Mechanistic Insights into Cooling-Rate-Governed Acicular Ferrite Transformation Kinetics and Strengthening-Toughening Synergy in EH36 Heavy Steel Plate
Highlights
- An optimal cooling window of 3–7 °C/s maximizes acicular ferrite content and aspect ratio in heavy steel.
- Core–shell inclusions promote acicular ferrite nucleation via interfacial energy reduction and radial growth.
- Interlocking ferrite enhances hardness by 14% via grain refinement and high dislocation density.
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Methods
2.3. Microstructural Characterization
2.4. Microhardness Measurement
3. Results
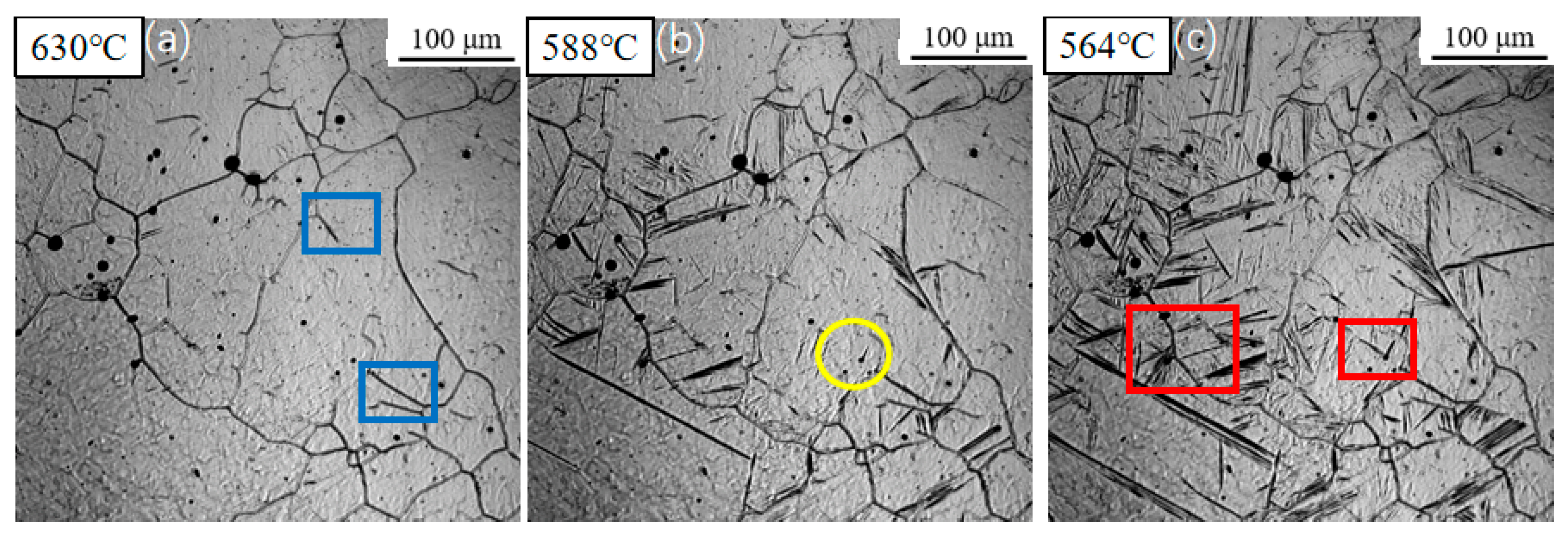
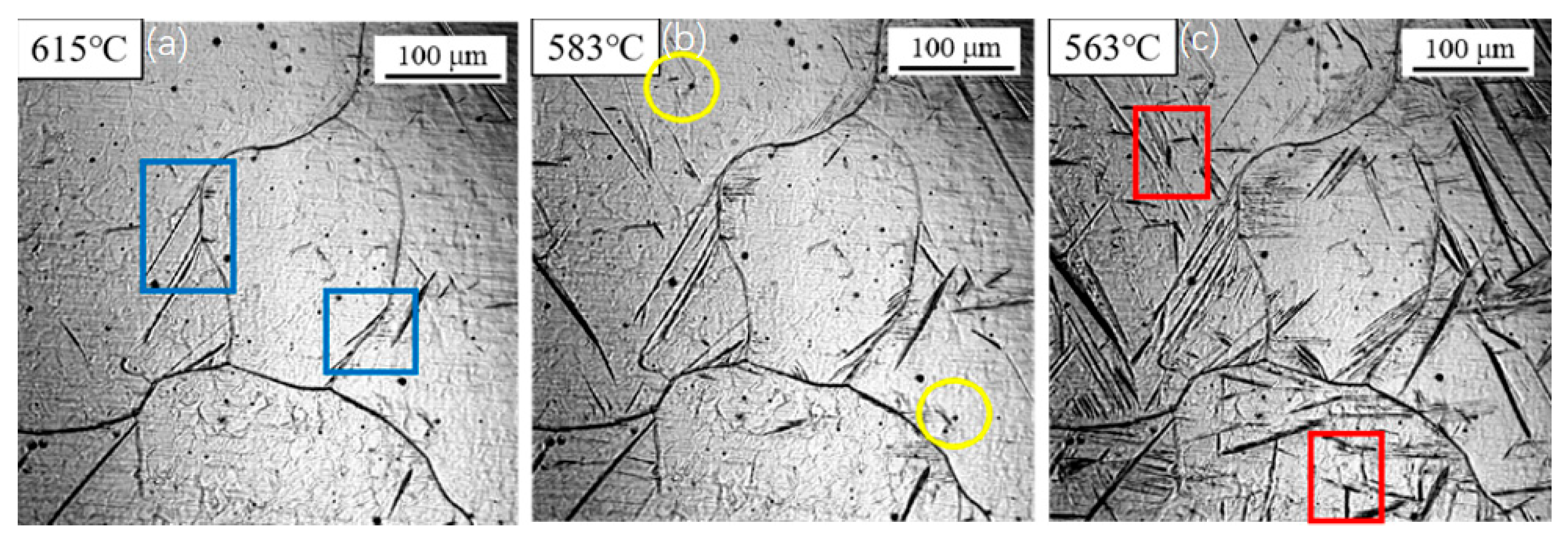
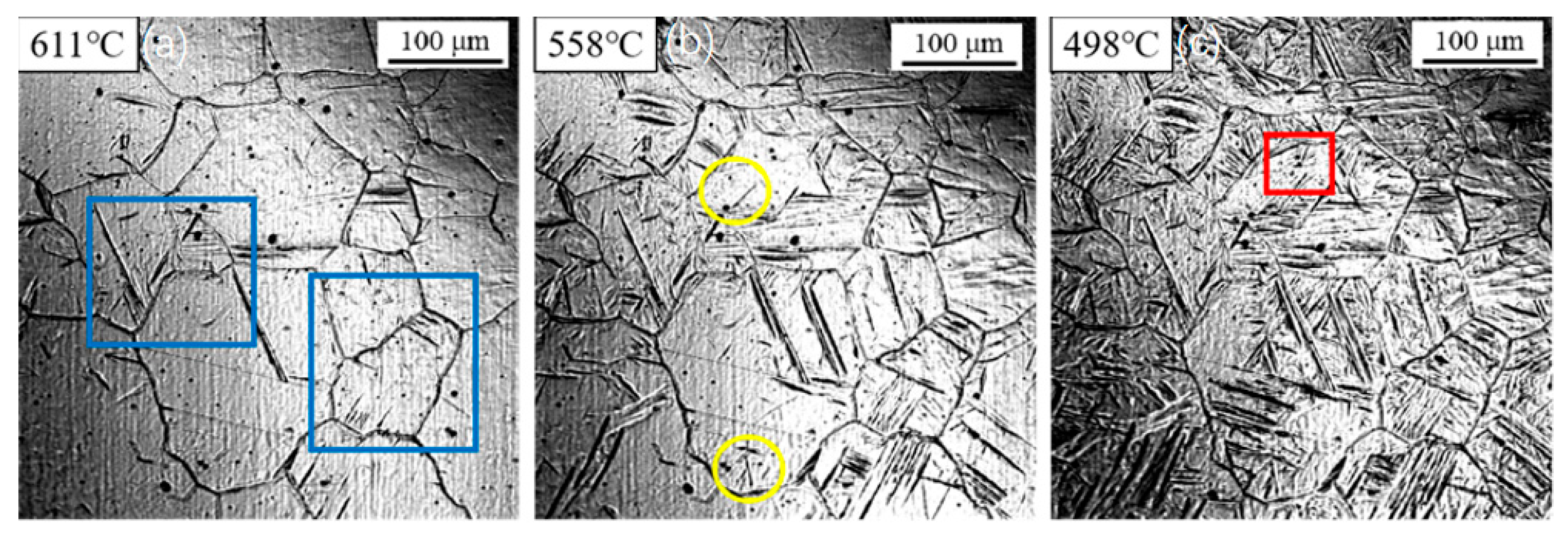
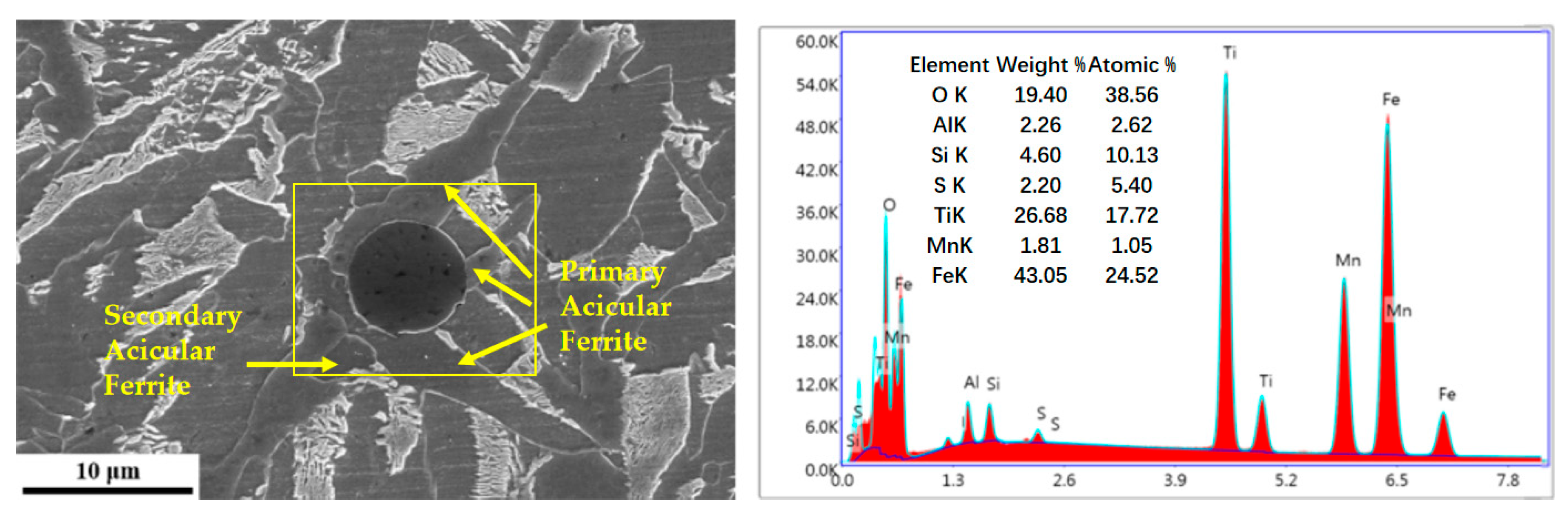
3.1. Dynamic Observation of Acicular Ferrite Nucleation and Growth
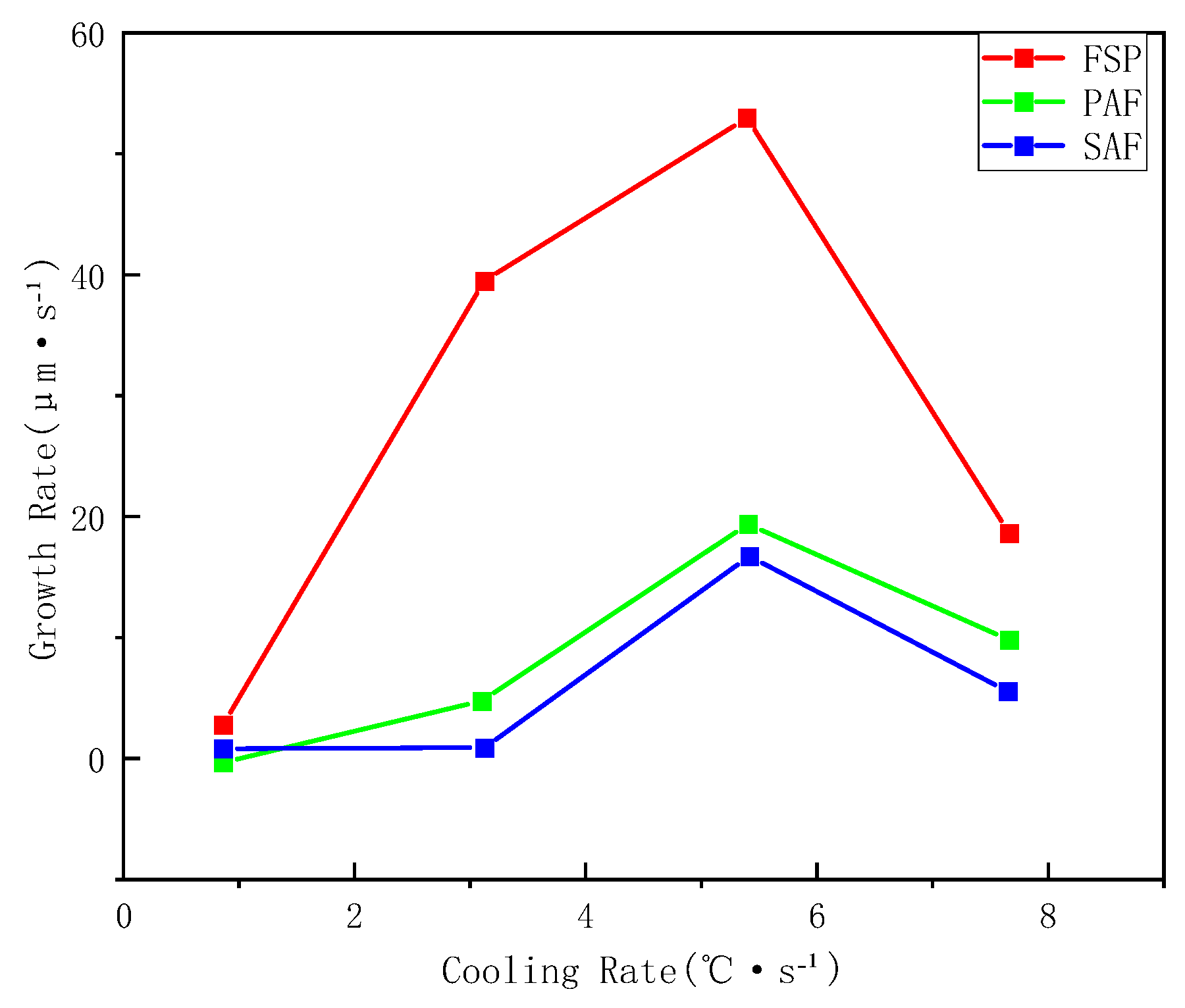
3.2. Influence of Cooling Rate on the Transformation Kinetics of Acicular Ferrite
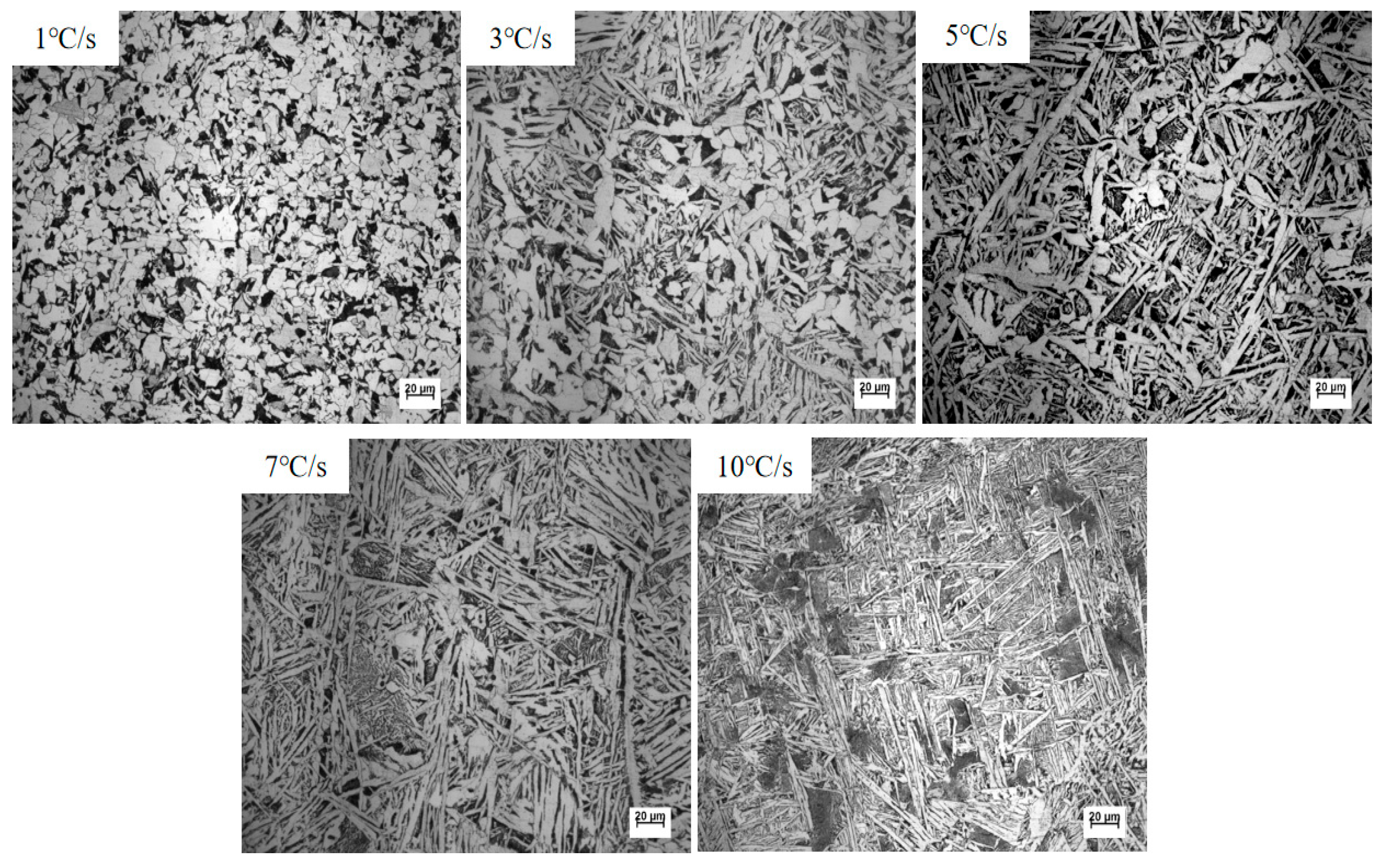
3.3. Evolution of Microstructure
3.4. Microhardness Evaluation and Discussion
4. Discussion
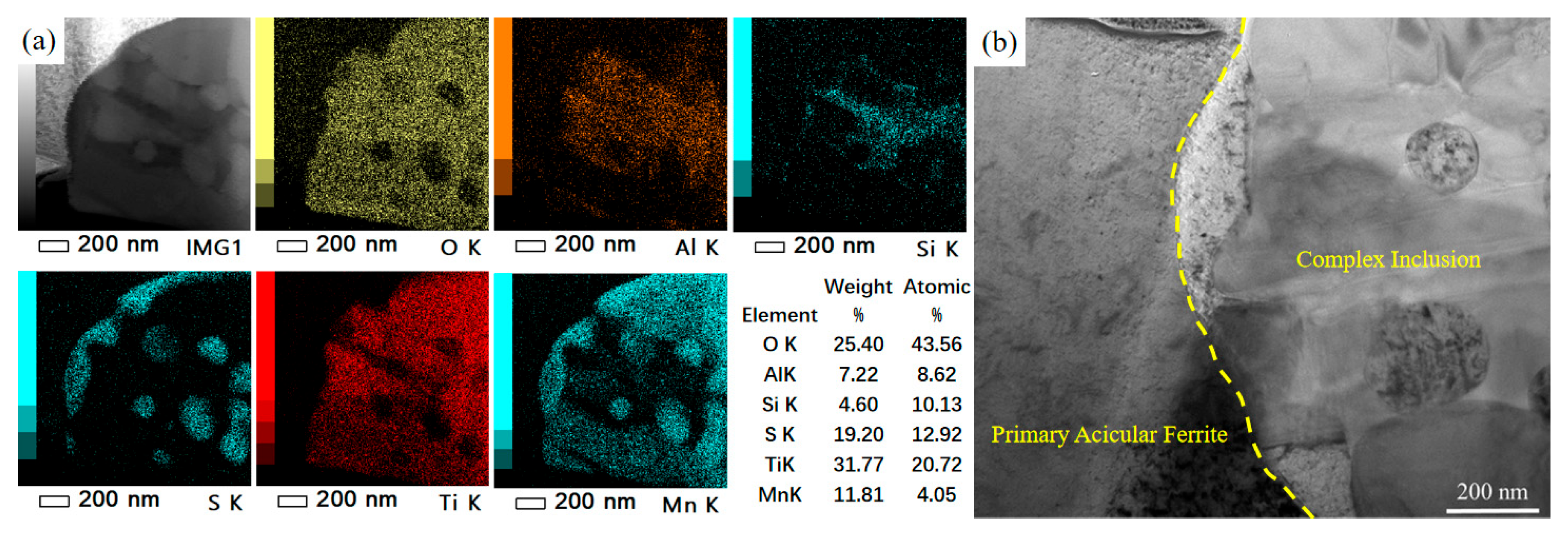
4.1. Inclusion Characteristics and Their Influence on Acicular Ferrite Nucleation
4.2. Dislocation Structure and Strengthening Mechanisms
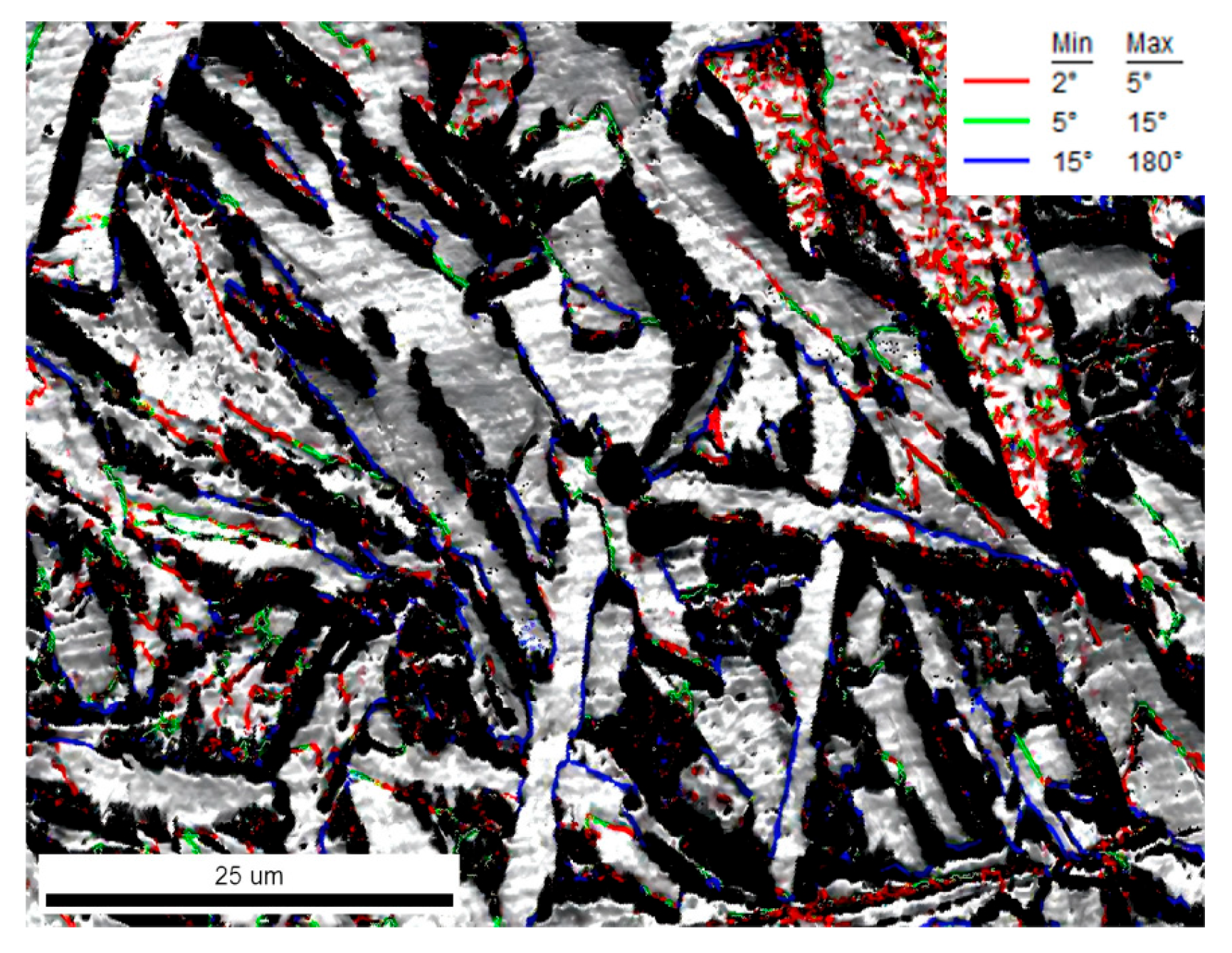
4.3. Grain Boundary Characteristics and Toughening Mechanism
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, J.; Zhu, K.; Wang, G. Progress in the technological development of oxide metallurgy for manufacturing steel plates with excellent HAZ toughness. Baosteel Tech. Res. 2008, 2, 43. [Google Scholar]
