When a Small Amount of Comonomer Is Enough: Tailoring the Critical Solution Temperature of LCST-Type Thermoresponsive Random Copolymers by PEG Methyl Ether Methacrylate with 1100 g/mol Molecular Weight
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Homopolymers and Copolymers
2.3. Characterization Methods
2.3.1. Gel Permeation Chromatography (GPC)
2.3.2. Proton Nuclear Magnetic Resonance (1H NMR)
2.3.3. Turbidimetric Measurements
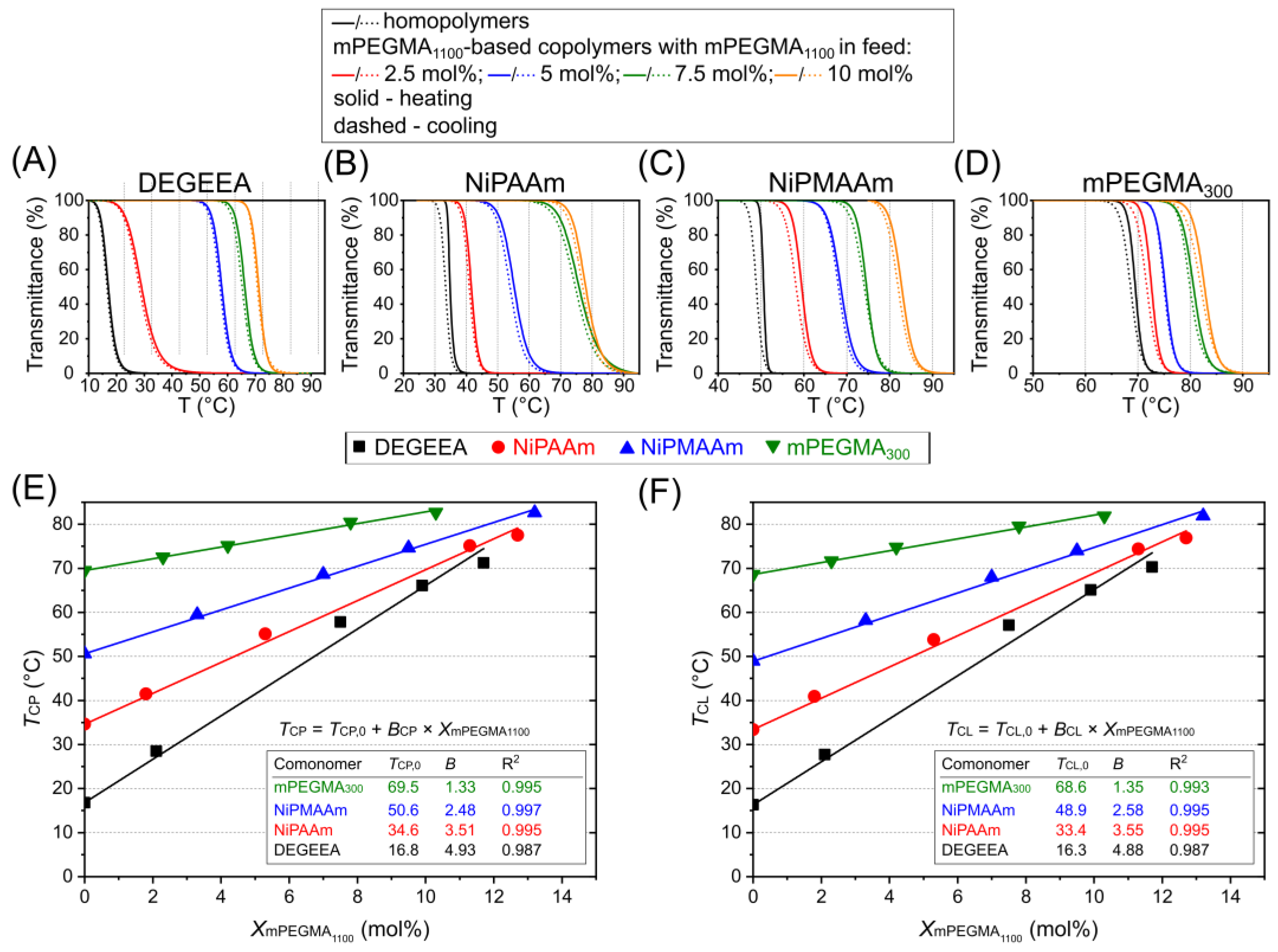
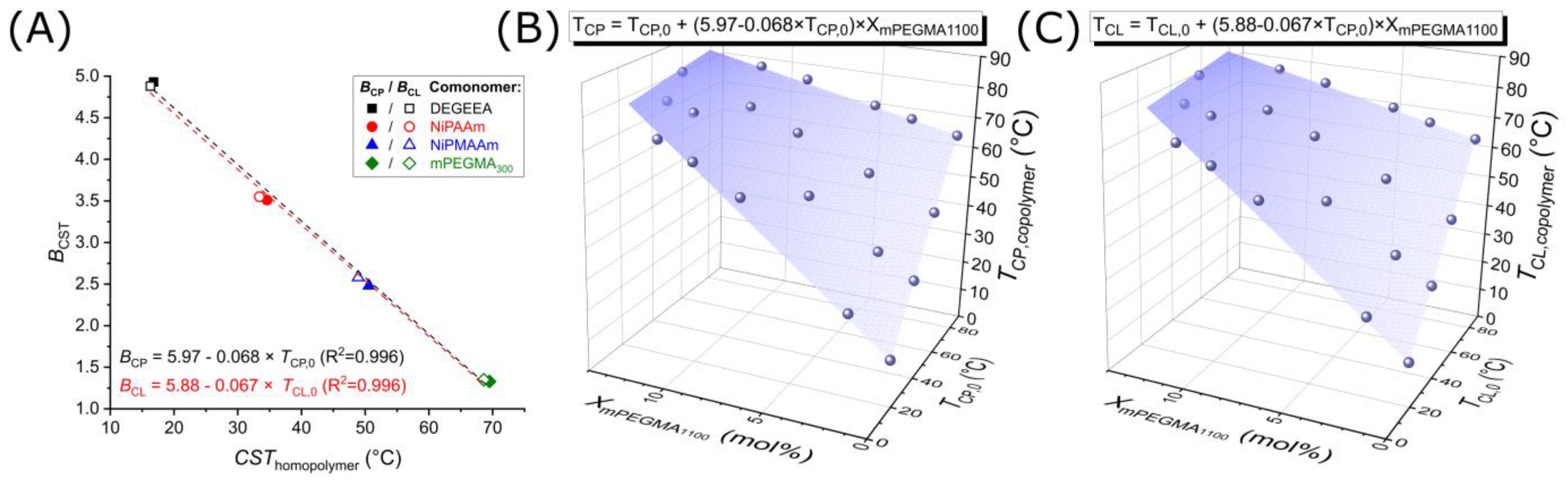
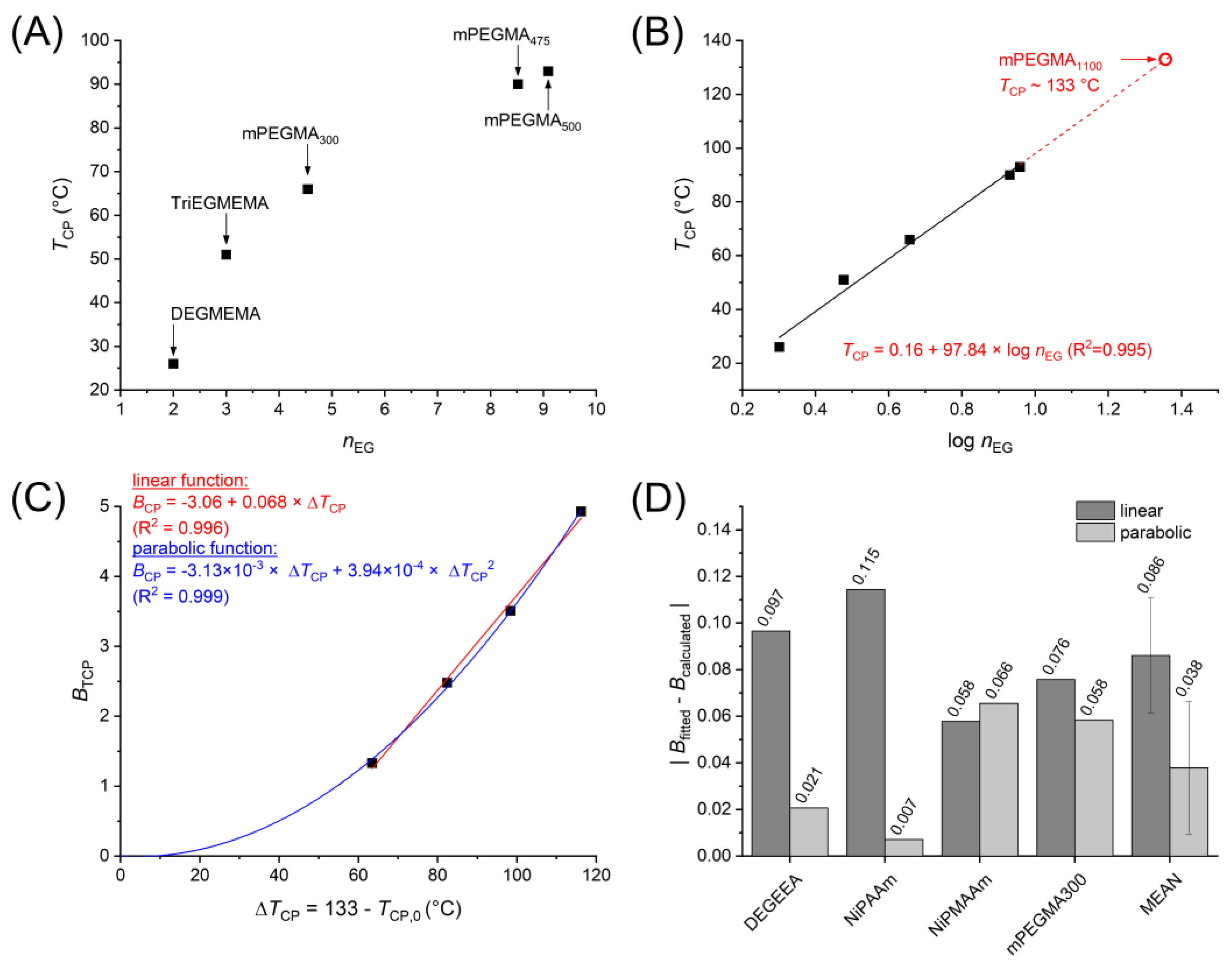
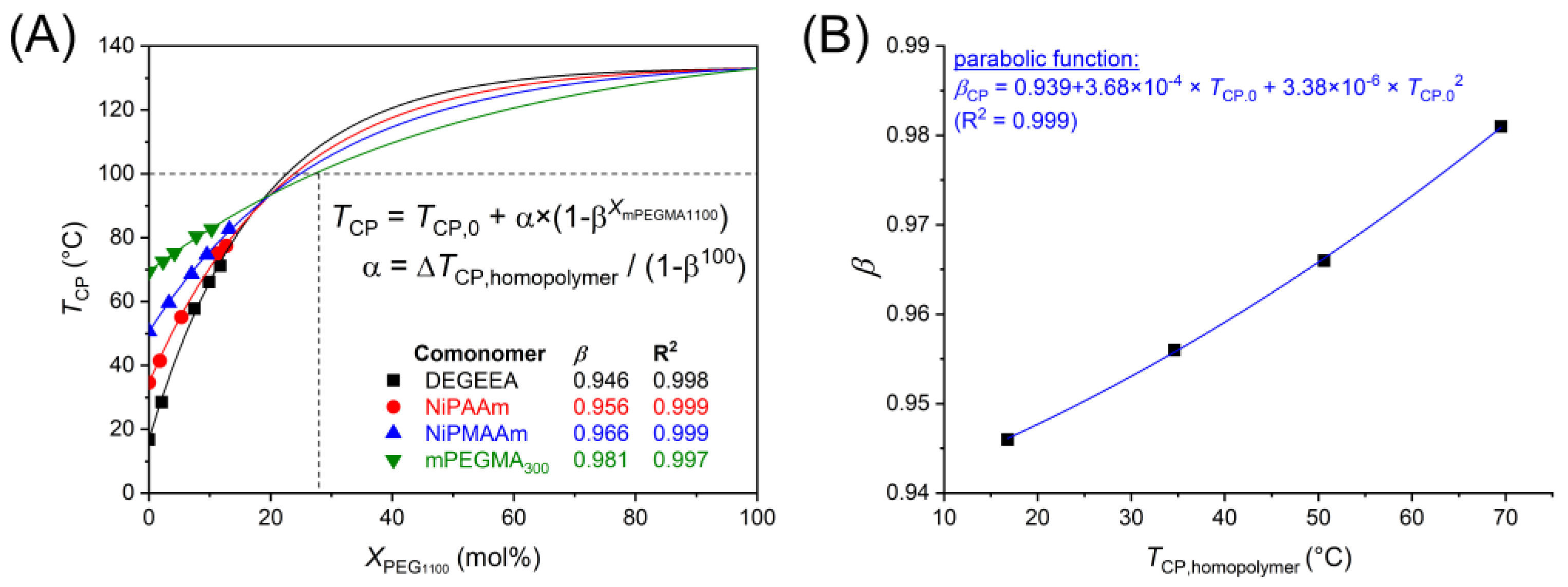
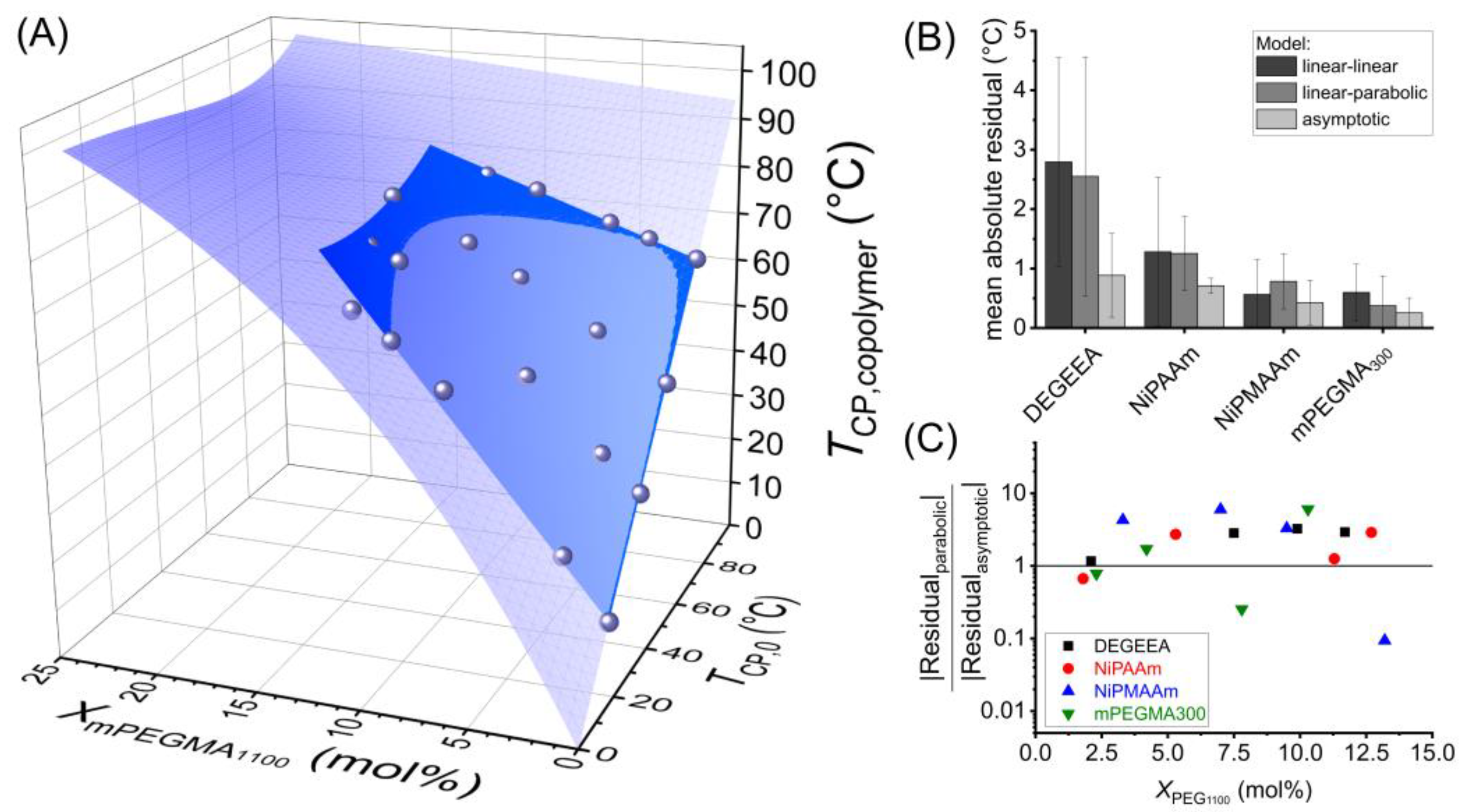
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ward, M.A.; Georgiou, T.K. Thermoresponsive Polymers for Biomedical Applications. Polymers 2011, 3, 1215–1242. [Google Scholar] [CrossRef]
- Doberenz, F.; Zeng, K.; Willems, C.; Zhang, K.; Groth, T. Thermoresponsive polymers and their biomedical application in tissue engineering—A review. J. Mater. Chem. B 2020, 8, 607–628. [Google Scholar] [CrossRef] [PubMed]
- Sponchioni, M.; Capasso Palmiero, U.; Moscatelli, D. Thermo-responsive polymers: Applications of smart materials in drug delivery and tissue engineering. Mater. Sci. Eng. C 2019, 102, 589–605. [Google Scholar] [CrossRef]
- Bordat, A.; Boissenot, T.; Nicolas, J.; Tsapis, N. Thermoresponsive polymer nanocarriers for biomedical applications. Adv. Drug Delivery Rev. 2019, 138, 167–192. [Google Scholar] [CrossRef]
