Challenges in Nanofiber Formation from NADES-Based Anthocyanin Extracts: A Physicochemical Perspective
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Solvent Selection and Preparation
2.3. Antocyanins Extraction
2.4. Total Polyphenol Content (TPC) Determination
2.5. Total Anthocyanin Content Determination (TAC)
2.6. Electrospinning Solutions Preparation
2.7. The Rheological Properties of Electrospinning Solutions
2.8. Electrospinning Process
2.9. FTIR Characterization
2.10. Scanning Electron Microscopy
2.11. Preparation of Alginate-Based Films
2.12. Verification of the Usefulness of Freshness Indicators in Food Testing
3. Results
3.1. Total Polyphenol and Anthocyanin Content in Red Cabbage Extracts
3.2. FTIR Characterization
3.3. Electrospinning of PEO/Alginate Polymers with Red Cabbage Extracts
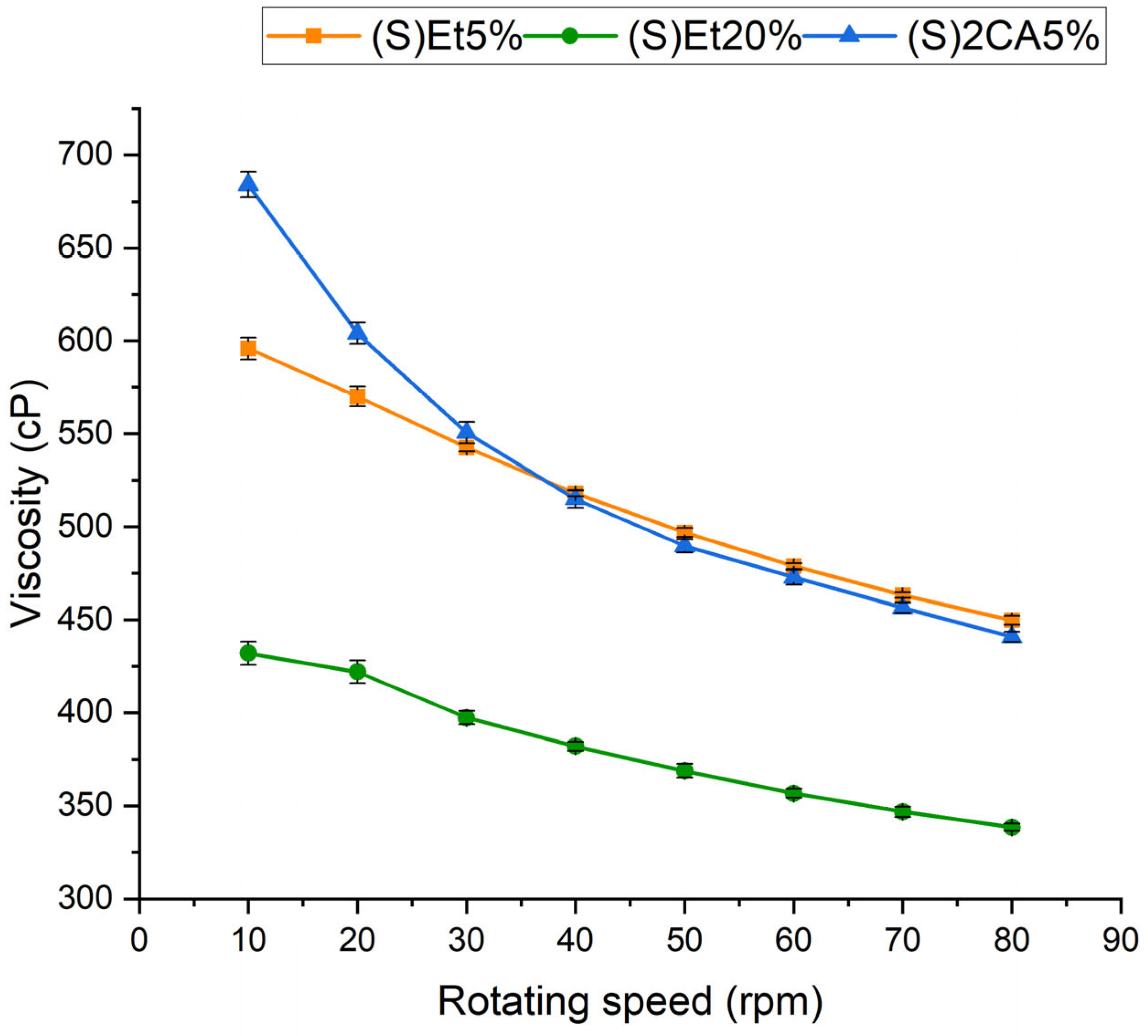
3.3.1. Viscosity of Electrospinning Solutions
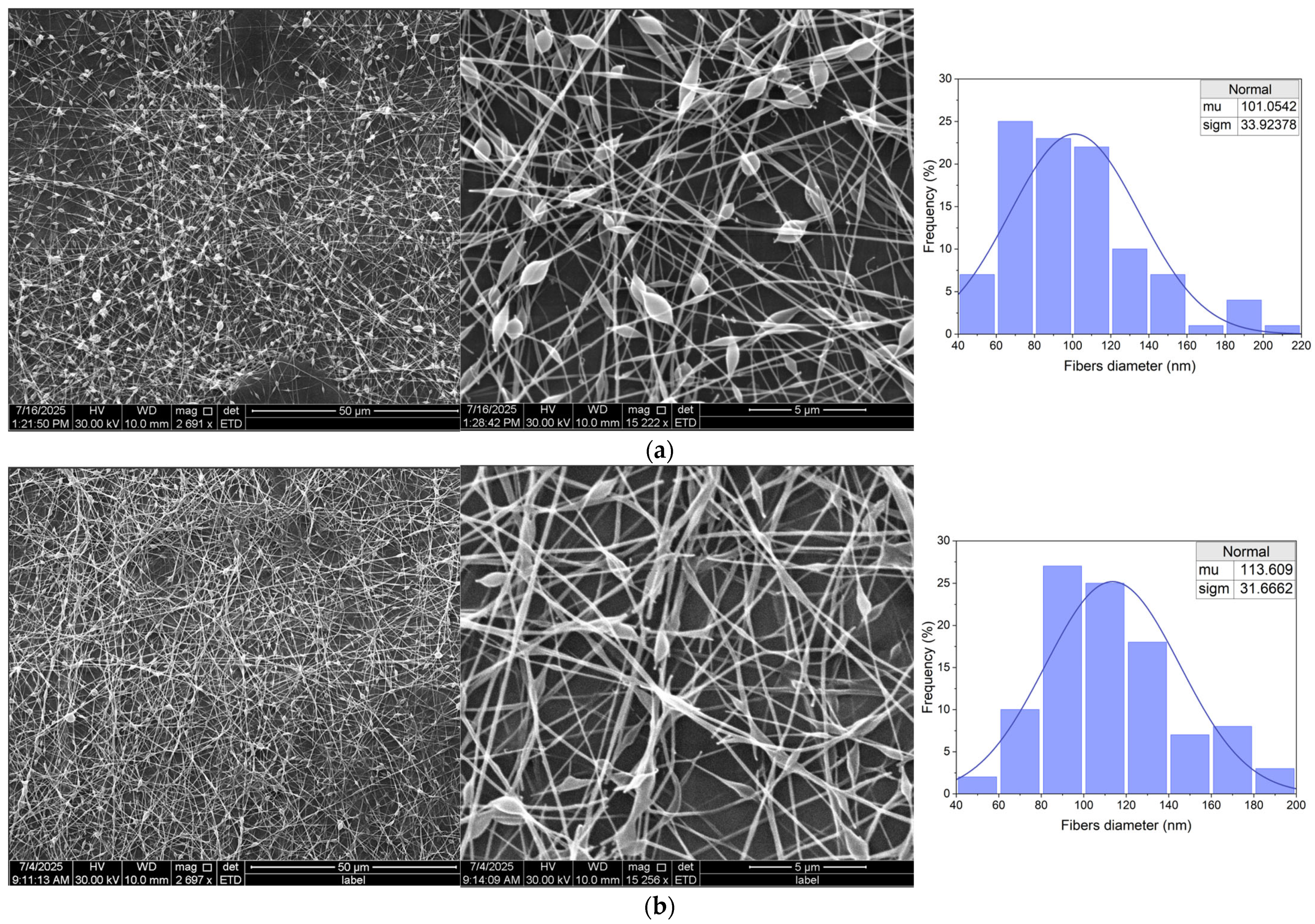
3.3.2. Fibers Morphology
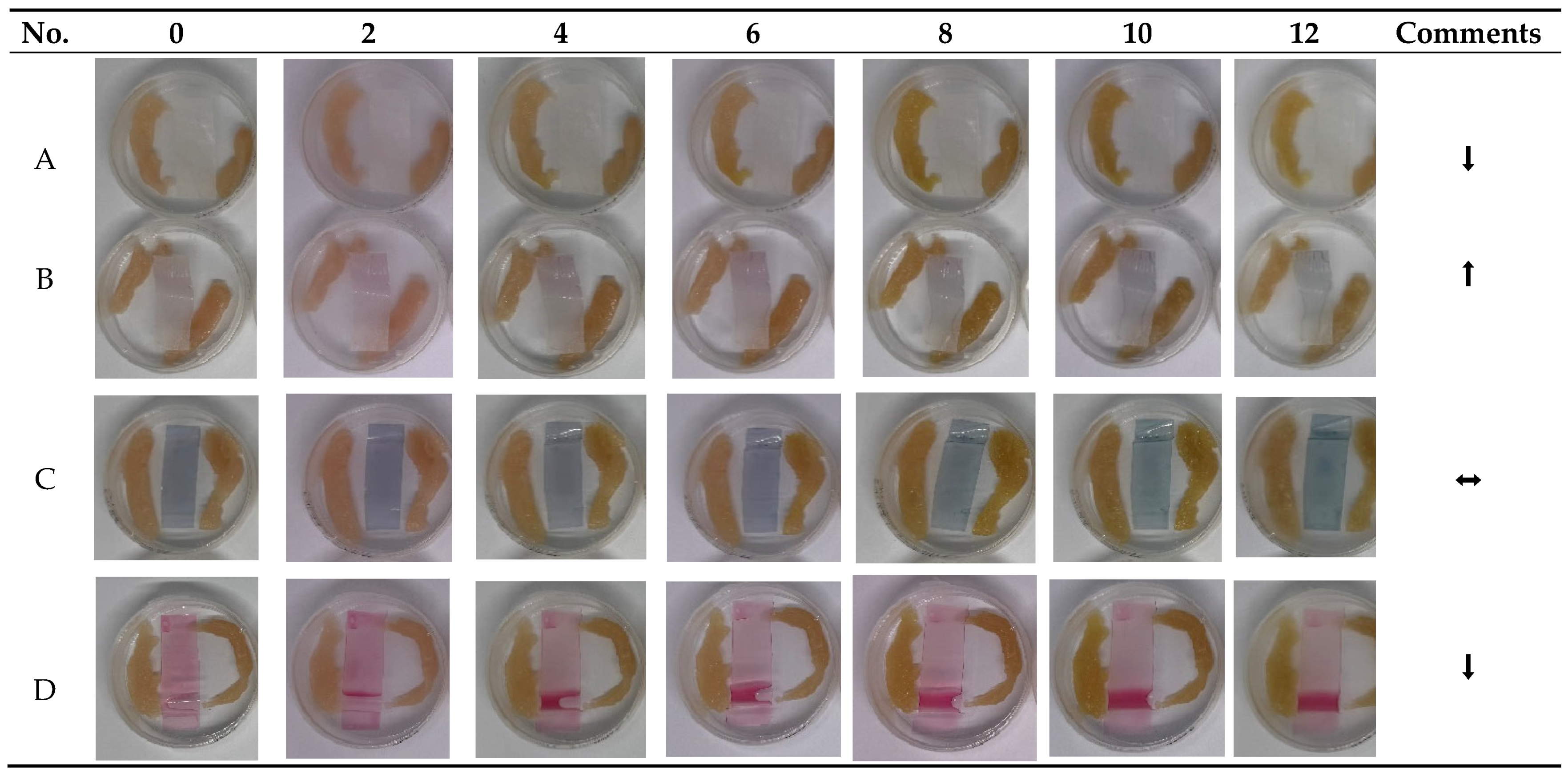
3.4. Evaluation of Indicator Performance on Deteriorating Chicken Meat
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Alg | Sodium alginate |
| ChCl | Choline chloride |
| HBA | Hydrogen bond acceptor |
| HBD | Hydrogen bond donor |
| NADES | Natural deep eutectic solvent |
| PEO | Polyethylene oxide |
| SEM | Scanning electron microscope |
| TAC | Total anthocyanin content |
| TPC | Total phenolic content |
References
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors Affecting Their Stability and Degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Khan, R.A. Anthocyanins: Traditional Uses, Structural and Functional Variations, Approaches to Increase Yields and Products’ Quality, Hepatoprotection, Liver Longevity, and Commercial Products. Int. J. Mol. Sci. 2022, 23, 2149. [Google Scholar] [CrossRef]
- Wang, H.; Sun, S.; Zhou, Z.; Qiu, Z.; Cui, X. Rapid Analysis of Anthocyanin and Its Structural Modifications in Fresh Tomato Fruit. Food Chem. 2020, 333, 127439. [Google Scholar] [CrossRef] [PubMed]
