Electrodeposition of Amorphous Cobalt–Phosphorus Coating
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials, Chemicals, and Consumables
2.2. Electrodeposition
- Cleaning in isopropanol (alcohol) in a sonicator (model UR 1 from Retsch GmbH, Haan, Germany, operation frequency 35 kHz, volume 5.7 L) for 15 min.
- Polishing on aluminum oxide flexible foams (3M, Saint Paul, MN, USA): “fine” (grit 320 to 400), “superfine” (grit 500 to 600), and “microfine” (grit 1200 to 1500).
- Cleaning in isopropanol in a sonicator for 15 min.
- Chemical polishing in a concentrated acid solution consisting of H3PO4, HNO3, and CH3COOH (1:1:1 vol. ratio).
- Cleaning with deionized (DI) water.
- Drying with a cold-air fan.
2.3. Coating Characterization
3. Results and Discussion
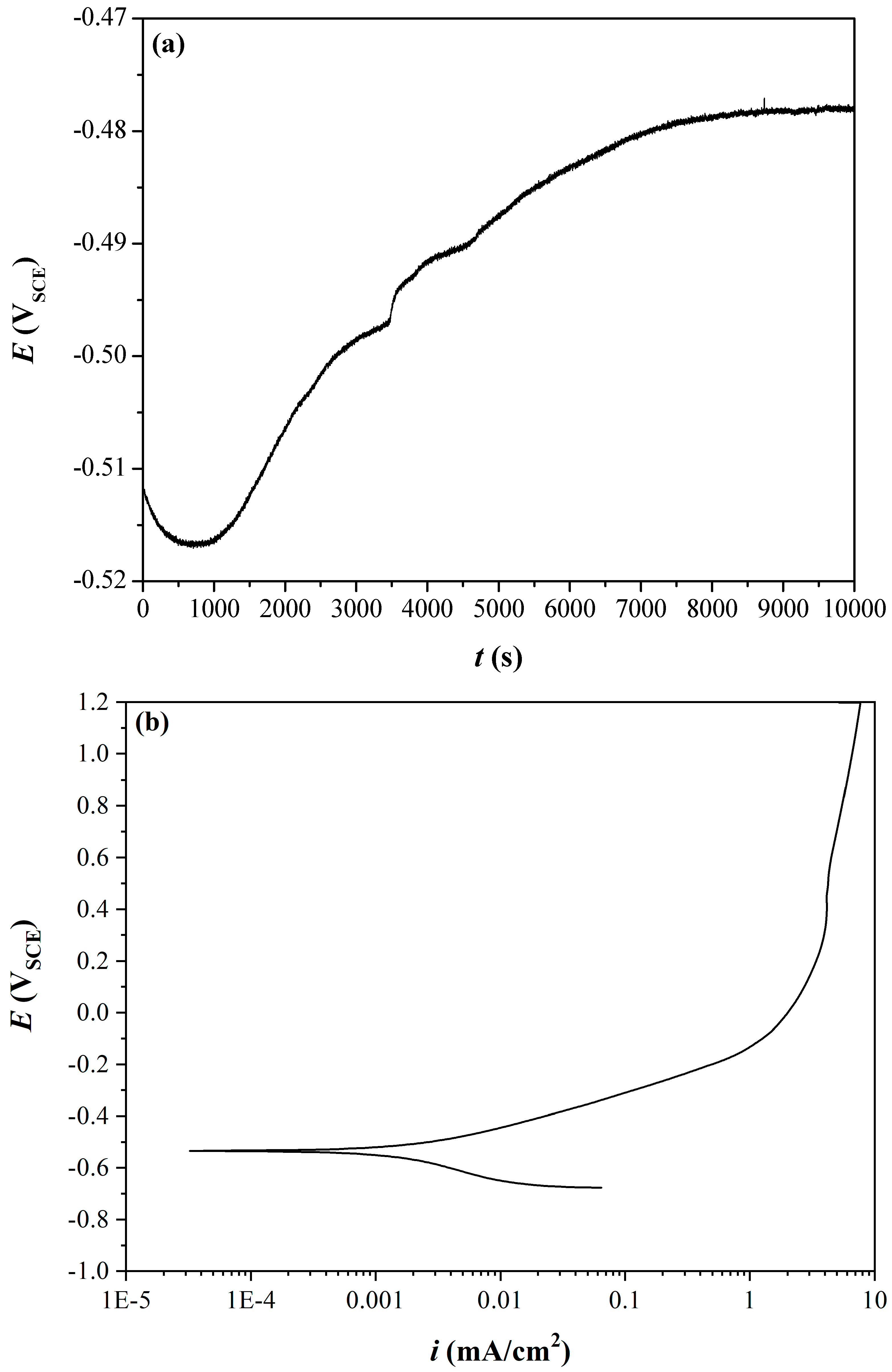
3.1. Coating Electrochemistry and Deposition Potential
3.2. Coating’s Surface Morphology, Surface Roughness, and Thickness
3.3. Chemical Composition of the Coatings
3.4. Corrosion Resistance of the Coatings
3.5. Hardness, Young’s Modulus, Adhesion, and Friction Properties, of the CoP Coating
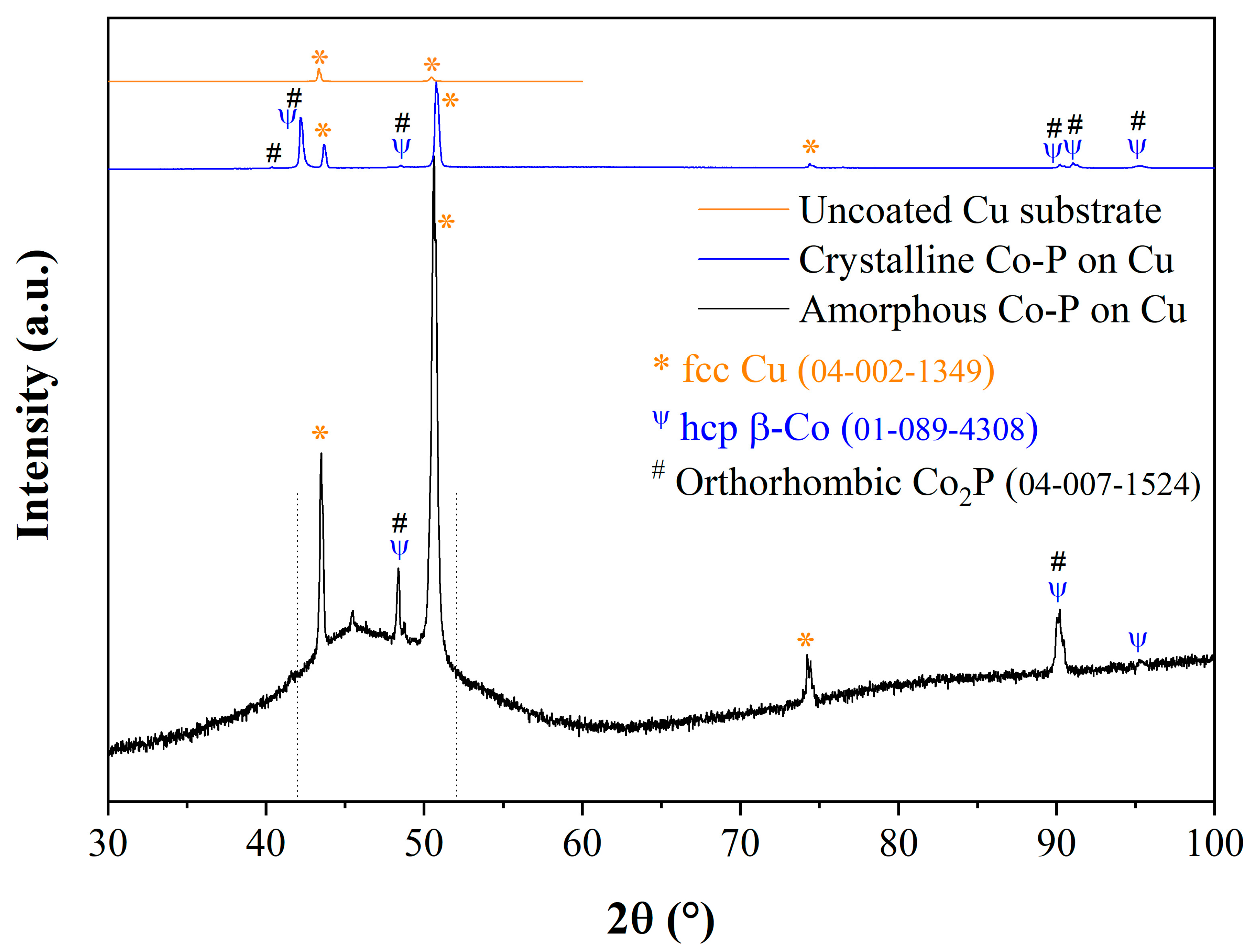
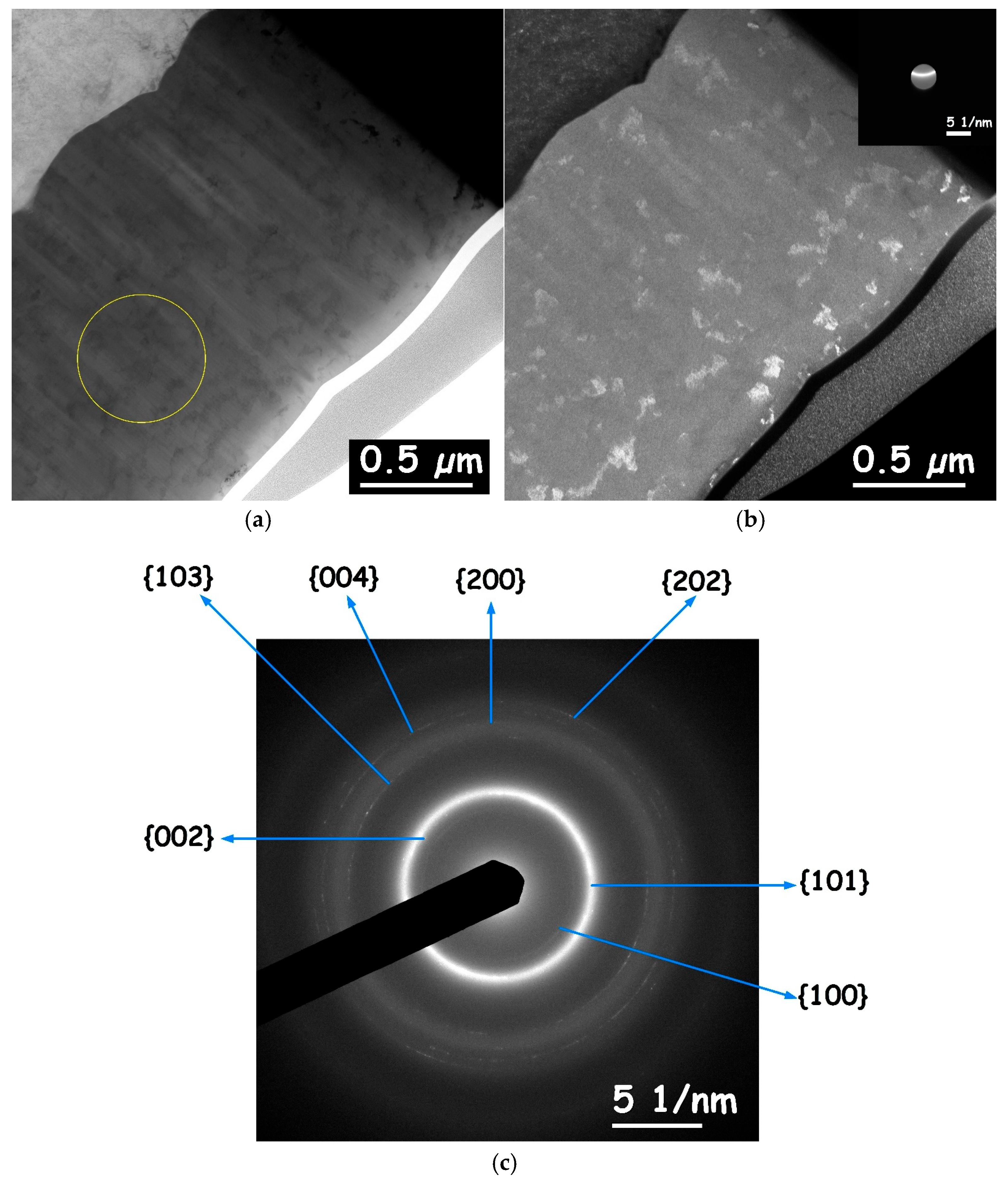
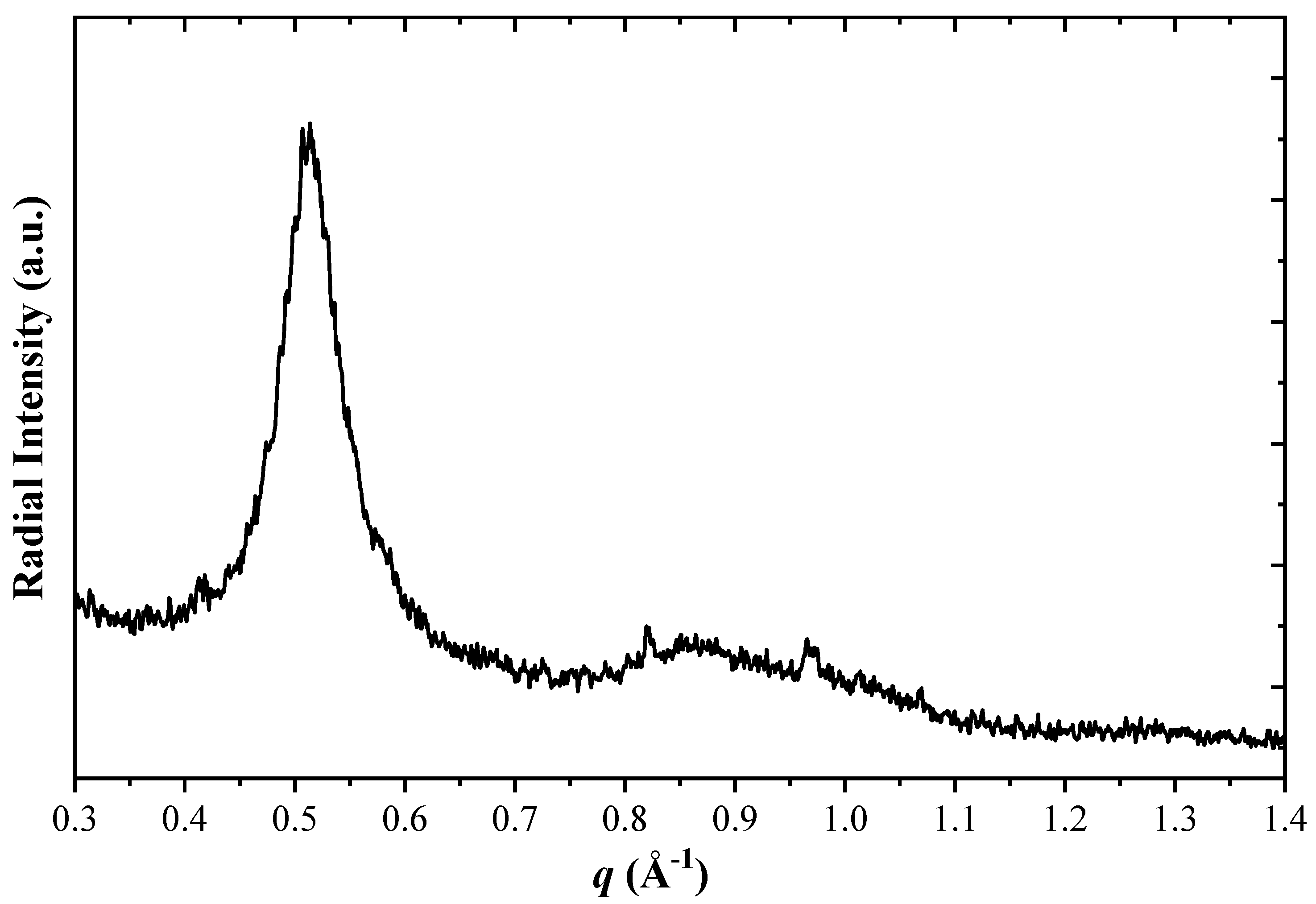
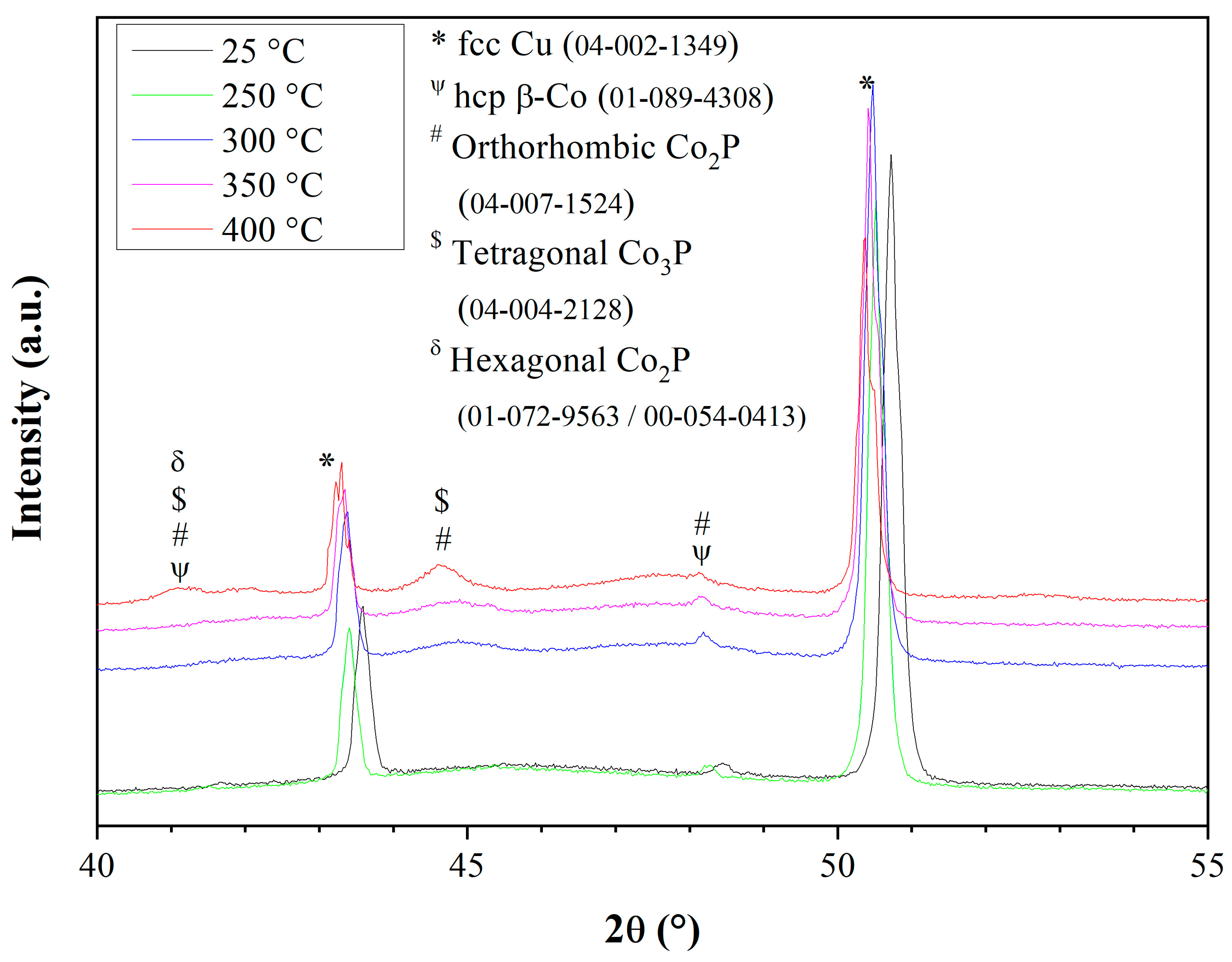
3.6. Microstructure of CoP Coatings
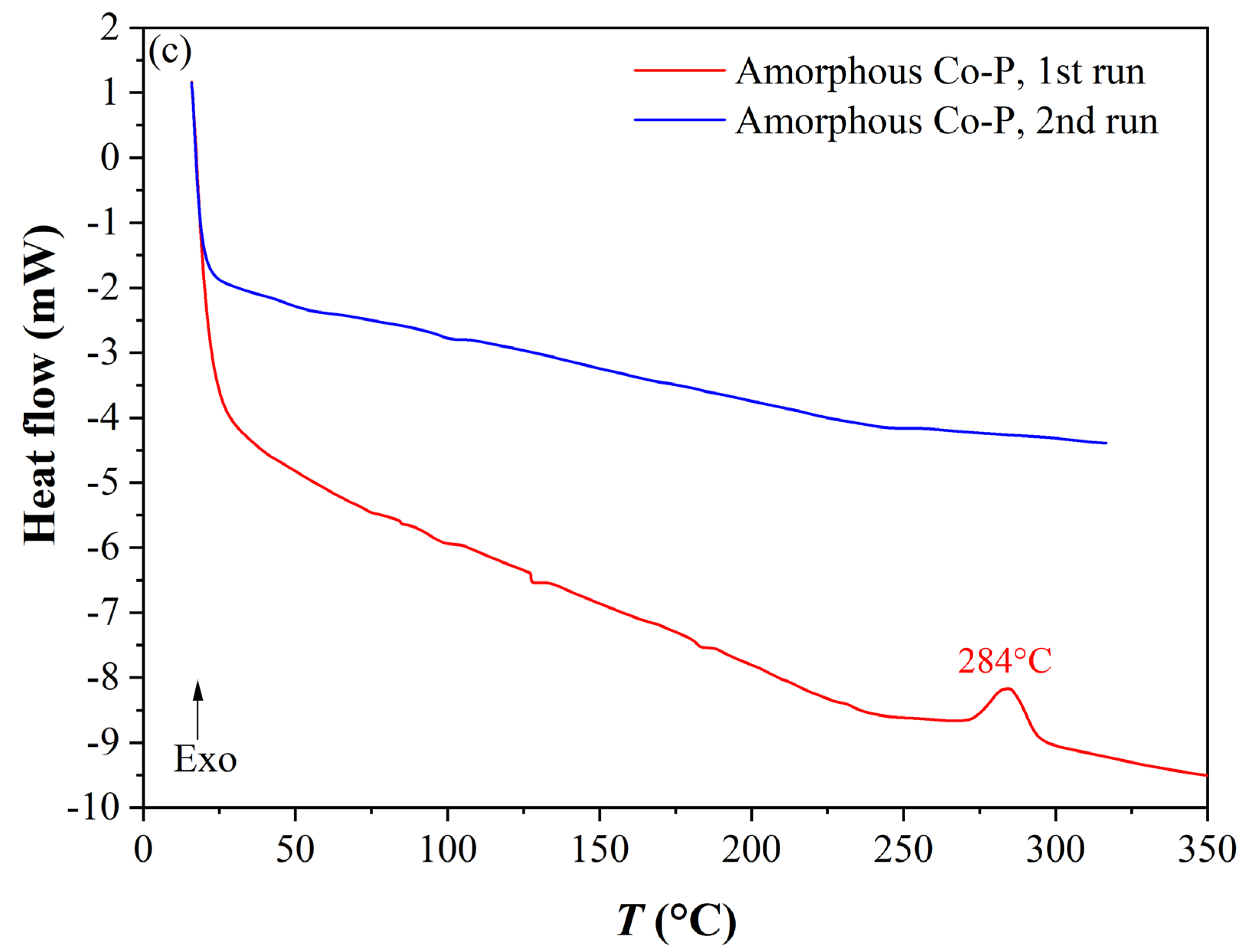
3.7. Thermal Stability of the CoP Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hook, J.R.; Hall, H.E. Solid State Physics; Wiley: Chichester, UK, 1991; pp. 18–46. [Google Scholar]
- Greer, A.L. Metallic Glasses. In Physical Metallurgy, 5th ed.; Laughlin, D.E., Hono, K., Eds.; Elsevier: Oxford, UK, 2014; Volume 1, pp. 305–385. [Google Scholar]
- Greer, A.L.; Costa, M.B.; Houghton, O.S. Metallic glasses. MRS Bull. 2023, 48, 1054–1061. [Google Scholar] [CrossRef]
- Li, R.; Hou, Y.; Dong, Q.; Su, P.; Ju, P.; Liang, J. Wear and corrosion resistance of Co–P coatings: The effects of current modes. RSC Adv. 2018, 8, 895–903. [Google Scholar] [CrossRef]
- Barzegar, M.; Allahkaram, S.R.; Naderi, R.; Ghavidel, N. Effect of phosphorus content and heat treatment on the structure, hardness and wear behavior of Co–P coatings. Wear 2019, 422–423, 35–43. [Google Scholar] [CrossRef]
- Sheikholeslam, M.A.; Raeissi, K.; Enayati, M.H. Study on corrosion behaviour of nanocrystalline and amorphous Co–P electrodeposits. Trans. Inst. Met. Finish. 2010, 88, 324–329. [Google Scholar] [CrossRef]
- Alanazi, N.M.; El-Sherik, A.M.; Alamar, S.H.; Shen, S. Influence of residual stresses on corrosion and wear behavior of electrodeposited nanocrystalline cobalt-phosphorus coatings. Int. J. Electrochem. Sci. 2013, 8, 10350–10358. [Google Scholar] [CrossRef]
- Ciudad, D.; Prieto, J.L.; Lucas, I.; Aroca, C.; Sánchez, P. Optimization of magnetic properties of electrodeposited CoP multilayers for sensor applications. J. Appl. Phys. 2007, 101, 043907. [Google Scholar] [CrossRef]
- Sinnecker, J.P.; Knobel, M.; Pirota, K.R.; Garcia, J.M.; Asenjo, A.; Vázquez, A. Frequency dependence of magnetoimpedance in amorphous CoP layers. J. Appl. Phys. 2000, 87, 4825–4827. [Google Scholar] [CrossRef]