- Tanga, S.; Aucamp, M.; Ramburrun, P. Injectable Thermoresponsive Hydrogels for Cancer Therapy: Challenges and Prospects. Gels 2023, 9, 418. [Google Scholar] [CrossRef] [PubMed]
- Musarurwa, H.; Tavengwa, N.T. Thermo-responsive polymers and advances in their applications in separation science. Microchem. J. 2022, 179, 107554. [Google Scholar] [CrossRef]
- Kasza, G.; Stumphauser, T.; Bisztrán, M.; Szarka, G.; Hegedüs, I.; Nagy, E.; Iván, B. Thermoresponsive Poly(N,N-diethylacrylamide-co-glycidyl methacrylate) Copolymers and Its Catalytically Active α-Chymotrypsin Bioconjugate with Enhanced Enzyme Stability. Polymers 2021, 13, 987. [Google Scholar] [CrossRef]
- Kasza, G.; Fábián, Á.; Fecske, D.; Kardos, A.; Mészáros, R.; Iván, B. Hyperbranched polyglycerol grafted poly(N,N-diethylacrylamide) thermoresponsive copolymers as biocompatible, highly efficient encapsulation and sustained release systems of curcumin. Eur. Polym. J. 2024, 219, 113378. [Google Scholar] [CrossRef]
- Hechenbichler, M.; Laschewsky, A.; Gradzielski, M. Poly(N,N-bis(2-methoxyethyl) acrylamide), a thermoresponsive non-ionic polymer combining the amide and the ethyleneglycolether motifs. Colloid Polym. Sci. 2021, 299, 205–219. [Google Scholar] [CrossRef]
- Diehl, C.; Schlaad, H. Thermo-Responsive Polyoxazolines with Widely Tuneable LCST. Macromol. Biosci. 2009, 9, 157–161. [Google Scholar] [CrossRef]
