Catalytic Combustion of Methane over Pd-Modified La-Ce-Zr-Al Catalyst
Abstract
1. Introduction
2. Materials and Methods
2.1. Catalysts Preparation
2.2. Characterization Techniques
2.3. Catalytic Activity Measurement
3. Results and Discussion
3.1. Catalytic Tests on Pd/La-Ce-Zr-Al and Pd/La-Ce-Zr-Alc Catalysts
3.2. Physico-Chemical Characterization of the Catalysts
3.3. O2–Temperature-Programmed Desorption (TPD) and CH4-Temperature-Programmed Reduction (TPR)
3.4. Reaction Kinetics of Pd/La-Ce-Zr-Al Catalyst
3.5. Reactor Modeling
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, P.; Yadav, D.; Pandian, S. 5—Link between air pollution and global climate change. In Global Climate Change; Elsevier: Amsterdam, The Netherlands, 2021; pp. 79–108. [Google Scholar] [CrossRef]
- Ramanthan, V.; Feng, Y. Air pollution, greenhouse gases and climate change: Global and regional perspectives. Atmos. Environ. 2009, 43, 37–50. [Google Scholar] [CrossRef]
- Available online: https://www.eea.europa.eu/en/topics/in-depth/air-pollution (accessed on 9 April 2025).
- Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32021R1119 (accessed on 30 June 2021).
- Lee, Y.G.; Lee, P.H.; Choi, S.M.; An, M.H.; Jang, A.S. Effects of Air Pollutants on Airway Diseases. Int. J. Environ. Res. Public Health 2021, 18, 9905. [Google Scholar] [CrossRef]
- Afifa; Arshad, K.; Hussain, N.; Ashraf, M.H.; Saleem, M.Z. Air pollution and climate change as grand challenges to sustainability. Sci. Total Environ. 2024, 928, 172370. [Google Scholar] [CrossRef]
- Shindell, D.; Faluvegi, G.; Koch, D.; Schmidt, G.; Unger, N.; Bauer, S. Improved attribution of climate forcing to emissions. Science 2009, 326, 716–718. [Google Scholar] [CrossRef] [PubMed]
- Arendt, E.; Maione, A.; Klisinska, A.; Sanz, O.; Montes, M.; Suarez, S.; Blanco, J.; Ruiz, P. Structuration of LaMnO3 perovskite catalysts on ceramic and metallic monoliths: Physico-chemical characterization and catalytic activity in methane combustion. Appl. Catal. A Gen. 2008, 339, 1–14. [Google Scholar] [CrossRef]
- Barrera, A.; Fuentes, S.; Diaz, G.; Gomez-Cortes, A.; Tzompantzi, F.; Molina, J.C. Methane oxidation over Pd catalysts supported on binary Al2O3–La2O3 oxides prepared by the sol–gel method. Fuel 2012, 93, 136–141. [Google Scholar] [CrossRef]
- Setiawan, A.; Kennedy, E.M.; Dlugogorski, B.Z.; Adesina, A.A.; Stockenhuber, M. The stability of Co3O4, Fe2O3, Au/Co3O4 and Au/Fe2O3 catalysts in the catalytic combustion of lean methane mixtures in the presence of water. Catal. Today 2015, 258, 276–283. [Google Scholar] [CrossRef]
