Multiscale Investigation of Interfacial Behaviors in Rubber Asphalt–Aggregate Systems Under Salt Erosion: Insights from Laboratory Tests and Molecular Dynamics Simulations
Abstract
1. Introduction
2. Materials and Methods
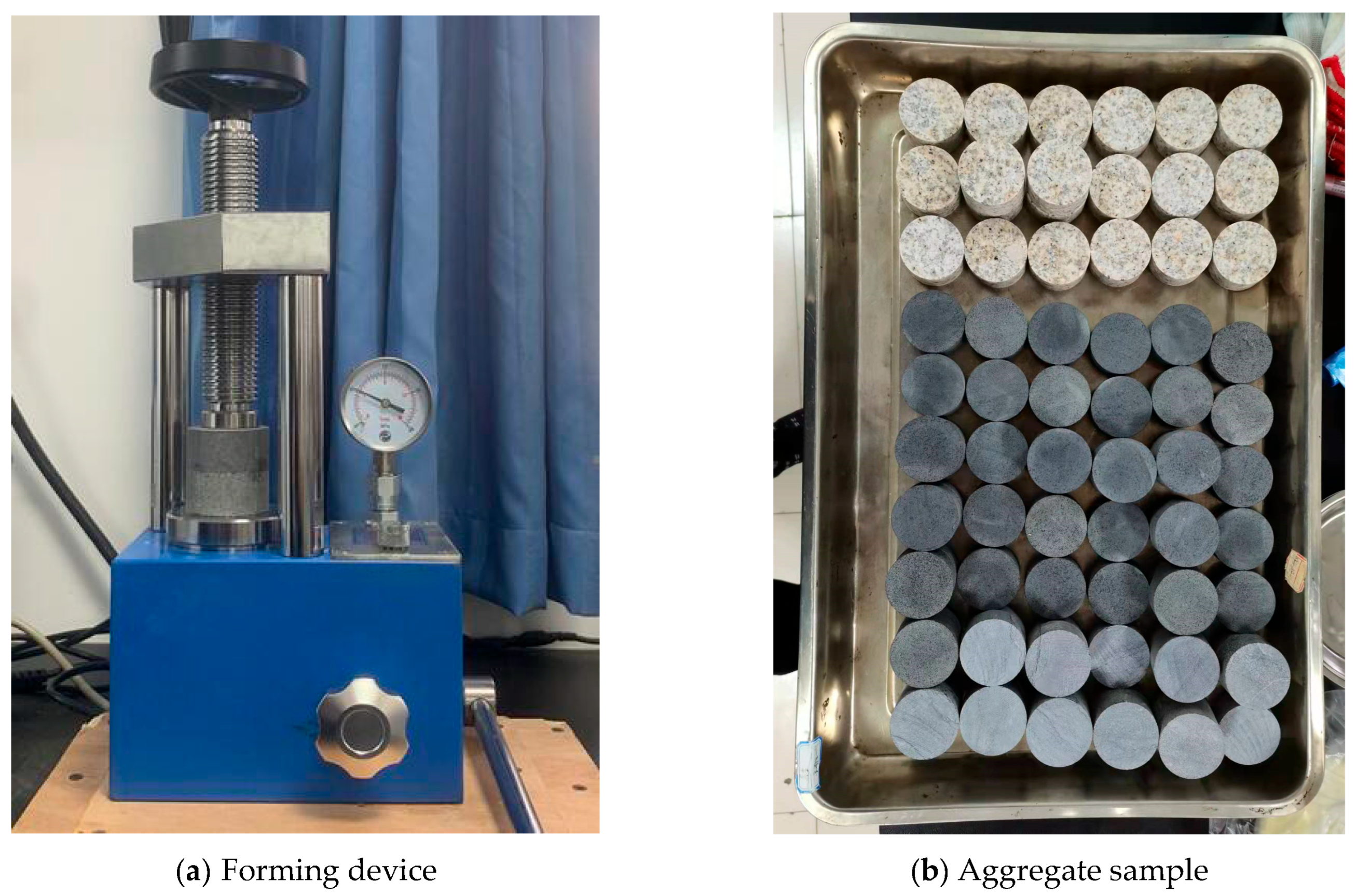
2.1. Raw Materials
2.2. Test Method
2.2.1. Simulation of the Salt–Freeze–Thaw Cycles
2.2.2. Macroscopic Pull-Out Test of Rubber Asphalt–Aggregate
2.2.3. Characterization of Microscopic Properties for Rubber Asphalt
AFM Test
FTIR Test
2.2.4. Simulation of Molecular Behavior at the Asphalt–Aggregate Interface
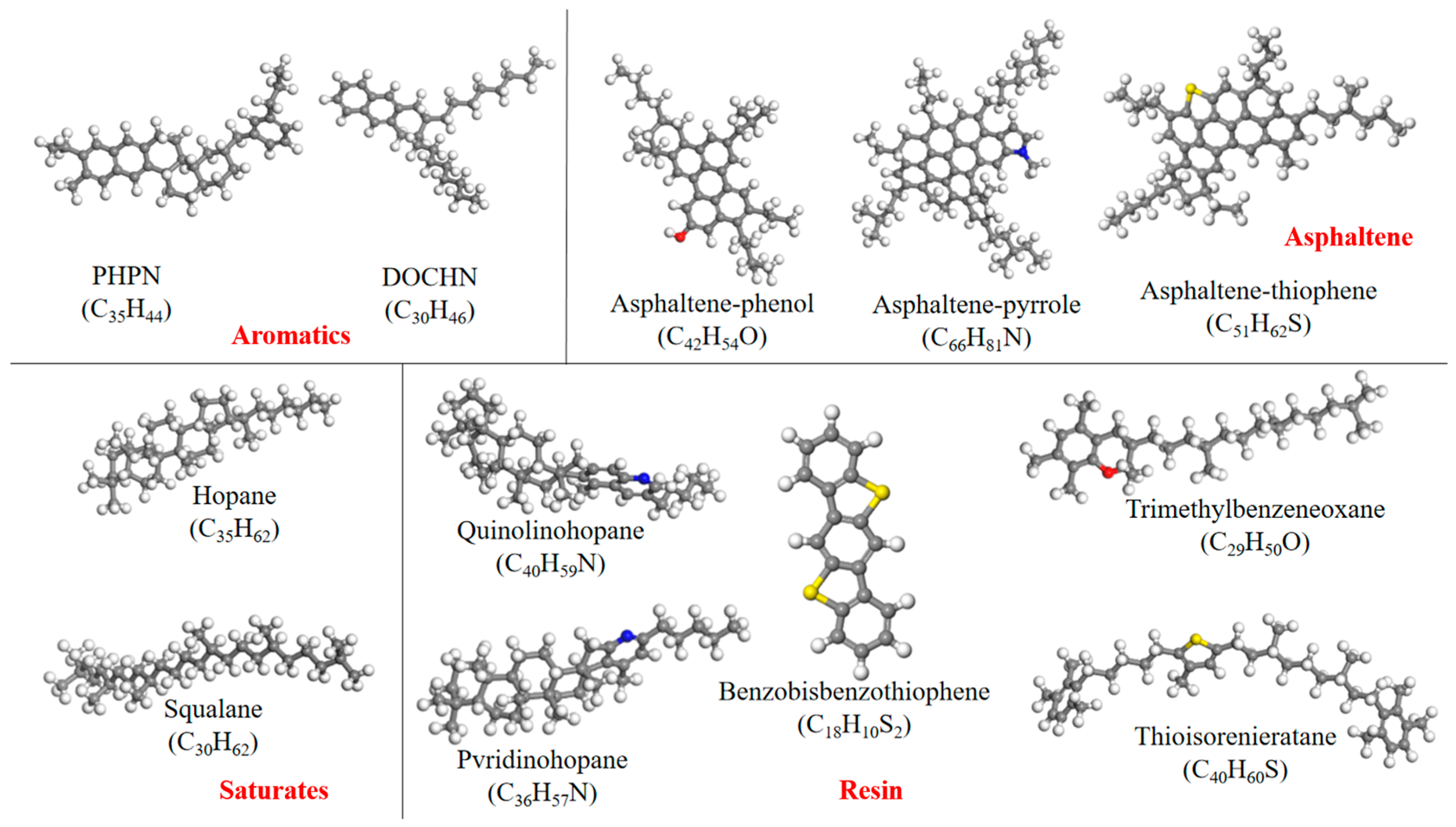
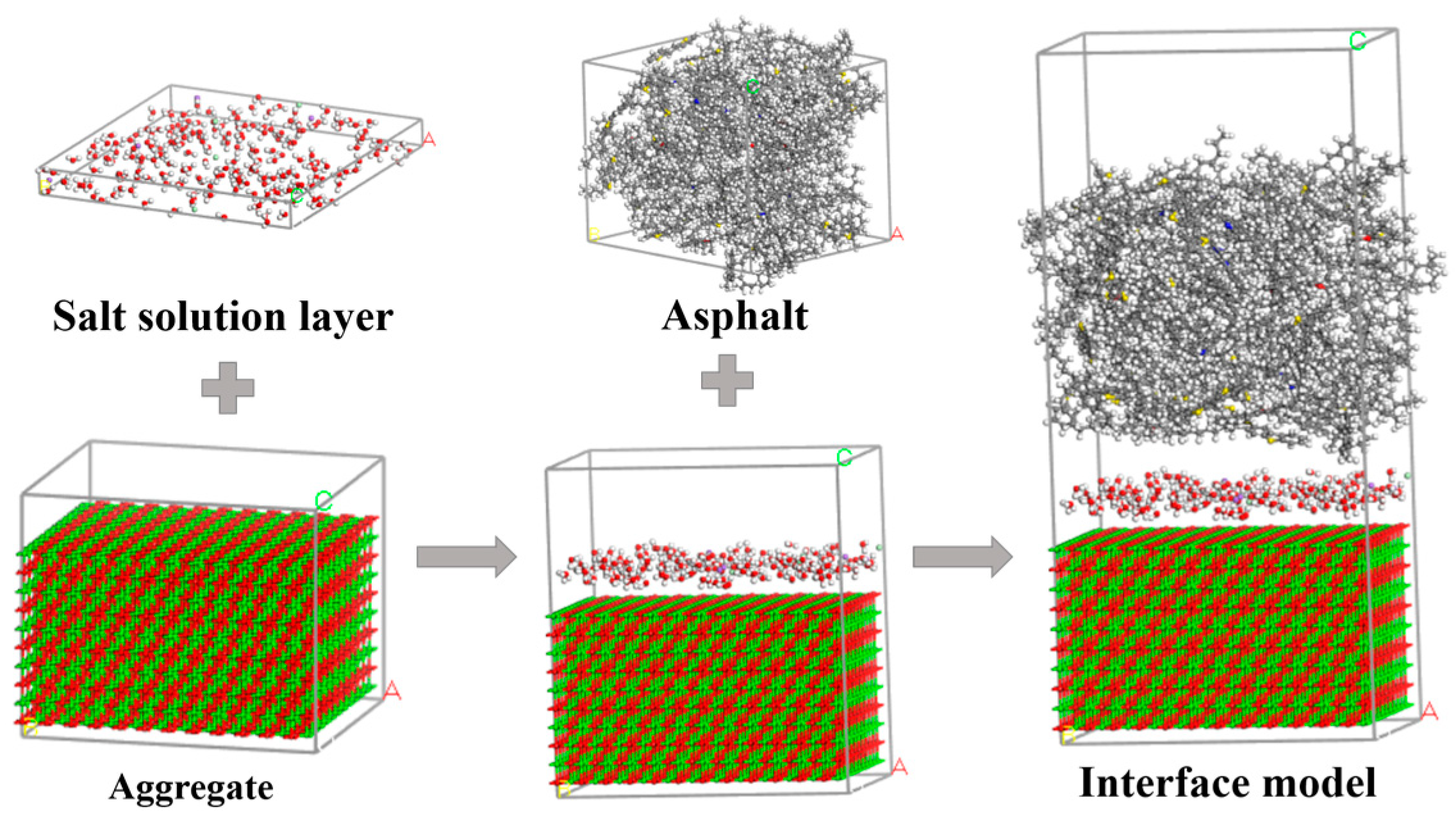
Interfacial Modeling of Asphalt and Aggregate
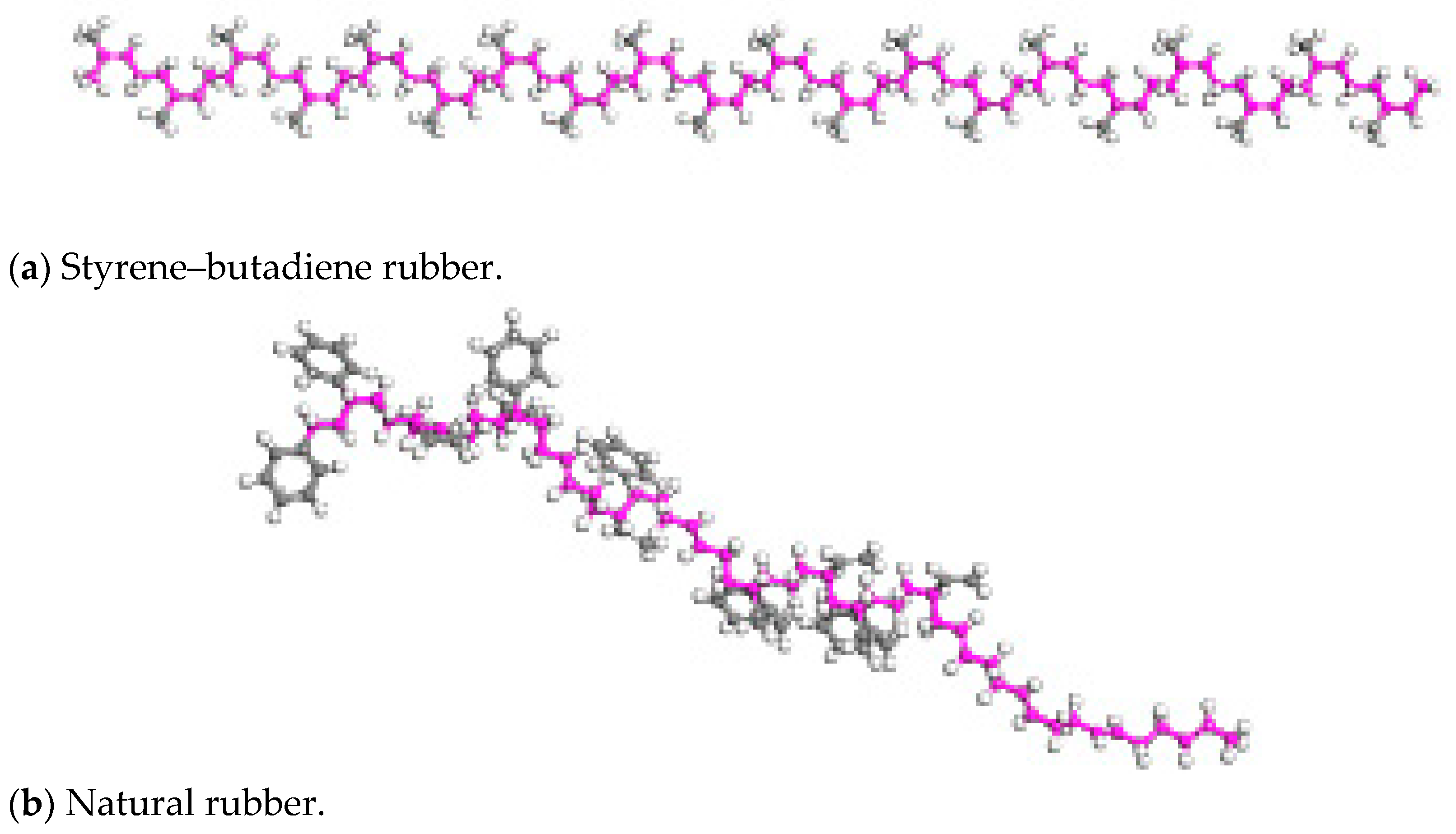
Rubber Asphalt Molecular Model
Aggregate Molecular Model
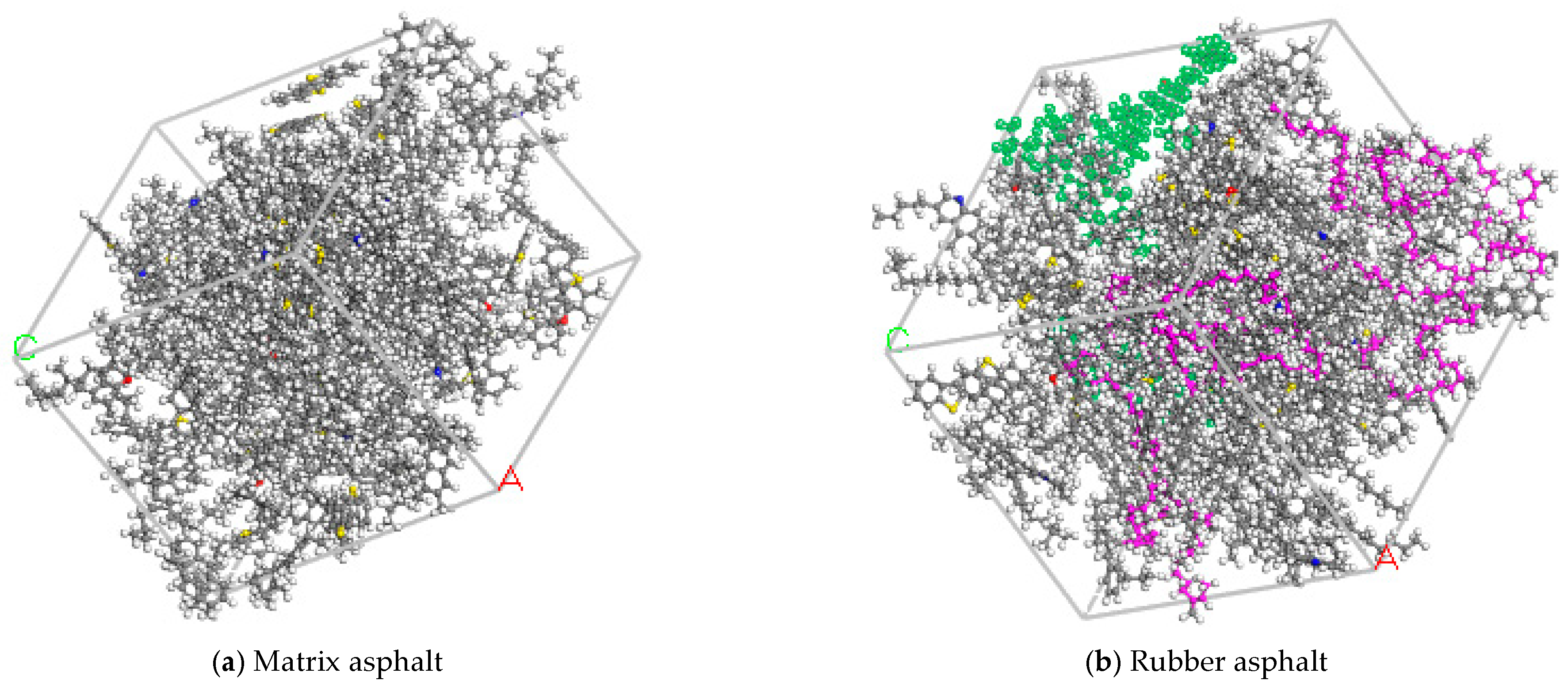
Interfacial Model Between Asphalt and Aggregate
Evaluation Indicators for Interfacial Behavior
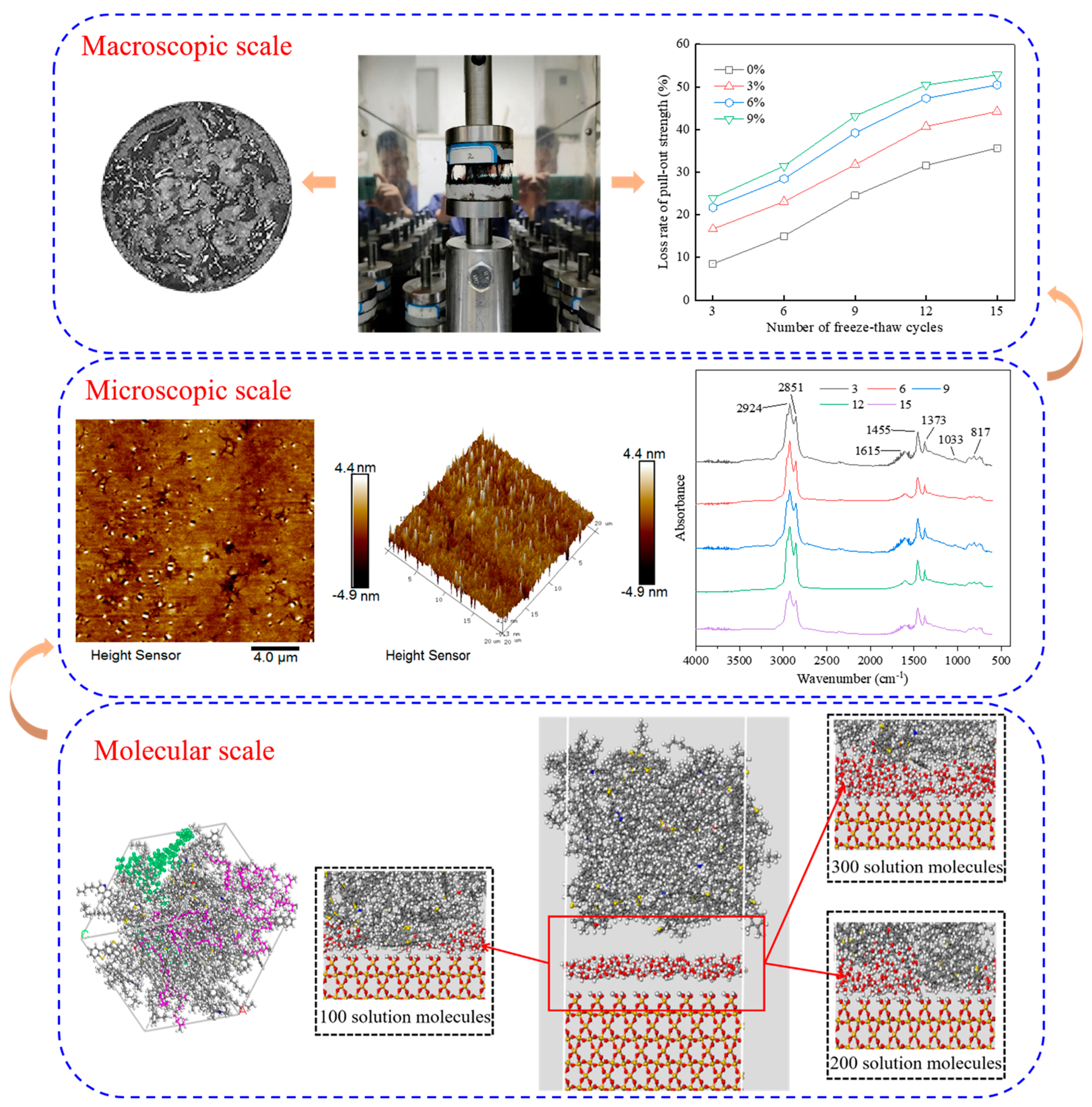
3. Results and Discussion
3.1. Interfacial Pull-Out Properties Between Rubber Asphalt and Aggregate
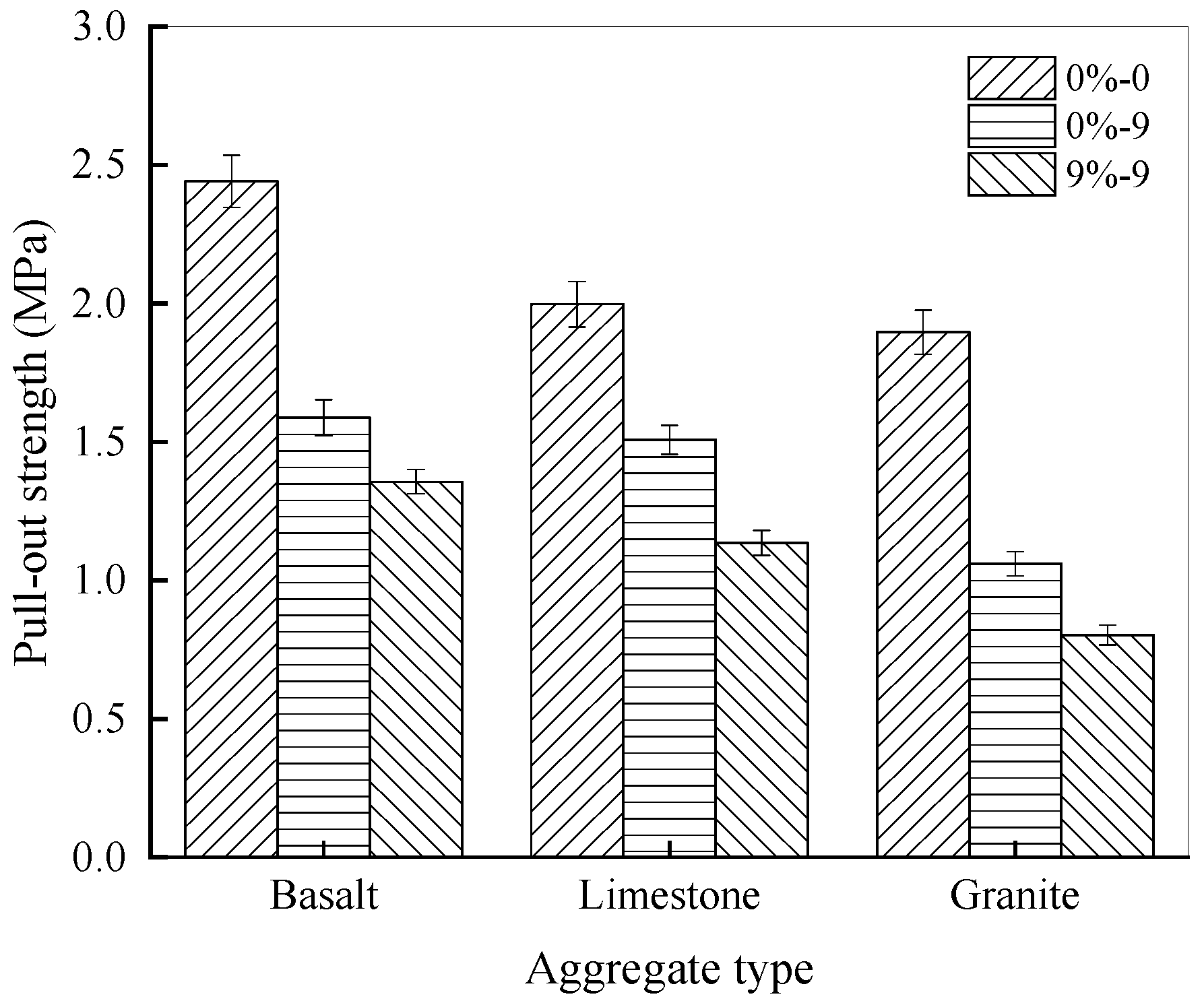
3.1.1. Pull-Out Properties Under Different Aggregate Types
3.1.2. Pull-Out Properties at Different Salt Solution Concentrations
3.2. Interfacial Salt Damage Mechanism Between Rubber Asphalt and Aggregate
3.2.1. Microscopic Properties of Rubber Asphalt
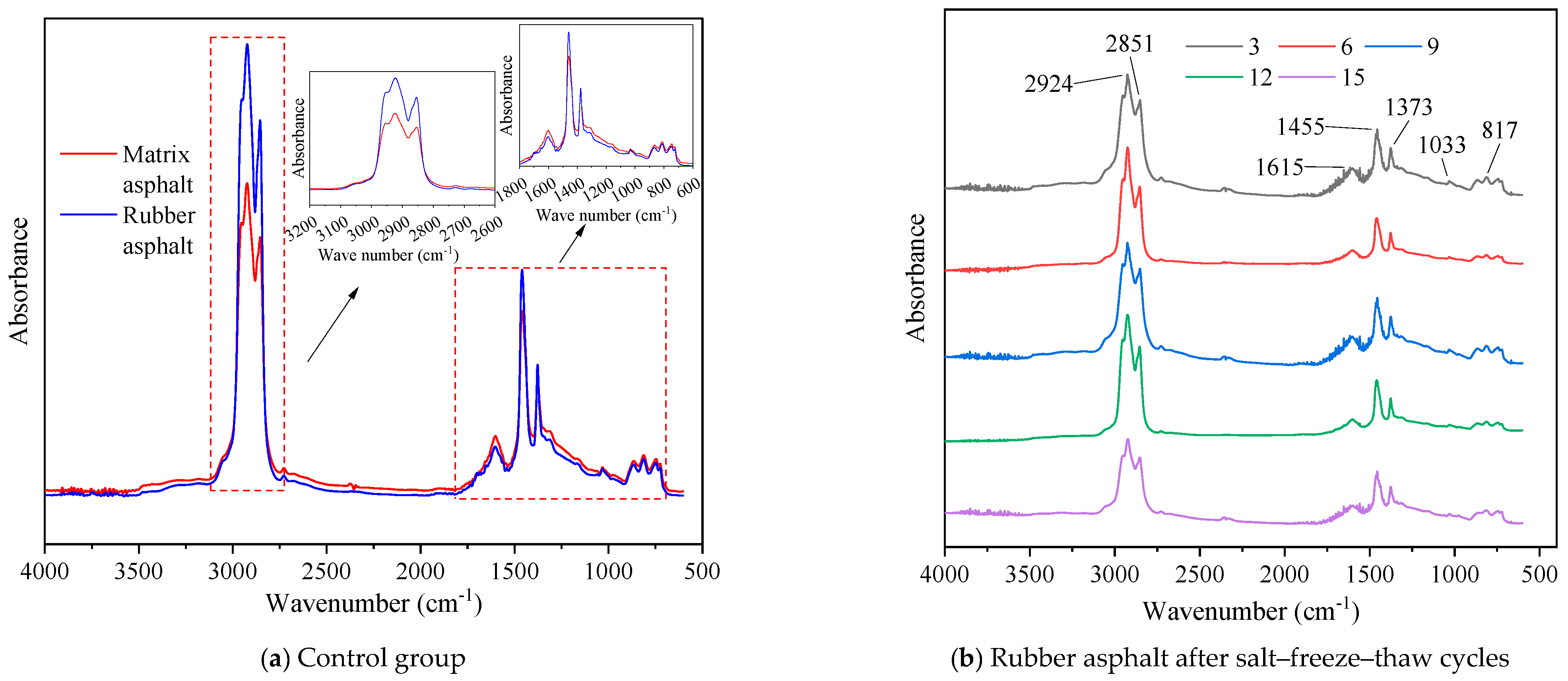
Microscopic Morphology of Rubber Asphalt After Salt–Freeze–Thaw Cycles
Chemical Functional Groups of Rubber Asphalt After Salt–Freeze–Thaw Cycles
3.2.2. Molecular Behavior at the Asphalt–Aggregate Interface
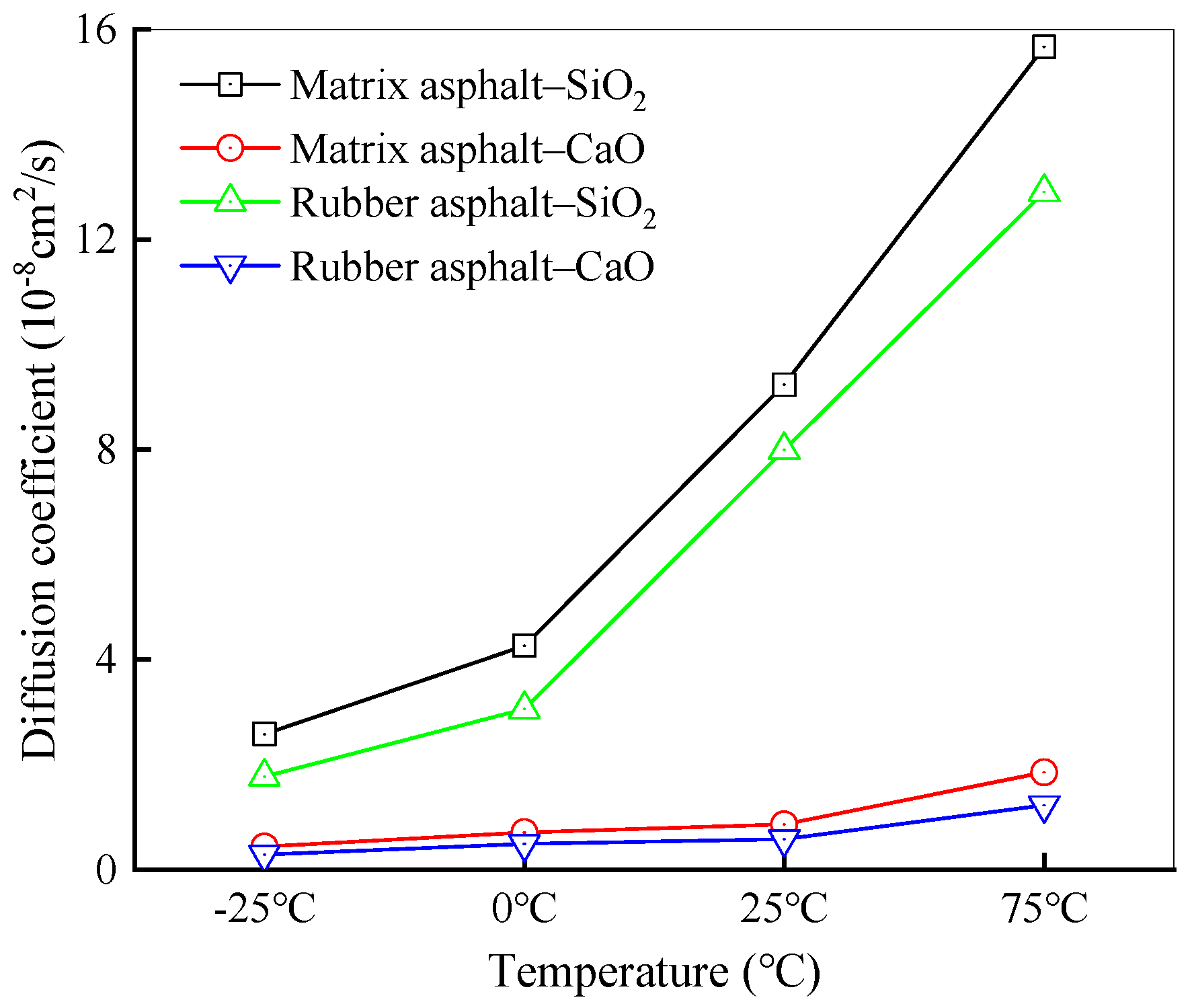
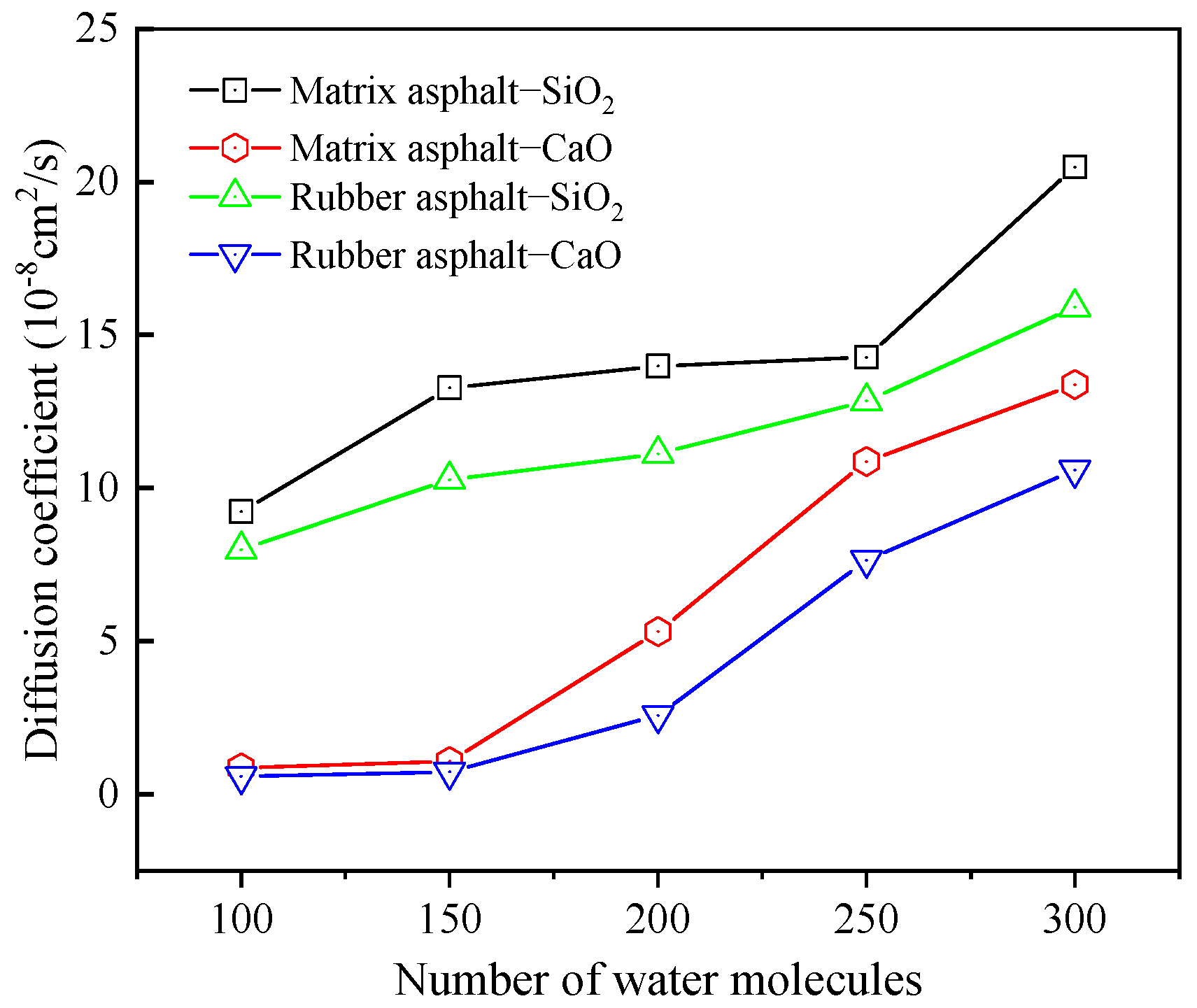
Diffusion Behavior of H2O
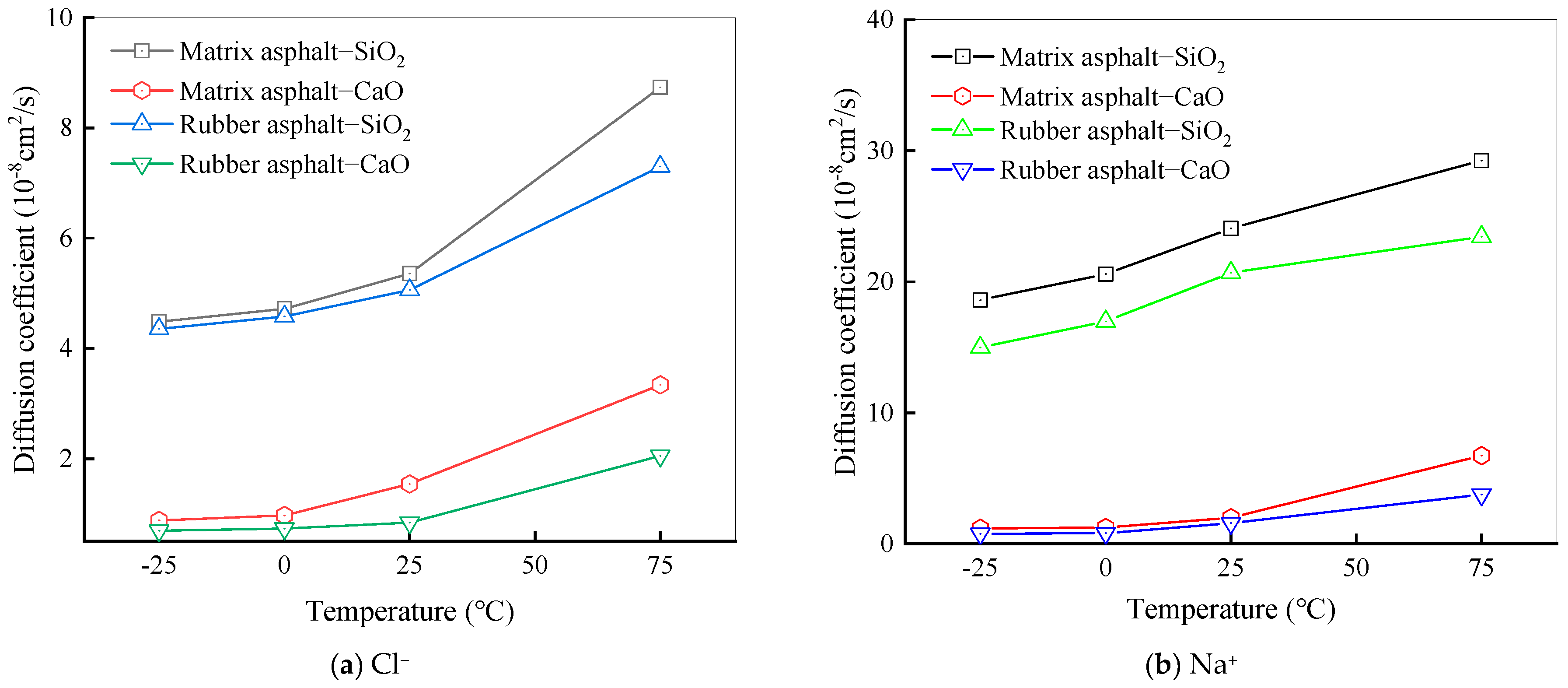
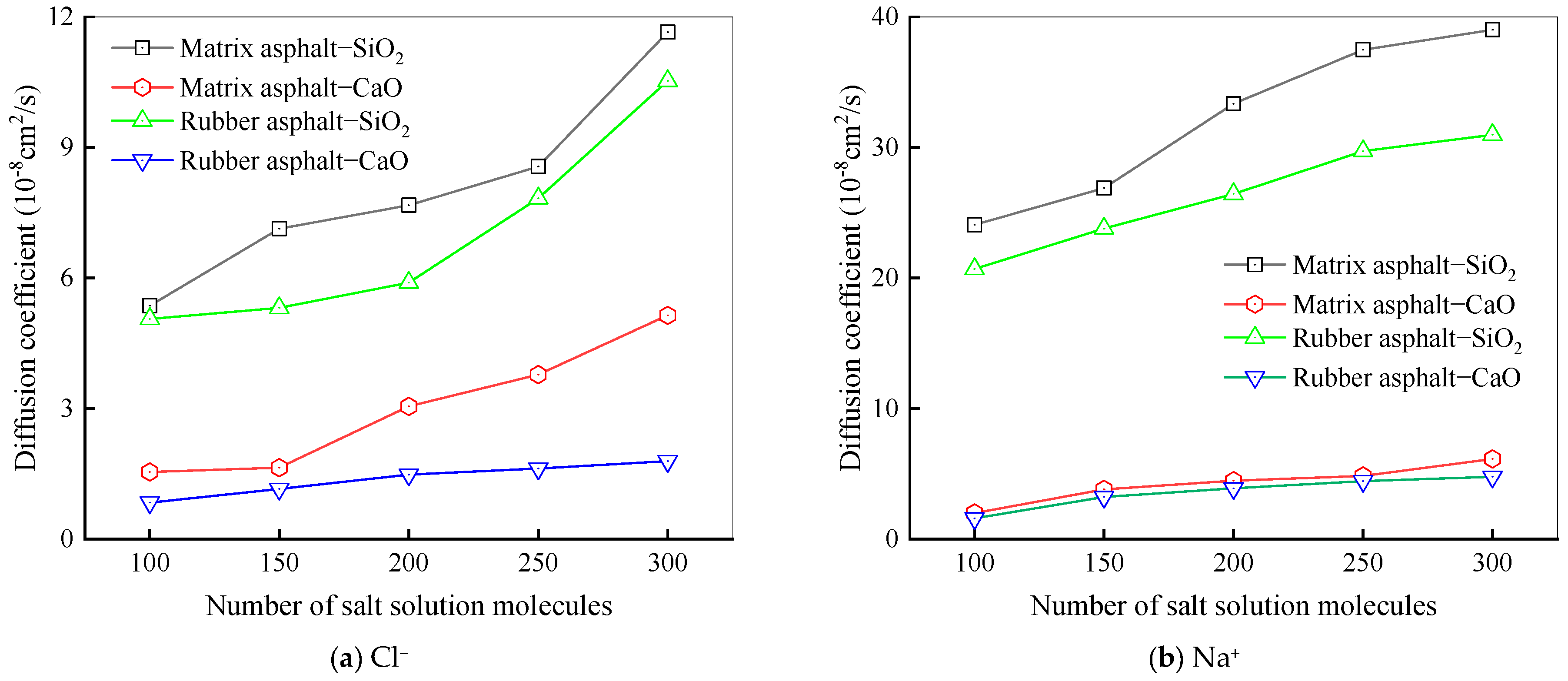
Diffusion Behavior of Na and Cl Ions
Diffusion Behavior of Four Components in Asphalt
3.2.3. Damage Mechanism of Asphalt–Aggregate Under Salt Erosion Conditions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guan, B.; Xue, X.; Tian, H.; Wang, Z.; Qiu, W.; Xu, Z. Investigation of moisture and salt-erosion resistance of asphalt-calcined bauxite systems. J. Mater. Civ. Eng. 2023, 35, 0004553. [Google Scholar] [CrossRef]
- Meng, Y.; Hu, C.; Tang, Y.; Großegger, D.; Qin, W. Investigation on the erosion mechanism of simulated salt conditions on bitumen. Constr. Build. Mater. 2022, 334, 127267. [Google Scholar] [CrossRef]
- Su, B.; Meng, Y.; Hu, S.; Qin, Y. Study on self-healing performance of asphalt under sodium salt erosion. Case Stud. Constr. Mater. 2024, 20, e02921. [Google Scholar] [CrossRef]
