Study on Mechanical Strength and Resistance to Freeze–Thaw Cycles and Chloride Ions of Fly Ash Mortar Mixed with Limestone Powder Cured Under Low Temperature
Abstract
1. Introduction
2. Experimental Procedure
2.1. Materials
2.2. Specimen Preparation
2.3. Test Methods
2.3.1. Compressive Strength
2.3.2. Freeze–Thaw Resistance
2.3.3. Chloride Diffusion Coefficient
2.3.4. Characterizations
2.3.5. Environmental Evaluation
3. Results and Discussion
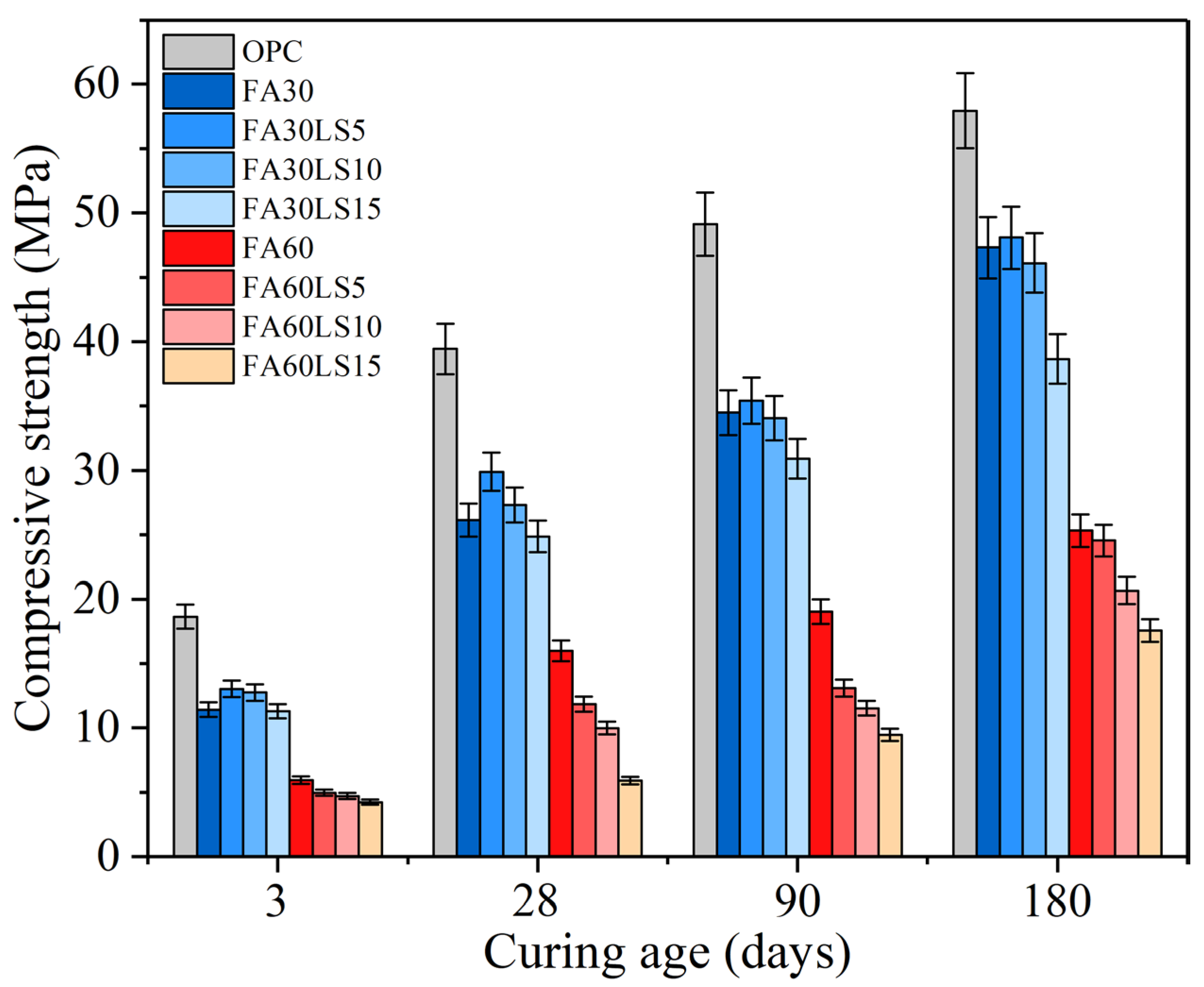
3.1. Compressive Strength
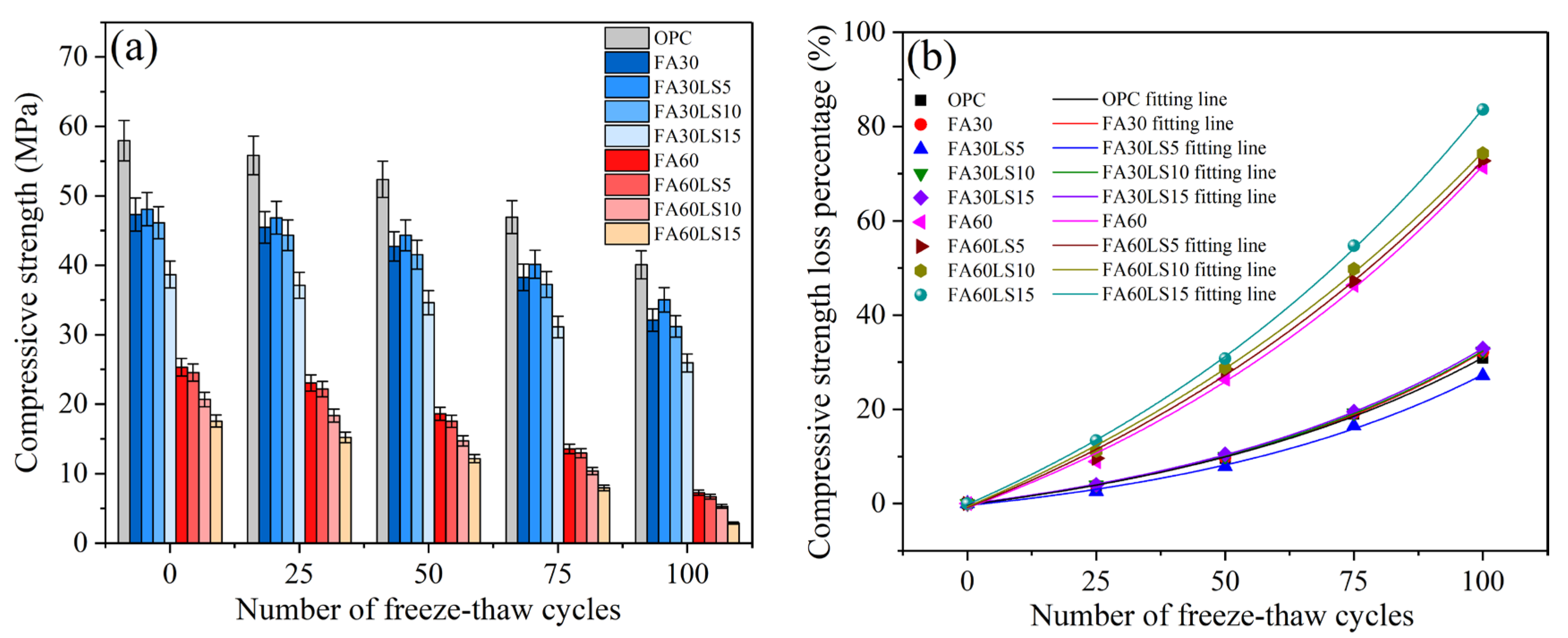
3.2. Compressive Strength After Freeze–Thaw Cycles
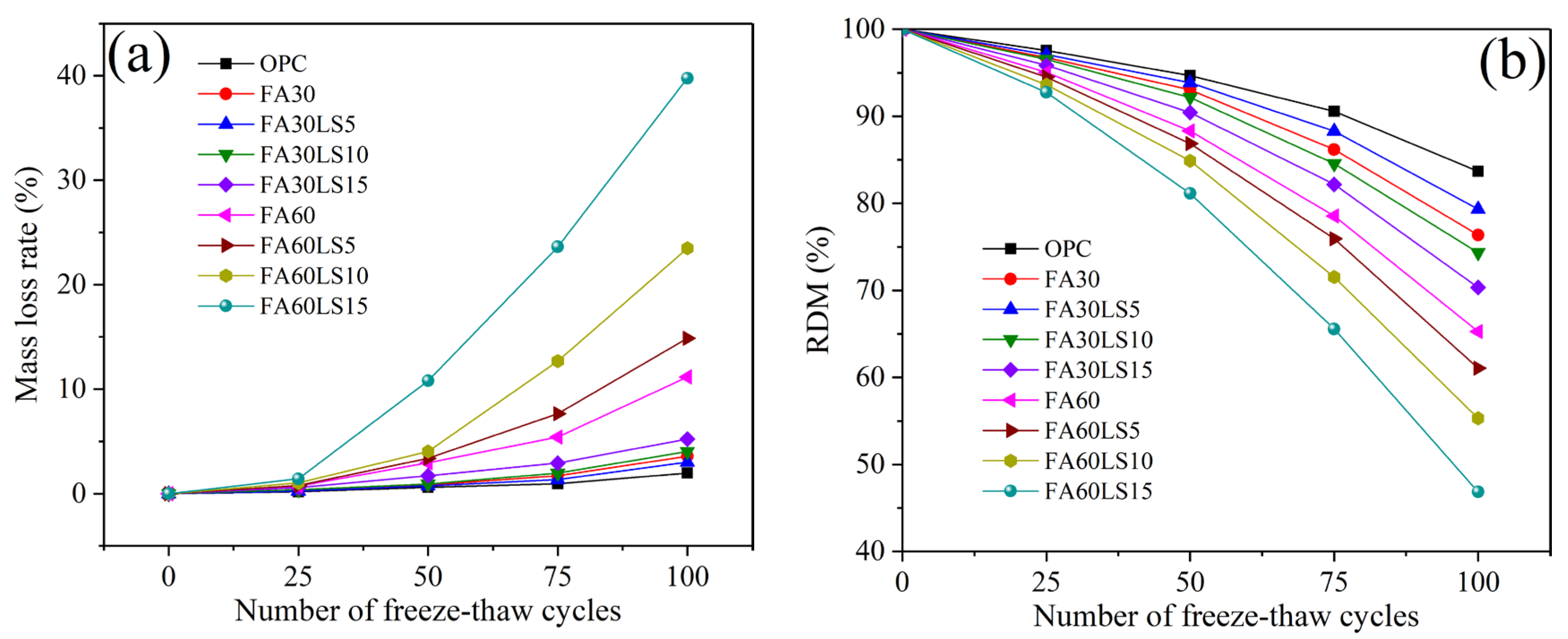
3.3. Mass Loss Rate and RDM After Freeze–Thaw Cycles
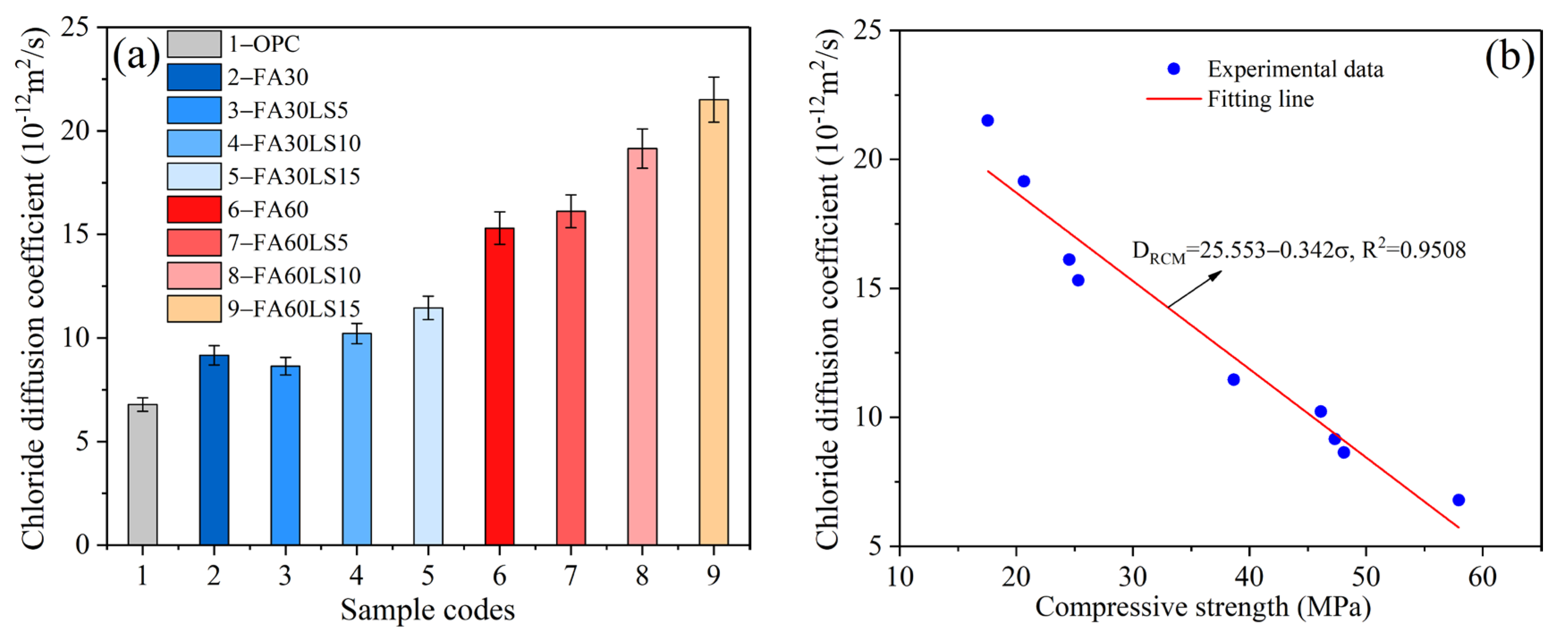
3.4. Chloride Diffusion Coefficient