- Seeburg, D.; Liu, D.J.; Radnik, J.; Atia, H.; Pohl, M.M.; Schneider, M.; Martin, A.; Wohlrab, S. Structural changes of highly active Pd/MeOx (Me = Fe, Co, Ni) during catalytic methane combustion. Catalysts 2018, 8, 42. [Google Scholar] [CrossRef]
- Stoian, M.; Rogé, V.; Lazar, L.; Maurer, T.; Védrine, J.C.; Marcu, I.-C.; Fechete, I. Total Oxidation of methane on oxide and mixed oxide ceria-containing. Catalysts. Catalysts 2021, 11, 427. [Google Scholar] [CrossRef]
- He, L.; Fan, Y.L.; Bellettre, J.; Yue, J.; Luo, L.G. A review on catalytic methane combustion at low temperatures: Catalysts, mechanisms, reaction conditions and reactor designs. Renew. Sustain. Energy Rev. 2020, 119, 109589. [Google Scholar] [CrossRef]
- Jiang, D.; Khivantsev, K.; Wang, Y. Low-Temperature methane oxidation for efficient emission control in natural gas vehicles: Pd and Beyond. Catalysts 2020, 10, 23, 14304–14314. [Google Scholar] [CrossRef]
- Centi, G. Supported palladium catalysts in environmental catalytic technologies for gaseous emissions. J. Mol. Catal. A Chem. 2001, 173, 287–312. [Google Scholar] [CrossRef]
- Xiao, L.; Sun, K.; Xu, X. Catalytic combustion of methane over CeO2–MOx(M = La3+, Ca2+) solid solution promoted Pd/c-Al2O3 Catalysts. Acta Phys.–Chim. Sin. 2008, 24, 2108–2113. [Google Scholar] [CrossRef]
- Tao, J.; Liu, Y.; Deng, J.; Jing, L.; Hou, Z.; Wei, L.; Wang, Z.; Dai, H. Methane combustion over zeolite-supported palladium-based catalysts. Catalysts 2023, 13, 1251. [Google Scholar] [CrossRef]
- Yue, B.; Zhou, R.; Wang, Y.; Zheng, X. Effect of rare earths (La, Pr, Nd, Sm, and Y) on the methane combustion over Pd/Ce–Zr/Al2O3 catalysts. Appl. Catal. A Gen. 2005, 295, 31–39. [Google Scholar] [CrossRef]
- Arai, H.; Fukuzawa, H. Research and development on high temperature catalytic combustion. Catal. Today 1995, 26, 217–221. [Google Scholar] [CrossRef]
- Furuya, T.; Sasaki, K.; Hanakata, Y.; Ohhashi, T.; Yamada, M.; Tsuchiya, T.; Furuse, Y. Development of a hybrid catalytic combustor for a 1300◦C class gas turbine. Catal. Today 1995, 26, 345–350. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, B.; Chen, Y.; Ren, C.; Chen, Y. Rare earths (Ce, Y, Pr) modified Pd/La2O3-ZrO2-Al2O3 catalysts used in lean-burn natural gas fueled vehicles. J. Rare Earth 2017, 35, 1077–1082. [Google Scholar] [CrossRef]