- Zannou, O.; Koca, I.; Ibrahim, S.A. Acidic Deep Eutectic Solvent as a Greener Medium for Highly Efficient Extraction of Anthocyanins from Blackberry Fruit: Optimization, Stability and Purification with Two-Aqueous Phase Method. Microchem. J. 2024, 205, 111291. [Google Scholar] [CrossRef]
- Chen, L.; Wang, W.; Wang, W.; Zhang, J. Effect of Anthocyanins on Colorimetric Indicator Film Properties. Coatings 2023, 13, 1682. [Google Scholar] [CrossRef]
- Chung, C.; Rojanasasithara, T.; Mutilangi, W.; McClements, D.J. Stability Improvement of Natural Food Colors: Impact of Amino Acid and Peptide Addition on Anthocyanin Stability in Model Beverages. Food Chem. 2017, 218, 277–284. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, J.; Li, L.; Ren, J.; Lu, J.; Luo, F. Advances in Embedding Techniques of Anthocyanins: Improving Stability, Bioactivity and Bioavailability. Food Chem. X 2023, 20, 100983. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; He, Y.; Fan, K. Recent Advances in Stability Improvement of Anthocyanins by Efficient Methods and Its Application in Food Intelligent Packaging: A Review. Food Biosci. 2023, 56, 103164. [Google Scholar] [CrossRef]
- Xue, H.; Zhao, J.; Wang, Y.; Shi, Z.; Xie, K.; Liao, X.; Tan, J. Factors Affecting the Stability of Anthocyanins and Strategies for Improving Their Stability: A Review. Food Chem. X 2024, 24, 101883. [Google Scholar] [CrossRef]
- Khalafi, N.; Gharachorloo, M.; Ganjloo, A.; Yousefi, S. Release Kinetics, Color Stability and Antioxidant Activity of Red Cabbage Anthocyanins Encapsulated in Zein Electrospun Nanoribbons. J. Food Meas. Charact. 2024, 18, 1363–1371. [Google Scholar] [CrossRef]
- Panić, M.; Gunjević, V.; Cravotto, G.; Radojčić Redovniković, I. Enabling Technologies for the Extraction of Grape-Pomace Anthocyanins Using Natural Deep Eutectic Solvents in up-to-Half-Litre Batches Extraction of Grape-Pomace Anthocyanins Using NADES. Food Chem. 2019, 300, 125185. [Google Scholar] [CrossRef] [PubMed]
- Meenu, M.; Bansal, V.; Rana, S.; Sharma, N.; Kumar, V.; Arora, V.; Garg, M. Deep Eutectic Solvents (DESs) and Natural Deep Eutectic Solvents (NADESs): Designer Solvents for Green Extraction of Anthocyanin. Sustain. Chem. Pharm. 2023, 34, 101168. [Google Scholar] [CrossRef]
