Synthesis of Poly(Lactic Acid-co-Arginine) and Construction of Its Ternary Phase Diagram for Nonsolvent Induced Phase Separation
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Synthesis of PLAA
2.3. Characterization of PLAA
2.4. Preparation of “PLAA/CHCl3/C6H14” Ternary System
3. Results and Discussion
3.1. Molecular Weight and Polymerization Mechanism of PLAA
3.2. Chemical Structure Characterization of PLAA
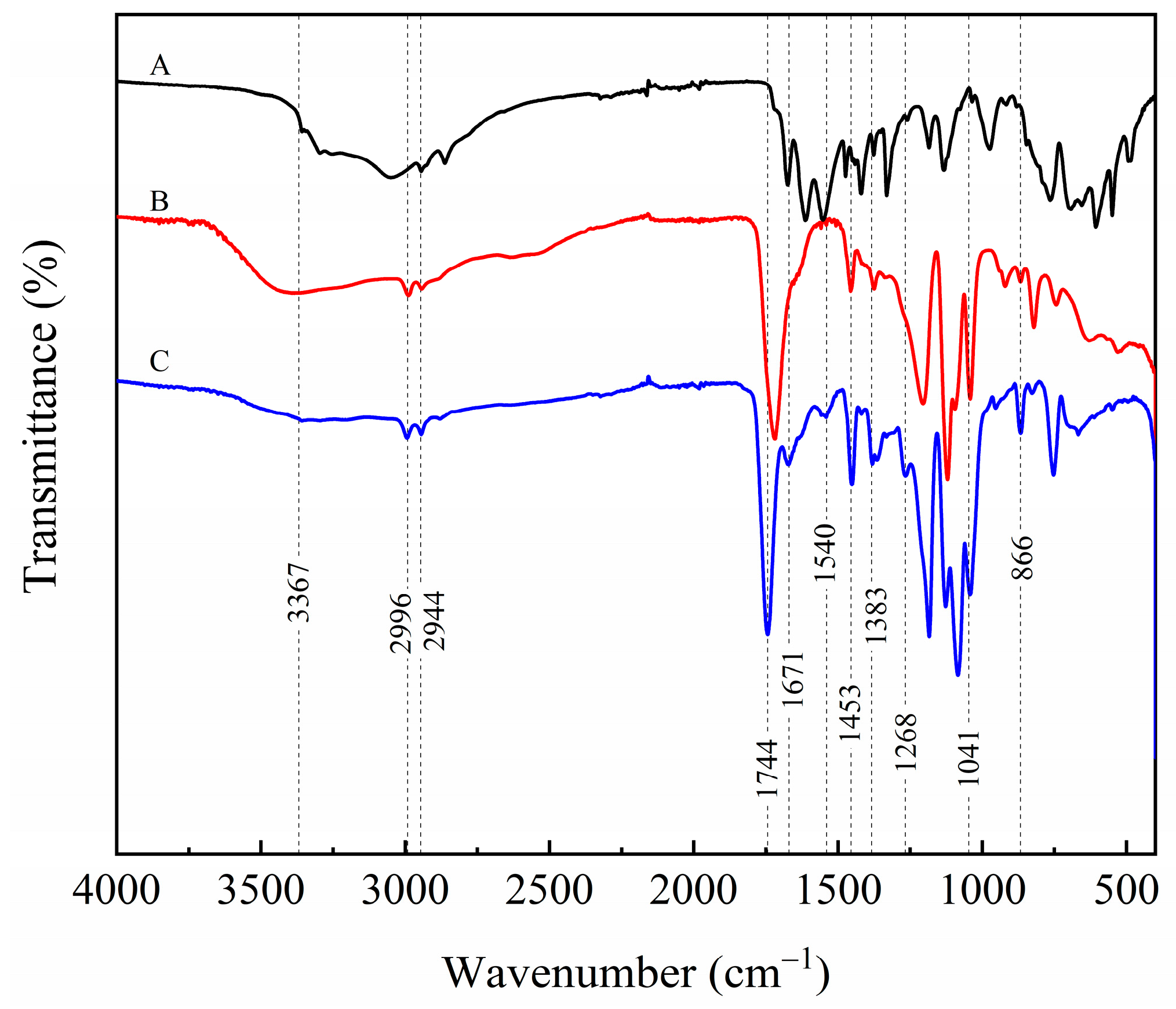
3.2.1. FTIR Characterization of PLAA
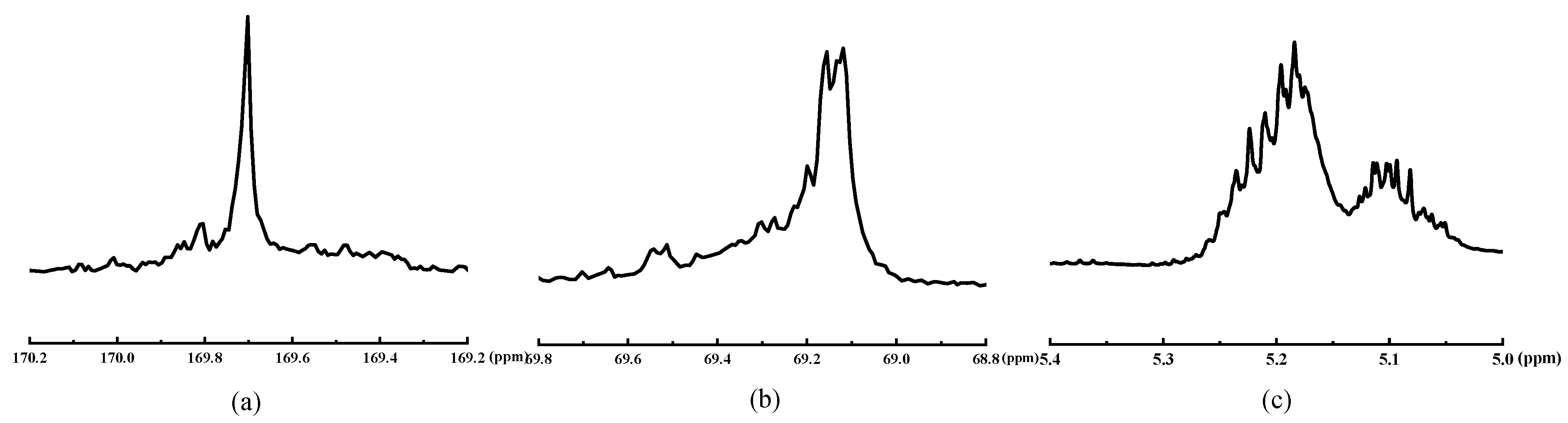
3.2.2. The 1H-NMR and 13C-NMR Spectra of PLAA
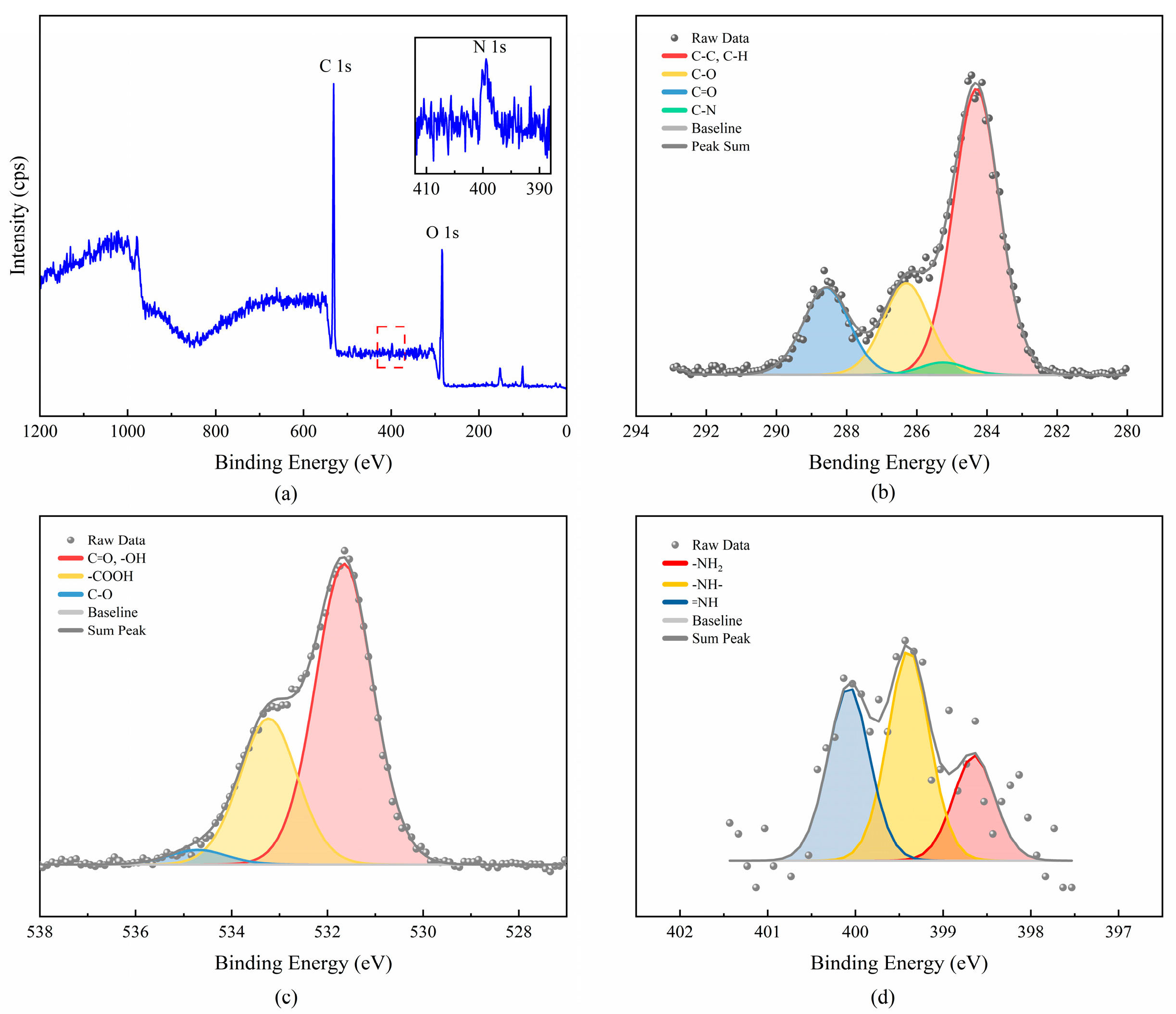
3.2.3. XPS Analysis of PLAA
3.2.4. Racemization of Lactic Acid Segments in PLAA
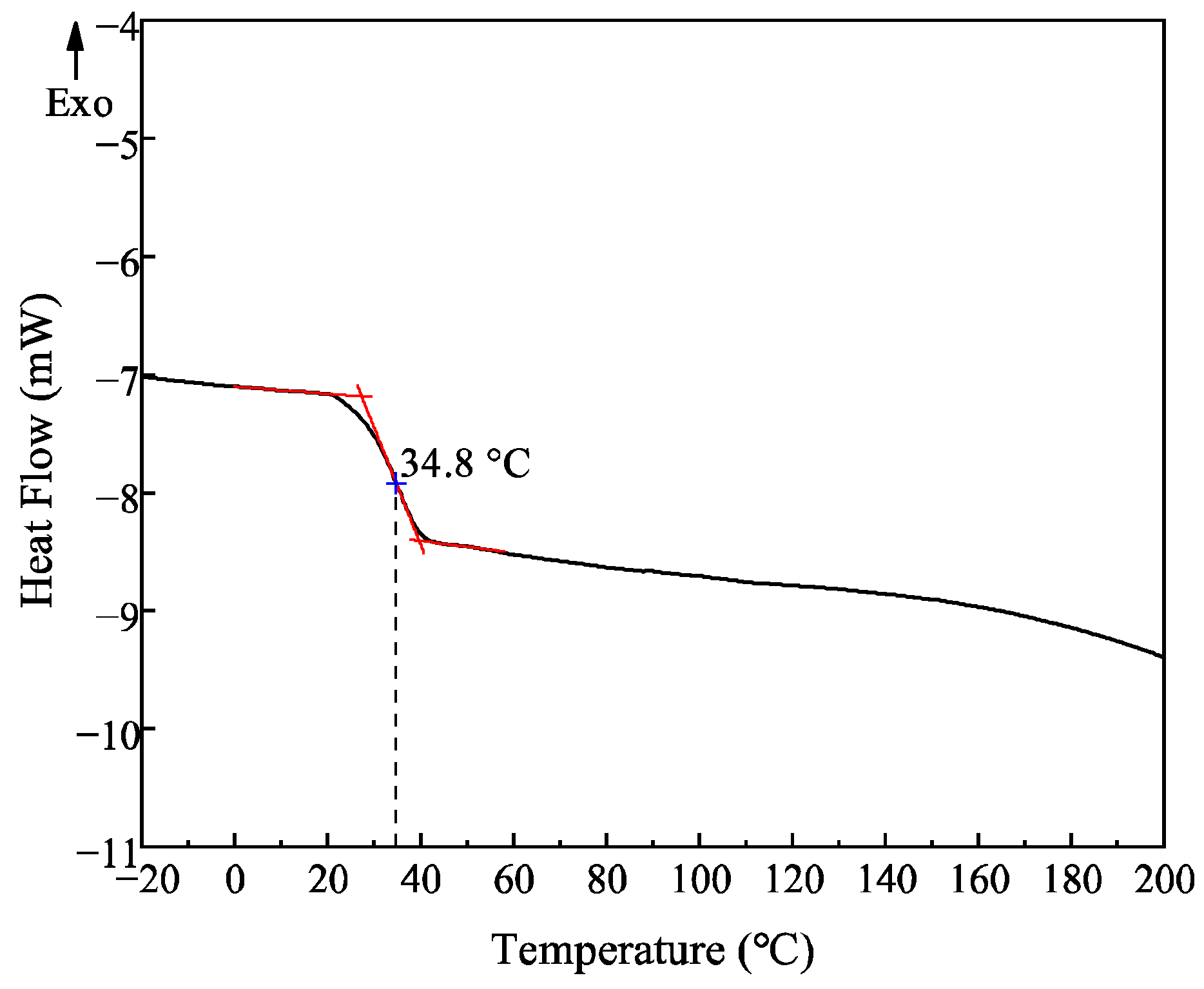
3.3. DSC Analysis of PLAA
3.4. Hydrophilic Property Analysis of PLAA
3.5. Construction of “PLAA/CHCl3/C6H14” Ternary Phase Diagram
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stefaniak, K.; Masek, A. Green Copolymers Based on Poly(Lactic Acid)—Short Review. Materials 2021, 14, 5254. [Google Scholar] [CrossRef]
- Mazur, K.E.; Witko, T.W.; Kośmider, A.; Kuciel, S.T. Development and Mechanical Characterization of Environmentally Friendly PLA/Crop Waste Green Composites. Materials 2025, 18, 3608. [Google Scholar] [CrossRef]