- Kirila, T.; Smirnova, A.; Aseyev, V.; Tenkovtsev, A.; Tenhu, H.; Filippov, A. Self-Organization in Dilute Aqueous Solutions of Thermoresponsive Star-Shaped Six-Arm Poly-2-Alkyl-2-Oxazines and Poly-2-Alkyl-2-Oxazolines. Polymers 2021, 13, 1429. [Google Scholar] [CrossRef]
- Glaive, A.S.; Amiel, C.; Volet, G. Synthesis and thermoresponsive behavior of double hydrophilic graft copolymer based on poly(2-methyl-2-oxazoline) and poly(2-ethyl-2-oxazoline). Eur. Polym. J. 2022, 179, 111504. [Google Scholar] [CrossRef]
- Bi, P.; Zhu, X.; Tian, L.; Han, J.; Zhang, W.; Wang, T. Preparation and Performance Study of HTPB-g-(PNIPAM/PEG) Thermoresponsive Polymer Brush. Polymers 2024, 16, 1248. [Google Scholar] [CrossRef]
- Lutz, J.-F.; Akdemir, Ö.; Hoth, A. Point by point comparison of two thermosensitive polymers exhibiting a similar LCST: Is the age of poly(NIPAM) over? J. Am. Chem. Soc. 2006, 128, 13046–13047. [Google Scholar] [CrossRef]
- Jones, J.A.; Novo, N.; Flagler, K.; Pagnucco, C.D.; Carew, S.; Cheong, C.; Kong, X.Z.; Burke, N.A.D.; Stover, H.D.H. Thermoresponsive copolymers of methacrylic acid and poly(ethylene glycol) methyl ether methacrylate. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 6095–6104. [Google Scholar] [CrossRef]
- Becer, C.R.; Hahn, S.; Fijten, M.W.; Thijs, H.M.; Hoogenboom, R.; Schubert, U.S. Libraries of methacrylic acid and oligo(ethylene glycol) methacrylate copolymers with LCST behavior. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 7138–7147. [Google Scholar] [CrossRef]
