Study on Decarburization and Mechanical Properties of Ultra-Low Carbon Steel by Enlarged Vacuum Chamber Volume
Abstract
1. Introduction
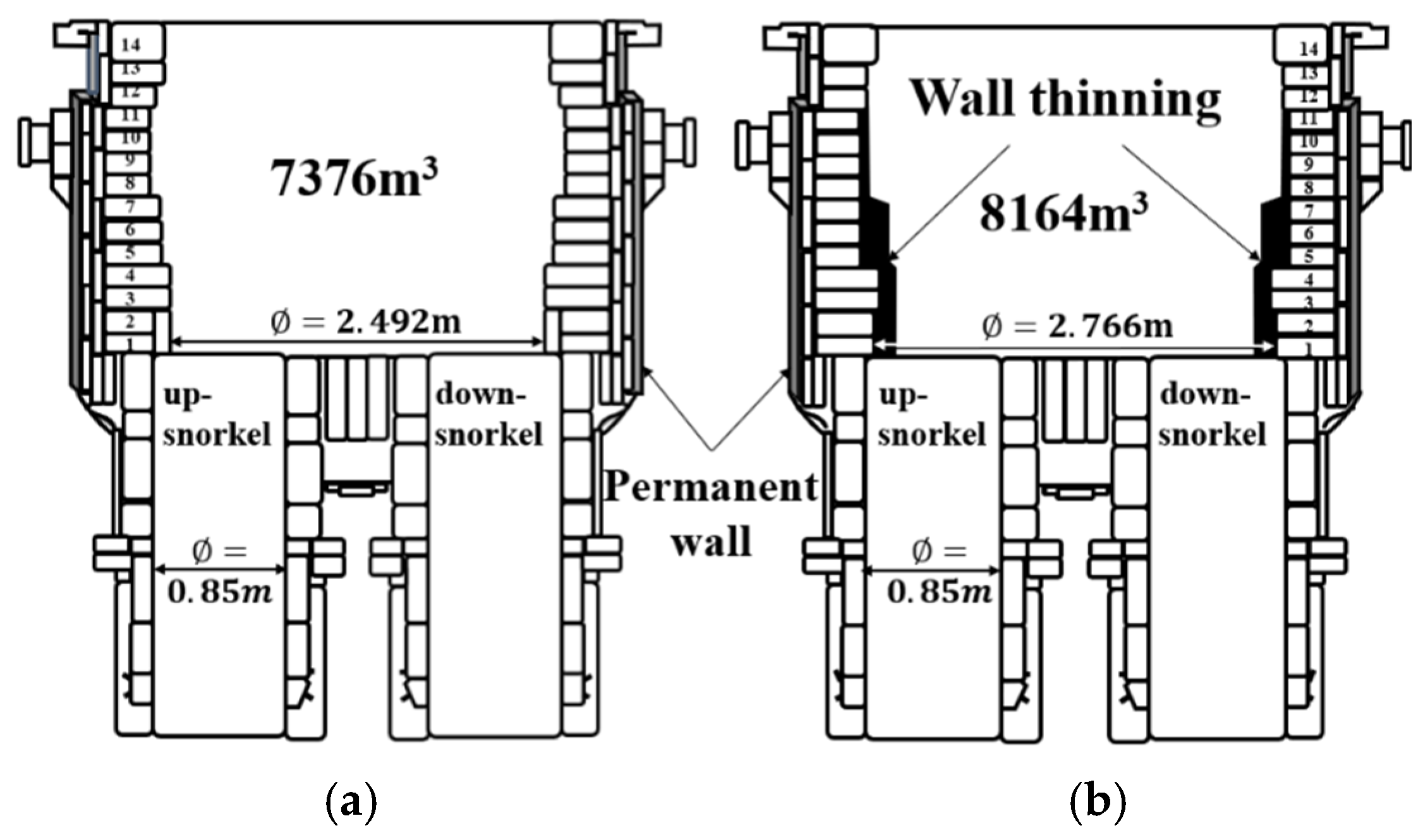
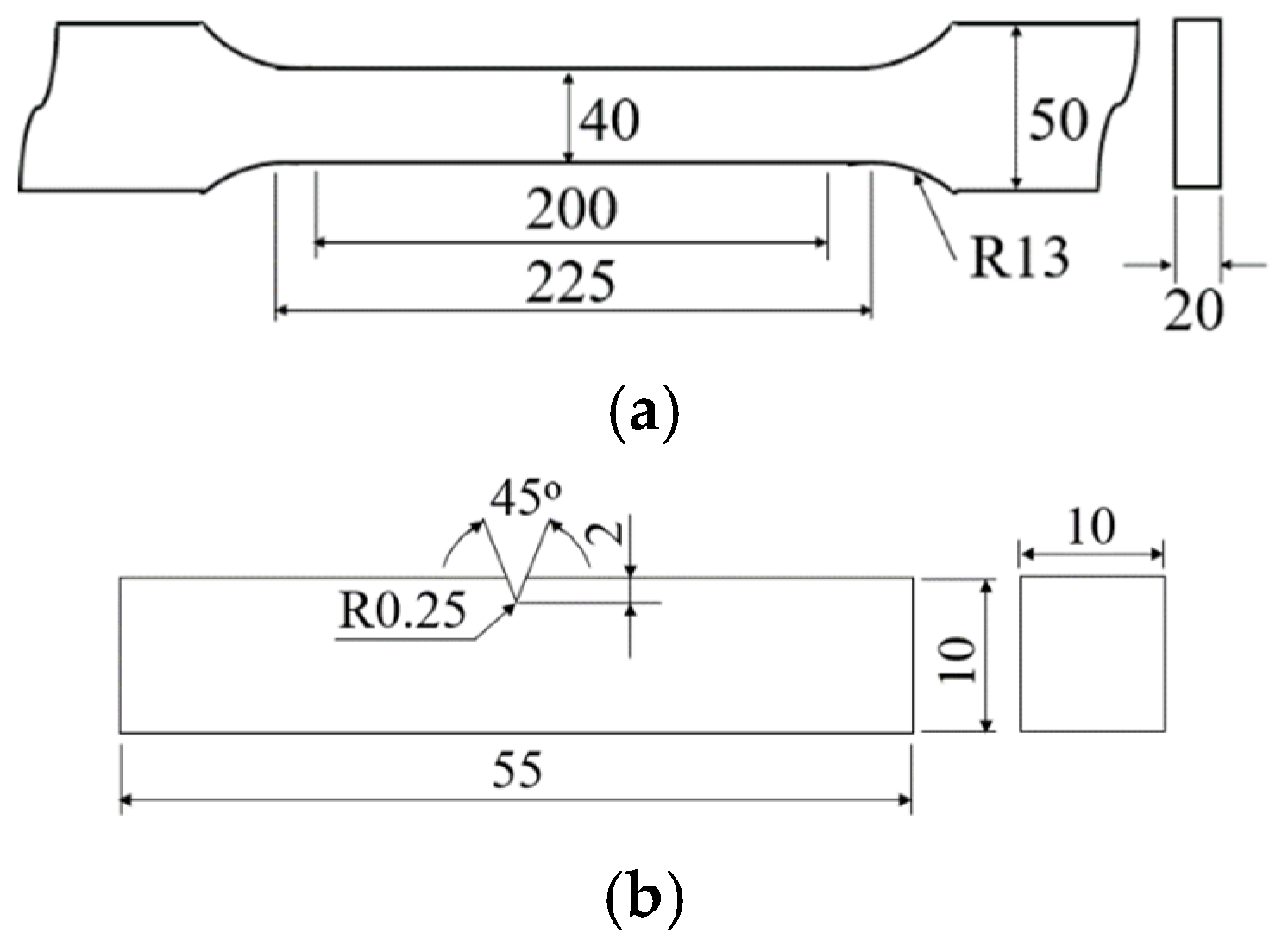
2. Experimental Methods
3. Results and Discussion
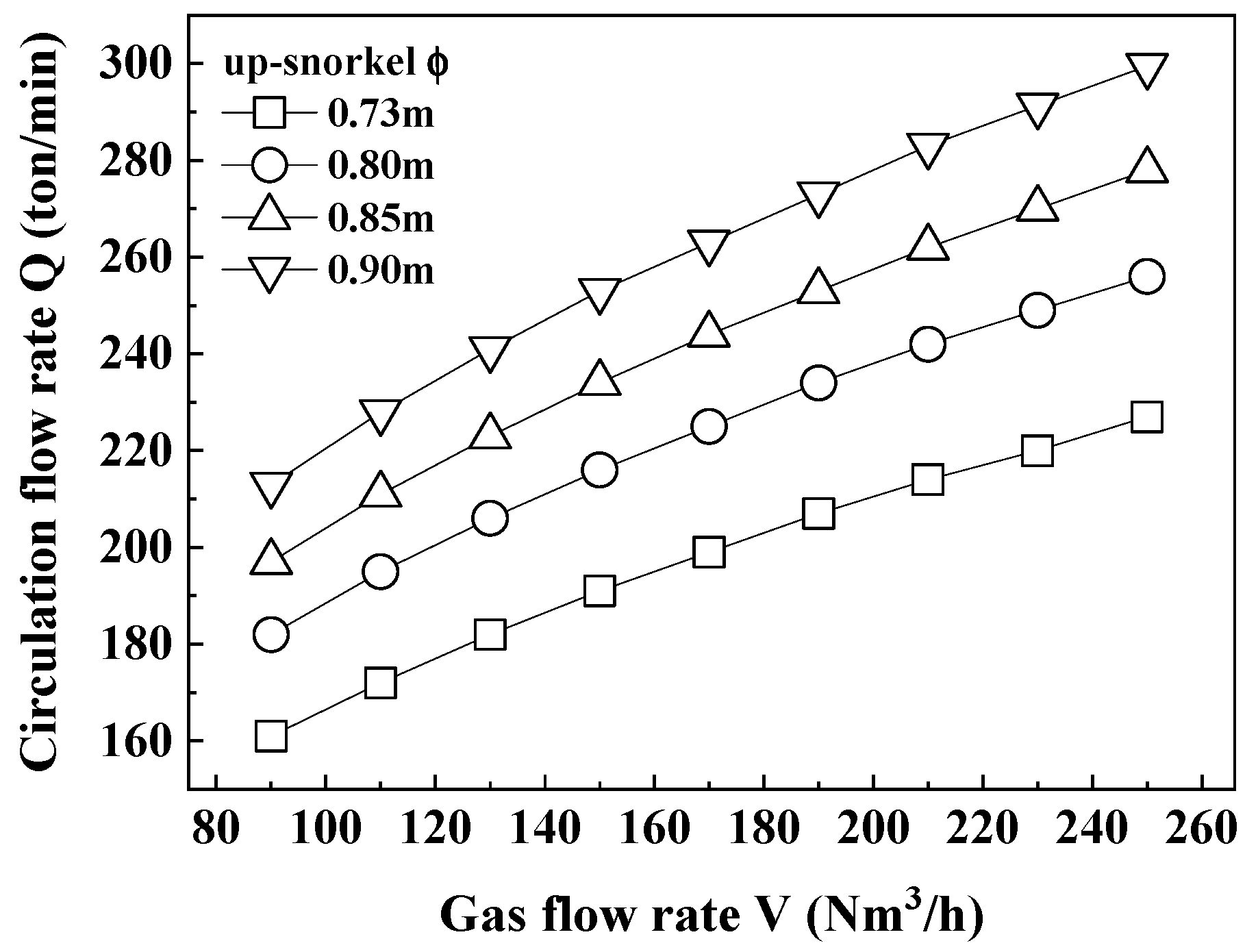
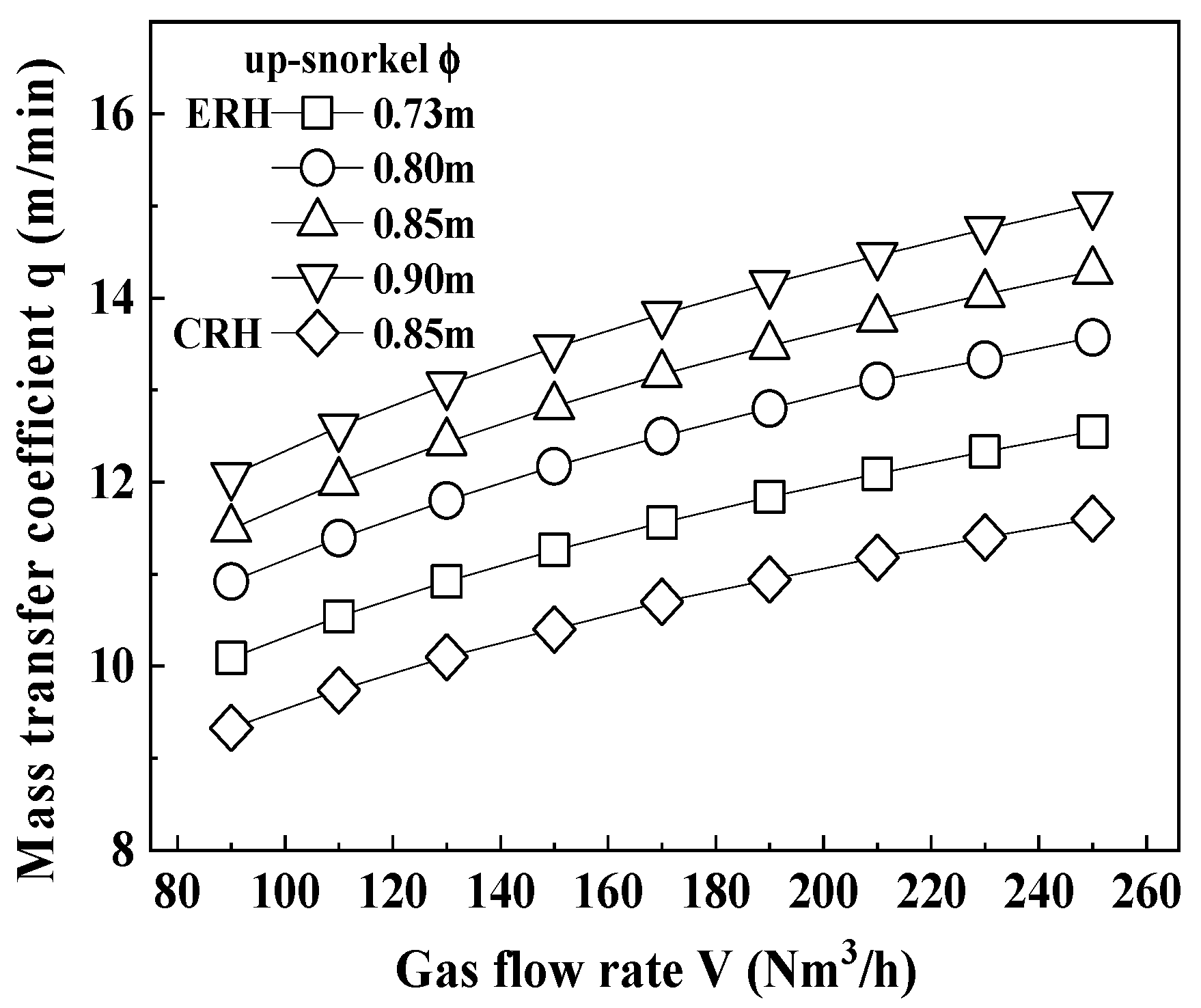
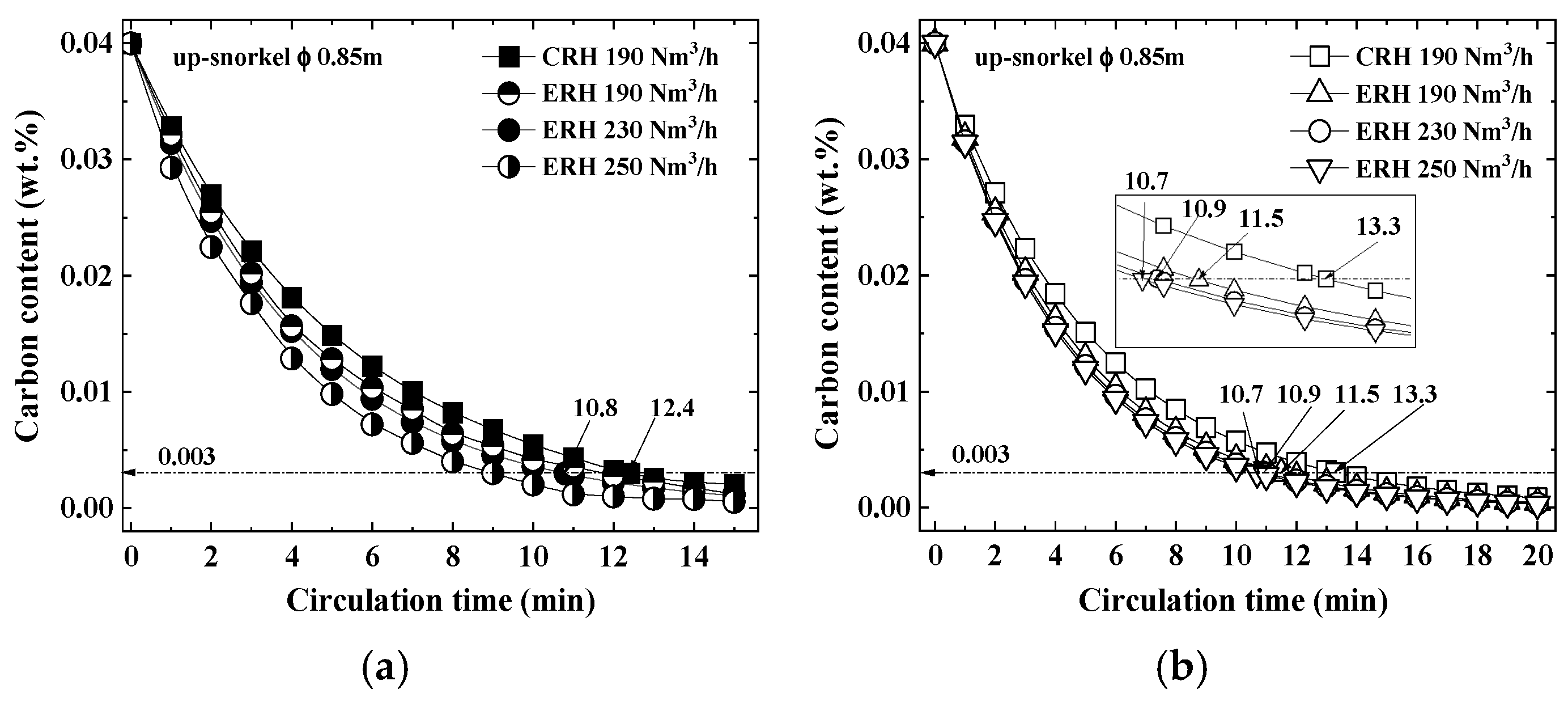
3.1. Carbon Content According to Circulation Gas Flow Rate
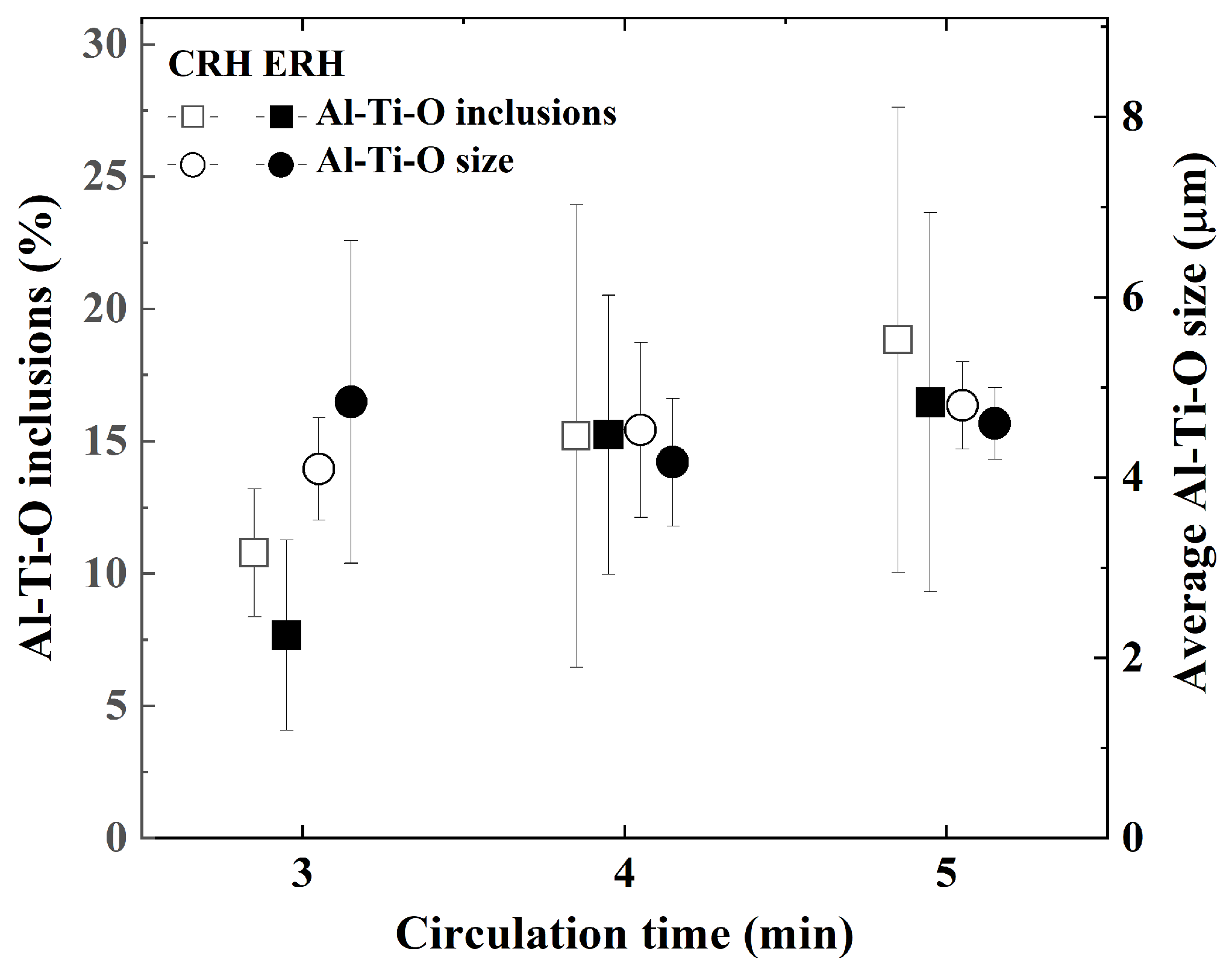
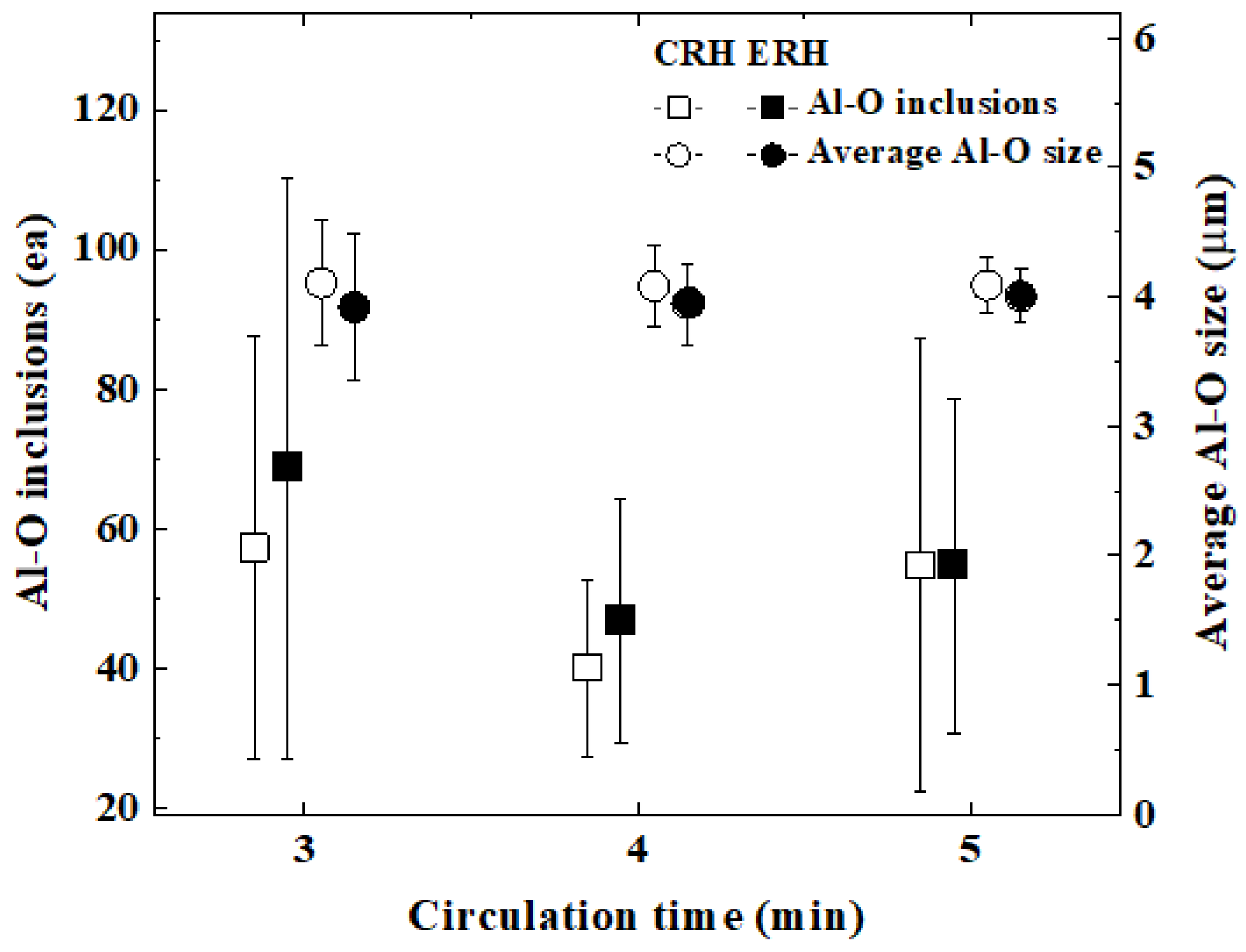
3.2. Inclusions According to Circulation Time
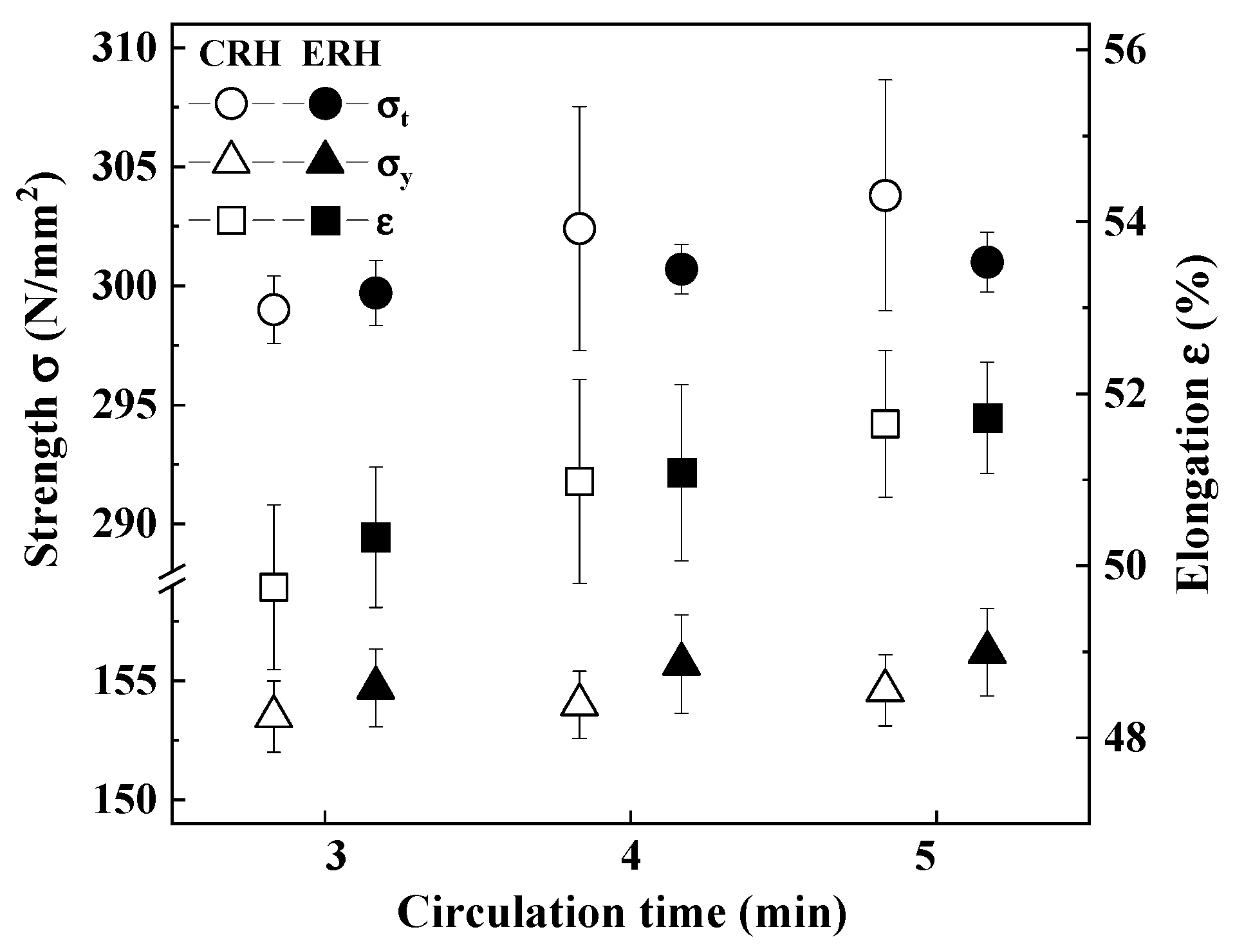
3.3. Mechanical Properties
3.4. Observation of Inclusions
3.5. Reduce Production Costs
4. Conclusions
- (1)
- Both CRH and ERH showed increased decarburization with prolonged circulation times at the same circulation gas flow rate (190 Nm3/h), while ERH exhibited greater decarburization than CRH, especially as the circulation gas flow rate increased. The highest decarburization occurred with a circulation gas flow rate of 250 Nm3/h, leading to a reduced lifespan of the up-snorkel and RH lower firebrick. Therefore, the optimal circulation gas flow rate was determined to be 230 Nm3/h.
- (2)
- The experimental decarburization times required to reach the 0.003 wt% carbon content in ultra-low carbon steel were 12.4 min for CRH and 10.8 min for ERH, with ERH showing a reduction of 1.6 min. Conversely, the calculated decarburization times were 13.3 min for CRH and 10.9 min for ERH, with ERH achieving a reduction of 2.4 min. As a result, the calculated time was 0.8 min longer than the experimental time.
- (3)