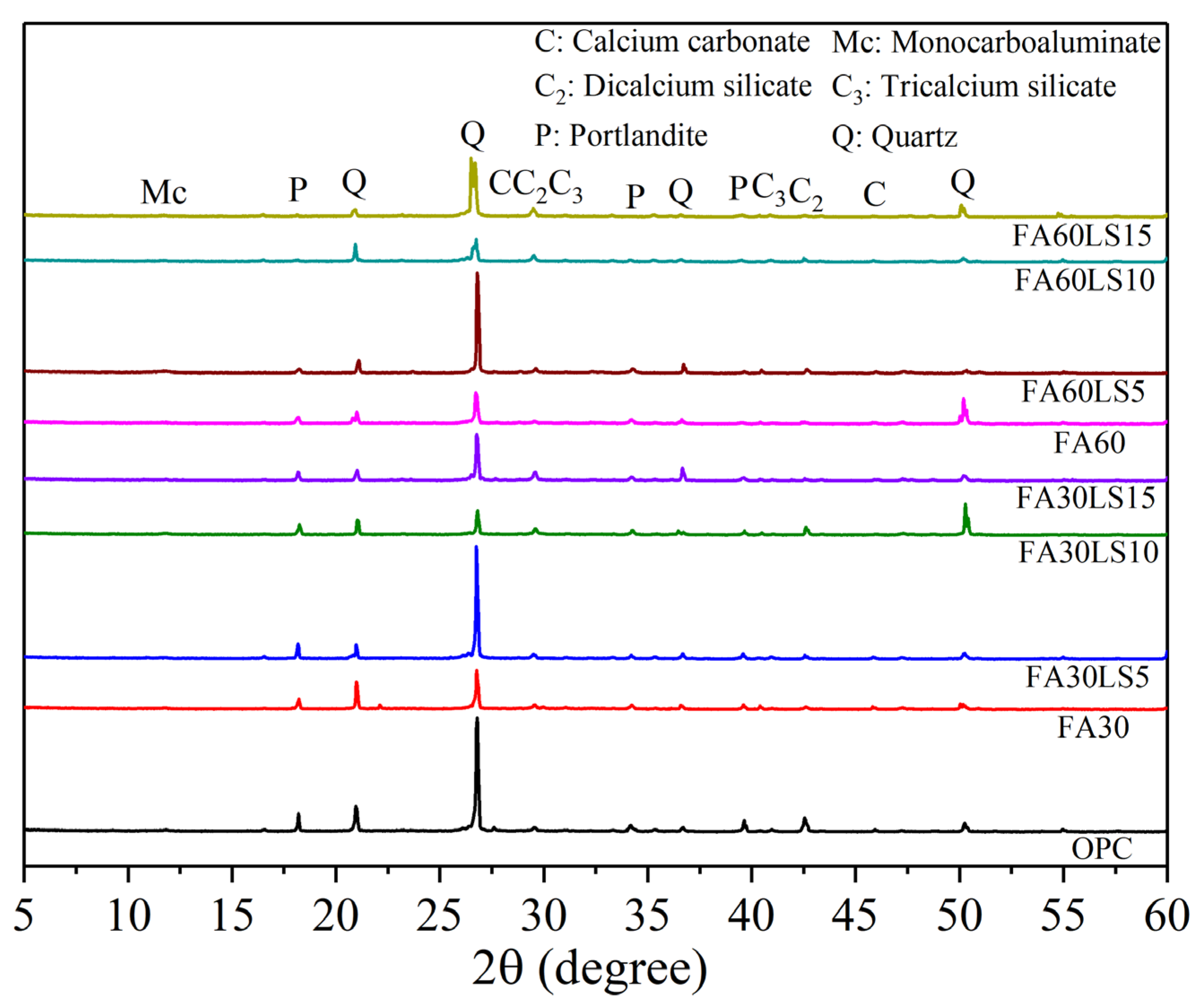
3.5. XRD
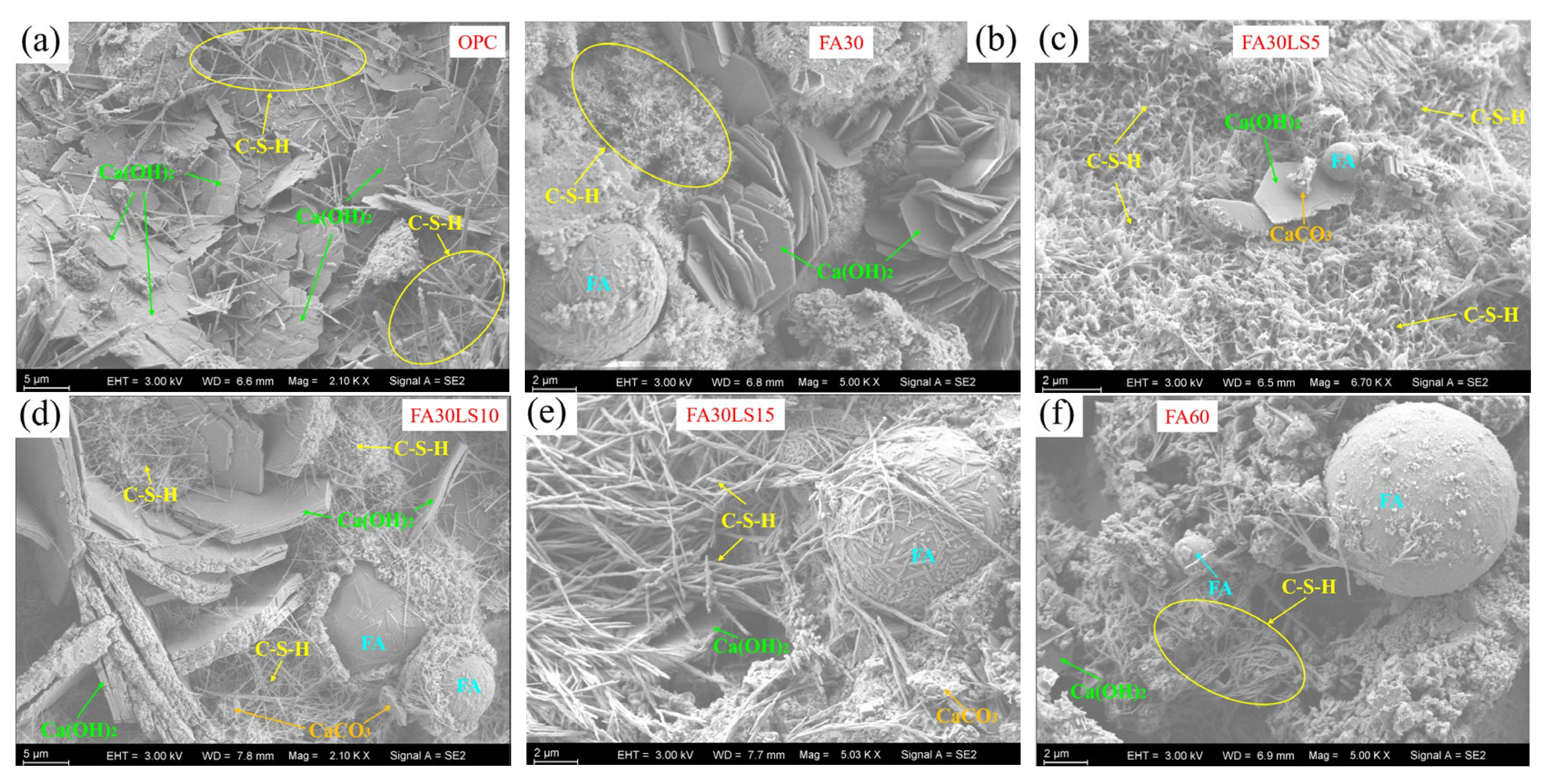
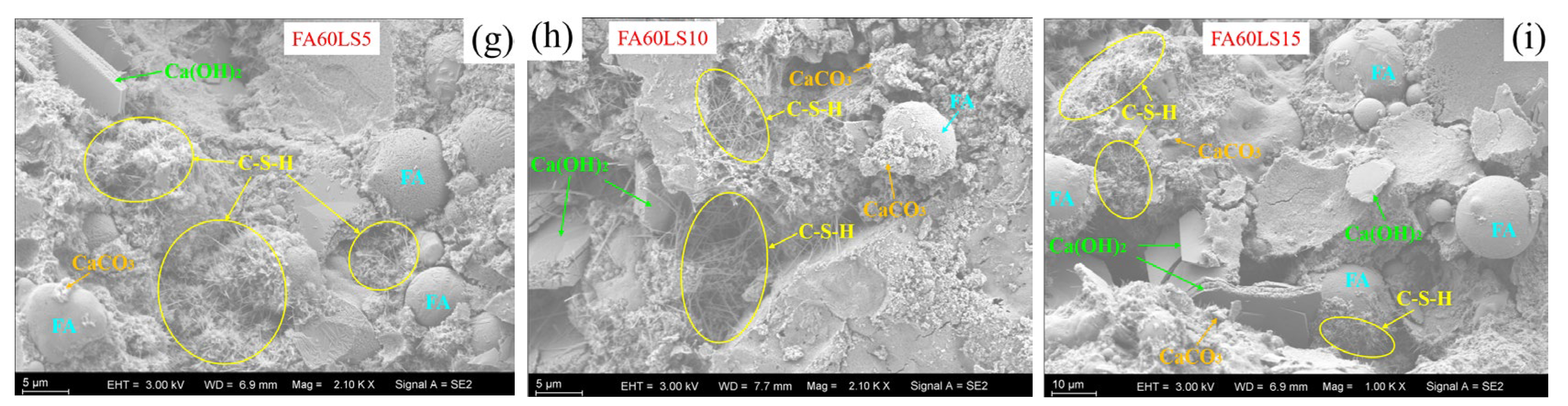
3.6. SEM
3.7. TG
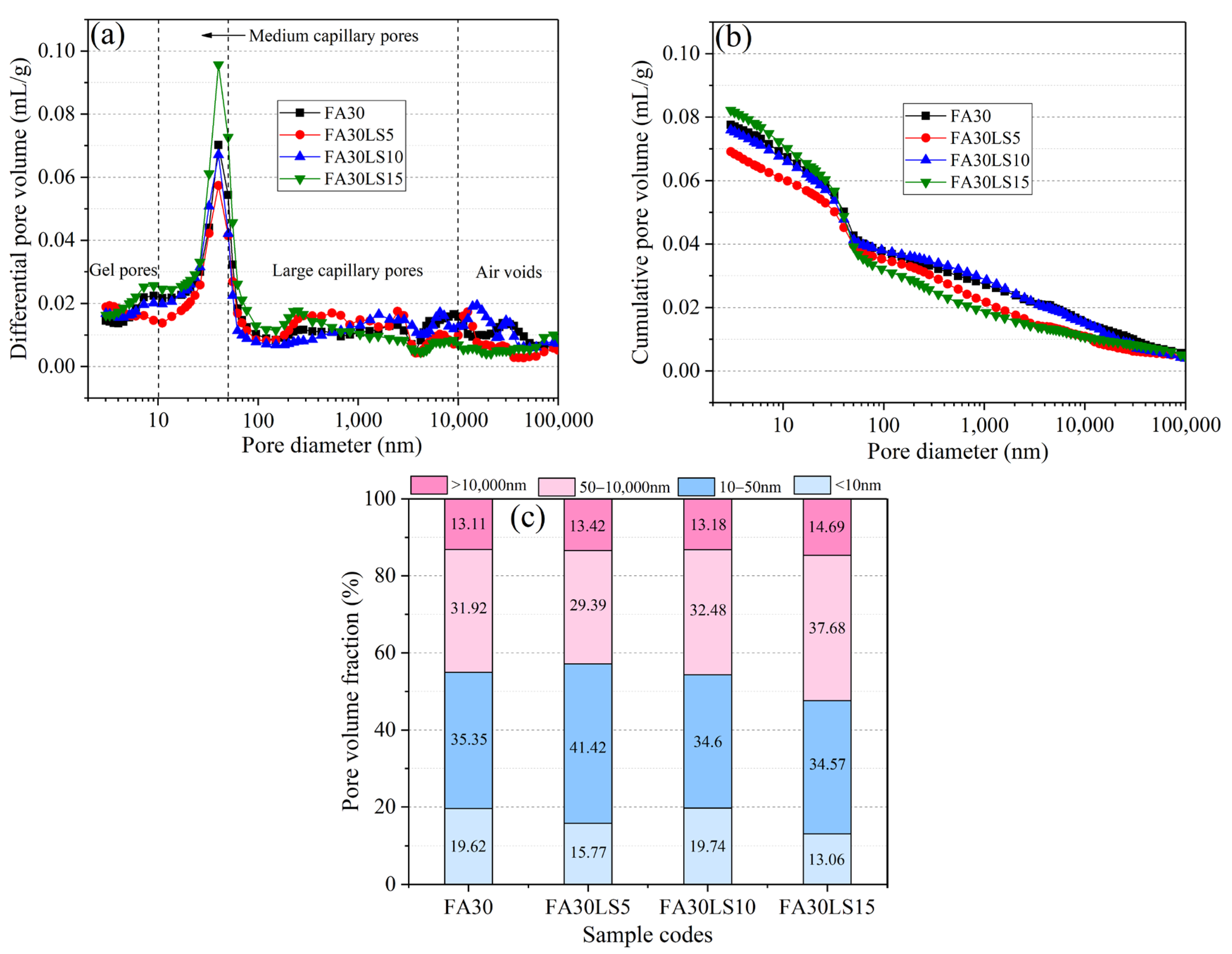
3.8. MIP
3.9. Relationship Between Mechanical Strength and Environmental Effect
4. Conclusions
- (1)
- For the mortar containing 30% FA, the addition of 5% and 10% LS can improve the compressive strength of FA mortar at 3 d and 28 d, and only 5% LS can improve the strength of FA mortar at 90 d and 180 d. Adding 5% LS can increase the compressive strength of FA mortar by 14.31% at 3 d and 1.63% at 180 d. However, 15% LS will reduce the compressive strength of FA mortar from 3 d to 180 d. For the mortar containing 60% LS, adding LS does not increase the strength of FA mortar from 3 d to 180 d.