- Tung, M.T.; Hang, L.T.T.; Tuan, L.A.; Nghi, N.H.; Phan, M.H. Influence of electrodeposition parameters on the magnetic and magneto-impedance properties of CoP/Cu wires. Phys. B Condens. Matter. 2014, 442, 16–20. [Google Scholar] [CrossRef]
- Hüller, K.; Sydow, M.; Dietz, G. Magnetic anisotropy, magnetostriction and intermediate range order in Co-P alloys. J. Magn. Magn. Mater. 1985, 53, 269–274. [Google Scholar] [CrossRef]
- Kohn, A.; Eizenberg, M.; Shacham-Diamand, Y. The role of microstructure in nanocrystalline conformal Co0.9W0.02P0.08 diffusion barriers for copper metallization. Appl. Surf. Sci. 2003, 212–213, 367–372. [Google Scholar] [CrossRef]
- Safavi, M.S.; Walsh, F.C. Electrodeposited Co–P alloy and composite coatings: Progress towards replacing hard chromium deposits. Surf. Coat. Technol. 2021, 422, 127564. [Google Scholar] [CrossRef]
- Bai, N.; Li, Q.; Mao, D.; Li, D.; Dong, H. One-step electrodeposition of Co/CoP film on Ni foam for efficient hydrogen evolution in alkaline solution. ACS Appl. Mater. Interfaces 2016, 8, 29400–29407. [Google Scholar] [CrossRef]
- Yu, X.; Wang, M.; Gong, X.; Guo, Z.; Wang, Z.; Jiao, S. Self-supporting porous CoP-based films for ultrastable overall water electrolysis. Adv. Energy Mater. 2018, 8, 1802445. [Google Scholar] [CrossRef]
- Anantharaj, S.; Ede, S.R.; Sakthikumar, K.; Karthick, K.; Mishra, S.; Kundu, S. Recent trends in electrochemical water splitting with sulfide, selenide and phosphide catalysts of Fe, Co and Ni: A review. ACS Catal. 2016, 6, 8069–8097. [Google Scholar] [CrossRef]
- Ha, D.-H.; Han, B.; Risch, M.; Giordano, L.; Yao, K.P.C.; Karayaylali, P.; Shao-Horn, Y. Activity and stability of cobalt phosphides for hydrogen evolution upon water splitting. Nano Energy 2016, 29, 37–45. [Google Scholar] [CrossRef]
- Su, L.; Cui, X.; He, T.; Zeng, L.; Tian, H.; Song, Y.; Qi, K.; Xia, B.Y. Surface reconstruction of cobalt phosphide nanosheets by electrochemical activation for enhanced alkaline HER. Chem. Sci. 2019, 10, 2019–2024. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Lin, Y.; Chen, Y.; Liu, Y.; Liu, C. Cobalt phosphide-based electrocatalysts: Synthesis and phase catalytic activity comparison for HER. J. Mater. Chem. A 2016, 4, 4745–4754. [Google Scholar] [CrossRef]
- Hu, G.; Tang, Q.; Jiang, D.-E. CoP for hydrogen evolution: Implications from hydrogen adsorption on CoP surfaces. Phys. Chem. Chem. Phys. 2016, 18, 33380–33386. [Google Scholar] [CrossRef]
- Men, Y.; Li, P.; Zhou, J.; Chen, S.; Luo, W. Descriptor for alkaline HER on CoP via transition-metal doping. Cell Rep. Phys. Sci. 2020, 1, 100136. [Google Scholar] [CrossRef]
- Ruythooren, W.; de Wit, E.; De Boeck, J.; Celis, J.-P. Electrodeposited CoP: Structural and magnetic properties. In Magnetic Materials, Processes, and Devices VI; The Electrochemical Society: Pennington, NJ, USA, 2001; pp. 309–316. [Google Scholar]
- Brenner, A.; Couch, D.E.; Williams, E.K. Electrodeposition of alloys of phosphorus with nickel or cobalt. J. Res. Natl. Bur. Stand. 1950, 44, 109. [Google Scholar] [CrossRef]
- Brenner, A. Electrodeposition of Alloys: Principles and Practice; Academic Press: New York, NY, USA, 1963; Volume II. [Google Scholar]