- There were no significant differences in inclusions characteristics between CRH and ERH at circulation times of 3, 4, and 5 min. However, the mechanical properties of ERH proved to be slightly superior at 4 and 5 min, leading to a decision based on economic feasibility. Hence, the expansion of the lower area of the RH degasser was to improve productivity and contribute to a reduction in production costs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yuan, Q.; Ren, J.; Tang, E.; Li, Z.; Mo, J.; Zhao, S.; Xu, Z. Rapid annealing of an ultralow carbon steel: In-depth exploration of the microstructure-mechanical properties evolution. J. Mater. Res. Technol. 2023, 27, 1097–1113. [Google Scholar] [CrossRef]
- Li, C.; Duan, R.; Fu, W.; Gao, H.; Wang, D.; Di, X. Improvement of mechanical properties for low carbon ultra-high strength steel strengthened by Cu-rich multistructured precipitation via modification to bainite. Mater. Sci. Eng. A 2021, 817, 141337. [Google Scholar] [CrossRef]
- You, D.; Bernhard, C.; Viertauer, A.; Linzer, B. Simulation of the Refining Process of Ultra-Low Carbon (ULC) Steel. Crystals 2021, 11, 893. [Google Scholar] [CrossRef]
- Park, J.H.; Kang, Y.B. Reoxidation Phenomena of Liquid Steel in Secondary Refining and Continuous Casting Processes: A Review. Steel Res. Int. 2024, 95, 2300598. [Google Scholar] [CrossRef]
- Liu, Z.; Li, W.; Shao, X.; Kang, Y.; Li, Y. An Ultra-low-Carbon Steel with Outstanding Fish-Scaling Resistance and Cold Formability for Enameling Applications. Metall. Mater. Trans. A 2019, 50, 1805–1815. [Google Scholar] [CrossRef]
- Paul, K.; Raj, A.; Biswas, P.; Manikandan, G.; Verma, R.K. Tensile flow behavior of ultra low carbon, low carbon and micro alloyed steel sheets for auto application under low to intermediate strain rate. Mater. Des. 2014, 57, 211–217. [Google Scholar] [CrossRef]
- Dutta, S.; Panda, A.K.; Mitra, A.; Chatterjee, S.; Roy, R.K. Microstructural evolution, recovery and recrystallization kinetics of isothermally annealed ultra low carbon steel. Mater. Res. Express 2020, 7, 016554. [Google Scholar] [CrossRef]
