Influence of Fe Vacancy on the Bonding Properties of γ-Fe (111)/α-Al2O3 (0001) Interfaces: A Theoretical Study
Abstract
1. Introduction
2. Calculation Method and Details
2.1. Calculation Settings
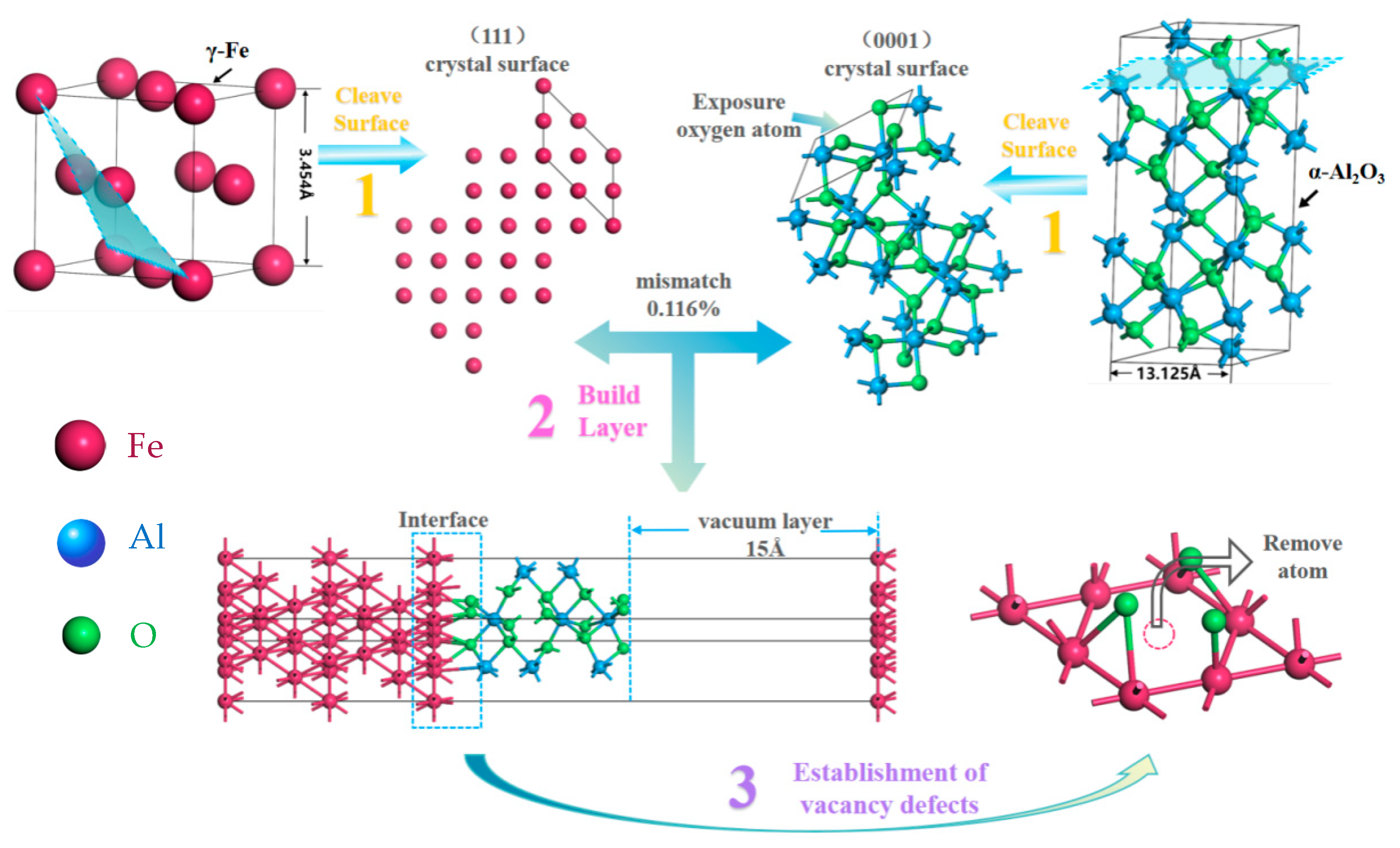
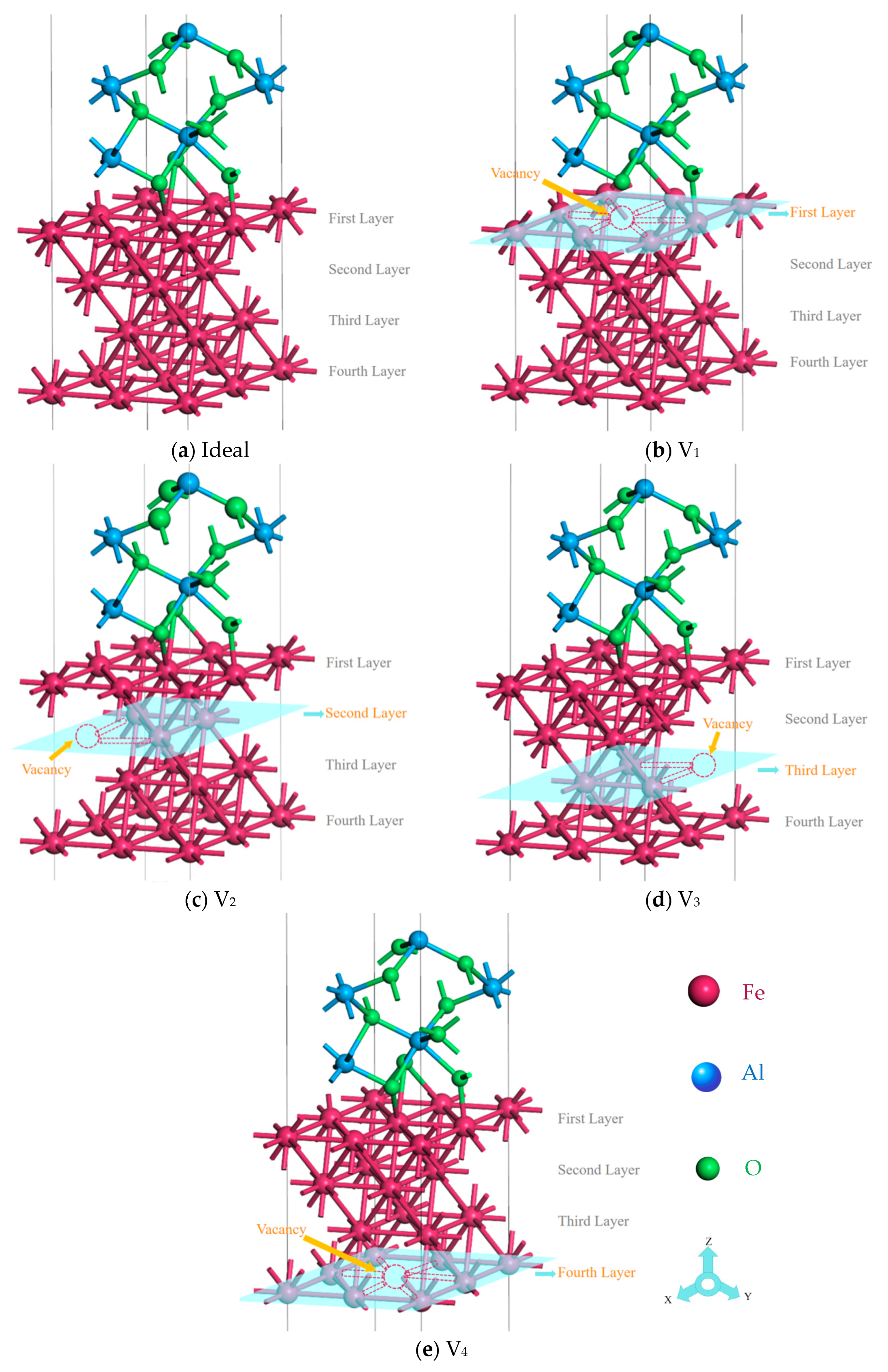