- (2)
- For the mortar containing 30% FA, the addition of 5% LS results in a 0.58% reduction in the mass loss rate and an increase of 2.98% in RDM compared to FA mortar after 100 freeze–thaw cycles. However, the addition of 10% and 15% LS will lead to an increase in mass loss rates and a decrease in RDM of FA mortars. For the mortar containing 60% LS, with the addition of LS from 5% to 15%, the mass loss rate increases sharply, while the RDM decreases sharply.
- (3)
- The mortar without FA and LS can obtain the most C-S-H gels. For the mortar containing 30% FA, the addition of 5% LS can significantly promote the hydration products of C-S-H, while 10% and 15% will gradually decrease the hydration products of C-S-H. For the mortar containing 60% FA, the increasing dosage of LS will lead to the gradual decrease in C-S-H gels.
- (4)
- The FA mortar containing 5% LS and 15% LS can result in the smallest cumulative pore volume and the largest cumulative pore volume, respectively. Meanwhile, the FA mortar containing 5% LS can obtain the minimum total amount of large capillary pores and air pores. However, 10% and 15% LS will lead to the total amount of large capillary pores and air pores of FA mortar gradually increasing.
- (5)
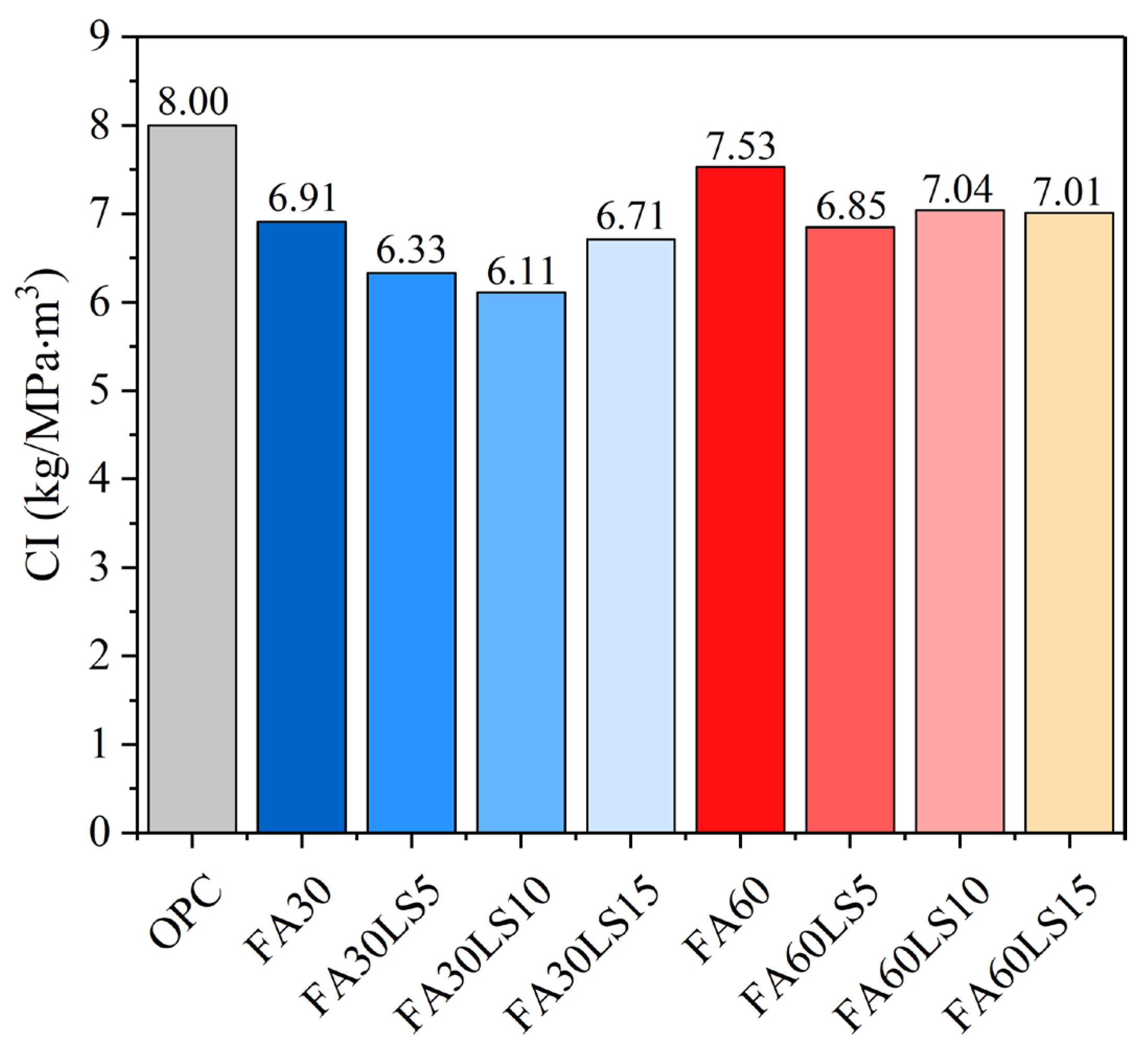
- The addition of FA and LS can improve the environmental friendliness of mortar. For the mortar containing 30% FA, with an increase in LS dosage, the CI value first decreases and then increases, and it reaches the minimum when the LS dosage is 10%. For the mortar containing 60% FA, adding different dosages of LS to FA mortar can all reduce the CI values, and when LS dosage is 5%, the CI value of FA mortar reaches the minimum.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dhahir, M.K.; Marx, S. Stress development and deformation characteristics of chemically prestressed high-strength concrete plates reinforced with different ratios of carbon textile reinforcement. Eng. Struct. 2025, 337, 120500. [Google Scholar] [CrossRef]
- Liu, Z.; Rupnow, T.; Milla, J.; Cooper III, S.B. Aggregate alkali–silica reactivity and mitigation effectiveness evaluation with miniature concrete prism test. Mag. Concr. Res. 2025, 77, 819–824. [Google Scholar] [CrossRef]
- Sathurshan, M.; Derakhshan, H.; Thamboo, J.; Gill, J.; Inglis, C.; Zahra, T. Axial stress-strain models for grouted dry-stack concrete block masonry. Structures 2025, 79, 109385. [Google Scholar] [CrossRef]
- Clark, G.; Davis, M.; Kumar, A. Multi-measure pathways for achieving carbon-neutral cement production. Sustain. Prod. Consum. 2025, 57, 355–374. [Google Scholar] [CrossRef]
- Ojeda-Paredes, A.Y.; Mitsos, A.; Dahmen, M. Retrofitting Ordinary Portland cement production for reduced greenhouse gas emissions. Comput. Chem. Eng. 2025, 201, 109200. [Google Scholar] [CrossRef]
- Sim, Y.; Oh, S.; Song, C.; Lee, S.-C.; Hong, G. Utilization of steel slag for sustainable concrete blocks: Laboratory study on water absorption, flexural strength, carbon dioxide capture, and volumetric expansion. J. Build. Eng. 2025, 111, 113335. [Google Scholar] [CrossRef]
- Bušić, R.; Gazić, G.; Guljaš, I.; Miličević, I. Experimental determination of double-K fracture parameters for self-compacting concrete with waste tire rubber and silica fume. Theor. Appl. Fract. Mech. 2025, 139, 105021. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, C.; Mazlan, D.; Kumar, S.S.N. Sustainable thermoelectric energy harvesting in fly ash bamboo fiber reinforced concrete for smart infrastructure. Energy Build. 2025, 343, 115927. [Google Scholar] [CrossRef]
- Oh, S.; Oh, G.; Hong, G.; Choi, Y.-C.; Choi, S. Thermomechanical properties of high-volume fly ash concrete for application in mass concrete. Case Stud. Constr. Mater. 2025, 22, e04681. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, C.; Shen, D.; Nie, D.; Qin, X.; An, X. Effects of fly ash content on the macro-performance and microstructure of self-compacting concrete using tuff powder under freeze-thaw cycles. Case Stud. Constr. Mater. 2025, 23, e04926. [Google Scholar] [CrossRef]
- Paulpandian, M.K.S.; Neves, R. Residual service life of reinforced fly ash recycled aggregate concrete structures subject to carbonation. J. Build. Eng. 2025, 99, 111553. [Google Scholar] [CrossRef]