- Chen, K.; Luo, Y.; Du, J.; Gu, Q.; Liu, G.; Yuan, L.; Li, H. Study of the Submerged Entry Nozzle Failure during Casting of Ti-Bearing Ultra-Low Carbon Steel. Steel Res. Int. 2025, 2500198. [Google Scholar] [CrossRef]
- Pan, C.; Hu, X.; Lin, P.; Chou, K. Effects of Ti and Al addition on the Formation and Evolution of Inclusions in Fe-17Cr-9Ni Austenite Stainless Steel. Metall. Mater. Trans. B 2020, 51, 3039–3050. [Google Scholar] [CrossRef]
- Park, Y.J.; Kang, Y.B. Evolution of Al-Ti Complex Oxide Inclusions in Interstitial-Free Steel Analyzed Using CALPHAD, SEM, EDS, EBSD, and CSLM. Metall. Mater. Trans. B 2025, 56, 237–268. [Google Scholar] [CrossRef]
- Ling, H.; Zhang, L. Investigation on the Fluid Flow and Decarburization Process in the RH Process. Metall. Mater. Trans. B 2018, 49, 2709–2721. [Google Scholar] [CrossRef]
- Zhan, D.; Zhang, Y.-P.; Jiang, Z.H.; Zhang, H.S. Model for Ruhrstahl–Heraeus (RH) decarburization process. J. Iron Steel Res. Int. 2018, 25, 409–416. [Google Scholar] [CrossRef]
- Peng, K.; Liu, C.; Zhang, L.; Sun, Y. Numerical Simulation of Decarburization Reaction with Oxygen Blowing During RH Refining Process. Metall. Mater. Trans. B 2022, 53, 2004–2017. [Google Scholar] [CrossRef]
- Zhang, W.; Zheng, W.; Yan, W.; Li, G. Formation mechanism of interface reaction layer between microporous magnesia refractories and molten steel and its effect on steel cleanliness. J. Iron Steel Res. 2023, 30, 1743–1754. [Google Scholar] [CrossRef]
- Lyu, Z.; Gu, C.; Wang, Z.; Bao, Y. In-depth analysis on the mechanism and reaction efficiency of hydrogen deoxidation in ultra-low carbon steel. Surf. Interfaces 2024, 55, 105284. [Google Scholar] [CrossRef]