- Long, Z.; Guo, N.; Tang, X.; Ding, Y.; You, L.; Xu, F. Microstructural evolution of asphalt induced by chloride salt erosion. Constr. Build. Mater. 2022, 343, 128056. [Google Scholar] [CrossRef]
- Tan, F.; Qin, J.; Zeng, F.; Nong, T.; Lin, X.; Li, H. Effect of chloride corrosion on the adhesion performance of asphalt aggregate interface. Int. J. Adhes. Adhes. 2025, 140, 104007. [Google Scholar] [CrossRef]
- Xiong, R.; Chu, C.; Qiao, N.; Wang, L.; Yang, F.; Sheng, Y.; Guan, B.; Niu, D.; Geng, J.; Chen, H. Performance evaluation of asphalt mixture exposed to dynamic water and chlorine salt erosion. Constr. Build. Mater. 2019, 201, 121–126. [Google Scholar] [CrossRef]
- Zhang, K.; Luo, Y.; Xie, W.; Wu, J. Evaluation of road performance and adhesive characteristic of asphalt binder in salt erosion environment. Mater. Today Commun. 2020, 25, 101593. [Google Scholar] [CrossRef]
- Zhang, R.; Tang, N.; Deng, X.; Zhu, H.; Su, C.; Xi, Y. Erosion mechanism of sea salt solution on the performance of SBS-modified asphalt mixtures. Int. J. Pavement Eng. 2023, 24, 2120991. [Google Scholar] [CrossRef]
- Wang, F.; Qin, X.; Pang, W.; Wang, W. Performance deterioration of asphalt mixture under chloride salt erosion. Materials 2021, 14, 3339. [Google Scholar] [CrossRef]
- Jiang, Q.; Liu, W.; Wu, S. Experimental investigation on the rheological properties and erosion mechanisms of chloride salt-modified asphalt mortar. J. Mater. Civ. Eng. 2024, 36, 04023549. [Google Scholar] [CrossRef]
- Hu, C.; Zhou, Z.; Luo, Y. Study on the effect and mechanism of different snowmelt agents on asphalt. J. Test. Eval. 2023, 51, 4404–4418. [Google Scholar] [CrossRef]
- Busang, S.; Maina, J. Laboratory simulation and mechanical performance of asphalt materials under the action of saline. Constr. Build. Mater. 2021, 313, 125387. [Google Scholar] [CrossRef]
- Gilani, V.N.M.; Hosseinian, S.M.; Hamedi, G.H.; Safari, D. Presentation of predictive models for two-objective optimization of moisture and fatigue damages caused by deicers in asphalt mixtures. J. Test. Eval. 2021, 49, 4437–4458. [Google Scholar] [CrossRef]
- Behbahani, H.; Hamedi, G.H.; Gilani, V.N.M. Predictive model of modified asphalt mixtures with nano hydrated lime to increase resistance to moisture and fatigue damages by the use of deicing agents. Constr. Build. Mater. 2020, 265, 120353. [Google Scholar] [CrossRef]
- Mackiewicz, P.; Maczka, E. The impact of water and road salt with anti-caking agent on the stiffness of select mixes used for the road surface. Materials 2021, 14, 1345. [Google Scholar] [CrossRef]