2.2. Model Building
3. Results and Discussion
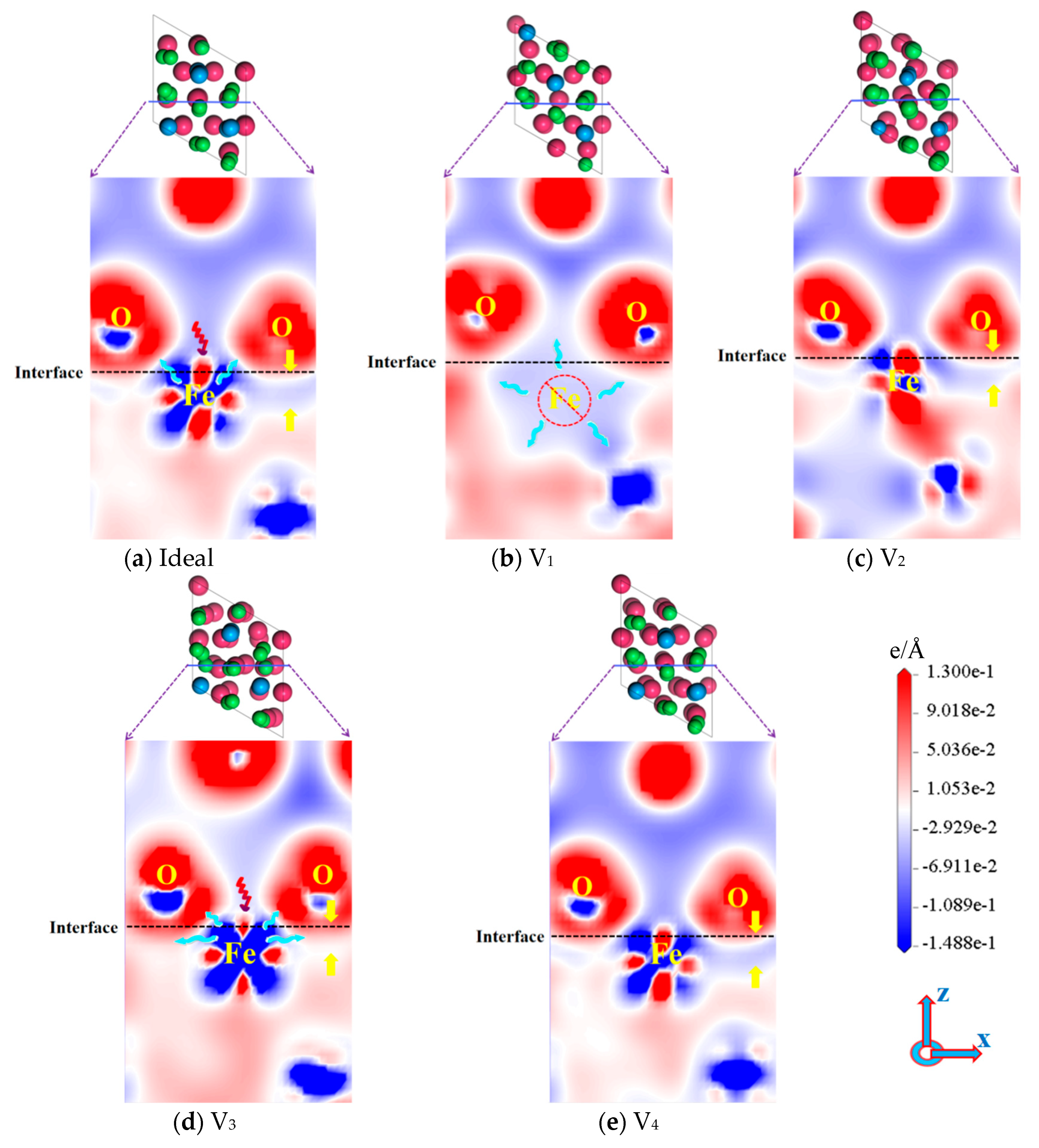
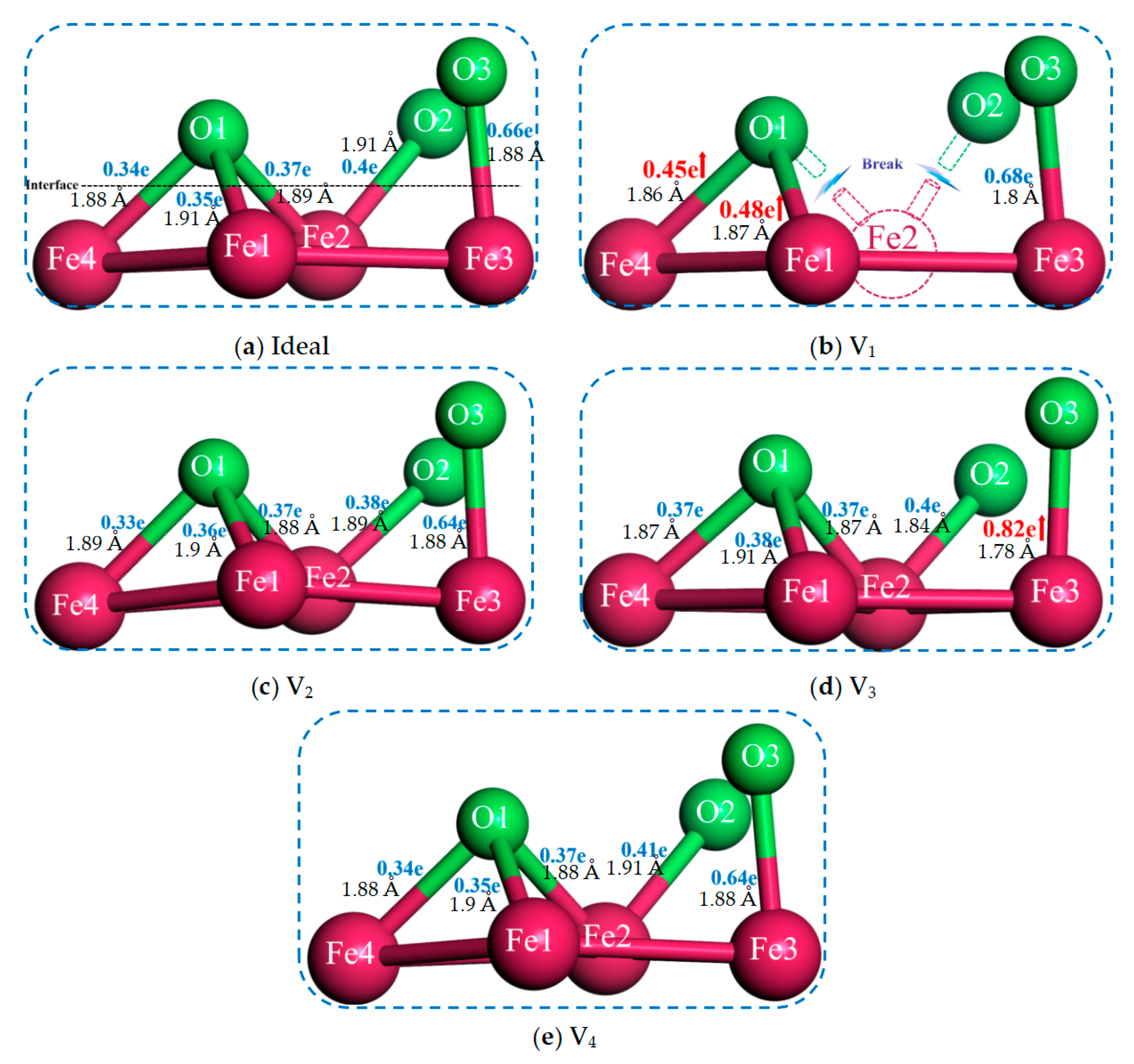
3.1. Bonding Strength of the Interface
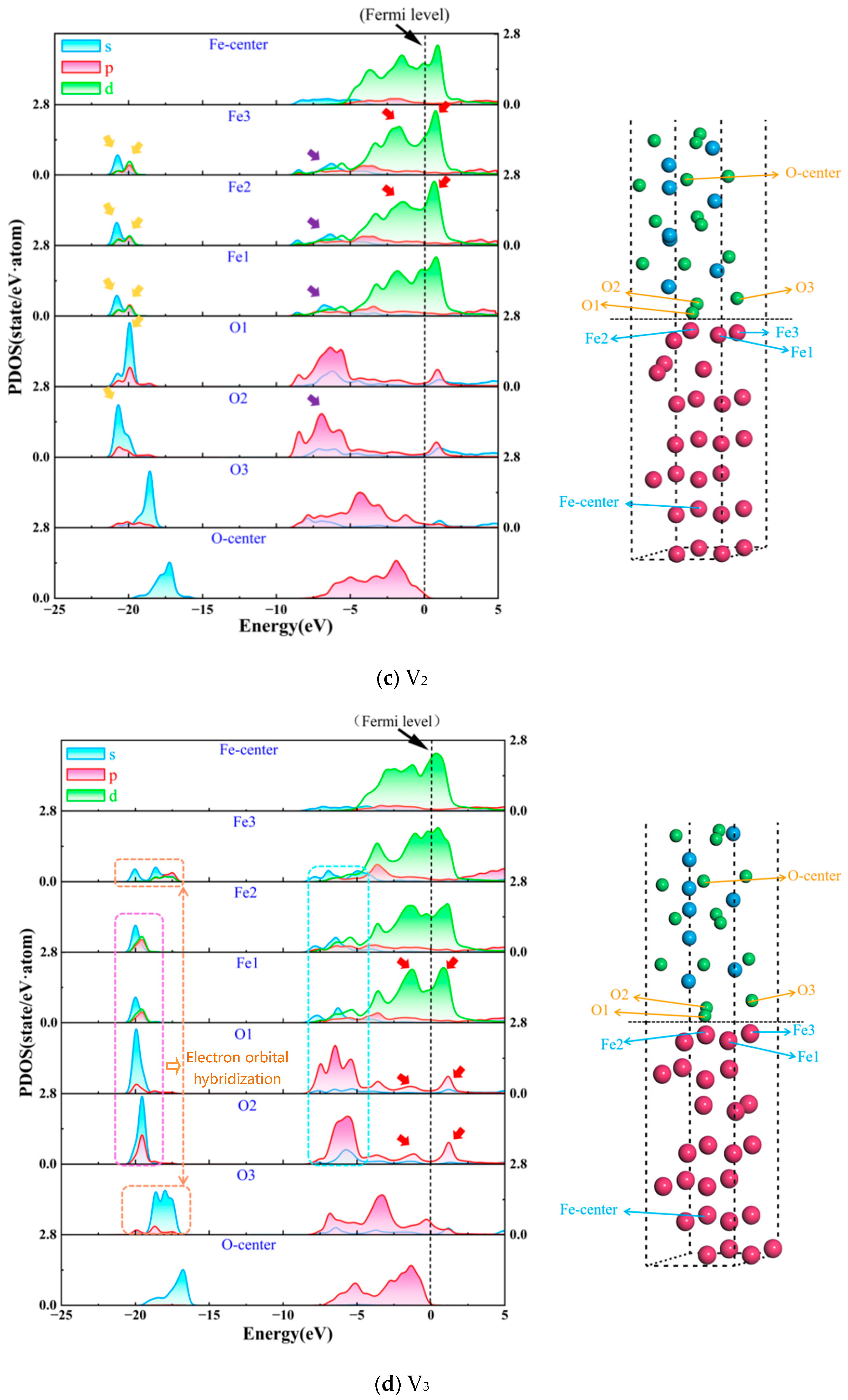
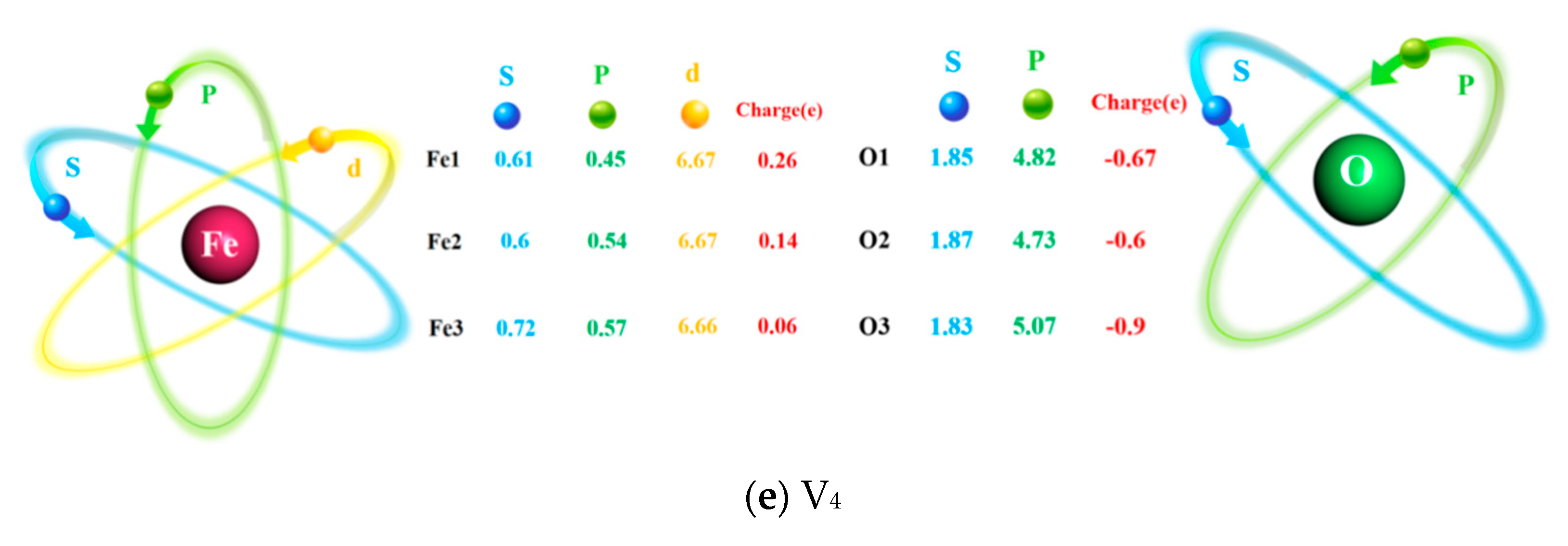
3.2. Electronic Properties of Interface
