Optimized Optical and Thermal Properties of Al-Pigmented Low-Emissivity Coatings by CuCr2O4 Powder
Abstract
1. Introduction
2. Experimental and Characterization
2.1. Experimental Materials
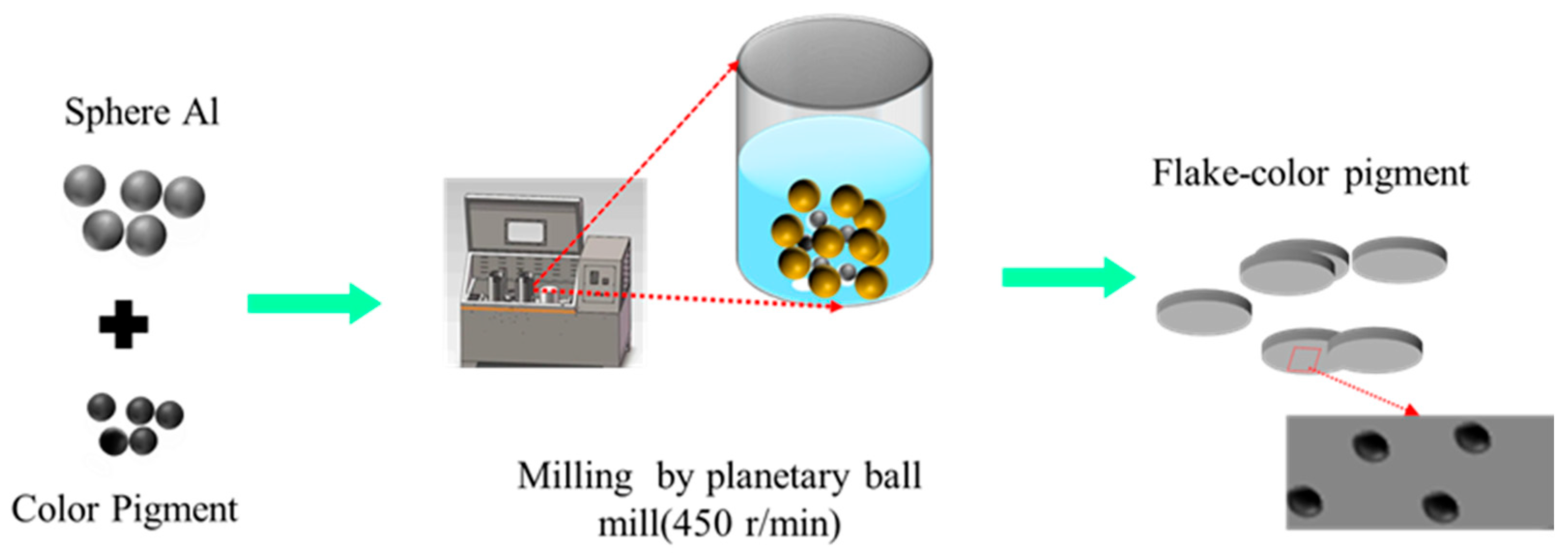
2.2. Preparation of Composite Pigments and Coatings
2.3. Characterization and Testing
3. Results and Discussion
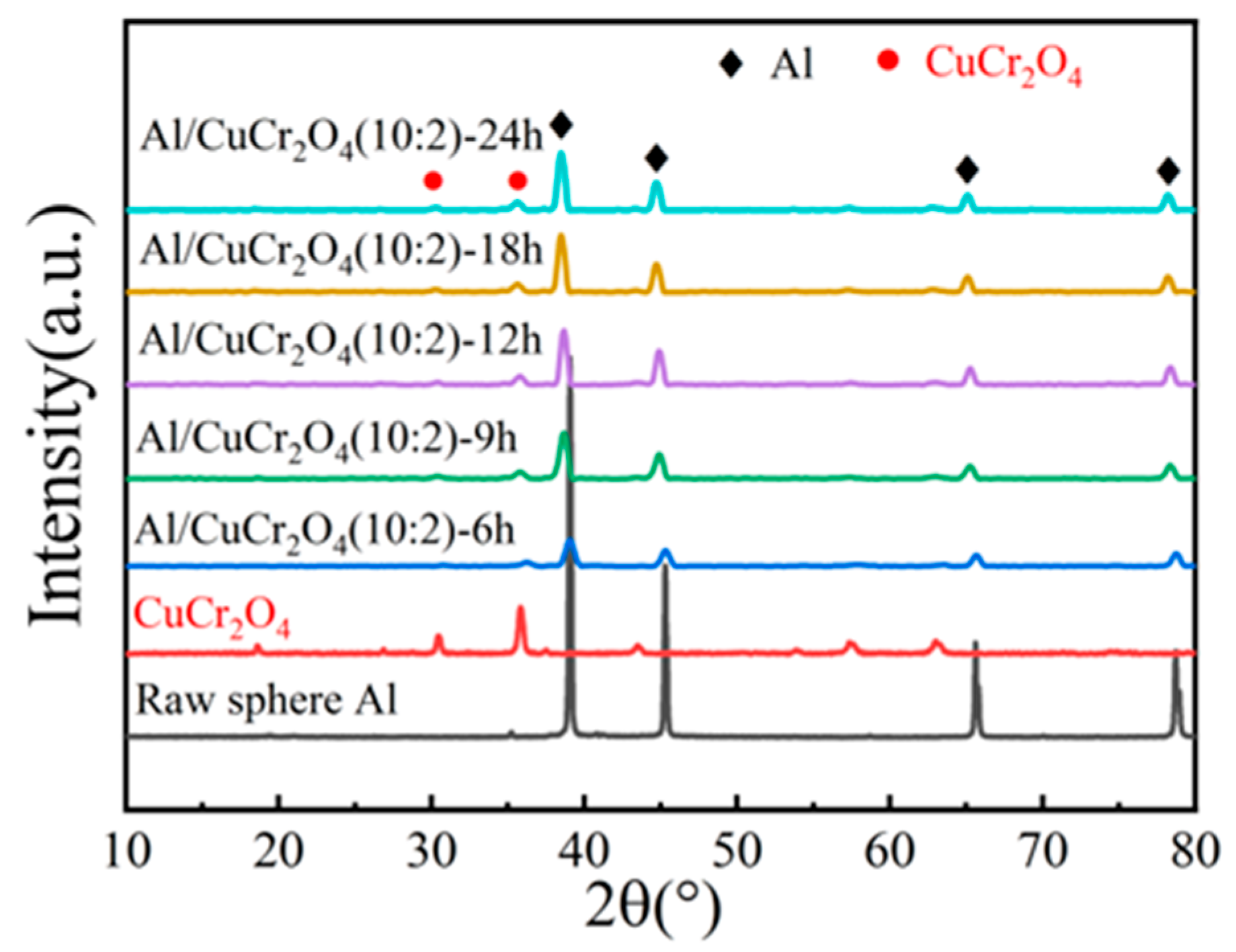
3.1. The XRD Patterns of Composite Powders
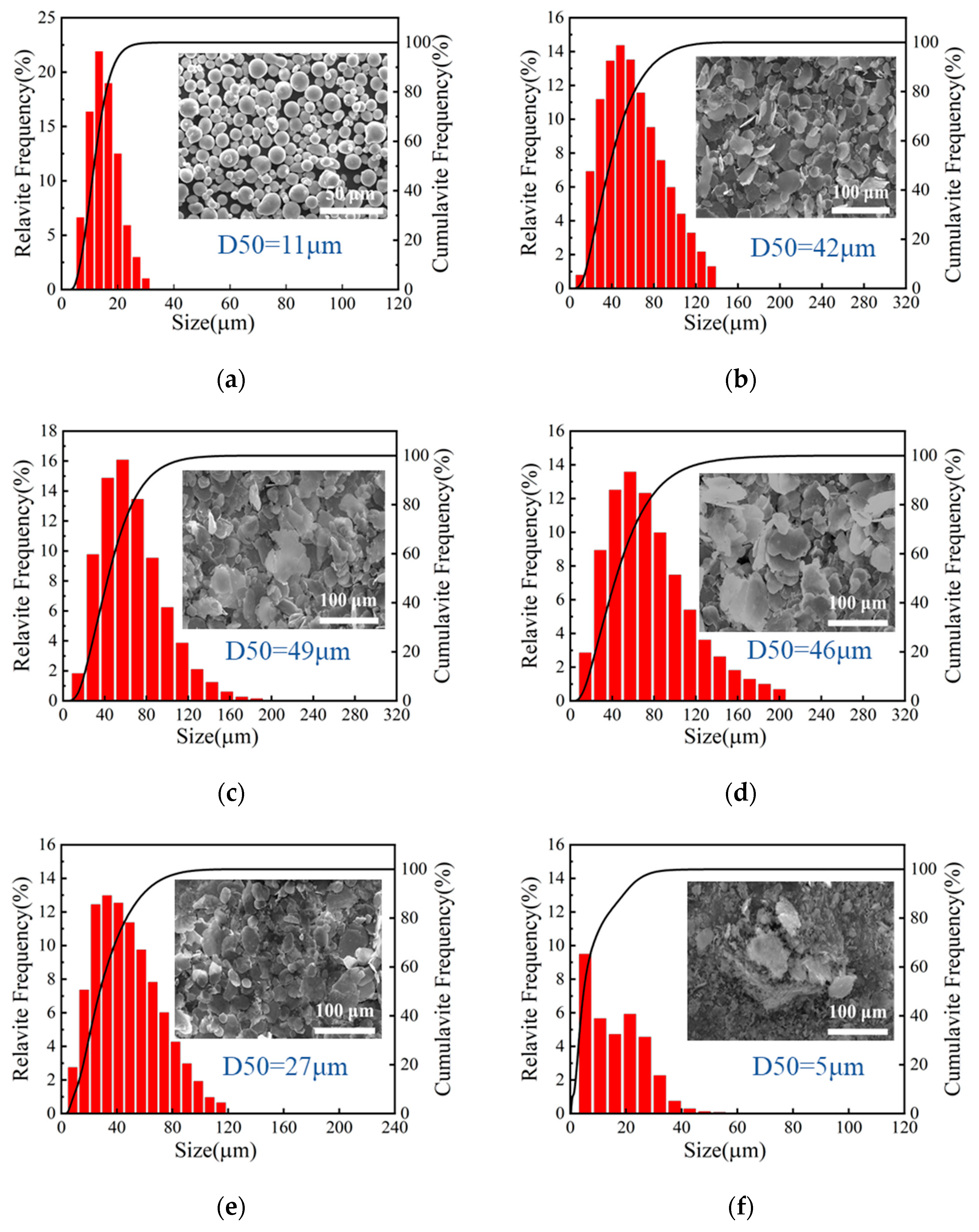
3.2. The Morphology and Size Distribution of Composite Powders
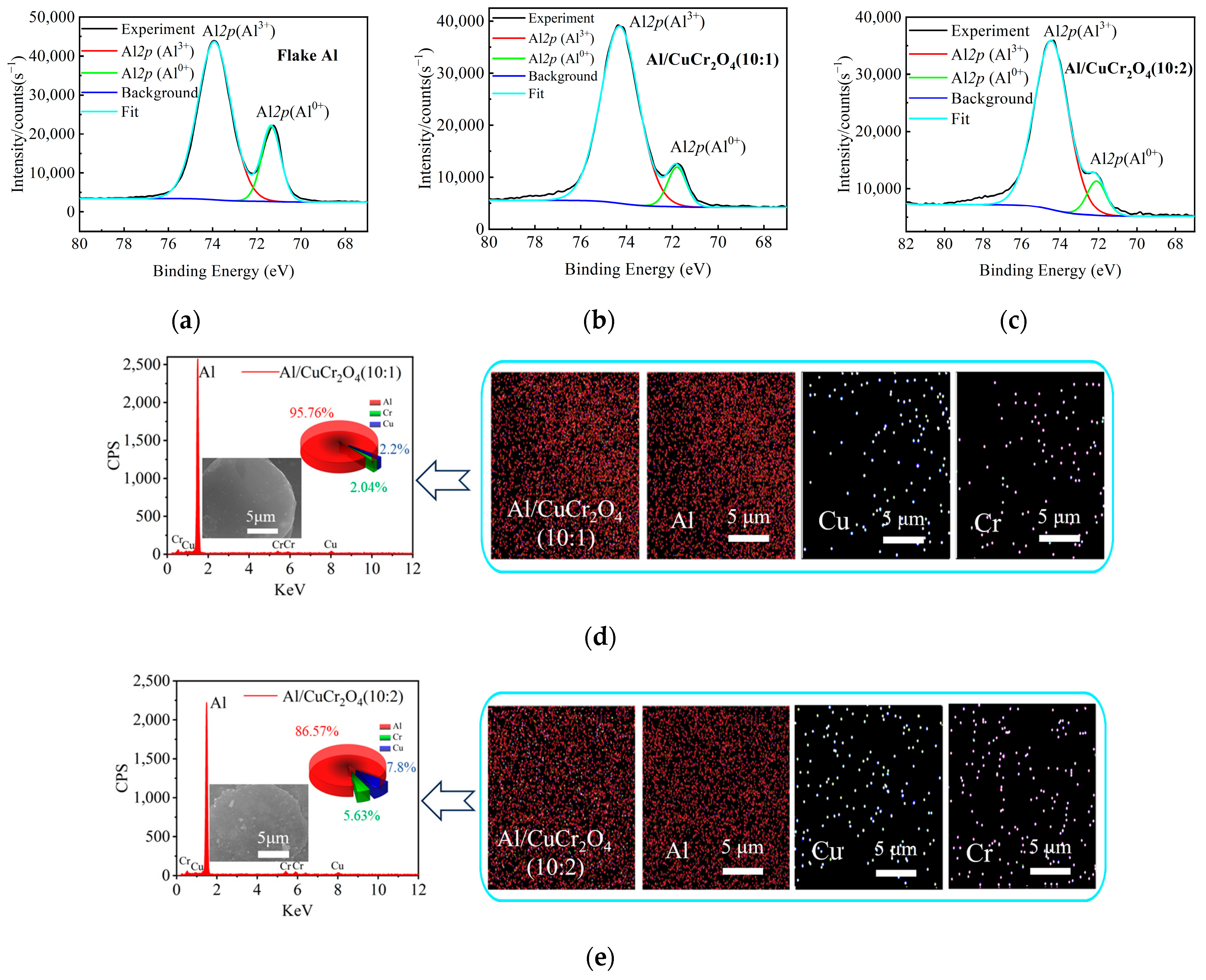
3.3. The XPS and EDS Analysis of Composite Powders
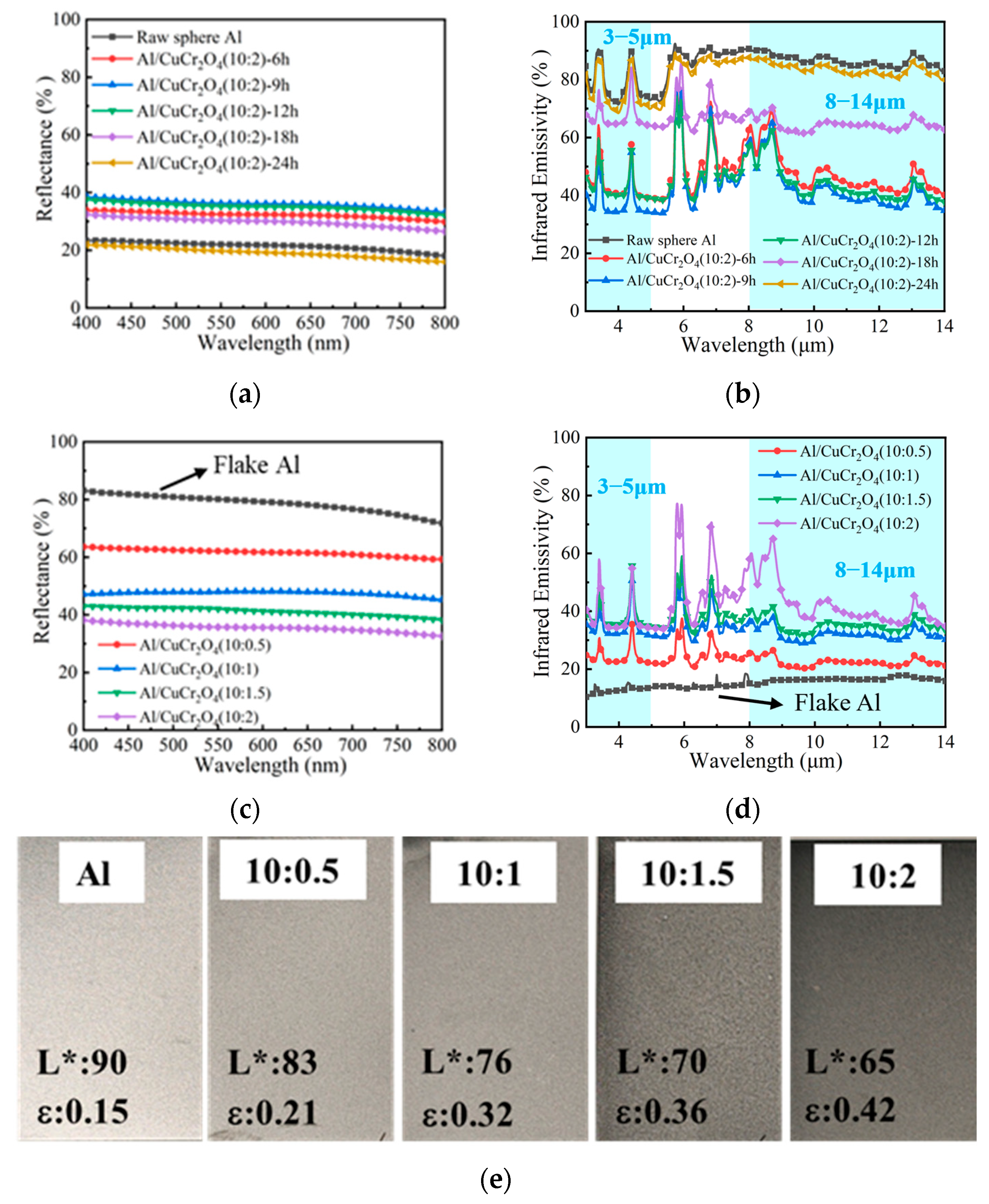
3.4. The Optical and Infrared Properties of Composite Coatings
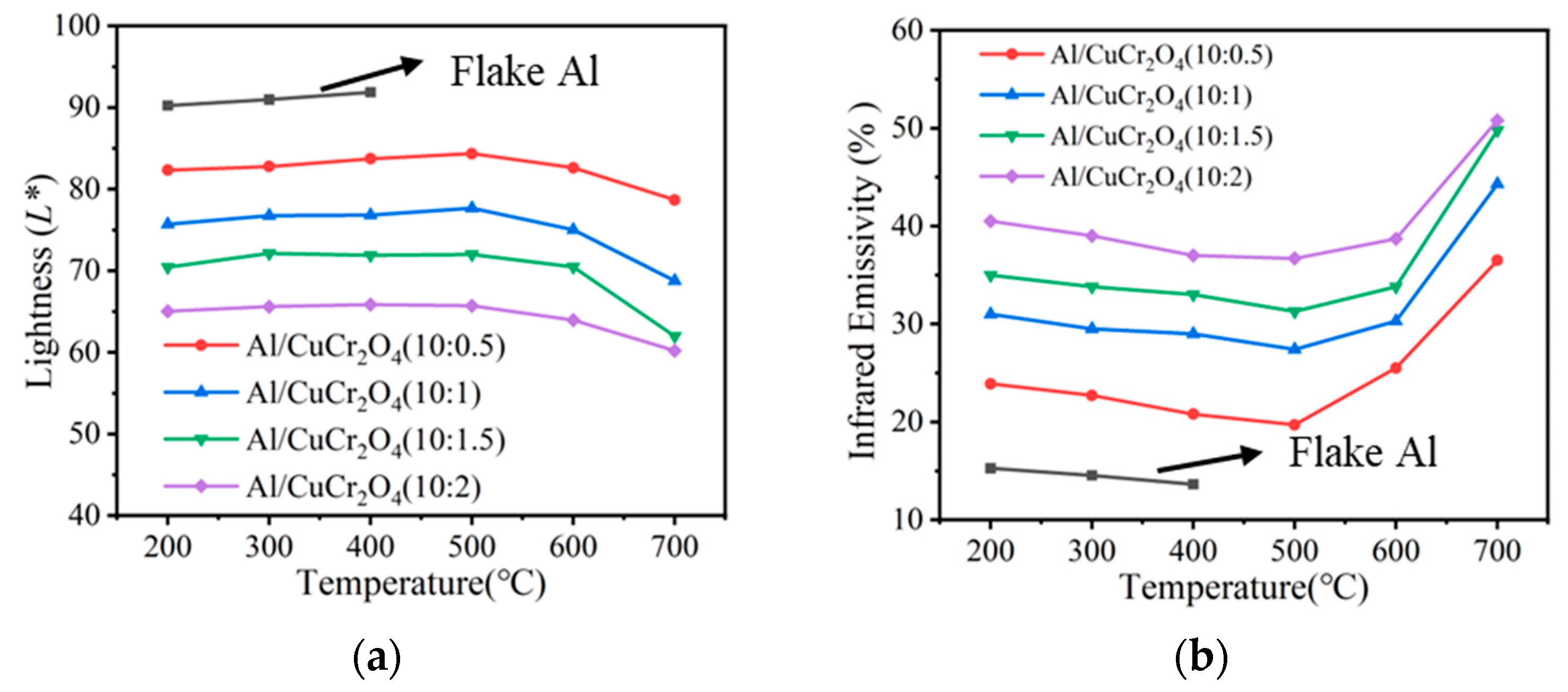
3.5. The Thermal Resistance of Composite Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fang, S.; Wang, W.; Yu, X.; Xu, H.; Zhong, Y.; Sui, X.; Zhang, L.; Mao, Z. Preparation of ZnO:(Al, La)/polyacrylonitrile (PAN) nonwovens with low infrared emissivity via electrospinning. Mater. Lett. 2015, 143, 120–123. [Google Scholar] [CrossRef]
