Exosomes Derived from Mg-Preconditioned Bone Mesenchymal Stem Cells Promote Angiogenesis and Osteogenesis for Osteonecrosis Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
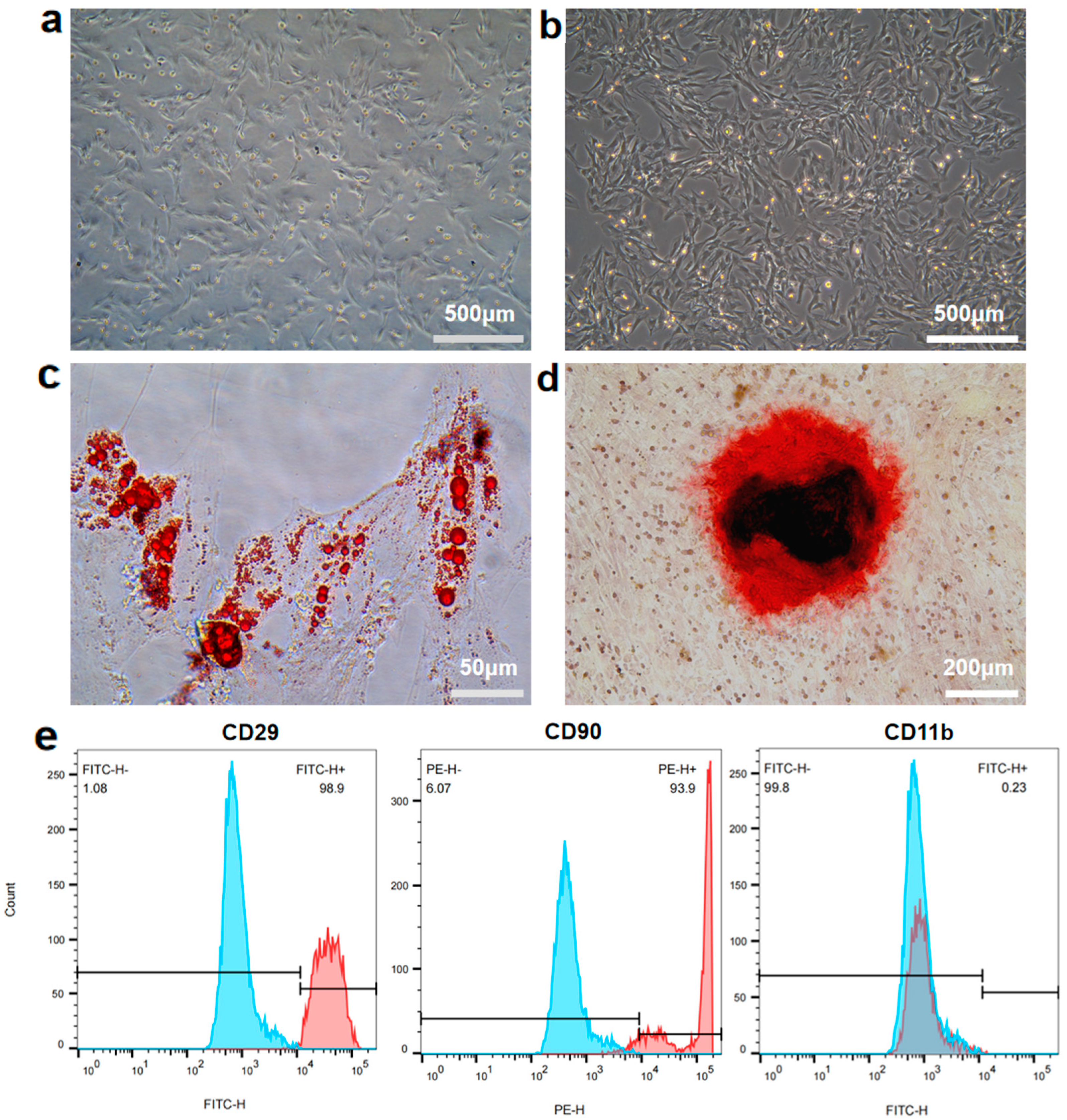
2.2. Isolation and Characterization of rBMSCs
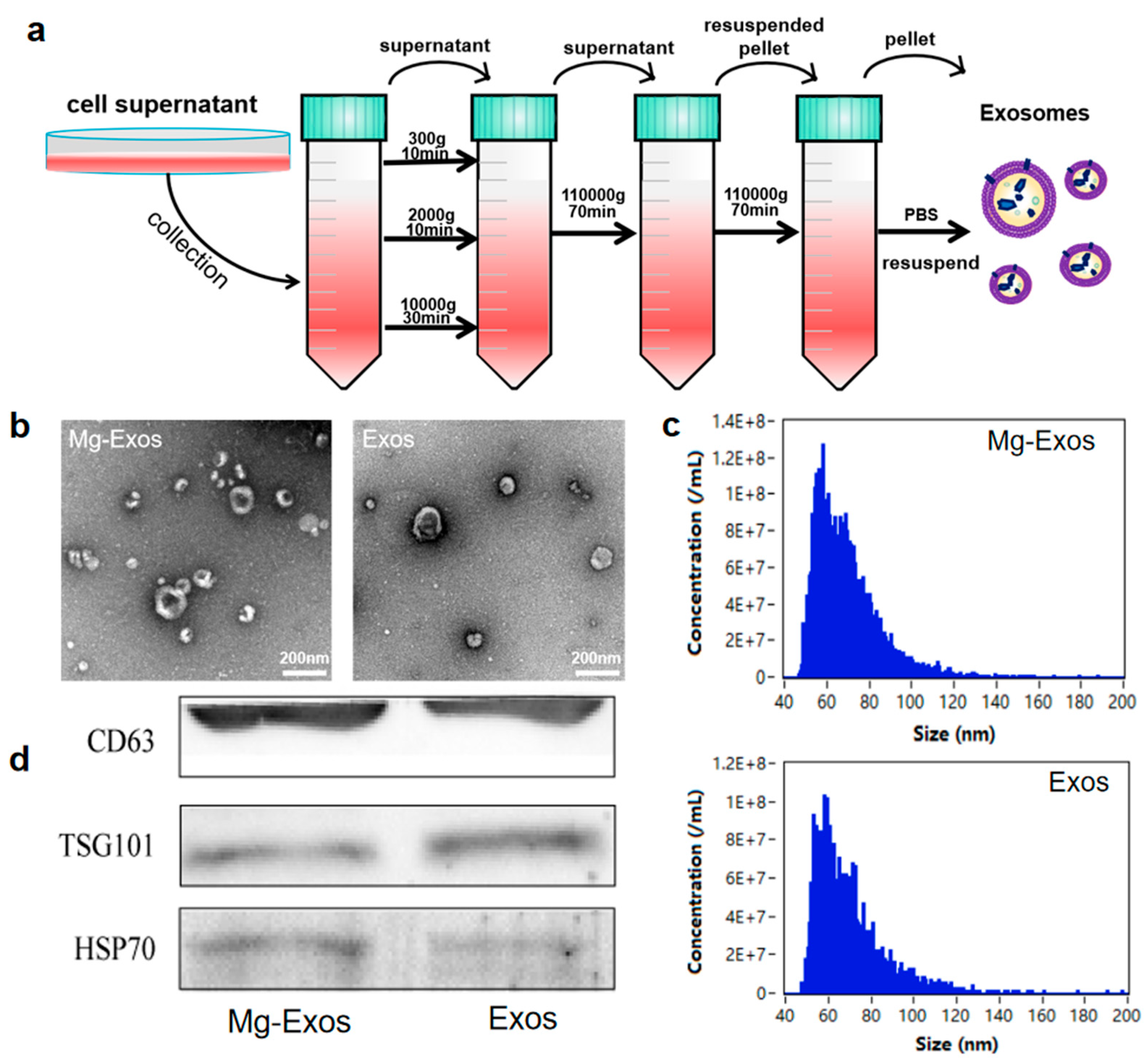
2.3. Isolation of Exosomes
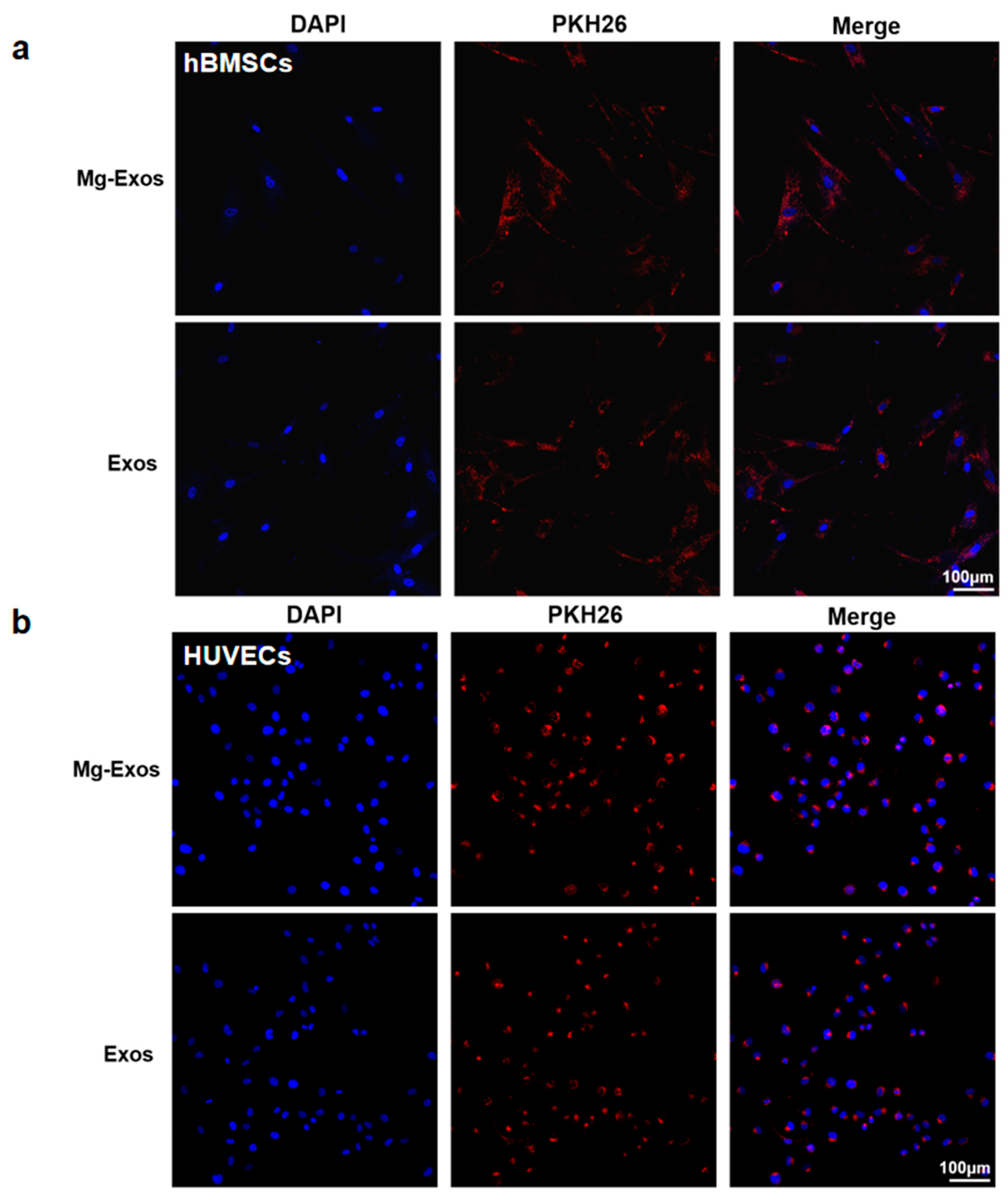
2.4. Characterization and Internalization of Exosomes
2.5. Cell Proliferation
2.6. Wound Healing Assay
2.7. Transwell Migration Assay
2.8. Tube Formation Assay
2.9. ALP Staining
2.10. ARS Staining
2.11. RT-qPCR Analysis
2.12. Statistical Analysis
3. Results and Discussion
3.1. Isolation and Characterization of rBMSCs
3.2. Characterization and Internalization of Exosomes
3.3. Mg-Exos Promoted Angiogenesis of HUVECs
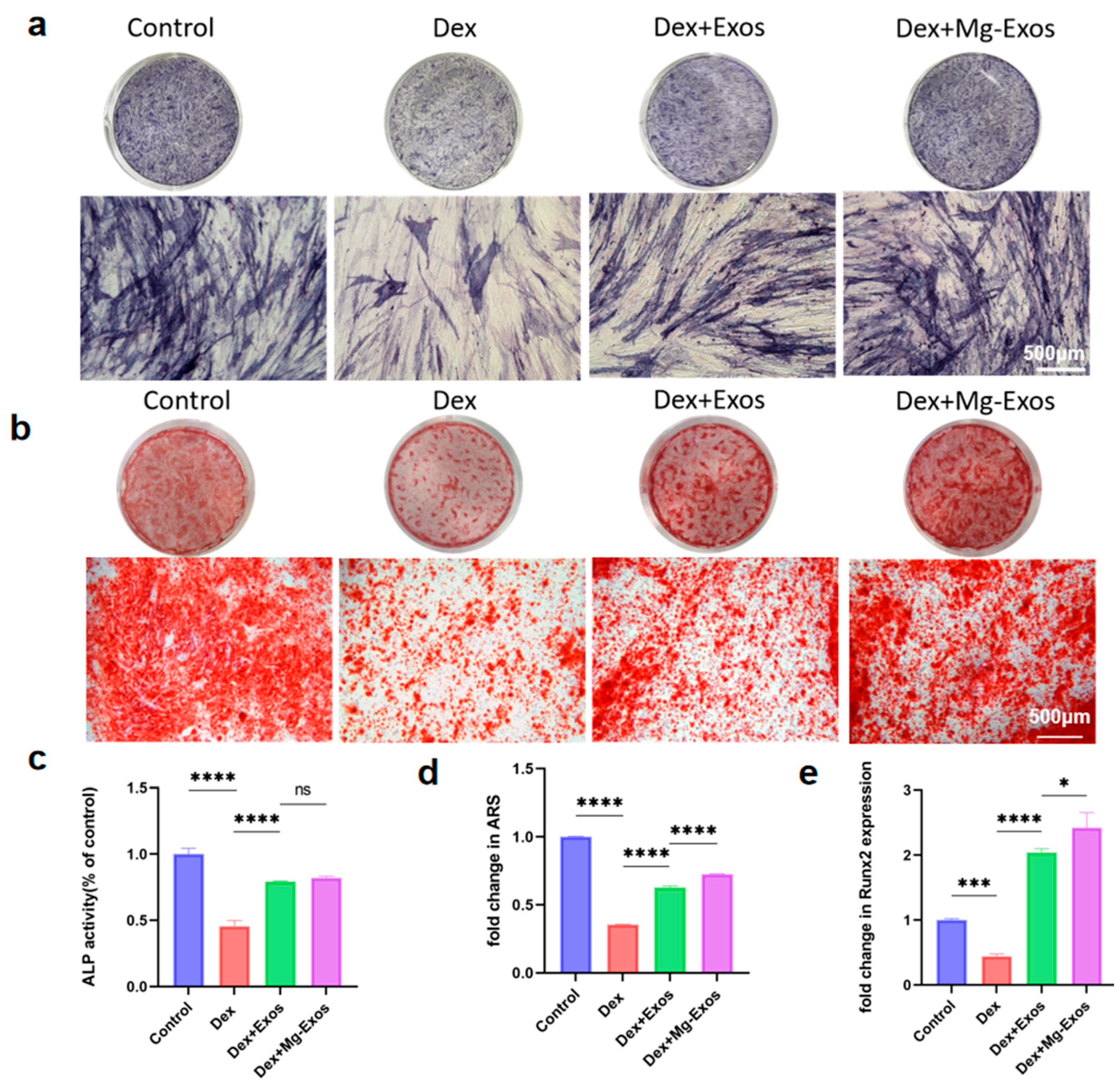
3.4. Mg-Exos Promoted Osteogenesis of hBMSCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- van der Goes, M.C.; Strehl, C.; Buttgereit, F.; Bijlsma, J.W.; Jacobs, J.W. Can Adverse Effects of Glucocorticoid Therapy Be Prevented and Treated? Expert Opin. Pharmacother. 2016, 17, 2129–2133. [Google Scholar] [CrossRef][Green Version]
- Motta, F.; Timilsina, S.; Gershwin, M.E.; Selmi, C. Steroid-Induced Osteonecrosis. J. Transl. Autoimmun. 2022, 5, 100168. [Google Scholar] [CrossRef]
- Chang, C.; Greenspan, A.; Gershwin, M.E. The Pathogenesis, Diagnosis and Clinical Manifestations of Steroid-Induced Osteonecrosis. J. Autoimmun. 2020, 110, 102460. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Han, L.; Wang, B.; Wang, P.; Wang, Y.; Wang, R.; Lv, X.; Feng, Y. The Role of Piezo1 in Bone Marrow Stem Cells in Response to Elevated Intraosseous Pressure on Regulating Osteogenesis and Angiogenesis of Steroid-Induced Osteonecrosis of the Femoral Head. J. Orthop. Transl. 2025, 51, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Shi, K.; Long, J.; Liu, Y.; Meng, X.; Huang, C.; Hao, J.; Li, L.; Zhao, Y.; Ye, T.; et al. PDGF-BB Improves Cortical Bone Quality through Restoring the Osteogenic Microenvironment in the Steroid-Associated Osteonecrosis of Rabbits. J. Orthop. Transl. 