- Ignatova, K.; Marcheva, Y. Kinetics of electrodeposition of NiP, CoP and NiCoP coatings vs. sodium hypophosphite concentration. J. Chem. Technol. Metall. 2018, 53, 549–555. [Google Scholar]
- Brenner, A.; Riddell, G.E. Nickel plating on steel by chemical reduction. J. Res. Natl. Bur. Stand. 1946, 37, 31–34. [Google Scholar] [CrossRef]
- Brenner, A.; Riddell, G.E. Deposition of nickel and cobalt by chemical reduction. J. Res. Natl. Bur. Stand. 1947, 39, 385–395. [Google Scholar] [CrossRef]
- Da Silva, R.C.; Pasa, A.A.; Mallett, J.J.; Schwarzacher, W. Surface-morphology evolution during electrodeposition of amorphous CoP films. Surf. Sci. 2005, 576, 212–216. [Google Scholar] [CrossRef]
- Lucas, I.; Ciudad, D.; Plaza, M.; Ruiz-Gomez, S.; Aroca, C.; Perez, L. Assessment of layer thickness and interface quality in CoP electrodeposited multilayers. ACS Appl. Mater. Interfaces 2016, 8, 18930–18934. [Google Scholar] [CrossRef]
- Eliaz, N.; Gileadi, E. Physical Electrochemistry: Fundamentals, Techniques, and Applications, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2019; p. 480. ISBN 978-3-527-34139-9. [Google Scholar]
- Eliyahu, D.; Gileadi, E.; Galun, E.; Eliaz, N. AFM-based meniscus-confined three-dimensional electrodeposition. Adv. Mater. Technol. 2020, 5, 1900827. [Google Scholar] [CrossRef]
- Eliyahu, D.; Eliaz, N.; Gileadi, E. Meniscus-Confined Three-Dimensional Electrodeposition. U.S. Patent 12,202,193, 1 January 2025. [Google Scholar]
- Yan, X.; Sun, H.; Chen, S.; Liu, F.; Cheng, F.; Chen, J. Electrodeposition of (hydro)oxides for oxygen-evolution electrodes: A versatile route. Chem. Sci. 2020, 11, 12354–12369. [Google Scholar] [CrossRef]
- Chiu, C.-W.; Sun, I.-W.; Chen, P.-Y. Electrodeposition and characterization of CoP compounds from the hydrophilic ionic liquid [Bmpyrr][DCA]. J. Electrochem. Soc. 2017, 164, H5018–H5025. [Google Scholar] [CrossRef]
- Kim, J.; Jang, Y.J.; Jang, Y.H. Electrodeposition of stable noble-metal-free Co–P electrocatalysts for HER. Materials 2023, 16, 593. [Google Scholar] [CrossRef]
- Wang, T.; Jiang, Y.; Zhou, Y.; Du, Y.; Wang, C. In situ electrodeposition of CoP nanoparticles on carbon-nanomaterial-doped PPS flexible electrodes for HER. Appl. Surf. Sci. 2018, 442, 340–347. [Google Scholar] [CrossRef]
- Deng, R.; Li, X.; Wang, M.; Zhang, Q. Electrodeposited cobalt phosphide film with regulated interfacial structure and wetting for enhanced hydrogen evolution performance: Effect of saccharin. Appl. Surf. Sci. 2023, 616, 156456. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, L.; Dharan, G.; Ho, G.W. Electrodeposited cobalt phosphide superstructures for solar-driven thermoelectrocatalytic overall water splitting. J. Mater. Chem. A 2017, 5, 16580–16584. [Google Scholar] [CrossRef]
- Li, T.-T.; Zhou, Q.; Qian, J.; Hu, Y.; Zheng, Y.-Q. Electrodeposited CoP film for enhanced PEC water oxidation with an α-Fe2O3 photoanode. Electrochim. Acta 2019, 307, 92–99. [Google Scholar] [CrossRef]