- Fettis, G. Automotive Paints and Coatings, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Uemoto, K.L.; Sato, N.M.N.; John, V.M. Estimating thermal performance of cool colored paints. Energy Build. 2010, 42, 17–22. [Google Scholar] [CrossRef]
- Qi, L.; Weng, X.; Yuan, L.; Wei, B.; Wu, X.; Huang, G.; Du, X.; Liu, H. Improved thermal expansion performance of Aluminum/polysiloxane/glass coatings with low infrared emissivity by zinc powder. Infrared Phys. Technol. 2020, 110, 103458. [Google Scholar] [CrossRef]
- Maile, F.J.; Pfaff, G.; Reynders, P. Effect pigments—Past, present and future. Prog. Org. Coat. 2005, 54, 150–163. [Google Scholar] [CrossRef]
- Gunde, M.K.; Kunaver, M. Infrared Reflection–Absorption Spectra of Metal-Effect Coatings. Appl. Spectrosc. 2003, 57, 1266. [Google Scholar] [CrossRef] [PubMed]
- Manara, J.; Reidinger, M.; Rydzek, M.; Arduini-Schuster, M. Polymer-based pigmented coatings on flexible substrates with spectrally selective characteristics to improve the thermal properties. Prog. Org. Coat. 2011, 70, 199–204. [Google Scholar] [CrossRef]
- Qi, L.; Xiang, Z.; Weng, X.; Wang, Y.; Wu, X.; Yuan, L. Effect of high-temperature compositional and structural evolution of the polysiloxane coating with glass/Al powder on the infrared and mechanical properties. Polym. Degrad. Stab. 2023, 215, 110461. [Google Scholar] [CrossRef]
- Yuan, L.; Weng, X.; Du, W. Optical and magnetic properties of Al/Fe3O4 core-shell low infrared emissivity pigments. J. Alloys Compd. 2014, 583, 125–132. [Google Scholar] [CrossRef]
- Yuan, L.; Weng, X.; Xie, J.; Du, W.; Deng, L. Solvothermal synthesis and visible/infrared optical properties of Al/Fe3O4 core–shell magnetic composite pigments. J. Alloys Compd. 2013, 580, 108–113. [Google Scholar] [CrossRef]
