Effect of Homogenization Treatment on Microstructure, Dendritic Segregation, and Primary Carbides in H13 Steel
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
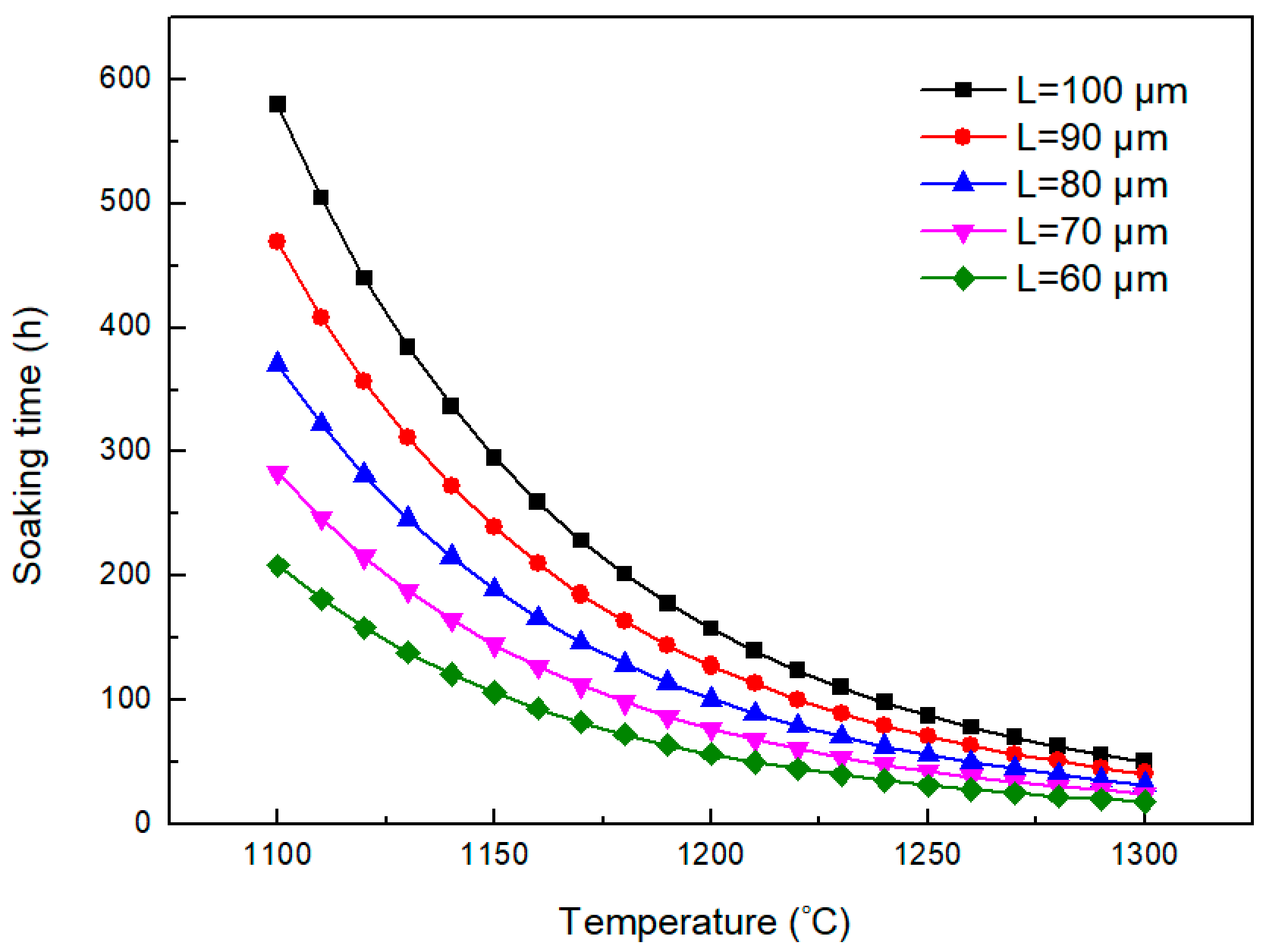
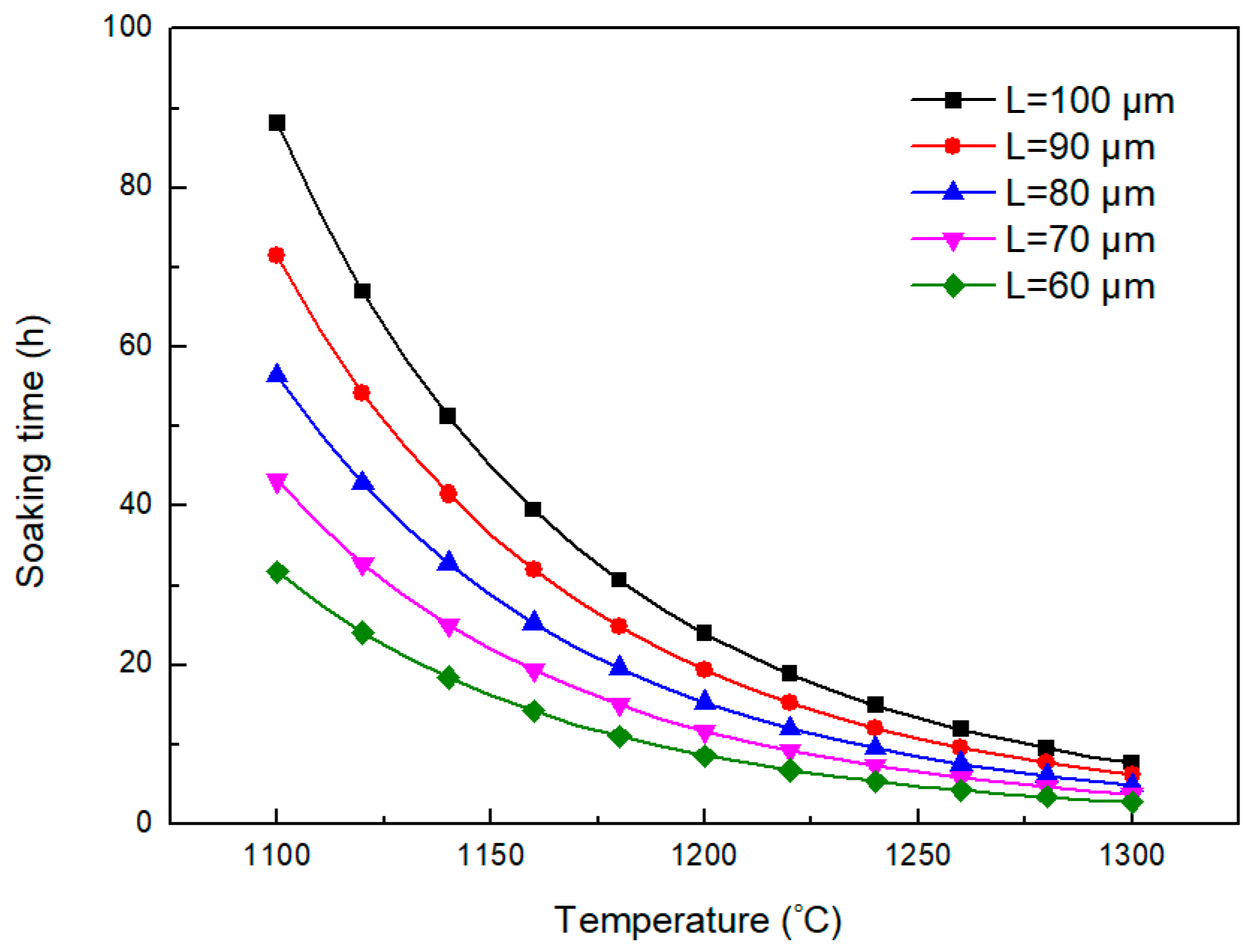
3.1. Evolution of Dendritic Structures During the Homogenization Process
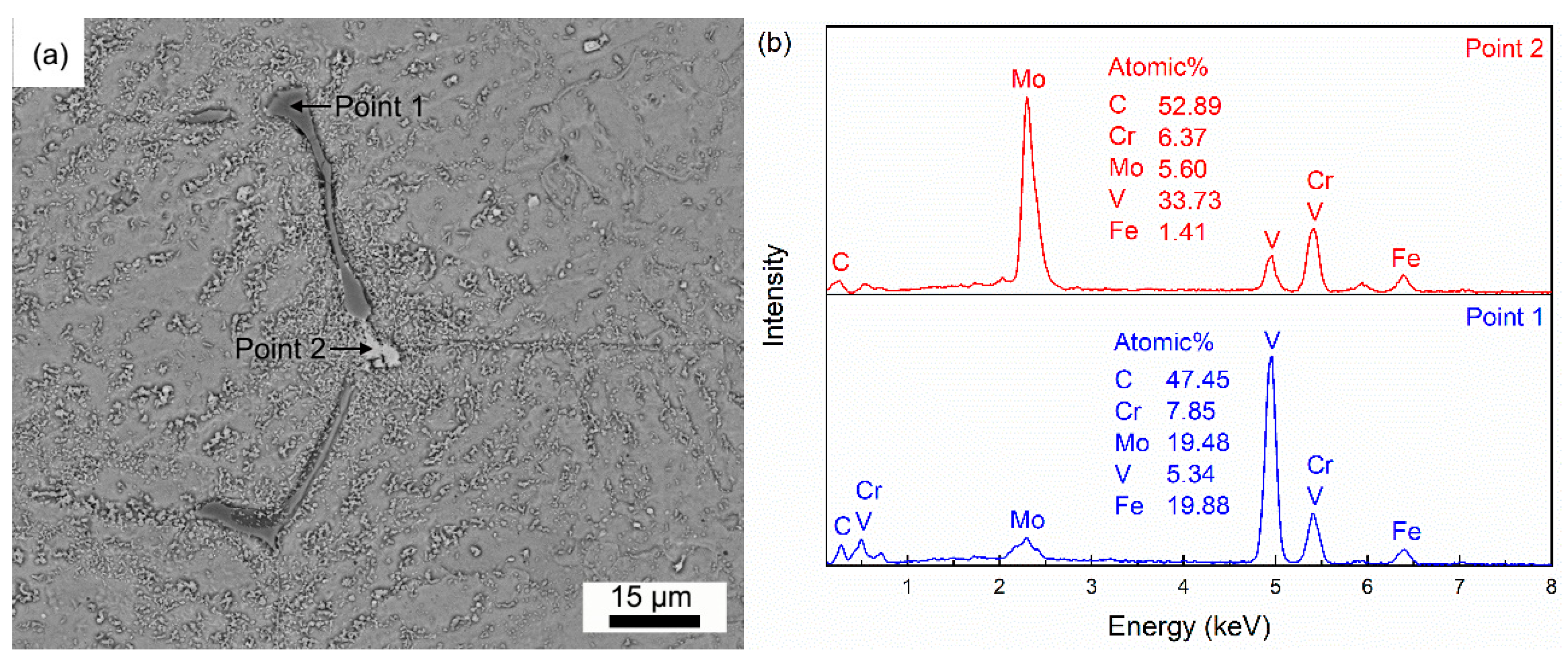
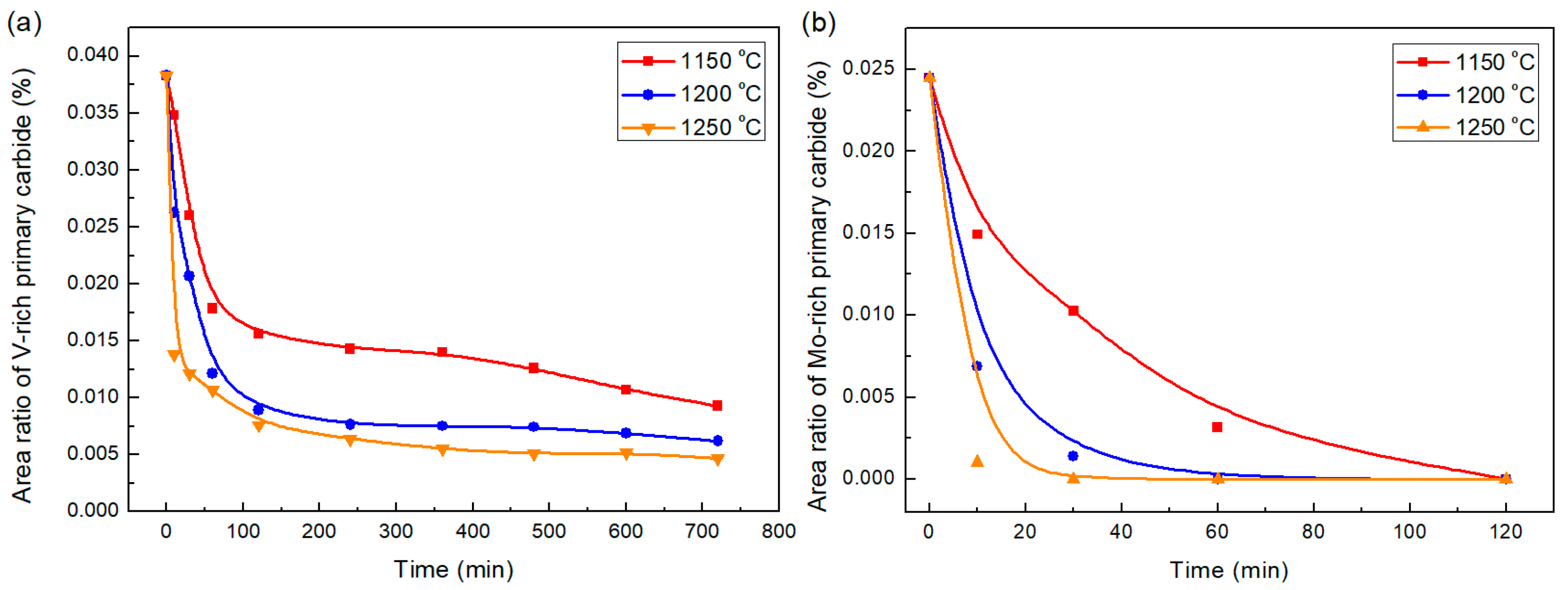
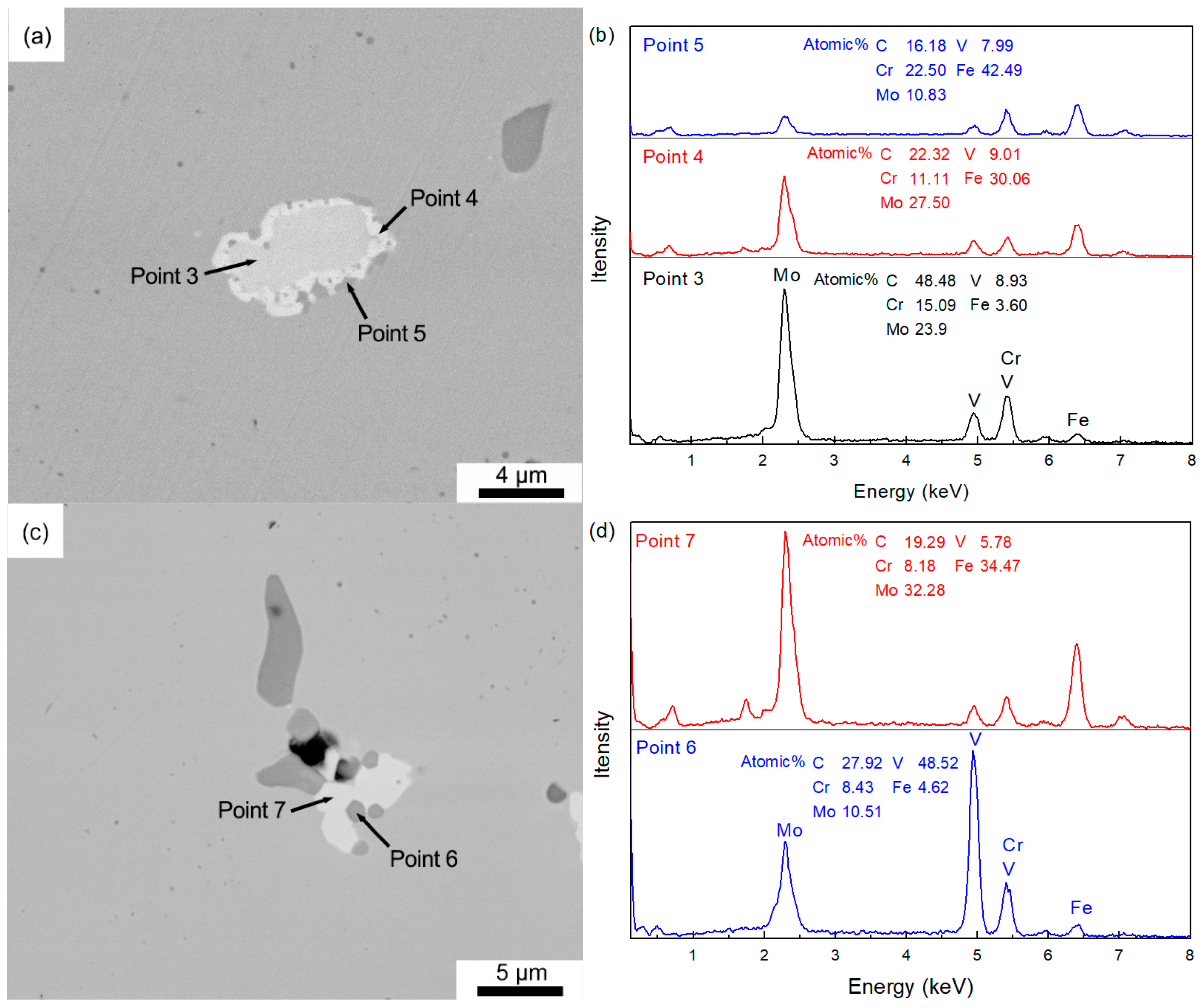
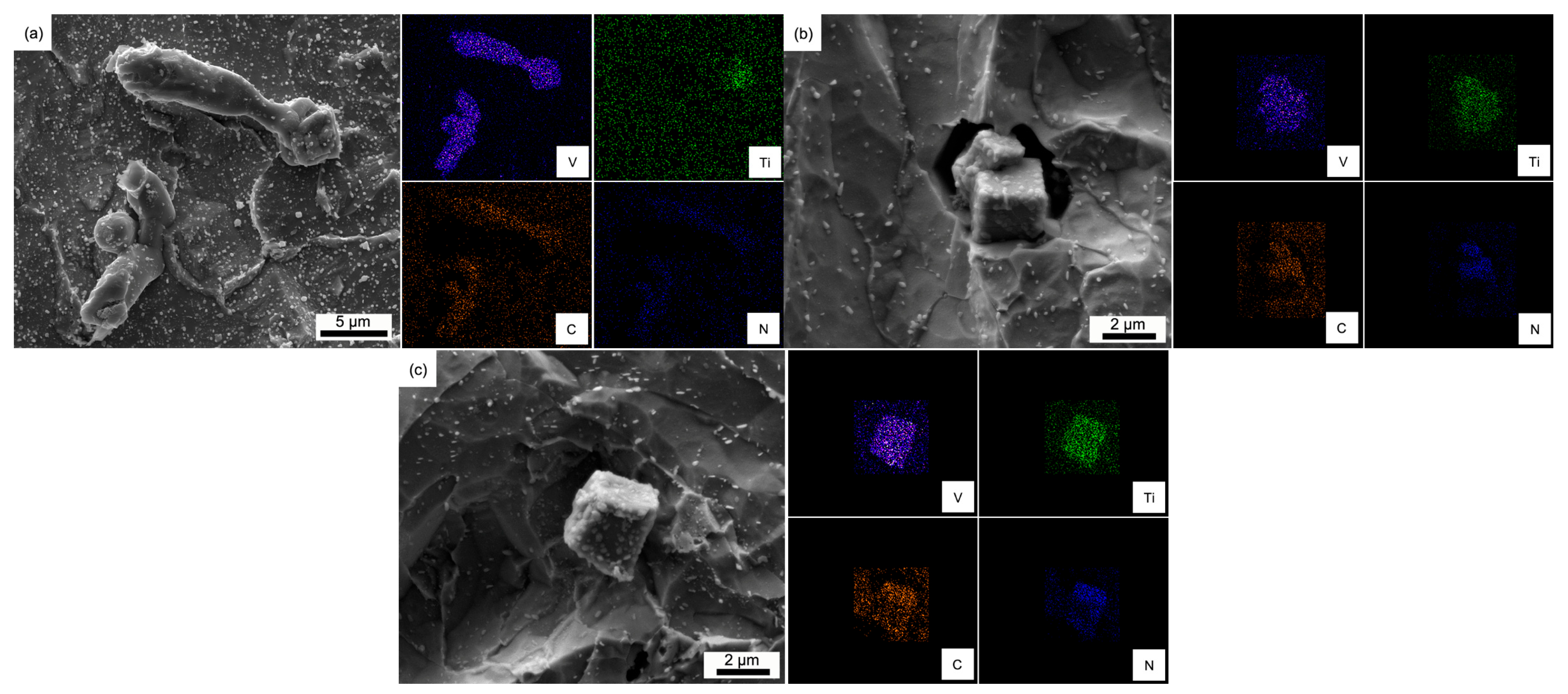
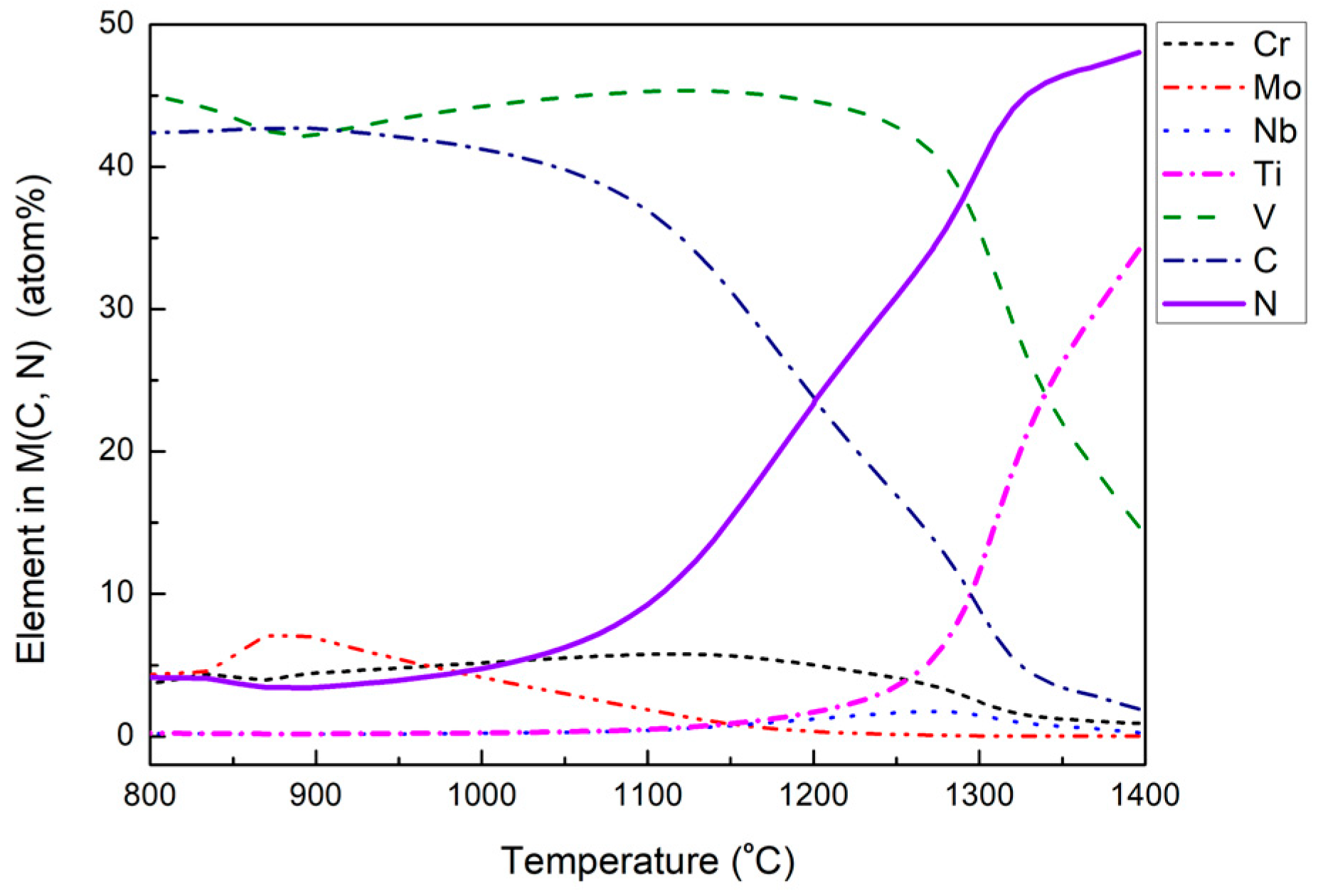
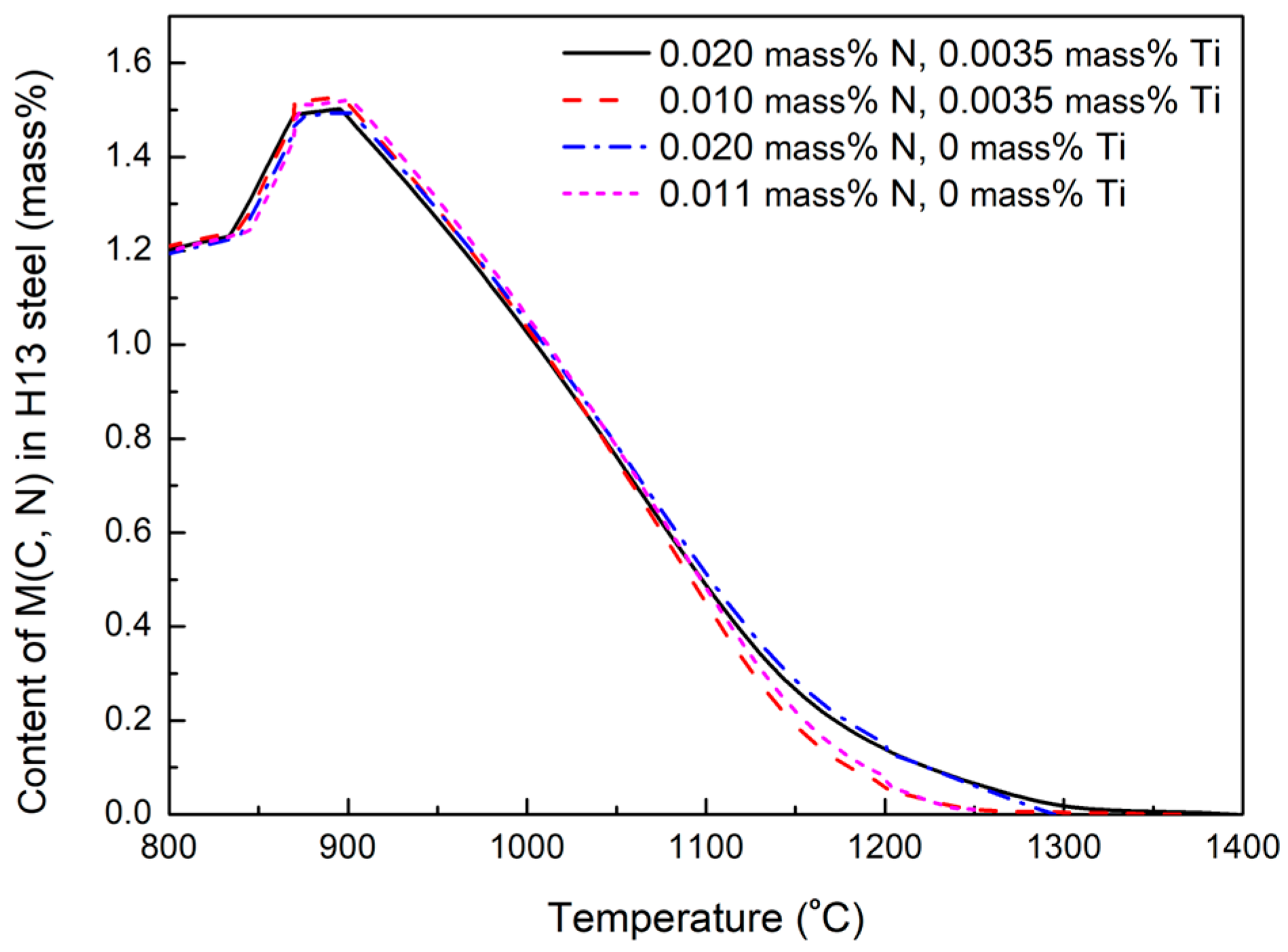
3.2. Dissolution Behavior of Primary Carbides
3.3. Grain Growth Behavior of H13 Steel During Homogenization Process
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, J.; Zhang, Z.; Xie, J. Improving strength and ductility of H13 die steel by pre-tempering treatment and its mechanism. Mater. Sci. Eng. A 2019, 752, 101–114. [Google Scholar] [CrossRef]
- Chen, H.; Li, S.; Ren, Y.; Hou, X.; Yang, H.; Zhang, S. Thermo-mechanical fatigue behavior and microstructure evolution of 4Cr5Mo3V hot work die steel. Int. J. Fatigue 2024, 183, 108263. [Google Scholar] [CrossRef]