- McCarthy, M.J.; Hope, T.A.; Csetenyi, L.J. Mechanical processing of wet stored fly ash for use as a cement component in concrete. Mag. Concr. Res. 2024, 76, 1424–1438. [Google Scholar] [CrossRef]
- Song, Q.; Zou, Y.; Zhang, P.; Xu, S.; Yang, Y.; Bao, J.; Xue, S.; Liu, J.; Gao, S.; Lin, L. Novel high-efficiency solid particle foam stabilizer: Effects of modified fly ash on foam properties and foam concrete. Cem. Concr. Compos. 2025, 155, 105818. [Google Scholar] [CrossRef]
- Singh, G.V.P.B.; Prasad, V.D. Environmental impact of concrete containing high volume fly ash and ground granulated blast furnace slag. J. Clean. Prod. 2024, 448, 141729. [Google Scholar] [CrossRef]
- Zhang, M.; Lv, H.; Jiang, Q.; Wu, Y.; Yang, Y.; Dai, H. Study on flexural behavior of laminated slabs constructed with composite limestone powder-tailings mixed sand concrete. Structures 2025, 77, 109169. [Google Scholar] [CrossRef]
- Wu, C.; Meng, L.; Wang, D.; Zhang, H.; Ke, L.; Ma, Z. Performance of sulfate species on limestone powder concrete under low temperature pulse current. Phys. Chem. Earth Parts A/B/C 2025, 138, 103850. [Google Scholar] [CrossRef]
- Radović, A.; Carević, V.; Marinković, S.; Plavšić, J.; Tešić, K. Prediction model for calculation of the limestone powder concrete carbonation depth. J. Build. Eng. 2024, 86, 108776. [Google Scholar] [CrossRef]
- Elgalhud, A.A.; Dhir, R.K.; Ghataora, G. Limestone addition effects on concrete porosity. Cem. Concr. Compos. 2016, 72, 222–234. [Google Scholar] [CrossRef]
- Jin, W.Z.; Tang, X.D.; Bai, Z.P.; Yang, H.; Chen, Z.Y.; Wang, L.; Zhang, L.; Jiang, L.H. Effect of Curing Temperature on Mechanical Strength and Thermal Properties of Hydraulic Limestone Powder Concrete. J. Mater. Eng. Perform. 2024, 33, 11214–11230. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Lou, G.; Yao, T. Effect of limestone powder on mechanical properties of concrete based on Griffith’s microcracking theory. Constr. Build. Mater. 2024, 449, 138413. [Google Scholar] [CrossRef]
- Jin, W.Z.; Jiang, L.H.; Han, L.; Huang, H.M.; Zhi, F.F.; Yang, G.H.; Niu, Y.L.; Chen, L.; Wang, L.; Chen, Z.Y. Influence of curing temperature on freeze-thaw resistance of limestone powder hydraulic concrete. CASE Stud. Constr. Mater. 2022, 17, e01322. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Q.; Shang, H.; Wang, J.; Song, N. Development of sustainable ultra-high-performance concrete (UHPC) by synergistic utilization of red mud and limestone powder. J. Build. Eng. 2024, 90, 109372. [Google Scholar] [CrossRef]
- Jin, W.Z.; Jiang, L.H.; Han, L.; Gu, Y.; Guo, M.Z.; Gao, S.; Zhang, L.; Liu, M.W. Influence of Calcium Leaching on Mechanical and Physical Properties of Limestone Powder-Cement Pastes Cured under Different Temperatures. J. Mater. Civ. Eng. 2022, 34, 04022214. [Google Scholar] [CrossRef]
- Wang, C.; Jin, Z.; Liu, G.; Dong, W.; Pang, B.; Ding, X. Mechanisms of chloride transport in low carbon marine concrete: An alkali-activated slag system with high limestone powder. J. Build. Eng. 2023, 72, 106539. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, H.; Gu, C.; Shao, C.; Jiang, Z.; Wang, Y.; Chen, X. Analysis of concrete damage evolution in cold regions under combined freeze-thaw cycle-crack effect. Constr. Build. Mater. 2024, 456, 139296. [Google Scholar] [CrossRef]
- Miao, Y.; Yu, W.; Jin, L.; Wang, L.; Lin, J.; Li, Y.; Lu, Z.; Jiang, J. Effect of shrinkage-induced initial damage on the frost resistance of concrete in cold regions. Eng. Fract. Mech. 2024, 312, 110652. [Google Scholar] [CrossRef]
- Pan, D.; Niu, D.; Li, Z. Corrosion products of low-alloy steel bars and their induction of cracking in seawater sea-sand concrete cover. Constr. Build. Mater. 2023, 389, 131800. [Google Scholar] [CrossRef]
- Zhou, J.; Shang, H.; Fan, L.; Wang, Z.; Huang, Y.; Wang, Y. Bond and corrosion behavior of steel bar embedded in cracked concrete exposed to marine environment. J. Build. Eng. 2025, 104, 112190. [Google Scholar] [CrossRef]