- Theodorou, A.; Raptis, V.; Baltzaki, C.I.M.; Manios, T.; Harmandaris, V.; Velonia, K. Synthesis and Modeling of Poly(L-lactic acid) via Polycondensation of L-Lactic Acid. Polymers 2023, 15, 4569. [Google Scholar] [CrossRef]
- Vayshbeyn, L.I.; Mastalygina, E.E.; Olkhov, A.A.; Podzorova, M.V. Poly(lactic acid)-Based Blends: A Comprehensive Review. Appl. Sci. 2023, 13, 5148. [Google Scholar] [CrossRef]
- Shen, Y.; Tu, T.; Yi, B.; Wang, X.; Tang, H.; Liu, W.; Zhang, Y. Electrospun Acid-neutralizing Fibers for the Amelioration of Inflammatory Response. Acta Biomater. 2019, 97, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Ramot, Y.; Haim-Zada, M.; Domb, A.J.; Nyska, A. Biocompatibility and Safety of PLA and Its Copolymers. Adv. Drug Deliv. Reviews 2016, 107, 153–162. [Google Scholar] [CrossRef]
- Ma, J.; Yu, P.; Xia, B.; An, Y.; Wang, Z. Synthesis of a Biodegradable and Environmentally Friendly Shale Inhibitor Based on Chitosan-grafted l-Arginine for Wellbore Stability and the Mechanism Study. ACS Appl. Bio Mater. 2019, 2, 4303–4315. [Google Scholar] [CrossRef]
- Yao, J.; Zhang, S.; Li, W.; Li, Y. Miscibility Evaluation of Poly(l-lactic acid)/Poly(lactic acid-co-lysine) Blends. J. Appl. Biomater. Funct. Mater. 2016, 14, e230–e239. [Google Scholar] [CrossRef]