- Wen, T.; Yang, F.; Wang, J.; Yang, H.; Fu, J.; Ji, S. Ultrastrong and ductile synergy of additively manufactured H13 steel by tuning cellular structure and nano-carbides through tempering treatment. J. Mater. Res. Technol. 2022, 22, 157–168. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Song, K.-X.; Zhang, Y.-M.; Wang, G.-X. Microstructure evolution and fracture mechanism of H13 steel during high temperature tensile deformation. Mater. Sci. Eng. A 2019, 746, 127–133. [Google Scholar] [CrossRef]
- Hu, X.; Li, L.; Wu, X.; Zhang, M. Coarsening behavior of M23C6 carbides after ageing or thermal fatigue in AISI H13 steel with niobium. Int. J. Fatigue 2006, 28, 175–182. [Google Scholar] [CrossRef]
- Han, Y.; Li, C.; Ren, J.; Qiu, C.; Zhang, Y.; Wang, J. Dendrite Segregation Changes in High Temperature Homogenization Process of As-cast H13 Steel. ISIJ Int. 2019, 59, 1893–1900. [Google Scholar] [CrossRef]
- Zhu, H.; Li, H.; He, Z.; Feng, H.; Jiang, Z.; He, T. Effect of Pressure on Dendrite Structure and Characteristics of Carbides during Solidification Process of H13 Die Steel Ingot. ISIJ Int. 2021, 61, 1889–1898. [Google Scholar] [CrossRef]
- Mao, M.-T.; Guo, H.-J.; Wang, F.; Sun, X.-L. Chemical composition and structural identification of primary carbides in as-cast H13 steel. Int. J. Miner. Met. Mater. 2019, 26, 839–848. [Google Scholar] [CrossRef]
