical Fine Metal Powders by Centrifugal Atomization Based on Mono-Sized Droplets. Powder Metall. Met. Ceram. 2020, 59, 239–248. [Google Scholar] [CrossRef]
- Filho, W.A.S.; Silveira, G.M.S.; Costa, J.F.M.; Mendes, M.C.; de Souza, L.F.G.; Jorge, J.C.F. Microstructure and impact toughness of high strength steel weld metals deposited by MCAW-RE process using different shielding gases. Int. J. Adv. Manuf. Technol. 2021, 115, 3105–3120. [Google Scholar] [CrossRef]
- Pijuan, J.; Cegarra, S.A.; Dosta, S.; Albaladejo-Fuentes, V.; Riera, M.D. Centrifugal Atomization of Glass-Forming Alloy Al86Ni8Y4.5La1.5. Materials 2022, 15, 8159. [Google Scholar] [CrossRef]
- Eslamian, M.; Rak, J.; Ashgriz, N. Preparation of aluminum/silicon carbide metal matrix composites using centrifugal atomization. Powder Technol. 2008, 184, 11–20. [Google Scholar] [CrossRef]
- Ciurans-Oset, M.; Mouzon, J.; Akhtar, F. Flexible Production of Spherical Powders of Ti-V-Zr-Nb-Mo-Hf-Ta-W Refractory High-Entropy Alloys by Plasma-Assisted Centrifugal Atomization. Adv. Mater. Technol. 2025, 10, 2401643. [Google Scholar] [CrossRef]


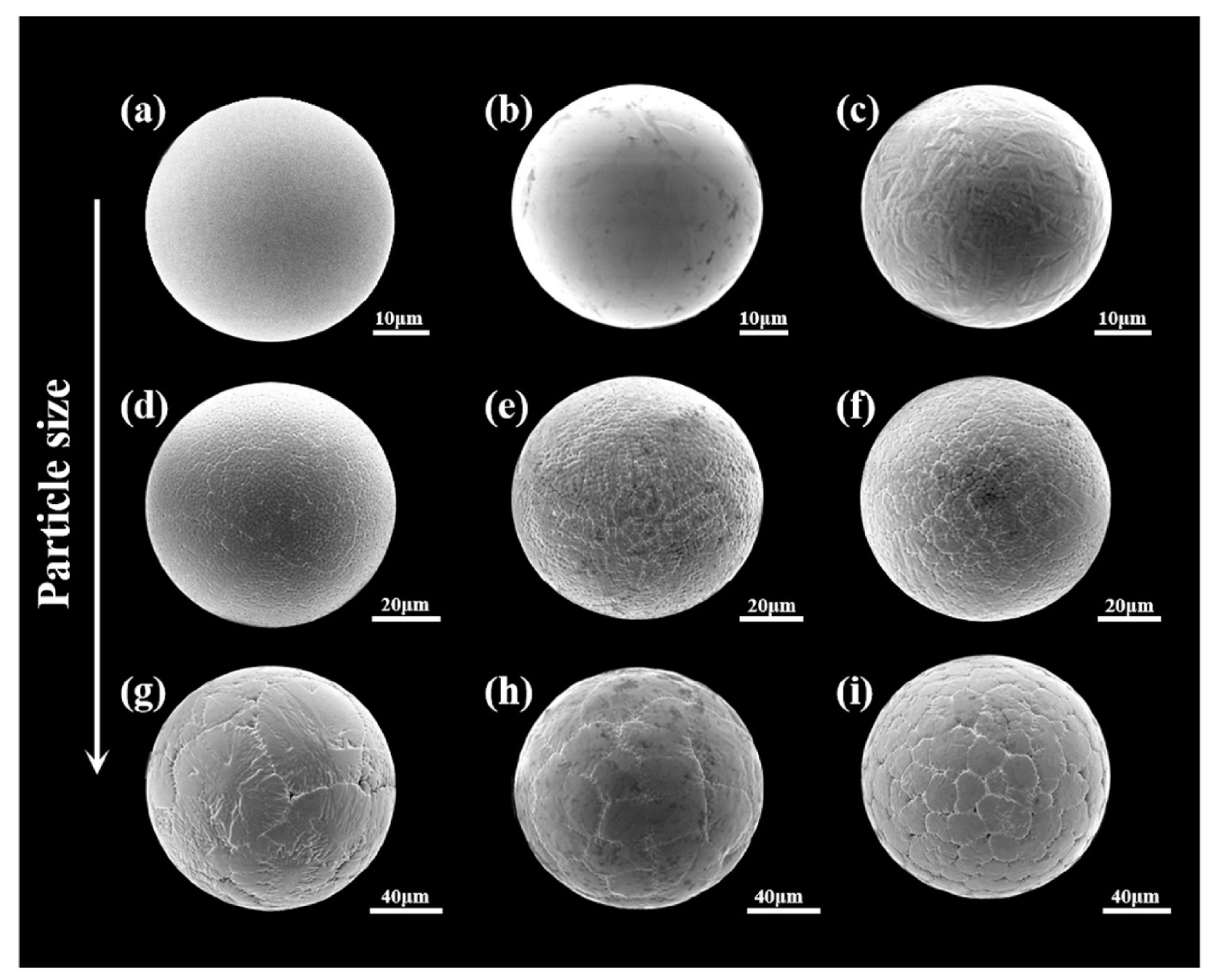
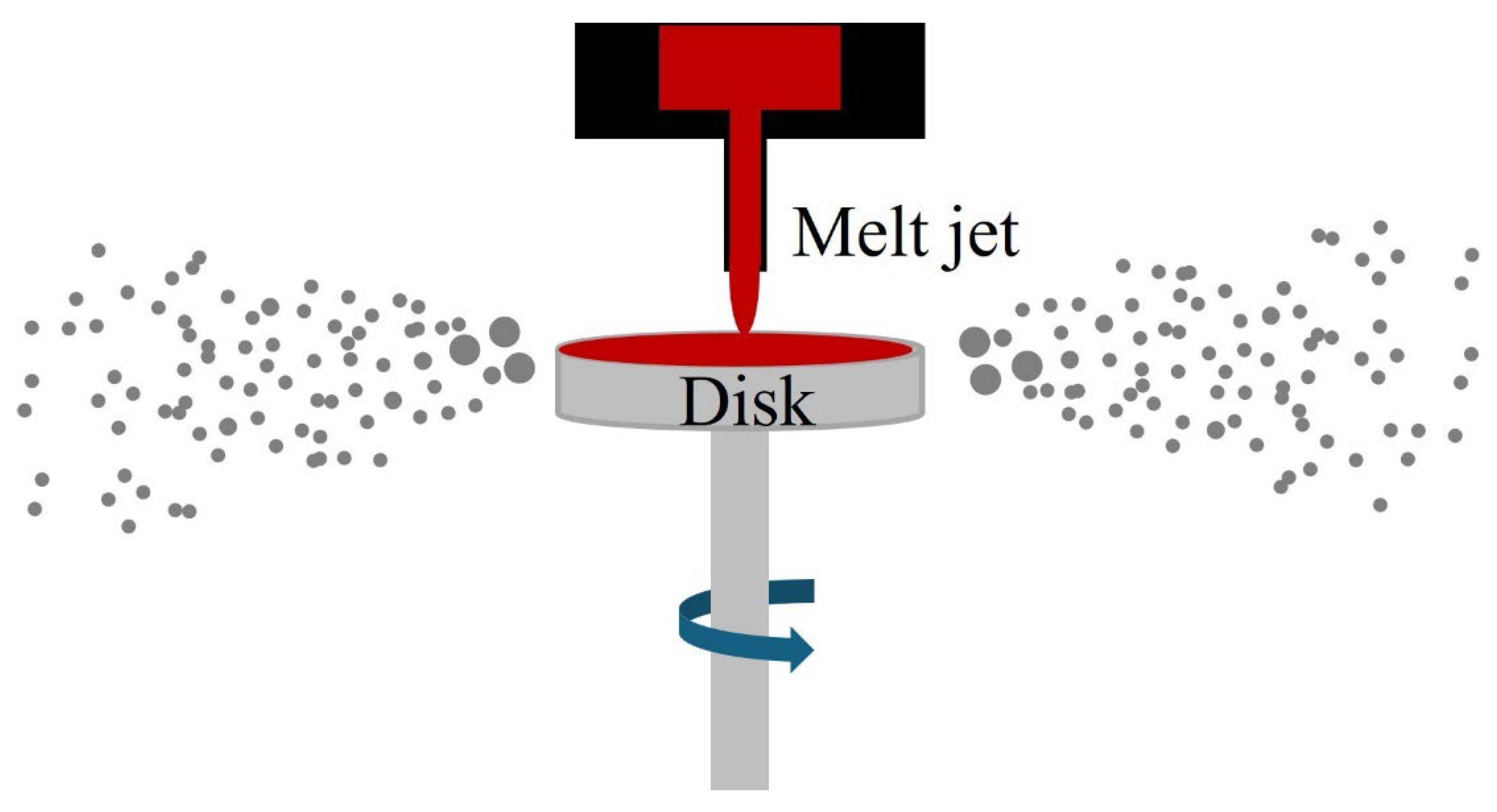
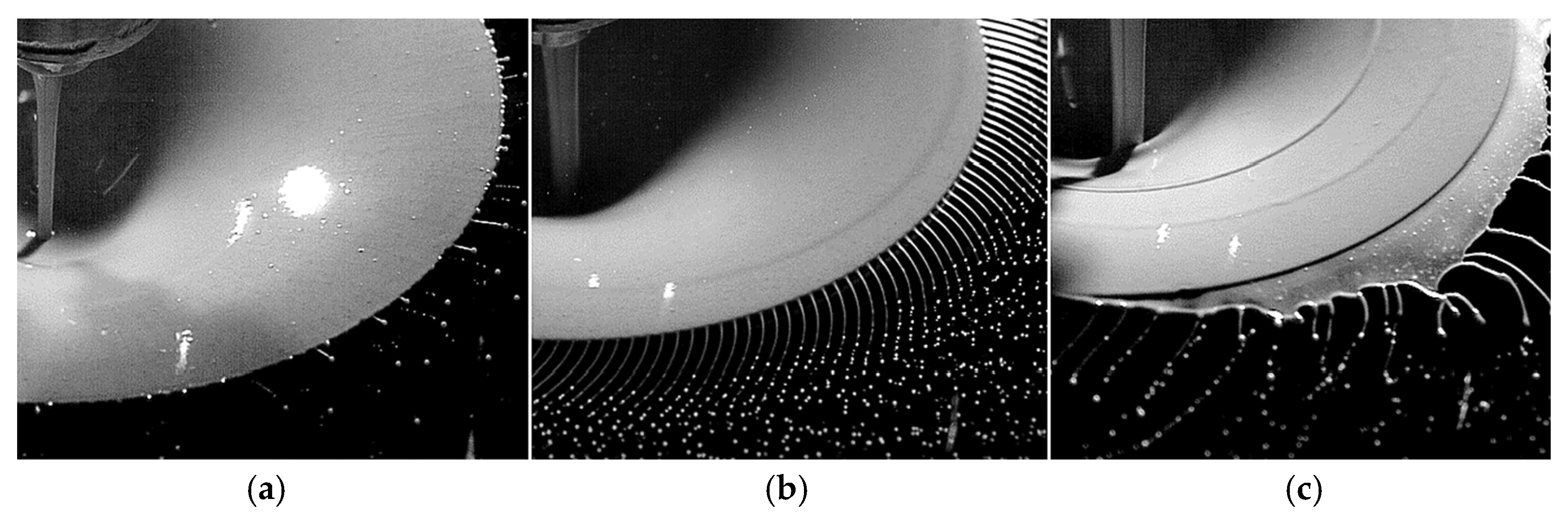
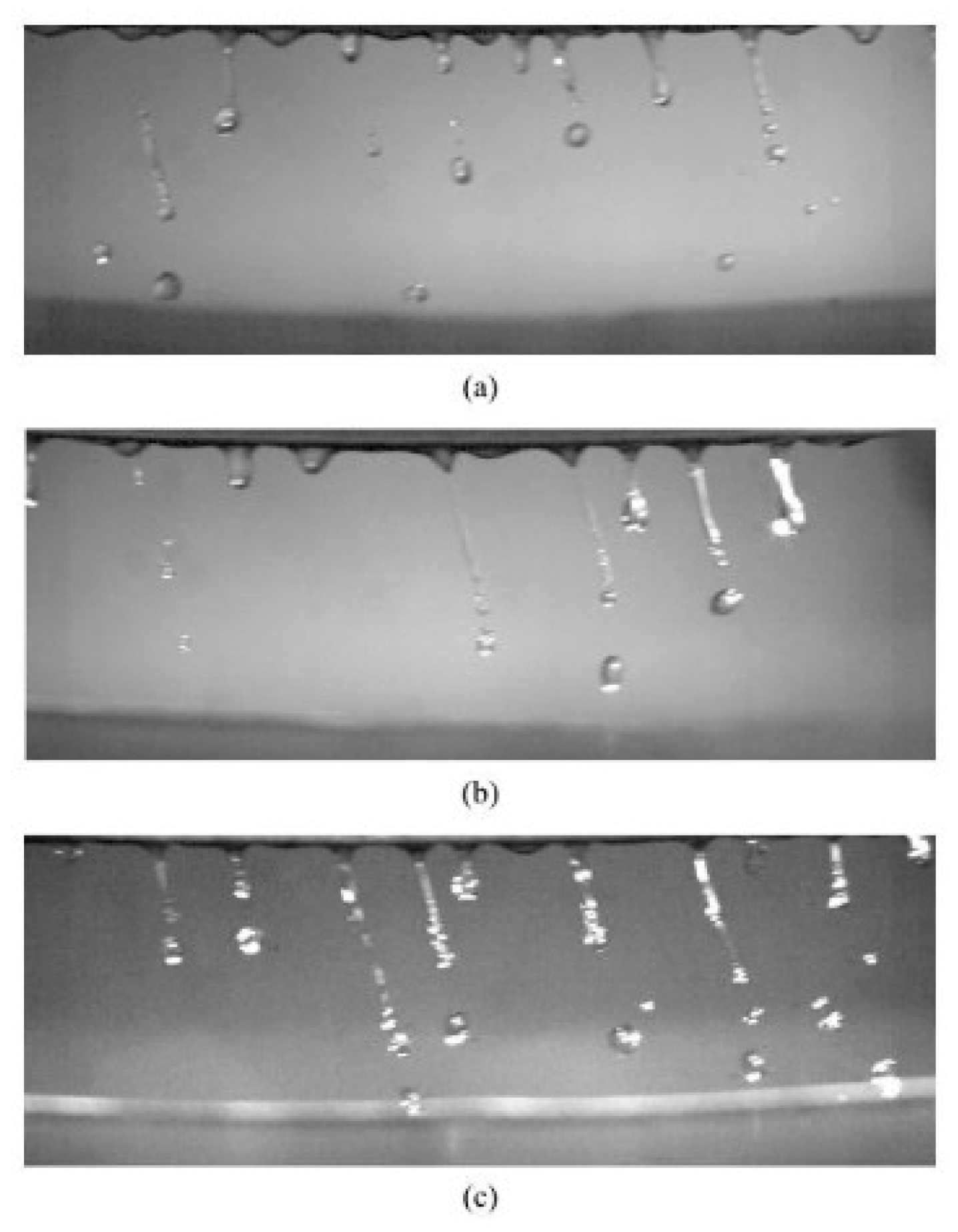