- Ozawa, Y.; Tochihara, Y.; Watanabe, A.; Nagai, M.; Omi, S. Stabilizing effect of Nd2O3, La2O3 and ZrO2 on Pt·PdO/Al2O3 during catalytic combustion of methane. Appl. Catal. A Gen. 2004, 258, 261–267. [Google Scholar] [CrossRef]
- Zhang, B.; Li, D.; Wang, X. Catalytic performance of La–Ce–O mixed oxide for combustion of methane. Catal. Today 2010, 158, 348–353. [Google Scholar] [CrossRef]
- Gil, S.; Garcia-Vargas, M.J.; Liotta, F.L.; Pantaleo, G.; Ousmane, M.; Retailleau, L.; Giroir-Fendler, A. Catalytic oxidation of propene over Pd catalysts supported on CeO2, TiO2, Al2O3 and M/Al2O3 Oxides (M = Ce, Ti, Fe, Mn). Catalysts 2015, 5, 671–689. [Google Scholar] [CrossRef]
- Martínez-Arias, A.; Fernández-García, M.; Hungría, A.; Iglesias-Juez, A.; Duncan, K.; Smith, R.; Anderson, J.; Conesa, J.; Soria, J. Effect of thermal sintering on light-off performance of Pd/(Ce,Zr)Ox/Al2O3 three-way catalysts: Model gas and engine tests. J. Catal. 2001, 204, 238–248. [Google Scholar] [CrossRef]
- Vlaic, G.; Fornasiero, P.; Geremia, S.; Kaspar, J.; Graziani, M. Relationship between the zirconia-promoted reduction in the Rh-Loaded Ce0.5Zr0.5O2 mixed oxide and the Zr–O local structure. J. Catal. 1997, 168, 386–392. [Google Scholar] [CrossRef]
- Jeong, M.; Nunotani, N.; Moriyama, N.; Imanaka, N. High methane combustion activity of PdO/CeO2–ZrO2–NiO/Al2O3 catalysts. J. Asian Ceram. Soc. 2016, 4, 259–262. [Google Scholar] [CrossRef]
- Mortensen, L.R.; Noack, H.-D.; Pedersen, K.; Dunstan, M.; Wilhelm, F.; Rogalev, A.; Pedersen, K.; Mielby, J.; Mossin, S. Understanding the reversible and irreversible deactivation of methane oxidation catalysts. Appl. Catal. B-Environ. Energy 2024, 344, 123646. [Google Scholar] [CrossRef]
- Mortensen, R.L.; Noack, H.-D.; Pedersen, K.; Mossin, S.; Mielby, J. Recent Advances in complete methane oxidation using zeolite-supported metal nanoparticle catalysts. ChemCatChem 2022, 14, e202101924. [Google Scholar] [CrossRef]
- Pecchi, G.; Reyes, P.; López, T.; Gómez, R.; Moreno, A.; Fierro, J.L.G.; Martínez-Arias, A. Catalytic combustion of methane on Fe-TiO2 catalysts prepared by sol-gel method. J. Sol-Gel Sci. Tehnol. 2003, 27, 205–214. [Google Scholar] [CrossRef]