- Krauss, G. Solidification, segregation, and banding in carbon and alloy steels. Met. Mater. Trans. B 2003, 34, 781–792. [Google Scholar] [CrossRef]
- Wang, X.; Li, G.; Liu, Y.; Wang, F.; Wang, Q. Cerium Addition Effect on Modification of Inclusions, Primary Carbides and Microstructure Refinement of H13 Die Steel. ISIJ Int. 2021, 61, 1850–1859. [Google Scholar] [CrossRef]
- Ma, D.-S.; Zhou, J.; Chen, Z.-Z.; Zhang, Z.-K.; Chen, Q.-A.; Li, D.-H. Influence of thermal homogenization treatment on structure and impact toughness of H13 ESR steel. J. Iron Steel Res. Int. 2009, 16, 56–60. [Google Scholar] [CrossRef]
- Yao, D.; Li, J.; Li, J.; Zhu, Q. Effect of Cold Rolling on Morphology of Carbides and Properties of 7Cr17MoV Stainless Steel. Mater. Manuf. Process. 2014, 30, 111–115. [Google Scholar] [CrossRef]
- Fukaura, K.; Yokoyama, Y.; Yokoi, D.; Tsujii, N.; Ono, K. Fatigue of cold-work tool steels: Effect of heat treatment and carbide morphology on fatigue crack formation, life, and fracture surface observations. Metall. Mater. Trans. A 2004, 35, 1289–1300. [Google Scholar] [CrossRef]
- Qi, Y.-F.; Li, J.; Shi, C.-B.; Zhang, Y.; Zhu, Q.-T.; Wang, H. Effect of directional solidification of electroslag remelting on the microstructure and primary carbides in an austenitic hot-work die steel. J. Mech. Work. Technol. 2017, 249, 32–38. [Google Scholar] [CrossRef]
