Characterization of Lignocellulosic Byproducts from the Portuguese Forest: Valorization and Sustainable Use
Highlights
- Portuguese forest byproducts have been assessed as novel bioadsorbents.
- Physicochemical profiling highlights adsorption key factors.
- There is promising use in wastewater treatment applications.
- Supports sustainable and circular economy practices.
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
- branches of maritime pine and stone pine obtained as residues from tree pruning and collected in Viseu, Portugal; the branches had diameters between 1 and 6 cm, and were separated by diameter in two classes: large (4–6 cm) and small (1–3 cm) branches, and processed whole, i.e., wood and bark. The samples were identified as the following: MPLB (maritime pine large branches), MPSB (maritime pine small branches), MPN (maritime pine needles), SPLB (stone pine large branches), SPSB (stone pine small branches), and SPN (stone pine needles).
- Acacia branches obtained from harvested trees, resulting from control and removal operations made in the region of Viseu; the material was processed in the same way as the pine samples, including wood and bark, in two diameter classes: 1–3 and 4–6 cm. The samples were identified as the following: ALB (acacia large branches), ASB (acacia small branches) and AL (acacia leaves).
2.2. Chemical Composition
2.3. Ash Content and Composition
2.4. Point of Zero Charge
2.5. TGA, XRD, SEM-EDS and ATR-FTIR Analysis
3. Results
3.1. Chemical Composition, Ash, and Cationic Content
3.2. Point of Zero Charge
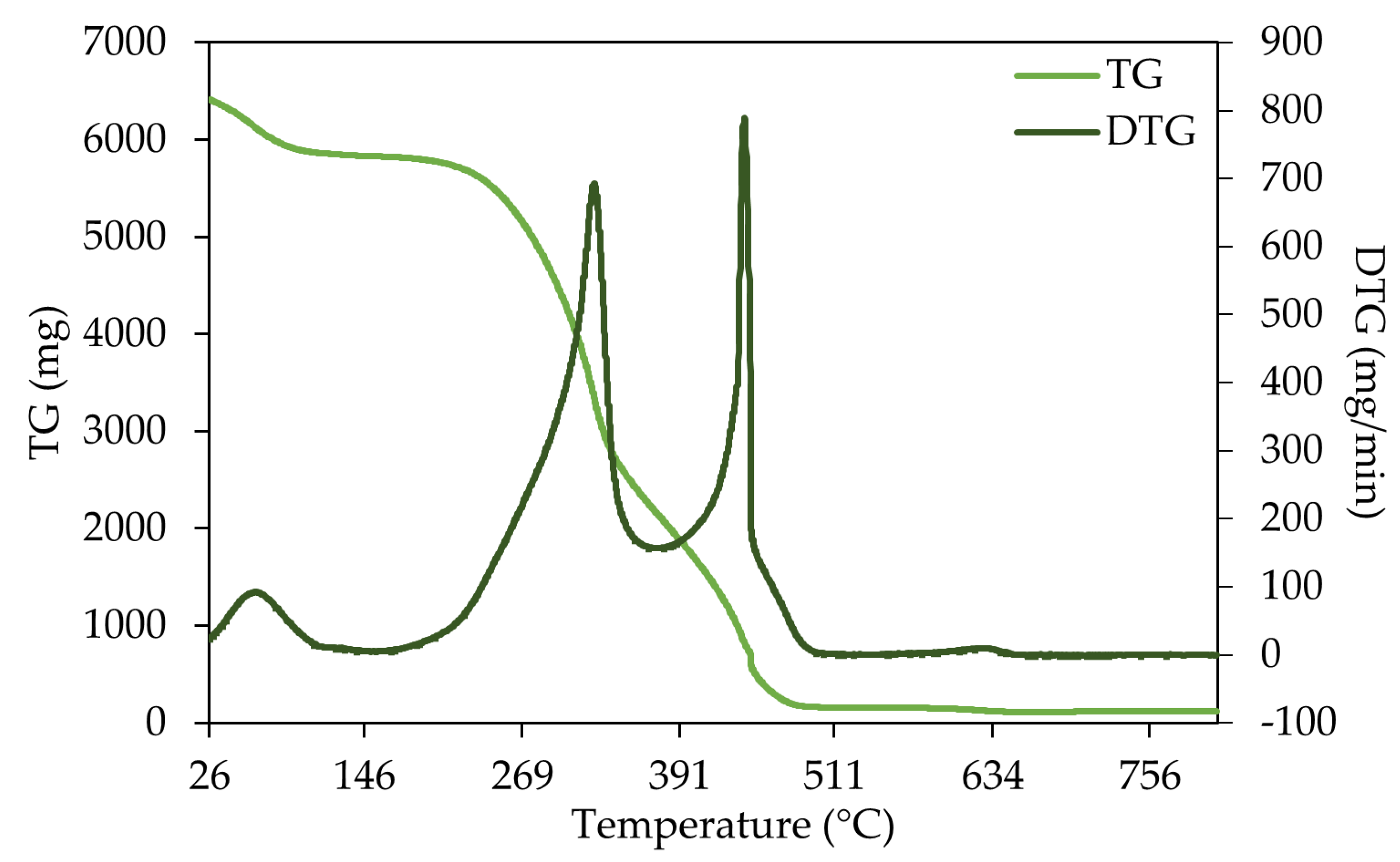
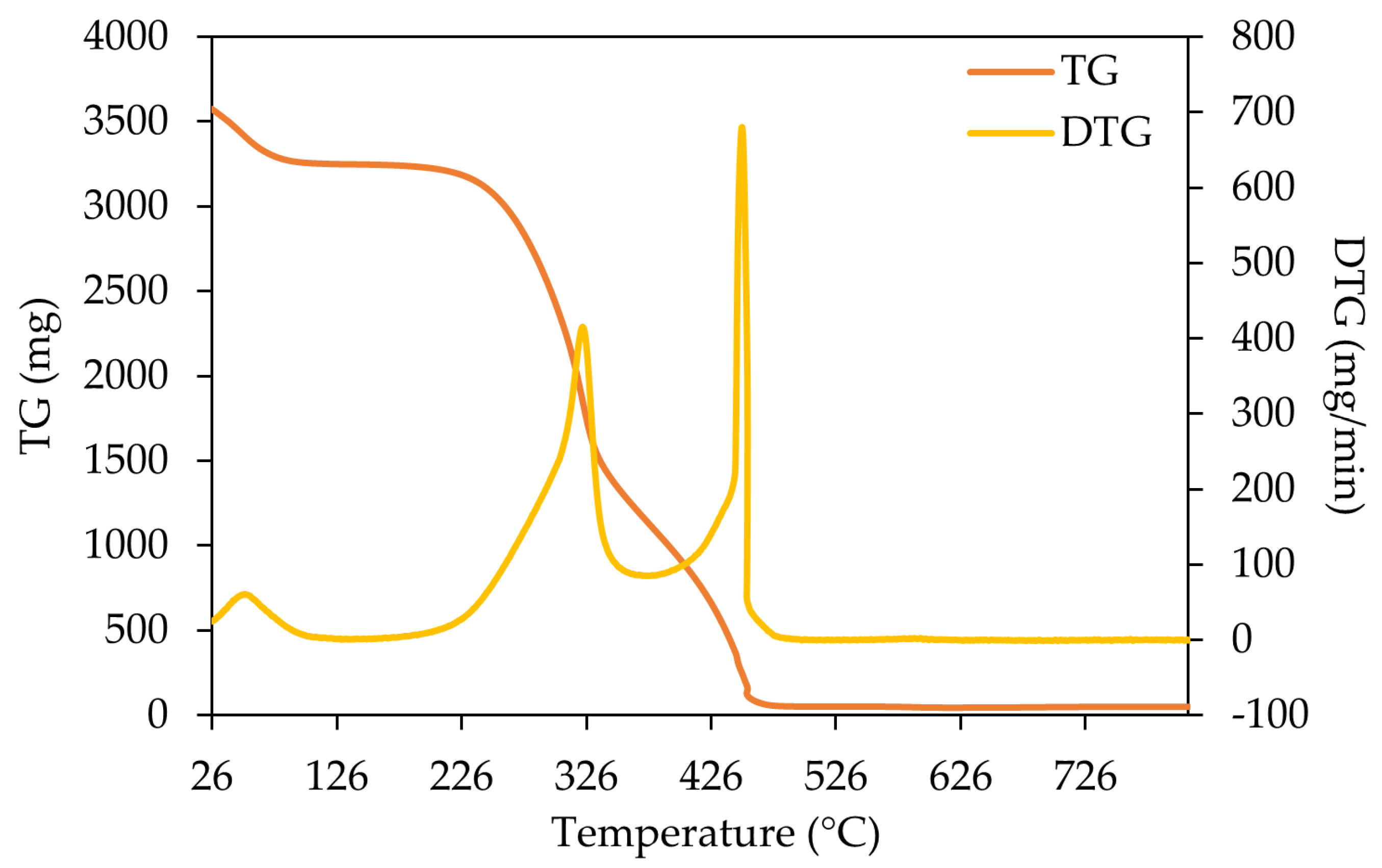
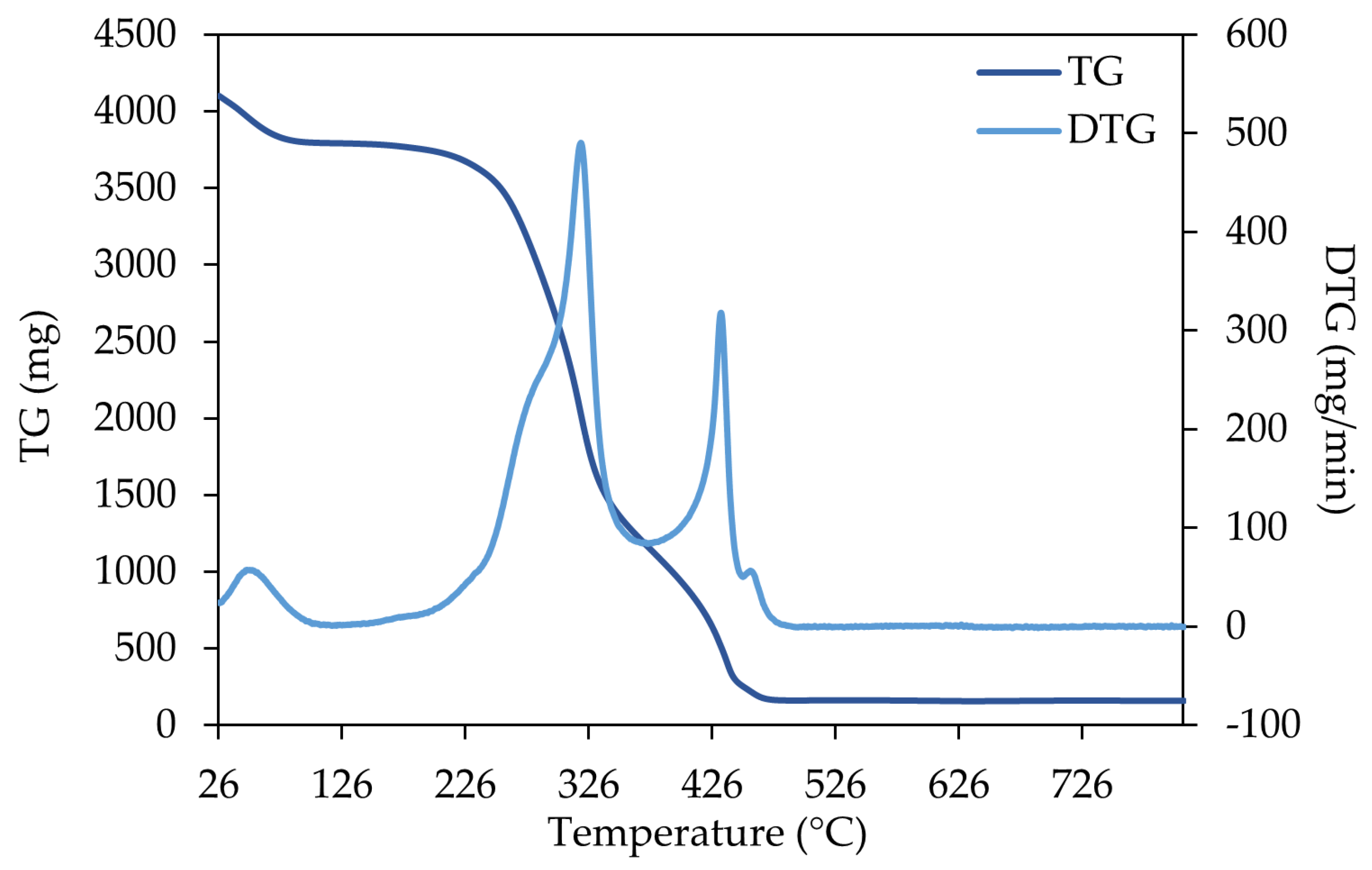
3.3. Thermogravimetric Analysis (TGA)
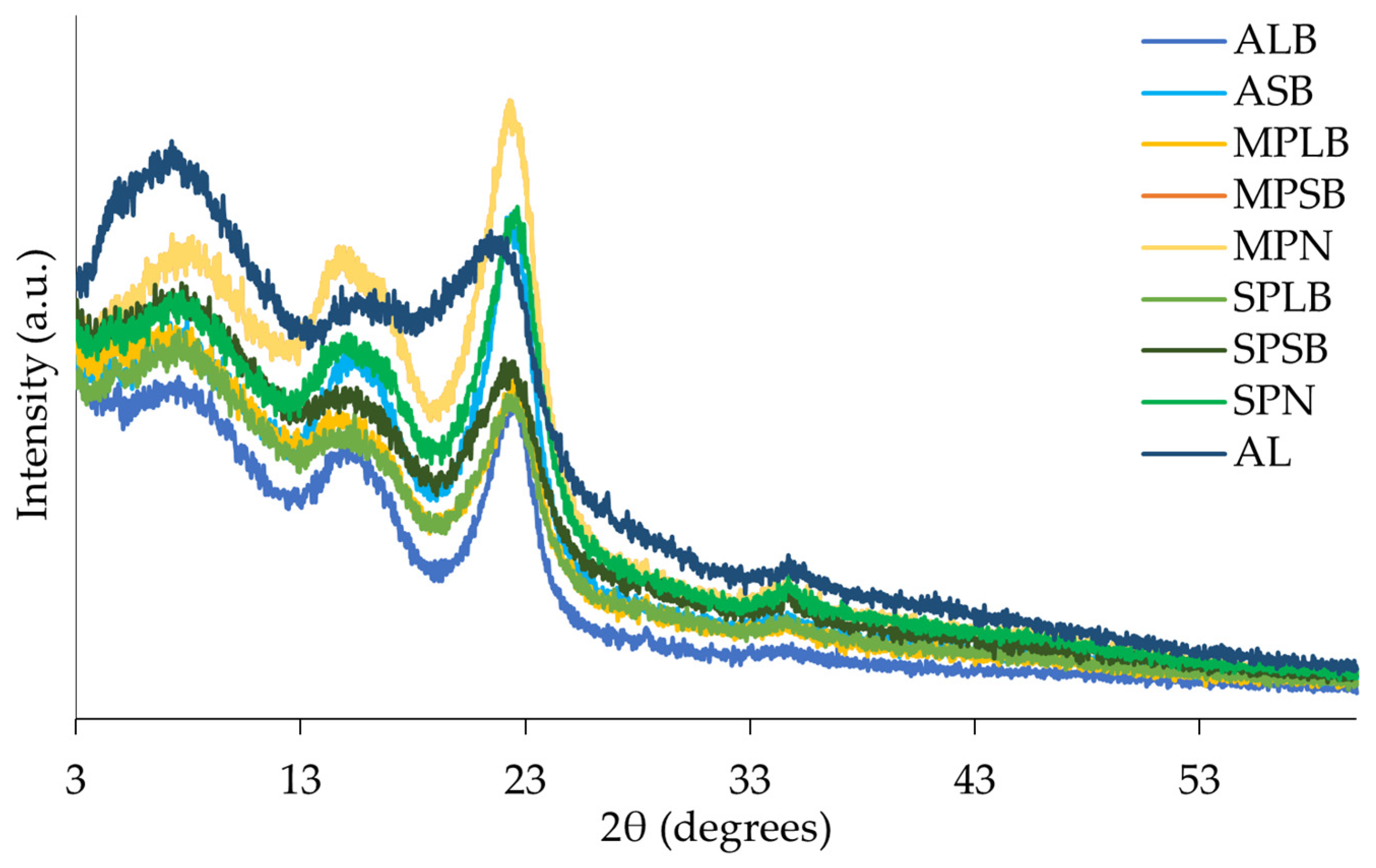
3.4. Powder X-Ray Diffraction (XRD)
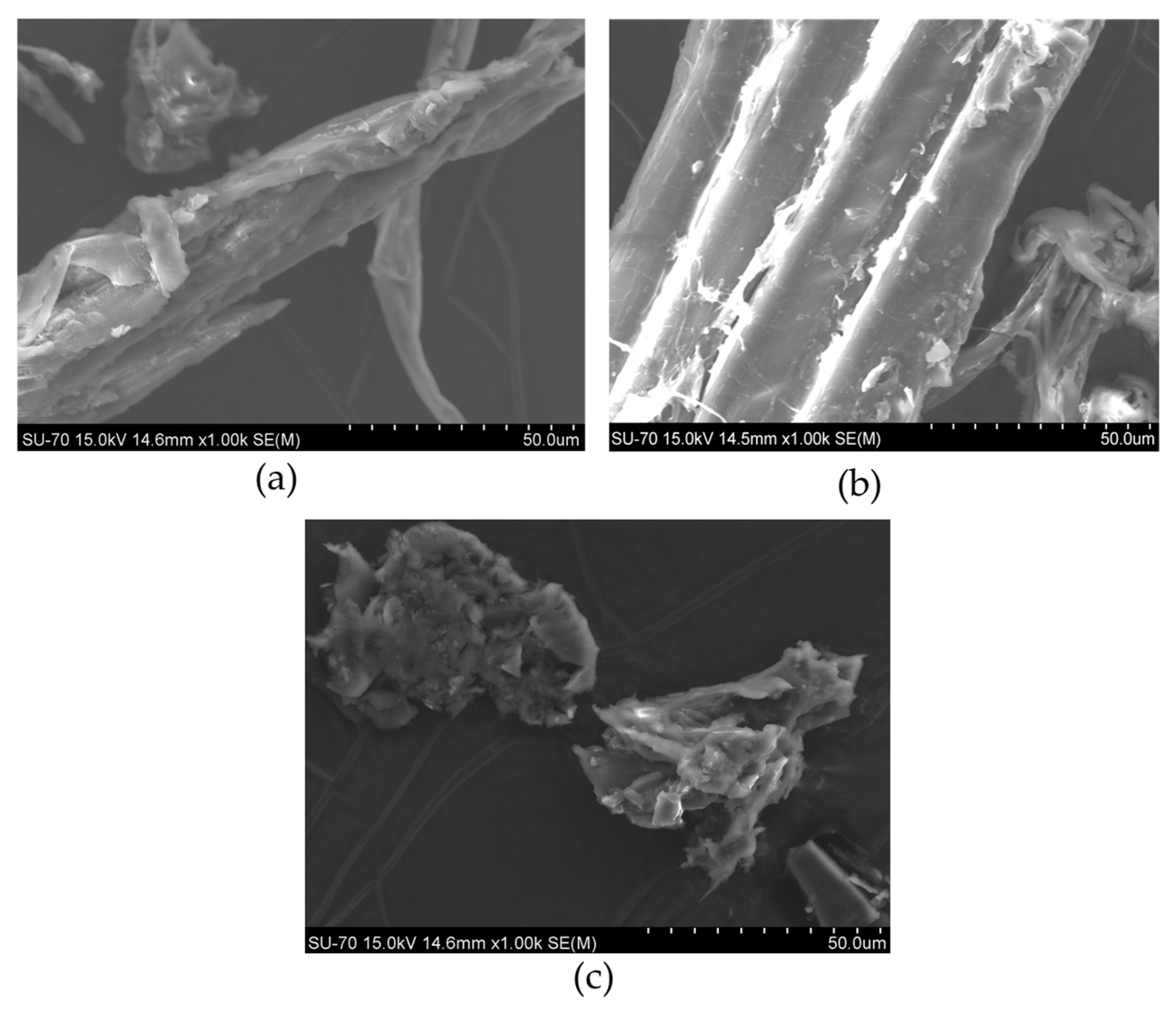
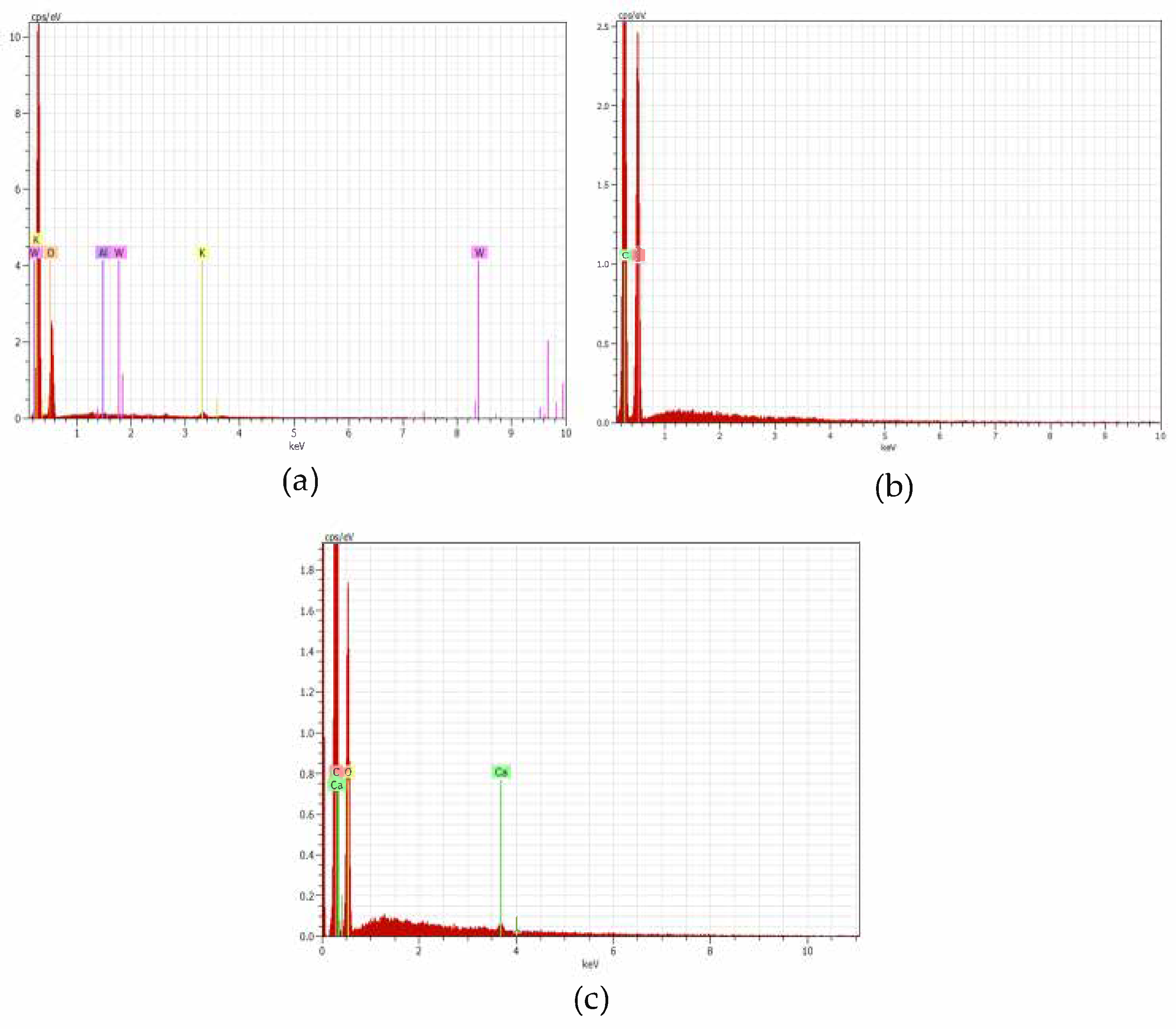
3.5. Scanning Electron Microscopy–Energy Dispersive X-Ray Spectroscopy (SEM-EDS) Analysis
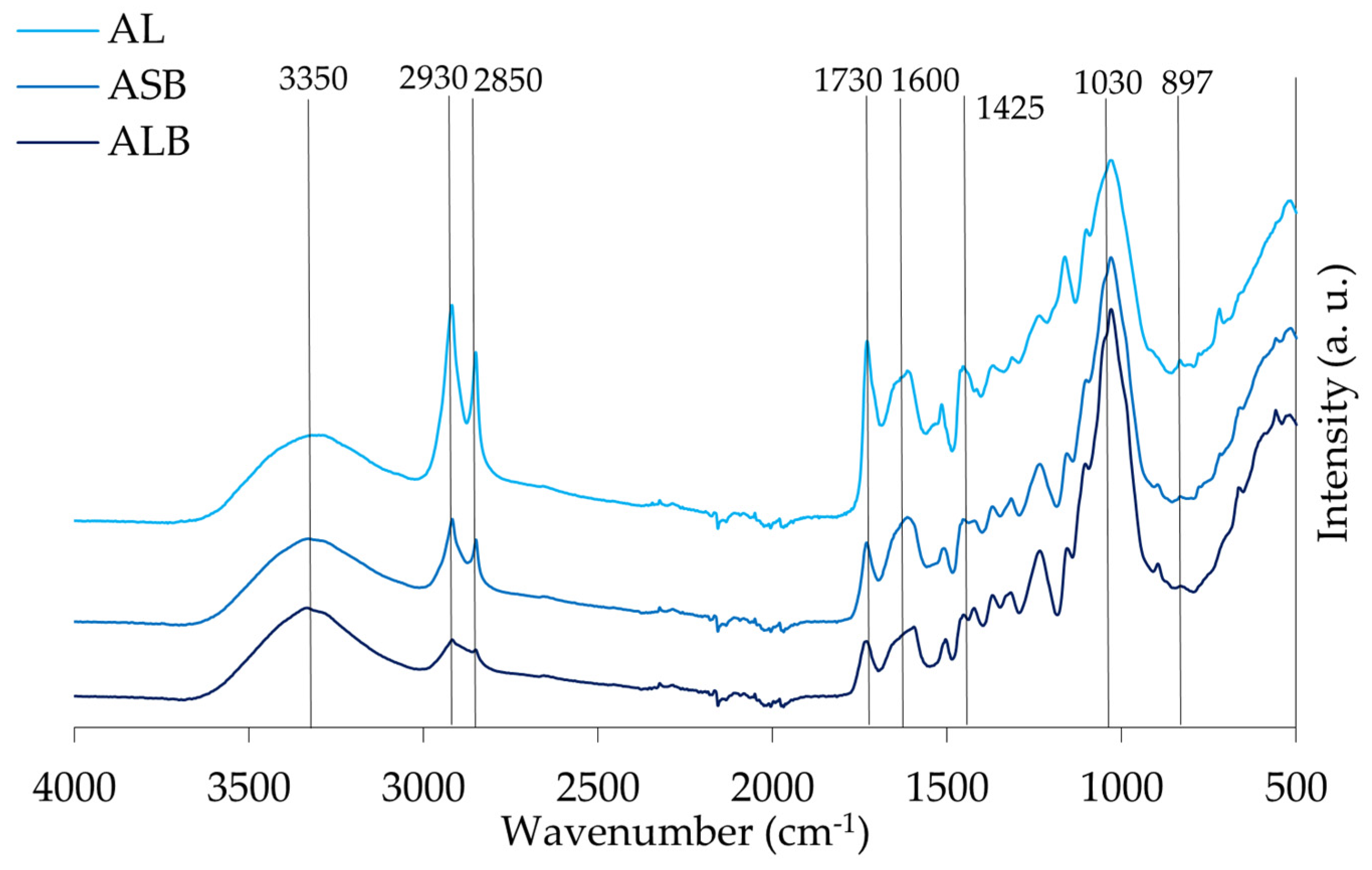
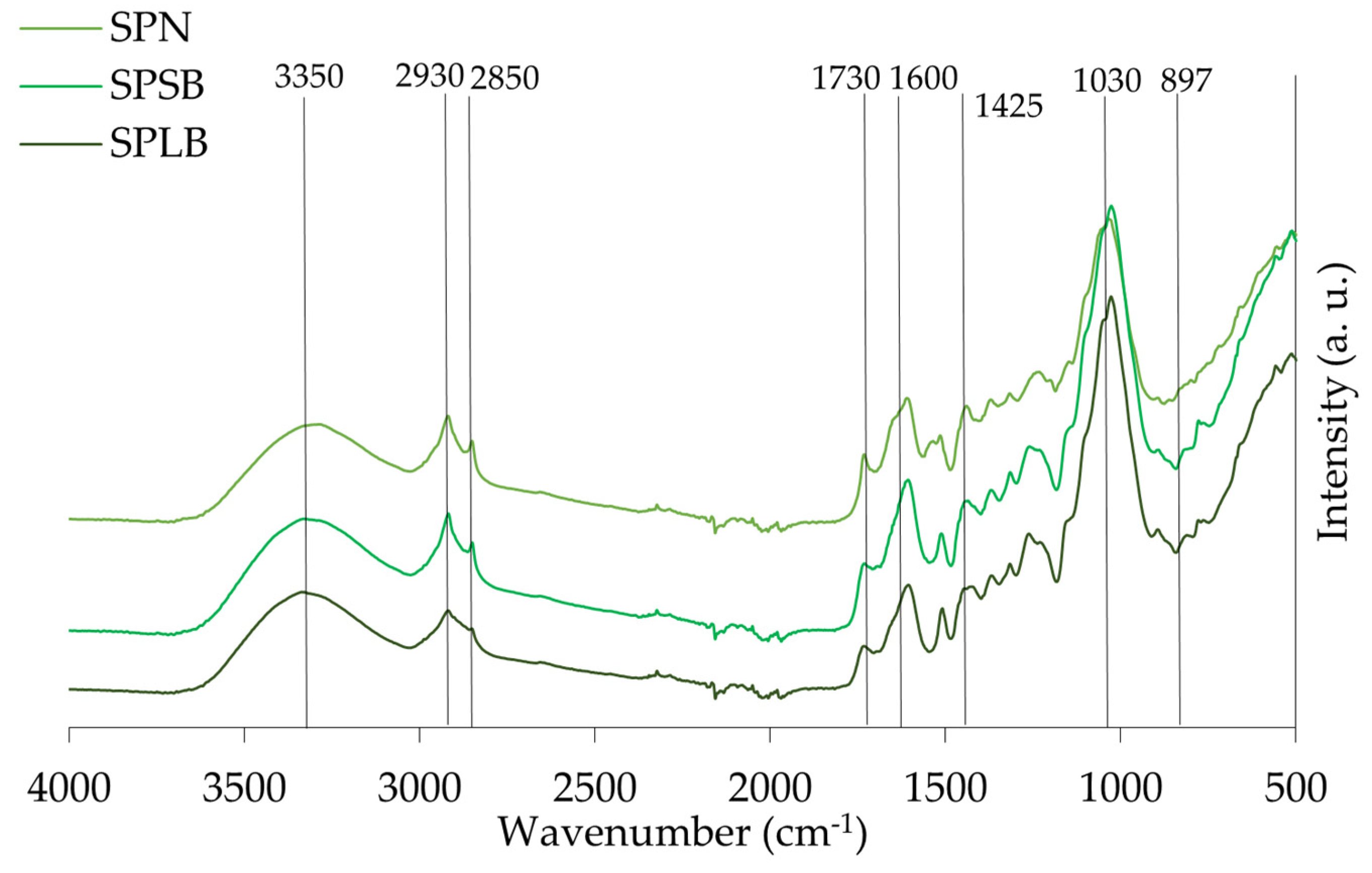
3.6. Transform Infrared Spectroscopy (ATR-FTIR) Characterization
4. Discussion
4.1. Chemical Composition
4.2. Point of Zero Charge (pHpzc)
4.3. TGA, XRD, SEM-EDS, and ATR-FTIR
4.3.1. Thermogravimetric Analysis (TGA)
4.3.2. Powder X-Ray Diffraction (XRD)
4.3.3. Scanning Electron Microscopy–Energy Dispersive X-Ray Spectroscopy (SEM-EDS)
4.3.4. Transform Infrared Spectroscopy (ATR-FTIR)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ICNF. Perfil Florestal—Portugal; Instituto da Conservação da Natureza e das Florestas: Oeiras, Portugal, 2021; p. 4. [Google Scholar]
- Nunes, L.J.R.; Meireles, C.I.R.; Pinto Gomes, C.J.; Almeida Ribeiro, N.M.C. Historical Development of the Portuguese Forest: The Introduction of Invasive Species. Forests 2019, 10, 974. [Google Scholar] [CrossRef]