- Algebraistova, P.Y.; Basko, A.V.; Ilyasova, A.N.; Lebedeva, T.N.; Mironov, A.V.; Pochivalov, K.V.; Popov, V.K. Phase Equilibria and Structure Formation in the Polylactic-co-Glycolic Acid/Tetraglycol/Water Ternary System. Polymers 2023, 15, 1281. [Google Scholar] [CrossRef] [PubMed]
- Rezabeigi, E.; Wood-Adams, P.M.; Drew, R.A.L. Isothermal Ternary Phase Diagram of the Polylactic Acid-dichloromethane-hexane System. Polymer 2014, 55, 3100–3106. [Google Scholar] [CrossRef]
- Wang, B.; Yao, J.; Wang, H.; Wang, M. Construction of a Ternary System: A Strategy for the Rapid Formation of Porous Poly(lactic acid) Fibers. RSC Adv. 2022, 12, 6476–6483. [Google Scholar] [CrossRef]
- Qi, Z.; Yu, H.; Chen, Y.; Zhu, M. Highly Porous Fibers Prepared by Electrospinning a Ternary System of Nonsolvent/Solvent/Poly(l-lactic acid). Mater. Lett. 2009, 63, 415–418. [Google Scholar] [CrossRef]
- Kang, J.; Hwang, J.-Y.; Huh, M.; Yun, S.I. Porous Poly(3-Hydroxybutyrate) Scaffolds Prepared by Non-Solvent-Induced Phase Separation for Tissue Engineering. Macromol. Res. 2020, 28, 835–843. [Google Scholar] [CrossRef]
- Rarima, R.; Unnikrishnan, G. Poly(Lactic Acid)/Gelatin Foams by Non-Solvent Induced Phase Separation for Biomedical Applications. Polym. Degrad. Stab. 2020, 177, 109187. [Google Scholar] [CrossRef]