2025, 52, 97–115. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Miao, Y.; Liu, K.; Xue, F.; Zhu, B.; Zhang, C.; Li, G. Evolutionary Course of the Femoral Head Osteonecrosis: Histopathological—Radiologic Characteristics and Clinical Staging Systems. J. Orthop. Transl. 2022, 32, 28–40. [Google Scholar] [CrossRef]
- Cao, H.; Guan, H.; Lai, Y.; Qin, L.; Wang, X. Review of Various Treatment Options and Potential Therapies for Osteonecrosis of the Femoral Head. J. Orthop. Transl. 2016, 4, 57–70. [Google Scholar] [CrossRef]
- Caplan, A.I. Adult Mesenchymal Stem Cells for Tissue Engineering versus Regenerative Medicine. J. Cell. Physiol. 2007, 213, 341–347. [Google Scholar] [CrossRef]
- Loebel, C.; Burdick, J.A. Engineering Stem and Stromal Cell Therapies for Musculoskeletal Tissue Repair. Cell Stem Cell 2018, 22, 325–339. [Google Scholar] [CrossRef]
- Gao, X.; Ruzbarsky, J.J.; Layne, J.E.; Xiao, X.; Huard, J. Stem Cells and Bone Tissue Engineering. Life 2024, 14, 287. [Google Scholar] [CrossRef]
- Wang, Y.; Wen, J.; Lu, T.; Han, W.; Jiao, K.; Li, H. Mesenchymal Stem Cell-Derived Extracellular Vesicles in Bone-Related Diseases: Intercellular Communication Messengers and Therapeutic Engineering Protagonists. Int. J. Nanomed. 2024, 19, 3233–3257. [Google Scholar] [CrossRef]
- Li, Z.; Li, Q.; Tong, K.; Zhu, J.; Wang, H.; Chen, B.; Chen, L. BMSC-Derived Exosomes Promote Tendon-Bone Healing after Anterior Cruciate Ligament Reconstruction by Regulating M1/M2 Macrophage Polarization in Rats. Stem Cell Res. Ther. 2022, 13, 295. [Google Scholar] [CrossRef]
- Dabrowska, S.; Andrzejewska, A.; Janowski, M.; Lukomska, B. Immunomodulatory and Regenerative Effects of Mesenchymal Stem Cells and Extracellular Vesicles: Therapeutic Outlook for Inflammatory and Degenerative Diseases. Front. Immunol. 2021, 11, 591065. [Google Scholar] [CrossRef] [PubMed]