- ICNF. 6° Inventário Florestal Nacional—Relatório Final; IFN; Instituto da Conservação da Natureza e das Florestas: Lisboa, Portugal, 2019. [Google Scholar]
- Sousa, J.L.C.; Ramos, P.A.B.; Freire, C.S.R.; Silva, A.M.S.; Silvestre, A.J.D. Chemical Composition of Lipophilic Bark Extracts from Pinus pinaster and Pinus pinea Cultivated in Portugal. Appl. Sci. 2018, 8, 2575. [Google Scholar] [CrossRef]
- Costa, R.A.; Lourenço, A.; Patrício, H.; Quilhó, T.; Gominho, J. Valorization of Pine Nut Industry Residues on a Biorefinery Concept. Waste Biomass Valor 2023, 14, 4081–4099. [Google Scholar] [CrossRef]
- Observatório Técnico Independente; Castro Rego, F.; Fernandes, P.; Sande Silva, J.; Azevedo, J.; Moura, J.M.; Oliveira, E.; Cortes, R.; Viegas, D.X.; Caldeira, D.; et al. Redução Do Risco De Incêndio Através Da Utilização De Biomassa Lenhosa Para Energia; Assembleia da República: Lisboa, Portugal, 2020; p. 22. [Google Scholar]
- Fabre, E.; Vale, C.; Pereira, E.; Silva, C.M. Sustainable Water Treatment: Use of Agricultural and Industrial Wastes to Remove Mercury by Biosorption. Water Air Soil Pollut. 2021, 232, 284. [Google Scholar] [CrossRef]
- Correia, R.; Quintela, J.C.; Duarte, M.P.; Gonçalves, M. Insights for the Valorization of Biomass from Portuguese Invasive Acacia Spp. in a Biorefinery Perspective. Forests 2020, 11, 1342. [Google Scholar] [CrossRef]
- Raposo, M.A.M.; Pinto Gomes, C.J.; Nunes, L.J.R. Evaluation of Species Invasiveness: A Case Study with Acacia Dealbata Link. on the Slopes of Cabeça (Seia-Portugal). Sustainability 2021, 13, 11233. [Google Scholar] [CrossRef]