4. Conclusions
- (1)
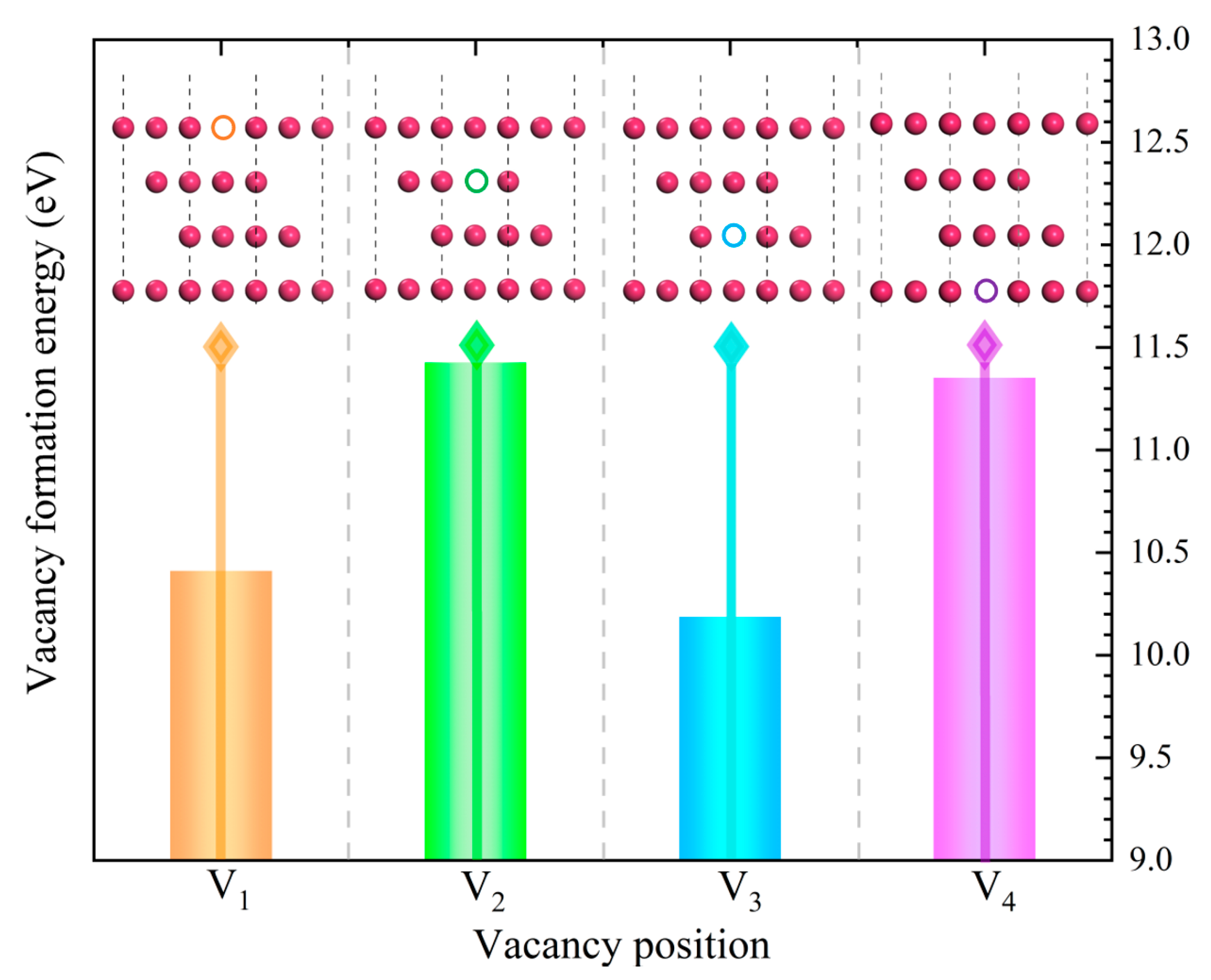
- In contrast to the ideal Fe/Al2O3 interface, the bonding strength of the V1 interface decreased by about 12%, while the bonding strength of the V3 interface increased by nearly 18%. Vacancies progressively change from bond-weakening to bond-strengthening as they move inward from the first to third atomic layers, becoming ineffective beyond this depth. Given the importance of reducing vacancy defects in the first layer of the Fe matrix for improving the interfacial bonding strength of Fe-Al2O3 composites, several strategies could be explored in future studies. These might include controlling irradiation-induced damage during experiments. The introduction of a protective atmosphere, such as helium, may also be beneficial to suppress vacancy formation and thereby optimize interfacial performance.