- Ogata, K.; Katagiri, W.; Hibi, H. Secretomes from Mesenchymal Stem Cells Participate in the Regulation of Osteoclastogenesis in Vitro. Clin. Oral. Investig. 2017, 21, 1979–1988. [Google Scholar] [CrossRef]
- Lukomska, B.; Stanaszek, L.; Zuba-Surma, E.; Legosz, P.; Sarzynska, S.; Drela, K. Challenges and Controversies in Human Mesenchymal Stem Cell Therapy. Stem Cells Int. 2019, 2019, 9628536. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xiong, J.; Yang, L.; Zhang, J.; Sun, S.; Liang, Y. Cell-Free Exosome-Laden Scaffolds for Tissue Repair. Nanoscale 2021, 13, 8740–8750. [Google Scholar] [CrossRef]
- Bei, H.P.; Hung, P.M.; Yeung, H.L.; Wang, S.; Zhao, X. Bone-a-Petite: Engineering Exosomes towards Bone, Osteochondral, and Cartilage Repair. Small 2021, 17, 2101741. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA Delivery by Extracellular Vesicles in Mammalian Cells and Its Applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef]
- Rani, S.; Ryan, A.E.; Griffin, M.D.; Ritter, T. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Toward Cell-Free Therapeutic Applications. Mol. Ther. 2015, 23, 812–823. [Google Scholar] [CrossRef]
- Zhang, L.; Jiao, G.; Ren, S.; Zhang, X.; Li, C.; Wu, W.; Wang, H.; Liu, H.; Zhou, H.; Chen, Y. Exosomes from Bone Marrow Mesenchymal Stem Cells Enhance Fracture Healing through the Promotion of Osteogenesis and Angiogenesis in a Rat Model of Nonunion. Stem Cell Res. Ther. 2020, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Deng, G.; She, H.; Bai, F.; Xiang, B.; Zhou, J.; Zhang, S. Polydopamine-Coated Biomimetic Bone Scaffolds Loaded with Exosomes Promote Osteogenic Differentiation of BMSC and Bone Regeneration. Regen. Ther. 2023, 23, 25–36. [Google Scholar] [CrossRef]
- Li, H.; Liu, H.; Zhou, Y.; Cheng, L.; Wang, B.; Ma, J. The Multifaceted Roles of Extracellular Vesicles in Osteonecrosis of the Femoral Head. J. Orthop. Transl. 2025, 52, 70–84. [Google Scholar] [CrossRef]