- Liu, W.; Huang, N.; Yang, J.; Peng, L.; Li, J.; Chen, W. Characterization and Application of Porous Polylactic Acid Films Prepared by Nonsolvent-Induced Phase Separation Method. Food Chem. 2022, 373, 131525. [Google Scholar] [CrossRef]
- Ferruti, F.; Alongi, J.; Manfredi, A.; Ranucci, E.; Ferruti, P. Controlled Synthesis of Linear Polyamidoamino Acids. Polymers 2019, 11, 1324. [Google Scholar] [CrossRef]
- Zhan, S.; Wan, Z.; Zhao, Y.; Wang, J.; Li, Z. Ring-Opening Dispersion Polymerization of L-Lactide Initiated by L-Arginine in Supercritical Carbon Dioxide. Polym. Degrad. Stab. 2020, 171, 109049. [Google Scholar] [CrossRef]
- Tan, H.; Zhang, Y.; Sun, L.; Sun, Y.; Dang, H.; Yang, Y.; Jiang, D. Preparation of Nano Sustained-Release Fertilizer Using Natural Degradable Polymer Polylactic Acid by Coaxial Electrospinning. Int. J. Biol. Macromol. 2021, 193, 903–914. [Google Scholar] [CrossRef]
- Ma, Y.; Li, J. An Antibacterial Coaxial Electrospun Polylactic Acid/Silk Fibroin Loaded with Nano-ZnO and Voltaren. Mater. Lett. 2022, 325, 132804. [Google Scholar] [CrossRef]