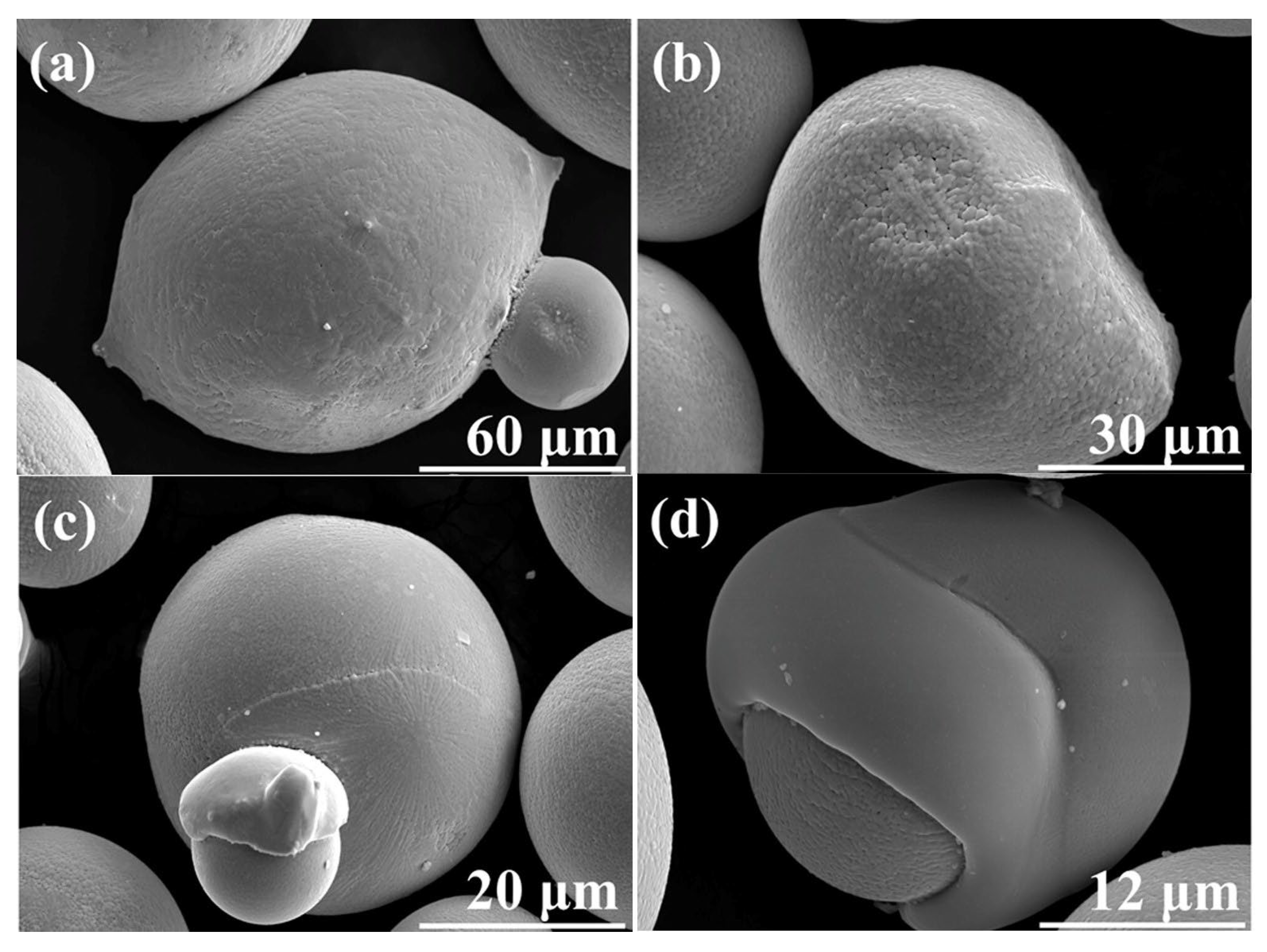

| Type | Particle Size (μm) | Flowability (s/50 g) | Apparent Density (g/cm3) | Tap Density (g/cm3) | Ref. |
|---|---|---|---|---|---|
| Ti6Al4V | 60–120 | 29.6 | 2.59 | - | [20] |
| W | 45–150 | 6 | 11.35 | 12.5 | [21] |
| W-0.3%La2O3 | 45–150 | 6.4 | 10.91 | 11.62 | |
| W-1%La2O3 | 45–150 | 6.33 | 10.85 | 11.24 | |
| Ni-11Mo-8Al-3Ta-2Cr-1Re | <50 | 13.7 | 4.83 | 5.15 | [13] |
| 50–150 | 12.2 | 4.86 | 5.24 | ||
| S800 Ag | 23.08–51.04 | - | 5.92 | 6.2 | [22] |
| Ti6Al4V | <45 | 23.17 | - | - | [23] |
| 100–150 | 26.61 | ||||
| 178–250 | 28.48 | ||||
| Ti-28at.%Ta | 30–260 | 15.5 | - | - | [24] |
| IN718 | 26.5–39.9 | 12.4 | 4.84 | - | [25] |
| EP648 | 55–110 | 17.08 | 4.8 | - | [26] |
| 110–170 | 18.36 | 4.84 | |||
| IC21 | <53 | 14.47 | 4.72 | 5.24 | [19] |
| 53–106 | 14.92 | 4.76 | 5.21 | ||
| K390 | <150 | 13.51 | 4.89 | 6.13 | [27] |
| T15 high speed steel | <250 | 11.7 | 5.17 | 5.5 | [28] |
| Type | Average Particle Size (μm) | Rotational Speed (rpm) | Diameter of the Electrode (mm) | Arc Current (A) | Gas Type | Gas Flow Rate (L/min) | Ref. |
|---|---|---|---|---|---|---|---|
| Ti64 | ~550 | 7000 | 15 | 80 | Ar | - | [29] |
| ~450 | 9000 | ||||||
| ~400 | 11,700 | ||||||
| ~500 | 7000 | 20 | |||||