- Lou, H.; Wang, C.; Wang, B.; Wang, G. Inclusion evolution behavior of Ti–Mg oxide metallurgy steel and its effect on a hgh heat input welding HAZ. Metals 2018, 8, 534. [Google Scholar] [CrossRef]
- Guo, W.; Xiao, P.; Yao, J.; Liu, Z.; Wang, C.; Cao, Y.; Chen, M. Development and Practice of Series Hull Stell with Large Heat Input Welding. Wide Heavy Platee 2023, 29, 13–16. [Google Scholar]
- Niu, J.; Liu, D.; Chen, H. Microstructure and Mechanical Properties of TMCP Processed ABS-EQ47 Plate Steel for Offshore Platform. Iron Steel 2013, 48, 64–70. [Google Scholar]
- Chen, H.; Liu, D. Microstructure and Properties of simulated heat affected zone of TMCP processed E500 heavy plate steel for offshore platform. Iron Steel 2015, 50, 87–94. [Google Scholar]
- Song, X.; Li, W.; Bing, C.; Yu, T.; Bi, J.T. Microstructures Evolution and Strengthening-Toughening Mechanism of S460G1 Steel Heavy Plate During Quenching and Tempering. Mater. Mech. Eng. 2022, 46, 20–30. [Google Scholar]
- Fu, T.L.; Han, Y.; Deng, X.T.; Liu, G.H.; Wang, G.D. Research progress and application of roller quenching technology for high-grade plate. Steel Roll. 2022, 39, 60–66. [Google Scholar]
- Tuo, C.D.; Gao, Q.; Zhang, Y.W.; Shi, S.H.; Feng, Z. Effect of quenching process on microstructure and properties of a 500 MPa grade hot-rolled offshore engineering steel. Heat Treat. Met. 2025, 50, 110–118. [Google Scholar]