- Zamir, S.S.; Fathi, B.; Ajji, A.; Robert, M.; Elkoun, S. Biodegradation of Modified Starch/Poly Lactic Acid Nanocomposite in Soil. Polym. Degrad. Stab. 2022, 199, 109902. [Google Scholar] [CrossRef]
- Peng, H.; Xiao, Y.; Mao, X.; Chen, L.; Crawford, R.; Whittaker, A.K. Amphiphilic Triblock Copolymers of Methoxy-Poly(ethylene glycol)-b-Poly(l-lactide)-b-Poly(l-lysine) for Enhancement of Osteoblast Attachment and Growth. Biomacromolecules 2009, 10, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Khouri, N.G.; Bahú, J.O.; Blanco-Llamero, C.; Severino, P.; Concha, V.O.C.; Souto, E.B. Polylactic Acid (PLA): Properties, Synthesis, and Biomedical Applications—A Review of the Literature. J. Mol. Struct. 2024, 1309, 138243. [Google Scholar] [CrossRef]
- Kasperczyk, J. Microstructural Analysis of Poly[(l,l-lactide)–co–(glycolide)] by 1H and 13C n.m.r. Spectroscopy. Polymer 1996, 37, 201–203. [Google Scholar] [CrossRef]




| C6H14/CHCl3 (v/v) | PLAA in CHCl3 Concentration (wt%) | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.25 | 0.5 | 0.75 | 1 | 2 | 2.25 | 2.5 | 2.75 | 3 | 4 | 6 | 7 | 10 | 12 | 15 | 15.25 | 15.5 | 15.75 | 16 | 17 | 19 | 25 | 30 | 35 | |
| 0.25 | S | S | S | S | ||||||||||||||||||||
| 0.5 | S | S | L | L | L | L | L | |||||||||||||||||
| 0.6 | S | L | L | |||||||||||||||||||||