- (2)
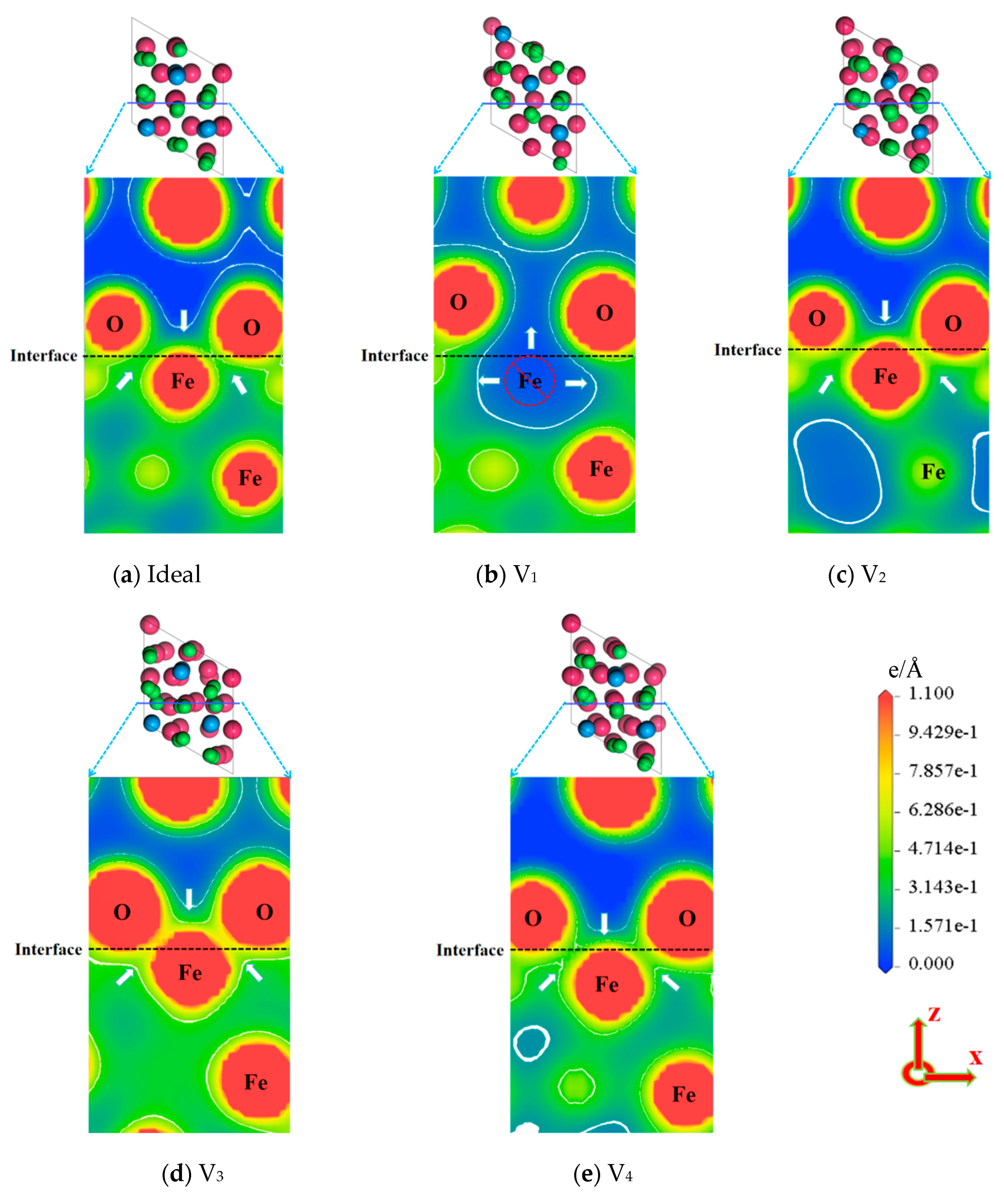
- The existence of vacancies at various positions will induce the relaxation behavior of atoms at the interface, which creates the bond-breaking and bond-forming reactions between atoms at the Fe/Al2O3 interface. This alters the environment of the interface region and directly affects the interfacial bonding performance.
- (3)
- Vacancy defects at the Fe/Al2O3 interface promote electronic redistribution and enhance interatomic orbital hybridization, resulting in a charge-sharing region. The charge density in this region varies with the vacancy type, being lowest in the V1 structure and highest in V3. In other words, the higher the charge concentration at the interface, the stronger the binding strength at the interface. This charge concentration shows a positive correlation with the interfacial bonding strength. Furthermore, increased orbital hybridization strengthens the interfacial bonding, which fundamentally explains how vacancy defects modulate the bonding properties of the interface.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cai, X.C.; Liang, J.J.; Xu, L.D.; Sun, B.R.; Chen, Z.; Wen, K.K.; Jin, S.B.; Sha, G.; Shen, T.D. Ultrastrong nanocrystalline oxide-dispersion-strengthened ferritic alloy with exceptional thermal stability. Mater. Sci. Eng. A 2021, 821, 141616. [Google Scholar] [CrossRef]
- Xu, L.D.; Cai, X.C.; Wen, K.K.; Wu, Y.; Zhu, H.H.; Xin, S.W.; Sun, B.R.; Shen, T.D. Superior creep resistance of a microstructurally stable nanocrystalline oxidedispersion-strengthened ferritic alloy. Scr. Mater. 2024, 252, 116283. [Google Scholar] [CrossRef]
- Zhang, S.H.; Hayashi, S.; Ukai, S.; Oono, N.H. Effect of Co addition on the high temperature oxidation behavior of oxide-dispersion-strengthened FeCrAl alloys. Corros. Sci. 2021, 184, 109391. [Google Scholar] [CrossRef]
- Wilms, M.B.; Rittinghaus, S.; Goßling, M.; Gökce, B. Additive manufacturing of oxidedispersion strengthened alloys: Materials, synthesis and manufacturing. Prog. Mater. Sci. 2023, 133, 101049. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Zhang, J.X.; Wu, Y.X.; Lu, T.Y.; Wu, H.Y.; Jia, B.R.; Qin, M.L.; Qu, X.H. Microstructure and magnetic properties of Fe-Al2O3 nanocomposites prepared by a versatile facile combustion-based route. Ceram. Int. 2025, 51, 4780–4785. [Google Scholar] [CrossRef]
- Rangel, W.M.; Santa, R.A.A.B.; Riella, H.G. A facile method for synthesis of nanostructured copper (II) oxide by coprecipitation. J. Mater. Res. Technol. 2020, 9, 994–1004. [Google Scholar] [CrossRef]
- Shakib, S.E.; Raiszadeh, R.; Vahdati-Khaki, J. A self-propagating high-temperature synthesis process for the fabrication of Fe(Cr)-Al2O3 nanocomposite. Int. J. Miner. Metall. Mater. 2019, 26, 775–786. [Google Scholar] [CrossRef]
- Guo, Z.M.; Liu, Q.; Yang, W.W.; Guo, L.C. Densification and properties of ODS-iron based alloy. Rare Met. 2012, 31, 339–342. [Google Scholar] [CrossRef]
- Santos, A.; Ardisson, J.D.; Viegas, A.D.C.; Schmidt, J.E.; Persiano, A.I.C.; Macedo, W.A.A. Structure and magnetism of granular Fe-Al2O3. J. Magn. Magn. Mater. 2001, 226-230, 1861–1863. [Google Scholar] [CrossRef]
- Aghili, S.E.; Enayati, M.H.; Karimzadeh, F. Fabrication of bulk (Fe, Cr) 3Al/Al2O3 intermetallic matrix nanocomposite through mechanical alloying and sintering. Acta Metall. Sin. 2016, 29, 911–919. [Google Scholar] [CrossRef]
- Brendan, D.; Amanda, L.; Jinsuo, Z. A Method for Inhibiting Corrosion of Type 316L Stainless Steel in High-Temperature Molten Chloride Salt. Nucl. Sci. Eng. 2024, 198, 749–753. [Google Scholar]