| ~410 | 9000 | ||||||
| ~325 | 11,700 | ||||||
| ~400 | 7000 | 25 | |||||
| ~350 | 9000 | ||||||
| ~250 | 11,700 | ||||||
| Ti64 | ~580 | 7000 | 15 | 50 | Ar | - | [30] |
| ~600 | 70 | ||||||
| ~650 | 90 | ||||||
| ~378 | - | 15 | 80 | Ar | 0 | ||
| ~370 | 50 | ||||||
| ~350 | 100 | ||||||
| ~380 | 150 | ||||||
| ~340 | He | 0 | |||||
| ~325 | 50 | ||||||
| ~348 | 100 | ||||||
| ~350 | 150 | ||||||
| Ti64 | ~640 | 7000 | 15 | 80 | Ar | - | [31] |
| ~480 | 9000 | ||||||
| ~380 | 11,700 | ||||||
| ~500 | 7000 | 20 | |||||
| ~430 | 9000 | ||||||
| ~350 | 11,700 | ||||||
| ~450 | 7000 | 25 | |||||
| ~330 | 9000 | ||||||
| ~280 | 11,700 | ||||||
| 371.3 | 11,700 | 15 | 0 | ||||
| 384.4 | 70 | ||||||
| 421.7 | 160 | ||||||
| SUS316 | ~320 | 7000 | 20 | - | |||
| ~260 | 9000 | ||||||
| ~200 | 11,700 | ||||||
| ~280 | 7000 | 20 | 50 | ||||
| ~350 | 70 | ||||||
| ~365 | 90 | ||||||
| Ti6Al4V | 240 (D50) | 8000 | 75 | - | Ar | - | [17] |
| 226 (D50) | 10,000 | ||||||
| 185 (D50) | 12,000 | ||||||
| 156 (D50) | 14,000 | ||||||
| Ti-45Al-7Nb-0.3W | 46–150 (full range) | 15,000–16,000 | 75 | 1100 | Ar | - | [33] |
| Ti-48Al-2Nb-2Cr | 25–150 (full range) | 32,000–42,000 | 30 | 650–700 | - | - | [16] |
| Ti-28 at.%Ta | 30–260 (full range) | 12,000 | 50 | 1100 | - | - | [24] |