- Muñoz, H.J.; Korili, S.A.; Gil, A. Progress and recent strategies in the synthesis and catalytic applications of perovskites based on lanthanum and aluminum. Materials 2022, 15, 3288. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Shan, W.; He, H. Comparative investigation of methane combustion over palladium-based catalysts supported on mesoporous MgAl2O4 and Al2O3. Fuel 2023, 340, 127493. [Google Scholar] [CrossRef]
- Zhou, K.B.; Chen, H.D.; Tian, Q.; Hao, Z.P.; Shen, D.X.; Xu, X.B. Pd-containing perovskite-type oxides used for three-way catalysts. J. Mol. Catal. A Chem. 2002, 189, 225–232. [Google Scholar] [CrossRef]
- Chen, J.; Arandiyan, H.; Gao, X.; Li, J. Recent advances in catalysts for methane combustion. Catal. Surv. Asia 2015, 19, 140–171. [Google Scholar] [CrossRef]
- Ciambelli, P.; Palma, V.; Palo, E. Comparison of ceramic honeycomb monolith and foam as Ni catalyst carrier for methane autothermal reforming. Catal. Today 2010, 155, 92–100. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H.; Hu, Z.; Yao, M.; Li, Y. A Review on the Pd-based three-way catalyst. Catal. Rev. 2014, 57, 79–144. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, S.; Tang, X.; Chen, C.; Yi, H. Stainless steel catalyst for air pollution control: Structure, properties, and activity. Environ. Sci. Pollut. Res. 2022, 29, 55367–55399. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Lu, j.; Fedeyko, j.; Raj, A. Zeolite supported Pd catalysts for the complete oxidation of methane: A critical review. Appl. Catal. A Gen. 2022, 633, 118534. [Google Scholar] [CrossRef]
- Chen, L.; Liu, G.; Feng, N.J.; Yu, J.H.; Meng, J.; Fang, F.; Zhao, P.; Wang, L.; Wan, H.; Guan, G.F. Effect of calcination temperature on structural properties and catalytic soot combustion activity of MnOx/wire-mesh monoliths. Appl. Surf. Sci. 2019, 467–468, 1088–1103. [Google Scholar] [CrossRef]
- Labhsetwar, N.; Doggali, P.; Rayalu, S.; Yadav, R.; Mistuhashi, T.; Haneda, H. Ceramics in environmental catalysis: Applications and possibilities. Chin. J. Catal. 2012, 33, 1611–1621. [Google Scholar] [CrossRef]
- Yoldas, B.E. Alumina gels that form porous transparent Al2O3. J. Mater. Sci. 1975, 10, 1856–1860. [Google Scholar] [CrossRef]
- Gomez-Serrano, V.; Gonzalez-Garcia, C.; Gonzalez-Martın, M. Nitrogen adsorption isotherms on carbonaceous materials, Comparison of BET and Langmuir surface areas. Powder. Technol. 2001, 116, 103–108. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I.Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, Z.; Xiao, M.; Zhang, C.; Ruan, L.; Zhang, Y.; Zhong, Y.; Yan, Y.; Yu, Y.; He, H. Catalytic performance of Pd catalyst supported on CeO2 or ZrO2 modified beta zeolite for methane oxidation. J. Environ. Sci. 2025, 152, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.A.; Hao, J.; Farooq, A. Catalytic performance of Pd catalyst supported on Zr:Ce modified mesoporous silica for methane oxidation. Chem. Eng. J. 2020, 397, 125489. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area and Porosity; Academia Press: London, UK, 1991; pp. 111–194. [Google Scholar]
- Amairia, C.; Fessi, S.; Ghorbel, A.; Rives, A. Methane oxidation behaviour over sol–gel derived Pd/Al2O3-ZrO2 materials: Influence of the zirconium precursor. J. Mol. Catal. A Chem. 2010, 332, 25–31. [Google Scholar] [CrossRef]
- Niu, C.-M.; Rieger, P.H.; Dwight, K.; Wold, A. Preparation and properties of the system, CuxPd1−xO (0≦x≦0.175). J. Solid State Chem. 1990, 86, 175–179. [Google Scholar] [CrossRef]
- Zhou, R.; Zhao, B.; Yue, B. Effects of CeO2-ZrO2 present in Pd/Al2O3 catalysts on the redox behavior of PdOx and their combustion activity. J. Catal. 1999, 187, 275–284. [Google Scholar] [CrossRef]
- Wenge, L.; Deyong, G.; Xin, X. Research progress of palladium catalysts for methane combustion. China Petrol. Proc. Petrochem. Technol. Rev. 2012, 14, 1–9. [Google Scholar] [CrossRef]
- de la Peña O’Shea, V.A.; Alvarez-Galvan, M.C.; Fierro, J.L.G.; Arias, P.L. Influence of feed composition on the activity of Mn and PdMn/Al2O3 catalysts for combustion of formaldehyde/methanol. Appl. Catal. B 2005, 57, 191–199. [Google Scholar] [CrossRef]
- Colussi, S.; Gayen, A.; Farnesi Camellone, M.; Boaro, M.; Llorca, J.; Fabris, S.; Trovarelli, A. Nanofaceted Pd–O Sites in Pd–Ce surface superstructures: Enhanced sctivity in satalytic sombustion of methane. Angew. Chem. Int. Ed. 2009, 48, 8481–8484. [Google Scholar] [CrossRef]
- Colussi, S.; Gayen, A.; Boaro, M.; Llorca, J.; Trovarelli, A. Influence of different palladium precursors on the properties of solution-Combustion-Synthesized Palladium/Ceria Catalysts for Methane Combustion. ChemCatChem 2015, 7, 2222–2229. [Google Scholar] [CrossRef]
- Scanlon, D.O.; Morgan, B.J.; Watson, G.W. The origin of the enhanced oxygen storage capacity of Ce1−x(Pd/Pt)xO2. Phys. Chem. Chem. Phys. 2011, 13, 4279–4284. [Google Scholar] [CrossRef]
- Khader, M.M.; Al-Marri, M.J.; Ali, S.; Abdelmoneim, A.G. Active and stable methane oxidation nano-catalyst with highly-ionized palladium species prepared by solution combustion synthesis. Catalysts 2018, 8, 66. [Google Scholar] [CrossRef]