- Gao, L.; Shi, Z.; Yang, Y.; Li, D.; Zhang, G.; McLean, A.; Chattopadhyay, K. Mathematical Modeling of Decarburization in Levitated Fe-Cr-C Droplets. Metall. Mater. Trans. B 2018, 49, 1985–1994. [Google Scholar] [CrossRef]
- Li, P.; Wu, Q.; Hu, W.; Ye, J. Mathematical simulation of behavior of carbon and oxygen in RH decarburization. J. Iron Steel Res. Int. 2015, 22, 63–67. [Google Scholar] [CrossRef]
- Xin, Z.; Lin, W.; Zhang, J.; Liu, Q. Study on Decarburization Behavior in BOF Steelmaking Based on Multi-Zone Reaction Mechanism. Materials 2025, 18, 4599. [Google Scholar] [CrossRef]
- Shen, J.; Zhangm, Q.; Tian, S.; Li, X.; Liu, J.; Tian, J. The role of hydrogen in iron and steel production: Development trends, decarbonization potentials, and economic impacts. Int. J. Hydrogen Energy 2024, 92, 1409–1422. [Google Scholar] [CrossRef]
- Sakker, T.R.; Ethen, D.Z.; Nanda, S. Decarbonization of Metallurgy and Steelmaking Industries Using Biochar: A Review. Chem. Eng. Technol. 2024, 47, e202400217. [Google Scholar] [CrossRef]
- Putera, A.D.P.; Putra, H.P.; Karuana, F.; Ghazidin, H.; Kuswa, F.M.; Suyatno, S.; Aziz, M.; Prismantoko, A. Toward decarbonization in steelmaking: A review on direct reduced iron using biomass-based syngas. Sustain. Energy Technol. Assess. 2025, 82, 104554. [Google Scholar] [CrossRef]
- Suzuki, K.; Mori, K.; Kitagawa, T.; Shibayama, S. Rate of Removal of Carbon and Oxygen from Liquid Iron. Tetsu-to-Hagane 1976, 62, 354–361. [Google Scholar] [CrossRef][Green Version]
- Yamaguchi, K.; Kishimoto, Y.; Sakuraya, T.; Fujii, T.; Aratani, M.; Nishikawa, H. Effect of Refining Conditions Decarburization Reaction in RH for Ultra Low Carbon Steel on Degasser. ISIJ Int. 1992, 32, 126–135. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, W.; Tong, Q.; Wang, L. Effects of Temperature and Oxygen Concentration on the Characteristics of Decarburization of 55SiCr Spring Steel. ISIJ Int. 2014, 54, 1920–1926. [Google Scholar] [CrossRef]