| IN600 | 70–130 (full range) | - | - | - | Ar | - | [34] |
| S800 Ag | 15–60 (full range) | 25,000–37,000 | 30 | 500–700 | Ar | - | [22] |
| Co31.5Cr7Fe30Ni31.5 | 20–106 (full range) | 23,000–32,000 | 29 | 760 | - | [35] | |
| 316H | 51.3 (D50) | 10,000–35,000 | 50 | 1200–1550 | Ar | - | [36] |
| Type | Average Powder Size (μm) | Rotating Atomizer | Melt Feed Rate (kg/h) | Diameter of Atomizer (mm) | Rotating Speed (rpm) | Melt Temperature (°C) | Ref. |
|---|---|---|---|---|---|---|---|
| Al | 107.9 (D50) | 12° tapered | 2.94 mL/s | 59 | 18,000 | - | [40] |
| 99.1 (D50) | 24,000 | ||||||
| 90.2 (D50) | 33,000 | ||||||
| 80.2 (D50) | 39,000 | ||||||
| 266.8 (D50) | R90 curved | 5.33 mL/s | 59 | 6000 | - | ||
| 179 (D50) | 12,000 | ||||||
| 147.7 (D50) | 18,000 | ||||||
| 116 (D50) | 24,000 | ||||||
| 85.5 (D50) | R80 curved | 2.94 mL/s | 59 | 24,000 | - | ||
| 99.7 (D50) | Flat | ||||||
| Cu | 88.3 (D50) | Disk | 75 | 40 | 15,000 | 1200 | [41] |
| 72.4 (D50) | 25,000 | ||||||
| 68.1 (D50) | 35,000 | ||||||
| SAC305 | ~90/~95 | Flat disk | 32 | 50 | 10,000 | 318 | [42] |
| ~65/~67 | 15,000 | ||||||
| ~60/~70 | 20,000 | ||||||
| ~45/~50 | 25,000 | ||||||
| ~44/~49 | 30,000 | ||||||
| ~50/~56 | 17.7 | 20,000 | |||||
| ~60/~70 | 31.3 | ||||||