- Wu, J.; Wu, J.; Liu, Z.; Gong, Y.; Feng, D.; Xiang, W.; Fang, S.; Chen, R.; Wu, Y.; Huang, S.; et al. Mesenchymal Stem Cell–Derived Extracellular Vesicles in Joint Diseases: Therapeutic Effects and Underlying Mechanisms. J. Orthop. Transl. 2024, 48, 53–69. [Google Scholar] [CrossRef]
- Sadeghi, S.; Tehrani, F.R.; Tahmasebi, S.; Shafiee, A.; Hashemi, S.M. Exosome Engineering in Cell Therapy and Drug Delivery. Inflammopharmacol 2023, 31, 145–169. [Google Scholar] [CrossRef]
- Wu, Z.; He, D.; Li, H. Bioglass Enhances the Production of Exosomes and Improves Their Capability of Promoting Vascularization. Bioact. Mater. 2021, 6, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Miceli, V.; Bulati, M.; Iannolo, G.; Zito, G.; Gallo, A.; Conaldi, P.G. Therapeutic Properties of Mesenchymal Stromal/Stem Cells: The Need of Cell Priming for Cell-Free Therapies in Regenerative Medicine. Int. J. Mol. Sci. 2021, 22, 763. [Google Scholar] [CrossRef]
- Lian, M.; Qiao, Z.; Qiao, S.; Zhang, X.; Lin, J.; Xu, R.; Zhu, N.; Tang, T.; Huang, Z.; Jiang, W.; et al. Nerve Growth Factor-Preconditioned Mesenchymal Stem Cell-Derived Exosome-Functionalized 3D-Printed Hierarchical Porous Scaffolds with Neuro-Promotive Properties for Enhancing Innervated Bone Regeneration. ACS Nano 2024, 18, 7504–7520. [Google Scholar] [CrossRef]
- Liu, L.; Yu, F.; Chen, L.; Xia, L.; Wu, C.; Fang, B. Lithium-Containing Biomaterials Stimulate Cartilage Repair through Bone Marrow Stromal Cells-Derived Exosomal miR-455-3p and Histone H3 Acetylation. Adv. Healthc. Mater. 2023, 12, 2202390. [Google Scholar] [CrossRef] [PubMed]
- Yuan, N.; Ge, Z.; Ji, W.; Li, J. Exosomes Secreted from Hypoxia-Preconditioned Mesenchymal Stem Cells Prevent Steroid-Induced Osteonecrosis of the Femoral Head by Promoting Angiogenesis in Rats. BioMed Res. Int. 2021, 2021, e6655225. [Google Scholar] [CrossRef]