- Kuwabara, T.; Umezawa, K.; Mori, K.; Watanabe, H. Investigation of Behavior in RH-reactor and Its Operation Improvement. Trans. Lron Steel Inst. Jpn. 1988, 28, 305–314. [Google Scholar] [CrossRef]
- Ono, H.; Tajiri, H.; Usui, T.; Tanaka, T.; Marukawa, K. Rate Enhancement of the Degassing Reaction by the Enlargement of RH and DH Reactors. Tetsu-to-Hagane 2003, 89, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.F.; Luo, Z.G.; Meng, F.X.; Zou, Z.S.; Li, Y.H.; Zhao, A.C.; Gui, H.L. Physical Simulation on Molten Steel Flow Characteristics of RH Vacuum Chamber with Arched Snorkels. Adv. Mater. Sci. Eng. 2020, 2020, 6525096. [Google Scholar] [CrossRef]
- Basu, S.; Choudhary, S.K.; Girase, N.U. Nozzle Clogging Behaviour of Ti-bearing Al-killed Ultra Low Carbon Steel. ISIJ Int. 2004, 44, 1653–1660. [Google Scholar] [CrossRef]
- Cha, W.Y.; Kim, W.Y. In-situ Observation of Inclusion Formation Behavior in Ultra-Low Carbon Steel with Al–Ti Complex Deoxidation. ISIJ Int. 2025, 65, 1631–1641. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, M.; Bao, Y. The Research of Low-Oxygen Control and Oxygen Behavior during RH Process in Silicon-Deoxidization Bearing Steel. Metals 2019, 9, 812. [Google Scholar] [CrossRef]
- Heo, J.; Kim, T.W.; Jung, S.J.; Han, S. Real-Time Prediction Model of Carbon Content in RH Process. Appl. Sci. 2022, 12, 10753. [Google Scholar] [CrossRef]
- Pindar, S.; Pande, M.M. Assessment of Si-O equilibria and nonmetallic inclusion characteristics in high silicon steels. Steel Res. Int. 2023, 94, 2300115. [Google Scholar] [CrossRef]
- Pindar, S.; Pande, M.M. Investigation of Inclusion Characteristics in Ferrosilicon Killed High Silicon Steels. Steel Res. Int. 2024, 95, 2400331. [Google Scholar] [CrossRef]