- Agarwal, A.; Upadhyay, U.; Sreedhar, I.; Singh, S.A.; Patel, C.M. A Review on Valorization of Biomass in Heavy Metal Removal from Wastewater. J. Water Process Eng. 2020, 38, 101602. [Google Scholar] [CrossRef]
- Barros, D.; Fernandes, É.; Jesus, M.; Barros, L.; Alonso-Esteban, J.I.; Pires, P.; Vaz Velho, M. The Chemical Characterisation of the Maritime Pine Bark Cultivated in Northern Portugal. Plants 2023, 12, 3940. [Google Scholar] [CrossRef] [PubMed]
- Braghiroli, F.L.; Passarini, L. Valorization of Biomass Residues from Forest Operations and Wood Manufacturing Presents a Wide Range of Sustainable and Innovative Possibilities. Curr. For. Rep. 2020, 6, 172–183. [Google Scholar] [CrossRef]
- Luzardo, F.H.M.; Velasco, F.G.; Alves, C.P.; Correia, I.K.D.S.; Cazorla, L.L. Chemical Characterization of Agroforestry Solid Residues Aiming Its Utilization as Adsorbents for Metals in Water. Rev. Bras. Eng. Agríc. Ambient. 2015, 19, 77–83. [Google Scholar] [CrossRef]
- Gundogdu, A.; Ozdes, D.; Duran, C.; Bulut, V.N.; Soylak, M.; Senturk, H.B. Biosorption of Pb(II) Ions from Aqueous Solution by Pine Bark (Pinus brutia Ten.). Chem. Eng. J. 2009, 153, 62–69. [Google Scholar] [CrossRef]
- Junior, A.C.G.; Strey, L.; Lindino, C.A.; Nacke, H.; Schwantes, D.; Seidel, E.P. Applicability of the Pinus Bark (Pinus elliottii) for the Adsorption of Toxic Heavy Metals from Aqueous Solutions. Acta Scientiarum. Technol. 2012, 34, 79–87. [Google Scholar] [CrossRef]