- Li, C.; Zhang, W.; Nie, Y.; Du, X.; Huang, C.; Li, L.; Long, J.; Wang, X.; Tong, W.; Qin, L.; et al. Time-Sequential and Multi-Functional 3D Printed MgO2/PLGA Scaffold Developed as a Novel Biodegradable and Bioactive Bone Substitute for Challenging Postsurgical Osteosarcoma Treatment. Adv. Mater. 2024, 36, 2308875. [Google Scholar] [CrossRef]
- Zhang, W.; Li, L.; Wang, Z.; Nie, Y.; Yang, Y.; Li, C.; Zhang, Y.; Jiang, Y.; Kou, Y.; Zhang, W.; et al. Injectable and Adhesive MgO2-Potentiated Hydrogel with Sequential Tumor Synergistic Therapy and Osteogenesis for Challenging Postsurgical Osteosarcoma Treatment. Biomaterials 2025, 315, 122959. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Zhang, W.; Deng, J.; Nie, Y.; Du, X.; Qin, L.; Lai, Y. 3D-Printed NIR-Responsive Shape Memory Polyurethane/Magnesium Scaffolds with Tight-Contact for Robust Bone Regeneration. Bioact. Mater. 2021, 16, 218–231. [Google Scholar] [CrossRef]
- Bai, L.; Song, P.; Su, J. Bioactive Elements Manipulate Bone Regeneration. Biomater. Transl. 2023, 4, 248–269. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, S.; Cheng, L.; Lin, Z.; Zeng, M.; Ruan, Z.; Sun, B.; Luo, Z.; Tang, Y.; Long, H. Mg2+-mediated Autophagy-dependent Polarization of Macrophages Mediates the Osteogenesis of Bone Marrow Stromal Stem Cells by Interfering with Macrophage-derived Exosomes Containing miR-381. J. Orthop. Res. 2022, 40, 1563–1576. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Y.; Qi, G. Evaluation of Isolation Methods and Culture Conditions for Rat Bone Marrow Mesenchymal Stem Cells. Cytotechnology 2013, 65, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Liu, N.; Bao, C.; Yang, D.; Ma, G.; Yi, W.; Xiao, G.; Cao, H. Mesenchymal Stem Cells in Fibrotic Diseases—The Two Sides of the Same Coin. Acta Pharmacol. Sin. 2023, 44, 268–287. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Huang, M.; Shi, Q.; Du, T.; Cao, Y. Cultivation and Identification of Rat Bone Marrow-derived Mesenchymal Stem Cells. Mol. Med. Rep. 2014, 10, 755–760. [Google Scholar] [CrossRef]