- Yang, T.; Su, H.; Luo, X.B.; Chai, F.; Zhang, Z.Y. Cross section microstructure and homogeneity of mechanical properties of as quenched high strength ship steel. J. Iron Steel Res. 2017, 29, 583–589. [Google Scholar]
- Meng, T.Y.; Zhu, L.G.; Wang, Y.; Pao, Z.L.; Huo, J.X. Development and research status of oxide nucleation based on two-step nucleation theory. Contin. Cast. 2024, 43, 12–24, + 40. [Google Scholar]
- Li, H.R.; Sun, L.G.; Zhang, X.; Wang, B.; Zhang, C.J. Mechanism of Mg treatment on pinning particles for shipbuilding steel. Iron Steel 2024, 59, 138–150. [Google Scholar]
- Zhang, X.Y.; Hu, K.; Wang, T.S.; Li, Z.; Hao, J.J.; Wang, C. Effect of Ti-Mg Deoxidation on Microstructure and Properties of Electro-Gas Vertical Welding Joint of Low Carbon Steel Plate. Hot Work. Technol. 2024, 53, 39–43. [Google Scholar]
- Zang, Y.Q.; Zang, Y.H.; Yang, J. Effect of Mg treatment on secondary phase particls, microstructure and toughness in the HAZ of steel plate. Steelmaking 2023, 39, 1–17, + 35. [Google Scholar]
- Zhao, P. Analysis and discussion on oxides metallurgy. China Met. 2022, 32, 1–6. [Google Scholar]
- Wang, J.H.; Yo, H.Q.; Zhao, P.L.; Li, C.; Li, Y.Z. High temperature deformation behavior of Q355NE H-shaped steel for marine engineering and its influence on microstructure. J. Iron Steel Res. 2024, 36, 766–775. [Google Scholar]
- Sun, L.G.; Ma, Z.Y.; Sun, Y.M.; Li, M.D.; Zhu, L.G. Study on IAF induced by inclusions in magnesium-treated shipbuilding steel. Steelmaking 2022, 38, 80–86. [Google Scholar]
- Lu, X.H.; Gao, S.; Zhang, C.Y. Microstructure and mechanical properties of an Nb-microalloyed EH47 steel. Baosteel Tech. Res. 2023, 17, 15–28. [Google Scholar]