- Mao, M.; Guo, H.; Wang, F.; Sun, X. Effect of Cooling Rate on the Solidification Microstructure and Characteristics of Primary Carbides in H13 Steel. ISIJ Int. 2019, 59, 848–857. [Google Scholar] [CrossRef]
- Li, Q.; Xia, Z.; Guo, Y.; Shen, Z.; Zheng, T.; Ding, B.; Zhong, Y. Carbides Modification and Mechanical Properties Enhancement of Cr12MoV Die Steel by Magnetically Controlled Electroslag Remelting. Met. Mater. Trans. B 2021, 52, 1495–1507. [Google Scholar] [CrossRef]
- Du, G.; Li, J.; Wang, Z.-B. Control of Carbide Precipitation During Electroslag Remelting-Continuous Rapid Solidification of GCr15 Steel. Met. Mater. Trans. B 2017, 48, 2873–2890. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Z.; Geng, X.; Chen, M.; Peng, L. Evolution Mechanism of Inclusions in H13 Steel with Rare Earth Magnesium Alloy Addition. ISIJ Int. 2019, 59, 1552–1561. [Google Scholar] [CrossRef]
- Huang, Y.; Cheng, G.; Li, S.; Dai, W. Effect of Cerium on the Behavior of Inclusions in H13 Steel. Steel Res. Int. 2018, 89, 1800371. [Google Scholar] [CrossRef]
- Han, Y.; Li, C.; He, S.; Gao, C.; Chen, S.; Li, E. Effect of Homogenisation Temperature on the Microstructure and Microhardness of As-Cast H13 Steel. Met. Mater. Int. 2021, 28, 755–769. [Google Scholar] [CrossRef]