- Liu, Y.F.; Xie, J.L.; Luo, M.; Peng, B.; Deng, L.J. Synthesis and characterization of magnetic Al/NiO composite pigments with low infrared emissivity. Mater. Sci. Forum 2017, 898, 1561–1568. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, J.; Luo, M.; Jian, S.; Peng, B.; Deng, L. The synthesis and characterization of Al/Co3O4 magnetic composite pigments with low infrared emissivity and low lightness. Infrared Phys. Technol. 2017, 83, 88–93. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, J.; Luo, M.; Peng, B.; Xu, C.; Deng, L. The synthesis and optical properties of Al/MnO2 composite pigments by ball-milling for low infrared emissivity and low lightness. Prog. Org. Coat. 2017, 108, 30–35. [Google Scholar] [CrossRef]
- Wu, G.; Yu, D. Preparation and characterization of a new low infrared-emissivity coating based on modified aluminum. Prog. Org. Coat. 2013, 76, 107–112. [Google Scholar] [CrossRef]
- Luoyang Institute of Advanced Technology. Temperature-Resistant Infrared Low-Emissivity Coating and Preparation Method Thereof. CN109423196A, 5 March 2019.
- Hu, C.; Xu, G.; Shen, X.; Shao, C.; Yan, X. The epoxy-siloxane/Al composite coatings with low infrared emissivity for high temperature applications. Appl. Surf. Sci. 2010, 256, 3459–3463. [Google Scholar] [CrossRef]
- Guo, T.; Xu, G.Y.; Chen, Y.; Hu, C.; Wang, Y. Preparation of heat-resistant coatings with low infrared emissivity and mechanism. Materials Reports 2011, 25, 96–99. [Google Scholar]
- Mou, W.; Li, H.-M.; Xu, J. Research on the oxidation characteristics of aluminum flakes of DSC/DTG method. J. Saf. Environ. 2013, 2, 45. [Google Scholar]
- Hu, C.; Xu, G.; Shen, X. Preparation and characteristics of thermal resistance polysiloxane/Al composite coatings with low infrared emissivity. J. Alloys Compd. 2009, 486, 371–375. [Google Scholar] [CrossRef]
- Zhao, J.; Luo, W.; Qi, L.; Yuan, L.; Huang, G.; Huang, Y.; Weng, X. The high-temperature resistance properties of polysiloxane/Al coatings with low infrared emissivity. Coatings 2018, 8, 125. [Google Scholar] [CrossRef]