- Yang, Y.K.; Zhu, J.Y.; Li, X.M.; Wang, Y.; Zhan, D.P. A review of research on banded structure control in low carbon alloy steel. Iron Steel 2023, 58, 1–10. [Google Scholar]
- Zhu, W.T.; Cui, J.J.; Chen, Z.Y.; Zhao, Y.; Chen, L.Q. Correlation of Microstructure Feature with Impact Fracture Behavior in a TMCP Processed High Strength Low Alloy Construction Steel. Acta Metall. Sin. (Engl. Lett.) 2022, 35, 527–536. [Google Scholar] [CrossRef]
- Yao, H.; Ren, Q.; Zhang, L.F. Review on the control of the acicular ferrite in high strength low alloy steels. Steelmaking 2022, 38, 1–10, + 24. [Google Scholar]
- Guo, H.; Yang, S.F.; Wang, T.T.; Yuan, H.; Zhang, Y.L.; Li, J.S. Microstructure evolution and acicular ferrite nucleation in inclusion-engineered steel with modified MgO@C nanoparticle addition. J. Mater. Sci. Amp. Technol. 2022, 99, 277–287. [Google Scholar] [CrossRef]
- Huang, Z.J.; Deng, Z.J. Analysis of inclusions and precipitates in tough weld metal of X80 steel. Baosteel Tech. Res. 2021, 15, 12–20. [Google Scholar]
- Yao, H.; Liu, C.J.; Zhang, L.F. Effect of austenite grain on the acicular ferrite transformation in Ti-Z treated steel. Chin. J. Eng. 2023, 45, 907–914. [Google Scholar]
- Song, F.Y.; Zhou, L.H.; Lun, W.S.; Hang, Z.Y.; Bai, X.Y. Needle ferrite grain growth behavior in weld metal. Trans. China Weld. Inst. 2021, 42, 23–28, + 99. [Google Scholar]
- Li, B.; Liu, Q.Y.; Jia, S.J.; Chen, J. Characteristic of microstructure transformation of low-carbon microalloyed steel during continuous cooling. Heat Treat. Met. 2018, 43, 18–24. [Google Scholar]