| ~70/~80 | 64.7 | ||||||
| ~130/145 | 133.6 | ||||||
| ~110/~130 | Flat disk | 63.3 | 30 | ||||
| ~105/~115 | 40 | ||||||
| ~70/~80 | 50 | ||||||
| ~108/~112 | Cup | 63.3 | 30 | 20,000 | |||
| ~85/~90 | 40 | ||||||
| ~70/~75 | 50 | ||||||
| Al-4%Cu | 113 (D50 with Ar) | Disk | - | 45 | 40,000 | 776.85 | [43] |
| 107 (D50 with Ar) | 576.85 | ||||||
| 119 (D50 with He) | 776.85 | ||||||
| 104 (D50 with He) | 576.85 | ||||||
| Nd-Fe-B alloy | 320 (thickness) | Wheel | - | - | 210 | ~1452 | [44] |
| 120 (thickness) | 280 | ||||||
| Ca | 126.1/123.8 (D50) 168.2/181.8 (D50) | Cup | - | 51 | 20,000 | - | [45] |
| Semisteel | 550 | Cup | 1.3 × 10−5 m3/s | 110 | 600 | 1450 | [46] |
| ~525 | 800 | ||||||
| 490 | 1000 | ||||||
| Gd | 216 (D50) | Disk | - | 41 | 8000 | 1433 | [47] |
| Zn | ~275 (D50) | Disk | 50.47 | 40 | 10,000 | 550 | [48] |
| ~250 (D50) | 15,000 | ||||||
| ~200 (D50) | 20,000 | ||||||
| ~150 (D50) | 25,000 | ||||||
| ~125 (D50) | 30,000 | ||||||
| ~160 (D50) | 30 | ||||||
| ~110 (D50) | 50 | ||||||
| ~95 (D50) | 60 | ||||||
| ~100 (D50) | 81.66 | ||||||
| ~105 (D50) | 121.38 |
| Type | Particle Size (μm) | SDAS (μm) | Cooling Rate (K/s) | Atmosphere | Ref. |
|---|---|---|---|---|---|
| Al0.5CoCrFeNi | 25–50 | 0.14–0.37 | 105–106 | Argon | [12] |