- Li, X.; Qian, X.; Xu, Y.; Duan, F.; Yu, Q.; Wang, J.; Chen, L.; Dan, Y.; Cheng, X. Electrodeposited cobalt phosphides with hierarchical nanostructure on carbon membranes. J. Alloys Compd. 2020, 830, 154624. [Google Scholar] [CrossRef]
- Yu, L.; Wu, P.; Tian, T.; He, X.; Fan, M.; Cui, L. Crystalline/amorphous CoP@Ni/Fe–P as a boosted electrocatalyst for full water splitting. Dalton Trans. 2023, 52, 11941–11948. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; You, T.; Liu, X.; Deng, K.; Liu, P.; Tian, W.; Ji, J. Coupled plasma etching and electrodeposition of CoP/NiO nanosheets with surface reconstruction for water splitting. J. Mater. Chem. A 2024, 12, 9830–9840. [Google Scholar] [CrossRef]
- Duan, D.; Guo, D.; Gao, J.; Liu, S.; Wang, Y. Electrodeposition of cobalt–iron bimetal phosphide on Ni foam as a high-performance bifunctional electrocatalyst. J. Colloid Interface Sci. 2022, 621, 346–356. [Google Scholar] [CrossRef]
- Liu, J.; Chen, G.; Xiao, J.; Zhang, Y. Co–P/Fe3O4 composite film on nickel foam prepared by one-step electrodeposition for efficient water splitting. Int. J. Hydrogen Energy 2024, 62, 803–811. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.; Ahn, S.H. Electrodeposited amorphous Co–P–B ternary catalyst for HER. J. Mater. Chem. A 2018, 6, 6282–6290. [Google Scholar] [CrossRef]
- Yang, M.; Wang, Y.; Gu, Y.; Xue, Z.; Shi, J.; An, W.; Rui, Y. Electro-deposited copper nanoclusters on leaf-shaped cobalt phosphide (Co2P@Cu/NF) for boosted HER. J. Alloys Compd. 2022, 902, 163771. [Google Scholar] [CrossRef]
- Mondal, K.; Sathithsuksanoh, N.; Lalvani, S.B. Electrodeposition and characterization of films of CoP. Plating Surf. Finish. 2005, 2, 42–44. [Google Scholar]
- Sudagar, J.; Tamilarasan, R.; Sanjith, U.; Rajendran, R.; Kumar, R. Electroless deposition of nanolayered metallic coatings. In Nanoscaled Films and Layers; Nánai, L., Ed.; IntechOpen: London, UK, 2017; pp. 27–50. [Google Scholar] [CrossRef]
- Hao, Q.Q.; Hu, M.; Xie, Z.-X.; Ma, X.; Wang, W.; Ren, H.-P. Impact of coordination features of Co(II)–glycine complex on the surface sites of Co/SiO2 for Fischer–Tropsch synthesis. Catalysts 2020, 10, 1295. [Google Scholar] [CrossRef]
- Koizumi, N.; Mochizuki, T.; Yamada, M. Preparation of highly active catalysts for ultra-clean fuels. Catal. Today 2009, 141, 34–42. [Google Scholar] [CrossRef]
- Reed, S.J.B. Electron probe microanalysis. In Microprobe Techniques in the Earth Sciences; Potts, P.J., Bowles, J.F.W., Reed, S.J.B., Cave, M.R., Eds.; Chapman & Hall: London, UK, 1995; pp. 49–89. [Google Scholar]
- ASTM International. ASTM D3359-23: Standard Test Methods for Rating Adhesion by Tape Test; ASTM International: West Conshohocken, PA, USA, 2023. [Google Scholar] [CrossRef]
- ASTM D4541; Standard Test Method for Pull-Off Strength of Coatings Using Portable Adhesion Testers. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM D7234; Standard Test Method for Pull-Off Adhesion Strength of Coatings on Concrete Using Portable Pull-Off Adhesion Testers. ASTM International: West Conshohocken, PA, USA, 2021.
- ISO 4624; Paints and varnishes — Pull-off test for adhesion. International Organization for Standardization: Geneva, Switzerland, 2016.