- Shao, Y.; Liu, C.X.; Yan, Z.S.; Li, H.J.; Liu, Y.C. Formation mechanism and control methods of acicular ferrite in HSLA steels: A review. J. Mater. Sci. Amp. Technol. 2018, 34, 737–744. [Google Scholar] [CrossRef]
- Liu, Z.H.; Qu, Z.G.; Wang, C.; Fan, M.D.; Yang, H.F.; Zhao, H.M. Research and development of 30.2mm thick X80M plate. Steel Roll. 2023, 40, 125–128, + 134. [Google Scholar]
- Zhu, L.G.; Zhang, Q.J. Fundamental research of the microalloying theory based on oxide metallurgy technology. Chin. J. Eng. 2022, 44, 1529–1537. [Google Scholar]
- Liu, G.Y.; Ge, L.; Zhou, Y.D.; Zhao, P.Y. Research and Development of 420 MPa Grade UItra-high Strength and High Toughness Steel for Offshore Engineering. Wide Heavy Plate 2021, 27, 30–33. [Google Scholar]
- Shim, J.-H.; Lee, S.-C.; Lee, B.-J.; Suh, J.-Y.; Cho, Y.W. Molecular dynamics simulation of the crystallization of a liquid gold nanoparticle. J. Cryst. Growth 2003, 250, 558–564. [Google Scholar] [CrossRef]
- Wang, C.; Yuan, G.; Wang, G.D. Hot-rolled intrinsically fine-grained steel technology based on precipitation control and its application. Iron Steel 2023, 58, 167–177. [Google Scholar]
- Wang, W.L.; Wang, L.K.; Li, P.S. Kinetics of austenite growth and bainite transformation during reheating and cooling treatments of high strength microalloyed steel produced by sub-rapid solidification. Int. J. Miner. Metall. Mater. 2023, 30, 354–364. [Google Scholar] [CrossRef]