- Fang, S.; Li, Y.; Chen, P. Osteogenic Effect of Bone Marrow Mesenchymal Stem Cell-Derived Exosomes on Steroid-Induced Osteonecrosis of the Femoral Head. Drug Des. Dev. Ther. 2018, 13, 45–55. [Google Scholar] [CrossRef]
- Díaz-Tocados, J.M.; Herencia, C.; Martínez-Moreno, J.M.; Montes de Oca, A.; Rodríguez-Ortiz, M.E.; Vergara, N.; Blanco, A.; Steppan, S.; Almadén, Y.; Rodríguez, M.; et al. Magnesium Chloride Promotes Osteogenesis through Notch Signaling Activation and Expansion of Mesenchymal Stem Cells. Sci. Rep. 2017, 7, 7839. [Google Scholar] [CrossRef]
- Bernardini, D.; Nasulewic, A.; Mazur, A.; Maier, J.A.M. Magnesium and Microvascular Endothelial Cells: A Role in Inflammation and Angiogenesis. Front. Biosci. 2005, 10, 1177–1182. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, J.; Zhang, X.; Liang, G.; Xu, T.; Niu, W. Three-Dimensional Printed Mg-Doped β-TCP Bone Tissue Engineering Scaffolds: Effects of Magnesium Ion Concentration on Osteogenesis and Angiogenesis In Vitro. Tissue Eng. Regen. Med. 2019, 16, 415–429. [Google Scholar] [CrossRef]
- Lai, Y.; Li, Y.; Cao, H.; Long, J.; Wang, X.; Li, L.; Li, C.; Jia, Q.; Teng, B.; Tang, T.; et al. Osteogenic Magnesium Incorporated into PLGA/TCP Porous Scaffold by 3D Printing for Repairing Challenging Bone Defect. Biomaterials 2019, 197, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Weng, J.; Zhou, B.; Zhang, W.; Li, G.; Chen, Y.; Qi, T.; Zhu, Y.; Yu, F.; Zeng, H. Magnesium Ions Promote In Vitro Rat Bone Marrow Stromal Cell Angiogenesis through Notch Signaling. Biol. Trace Elem. Res. 2023, 201, 2823–2842. [Google Scholar] [CrossRef]
- Yoshizawa, S.; Brown, A.; Barchowsky, A.; Sfeir, C. Magnesium Ion Stimulation of Bone Marrow Stromal Cells Enhances Osteogenic Activity, Simulating the Effect of Magnesium Alloy Degradation. Acta Biomater. 2014, 10, 2834–2842. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Chen, H.; Huang, P.; Qi, J.; Qian, N.; Deng, L.; Guo, L. Glucocorticoids Impair Bone Formation of Bone Marrow Stromal Stem Cells by Reciprocally Regulating microRNA-34a-5p. Osteoporos. Int. 2016, 27, 1493–1505. [Google Scholar] [CrossRef]