- Li, Z.; Cui, R.; Liu, W.; Wang, M.; Li, L.; Liu, F.; Du, B.; Song, L. Application of Green Deep Eutectic Solvents for Anthocyanins Extraction from Grape Pomace: Optimization, Stability, Antioxidant Activity, and Molecular Dynamic Simulation. LWT 2024, 211, 116878. [Google Scholar] [CrossRef]
- Jeong, K.M.; Zhao, J.; Jin, Y.; Heo, S.R.; Han, S.Y.; Yoo, D.E.; Lee, J. Highly Efficient Extraction of Anthocyanins from Grape Skin Using Deep Eutectic Solvents as Green and Tunable Media. Arch. Pharm. Res. 2015, 38, 2143–2152. [Google Scholar] [CrossRef]
- Bu, F.; Zhao, Y.; Li, B.; Zhang, X.; Li, J. The Effect of Choline Chloride-Butanediol Based Deep Eutectic Solvents on Ultrasound-Assisted Extraction, Antioxidant Activity and Stability of Anthocyanins Extracted from Perilla frutescens (L.) Britt. Sustain. Chem. Pharm. 2023, 32, 101000. [Google Scholar] [CrossRef]
- Prakash, S.; Goswami, A.; Patil, R.; Mitra, A.; Kutty, N.N. An Eco-Friendly Approach to Extract Anthocyanins from Rose Flowers Using Natural Deep Eutectic Solvents. Ind. Crops Prod. 2024, 210, 118059. [Google Scholar] [CrossRef]
- Pires, I.V.; da Silva, L.H.M.; Rodrigues, A.M.d.C.; Saldaña, M.D.A. Natural Deep Eutectic Solvents for Anthocyanin Extraction from Agricultural Sources: Process Parameters, Economic and Environmental Analysis, and Industrial Challenges. Compr. Rev. Food Sci. Food Saf. 2024, 23, e70057. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Guo, S.; Xu, Y.; Ettoumi, F.; Fang, J.; Yan, X.; Xie, Z.; Luo, Z.; Cheng, K. Valorization and Protection of Anthocyanins from Strawberries (Fragaria×ananassa Duch.) by Acidified Natural Deep Eutectic Solvent Based on Intermolecular Interaction. Food Chem. 2024, 447, 138971. [Google Scholar] [CrossRef]
- Khalafi, N.; Zandi, M. Utilizing Natural Pigments in Electrospun Nanofibers: Sustainable and Smart Food Packaging Solutions Driven by Nanotechnology. Appl. Food Res. 2025, 5, 101220. [Google Scholar] [CrossRef]