- Meng, E.; Yang, G.W.; Han, R.Y.; Xu, Y.W.; Fu, Z.X.; Xu, D.M. Transformation behavior of Ti-Mo-V microalloyed ultra-high strength steel. Iron Steel 2024, 59, 143–152, + 182. [Google Scholar]
- Qiao, J.L.; Guo, F.H.; Shi, P.Z.; Cao, R.H.L.; Xiong, C.G.; Xu, L.J. Precipitation behavior of composite precipitates in Nb-Ti Micro-alloy EH36 offshore steel. Iron Steel Vanad. Titan. 2024, 45, 122–130. [Google Scholar]
- Zhou, C.; Ye, Q.B.; Yan, L. Effect of ultra-fast cooling on microstructure and properties of high strength steel for shipbuilding. Trans. Mater. Heat Treat. 2014, 35, 161–166. [Google Scholar]
- Zou, L.L.; Yang, X.S.; Huang, C.D.; Zhao, C.L.; Guo, X.L. Effect of cooling rate on phase transformation behavior of a non-quenched and tempered steel. Heat Treat. Met. 2025, 50, 188–195. [Google Scholar]
- Xiong, J.; Lan, H.F.; Li, L.X.; Tang, S.; Liu, Z.Y.; Wang, G.D. Effect of Cr on the Isothermal Decomposition Behavior of Austenite in Low-Carbon Ti Bearing Microalloyed Steel. Acta Metall. Sin. 2025, 301. [Google Scholar] [CrossRef]
- Zhang, Z.R.; Chen, C.; Wang, Y. Ferrite characteristics of low-nickel 316L stainless steel slab and effect of cooling rate on it. Iron Steel 2025, 1–13. [Google Scholar] [CrossRef]
- Xie, X.; Wan, Y.B.; Zhong, M.; Zou, X.D.; Wang, C. Optimizing Microstructures and Mechanical Properties of Electro-Gas Welded Metals for EH36 Shipbuilding Steel Treated by CaF2-TiO2 Fluxes. Acta Metall. Sin. 2025, 61, 998–1010. [Google Scholar]
- Sun, Q.; Du, Y.; Wang, X.N.; Liu, T.; Tao, Z.; Li, L.; Liao, Z.H.; Du, L.X. Study on Argon-rich Mixed Gas Shielded Welding Process of Medium-Mn Low-temperature Steel. Electr. Weld. Mach. 2024, 54, 46–54. [Google Scholar]