- Fernández, G.A.; Muñoz-Sánchez, B.; Nieto-Maestre, J.; García-Romero, A. High temperature corrosion behavior on molten nitrate salt-based nanofluids for CSP plants. Renew. Energy 2019, 130, 902–909. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, X.; Li, W.; Ai, T.T.; Dong, H.F.; Yang, Z.R. Study of Mo3NiB3 and Mo2NiB2 cermets by first-principles calculation and experiment. Next Mater. 2025, 7, 100324. [Google Scholar] [CrossRef]
- Zhang, H.R.; Li, J.; Bian, Y.; Cen, W.L.; Yin, D.Q. The energetic, electronic and mechanical properties of metal (Fe/Co/Ni/Cu)/ceramics (TiN) interfaces: A first-principles study. Mater. Today Commun. 2025, 42, 111281. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.; Xiao, B.; Zheng, Q.L.; Gao, Y.M.; Zhao, S.Y.; Wang, Z.C. First-principles calculation on the adhesion strength, fracture mechanism, interfacial bonding of the NiTi (111)//α-Al2 O3 (0001) interfaces. Mater. Des. 2019, 183, 108119. [Google Scholar] [CrossRef]
- Hocker, S.; Schmauder, S.; Bakulin, A.; Kulkova, S. Ab initio investigation of tensile strengths of metal (111)/α-Al2O3 (0001) interfaces. Philos. Mag. 2014, 94, 265–284. [Google Scholar] [CrossRef]
- Shi, S.; Tanaka, S.; Kohyama, M. First-principles study of the tensile strength and failure of α-Al2O3 (0001)/Ni(111) interfaces. Phys. Rev. 2007, 76, 075431. [Google Scholar] [CrossRef]
- Xie, H.N.; Zhao, N.Q.; Shi, C.S.; He, C.N.; Liu, E.Z. Effects of active elements on adhesion of the Al2O3/Fe interface: A first principles calculation. Comput. Mater. Sci. 2021, 188, 110226. [Google Scholar] [CrossRef]
- Peng, M.M.; Lai, W.S. Interaction between vacancies and the α-Fe/Y2O3 interface: A first-principles study. Nucl. Instrum. Methods Phys. Res. 2015, 352, 67–71. [Google Scholar] [CrossRef]
- Li, R.W.; Chen, Q.C.; Liang, O.Y.; Ding, Y.L. Adhesion strength and bonding mechanism of γ-Fe (111)/α-Al2O3 (0001) interfaces with different terminations. J. Alloys Compd. 2021, 870, 159529. [Google Scholar] [CrossRef]
- Li, R.W.; Chen, Q.C.; Zhang, Z.L.; Liang, O.Y.; Zhang, Y.J.; Nie, B.J.; Ding, Y.L. Revealing the atomic-scale structure and the fracture mechanism of the α-Al2O3/γ-Fe ceramic-metal interface. J. Alloys Compd. 2021, 885, 161163. [Google Scholar]
- Li, R.W.; Chen, Q.C.; Ji, M.X.; Ding, Y.L. Exploring failure mode and enhancement mechanism of doped rare-earth elements iron-based/alumina-ceramic interface. Ceram. Int. 2022, 48, 7827–7835. [Google Scholar] [CrossRef]
- Li, R.W.; Chen, Q.C.; Liang, O.Y.; Zhang, Y.J.; Nie, B.J.; Ding, Y.L. Insight into the strengthening mechanism of α-Al2O3/γ-Fe ceramic-metal interface doped with Cr, Ni, Mg, and Ti. Ceram. Int. 2021, 47, 22810–22820. [Google Scholar]
- Zhang, S.W.; Zhao, X.L.; Zhang, J.Y.; Sheng, J.; Ren, J.Q.; Lu, X.F.; Tang, X.C. Investigation on the Influence of Vacancy and Alloying Element Content on the Performance of Fe/NbN Interface. Metals 2025, 15, 759. [Google Scholar] [CrossRef]
- Deng, Z.; Lu, J.; Li, H.; Wu, Y.; Xu, X.Y.; Miao, Q.; Zhang, W.X. First-principles study on adhesive work, electronic structures and mechanical properties of Cr/Ti-doped Cu(100)/diamond(100) interfaces. Diam. Relat. Mater. 2025, 151, 111861. [Google Scholar]
- Gao, Q.; Qiao, G.; Wang, W.; Ge, Y.X.; Ren, J.Q.; Li, W.; Yang, P.; Lu, X.F.; Qiao, J.S. The first-principles study of interfacial bonding strength and segregation behavior of alloyed elements at the η(MgZn2)/Al interface. Phys. Chem. Chem. Phys. 2024, 26, 17420. [Google Scholar] [CrossRef]
- Pei, X.; Yuan, M.; Zhou, P.; Yang, W.; Wang, Y.; Yin, L.Z.; Xu, Z.H.; Zhou, X.S.; Shen, X.Q. First principles study of interface bonding strength, stability, and tensile properties of Ti2AlC(0001)/Ti3Al(0001) interface. J. Phys. Chem. Solids 2025, 199, 112493. [Google Scholar] [CrossRef]