- Vannuchi, N.; Ramos, S.d.P.; Mazzo, T.M.; Longo, E.; Bonsanto, F.P.; Braga, A.R.C.; de Rosso, V.V. Natural Deep Eutectic Solvents (NADES)-Extracted Anthocyanins: Bioaccessibility in Electrospun PEO Microfibers. Food Res. Int. 2024, 177, 113898. [Google Scholar] [CrossRef]
- Stoica, R.; Ganciarov, M.; Constantinescu-Aruxandei, D.; Capră, L.; Șuică-Bunghez, I.R.; Senin, R.M.; Pricope, G.D.; Ivan, G.R.; Călin, C.; Oancea, F. Sustainable Recovery of Anthocyanins and Other Polyphenols from Red Cabbage Byproducts. Foods 2023, 12, 4157. [Google Scholar] [CrossRef]
- Wróbel, P.; Zwolińska, J.; Szopa, D.; Witek-Krowiak, A. Towards Enhanced Electrospinning of Alginate—Can Recent Strategies Overcome Limitations? A Review. Polymers 2025, 17, 2255. [Google Scholar] [CrossRef] [PubMed]
- Inácio, M.R.C.; De Lima, K.M.G.; Lopes, V.G.; Pessoa, J.D.C.; De Almeida Teixeira, G.H. Total Anthocyanin Content Determination in Intact Açaí (Euterpe oleracea Mart.) and Palmitero-Juçara (Euterpe edulis Mart.) Fruit Using near Infrared Spectroscopy (NIR) and Multivariate Calibration. Food Chem. 2013, 136, 1160–1164. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Nalatambi, S.; Mahaindran, A.; Tee, L.H.; Chua, B.L.; Oh, K.S.; Lam, W.H. Characterization of Choline Chloride-Based Deep Eutectic Solvent for Anthocyanin Extraction via Aqueous Two-Phase System: Physicochemical Properties and Liquid–Liquid Equilibrium Phase Diagram. Asia-Pac. J. Chem. Eng. 2023, 18, e2990. [Google Scholar] [CrossRef]
- da Silva, D.T.; Pauletto, R.; da Silva Cavalheiro, S.; Bochi, V.C.; Rodrigues, E.; Weber, J.; de Bona da Silva, C.; Morisso, F.D.P.; Barcia, M.T.; Emanuelli, T. Natural Deep Eutectic Solvents as a Biocompatible Tool for the Extraction of Blueberry Anthocyanins. J. Food Compos. Anal. 2020, 89, 103470. [Google Scholar] [CrossRef]
- Kaur, D.; Qadri, O.S. Ultrasound-Assisted Extraction of Syzygium Cumini Anthocyanins Using Natural Deep Eutectic Solvents. Food Bioprocess Technol. 2025, 18, 1821–1833. [Google Scholar] [CrossRef]
- Jauregi, P.; Esnal-Yeregi, L.; Labidi, J. Natural Deep Eutectic Solvents (NADES) for the Extraction of Bioactives: Emerging Opportunities in Biorefinery Applications. PeerJ Anal. Chem. 2024, 6, e32. [Google Scholar] [CrossRef]