- Zuo, R.; Kong, L.; Wang, M.; Wang, W.; Xu, J.; Chai, Y.; Guan, J.; Kang, Q. Exosomes Derived from Human CD34+ Stem Cells Transfected with miR-26a Prevent Glucocorticoid-Induced Osteonecrosis of the Femoral Head by Promoting Angiogenesis and Osteogenesis. Stem Cell Res. Ther. 2019, 10, 321. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, L.; Liu, H.; Zhang, J.; Tang, P. Exosomes Promote hFOB1.19 Proliferation and Differentiation via LINC00520. J. Orthop. Surg. Res. 2023, 18, 546. [Google Scholar] [CrossRef]
- Kong, L.; Zuo, R.; Wang, M.; Wang, W.; Xu, J.; Chai, Y.; Guan, J.; Kang, Q. Silencing MicroRNA-137-3p, Which Targets RUNX2 and CXCL12 Prevents Steroid-Induced Osteonecrosis of the Femoral Head by Facilitating Osteogenesis and Angiogenesis. Int. J. Biol. Sci. 2020, 16, 655–670. [Google Scholar] [CrossRef]
- Xu, W.-N.; Zheng, H.-L.; Yang, R.-Z.; Jiang, L.-S.; Jiang, S.-D. HIF-1α Regulates Glucocorticoid-Induced Osteoporosis Through PDK1/AKT/mTOR Signaling Pathway. Front. Endocrinol. 2019, 10, 922. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Cao, H.; Cui, X.; Fan, Y.; Wang, Q.; Zhang, X. Optimization of Exosome-Based Cell-Free Strategies to Enhance Endogenous Cell Functions in Tissue Regeneration. Acta Biomater. 2023, 171, 68–84. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liang, X.; Zheng, K.; Ge, G.; Chen, X.; Xu, Y.; Bai, J.; Pan, G.; Geng, D. Horizon of Exosome-Mediated Bone Tissue Regeneration: The All-Rounder Role in Biomaterial Engineering. Mater. Today Bio 2022, 16, 100355. [Google Scholar] [CrossRef]
- Zhu, Y.; Jia, Y.; Wang, Y.; Xu, J.; Chai, Y. Impaired Bone Regenerative Effect of Exosomes Derived from Bone Marrow Mesenchymal Stem Cells in Type 1 Diabetes. Stem Cells Transl. Med. 2019, 8, 593–605. [Google Scholar] [CrossRef]
- Zhu, D.; You, J.; Zhao, N.; Xu, H. Magnesium Regulates Endothelial Barrier Functions through TRPM7, MagT1, and S1P1. Adv. Sci. 2019, 6, 1901166. [Google Scholar] [CrossRef]