- Momčilović, M.; Purenović, M.; Bojić, A.; Zarubica, A.; Ranđelović, M. Removal of Lead(II) Ions from Aqueous Solutions by Adsorption onto Pine Cone Activated Carbon. Desalination 2011, 276, 53–59. [Google Scholar] [CrossRef]
- Taty-Costodes, V.C.; Fauduet, H.; Porte, C.; Delacroix, A. Removal of Cd(II) and Pb(II) Ions, from Aqueous Solutions, by Adsorption onto Sawdust of Pinus sylvestris. J. Hazard. Mater. 2003, 105, 121–142. [Google Scholar] [CrossRef]
- Macena, M.; Cruz-Lopes, L.; Grosche, L.; Esteves, B.; Santos-Vieira, I.; Pereira, H. Valorization of Pinecones as Biosorbents for Environmental Remediation of Zn-Contaminated Wastewaters. Environments 2025, 12, 284. [Google Scholar] [CrossRef]
- Terkhi, M.C.; Belhaine, A.; Abdelmalek, F.; Ghezzar, M.R.; Addou, A. Acacia saligna Leaves: A Potential New Low-Cost Adsorbent for Removal of Methylene Blue from Aqueous Solutions. Desalination Water Treat. 2023, 300, 178–191. [Google Scholar] [CrossRef]
- Adam, A.B.; Hyelalibia, A. Modification and Characterazation of Acacia Nilotica Leaves and Its Application in Water Treatment. Chem. Res. Technol. 2024, 1, 192–203. [Google Scholar] [CrossRef]
- T 204 Cm-17; Solvent Extractives of Wood and Pulp, Test Method. Technical Association of the Pulp & Paper Industry (TAPPI): Peachtree Corners, GA, USA, 2017.