- Zhang, K.; Li, W.; Han, F. Performance deterioration mechanism and improvement techniques of asphalt mixture in salty and humid environment. Constr. Build. Mater. 2019, 208, 749–757. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, J. Low-temperature crack resistance of stone matrix asphalt mixtures under chloride salt dry-wet cycles. Eng. Fract. Mech. 2024, 303, 110092. [Google Scholar] [CrossRef]
- Meng, Y.; Li, H.; Yang, X.; Li, G.; Li, Y.; Xu, G. Study on the adhesion properties of SBS modified asphalt-aggregate with polyphosphoric acid and sugarcane bagasse fiber under salt erosion. Constr. Build. Mater. 2025, 466, 140269. [Google Scholar] [CrossRef]
- Dan, H.; Zou, Z.; Zhang, Z.; Tan, J. Effects of aggregate type and SBS copolymer on the interfacial heat transport ability of asphalt mixture using molecular dynamics simulation. Constr. Build. Mater. 2020, 250, 118922. [Google Scholar] [CrossRef]
- Xu, H.; Xu, W.; Zheng, X.; Cao, K. A multistage analysis of asphalt binder nanocrack generation and self-Healing behavior based on molecular dynamics. Polymers 2022, 14, 3581. [Google Scholar] [CrossRef]
- Xu, W.; Qiu, X.; Xiao, S.; Hu, G.; Wang, F.; Yuan, J. Molecular dynamic investigations on the adhesion behaviors of asphalt mastic-aggregate interface. Materials 2020, 13, 5061. [Google Scholar] [CrossRef]
- Ding, G.; Yu, X.; Dong, F.; Ji, Z.; Wang, J. Using silane coupling agent coating on acidic aggregate surfaces to enhance the adhesion between asphalt and aggregate: A molecular dynamics simulation. Materials 2020, 13, 5580. [Google Scholar] [CrossRef]
- Tang, Y.; Fu, Z.; Raos, G.; Ma, F.; Zhao, P.; Hou, Y. Molecular dynamics simulation of adhesion at the asphalt-aggregate interface: A review. Surf. Interfaces 2024, 44, 103706. [Google Scholar] [CrossRef]
- Bhasin, A.; Bommavaram, R.; Greenfield, M.L.; Little, D.N. Use of molecular dynamics to investigate self-Healing mechanisms in asphalt binders. J. Mater. Civ. Eng. 2011, 23, 485–492. [Google Scholar] [CrossRef]
- Yi, J.; Pang, X.; Feng, D.; Pei, Z.; Xu, M.; Xie, S.; Huang, Y. Studies on surface energy of asphalt and aggregate at different scales and bonding property of asphalt-aggregate system. Road Mater. Pavement Des. 2018, 19, 1102–1125. [Google Scholar] [CrossRef]
- Xu, M.; Yi, J.; Feng, D.; Huang, Y.; Wang, D. Analysis of adhesive characteristics of asphalt based on atomic force microscopy and molecular dynamics simulation. ACS Appl. Mater. Interfaces 2016, 8, 12393–12403. [Google Scholar] [CrossRef]
- Menapace, I.; Masad, E. The influence of moisture on the evolution of the microstructure of asphalt binders with aging. Road Mater. Pavement Des. 2020, 21, 331–346. [Google Scholar] [CrossRef]