- ASTM International. ASTM G133-22: Standard Test Method for Linearly Reciprocating Ball-on-Flat Sliding Wear; ASTM International: West Conshohocken, PA, USA, 2022. [Google Scholar] [CrossRef]
- Yu, Y.; Song, Z.; Ge, H.; Wei, G.; Jiang, L. Electrochemical mechanism of cobalt film electrodeposition process. Mater. Res. Innov. 2016, 20, 280–284. [Google Scholar] [CrossRef]
- Zeinali-Rad, M.; Allahkaram, S.R.; Mahdavi, S. Effect of pH, surfactant, and heat treatment on morphology, structure, and hardness of electrodeposited Co–P coatings. J. Mater. Eng. Perform. 2015, 24, 3209–3217. [Google Scholar] [CrossRef]
- Sheikholeslam, M.A.; Enayati, M.H.; Raeissi, K. Characterization of nanocrystalline and amorphous cobalt–phosphorous electrodeposits. Mater. Lett. 2008, 62, 3629–3631. [Google Scholar] [CrossRef]
- Kosta, I.; Sarret, M.; Müller, C. Structure, microhardness and corrosion behaviour of nanostructured Co–P coatings obtained by direct-current and pulse plating. Electrochim. Acta 2013, 114, 819–826. [Google Scholar] [CrossRef]
- Chen, Z.; Luo, W.; Du, F.; Wang, R.; Li, L.; Zhou, Y.; Zou, Z. Room-temperature preparation of cobalt-based electrocatalysts through simple solution treatment for selectively high-efficiency hydrogen evolution reaction in alkaline or acidic medium. J. Nanomater. 2018, 2018, 8124920. [Google Scholar] [CrossRef]
- Ezhilselvi, V.; Seenivasan, H.; Bera, P.; Anandan, C. Characterization and corrosion behavior of Co and Co–P coatings electrodeposited from chloride bath. RSC Adv. 2014, 4, 46293–46304. [Google Scholar] [CrossRef]
- Kosta, I.; Vicenzo, A.; Müller, C.; Sarret, M. Mixed amorphous–nanocrystalline cobalt–phosphorous by pulse plating. Surf. Coat. Technol. 2012, 207, 443–449. [Google Scholar] [CrossRef]
- Baik, S.I.; Duhin, A.; Phillips, P.J.; Klie, R.F.; Gileadi, E.; Seidman, D.N.; Eliaz, N. Atomic-scale structural and chemical study of columnar and multilayer Re–Ni electrodeposited thermal barrier coatings. Adv. Eng. Mater. 2016, 18, 1133–1144. [Google Scholar] [CrossRef]
- Ibrahim, M.A.M.; Al Radadi, R.M. Role of glycine as a complexing agent in nickel electrodeposition from acidic sulphate bath. Int. J. Electrochem. Sci. 2015, 10, 4946–4971. [Google Scholar] [CrossRef]
- Fukunaka, Y.; Aikawa, S.; Asaki, Z. Fundamental study on electrodeposition of Co and Co–P films. J. Electrochem. Soc. 1994, 141, 1783–1791. [Google Scholar] [CrossRef]