- Browning, B.L. Methods of Wood Chemistry; Interscience Publishers: New York, NY, USA; London, UK; Sydney, Australia, 1967; Volume II. [Google Scholar]
- T 203 cm-22; Alpha-, Beta- and Gamma-Cellulose in Pulp. Technical Association of the Pulp & Paper Industry (TAPPI): Peachtree Corners, GA, USA, 2008.
- T 222 Om-21; Acid Insoluble Lignin in Wood and Pulp. Technical Association of the Pulp & Paper Industry (TAPPI): Peachtree Corners, GA, USA, 2002.
- Segal, L.; Creely, J.J.; Martin, A.E., Jr.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Pereira, P.H.; Voorwald, H.C.; Cioffi, M.O.; Pereira, M.L.D.S. Preparação e caracterização de materiais híbridos celulose/NbOPO4.nH2O a partir de celulose branqueada de bagaço de cana-de-açúcar. Polímeros 2012, 22, 88–95. [Google Scholar] [CrossRef]
- Ju, X.; Bowden, M.; Brown, E.E.; Zhang, X. An Improved X-Ray Diffraction Method for Cellulose Crystallinity Measurement. Carbohydr. Polym. 2015, 123, 476–481. [Google Scholar] [CrossRef]
- Barnette, A.L.; Lee, C.; Bradley, L.C.; Schreiner, E.P.; Park, Y.B.; Shin, H.; Cosgrove, D.J.; Park, S.; Kim, S.H. Quantification of Crystalline Cellulose in Lignocellulosic Biomass Using Sum Frequency Generation (SFG) Vibration Spectroscopy and Comparison with Other Analytical Methods. Carbohydr. Polym. 2012, 89, 802–809. [Google Scholar] [CrossRef]
- Santos, J.; Pereira, J.; Ferreira, N.; Paiva, N.; Ferra, J.; Magalhães, F.D.; Martins, J.M.; Dulyanska, Y.; Carvalho, L.H. Valorisation of Non-Timber by-Products from Maritime Pine (Pinus Pinaster, Ait) for Particleboard Production. Ind. Crops Prod. 2021, 168, 113581. [Google Scholar] [CrossRef]
- Ferreira, S.; Gil, N.; Queiroz, J.A.; Duarte, A.P.; Domingues, F.C. An Evaluation of the Potential of Acacia Dealbata as Raw Material for Bioethanol Production. Bioresour. Technol. 2011, 102, 4766–4773. [Google Scholar] [CrossRef]
- López-Hortas, L.; Rodríguez-González, I.; Díaz-Reinoso, B.; Torres, M.D.; Moure, A.; Domínguez, H. Tools for a Multiproduct Biorefinery of Acacia dealbata Biomass. Ind. Crops Prod. 2021, 169, 113655. [Google Scholar] [CrossRef]
- Lal, P.S.; Sharma, A.; Bist, V. Pine Needle—An Evaluation of Pulp and Paper Making Potential. J. For. Prod. Ind. 2013, 2, 42–47. [Google Scholar]
- Mertoglu Elmas, G.; Yılgor, N. Chemical and Thermal Characterizations of Pinus sylvestris and Pinus pinaster. BioResources 2020, 15, 3604–3620. [Google Scholar] [CrossRef]
- Gonultas, O.; Ucar, M.B. Chemical Characteristics of the Cone and Wood of Pinus pinea. Lignocellulose 2013, 2, 262–269. [Google Scholar]
- Nunes, E.; Quilhó, T.; Pereira, H. Anatomy and Chemical Composition of Pinus pinaster Bark. IAWA J. 1996, 17, 141–150. [Google Scholar] [CrossRef]
- Nunes, E.; Quilhó, T.; Pereira, H. Anatomy and Chemical Composition of Pinus pinea L. Bark. Ann. For. Sci. 1999, 56, 479–484. [Google Scholar] [CrossRef]
- Carreira, A.R.F.; Veloso, T.; Macário, I.P.E.; Pereira, J.L.; Ventura, S.P.M.; Passos, H.; Coutinho, J.A.P. The Role of Biomass Elemental Composition and Ion-Exchange in Metal Sorption by Algae. Chemosphere 2023, 314, 137675. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liang, N.; Jin, X.; Zhou, D.; Li, H.; Wu, M.; Pan, B. The Role of Ash Content on Bisphenol A Sorption to Biochars Derived from Different Agricultural Wastes. Chemosphere 2017, 171, 66–73. [Google Scholar] [CrossRef]
- Bakatula, E.N.; Richard, D.; Neculita, C.M.; Zagury, G.J. Determination of Point of Zero Charge of Natural Organic Materials. Environ. Sci. Pollut. Res. 2018, 25, 7823–7833. [Google Scholar] [CrossRef]
- Nordin, N.; Zakaria, Z.A.; Ahmad, W.A. Utilisation of Rubber Wood Shavings for the Removal of Cu(II) and Ni(II) from Aqueous Solution. Water Air Soil Pollut. 2012, 223, 1649–1659. [Google Scholar] [CrossRef]
- Abadian, S.; Shayesteh, H.; Rahbar-Kelishami, A. Effective Adsorption of Diclofenac Sodium from Aqueous Solution Using Cationic Surfactant Modified Cuminum Cyminum Agri-Waste: Kinetic, Equilibrium, and Thermodynamic Studies. Int. J. Phytoremediation 2023, 25, 840–850. [Google Scholar] [CrossRef]
- Naidu, R.; Bolan, N.S.; Kookana, R.S.; Tiller, K.G. Ionic-Strength and pH Effects on the Sorption of Cadmium and the Surface Charge of Soils. Eur. J. Soil Sci. 1994, 45, 419–429. [Google Scholar] [CrossRef]