- Nivedya, M.K.; Trottier, T.G.; Yu, X.; Tao, M.; Burnham, N.A.; Mallick, R.B. Microstructural evolution of asphalt binder under combined action of moisture and pressure. J. Transp. Eng. Part B Pavements 2019, 145, 06019001. [Google Scholar] [CrossRef]
- Ma, W.; Huang, T.; Guo, S.; Yang, C.; Ding, Y.; Hu, C. Atomic force microscope study of the aging/rejuvenating effect on asphalt morphology and adhesion performance. Constr. Build. Mater. 2019, 205, 642–655. [Google Scholar] [CrossRef]
- Hu, K.; Yu, C.; Yang, Q.; Chen, Y.; Chen, G.; Ma, R. Multi-scale enhancement mechanisms of graphene oxide on styrene-butadiene-styrene modified asphalt: An exploration from molecular dynamics simulations. Mater. Des. 2021, 208, 109901. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, Z. Investigation of the micro characteristics of asphalt mastics under dry-wet and freeze-thaw cycles in a coastal salt environment. Materials 2019, 12, 2627. [Google Scholar] [CrossRef]
- Zou, Y.; Amirkhanian, S.; Xu, S.; Li, Y.; Wang, Y.; Zhang, J. Effect of different aqueous solutions on physicochemical properties of asphalt binder. Constr. Build. Mater. 2021, 286, 122810. [Google Scholar] [CrossRef]
- Zhang, D.; Yu, P.; Liu, Z.; Liu, Z.; Chen, H.; Gao, Y. Influence of acidity and alkalinity of water environments on the water stability of asphalt mixture: Phase I—Molecular dynamics simulation. Constr. Build. Mater. 2024, 411, 134466. [Google Scholar] [CrossRef]
- Sonibare, K.; Rucker, G.; Zhang, L. Molecular dynamics simulation on vegetable oil modified model asphalt. Constr. Build. Mater. 2021, 270, 121687. [Google Scholar] [CrossRef]
- Wu, M.; You, Z. Molecular dynamics models to investigate the diffusion behavior of emulsified asphalt. Constr. Build. Mater. 2023, 409, 134061. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, Y.; Li, H.; Chen, W. A study on the microscopic properties of the oil-stone interfacial phase of a reclaimed asphalt mixture based on molecular dynamics simulation. Coatings 2023, 13, 1717. [Google Scholar] [CrossRef]
- Tan, Y.; Xie, J.; Song, J.; Xu, J.; Li, X. Interfacial interaction behavior of recycled asphalt pavement: Molecular dynamics simulation. Colloids Surf. A Physicochem. Eng. Asp. 2023, 676, 132194. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, H.; Gao, M.; Zhang, Q.; Sun, Q.; Dong, Q. Nano-microscopic analysis on the interaction of new and old asphalt mortar in recycled asphalt mixture. Chem. Phys. Lett. 2023, 825, 140593. [Google Scholar] [CrossRef]
- Luo, L.; Chu, L.; Fwa, T.F. Molecular dynamics analysis of oxidative aging effects on thermodynamic and interfacial bonding properties of asphalt mixtures. Constr. Build. Mater. 2021, 269, 121299. [Google Scholar] [CrossRef]