- Kosta, I.; Imaz, N.; Cinca, N.; García-Lecina, E.; Sarret, M.; Müller, C. Pulse plated CoP alloy as substitute for hard chromium electrodeposits. Trans. IMF 2012, 90, 252–258. [Google Scholar] [CrossRef]
- Jung, H.; Alfantazi, A. An Electrochemical impedance spectroscopy and polarization study of nanocrystalline Co and Co–P alloy in 0.1 M H2SO4 solution. Electrochim. Acta 2006, 51, 1806–1814. [Google Scholar] [CrossRef]
- Zor, S. Sulfathiazole as potential corrosion inhibitor for copper in 0.1 M NaCl. Prot. Met. Phys. Chem. Surf. 2014, 50, 530–537. [Google Scholar] [CrossRef]
- King, F.; Litke, C.D.; Quinn, M.J.; LeNeveu, D.M. The measurement and prediction of the corrosion potential of copper in chloride solutions as a function of oxygen concentration and mass-transfer coefficient. Corros. Sci. 1995, 37, 833–851. [Google Scholar] [CrossRef]
- Li, Z.; Gordon, R.G.; Farmer, D.B.; Lin, Y.; Vlassak, J. Nucleation and adhesion of ALD copper on cobalt adhesion layers and tungsten-nitride diffusion barriers. Electrochem. Solid-State Lett. 2005, 8, G182–G185. [Google Scholar] [CrossRef]
- Luo, W.; Long, N.; Peng, J.; Wang, W.; Jiang, Y.; Shen, W.; He, R.; Su, W.; Li, M. Alloy/interface-induced activation of metal-phosphorus bonds in Ni5Cu3/CoP for efficient water splitting. Appl. Surf. Sci. 2025, 697, 163025. [Google Scholar] [CrossRef]
- Okamoto, N.; Wang, F.; Watanabe, T. Adhesion of electrodeposited copper, nickel and silver films on copper, nickel and silver substrates. Mater. Trans. 2004, 45, 3330–3333. [Google Scholar] [CrossRef]
- Lu, N.; Cai, J.; Li, L. Dependence of interfacial adhesion of Co–P film on its microstructure. Surf. Coat. Technol. 2012, 206, 4822–4827. [Google Scholar] [CrossRef]
- McCrea, J. Advanced Nanocrystalline Cobalt Alloys and Composites as Alternatives for Chromium and Nickel Plating in Repair Operations. In Final Report, SERDP Project WP-2609; Integram Technologies Inc.: San Antonio, TX, USA, 2019. [Google Scholar]
- Yu, Y.; Song, Z.; Ge, H.; Wei, G. Preparation of Co–P films by ultrasonic electroless deposition at low initial temperature. Prog. Nat. Sci. Mater. Int. 2014, 24, 232–238. [Google Scholar] [CrossRef]
- Dinega, D.P.; Bawendi, M.G. A solution-phase chemical approach to a new crystal structure of cobalt. Angew. Chem. Int. Ed. 1999, 38, 1788–1791. [Google Scholar] [CrossRef]
- Morcos, B. Development of a Process for the Synthesis of Magnetic Metal Nanoparticles Using Ionic Liquids. Ph.D. Thesis, Université de Lyon, Lyon, France, 2018. [Google Scholar]
- Elliott, S.R. Physics of Amorphous Materials, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1990. [Google Scholar]