- Wang, T.; Li, F.; An, H.; Xue, W.; Wang, Y. Enhanced catalytic activity over palladium supported on ZrO2@C with NaOH-assisted reduction for decomposition of formic acid. RSC Adv. 2019, 9, 3359. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.D.; Riggs, W.M.; Davis, L.E.; Moulder, J.F.; Muilenberg, G.E. (Eds.) Handbook of X-Ray Photoelectron Spectroscopy; Perkin-Elmer Corporation, EdenPrairie: Waltham, MA, USA, 1978. [Google Scholar]
- Yu, X.; Li, G. XPS study of cerium conversion coating on theanodized 2024 aluminum alloy. J. Alloys Compd. 2004, 364, 193–198. [Google Scholar] [CrossRef]
- Burroughs, P.; Hamnett, A.; Orchard, A.F.; Thornton, G. Satellite structure in the X-ray photoelectron spectra of some binary and mixed oxides of lanthanum and cerium. J. Chem. Soc. Dalton Trans. 1976, 17, 1686–1698. [Google Scholar] [CrossRef]
- Su, S.C.; Carstens, J.N.; Bell, A.T. A study of the dynamics of Pd oxidation and PdO reduction by H2 and CH4. J. Catal. 1998, 176, 125–135. [Google Scholar] [CrossRef]
- Stasinska, B.; Machocki, A.; Antoniak, K.; Rotko, M.; Figueiredo, J.L.; Goncalves, F. Importance of palladium dispersion in Pd/Al2O3 catalysts for complete oxidation, of humid low-methane–air mixtures. Catal. Today 2008, 137, 329–334. [Google Scholar] [CrossRef]
- Todorova, S.; Naydenov, A.; Kolev, H.; Holgado, J.P.; Ivanov, G.; Kadinov, G.; Caballero, A. Mechanism of complete n-hexane oxidation on silica supported cobalt and manganese catalysts. Appl. Catal. A Gen. 2012, 413–414, 43–51. [Google Scholar] [CrossRef]
- Markova-Velichkova, M.; Lazarova, T.; Tumbalev, V.; Ivanov, G.; Kovacheva, D.; Stefanov, P.; Naydenov, A. Complete oxidation of hydrocarbons on YFeO3 and LaFeO3 catalysts. Chem. Eng. J. 2013, 231, 236–244. [Google Scholar] [CrossRef]
- Mars, P.; van Krevelen, D.W. Oxidations carried out by means of vanadium oxide catalysts. Spec. Suppl. Chem. Eng. Sci. 1954, 3, 41–59. [Google Scholar] [CrossRef]
- Vannice, M.A.; Hyun, S.H.; Kalpakci, S.H.; Liauh, W.C. Entropies of adsorption in heterogeneous catalytic reactions. J. Catal. 1979, 56, 358–362. [Google Scholar] [CrossRef]
- Vannice, M.A. Kinetics of Catalytic Reactions; Springer Science-Business Media, Inc.: New York, NY, USA, 2005. [Google Scholar]
- Belfiore, L.A. Transport Phenomena for Chemical Reactor Design; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003. [Google Scholar]