- Yu, H.; Ge, J.; Qian, G.; Shi, C.; Zhang, C.; Dai, W.; Xie, T.; Nian, T. Evaluation of the interface adhesion mechanism between SBS asphalt and aggregates under UV aging through molecular dynamics. Constr. Build. Mater. 2023, 409, 133995. [Google Scholar] [CrossRef]
- Jiao, B.; Pan, B.; Liu, F.; Yin, P.; Li, Z. Evaluating the interfacial properties between crumb rubber-modified asphalt and aggregates using molecular dynamics simulation methods. Constr. Build. Mater. 2023, 400, 132809. [Google Scholar]
- ASTM D5; Standard Test Method for Penetration of Bituminous Materials. ASTM: West Conshohocken, PA, USA, 2020.
- ASTM D113; Standard Test Method for Ductility of Asphalt Materials. ASTM: West Conshohocken, PA, USA, 2017.
- ASTM D36; Standard Test Method for Softening Point of Bitumen (Ring and Ball Apparatus). ASTM: West Conshohocken, PA, USA, 2014.
- ASTM D2196; Standard test methods for rheological properties of non-newtonian materials by rotational viscountess. ASTM: West Conshohocken, PA, USA, 2018.
- Zhang, K.; Yang, J.; Zhao, Y.; Xie, W.; Wang, Y. Interfacial bonding property and microscopic damage mechanism of bitumen-aggregate in salt-freeze-thawing environment. Mater. Today Commun. 2023, 36, 106664. [Google Scholar] [CrossRef]
- Zhang, K.; Xie, W.; Zhao, Y. Deterioration mechanism of adhesion property for SBS modified asphalt in salt-freeze-thawing environment. Int. J. Adhes. Adhes. 2023, 126, 103441. [Google Scholar] [CrossRef]
- Huang, X.; Wang, L. Evolution of low-temperature properties of warm-mix rubber-modified asphalt under freeze-thaw cycles. J. Mater. Civ. Eng. 2023, 35, 04022406. [Google Scholar] [CrossRef]
- Tian, Z.; Zhang, Z.; Tang, X.; Zhang, Y.; Gui, Z.; Tan, J.; Chang, Q. Understanding the effect of moisture on interfacial behaviors of geopolymer-aggregate interaction at molecular level. Constr. Build. Mater. 2023, 385, 131404. [Google Scholar] [CrossRef]
- Tian, Z.; Zhang, Z.; Liu, H.; Zheng, W.; Tang, X.; Gui, Z. Interfacial characteristics and mechanical behaviors of geopolymer binder with steel slag aggregate: Insights from molecular dynamics. J. Clean. Prod. 2022, 362, 132385. [Google Scholar] [CrossRef]
- Li, G.; Gu, Z.; Tan, Y.; Xing, C.; Zhang, J.; Zhang, C. Research on the phase structure of Styrene-Butadiene-Styrene modified asphalt based on molecular dynamics. Constr. Build. Mater. 2022, 326, 126933. [Google Scholar]
- Liu, K.; Xu, Y.; Wang, H.; Wang, L. Experimental and molecular dynamics study on the properties of amorphous poly alpha olefin (APAO) reinforcing styrene butadiene rubber (SBR) modified asphalt. Constr. Build. Mater. 2024, 443, 137844. [Google Scholar] [CrossRef]