- Peng, X.; Yimin, W.; Zijian, W.; Le, H. Distribution laws of freeze-thaw cycles and unsaturated concrete experiments in cold-region tunnels. Cold Reg. Sci. Technol. 2020, 172, 102985. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, M.; Pei, W.; Lai, Y.; Zhang, R.; Sun, J.; Zhao, T. Deterioration process and damage constitutive model of concrete under freeze-thaw circumstance of severely cold regions. Cold Reg. Sci. Technol. 2024, 226, 104290. [Google Scholar] [CrossRef]
- GB/T 17671-2021; Test Method of Cement Mortar Strength. China Architecture & Building Press: Beijing, China, 2021.
- GB/T 50082-2009; Standard for Test Methods of Long-Term Performance and Durability of Ordinary Concrete. China Architecture & Building Press: Beijing, China, 2009.
- Long, G.; Gao, Y.; Xie, Y. Designing more sustainable and greener self-compacting concrete. Constr. Build. Mater. 2015, 84, 301–306. [Google Scholar] [CrossRef]
- Purnell, P. The carbon footprint of reinforced concrete. Adv. Cem. Res. 2013, 25, 362–368. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, X.; Lv, Y.; Wang, J.; Bai, W.; Qiao, M.; Hu, K.; Liu, Q.; Song, C.; Jin, W. Study on workability and mechanical strength of low cement ultra-high performance concrete with ultrafine quartz powder as alternative material under high temperature curing. Case Stud. Constr. Mater. 2024, 21, e04074. [Google Scholar] [CrossRef]
- Müller, H.S.; Haist, M.; Vogel, M. Assessment of the sustainability potential of concrete and concrete structures considering their environmental impact, performance and lifetime. Constr. Build. Mater. 2014, 67, 321–337. [Google Scholar] [CrossRef]
- European Federation of Concrete Admixtures Associations (EFCA). 2010. Available online: http://www.efca.info/ (accessed on 19 October 2025).
- Chiaia, B.; Fantilli, A.P.; Guerini, A.; Volpatti, G.; Zampini, D. Eco-mechanical index for structural concrete. Constr. Build. Mater. 2014, 67, 386–392. [Google Scholar] [CrossRef]
- Cao, J.; Zou, Z.; Zeng, P. Mechanical properties of fly ash concrete after the coupling effects of sustained load and sulphate erosion. Constr. Build. Mater. 2025, 460, 139866. [Google Scholar] [CrossRef]
- De Weerdt, K.; Haha, M.B.; Le Saout, G.; Kjellsen, K.O.; Justnes, H.; Lothenbach, B. The effect of temperature on the hydration of composite cements containing limestone powder and fly ash. Mater. Struct. 2012, 45, 1101–1114. [Google Scholar] [CrossRef]
- Han, F.; Zhu, Z.; Li, Y.; Pu, S.; Zhang, Z. Effect of ultrafine limestone powder on the hydration heat and rheological properties of Portland cement paste. Powder Technol. 2025, 454, 120717. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, J.; Chen, Z. Hydration kinetics, strength, autogenous shrinkage, and sustainability of cement pastes incorporating ultrafine limestone powder. Case Stud. Constr. Mater. 2024, 20, e03149. [Google Scholar] [CrossRef]
- Her, S.-W.; Yang, K.-H.; Bae, S.-C.; Kwon, S.-J.; Wang, X.-Y. Effect of particle size distribution and content of limestone powder on compressive response of high-early-strength cement mortars. J. Build. Eng. 2024, 97, 110964. [Google Scholar] [CrossRef]
- Sezer, G.İ. Compressive strength and sulfate resistance of limestone and/or silica fume mortars. Constr. Build. Mater. 2012, 26, 613–618. [Google Scholar] [CrossRef]
- Huang, Z.; Sookree, E.W.; Mohamoud, A.H.; Wang, Z. Influence of Limestone Powder and Fly Ash on the Freezing and Thawing Resistance of Roller-Compacted Concrete. KSCE J. Civ. Eng. 2021, 25, 2501–2507. [Google Scholar] [CrossRef]
- Zeng, H.; Li, Y.; Zhang, J.; Chong, P.; Zhang, K. Effect of limestone powder and fly ash on the pH evolution coefficient of concrete in a sulfate-freeze–thaw environment. J. Mater. Res. Technol. 2022, 16, 1889–1903. [Google Scholar] [CrossRef]
- Jiang, J.; Wu, J.; Yang, Q.; Li, Q.; Lu, X.; Ye, Z. Hydration of Portland cement in the presence of triethanolamine and limestone powder: Mechanical properties and synergistic mechanism. Constr. Build. Mater. 2024, 438, 137323. [Google Scholar] [CrossRef]
- Zhou, Y.-F.; Li, J.-S.; Lu, J.-X.; Cheeseman, C.; Poon, C.S. Recycling incinerated sewage sludge ash (ISSA) as a cementitious binder by lime activation. J. Clean. Prod. 2020, 244, 118856. [Google Scholar] [CrossRef]
- Guo, M.Z.; Fei, X.J.; Pei, C.; Jin, W.Z. Environmentally-optimized photocatalytic NOX degradation in cement mortars utilizing recycled red brick and waste glass for sustainable management. J. Environ. Manag. 2025, 375, 124209. [Google Scholar] [CrossRef]