| 50–75 | 0.37–0.63 | ||||
| 75–100 | 0.63–0.89 | ||||
| >100 | 0.89 | ||||
| Al-4%Cu | ~165 | ~2 | 104–105 | Helium | [43] |
| ~2 | |||||
| ~4.2 | 103–104 | Argon | |||
| ~4.5 | |||||
| Ti-28Ta | ~50 | ~1.05 | - | - | [24] |
| ~60 | ~1.1 | ||||
| ~70 | ~1.1 | ||||
| ~85 | ~1.4 | ||||
| ~95 | ~1.4 | ||||
| ~100 | ~1.5 | ||||
| ~110 | ~1.6 | ||||
| Ni-11Mo-8Al-3Ta-2Cr-1Re | <150 | 2–4 | 105–107 | Ar/He mixture | [13] |
| Ti-60Ta | ~50 | ~1 | 104–106 | Argon | [58] |
| ~60 | ~1.1 | ||||
| ~70 | ~1.2 | ||||
| ~85 | ~1.4 | ||||
| ~100 | ~1.4 | ||||
| ~105 | ~1.5 | ||||
| ~110 | ~1.6 | ||||
| Ti-48Al-3Nb-1.5Ta | ~80 | ~2 | ~17,000 | Ar/He mixture | [59] |
| ~110 | ~2.5 | ~10,000 | |||
| ~135 | ~4.25 | ~8000 | |||
| ~170 | ~4.75 | ~6000 | |||
| AlCu4Mg1-SiC | ~47 | 0.85 | 10−3–109 | Argon | [60] |
| ~250 | 4.16 | ||||
| ~450 | 9.97 |
| Atomizer | Disintegration Mode | Critical Conditions | Practical Implications | Ref. |
|---|---|---|---|---|
| CA (disk) | Direct drop-Ligament | Large, spherical droplets. Coarse powder production or low-throughput applications. | [82] | |
| [72] | ||||
| Ligament | Spherical to slightly oval particles. Balance between speed and feed rate to stabilize ligaments. | [82] | ||
| Ligament-Full ligament | [72] | |||