- Zhan, Y.; Wu, H.; Song, W.; Zhu, L. Molecular dynamics study of the diffusion between virgin and aged asphalt binder. Coatings 2022, 12, 403. [Google Scholar] [CrossRef]
- Yao, H.; Dai, Q.; You, Z. Investigation of the asphalt-aggregate interaction using molecular dynamics. Pet. Sci. Technol. 2017, 35, 586–593. [Google Scholar]
- Dong, F.; Wang, S.; Yu, X.; Guo, Y.; Jin, Y.; Zhu, H.; Jiang, Y.; Lu, J. Investigation on the diffusion behavior of dry modified SBS at the asphalt-aggregate interface: Molecular simulation and experiments. J. Mater. Civ. Eng. 2024, 36, 04023564. [Google Scholar] [CrossRef]
- Huang, G.; Chen, Z.; Bi, Y.; Wang, S.; Zhang, J.; Falchetto, A.C. A comprehensive evaluation of crumb rubber modified asphalt-aggregate interfacial behavior and the effect of water: Molecular dynamics simulation and experimental investigation. Fuel 2024, 377, 132824. [Google Scholar] [CrossRef]
- Maryam, M.; Ghabchi, R. Mechanical, thermodynamical, and microstructural characterization of adhesion evolution in asphalt binder-aggregate interface subjected to calcium chloride deicer. Constr. Build. Mater. 2023, 408, 133663. [Google Scholar] [CrossRef]
- Li, S.; Ao, Z.; Qu, Y.; Shao, Z.; Fang, Z.; Cheng, M.; Xu, H. Degradation behaviors of asphalt concrete under chloride erosion-freeze-thaw cycles: Insights into microstructure evolution and mechanical property correlation. Constr. Build. Mater. 2025, 486, 142000. [Google Scholar] [CrossRef]
- Liang, S.; Liang, C.; Li, M.; Cui, H.; Wang, Z.; Wang, S. Investigation of nanoscale interfacial bonding properties in foamed asphalt cold recycled mixtures under chloride salt erosion. Case Stud. Constr. Mater. 2024, 21, e03390. [Google Scholar] [CrossRef]
- Guo, Q.; Li, G.; Gao, Y.; Wang, K.; Dong, Z.; Liu, F.; Zhu, H. Experimental investigation on bonding property of asphalt-aggregate interface under the actions of salt immersion and freeze-thaw cycles. Constr. Build. Mater. 2019, 206, 590–599. [Google Scholar] [CrossRef]
- Feng, X.; Ning, Z.; Mao, Y.; Yang, Y.; Yang, X. Mechanical damage characteristics of CR/PPA composite modified asphalt pavement under multi-factor coupling effect in the seasonally frozen region. Case Stud. Constr. Mater. 2023, 19, e02296. [Google Scholar] [CrossRef]
- Masad, E.; Roja, K.L. Assessment of aging behaviour of reclaimed asphalt binders: Multi-scale mechanical and microstructural characterization. Road Mater. Pavement Des. 2025, 26, 794–814. [Google Scholar] [CrossRef]