- Zeinalipour-Yazdi, C.D.; Loizidou, E.Z. An Experimental FTIR-ATR and Computational Study of H-Bonding in Ethanol/Water Mixtures. Chem. Phys. 2021, 550, 111295. [Google Scholar] [CrossRef]
- Patil, J.S.; Patil, P.O.; Nangare, S.N.; Mutha, R.E.; Bari, S.B.; Khan, Z.G. Green Extraction of Anthocyanins from Prunus Domestica Using Natural Deep Eutectic Solvents: Anti-Sickling Properties and Phenolic Impact Assessment. Food Anal. Methods 2024, 17, 1432–1445. [Google Scholar] [CrossRef]
- McDowell, S.A.C. Comparative Computational Study of Frequency Shifts and Infrared Intensity Changes in Model Binary Complexes with Red- and Blue-Shifting Hydrogen Bonds. Molecules 2025, 30, 106. [Google Scholar] [CrossRef]
- Burikov, S.; Dolenko, T.; Patsaeva, S.; Starokurov, Y.; Yuzhakov, V. Raman and IR Spectroscopy Research on Hydrogen Bonding in Water-Ethanol Systems. Mol. Phys. 2010, 108, 2427–2436. [Google Scholar] [CrossRef]
- Maylinda, E.V.; Rinadi, A.; Putri, E.A.; Fadillah, G.; Wayuningsih, S. Color Stability of Anthocyanins Copigmentation from Red Rice (Oryza sativa L.) Bran by Spectrophotometry UV-Vis. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Kota Surakarta, Indonesia, 15–16 October 2018; Institute of Physics Publishing: New York, NY, USA, 2019; Volume 578. [Google Scholar]
- Forgie, J.R.P.; Leclinche, F.; Dréan, E.; Dolez, P.I. Electrospinning of High-Performance Nanofibres: State of the Art and Insights into the Path Forward. Appl. Sci. 2023, 13, 12476. [Google Scholar] [CrossRef]
- Soltani, S.; Emadi, R.; Javanmard, S.H.; Kharaziha, M.; Rahmati, A. Shear-Thinning and Self-Healing Nanohybrid Alginate-Graphene Oxide Hydrogel Based on Guest-Host Assembly. Int. J. Biol. Macromol. 2021, 180, 311–323. [Google Scholar] [CrossRef]
- Gupta, P.; Elkins, C.; Long, T.E.; Wilkes, G.L. Electrospinning of Linear Homopolymers of Poly(Methyl Methacrylate): Exploring Relationships between Fiber Formation, Viscosity, Molecular Weight and Concentration in a Good Solvent. Polymer 2005, 46, 4799–4810. [Google Scholar] [CrossRef]