| Ligament-Sheet | A mix of spherical and irregular (satellite) particles. Increased satellite formation from sheet breakup. Optimize We to minimize unstable sheet formation. | [82] | ||
| [72] | ||||
| CA (cup) | Direct drop-Ligament | Suitable for a wide range of metal powders. Offers a good balance between yield and particle shape control. | [77] | |
| [75] | ||||
| Ligament-Sheet | Enables high production rates. High likelihood of satellite and fine powder formation. Use this mode intentionally for applications requiring fines but expect a broader size distribution. | [77] | ||
| [83] | ||||
| PREP | Direct drop | Highly spherical, satellite-free powders. Premium applications require superior flowability. | [29,31,78,79,80,81] | |
| Ligament | Ligaments breakup into droplets via Rayleigh–Plateau instability, which can lead to larger powder size and a broader particle size distribution | |||
| Sheet | Large and irregularly shaped droplets formed. It should be avoided for high-quality powder production. |
| Atomizer | Correlation | Disintegration Mode | Remarks | Ref. |
|---|---|---|---|---|
| PREP | Direct drop-ligament | [29,81] | ||
| PREP | Ligament | [78,82] | ||
| Disk | All modes | [97] | ||
| Slotted | ||||
| Arc-edge | - | |||
| Bulged block | ||||