- Ji, X.; Sun, E.; Zou, H.; Hou, Y.; Chen, B. Study on the multiscale adhesive properties between asphalt and aggregate. Constr. Build. Mater. 2020, 249, 118693. [Google Scholar] [CrossRef]
- Tang, Y.; Fu, Z.; Ma, F.; Zhao, P.; Hou, Y.; Jiang, X.; Peng, C. Effect of water molecular behavior on adhesion properties of asphalt-aggregate interface. Constr. Build. Mater. 2023, 402, 133028. [Google Scholar] [CrossRef]
- Luo, L.; Liu, Y.; Oeser, M.; Liu, P. A novel molecular modeling approach for simulating asphalt-aggregate debonding: Considering interfacial moisture migration. Constr. Build. Mater. 2023, 402, 133061. [Google Scholar] [CrossRef]
- Yao, H.; Dai, Q.; You, Z. Chemo-physical analysis and molecular dynamics (MD) simulation of moisture susceptibility of nano hydrated lime modified asphalt mixtures. Constr. Build. Mater. 2015, 101, 536–547. [Google Scholar] [CrossRef]
- Wang, R.; Zhao, Y. Influence of water molecule distribution on asphalt-aggregate interface debonding: Insights from adhesion energy and hydrogen bonding. Colloids Surf. A Physicochem. Eng. Asp. 2025, 719, 137034. [Google Scholar] [CrossRef]
- Wang, R.; Luo, L.; Zhao, Y.; Sun, Q.; Wang, Y. Effect of chloride salt migration at interfaces on asphalt-aggregate adhesion: A molecular dynamics study. Constr. Build. Mater. 2024, 428, 136310. [Google Scholar] [CrossRef]
- Cai, S.; Xu, S.; Tong, Z.; Fang, L.; Zhang, C.; Fu, D.; Ma, Z. Investigation on the evolution of asphalt molecular structure under the salt erosion environment based on molecular dynamics simulation. Constr. Build. Mater. 2024, 443, 137785. [Google Scholar] [CrossRef]
- Guo, F.; Pei, J.; Zhang, J.; Xue, B.; Sun, G.; Li, R. Study on the adhesion property between asphalt binder and aggregate: A state-of-the-art review. Constr. Build. Mater. 2020, 256, 119474. [Google Scholar] [CrossRef]
- Sun, Y.; Han, P.; Hu, M.; Qiao, Y. Insight into the salt damage mechanism to the asphalt-aggregate interface in recycled asphalt mixtures at different salt environments using molecular dynamics and DFT simulations. Constr. Build. Mater. 2024, 445, 137931. [Google Scholar] [CrossRef]







| Index | Tested Value | Standard | |
|---|---|---|---|
| Matrix Asphalt | Rubber Asphalt | ||
| Penetration (25 °C,100 g, 5 s, 0.1 mm) | 64 | 42.3 | ASTM D5 [42] |
| Ductility (15 °C, 5 mm/min, cm) | >100 | 10.47 | ASTM D113 [43] |
| Softening point/°C | 48.0 | 72.6 | ASTM D36 [44] |
| 135 °C Brookfield viscosity/Pa▪S | 1.872 | 9.125 | ASTM D2196 [45] |
| RTFO treated at 163C for 85 min | |||
| Penetration (25 °C, 100 g, 5 s, 0.1 mm) | 63.9 | 63.9 | ASTM D5 [42] |
| Ductility (15 °C, 5 mm/min, cm) | 8.5 | 8.5 | ASTM D113 [43] |
| Aggregate Type | Chemical Composition (%) | ||||