- Huang, Y.; Cheng, G.; Li, S.; Dai, W. Distribution Characteristics and Thermal Stability of Primary Carbide in Cast Ce-H13 Steel. ISIJ Int. 2020, 60, 267–275. [Google Scholar] [CrossRef]
- Torkar, M.; Vodopivec, F.; Petovar, S. Analysis of segregations in as-cast X40CrMoV51 steel. Mater. Sci. Eng. A 1993, 173, 313–316. [Google Scholar] [CrossRef]
- Shewmon, P. Diffusion in Solids; McGraw-Hill: New York, NY, USA, 1963; p. 52. [Google Scholar] [CrossRef]
- He, S.; Li, C.; Ren, J.; Han, Y. Investigation on Alloying Element Distribution in Cr8Mo2SiV Cold-Work Die Steel Ingot during Homogenization. Steel Res. Int. 2018, 89, 1800148. [Google Scholar] [CrossRef]
- Ning, A.-G.; Liu, Y.; Gao, R.; Yue, S.; Wang, M.-B.; Guo, H.-J. Effect of austenitizing condition on mechanical properties, microstructure and precipitation behavior of AISI H13 steel. J. Iron Steel Res. Int. 2022, 31, 143–156. [Google Scholar] [CrossRef]
- Yamada, W.; Matsumiya, T.; Ito, A. Development of simulation model for composition change of nonmetallic inclusions during solidification of steel. In Proceedings of the Sixth International Iron and Steel Congress, Nagoya, Japan, 21–26 October 1990; pp. 618–625. [Google Scholar]