- Liu, W.; Guo, S.; Tang, Z.; Wei, X.; Gao, P.; Wang, N.; Li, X.; Guo, Z. Magnesium Promotes Bone Formation and Angiogenesis by Enhancing MC3T3-E1 Secretion of PDGF-BB. Biochem. Biophys. Res. Commun. 2020, 528, 664–670. [Google Scholar] [CrossRef]
- Deng, L.; Liu, Y.; Wu, Q.; Lai, S.; Yang, Q.; Mu, Y.; Dong, M. Exosomes to Exosome-Functionalized Scaffolds: A Novel Approach to Stimulate Bone Regeneration. Stem Cell Res. Ther. 2024, 15, 407. [Google Scholar] [CrossRef]
- Ma, W.; Yang, Z.; Lu, M.; Ma, H.; Wu, C.; Lu, H. Hierarchically Structured Biomaterials for Tissue Regeneration. Microstructures 2024, 4, 2024014. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Cheng, L.; Du, Y.; Zhang, Y.; Wang, Z.; Nie, Y.; Long, J.; Li, C.; Zhang, Y.; Lai, Y.; et al. Exosomes Derived from Mg-Preconditioned Bone Mesenchymal Stem Cells Promote Angiogenesis and Osteogenesis for Osteonecrosis Treatment. Materials 2025, 18, 4687. https://doi.org/10.3390/ma18204687
Li L, Cheng L, Du Y, Zhang Y, Wang Z, Nie Y, Long J, Li C, Zhang Y, Lai Y, et al. Exosomes Derived from Mg-Preconditioned Bone Mesenchymal Stem Cells Promote Angiogenesis and Osteogenesis for Osteonecrosis Treatment. Materials. 2025; 18(20):4687. https://doi.org/10.3390/ma18204687
Chicago/Turabian StyleLi, Long, Luyao Cheng, Yuhan Du, Yuyang Zhang, Zetao Wang, Yangyi Nie, Jing Long, Cairong Li, Yuanchi Zhang, Yuxiao Lai, and et al. 2025. "Exosomes Derived from Mg-Preconditioned Bone Mesenchymal Stem Cells Promote Angiogenesis and Osteogenesis for Osteonecrosis Treatment" Materials 18, no. 20: 4687. https://doi.org/10.3390/ma18204687
APA StyleLi, L., Cheng, L., Du, Y., Zhang, Y., Wang, Z., Nie, Y., Long, J., Li, C., Zhang, Y., Lai, Y., & Zhang, W. (2025). Exosomes Derived from Mg-Preconditioned Bone Mesenchymal Stem Cells Promote Angiogenesis and Osteogenesis for Osteonecrosis Treatment. Materials, 18(20), 4687. https://doi.org/10.3390/ma18204687