- Zhu, B.; Liu, Q.; Zhao, D.; Ren, S.; Xu, M.; Yang, B.; Hu, B. Effect of Nozzle Blockage on Circulation Flow Rate in Up-Snorkel during the RH Degasser Process. Steel Res. Int. 2016, 87, 136–145. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, P.; Dong, J.; Liu, M.; Liu, Y.; Lai, C. Effects of Different RH Degasser Nozzle Layouts on the Circulating Flow Rate. Materials 2022, 15, 8476. [Google Scholar] [CrossRef]
- Wang, J.; Ni, P.; Chen, C.; Ersson, M.; Li, Y. Effect of gas blowing nozzle angle on multiphase flow and mass transfer during RH refining process. Int. J. Miner. Metall. Mater. 2023, 30, 844–856. [Google Scholar] [CrossRef]
- Jung, W.G.; Kwon, O.D.; Cho, M.K. Nozzle Clogging Mechanism in Continuous Casting for Titanium-Containing Steel. Korean J. Mater. Res. 2009, 19, 473–480. [Google Scholar] [CrossRef][Green Version]
- Yang, W.; Zhang, L.; Ren, Y.; Chen, W.; Liu, F. Formation and Prevention of Nozzle Clogging during the Continuous Casting of Steels: A Review. ISIJ Int. 2024, 64, 1–20. [Google Scholar] [CrossRef]
- Wang, J.; Xia, M.; Wu, J.; Jian, X.; Ge, C. Precise control of atomization initial stage to address nozzle clogging issue in the vacuum induction-melting gas atomization process. J. Mater. Res. Technol. 2024, 30, 1505–1517. [Google Scholar] [CrossRef]
- Li, Y.; He, W.; Zhao, C.; Liu, J.; Yang, Z.; Zhao, Y.; Yang, J. Mathematical Modeling of Transient Submerged Entry Nozzle Clogging and Its Effect on Flow Field, Bubble Distribution and Interface Fluctuation in Slab Continuous Casting Mold. Metals 2024, 14, 742. [Google Scholar] [CrossRef]
- Sohn, H.S.; Jung, K.H. Decarbonization Kinetics of Molten Iron by Ar+O2 Gas Bubbling. J. Korean Inst. Met. Mater. 2009, 47, 107–113. [Google Scholar]