- Xia, S.; Fan, R.; Wu, H.; Guo, Z.; Gao, P.; Wei, L.; Wang, M.; Han, L. Extraction of Anthocyanins from Black Bean Peel Based on Deep Eutectic Solvents and the Determination of Their Antioxidant Properties and Stability. Separations 2025, 12, 73. [Google Scholar] [CrossRef]
- Maimulyanti, A.; Nurhidayati, I.; Mellisani, B.; Putri, F.A.R.; Puspita, F.; Prihadi, A.R.; Maimulyanti, A.; Nurhidayati, I.; Mellisani, B.; Putri, F.A.R.; et al. Development of Natural Deep Eutectic Solvent (NADES) Based on Choline Chloride as a Green Solvent to Extract Phenolic Compound from Coffee Husk Waste. Arab. J. Chem. 2023, 16, 104634. [Google Scholar] [CrossRef]
- Al Fuhaid, L.; Nava-Ocampo, M.F.; Bucs, S.S.; Verpoorte, R.; Choi, Y.H.; Witkamp, G.J.; Farinha, A.S.F. Crystallization and Time-Dependent Changes in Betaine-Urea-Water Natural Deep Eutectic Solvents. J. Mol. Liq. 2023, 387, 122718. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Tailoring Properties of Natural Deep Eutectic Solvents with Water to Facilitate Their Applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef]
- Du, X.; Kong, B.; He, J.; Zhang, Q.; An, G.; Zhang, T.; Xia, X. Cryoprotective Effect of Water-Tailored Trehalose-Based Natural Deep Eutectic Solvents on Frozen-Thawed Mirror Carp (Cyprinus carpio L.) Surimi. Food Chem. 2023, 426, 136633. [Google Scholar] [CrossRef]
- Grillo, G.; Gunjević, V.; Radošević, K.; Redovniković, I.R.; Cravotto, G. Deep Eutectic Solvents and Nonconventional Technologies for Blueberry-Peel Extraction: Kinetics, Anthocyanin Stability, and Antiproliferative Activity. Antioxidants 2020, 9, 1069. [Google Scholar] [CrossRef] [PubMed]
- Airouyuwa, J.O.; Mostafa, H.; Ranasinghe, M.; Maqsood, S. Influence of Physicochemical Properties of Carboxylic Acid-Based Natural Deep Eutectic Solvents (CA-NADES) on Extraction and Stability of Bioactive Compounds from Date (Phoenix dactylifera L.) Seeds: An Innovative and Sustainable Extraction Technique. J. Mol. Liq. 2023, 388, 122767. [Google Scholar] [CrossRef]