- Meng, M.D.; An, T.B.; Wei, J.S.; Ma, C.Y.; Zou, Y. Microstructure and strength-toughness mechanism of 690 MPa grade ULCB deposited metals. Iron Steel 2022, 57, 149–156. [Google Scholar]
- Sun, L.G.; Lei, M.; Zhang, X.; Liu, Y.S.; Zhu, L.G. In situ research on microstructure refining effect of Mg Treatment for shipbuilding steel at high temperature. Iron Steel 2020, 55, 94–102. [Google Scholar]
- Chi, Y.G.; Chen, Y.L.; Xu, L.Y.; Yang, J. Effect of Ti content on welding heat affected zone of Ti-Ca deoxidized steel after high-heat input welding. China Metall. 2025, 35, 67–79. [Google Scholar]
- Wan, X.L.; Li, G.Q.; Wu, K.M. Microstructure characteristics and formation mechanism of acicular ferrite in high-strength low-alloy steels. J. Iron Steel Res. 2016, 28, 388. [Google Scholar]
- Wan, X.L.; Shen, Y.; Li, Y.; Cheng, L.; Li, G.Q.; Wu, K.M. Effect of oxides and C, Mn on nucleation of ferrite in low-alloy steels. Trans. Mater. Heat Treat. 2019, 40, 114–120. [Google Scholar]
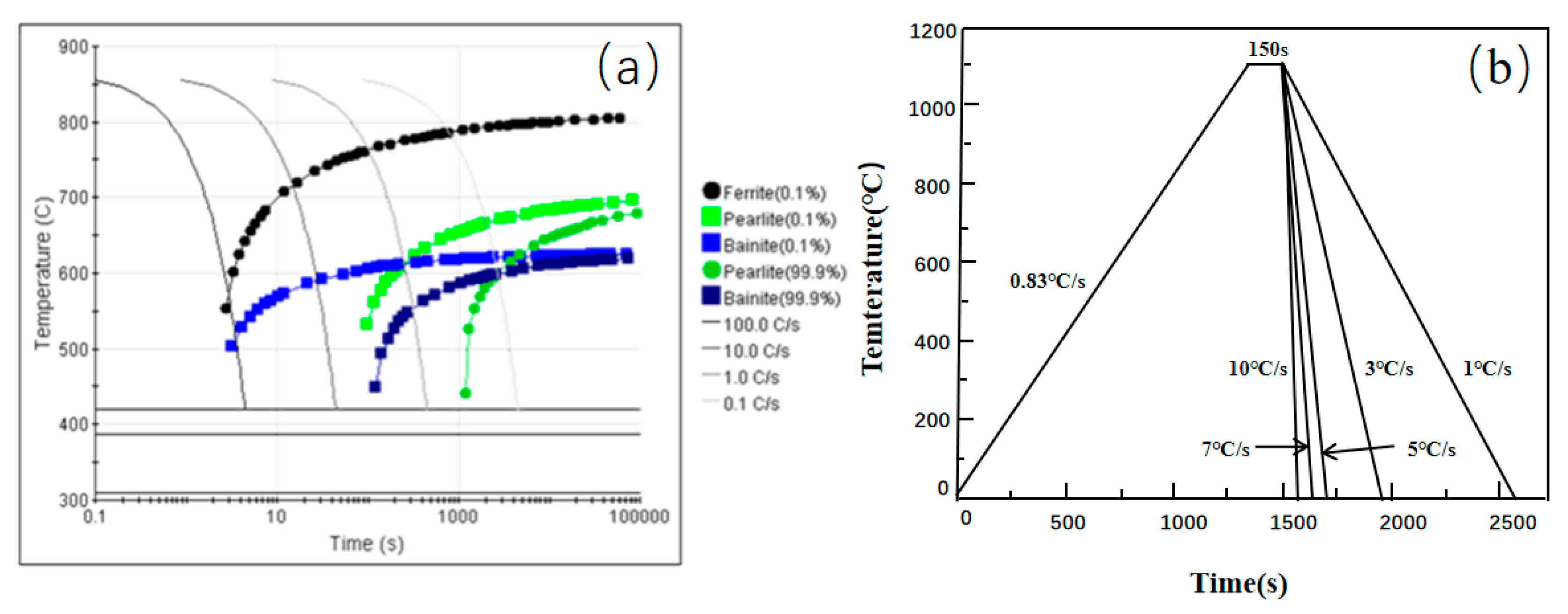
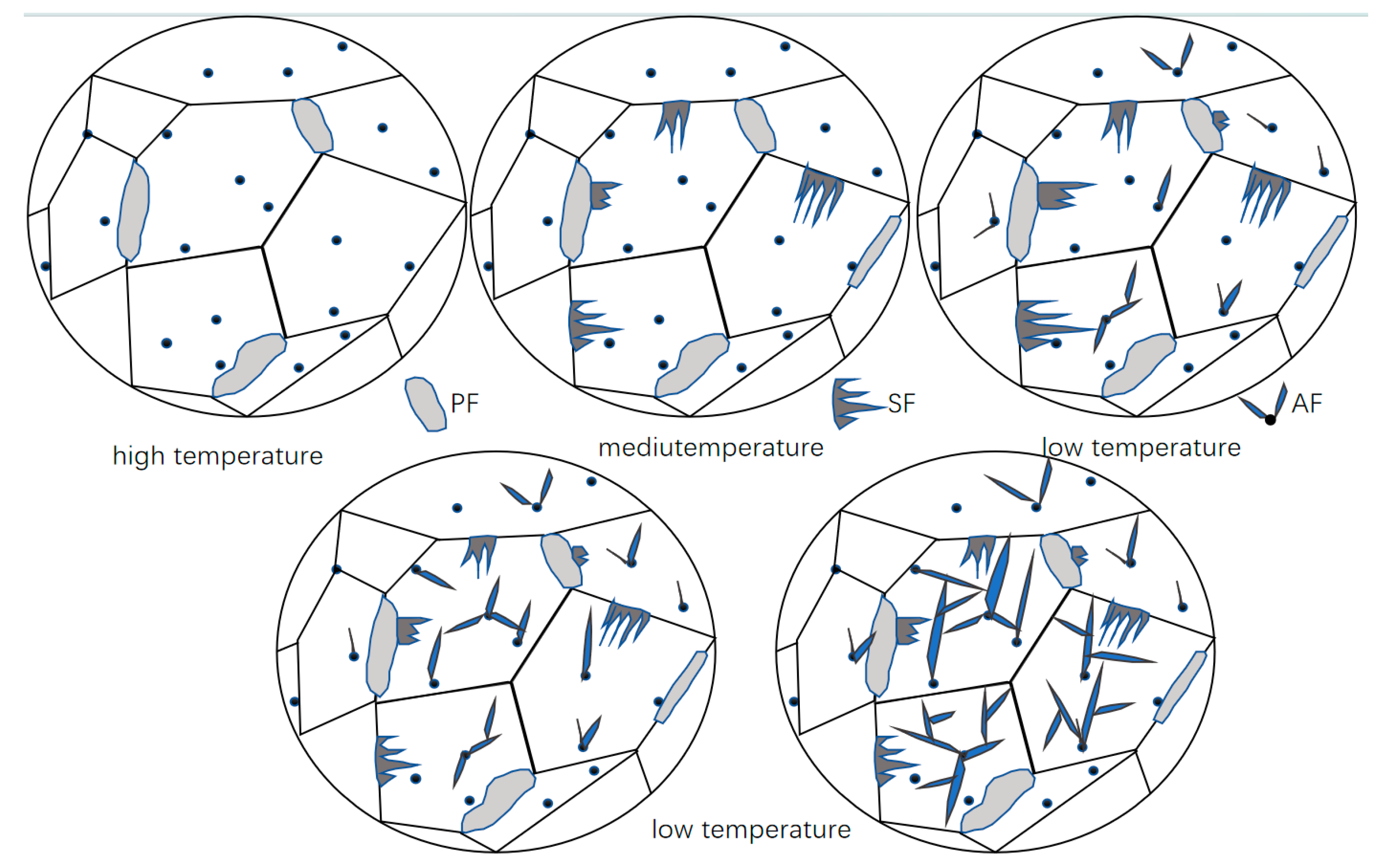
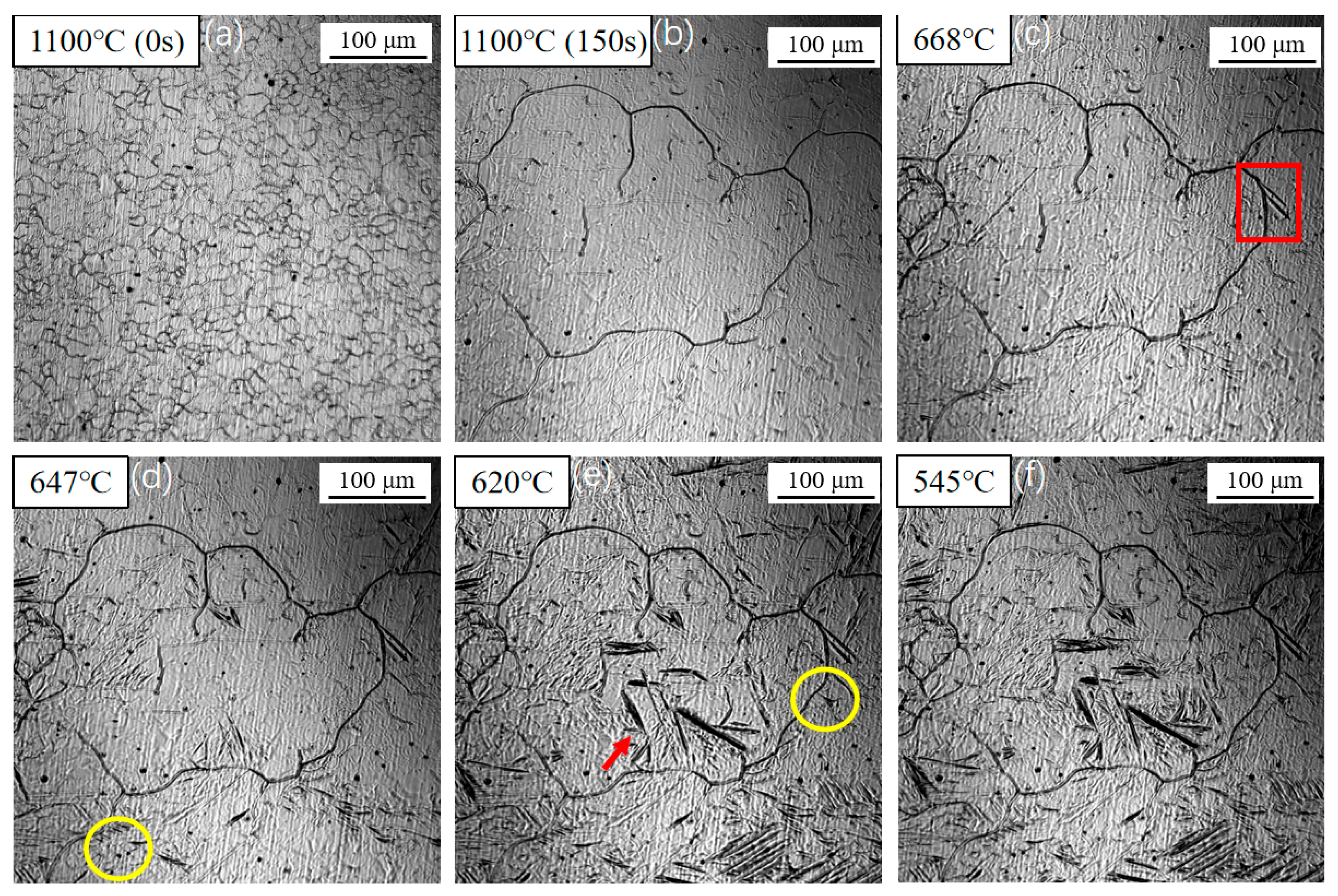

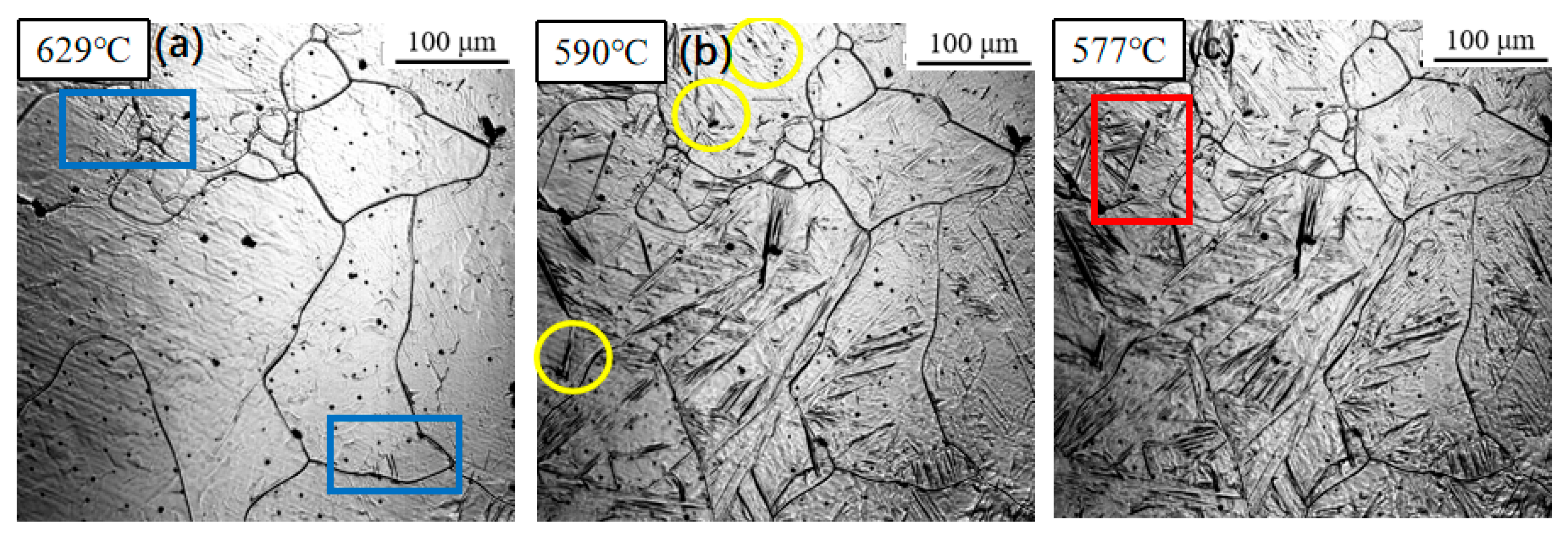




| Element | C | Si | Mn | Al | Ti | Nb | V | Mo | P | S |
|---|---|---|---|---|---|---|---|---|---|---|
| wt.% | 0.18 | 0.19 | 0.85 | 0.007 | 0.048 | 0.02 | 0.04 | 0.09 | 0.018 | 0.006 |
| Cooling Rate/(℃·S−1) | 1 | 3 | 5 | 7 | 10 | |
|---|---|---|---|---|---|---|
| Microstructure | ||||||
| FSP | 668 | 629 | 630 | 615 | 611 | |