- Levenspiel, O. Chemical Reactor Engineering, 3rd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1999. [Google Scholar]
- Nauman, E.B. Chemical Reactor Design, Optimization, and Scaleup; McGraw-Hill Companies: New York, NY, USA, 2002. [Google Scholar]
- Satterfield, C.N. Mass Transfer in Heterogeneous. Catalysis; MIT Press: Cambridge, MA, USA, 1970. [Google Scholar]
- Tomašić, V. Application of the monoliths in DeNOx catalysis. Catal. Tod. 2007, 119, 106–113. [Google Scholar] [CrossRef]
- Tomašić, S.; Gomzi, Z. Experimental and theoretical study of NO decomposition in a catalytic monolith reactor. Chem. Eng. Proc. 2004, 43, 765–774. [Google Scholar] [CrossRef]
- Tomašić, V.; Jović, F. State-of-the-art in the monolithic catalysts/reactors. Appl. Catal. Gen. 2006, 311, 112–121. [Google Scholar] [CrossRef]
- Armenta, M.A.; Maytorena, V.M.; Buentello-Montoya, D.A.; Arroyo, E.; Cota-Leal, M.; Yong, D.; Olivas, A. Effect of catalytic hydrodynamics over microagglomerates of Mn2O3 and PdO supported on γ-χ-Al2O3 for dimethyl ether production. Fuel 2022, 317, 123509. [Google Scholar] [CrossRef]
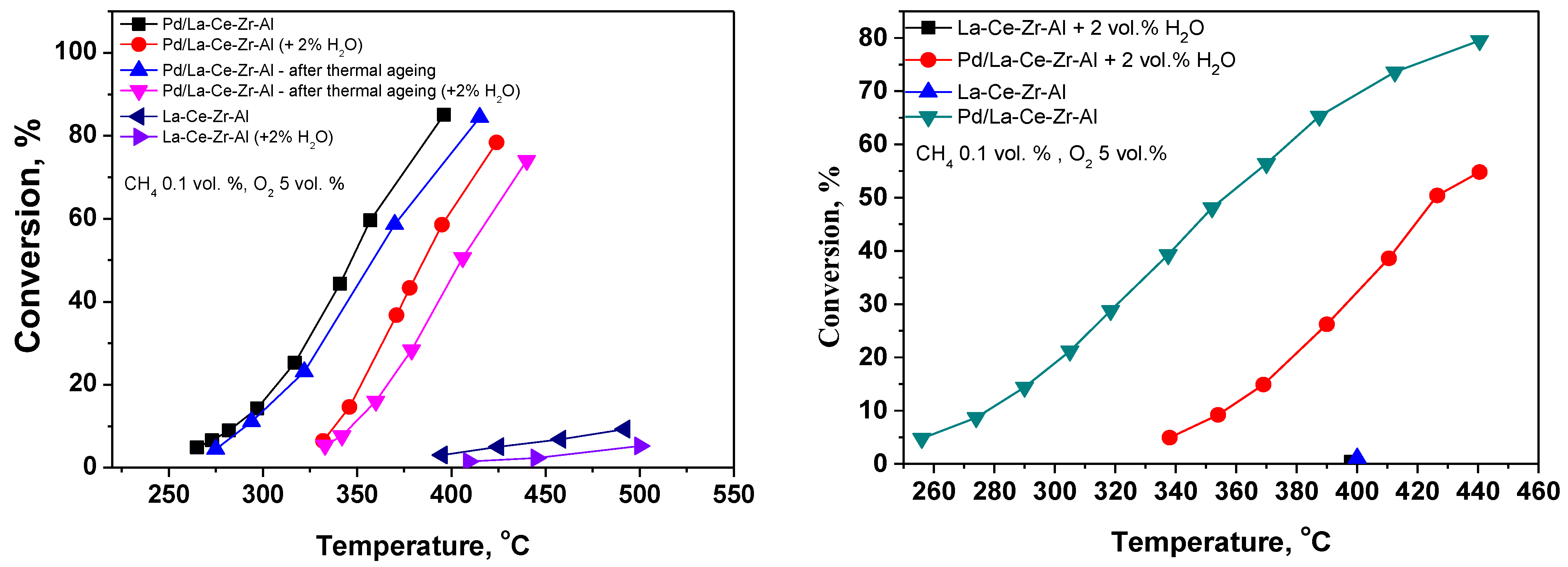
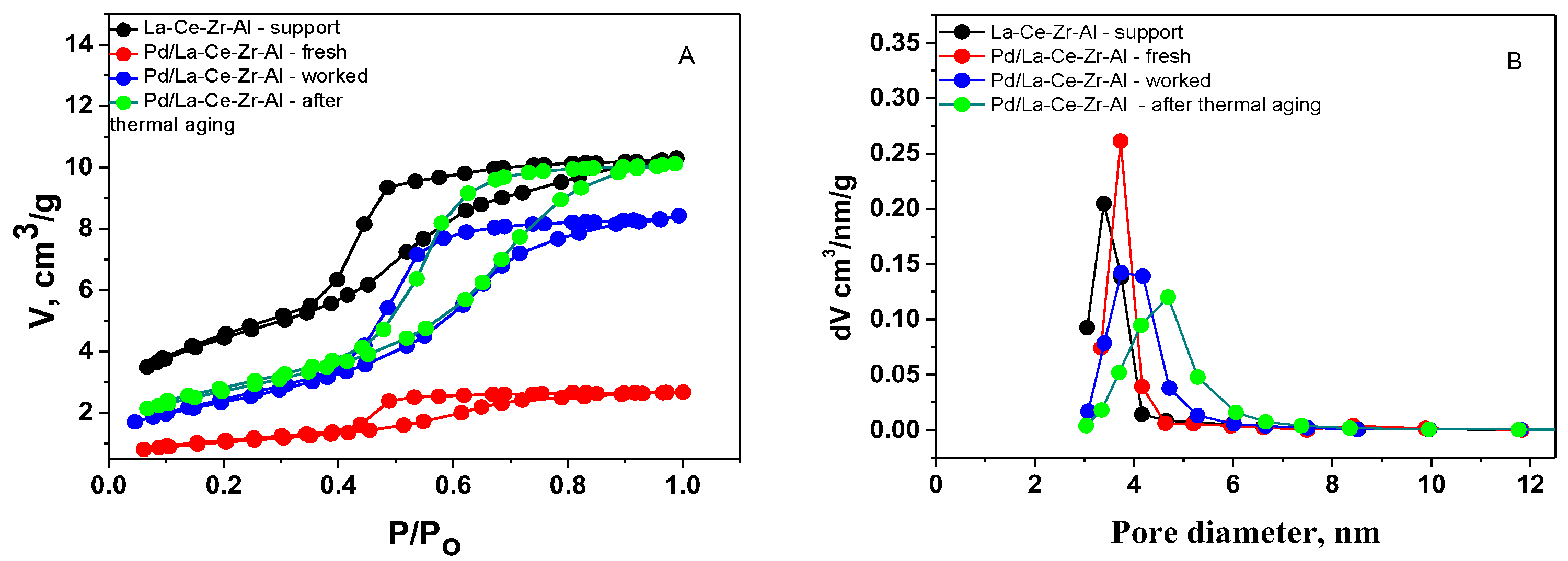
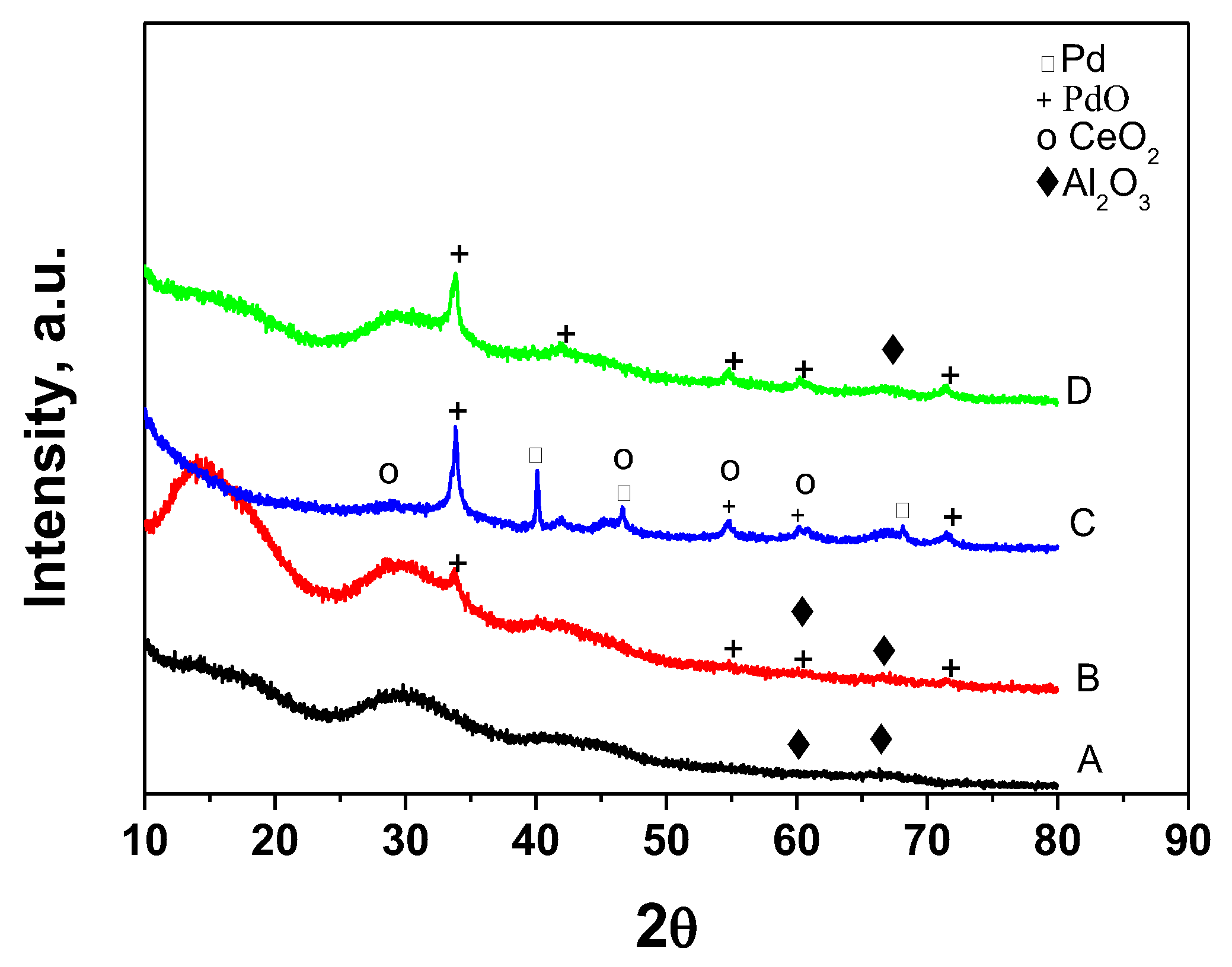
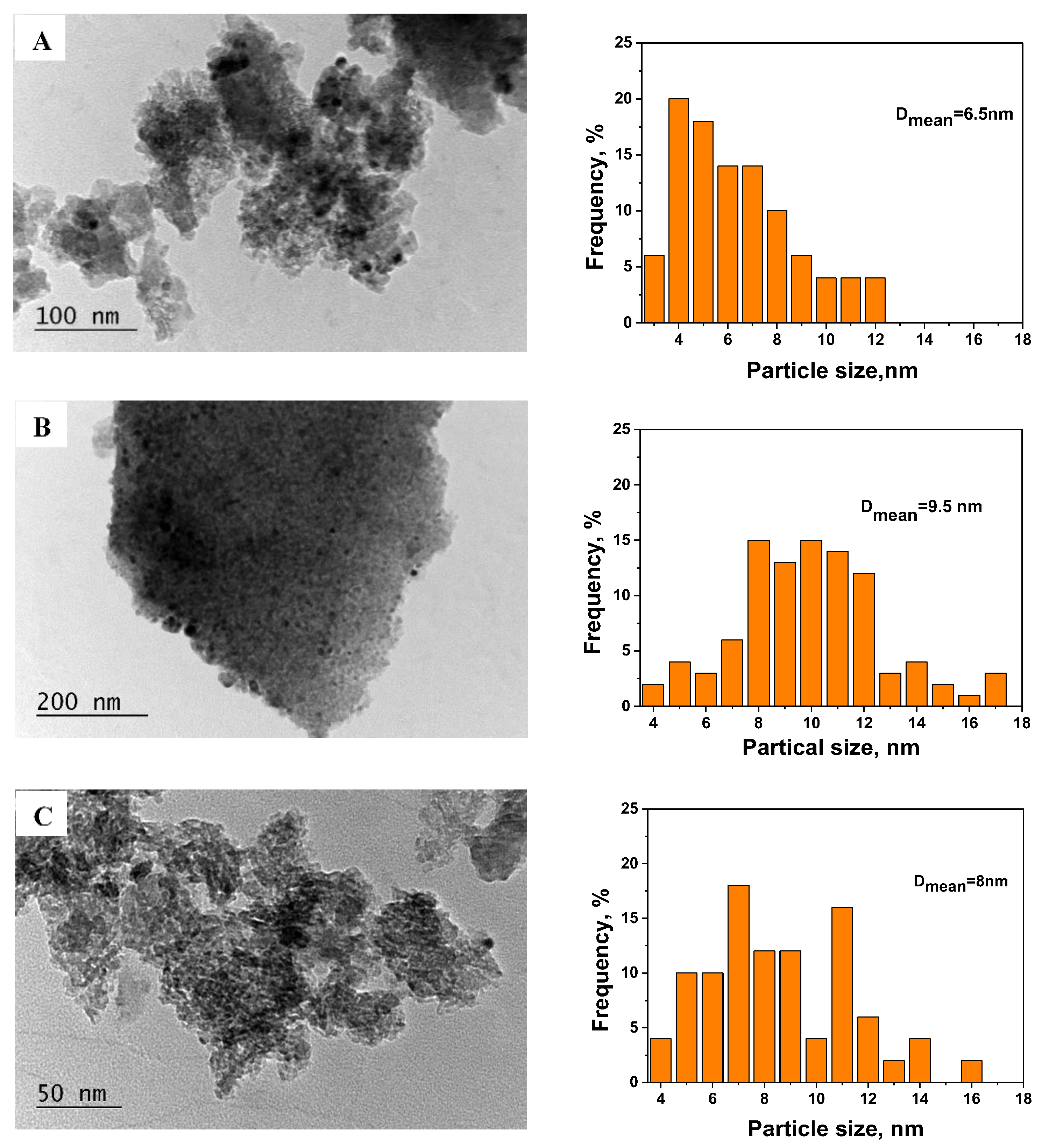
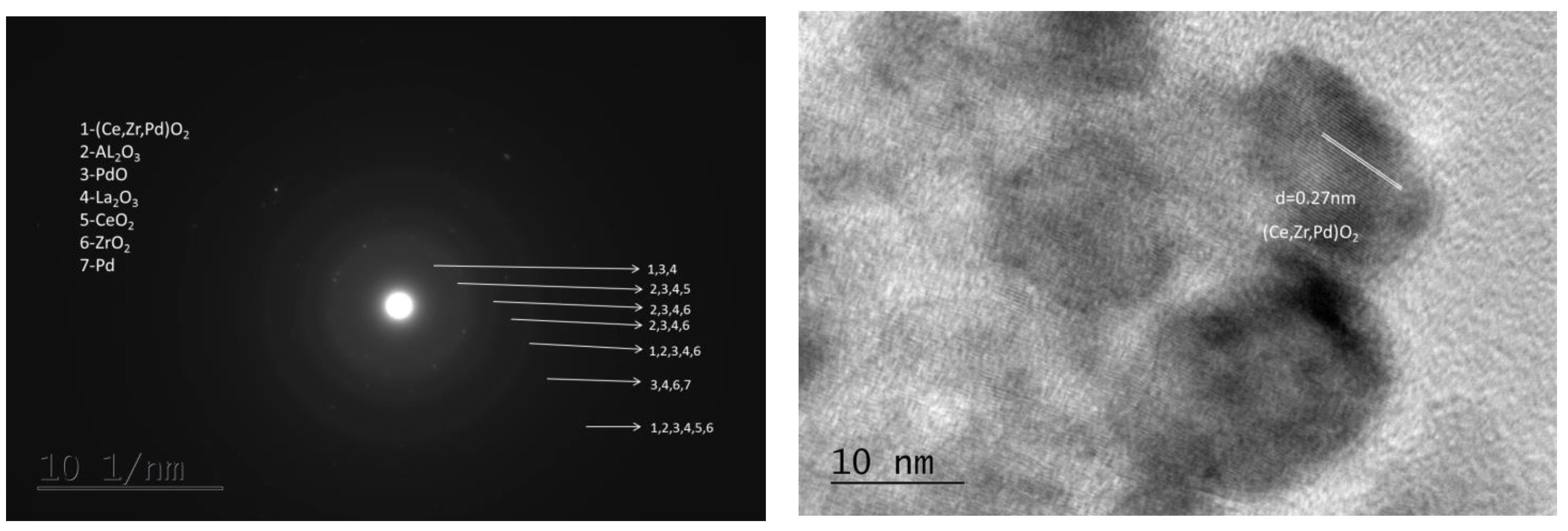
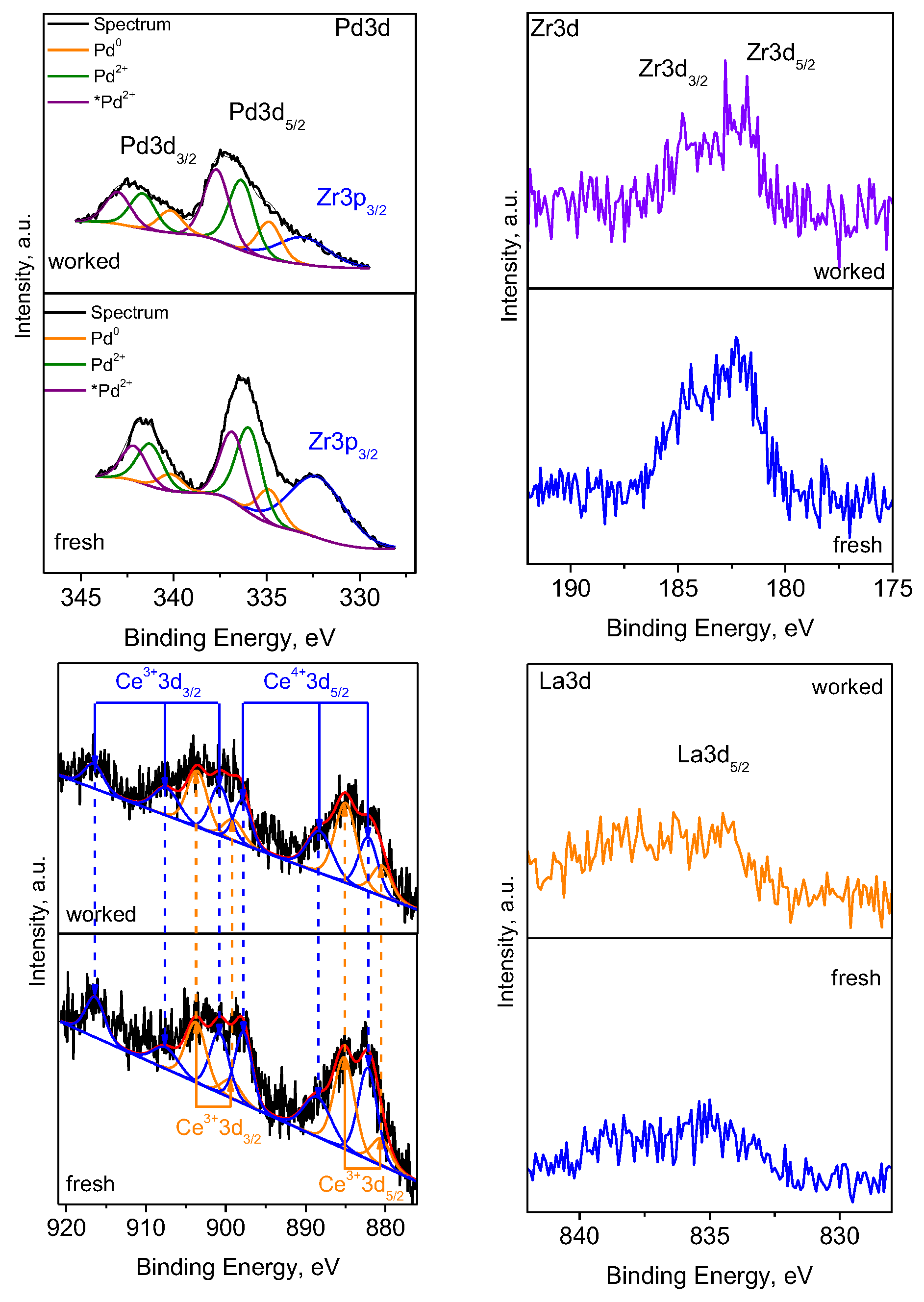
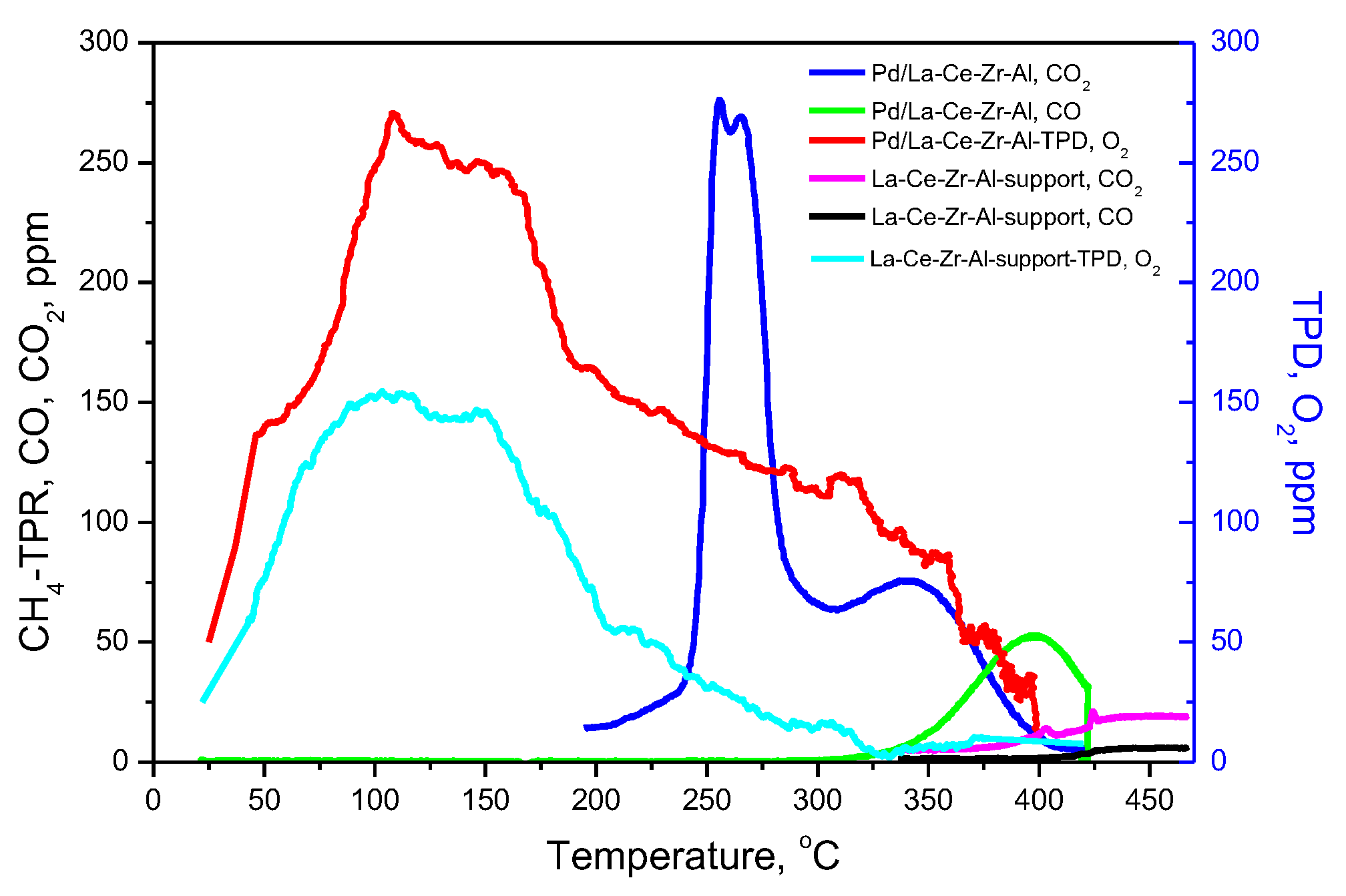
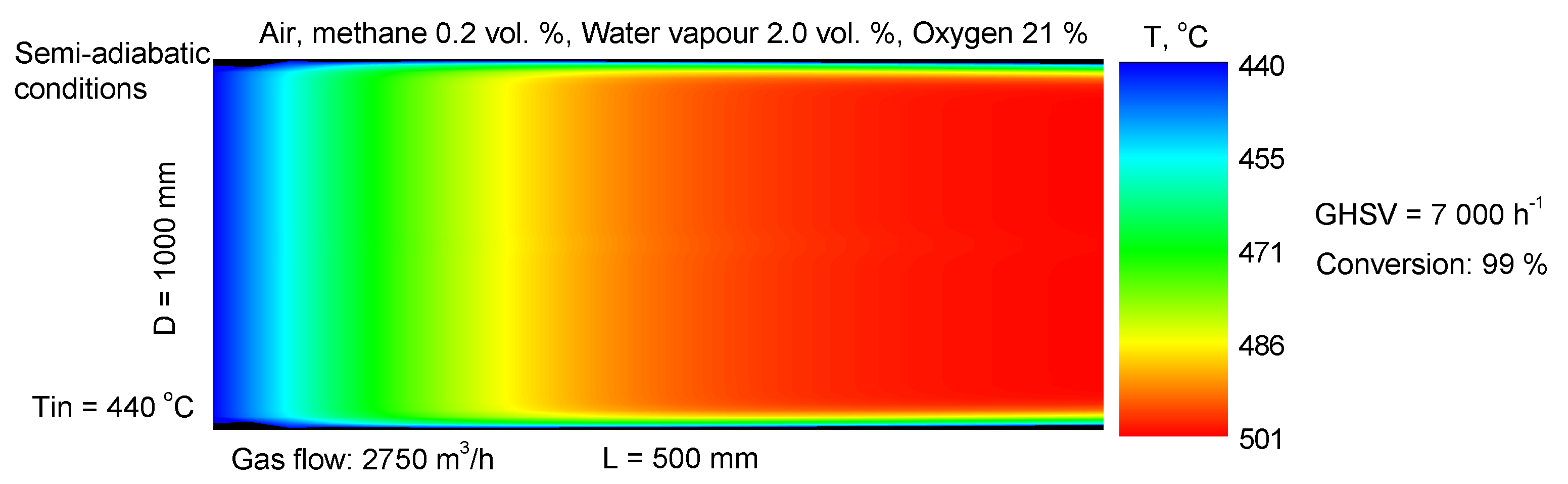
| Sample | SBET m2/g | Vt cm3/g | Dav nm |
|---|---|---|---|
| La-Ce-Zr-Al—support | 188 | 0.19 | 4.1 |
| Pd/La-Ce-Zr-Al—fresh | 158 | 0.18 | 4.6 |
| Pd/La-Ce-Zr-Al—worked | 115 | 0.17 | 6.0 |
| Pd/La-Ce-Zr-Al—thermal aged | 112 | 0.18 | 6.5 |
| Sample | Amorphous | γ-Al2O3 | (Ce, Zr, Pd)O2 Cubic Fm-3m | PdO Tetragonal P42/mmn | Pd Cubic Fm-3m |
|---|---|---|---|---|---|
| La-Ce-Zr-Al—support | 96% | broad diffuse peaks 4% | - <2 nm | - | - |
| Pd/La-Ce-Zr-Al—fresh | 91% | broad diffuse peaks 4% | - <2 nm | a = 3.042(7) Å c = 5.35(3) Å 4% 9nm | - |
| Pd/La-Ce-Zr-Al—worked | - | 86% 3 nm | 5.34(3) Å 8% 3 nm | a = 3.03(1) Å c = 5.36(3) Å 4% 18 nm | 3.89(1) Å 2% 68 nm |
| Pd/La-Ce-Zr—thermal aging | 96% | broad diffuse peaks 3% 4 nm | - <2 nm | a = 3.044(2) Å c = 5.340(4) Å 2% 19 nm | - |
| Samples | O | Pd | Ce | La | Zr | Al |
|---|---|---|---|---|---|---|
| Nominal preset | 40.2 | 2 | 6.5 | 3.4 | 5.9 | 42 |
| Pd/La-Ce-Zr-Al fresh | 56.5 | 6.6 | 1.2 | 0.5 | 3.6 | 31.6 |
| Pd/La-Ce-Zr-Al worked | 55.3 | 7.2 | 1.4 | 1.0 | 3.3 | 31.8 |