- Kareiva, A.; Beganskiene, A.; Senvaitiene, J.; Ramanaviciene, A.; Vaitkus, R.; Barkauskas, J.; Ramanavicius, A. Evaluation of carbon-based nanostructures suitable for the development of black pigments and glazes. Colloids Surf. A Physicochem. Eng. Asp. 2019, 580, 123718. [Google Scholar] [CrossRef]
- Krause, M.; Sonnenberg, J.; Munnik, F.; Grenzer, J.; Hübner, R.; Garcia-Valenzuela, A.; Gemming, S. Formation, structure, and optical properties of copper chromite thin films for high-temperature solar absorbers. Materialia 2021, 18, 101156. [Google Scholar] [CrossRef]
- Goldstein, J.I.; Newbury, D.E.; Echlin, P.; Joy, D.C.; Romig, A.D.; Lyman, C.E.; Fiori, C.; Lifshin, E. Scanning Electron Microscopy and X-Ray Microanalysis, 2nd ed.; Springer: Boston, MA, USA, 1992. [Google Scholar] [CrossRef]
- Bearden, J.A.; Burr, A.F. Reevaluation of X-ray atomic energy levels. Rev. Mod. Phys. 1967, 39, 125–142. [Google Scholar] [CrossRef]
- Yuan, L.; Weng, X.; Deng, L. Calculation of infrared properties of low emissivity coatings containing metallic flake pigments. Chin. Opt. Lett. 2013, 11 (Suppl. S1), 010104–310107. [Google Scholar] [CrossRef]
- Elmanovich, I.V.; Sizov, V.E.; Zefirov, V.V.; Kalinina, A.A.; Gallyamov, M.O.; Papkov, V.S.; Muzafarov, A.M. Chemical recycling of high-molecular-weight organosilicon compounds in supercritical fluids. Polymers 2022, 14, 5170. [Google Scholar] [CrossRef]
- Hayashi, T.; Shimojima, A. Self-healing materials based on dynamic properties of siloxane networks. J. Sol-Gel Sci. Technol. 2025, 1–15. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Matsuno, T.; Shimojima, A. Multilayered organosiloxane films with self-healing ability converted from block copolymer nanocomposites. Chem. Commun. 2025, 61, 3319–3322. [Google Scholar] [CrossRef] [PubMed]
- GB/T 9286-2021; Paints and Varnishes—Cross-Cut Test. China Standards Press: Beijing, China, 2021.