- Zhang, K.J.; Jin, W.Z.; Lv, Y.J.; Li, S.G.; Zhang, X.L.; Xiang, T.F.; Gu, C.K.; Bai, W.F.; Song, C.H.; Zhao, J. Study on mechanical, hydrophobic and corrosion resistant properties of methylsiloxan-based mortar modified by micro-nano supplementary cementitious materials. J. Mater. Res. Technol. JMRT 2025, 34, 1654–1670. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, Y.; Qin, F.; Sun, F.; Huang, Y. Mechanical properties of sustainable high strength ECC with substitution of cement by limestone powder. Case Stud. Constr. Mater. 2023, 19, e02434. [Google Scholar] [CrossRef]
- Lv, Y.J.; Luo, Y.Y.; Song, C.H.; Jin, W.Z.; Xiang, T.F.; Qiao, M.; Dang, J.T.; Bai, W.F.; Yang, Z.S.; Zhao, J. Effect of calcium stearate hydrophobic agent on the performance of mortar and reinforcement corrosion in mortar with cracks. Constr. Build. Mater. 2024, 450, 138684. [Google Scholar] [CrossRef]
- Mohammed, T.; Torres, A.; Aguayo, F.; Okechi, I.K. Evaluating carbonation resistance and microstructural behaviors of calcium sulfoaluminate cement concrete incorporating fly ash and limestone powder. Constr. Build. Mater. 2024, 442, 137551. [Google Scholar] [CrossRef]


| Samples | CaO | SiO2 | Al2O3 | Fe2O3 | MgO | SO3 | K2O | LOI |
|---|---|---|---|---|---|---|---|---|
| OPC | 62.58 | 20.39 | 5.43 | 3.76 | 1.73 | 2.28 | 0.78 | 3.05 |
| FA | 2.47 | 48.92 | 36.86 | 6.79 | 0.73 | 0.58 | 1.07 | 2.58 |
| LS | 56.34 | 0.37 | 0.19 | 0.18 | 1.69 | ̶ | ̶ | 41.23 |
| Samples | w/b | Water | Cement | FA | LS | Sand | SP |
|---|---|---|---|---|---|---|---|
| OPC | 0.35 | 194 | 554 | 0 | 0 | 1662 | 2.77 |
| FA30 | 0.35 | 194 | 387.8 | 166.2 | 0 | 1662 | 2.77 |
| FA30LS5 | 0.35 | 194 | 360.1 | 166.2 | 27.7 | 1662 | 2.77 |
| FA30LS10 | 0.35 | 194 | 332.4 | 166.2 | 55.4 | 1662 | 2.77 |
| FA30LS15 | 0.35 | 194 | 304.7 | 166.2 | 83.1 | 1662 | 2.77 |
| FA60 | 0.35 | 194 | 221.6 | 332.4 | 0 | 1662 | 2.77 |
| FA60LS5 | 0.35 | 194 | 193.9 | 332.4 | 27.7 | 1662 | 2.77 |
| FA60LS10 | 0.35 | 194 | 166.2 | 332.4 | 55.4 | 1662 | 2.77 |
| FA60LS15 | 0.35 | 194 | 138.5 | 332.4 | 83.1 | 1662 | 2.77 |
| Items | (kg/m3) | References |
|---|---|---|
| Cement | 0.83 | [34,35] |
| FA | 0.009 | [33,34] |
| LS | 0.017 | [33,36] |
| Sand | 0.001 | [33,36] |
| SP | 0.72 | [33,37] |
| Water | 0.0003 | [33,38] |
| Samples | A | B | C | R2 |
|---|---|---|---|---|
| OPC | 9.405 | 68.363 | −9.641 | 0.9993 |
| FA30 | 8.180 | 62.531 | −8.269 | 0.9998 |
| FA30LS5 | 6.969 | 62.255 | −7.370 | 0.9981 |
| FA30LS10 | 8.551 | 63.747 | −8.646 | 0.9999 |
| FA30LS15 | 8.926 | 64.640 | −9.012 | 0.9999 |
| FA60 | 37.771 | 93.262 | −38.686 | 0.9986 |
| FA60LS5 | 44.841 | 102.870 | −45.671 | 0.9982 |
| FA60LS10 | 51.232 | 110.620 | −51.879 | 0.9993 |
| FA60LS15 | 46.467 | 96.882 | −46.645 | 0.9999 |
| Samples | Total Porosity (%) | Average Pore Size (nm) |
|---|---|---|
| FA30 | 15.75 | 26.01 |
| FA30LS5 | 14.52 | 24.21 |
| FA30LS10 | 16.12 | 26.95 |
| FA30LS15 | 17.01 | 27.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Jin, W.; Li, J.; Huang, M.; Fang, P.; Zhao, Y. Study on Mechanical Strength and Resistance to Freeze–Thaw Cycles and Chloride Ions of Fly Ash Mortar Mixed with Limestone Powder Cured Under Low Temperature. Materials 2025, 18, 4814. https://doi.org/10.3390/ma18204814
Chen Q, Jin W, Li J, Huang M, Fang P, Zhao Y. Study on Mechanical Strength and Resistance to Freeze–Thaw Cycles and Chloride Ions of Fly Ash Mortar Mixed with Limestone Powder Cured Under Low Temperature. Materials. 2025; 18(20):4814. https://doi.org/10.3390/ma18204814
Chicago/Turabian StyleChen, Qingfeng, Weizhun Jin, Jingjing Li, Min Huang, Pengfei Fang, and Yuru Zhao. 2025. "Study on Mechanical Strength and Resistance to Freeze–Thaw Cycles and Chloride Ions of Fly Ash Mortar Mixed with Limestone Powder Cured Under Low Temperature" Materials 18, no. 20: 4814. https://doi.org/10.3390/ma18204814
APA StyleChen, Q., Jin, W., Li, J., Huang, M., Fang, P., & Zhao, Y. (2025). Study on Mechanical Strength and Resistance to Freeze–Thaw Cycles and Chloride Ions of Fly Ash Mortar Mixed with Limestone Powder Cured Under Low Temperature. Materials, 18(20), 4814. https://doi.org/10.3390/ma18204814