| Samples | Pd0 (%) | Pd2+ (%) | *Pd2+ (%) | Ce4+ (%) | Ce3+ (%) |
|---|---|---|---|---|---|
| Pd/La-Ce-Zr-Al fresh | 17.1 | 45.6 | 37.3 | 54.3 | 45.7 |
| Pd/La-Ce-Zr-Al worked | 19.7 | 40.0 | 40.3 | 40.0 | 60.0 |
| PWL | |||||||
|---|---|---|---|---|---|---|---|
| Ea | ko | m (CH4) | n (O2) | p (H2O) | RSS | R2 | |
| Pd/La-Ce-Zr-Al | 122.8 | 8.40 × 1010 | 1.02 | 0.01 | −0.33 | 15.5 | 0.999 |
| LH-DS-D: Water Compete with Oxygen and Methane | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ea | ko | −ΔHox | ko.ox | ΔHvoc | ko.voc | −ΔHwater-ox | ko.water-ox | −ΔHwater-red | ko.water-red | RSS | R2 | |
| Pd/La-Ce-Zr-Al | 143.8 | 3.03 × 1012 | 146.7 | 2.31 × 103 | 74.5 | 7.68 × 10−6 | 86.2 | 8.03 × 10−1 | 88.4 | 6.92 × 10−7 | 6.1 | 0.985 |
| Model: MVK-SDP, (Water Adsorbs on Oxidized and Reduced Sites, Slow Desorption of Products) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ea | ko | −ΔHox | ko.ox | −ΔHvoc | ko.voc | −ΔHwater-ox | ko.water-ox | −ΔHwater-red | ko.water-red | RSS | R2 | |
| Pd/La-Ce-Zr-Al | 120.3 | 2.43 × 1011 | 62.4 | 6.99 × 106 | 97.3 | 1.5 × 10−7 | 67.4 | 1.00 × 10−6 | 134.8 | 5.06 × 1011 | 5.0 | 0.995 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tumbalova, K.; Zlatanova, Z.; Velinova, R.; Shipochka, M.; Markov, P.; Kovacheva, D.; Spassova, I.; Todorova, S.; Ivanov, G.; Nihtianova, D.; et al. Catalytic Combustion of Methane over Pd-Modified La-Ce-Zr-Al Catalyst. Materials 2025, 18, 2319. https://doi.org/10.3390/ma18102319
Tumbalova K, Zlatanova Z, Velinova R, Shipochka M, Markov P, Kovacheva D, Spassova I, Todorova S, Ivanov G, Nihtianova D, et al. Catalytic Combustion of Methane over Pd-Modified La-Ce-Zr-Al Catalyst. Materials. 2025; 18(10):2319. https://doi.org/10.3390/ma18102319
Chicago/Turabian StyleTumbalova, Katerina, Zlatina Zlatanova, Ralitsa Velinova, Maria Shipochka, Pavel Markov, Daniela Kovacheva, Ivanka Spassova, Silviya Todorova, Georgi Ivanov, Diana Nihtianova, and et al. 2025. "Catalytic Combustion of Methane over Pd-Modified La-Ce-Zr-Al Catalyst" Materials 18, no. 10: 2319. https://doi.org/10.3390/ma18102319
APA StyleTumbalova, K., Zlatanova, Z., Velinova, R., Shipochka, M., Markov, P., Kovacheva, D., Spassova, I., Todorova, S., Ivanov, G., Nihtianova, D., & Naydenov, A. (2025). Catalytic Combustion of Methane over Pd-Modified La-Ce-Zr-Al Catalyst. Materials, 18(10), 2319. https://doi.org/10.3390/ma18102319