- Nakamura, O.; Numata, M.; Takatani, K. Numerical Simulation of Gas-liquid Circulation Flow of RH. Tetsu-to-Hagane 2015, 101, 123–128. [Google Scholar] [CrossRef][Green Version]
- Fan, Y.; Hu, X.; Zheng, Y.; Zhu, R.; Matsuura, H.; Chou, K. Pattern Optimization of O2–CO2 Mixed Injection for Decarburization Reactions During Steelmaking Process. J. Sustain. Metall. 2022, 8, 582–594. [Google Scholar] [CrossRef]
- Chanouian, S.; Ahlin, B.; Tilliander, A.; Ersson, M. A Fundamental Investigation of Decarburization Reactionsin the Argon–Oxygen Decarburization Converter UsingCoupled Computational Fluid Dynamics andThermodynamics Databases. Steel Res. Int. 2022, 93, 2200156. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Zhao, X.; Lei, S.; Dong, Q. Mathematical Model for Decarburization Process in RH Refining Process. ISIJ Int. 2014, 54, 1560–1569. [Google Scholar] [CrossRef]
- Liu, B.S.; Zhu, G.S.; Li, H.X.; Li, B.H.; Cui, Y.; Cui, A.M. Decarburization rate of RH refining for ultralow carbon steel. Int. J. Miner. Metall. Mater. 2010, 17, 22–27. [Google Scholar] [CrossRef]
- Yamamoto, K.I.; Yamamura, H.; Suwa, Y. Behavior of non-metallic inclusions in steel during hot deformation and the effects of deformed inclusions on local ductility. ISIJ Int. 2011, 12, 1987–1994. [Google Scholar] [CrossRef]
- Sun, M.; Jiang, Z.; Li, Y.; Chen, C.; Ma, S.; Ji, Y.; Wang, J.; Liu, H. Effect of Sulfur Content on the Inclusion and Mechanical Properties in Ce-Mg Treated Resulfurized SCr420H Steel. Metals 2022, 12, 136. [Google Scholar] [CrossRef]
- Kaijalainen, A.; Karjalainen, P.; Porter, D.; Suikkanen, P.; Kömi, J.; Kesti, K.; Saarinen, T. Effect of Inclusions on the Properties of Ultra-High-Strength Low-Alloy Steel with a Martensitic-Bainitic Microstructure. In Proceedings of the 8th International Conference on Clean Steel, Budapest, Hungary, 14–16 May 2012. [Google Scholar]