|---|---|---|---|---|---|
| SiO2 | CaO | Al2O3 | K2O | Fe2O3 | |
| Limestone | 8.1 | 65.2 | 3.6 | 3.0 | 2.4 |
| Basalt | 46.7 | 9.3 | 15.2 | 2.9 | 11.5 |
| Granite | 66.5 | 3.2 | 14.9 | 4.1 | 3.8 |
| Asphalt Type | Morphology Characteristic | |
|---|---|---|
| 2D | 3D | |
| Matrix asphalt | 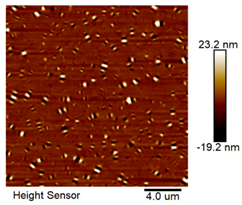 |  |
| Rubber asphalt | 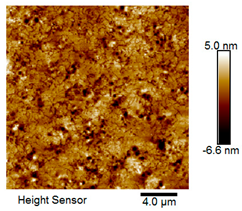 | 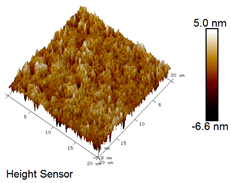 |
| Rubber asphalt after 3 freeze–thaw cycles | 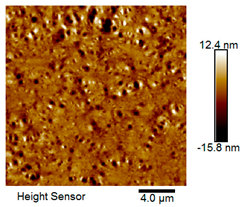 | 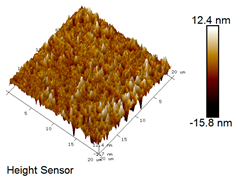 |
| Rubber asphalt after 6 freeze–thaw cycles | 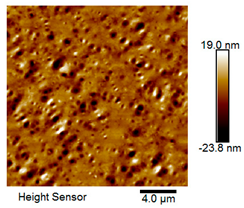 | 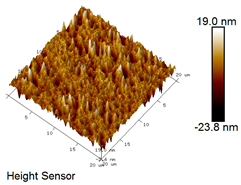 |
| Rubber asphalt after 9 freeze–thaw cycles | 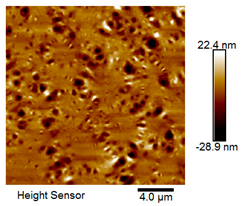 |  |
| Rubber asphalt after 12 freeze–thaw cycles | 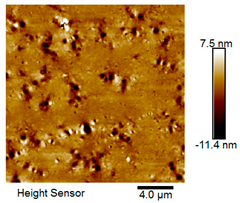 | 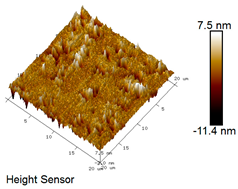 |
| Rubber asphalt after 15 freeze–thaw cycles | 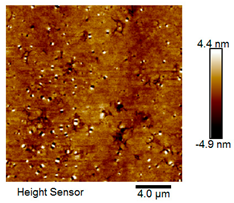 | 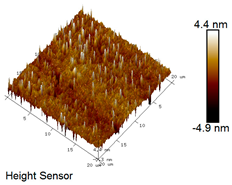 |
| Number of Salt Solution Molecules | Diffusion Coefficient (10−8 cm2/s) | |||
|---|---|---|---|---|
| Asphaltene | Aromatics | Saturates | Resin | |
| 100 | 2.55 | 2.74 | 2.91 | 2.26 |
| 150 | 2.95 | 3.98 | 3.05 | 3.01 |
| 200 | 3.27 | 5.06 | 5.45 | 4.38 |
| 250 | 5.82 | 6.99 | 7.96 | 6.44 |
| 300 | 7.65 | 8.54 | 10.16 | 9.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Si, Y.; Wang, S.; Li, P.; Zhang, K.; Zhu, Y. Multiscale Investigation of Interfacial Behaviors in Rubber Asphalt–Aggregate Systems Under Salt Erosion: Insights from Laboratory Tests and Molecular Dynamics Simulations. Materials 2025, 18, 4746. https://doi.org/10.3390/ma18204746
Li Y, Si Y, Wang S, Li P, Zhang K, Zhu Y. Multiscale Investigation of Interfacial Behaviors in Rubber Asphalt–Aggregate Systems Under Salt Erosion: Insights from Laboratory Tests and Molecular Dynamics Simulations. Materials. 2025; 18(20):4746. https://doi.org/10.3390/ma18204746
Chicago/Turabian StyleLi, Yun, Youxiang Si, Shuaiyu Wang, Peilong Li, Ke Zhang, and Yuefeng Zhu. 2025. "Multiscale Investigation of Interfacial Behaviors in Rubber Asphalt–Aggregate Systems Under Salt Erosion: Insights from Laboratory Tests and Molecular Dynamics Simulations" Materials 18, no. 20: 4746. https://doi.org/10.3390/ma18204746
APA StyleLi, Y., Si, Y., Wang, S., Li, P., Zhang, K., & Zhu, Y. (2025). Multiscale Investigation of Interfacial Behaviors in Rubber Asphalt–Aggregate Systems Under Salt Erosion: Insights from Laboratory Tests and Molecular Dynamics Simulations. Materials, 18(20), 4746. https://doi.org/10.3390/ma18204746