- Pan, A.Q.; Wang, W.Y.; Zhang, H.; Hao, S.M.; Xie, J.P. First-principles study on interfacial properties and the electronic structure of the Al(001)/MgAl2O4 (001) interface. J. Mater. Sci. 2024, 59, 2375–2389. [Google Scholar] [CrossRef]
- Guo, X.; Yang, P.; Zhang, J.; Zhou, J.T.; Ren, J.Q.; Lu, X.F. Effects of alloying element on the interface stability of fcc-Fe/NbC by first principles investigation. Vacuum 2024, 228, 113534. [Google Scholar] [CrossRef]
- Yang, J.; Huang, J.; Fan, D.; Chen, S.H.; Zhao, X.K. Comparative investigation on RE(La,Ce)AlO3 (100)/γ-Fe (100) interfaces: A first-principles calculation. Appl. Surf. Sci. 2016, 384, 207–216. [Google Scholar] [CrossRef]
- Shi, Z.; Liu, S.; Zhou, Y.; Yang, Q.X. First-principles calculation on the relationships of h-WC/γ-Fe interface. J. Phys. Chem. Solids 2018, 123, 11–18. [Google Scholar] [CrossRef]
- Xue, H.T.; Wei, X.; Guo, W.B.; Zhang, X.M. Bonding mechanism study of active Ti element and α-Al2O3 by using first-principle calculation. J. Alloys Compd. 2020, 820, 153070. [Google Scholar] [CrossRef]
- Evarestov, R.A.; Kotomin, E.A.; Senocrate, A.; Kremer, R.K.; Maier, J. First-principles comparative study of perfect and defective CsPbX3 (X=Br, I) crystals. Phys. Chem. Chem. Phys. 2020, 22, 3914–3920. [Google Scholar] [CrossRef]
- Hao, Z.; Mei-Hua, W.; Ke-Rong, M.; Kun, L.; Misko Vyacheslav, R.; Wen, Y. First-principles study of point defect diffusion in CoMn2O4 crystal. Electrochim. Acta 2023, 437, 141520. [Google Scholar]
- Startt, J.; Deo, C. Ab-initio study of point defects in Th and U alloy. J. Nucl. Mater. 2024, 595, 155034. [Google Scholar] [CrossRef]
- Tanaka, T.; Matsunaga, K.; Ikuhara, Y.; Yamamoto, T. First-principles study on structures and energetics of intrinsic vacancies in SrTiO3. Phys. Rev. B 2003, 68, 205213. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, W.B.; Liu, K.E.; Guo, Z.H.; Lu, S.L.; Zheng, Y.X.; Wu, X.Y.; Zhang, Q.J.; Wang, B.; Zhu, L.G. First-principles study on atomic formation and manganese diffusion in MnS/Mg-Ti-oxide complex inclusions in steel. Ceram. Int. 2024, 50, 55263–55274. [Google Scholar] [CrossRef]
- Kim, I.; Hunn, J.; Hashimoto, N.; Larson, D.; Lee, E. Defect and void evolution in oxide dispersion strengthened ferritic steels under 3.2 MeV Fe + ion irradiation with simultaneous helium injection. J. Nucl. Mater. 2000, 280, 264–274. [Google Scholar] [CrossRef]
- Rhee, Y.J. Electronic structures and optical properties of Fe2VAl; effect of hybridization. J. Korean Phys. Soc. 2013, 63, 1975–1979. [Google Scholar] [CrossRef]
- Fu, C.L.; Liang, J.N.; Cai, W.; Zhang, C.Y. The Electronic Structure of Hf-Doped Barium Titanate. Ferroelectrics 2012, 432, 1–7. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Li, R.; Chen, Q.; Kong, D.; Yang, H. Influence of Fe Vacancy on the Bonding Properties of γ-Fe (111)/α-Al2O3 (0001) Interfaces: A Theoretical Study. Materials 2025, 18, 4666. https://doi.org/10.3390/ma18204666
Zhang X, Li R, Chen Q, Kong D, Yang H. Influence of Fe Vacancy on the Bonding Properties of γ-Fe (111)/α-Al2O3 (0001) Interfaces: A Theoretical Study. Materials. 2025; 18(20):4666. https://doi.org/10.3390/ma18204666
Chicago/Turabian StyleZhang, Xiaofeng, Renwei Li, Qicheng Chen, Dehao Kong, and Haifeng Yang. 2025. "Influence of Fe Vacancy on the Bonding Properties of γ-Fe (111)/α-Al2O3 (0001) Interfaces: A Theoretical Study" Materials 18, no. 20: 4666. https://doi.org/10.3390/ma18204666
APA StyleZhang, X., Li, R., Chen, Q., Kong, D., & Yang, H. (2025). Influence of Fe Vacancy on the Bonding Properties of γ-Fe (111)/α-Al2O3 (0001) Interfaces: A Theoretical Study. Materials, 18(20), 4666. https://doi.org/10.3390/ma18204666