- Liu, X.Y.; Pan, Q.L.; Fan, X.; Bin He, Y.; Bin Li, W.; Liang, W.J. Microstructural evolution of Al–Cu–Mg–Ag alloy during homogenization. J. Alloys Compd. 2009, 484, 790–794. [Google Scholar] [CrossRef]
- Mao, M.; Wang, F.; Sun, X.; Guo, H. In situ research of partial melt in as-cast H13 steel at elevated temperature. Ironmak. Steelmak. 2018, 47, 159–167. [Google Scholar] [CrossRef]
- Lee, E.-S.; Park, W.-J.; Jung, J.Y.; Ahn, S. Solidification microstructure and M2C carbide decomposition in a spray-formed high-speed steel. Met. Mater. Trans. A 1998, 29, 1395–1404. [Google Scholar] [CrossRef]
- Chen, L.; Pei, J.; Li, F.; Zhang, Y.; Wang, M.; Ma, X. Decomposition Reaction of Metastable M2C Carbide in a Multi-Component Semi-High-Speed Steel. Met. Mater. Trans. A 2016, 47, 5662–5669. [Google Scholar] [CrossRef]
- Liu, W.; Guo, Y.; Cao, Y.; Wang, J.; Hou, Z.; Sun, M.; Xu, B.; Li, D. Transformation behavior of primary MC and M2C carbides in Cr4Mo4V steel. J. Alloys Compd. 2021, 889, 161755. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Wang, L.; Li, L. Study on Carbide in Forged and Annealed H13 Hot Work Die Steel. High Temp. Mater. Process. 2015, 34, 593–598. [Google Scholar] [CrossRef]