- Abdulhussain, R.; Adebisi, A.; Conway, B.R.; Asare-Addo, K. Electrospun Nanofibers: Exploring Process Parameters, Polymer Selection, and Recent Applications in Pharmaceuticals and Drug Delivery. J. Drug Deliv. Sci. Technol. 2023, 90, 105156. [Google Scholar] [CrossRef]
- Çaykara, T.; Demirci, S.; Eroǧlu, M.S.; Güven, O. Poly(Ethylene Oxide) and Its Blends with Sodium Alginate. Polymer 2005, 46, 10750–10757. [Google Scholar] [CrossRef]
- Vigani, B.; Rossi, S.; Milanesi, G.; Bonferoni, M.C.; Sandri, G.; Bruni, G.; Ferrari, F. Electrospun Alginate Fibers: Mixing of Two Different Poly(Ethylene Oxide) Grades to Improve Fiber Functional Properties. Nanomaterials 2018, 8, 971. [Google Scholar] [CrossRef]
- Jia, E.; Wang, C.; Su, L.; Liu, P.; Xu, J. Influence of Water on Polyvinyl Alcohol Sol–Gel Transition and Gel Spinning. J. Polym. Res. 2015, 22, 69. [Google Scholar] [CrossRef]
- Ryklin, D.; Demidova, M.; Azarchenko, V.; Skobova, N. Influence of Glycerin Adding on the Electrospun Nanofibers Diameter. AIP Conf. Proc. 2022, 2430, 030002. [Google Scholar] [CrossRef]
- Perez-Puyana, V.M.; Romero, A.; Guerrero, A.; Moroni, L.; Wieringa, P.A. Enabling Low Molecular Weight Electrospinning through Binary Solutions of Polymer Blends. Next Mater. 2025, 6, 100306. [Google Scholar] [CrossRef]
- Rutledge, G.C.; Fridrikh, S.V. Formation of Fibers by Electrospinning. Adv. Drug Deliv. Rev. 2007, 59, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Karimi Alavijeh, D.; Heli, B.; Ajji, A. Development of a Sensitive Colorimetric Indicator for Detecting Beef Spoilage in Smart Packaging. Sensors 2024, 24, 3939. [Google Scholar] [CrossRef]
- Liu, S.; Fu, Y.; Nian, S. Buffering Colour Fluctuation of Purple Sweet Potato Anthocyanins to Acidity Variation by Surfactants. Food Chem. 2014, 162, 16–21. [Google Scholar] [CrossRef]

| Solvent Code | Ethanol Content (wt%) | NADES Content (wt%) | NADES Composition |
|---|---|---|---|
| 0CA | 0% | 100% | ChCl:citric acid:water (1:2:6) |
| 1CA | 10% | 90% | ChCl:citric acid:water (1:2:6) |
| 2CA | 30% | 70% | ChCl:citric acid:water (1:2:6) |
| 3CA | 50% | 50% | ChCl:citric acid:water (1:2:6) |
| 1LA | 10% | 90% | ChCl:lactic acid (1:1) |
| 2LA | 30% | 70% | ChCl:lactic acid (1:1) |