- Kim, Y.C.; Bang, M.S.; Kim, J.C. Synthesis and Characterization of Poly(N-Isopropyl Acrylamide) Copolymer with Methoxy Polyethyleneglycol Monomethacrylate. J. Ind. Eng. Chem. 2006, 12, 446–454. [Google Scholar]
- Vlassi, E.; Papagiannopoulos, A.; Pispas, S. Star Polyelectrolytes with Mixed Arms of PDMAEMA and POEGMA: Self-Assembly and Coassembly with Insulin. Macromol. Chem. Phys. 2022, 223, 2200008. [Google Scholar] [CrossRef]
- Ward, M.A.; Georgiou, T.K. Thermoresponsive triblock copolymers based on methacrylate monomers: Effect of molecular weight and composition. Soft Matter 2012, 8, 2737–2745. [Google Scholar] [CrossRef]
- Zhang, Q.; Tosi, F.; Üǧdüler, S.; Maji, S.; Hoogenboom, R. Tuning the LCST and UCST Thermoresponsive Behavior of Poly(N,N-dimethylaminoethyl methacrylate) by Electrostatic Interactions with Trivalent Metal Hexacyano Anions and Copolymerization. Macromol. Rapid Commun. 2015, 36, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Salminen, L.; Karjalainen, E.; Aseyev, V.; Tenhu, H. Phase Separation of Aqueous Poly(diisopropylaminoethyl methacrylate) upon Heating. Langmuir 2021, 38, 5135–5148. [Google Scholar] [CrossRef]
- Wang, L.; Constantinou, A.P.; Li, Y.; Georgiou, T.K. A library of thermoresponsive diblock and statistical copolymers: Unravelling the effect of molar mass. Eur. Polym. J. 2024, 207, 112810. [Google Scholar] [CrossRef]