- Tervo, H.; Kaijalainen, A.; Pikkarainen, T.; Mehtonen, S.; Porter, D. Effect of impurity level and inclusions on the ductility and toughness of an ultra-high-strength steel. Mater. Sci. Eng. A 2017, 697, 184–193. [Google Scholar] [CrossRef]
- Yuan, B.; Liu, J.; Zeng, J.; Zhang, M.; Huang, J.; Yang, X. Evolution of Inclusions and Cleanliness in Ti-Bearing IF Steel Produced via the BOF–LF–RH–CC Process. Metals 2022, 12, 434. [Google Scholar] [CrossRef]



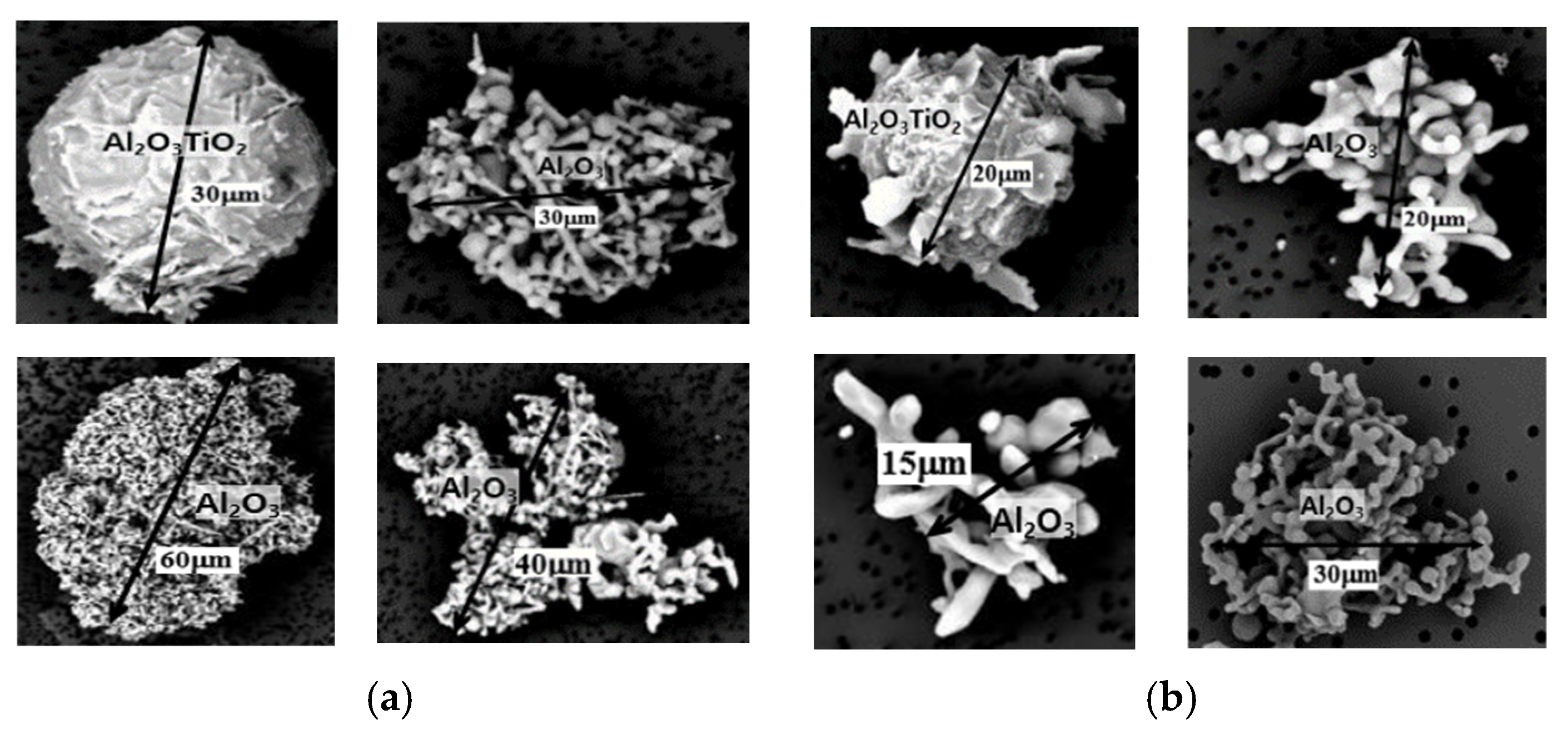

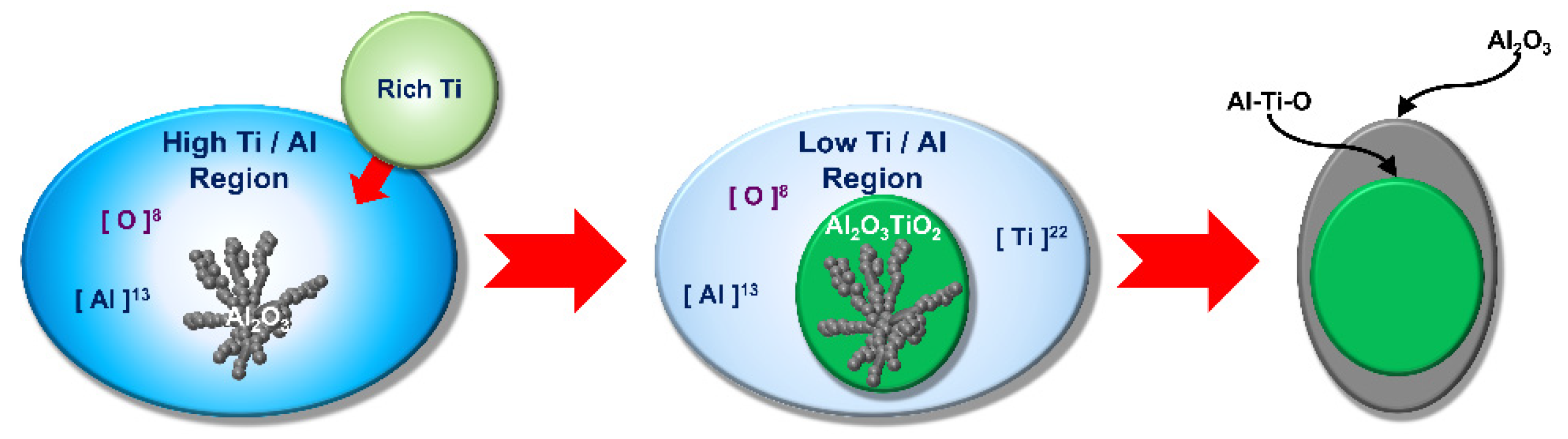
| Gas Flow Rate V (Nm3/h) | Circulation Flow Rate Q (ton/min) | Mass Transfer Coefficient q (m/min) | Decarburization Rate Constant Kc (min−1) | |
|---|---|---|---|---|
| CRH | 190 | 253 | 10.9 | 0.195 |
| ERH | 190 | 253 | 13.5 | 0.226 |
| 230 | 270 | 14.0 | 0.237 | |
| 250 | 278 | 14.3 | 0.242 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, K.; Yun, J.; Nam, K.; Kim, K. Study on Decarburization and Mechanical Properties of Ultra-Low Carbon Steel by Enlarged Vacuum Chamber Volume. Materials 2025, 18, 4891. https://doi.org/10.3390/ma18214891
Shin K, Yun J, Nam K, Kim K. Study on Decarburization and Mechanical Properties of Ultra-Low Carbon Steel by Enlarged Vacuum Chamber Volume. Materials. 2025; 18(21):4891. https://doi.org/10.3390/ma18214891
Chicago/Turabian StyleShin, Kihang, Jimin Yun, Kiwoo Nam, and Kwonhoo Kim. 2025. "Study on Decarburization and Mechanical Properties of Ultra-Low Carbon Steel by Enlarged Vacuum Chamber Volume" Materials 18, no. 21: 4891. https://doi.org/10.3390/ma18214891
APA StyleShin, K., Yun, J., Nam, K., & Kim, K. (2025). Study on Decarburization and Mechanical Properties of Ultra-Low Carbon Steel by Enlarged Vacuum Chamber Volume. Materials, 18(21), 4891. https://doi.org/10.3390/ma18214891