- Zhou, W.; Zhu, J.; Zhang, Z. Austenite Grain Growth Behaviors of La-Microalloyed H13 Steel and Its Effect on Mechanical Properties. Met. Mater. Trans. A 2020, 51, 4662–4673. [Google Scholar] [CrossRef]
- Yue, C.; Zhang, L.; Liao, S.; Gao, H. Kinetic Analysis of the Austenite Grain Growth in GCr15 Steel. J. Mater. Eng. Perform. 2009, 19, 112–115. [Google Scholar] [CrossRef]
- Li, Z.; Wen, Z.; Su, F.; Zhang, R.; Li, Z. Austenite grain growth behavior of a GCr15 bearing steel cast billet in the homogenization heat treatment process. J. Mater. Res. 2016, 31, 2105–2113. [Google Scholar] [CrossRef]
- Xu, Y.; Tang, D.; Song, Y.; Pan, X. Prediction model for the austenite grain growth in a hot rolled dual phase steel. Mater. Des. 2012, 36, 275–278. [Google Scholar] [CrossRef]
- Lee, S.-J.; Lee, Y.-K. Prediction of austenite grain growth during austenitization of low alloy steels. Mater. Des. 2008, 29, 1840–1844. [Google Scholar] [CrossRef]
- Najafkhani, F.; Kheiri, S.; Pourbahari, B.; Mirzadeh, H. Recent advances in the kinetics of normal/abnormal grain growth: A review. Arch. Civ. Mech. Eng. 2021, 21, 1–20. [Google Scholar] [CrossRef]



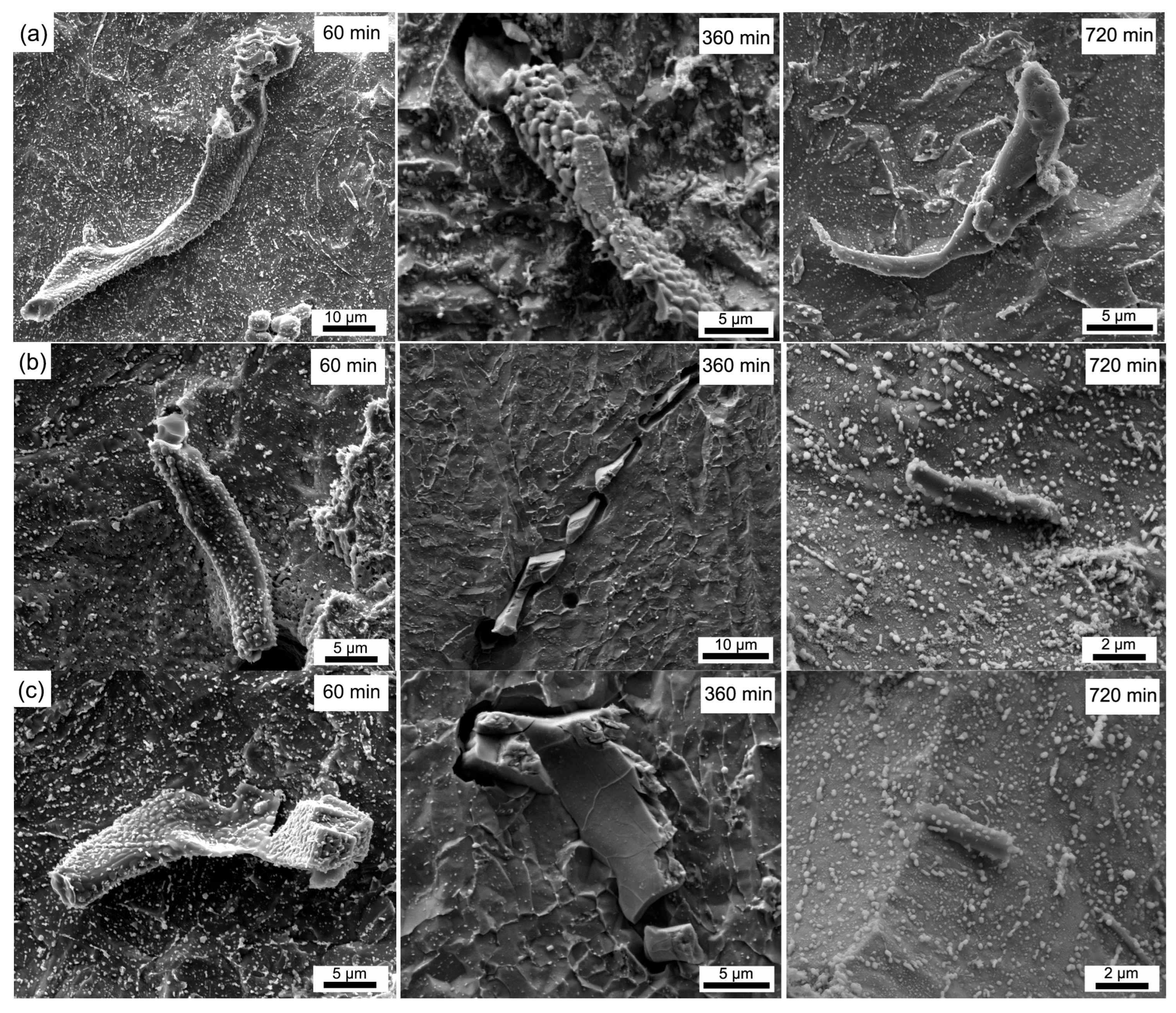
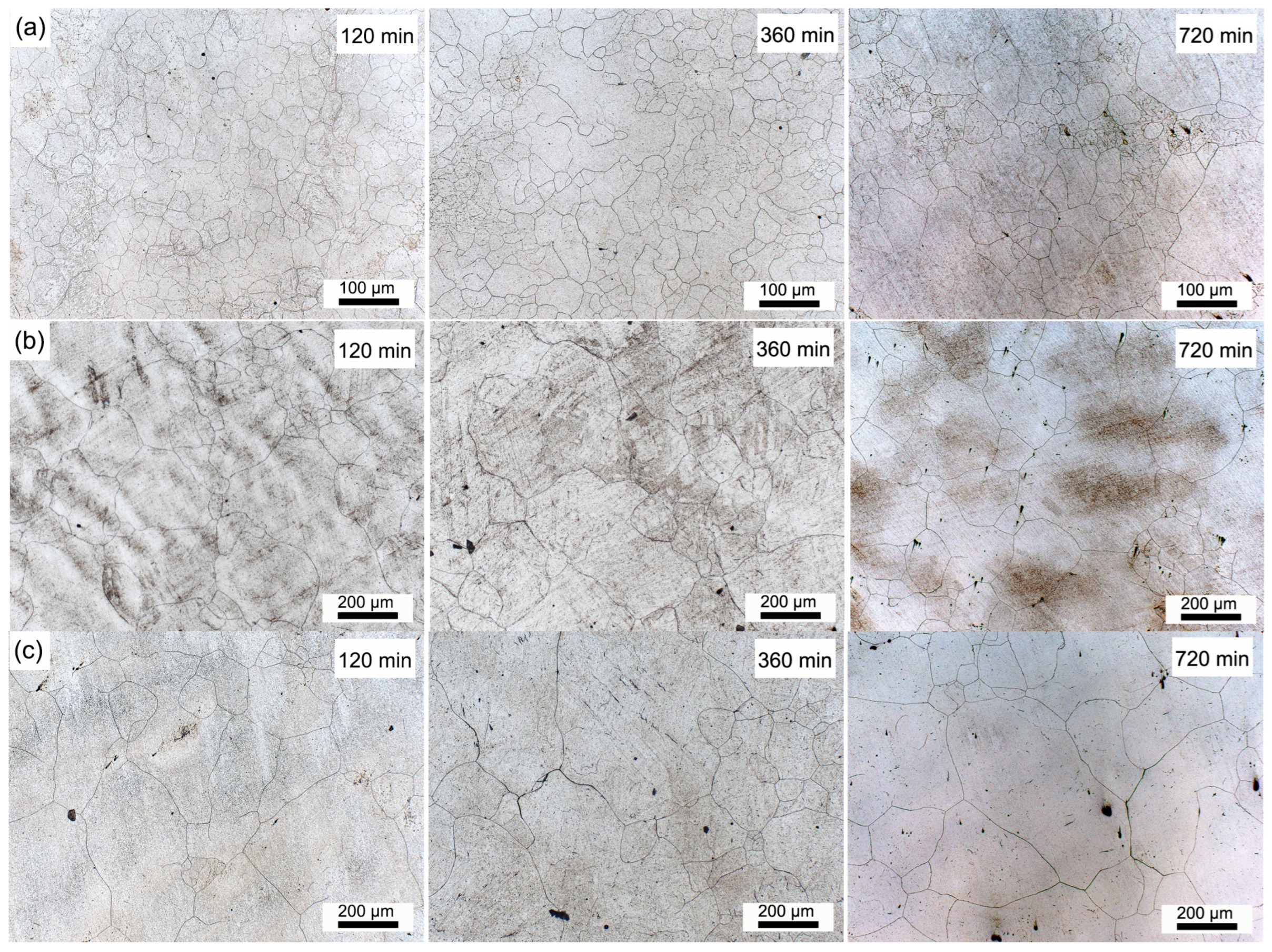
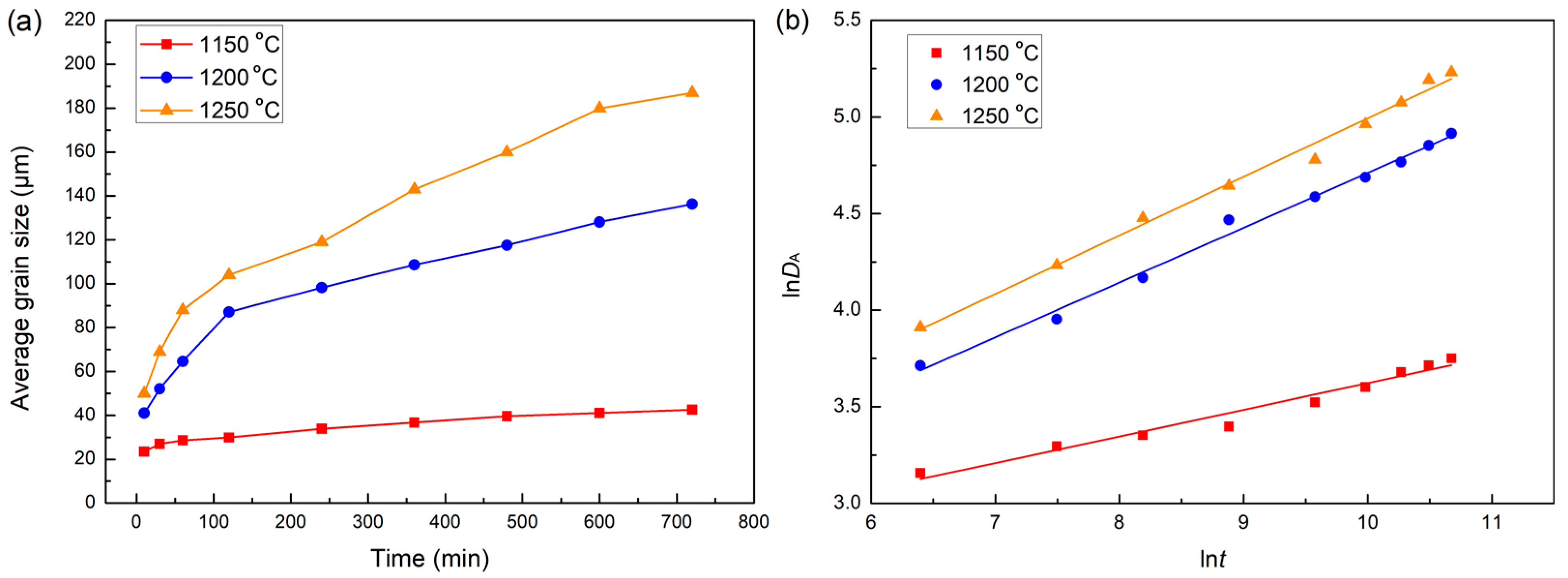