- Inoue, A. Stabilization of metallic supercooled liquid and bulk amorphous alloys. Acta Mater. 2000, 48, 279–306. [Google Scholar] [CrossRef]
- Kohn, A.; Eizenberg, M.; Shacham-Diamand, Y. Structure of electroless-deposited Co0.9 W0.02 P0.08 thin films and their evolution with thermal annealing. J. Appl. Phys. 2003, 94, 3810–3822. [Google Scholar] [CrossRef]
- Kohn, A.; Dean, J.; Kovacs, A.; Zeltser, A.; Carey, M.J.; Geiger, D.; Hrkac, G.; Schrefl, T.; Allwood, D. Exchange-bias in amorphous ferromagnetic and polycrystalline antiferromagnetic bilayers: Structural study and micromagnetic modeling. J. Appl. Phys. 2011, 109, 083924. [Google Scholar] [CrossRef]
- Döhl, R.; Macht, M.-P.; Naundorf, V. Measurement of the diffusion coefficient of cobalt in copper. Phys. Status Solidi A 1984, 86, 603–612. [Google Scholar] [CrossRef]
- Cebollada, F.; González, J.; de Julián, C. Detailed analysis of the crystallization of the Co–P amorphous system: Kinetics, influence of magnetic order, and formation of textures. Phys. Rev. B 1997, 56, 6056–6065. [Google Scholar] [CrossRef]
- Masui, K.; Maruno, S.; Miyoshi, N. Crystallization kinetics of electro-deposited amorphous Co–P alloys. J. Non-Cryst. Solids 1985, 70, 263–270. [Google Scholar] [CrossRef]
- Vijayan, S.; Luo, N.; Aindow, M. Microstructural stability and phase transformations in electrodeposited cobalt–phosphorus coatings. J. Alloys Compd. 2017, 719, 142–150. [Google Scholar] [CrossRef]
- Ishida, K.; Nishizawa, T. The Co–P (cobalt–phosphorus) system. Bull. Alloy Phase Diagr. 1990, 11, 555–560. [Google Scholar] [CrossRef]
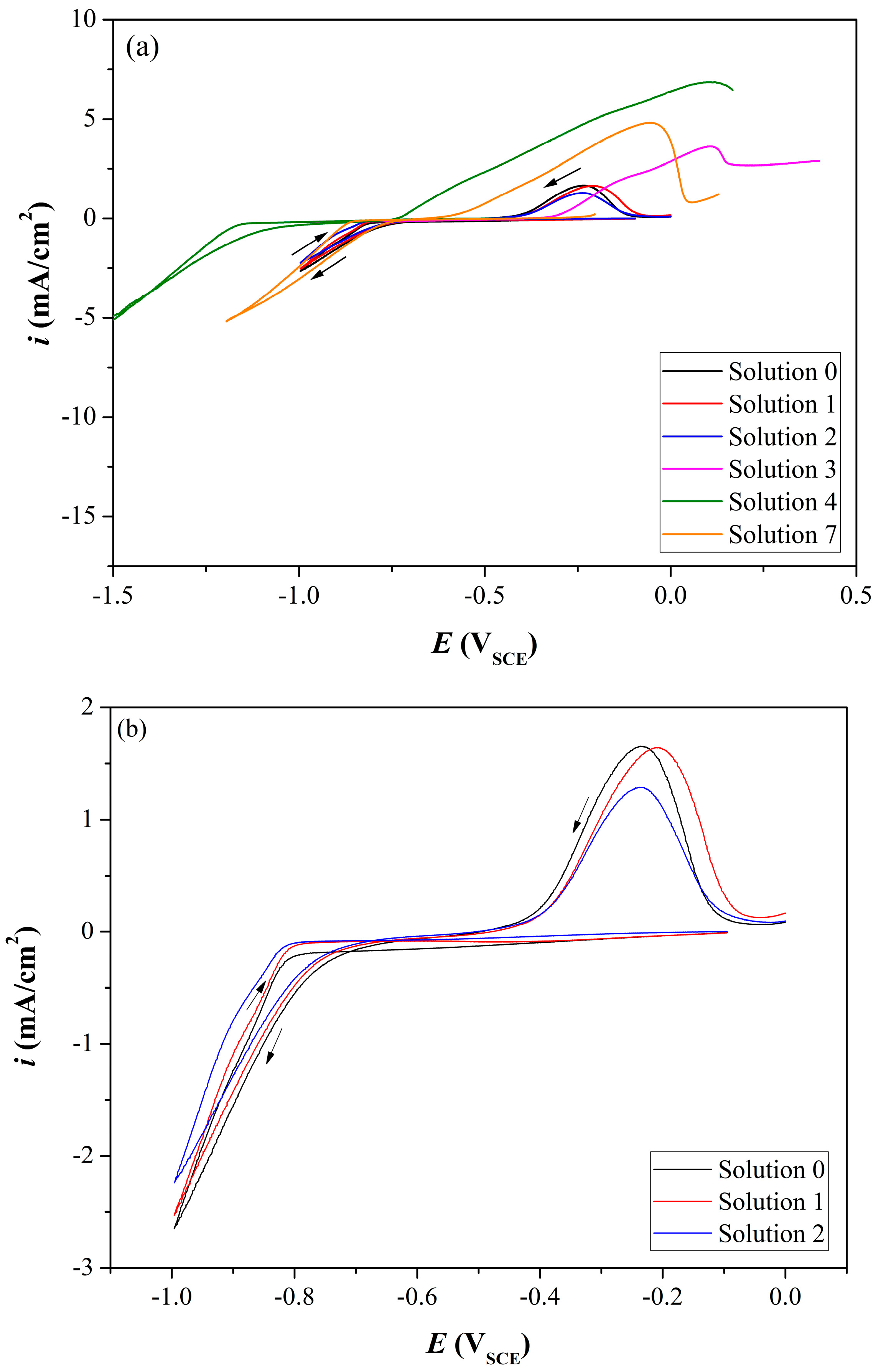

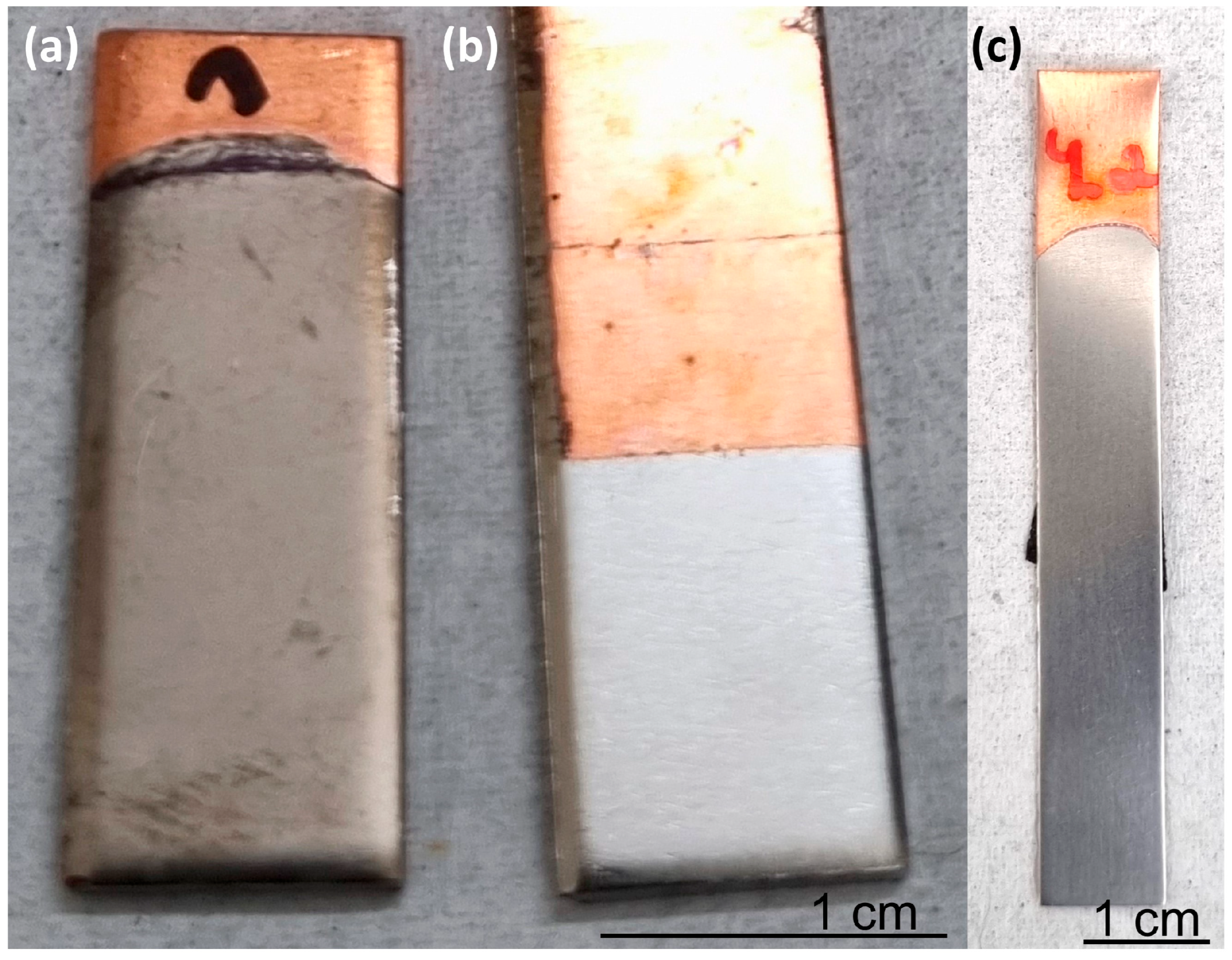
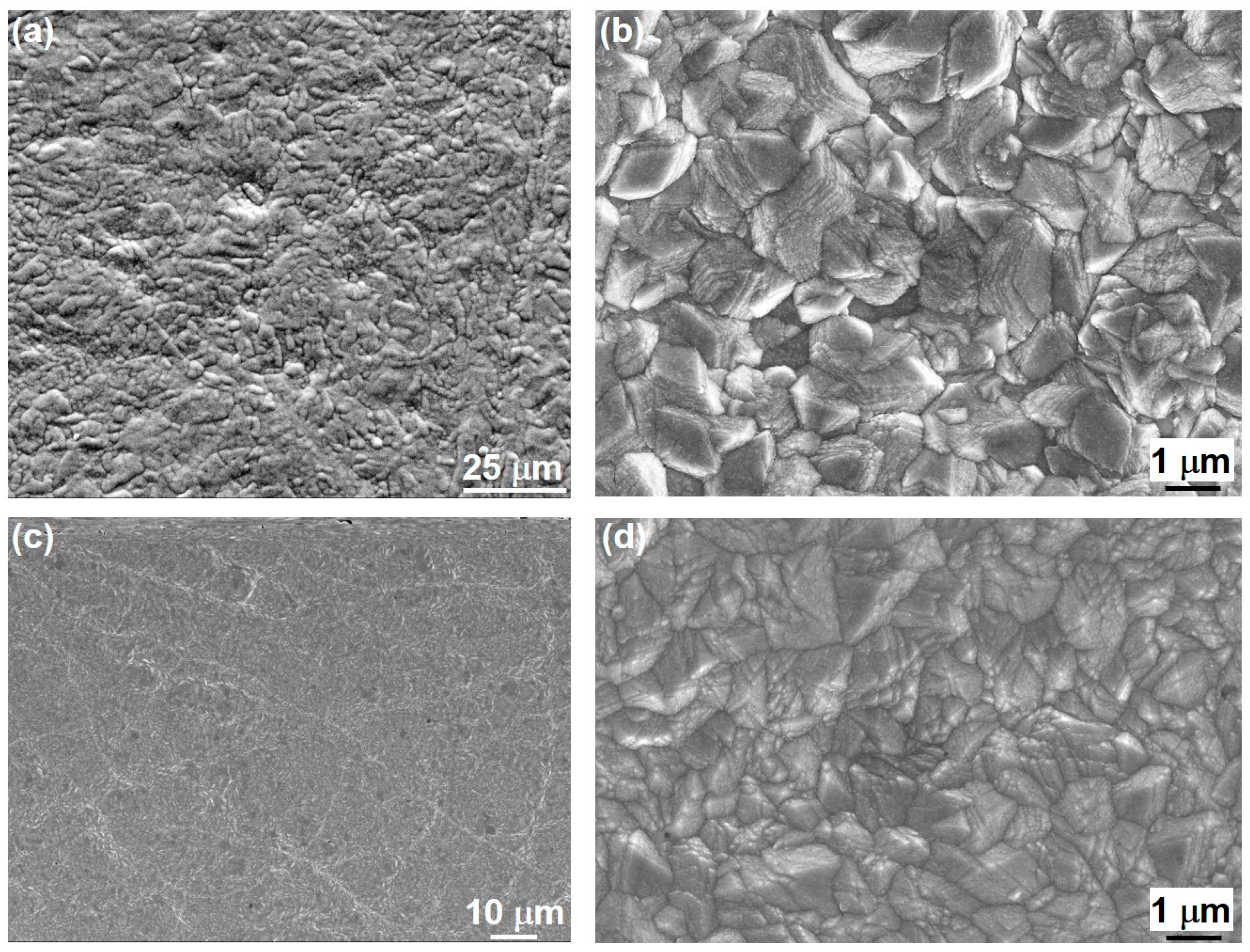
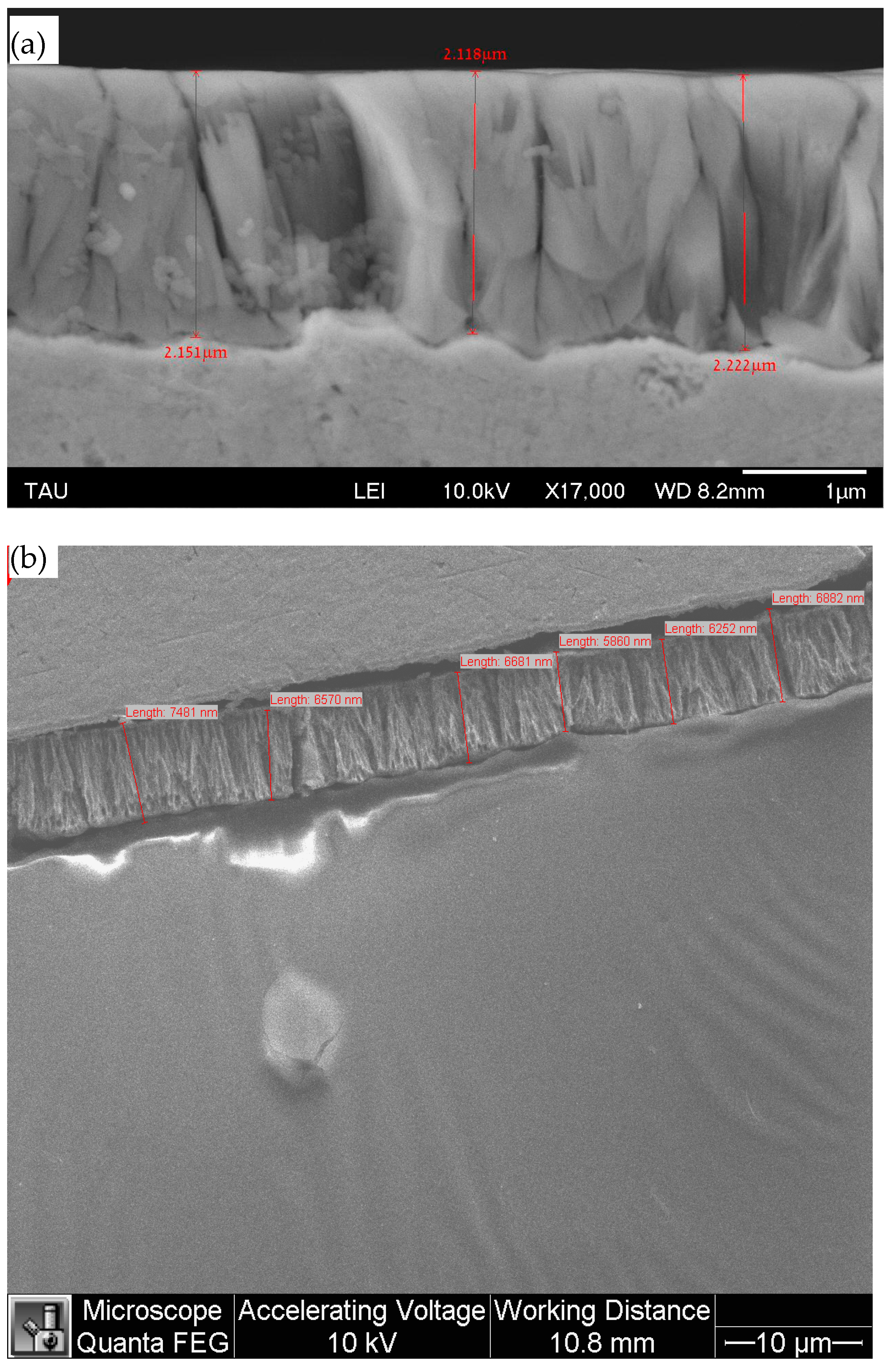

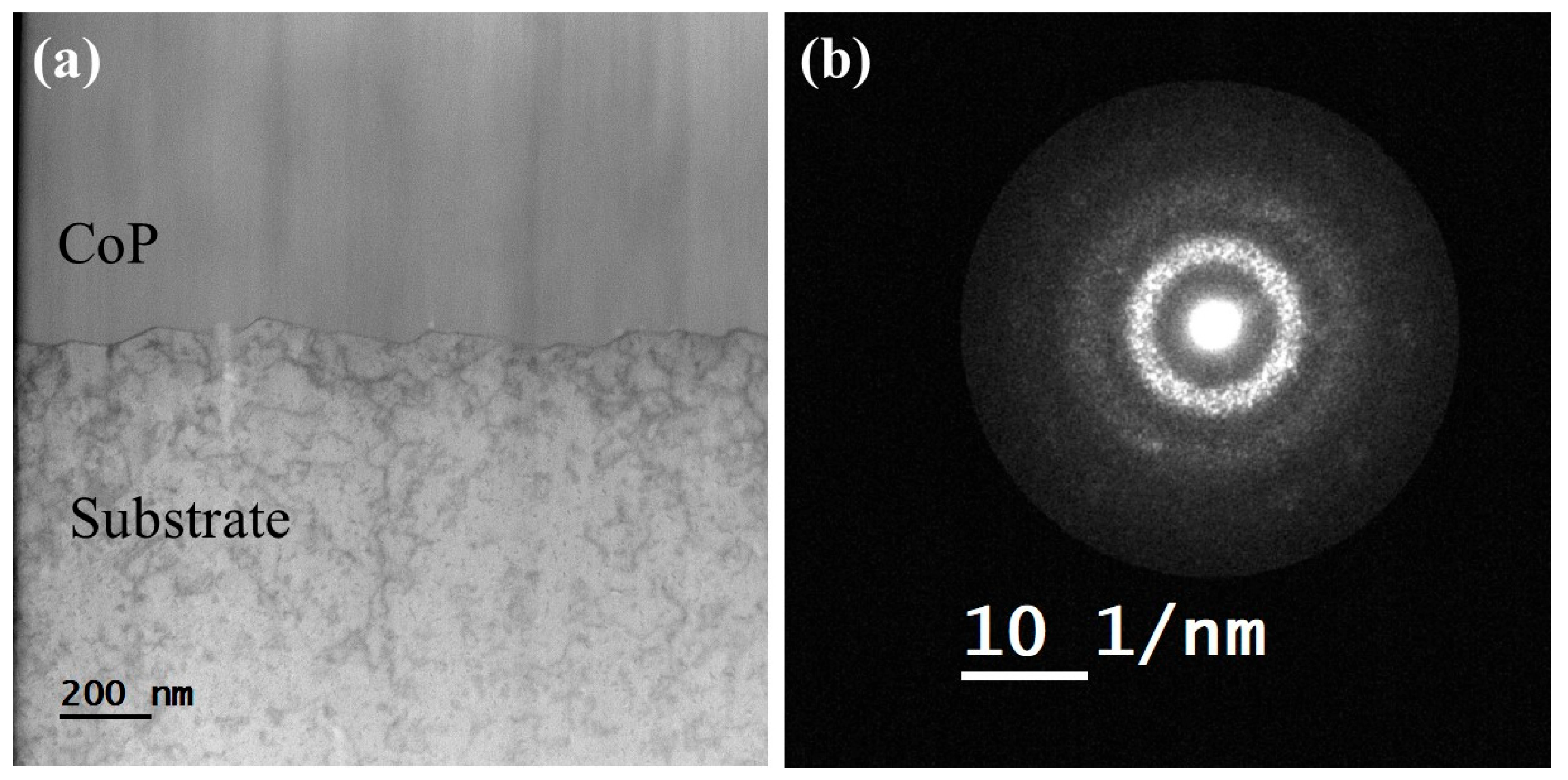
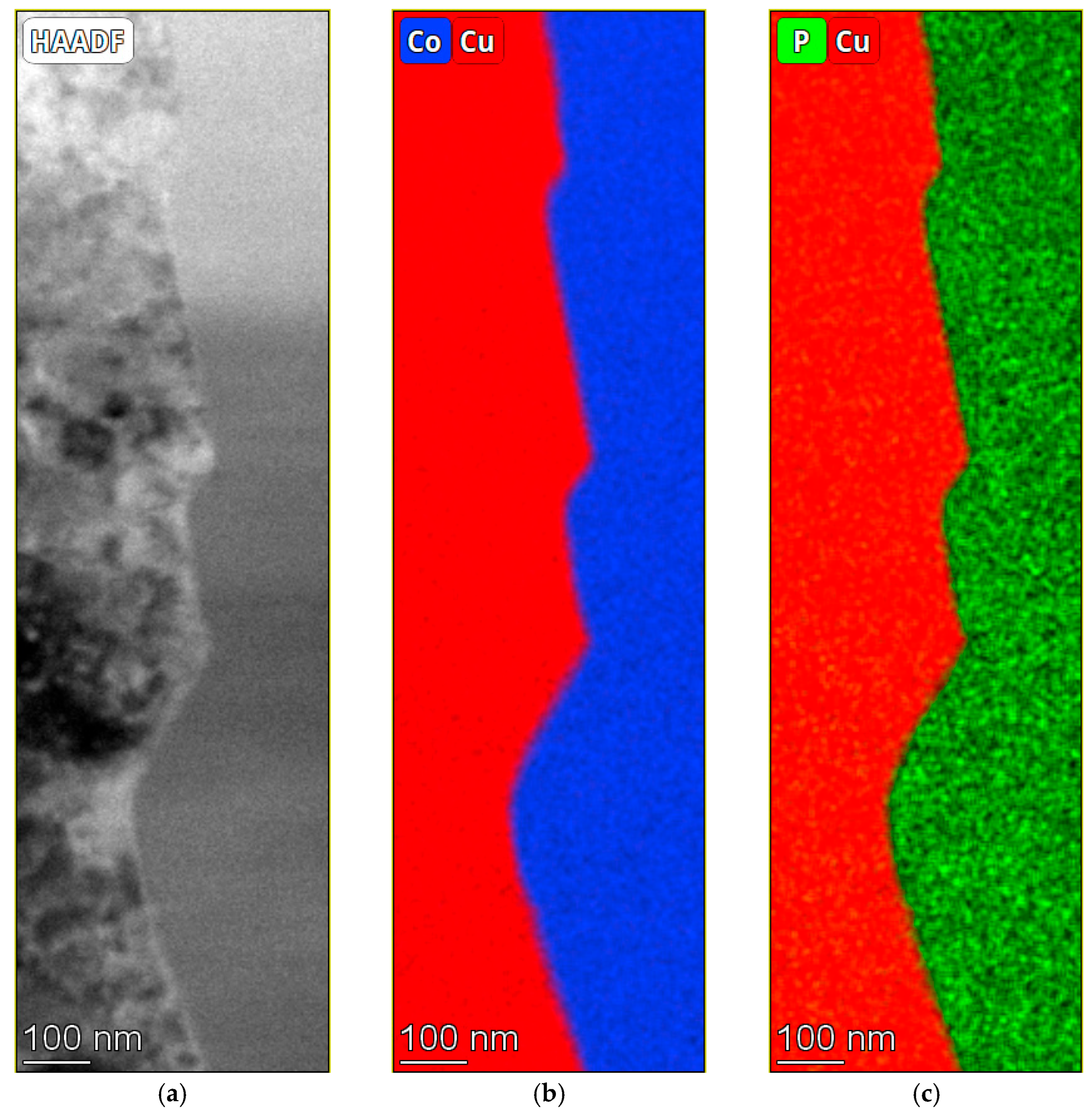
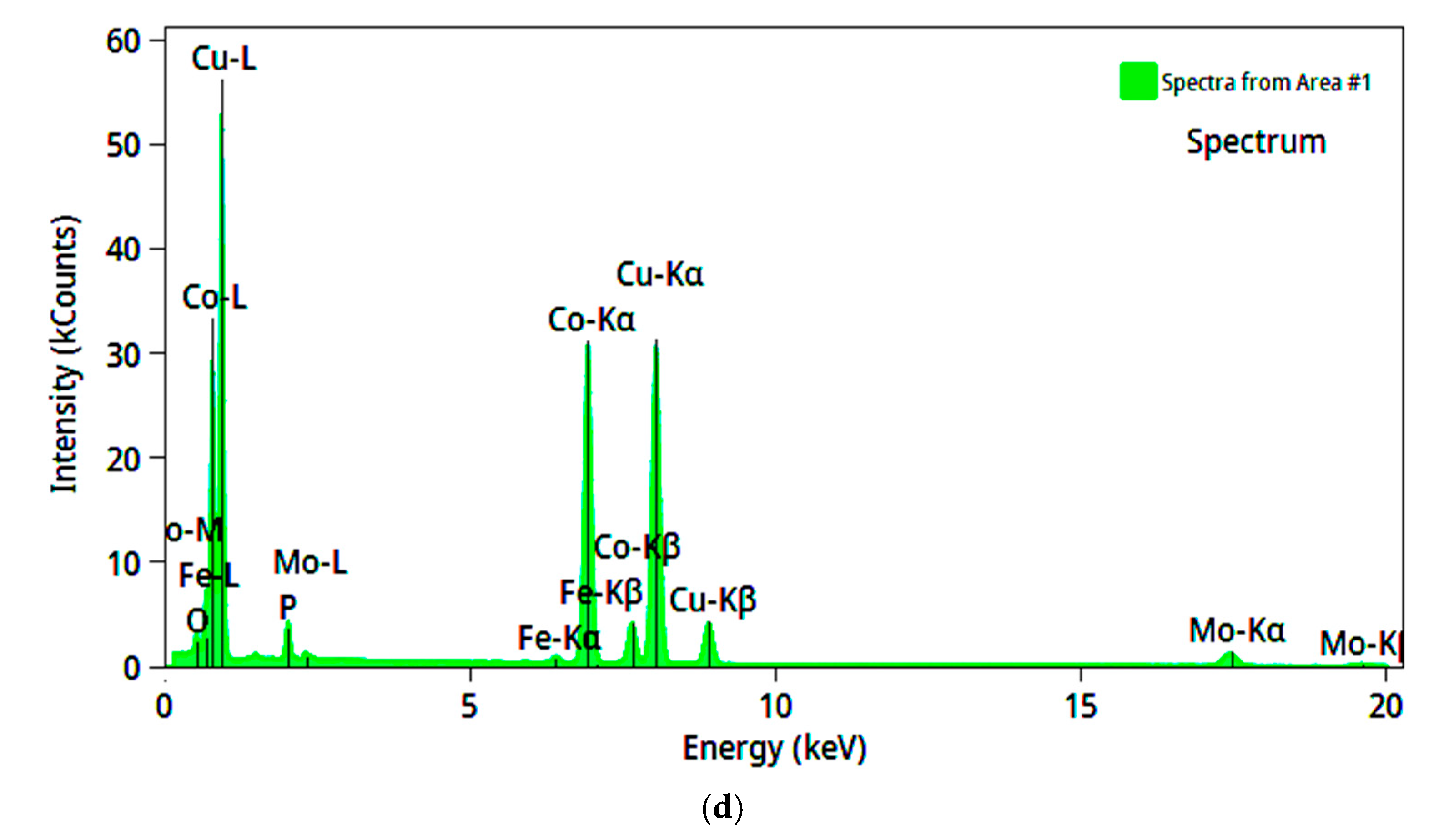

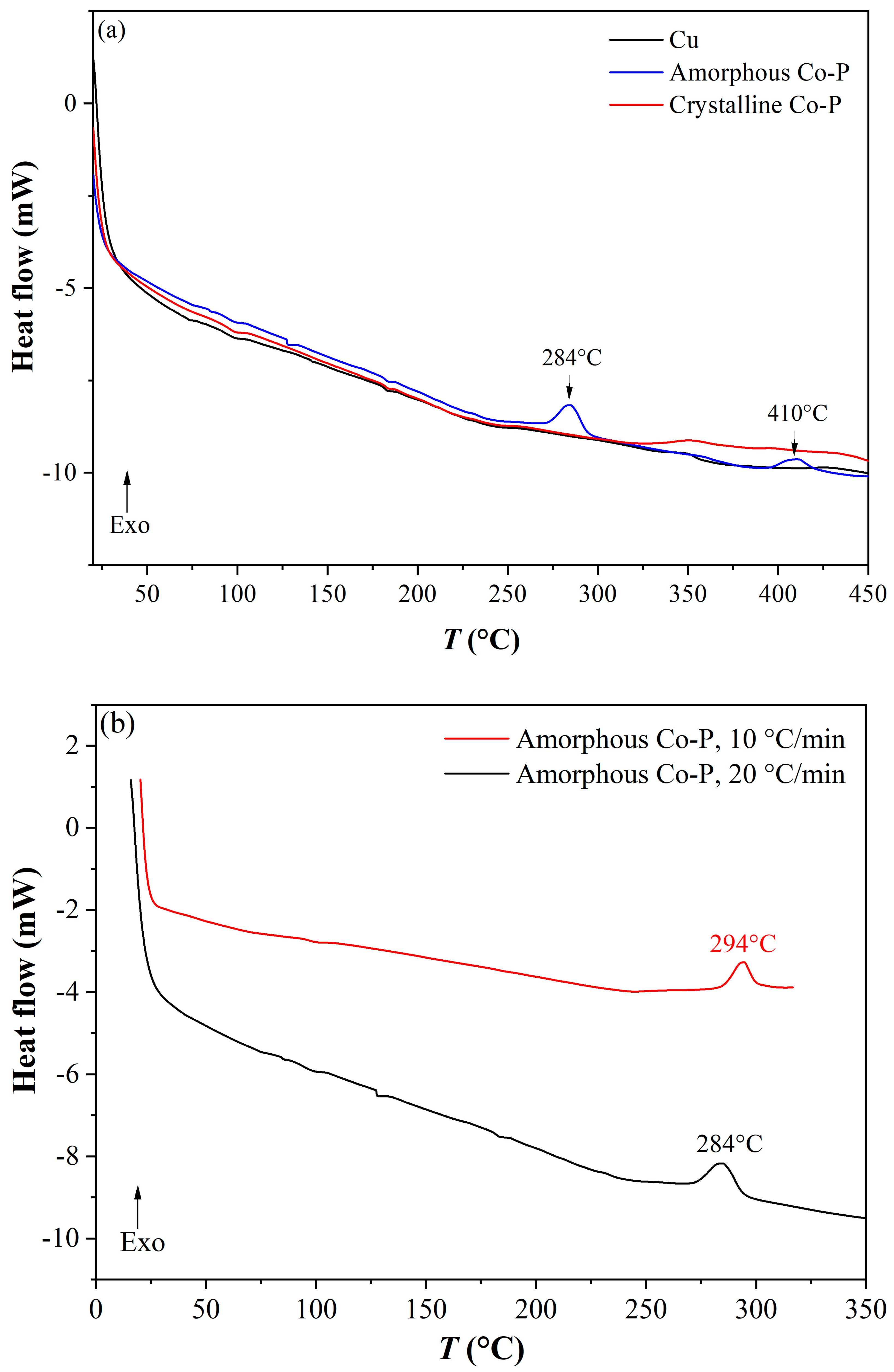
| Solution No. | CoSO4·7H2O [M] | H3PO4 [M] | H3PO3 [M] | Glycine [M] | pH |
|---|---|---|---|---|---|
| 0 | 0.25 | 0.102 | 0.122 | 0.3 | 3.6–3.8 |
| 1 | 0.25 | - | 0.122 | 0.3 | 3.72 |
| 2 | 0.25 | - | 0.122 | 0.6 | 3.83 |
| 3 | 0.25 | - | 0.122 | 0.6 | 9.71 |
| 4 | 0.10 | - | 0.122 | 0.6 | 9.71 |
| 7 | 0.10 | - | 0.122 | 0.6 | 6.19 |
| Sample | Ra [µm] |
|---|---|
| Uncoated Cu | 0.049 ± 0.002 |
| Amorphous CoP coating on Cu substrate | 0.046 ± 0.001 |
| Crystalline CoP coating on Cu substrate | 0.052 ± 0.001 |