- Cruz-Lopes, L.P.; Macena, M.; Esteves, B.; Guiné, R.P.F. Ideal pH for the Adsorption of Metal Ions Cr6+, Ni2+, Pb2+ in Aqueous Solution with Different Adsorbent Materials. Open Agric. 2021, 6, 115–123. [Google Scholar] [CrossRef]
- Macena, M.; Pereira, H.; Cruz-Lopes, L.; Grosche, L.; Esteves, B. Competitive Adsorption of Metal Ions by Lignocellulosic Materials: A Review of Applications, Mechanisms and Influencing Factors. Separations 2025, 12, 70. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of Hemicellulose, Cellulose and Lignin Pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Amaral, S.S.; de Carvalho Junior, J.A.; Costa, M.A.M.; Neto, T.G.S.; Dellani, R.; Leite, L.H.S. Comparative Study for Hardwood and Softwood Forest Biomass: Chemical Characterization, Combustion Phases and Gas and Particulate Matter Emissions. Bioresour. Technol. 2014, 164, 55–63. [Google Scholar] [CrossRef]
- Slopiecka, K.; Bartocci, P.; Fantozzi, F. Thermogravimetric Analysis and Kinetic Study of Poplar Wood Pyrolysis. Appl. Energy 2012, 97, 491–497. [Google Scholar] [CrossRef]
- Yao, F.; Wu, Q.; Lei, Y.; Guo, W.; Xu, Y. Thermal Decomposition Kinetics of Natural Fibers: Activation Energy with Dynamic Thermogravimetric Analysis. Polym. Degrad. Stab. 2008, 93, 90–98. [Google Scholar] [CrossRef]
- Xu, X.; Pan, R.; Chen, R. Comparative Thermal Degradation Behaviors and Kinetic Mechanisms of Typical Hardwood and Softwood in Oxygenous Atmosphere. Processes 2021, 9, 1598. [Google Scholar] [CrossRef]
- Veiga, T.R.L.A.; Lima, J.T.; Dessimoni, A.L.D.A.; Pego, M.F.F.; Soares, J.R.; Trugilho, P.F. Different plant biomass characterizations for biochar production. Cerne 2017, 23, 529–536. [Google Scholar] [CrossRef]
- Agarwal, U.P.; Ralph, S.A.; Baez, C.; Reiner, R.S.; Verrill, S.P. Effect of Sample Moisture Content on XRD-Estimated Cellulose Crystallinity Index and Crystallite Size. Cellulose 2017, 24, 1971–1984. [Google Scholar] [CrossRef]
- Ahvenainen, P.; Kontro, I.; Svedström, K. Comparison of Sample Crystallinity Determination Methods by X-Ray Diffraction for Challenging Cellulose I Materials. Cellulose 2016, 23, 1073–1086. [Google Scholar] [CrossRef]
- Melo, D.; do Nascimento, R.; Raulino, G.; Vidal, C.; Clecius, A. Adsorção: Aspectos Teóricos e Aplicações Ambientais, 2nd ed.; University Press: Fortaleza, Brazil, 2020; ISBN 978-65-990722-7-7. [Google Scholar]
- Popescu, C.-M.; Singurel, G.; Popescu, M.-C.; Vasile, C.; Argyropoulos, D.S.; Willför, S. Vibrational Spectroscopy and X-Ray Diffraction Methods to Establish the Differences between Hardwood and Softwood. Carbohydr. Polym. 2009, 77, 851–857. [Google Scholar] [CrossRef]
- Andersson, S.; Serimaa, R.; Paakkari, T.; SaranpÄÄ, P.; Pesonen, E. Crystallinity of Wood and the Size of Cellulose Crystallites in Norway Spruce (Picea abies). J. Wood Sci. 2003, 49, 531–537. [Google Scholar] [CrossRef]
- Jiang, Z.-H.; Yang, Z.; So, C.-L.; Hse, C.-Y. Rapid Prediction of Wood Crystallinity in Pinus elliotii Plantation Wood by Near-Infrared Spectroscopy. J. Wood Sci. 2007, 53, 449–453. [Google Scholar] [CrossRef]
- Kurita, K.; Sannan, T.; Iwakura, Y. Studies on Chitin. VI. Binding of Metal Cations. J. Appl. Polym. Sci. 1979, 23, 511–515. [Google Scholar] [CrossRef]
- Ali, M.M.; Sarkar, B.; Sarkar, B.; Bhattacharya, P.; Chatterjee, N.; Rana, S.; Rokunuzzaman, M.; Bhakta, J.N. Screening and Characterization of Novel Biosorbent for the Removal of Cadmium from Contaminated Water. Energy Nexus 2024, 13, 100278. [Google Scholar] [CrossRef]
- Coates, J.P. A Practical Approach to the Interpretation of Infrared Spectra. In Encyclopedia of Analytical Chemistry; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2000; pp. 10815–10837. [Google Scholar]
- Zhang, J.; Dong, X.; Wang, X.; Ye, Q.; Guo, Y.; Shi, X. Determination of Amino Acid Contents in Six Species of Pine Needles and Their Nutritional Value Evaluation. China Food Addit. 2023, 34, 281–290. [Google Scholar] [CrossRef]
- Heredia-Guerrero, J.A.; Benítez, J.J.; Domínguez, E.; Bayer, I.S.; Cingolani, R.; Athanassiou, A.; Heredia, A. Infrared Spectroscopy as a Tool to Study Plant Cuticles. Spectrosc. Eur. 2016, 28, 10–13. [Google Scholar]