| Disk/Cup | All modes | H is the depth of the atomizer. | [98] | |
| Disk | All modes | - | [99] | |
| Cup | All modes | [73] | ||
| Cup | Direct drop mode | - | [77] | |
| Disk | All modes | [100] | ||
| Disk | All modes | [101] | ||
| Disk | Sheet mode | [87] | ||
| Disk | Sheet mode | λopt is the optimal wavelength of the wave on the ligament | [102] | |
| PREP | Direct drop-ligament | [81] | ||
| Cup | All modes | [103] | ||
| Cup | All modes | [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Lu, X.; Shi, Q.; Zhao, Y. Review on Metal Powder Manufactured by Technologies Utilizing Centrifugal Force. Materials 2025, 18, 4905. https://doi.org/10.3390/ma18214905
Wu Z, Lu X, Shi Q, Zhao Y. Review on Metal Powder Manufactured by Technologies Utilizing Centrifugal Force. Materials. 2025; 18(21):4905. https://doi.org/10.3390/ma18214905
Chicago/Turabian StyleWu, Zhining, Xianke Lu, Qi Shi, and Yuyuan Zhao. 2025. "Review on Metal Powder Manufactured by Technologies Utilizing Centrifugal Force" Materials 18, no. 21: 4905. https://doi.org/10.3390/ma18214905
APA StyleWu, Z., Lu, X., Shi, Q., & Zhao, Y. (2025). Review on Metal Powder Manufactured by Technologies Utilizing Centrifugal Force. Materials, 18(21), 4905. https://doi.org/10.3390/ma18214905