- Szabó, Á.; Bencskó, G.; Szarka, G.; Iván, B. Thermoresponsive UCST-Type Behavior of Interpolymer Complexes of Poly(ethylene glycol) and Poly(poly(ethylene glycol) methacrylate) Brushes with Poly(acrylic acid) in Isopropanol. Macromol. Chem. Phys. 2017, 218, 1600466. [Google Scholar] [CrossRef]
- Szabó, Á.; Szanka, I.; Tolnai, G.; Szarka, G.; Iván, B. LCST-type thermoresponsive behaviour of interpolymer complexes of well-defined poly(poly(ethylene glycol) methacrylate)s and poly(acrylic acid) synthesized by ATRP. Polymer 2017, 111, 61–66. [Google Scholar] [CrossRef][Green Version]
- Narumi, A.; Sato, S.I.; Shen, X.; Kakuchi, T. Precision synthesis for well-defined linear and/or architecturally controlled thermoresponsive poly(N-substituted acrylamide)s. Polym. Chem. 2022, 13, 1293–1319. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, L.; Jiang, X. Freezing-resistant poly(N-isopropylacrylamide)-based hydrogel for thermochromic smart window with solar and thermal radiation regulation. J. Colloid. Interf. Sci. 2023, 652, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.Y.; Saunders, B.R. Thermally induced gelation of an oil-in-water emulsion stabilised by a graft copolymer. Chem. Commun. 2000, 24, 2461–2462. [Google Scholar] [CrossRef]