| Primary IAF | 647 | 590 | 588 | 583 | 558 | |
| Secondary IAF | 620 | 577 | 564 | 563 | 498 | |
| Cooling Rate/(°C·S−1) | 1 | 3 | 5 | 7 | 10 |
|---|---|---|---|---|---|
| Primary Ferrite/vol% | 15 | 34 | 42 | 24 | 16 |
| Secondary Ferrite/vol% | 11 | 28 | 32 | 20 | 9 |
| Cooling Rate/(°C·s−1) | 1 | 3 | 5 | 7 |
|---|---|---|---|---|
| Minimum | 1.79 | 1.61 | 1.97 | 2.40 |
| Maximum | 10.82 | 15.03 | 32.31 | 14.52 |
| Average | 4.51 | 5.66 | 5.97 | 6.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, C.; Wang, F.; Sang, R.; Zhang, Q. Mechanistic Insights into Cooling-Rate-Governed Acicular Ferrite Transformation Kinetics and Strengthening-Toughening Synergy in EH36 Heavy Steel Plate. Materials 2025, 18, 4661. https://doi.org/10.3390/ma18204661
Yan C, Wang F, Sang R, Zhang Q. Mechanistic Insights into Cooling-Rate-Governed Acicular Ferrite Transformation Kinetics and Strengthening-Toughening Synergy in EH36 Heavy Steel Plate. Materials. 2025; 18(20):4661. https://doi.org/10.3390/ma18204661
Chicago/Turabian StyleYan, Chunliang, Fengming Wang, Rongli Sang, and Qingjun Zhang. 2025. "Mechanistic Insights into Cooling-Rate-Governed Acicular Ferrite Transformation Kinetics and Strengthening-Toughening Synergy in EH36 Heavy Steel Plate" Materials 18, no. 20: 4661. https://doi.org/10.3390/ma18204661
APA StyleYan, C., Wang, F., Sang, R., & Zhang, Q. (2025). Mechanistic Insights into Cooling-Rate-Governed Acicular Ferrite Transformation Kinetics and Strengthening-Toughening Synergy in EH36 Heavy Steel Plate. Materials, 18(20), 4661. https://doi.org/10.3390/ma18204661