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Weng, X.; Wei, B.; Zhang, M.; Qi, L.; Wang, Y.; Yuan, L.; Qing, X.; Luo, W. Optimized Optical and Thermal Properties of Al-Pigmented Low-Emissivity Coatings by CuCr2O4 Powder. Materials 2025, 18, 4717. https://doi.org/10.3390/ma18204717
Ma X, Weng X, Wei B, Zhang M, Qi L, Wang Y, Yuan L, Qing X, Luo W. Optimized Optical and Thermal Properties of Al-Pigmented Low-Emissivity Coatings by CuCr2O4 Powder. Materials. 2025; 18(20):4717. https://doi.org/10.3390/ma18204717
Chicago/Turabian StyleMa, Xiaodong, Xiaolong Weng, Biao Wei, Min Zhang, Lun Qi, Yaqin Wang, Le Yuan, Xiaolong Qing, and Wei Luo. 2025. "Optimized Optical and Thermal Properties of Al-Pigmented Low-Emissivity Coatings by CuCr2O4 Powder" Materials 18, no. 20: 4717. https://doi.org/10.3390/ma18204717
APA StyleMa, X., Weng, X., Wei, B., Zhang, M., Qi, L., Wang, Y., Yuan, L., Qing, X., & Luo, W. (2025). Optimized Optical and Thermal Properties of Al-Pigmented Low-Emissivity Coatings by CuCr2O4 Powder. Materials, 18(20), 4717. https://doi.org/10.3390/ma18204717