- Virtanen, J.; Baron, C.; Tenhu, H. Grafting of Poly(N-isopropylacrylamide) with Poly(ethylene oxide) under Various Reaction Conditions. Macromolecules 2000, 33, 336–341. [Google Scholar] [CrossRef]
- Horgan, A.; Saunders, B.; Vincent, B.; Heenan, R.K. Poly(butyl methacrylate-g-methoxypoly(ethylene glycol)) and poly(methyl methacrylate-g-methoxypoly(ethylene glycol)) graft copolymers: Preparation and aqueous solution properties. J. Colloid. Interf. Sci. 2003, 262, 548–559. [Google Scholar] [CrossRef]
- Andersson, T.; Holappa, S.; Aseyev, V.; Tenhu, H. Complexation of linear and poly(ethylene oxide)-grafted poly (methacryl oxyethyl trimethylammonium chloride) with poly (ethylene oxide-block-sodium methacrylate). J. Polym. Sci. Pol. Chem. 2003, 41, 1904–1914. [Google Scholar] [CrossRef]
- Ali, M.M.; Stöver, H.D. Well-defined amphiphilic thermosensitive copolymers based on poly(ethylene glycol monomethacrylate) and methyl methacrylate prepared by atom transfer radical polymerization. Macromolecules 2004, 37, 5219–5227. [Google Scholar] [CrossRef]
- Lutz, J.-F.; Hoth, A. Preparation of ideal PEG analogues with a tunable thermosensitivity by controlled radical copolymerization of 2-(2-methoxyethoxy) ethyl methacrylate and oligo(ethylene glycol) methacrylate. Macromolecules 2006, 39, 893–896. [Google Scholar] [CrossRef]
- Fournier, D.; Hoogenboom, R.; Thijs, H.M.; Paulus, R.M.; Schubert, U.S. Tunable pH-and temperature-sensitive copolymer libraries by reversible addition− fragmentation chain transfer copolymerizations of methacrylates. Macromolecules 2007, 40, 915–920. [Google Scholar] [CrossRef]