| Sample | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Chemical Component (%) | ALB | ASB | AL | SPLB | SPSB | SPN | MPLB | MPSB | MPN |
| Total extractives | 4.4 | 2.8 | 7.7 | 12.6 | 13.9 | 14.1 | 5.5 | 10.0 | 18.8 |
| Dichloromethane extractives | 0.7 | 0.7 | 0.9 | 2.0 | 4.8 | 4.6 | 2.0 | 3.5 | 5.9 |
| Ethanol extractives | 2.8 | 1.2 | 4.2 | 9.5 | 6.9 | 6.7 | 2.3 | 4.2 | 8.1 |
| Water extractives | 0.9 | 0.9 | 2.7 | 1.1 | 2.2 | 2.7 | 1.3 | 2.3 | 4.8 |
| Total lignin | 34.5 | 39.2 | 53.9 | 31.5 | 31.2 | 30.1 | 38.1 | 36.6 | 29.0 |
| Klason lignin | 33.9 | 32.5 | 49.7 | 31.2 | 30.8 | 28.5 | 37.1 | 35.8 | 28.1 |
| Soluble lignin | 0.5 | 0.5 | 4.2 | 0.2 | 0.3 | 1.6 | 1.0 | 0.9 | 0.8 |
| Holocellulose | 78.9 | 73.0 | 57.1 | 66.8 | 59.6 | 57.1 | 79.2 | 73.7 | 64.4 |
| α-cellulose | 35.8 | 37.0 | 27.7 | 44.1 | 36.5 | 37.8 | 60.5 | 54.7 | 49.8 |
| Hemicellulose | 43.2 | 36.0 | 29.4 | 22.6 | 23.1 | 19.3 | 18.7 | 19.0 | 14.6 |
| Sample | Ash Content (%) | Cationic Content (mg g−1) | ||||
|---|---|---|---|---|---|---|
| Ca | Na | Mg | Zn | K | ||
| ALB | 1.1 | 0.81 | 0.23 | 0.49 | 0.002 | 1.77 |
| ASB | 2.3 | 1.42 | 0.17 | 1.01 | 0.01 | 2.72 |
| AL | 3.4 | 6.02 | 0.29 | 1.87 | 0.003 | 3.75 |
| SPLB | 1.3 | 3.23 | 0.07 | 0.60 | 0.0003 | 0.21 |
| SPSB | 2.1 | 5.93 | 0.26 | 1.24 | 0.002 | 0.70 |
| SPN | 4.2 | 7.11 | 0.61 | 1.35 | 0.002 | 1.08 |
| MPLB | 0.8 | 1.56 | 0.18 | 0.41 | 0.002 | 0.37 |
| MPSB | 1.6 | 1.72 | 0.17 | 0.63 | 0.002 | 0.64 |
| MPN | 3.5 | 0.98 | 0.14 | 0.61 | 0.001 | 0.32 |
| Sample | pHpzc |
|---|---|
| SPLB | 4.53 |
| SPSB | 4.70 |
| SPN | 3.95 |
| MPLB | 4.84 |
| MPSB | 3.95 |
| MPN | 4.44 |
| ALB | 4.90 |
| ASB | 5.29 |
| AL | 4.73 |
| Sample | IC (%) |
|---|---|
| ALB | 36.3 |
| ASB | 33.5 |
| AL | 19.6 |
| MPLB | 26.2 |
| MPSB | 23.1 |
| MPN | 34.6 |
| SPLB | 27.4 |
| SPSB | 25.5 |
| SPN | 31.0 |
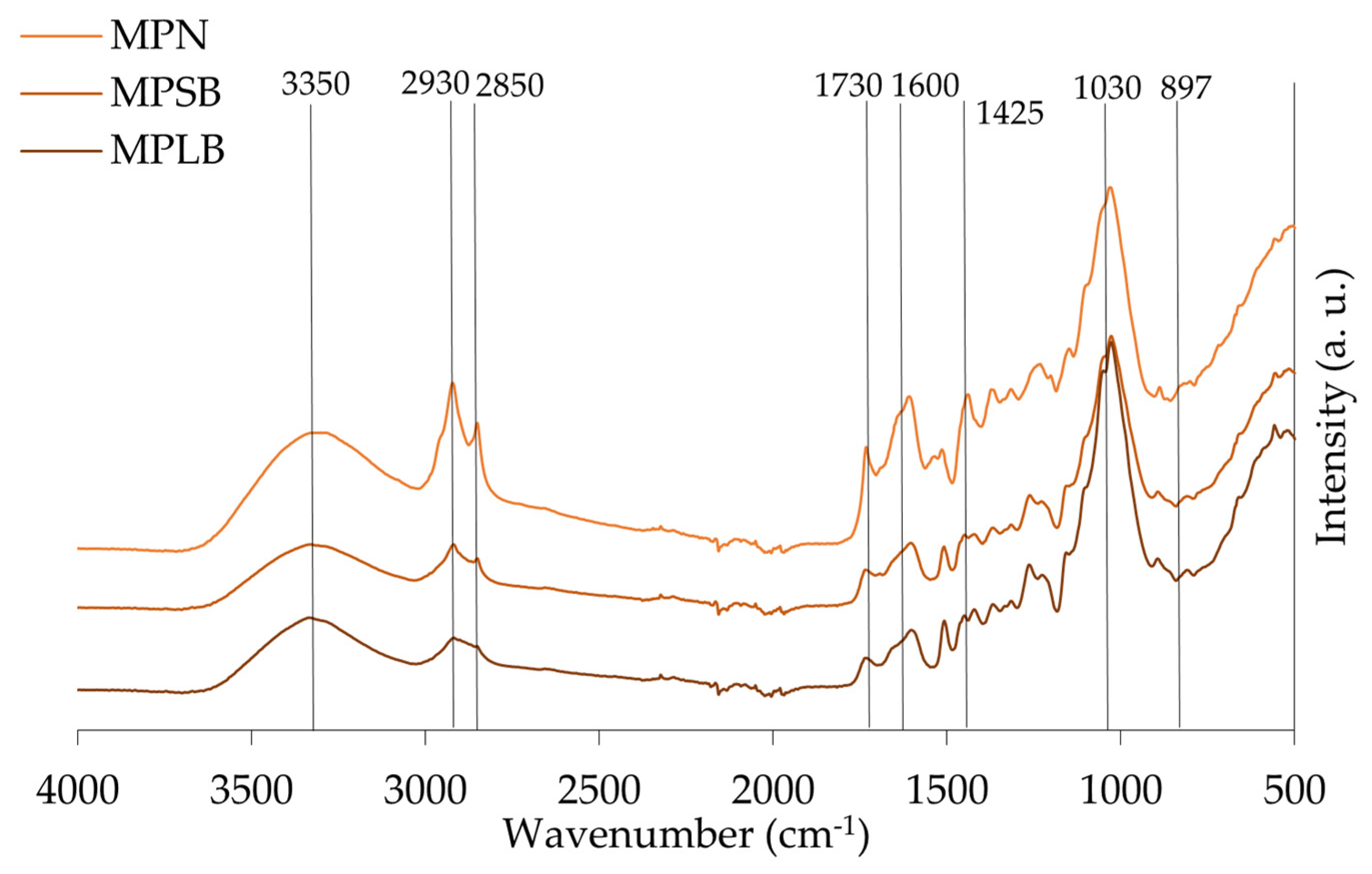
| Assignment | Peaks Identified in the Samples | |||
|---|---|---|---|---|
| Group | Range (cm−1) | ALB | MPLB | SPLB |
| O-H stretching of hydroxyl group | 3336 | 3331 | 3331 | 3337 |
| CH2 asymmetric and symmetric stretch from the methyl (CH3) and methylene (CH2) groups | 2916–2936 and 2843–2863 | 2918 2850 | 2919 2851 | 2919 2852 |
| C=O stretch in unconjugated ketones, carbonyls, and ester groups | 1738 | 1731 | 1733 | 1735 |
| Identified as C=O stretch and lignin peaks | 1603–1608 and 1508–1510 | 1607 1506 | 1607 1511 | 1604 1509 |
| C=C and C-H bond in plane deformation in lignin and hemicellulose | 1450–1453 | 1455 | - | 1450 |
| Symmetric stretching bands of the carboxyl group associated with CH deformation (methyl and methylene), CH2 bending vibrations, and CH3 stretching. | 1436–1319 | - | 1435 | - |
| C-OC aromatic ethers, asymmetric stretch | 1210–1310 | 1236 | 1263 | 1264 |
| C-O, CC, and C-CO stretch in cellulose, hemicellulose, and lignin | 1025–1035 | 1031 | 1028 | 1028 |
| Associated with stretching vibrations of C=O in carboxyl, as well as vibrations related to the aromatic skeleton, usually found in cellulose | 738–1614 | 897 | 895 | 895 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macena, M.; Cruz-Lopes, L.; Grosche, L.; Santos-Vieira, I.; Esteves, B.; Pereira, H. Characterization of Lignocellulosic Byproducts from the Portuguese Forest: Valorization and Sustainable Use. Materials 2025, 18, 4716. https://doi.org/10.3390/ma18204716
Macena M, Cruz-Lopes L, Grosche L, Santos-Vieira I, Esteves B, Pereira H. Characterization of Lignocellulosic Byproducts from the Portuguese Forest: Valorization and Sustainable Use. Materials. 2025; 18(20):4716. https://doi.org/10.3390/ma18204716
Chicago/Turabian StyleMacena, Morgana, Luísa Cruz-Lopes, Lucas Grosche, Isabel Santos-Vieira, Bruno Esteves, and Helena Pereira. 2025. "Characterization of Lignocellulosic Byproducts from the Portuguese Forest: Valorization and Sustainable Use" Materials 18, no. 20: 4716. https://doi.org/10.3390/ma18204716
APA StyleMacena, M., Cruz-Lopes, L., Grosche, L., Santos-Vieira, I., Esteves, B., & Pereira, H. (2025). Characterization of Lignocellulosic Byproducts from the Portuguese Forest: Valorization and Sustainable Use. Materials, 18(20), 4716. https://doi.org/10.3390/ma18204716