| 0.75 | S | L | L | L | L | P | P | P | ||||||||||||||||
| 1 | S | S | S | P | P | P | P | P | P | P | ||||||||||||||
| 1.15 | S | S | P | |||||||||||||||||||||
| 1.25 | S | S | S | P | P | |||||||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Yan, H.; Wang, B.; Shangguan, Z.; Yao, J. Synthesis of Poly(Lactic Acid-co-Arginine) and Construction of Its Ternary Phase Diagram for Nonsolvent Induced Phase Separation. Materials 2025, 18, 4816. https://doi.org/10.3390/ma18204816
Zhu Y, Yan H, Wang B, Shangguan Z, Yao J. Synthesis of Poly(Lactic Acid-co-Arginine) and Construction of Its Ternary Phase Diagram for Nonsolvent Induced Phase Separation. Materials. 2025; 18(20):4816. https://doi.org/10.3390/ma18204816
Chicago/Turabian StyleZhu, Yinying, Hongxia Yan, Bei Wang, Zihan Shangguan, and Junyan Yao. 2025. "Synthesis of Poly(Lactic Acid-co-Arginine) and Construction of Its Ternary Phase Diagram for Nonsolvent Induced Phase Separation" Materials 18, no. 20: 4816. https://doi.org/10.3390/ma18204816
APA StyleZhu, Y., Yan, H., Wang, B., Shangguan, Z., & Yao, J. (2025). Synthesis of Poly(Lactic Acid-co-Arginine) and Construction of Its Ternary Phase Diagram for Nonsolvent Induced Phase Separation. Materials, 18(20), 4816. https://doi.org/10.3390/ma18204816