| 3LA | 50% | 50% | ChCl:lactic acid (1:1) |
| Et | 50% | 50% (water) | Ethanol:water (1:1, v/v) |
| Solvent | TPC [mg GAE/mL] | TAC [mg C-3-G/mL] |
|---|---|---|
| (E)1CA | 0.765 ± 0.058 | 0.149 ± 0.015 |
| (E)2CA | 1.644 ± 0.172 | 0.312 ± 0.025 |
| (E)3CA | 1.273 ± 0.120 | 0.186 ± 0.012 |
| (E)1LA | 1.336 ± 0.115 | 0.184 ± 0.018 |
| (E)2LA | 1.223 ± 0.146 | 0.205 ± 0.009 |
| (E)3LA | 1.257 ± 0.109 | 0.047 ± 0.003 |
| (E)Et | 0.760 ± 0.051 | 0.014 ± 0.001 |
| Extract Type and Concentration in the Spinning Solution | Electrospinning Solution Code | Observations | Assessment of Electrospinning |
|---|---|---|---|
| (E)Et 5% | (S)Et5% | beads-on-string, smooth, continuous fibers, | (+++) stable |
| (E)Et 20% | (S)Et20% | beads-on-string, less smooth fibers, slight entanglement | (++) moderate |
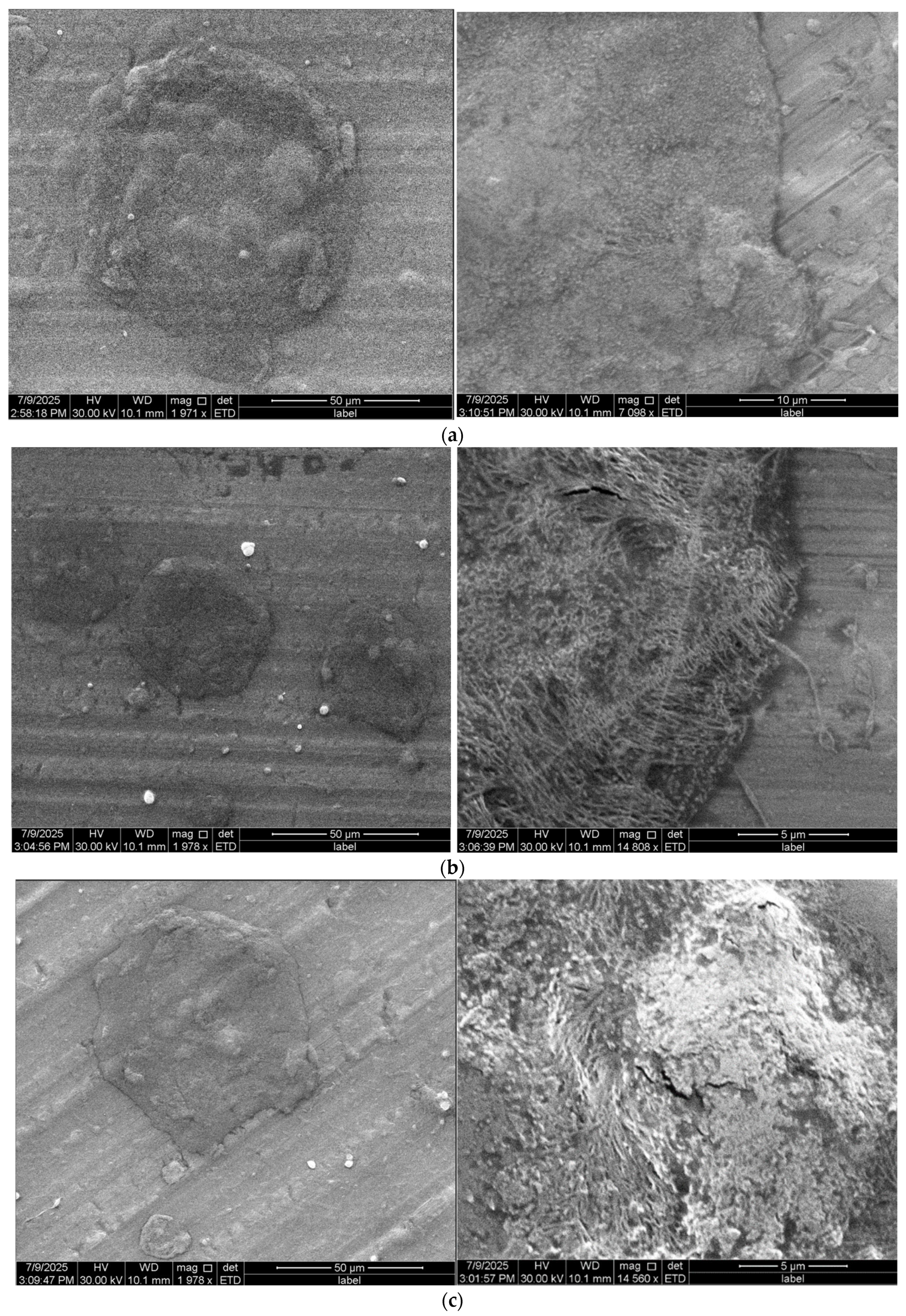
| (E)1CA 5% | (S)1CA5% | no fibers, amorphous, irregular structure | (−−−) lack of fibers |
| (E)2CA 5% | (S)2CA5% | no fibers, compact structure, aggregates | (−−−) lack of fibers |
| (E)3CA 5% | (S)3CA5% | adhesions, irregular mass, aggregates | (−−−) lack of fibers |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wróbel, P.; Latacz, K.; Chęcmanowski, J.; Witek-Krowiak, A. Challenges in Nanofiber Formation from NADES-Based Anthocyanin Extracts: A Physicochemical Perspective. Materials 2025, 18, 4502. https://doi.org/10.3390/ma18194502
Wróbel P, Latacz K, Chęcmanowski J, Witek-Krowiak A. Challenges in Nanofiber Formation from NADES-Based Anthocyanin Extracts: A Physicochemical Perspective. Materials. 2025; 18(19):4502. https://doi.org/10.3390/ma18194502
Chicago/Turabian StyleWróbel, Paulina, Katarzyna Latacz, Jacek Chęcmanowski, and Anna Witek-Krowiak. 2025. "Challenges in Nanofiber Formation from NADES-Based Anthocyanin Extracts: A Physicochemical Perspective" Materials 18, no. 19: 4502. https://doi.org/10.3390/ma18194502
APA StyleWróbel, P., Latacz, K., Chęcmanowski, J., & Witek-Krowiak, A. (2025). Challenges in Nanofiber Formation from NADES-Based Anthocyanin Extracts: A Physicochemical Perspective. Materials, 18(19), 4502. https://doi.org/10.3390/ma18194502