| Solution No. | Stirring | t [h] | Co [wt.%] | P [wt.%] | Co [at.%] | P [at.%] |
|---|---|---|---|---|---|---|
| 0 | Y | 1.2 | 94.83 ± 1.34 (n = 4) | 5.17 ± 1.34 (n = 4) | 90.62 ± 2.33 (n = 4) | 9.38 ± 2.33 (n = 4) |
| 1 | Y | 1.2 | 93.61 ± 0.46 (n = 16) | 6.39 ± 0.46 (n = 16) | 88.50 ± 0.77 (n = 16) | 11.50 ± 0.77 (n = 16) |
| 1 | N | 1.2 | 93.22 ± 1.76 (n = 2) | 2.66 ‡ ± 0.20 (n = 2) | ||
| 1 | Y | 4 | 92.80 ± 0.65 (n = 29) | 7.20 ± 0.65 (n = 29) | 87.14 ± 1.09 (n = 29) | 12.86 ± 1.09 (n = 29) |
| 1 | N | 4 | 94.53 (n = 1) | 5.47 (n = 1) 2.33 § | 90.09 (n = 1) | 9.91 (n = 1) |
| 2 | Y | 1.2 | 93.33 ± 1.22 (n = 11) | 6.67 ± 1.22 (n = 11) | 88.05 ± 2.06 (n = 11) | 11.95 ± 2.06 (n = 11) |
| 7 | Y | 1.2 | 98.30 ± 0.53 (n = 2) | 1.70 ± 0.53 (n = 2) | 96.80 ± 0.97 (n = 2) | 3.20 ± 0.97 (n = 2) |
| Sample Number | Coefficient of Friction | P [wt.%] |
|---|---|---|
| 4.3.1 § | 0.11 ± 0.02 | 7.19 ± 0.46 |
| 4.3.2 § | 0.14 ± 0.02 | 6.55 ± 0.36 |
| 4.3.3 § | 0.12 ± 0.01 | 6.91 ± 0.72 |
| 4.3.4 § | 0.15 ± 0.02 | 6.13 ± 0.42 |
| 3.6.2 | 0.16 ± 0.01 | 6.58 ± 0.17 |
| 3.5.1 | 0.17 ± 0.01 | 6.84 ± 0.07 |
| 3.6.1 | 0.14 ± 0.01 | 6.73 ± 0.23 |
| 3.3.2 | 0.12 ± 0.01 | 9.83 ± 0.65 |
| Microstructure | P | Co | ||
|---|---|---|---|---|
| [wt.%] | [at.%] | [wt.%] | [at.%] | |
| Amorphous | 7.83 ± 0.21 | 13.91 ± 0.35 | 92.17 ± 0.21 | 86.99 ± 0.35 |
| Crystalline | 4.30 ± 0.17 | 7.88 ± 0.30 | 95.70 ± 2.17 | 92.12 ± 0.30 |
| Microstructure | E [GPa] | H [GPa] | H [VHN] ‡ |
|---|---|---|---|
| Amorphous | 153 ± 9 § | 7.8 ± 0.7 | 795.6 |
| Crystalline | 167 ± 17 | 4.9 ± 0.8 | 449.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eliaz, N.; Weisman, G.; Kohn, A.; Levi, G.; Rosen, B.A.; Moshkovich, A.; Rapoport, L.S. Electrodeposition of Amorphous Cobalt–Phosphorus Coating. Materials 2025, 18, 4883. https://doi.org/10.3390/ma18214883
Eliaz N, Weisman G, Kohn A, Levi G, Rosen BA, Moshkovich A, Rapoport LS. Electrodeposition of Amorphous Cobalt–Phosphorus Coating. Materials. 2025; 18(21):4883. https://doi.org/10.3390/ma18214883
Chicago/Turabian StyleEliaz, Noam, Gal Weisman, Amit Kohn, George Levi, Brian A. Rosen, Alexey Moshkovich, and Lev S. Rapoport. 2025. "Electrodeposition of Amorphous Cobalt–Phosphorus Coating" Materials 18, no. 21: 4883. https://doi.org/10.3390/ma18214883
APA StyleEliaz, N., Weisman, G., Kohn, A., Levi, G., Rosen, B. A., Moshkovich, A., & Rapoport, L. S. (2025). Electrodeposition of Amorphous Cobalt–Phosphorus Coating. Materials, 18(21), 4883. https://doi.org/10.3390/ma18214883