| Average Size | Soaked at 1150 °C | Soaked at 1200 °C | Soaked at 1250 °C | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 60 min | 360 min | 720 min | 60 min | 360 min | 720 min | 60 min | 360 min | 720 min | |
| Amax-size (μm) | 9.93 | 7.06 | 6.55 | 6.11 | 4.97 | 4.38 | 5.88 | 4.72 | 3.69 |
| Amin-size (μm) | 4.33 | 4.09 | 3.86 | 3.86 | 3.23 | 3.03 | 3.75 | 3.16 | 2.88 |
| Max/Min-ratio | 2.29 | 1.73 | 1.70 | 1.58 | 1.54 | 1.45 | 1.57 | 1.49 | 1.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Yu, H.; He, S. Effect of Homogenization Treatment on Microstructure, Dendritic Segregation, and Primary Carbides in H13 Steel. Materials 2025, 18, 4785. https://doi.org/10.3390/ma18204785
Wang X, Yu H, He S. Effect of Homogenization Treatment on Microstructure, Dendritic Segregation, and Primary Carbides in H13 Steel. Materials. 2025; 18(20):4785. https://doi.org/10.3390/ma18204785
Chicago/Turabian StyleWang, Xijie, Huan Yu, and Sibo He. 2025. "Effect of Homogenization Treatment on Microstructure, Dendritic Segregation, and Primary Carbides in H13 Steel" Materials 18, no. 20: 4785. https://doi.org/10.3390/ma18204785
APA StyleWang, X., Yu, H., & He, S. (2025). Effect of Homogenization Treatment on Microstructure, Dendritic Segregation, and Primary Carbides in H13 Steel. Materials, 18(20), 4785. https://doi.org/10.3390/ma18204785