- Yamamoto, S.I.; Pietrasik, J.; Matyjaszewski, K. Temperature-and pH-responsive dense copolymer brushes prepared by ATRP. Macromolecules 2008, 41, 7013–7020. [Google Scholar] [CrossRef]
- Huang, X.; Du, F.; Cheng, J.; Dong, Y.; Liang, D.; Ji, S.; Lin, S.S.; Li, Z. Acid-sensitive polymeric micelles based on thermoresponsive block copolymers with pendent cyclic orthoester groups. Macromolecules 2009, 42, 783–790. [Google Scholar] [CrossRef]
- De, P.; Sumerlin, B.S. Precision control of temperature response by copolymerization of di(ethylene glycol) acrylate and an acrylamide comonomer. Macromol. Chem. Phys. 2013, 214, 272–279. [Google Scholar] [CrossRef]
- Micic, M.; Rogic Miladinovic, Z.; Suljovrujic, E. Tuning the thermoresponsive properties of poly(oligo(propylene glycol) methacrylate) hydrogels via gradient copolymerization with 2-hydroxyethyl methacrylate. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 18–27. [Google Scholar] [CrossRef]
- Nizardo, N.M.; Schanzenbach, D.; Schönemann, E.; Laschewsky, A. Exploring poly(ethylene glycol)-polyzwitterion diblock copolymers as biocompatible smart macrosurfactants featuring UCST-phase behavior in normal saline solution. Polymers 2018, 10, 325. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Tang, D.; Lv, H.; Wang, N.; Yang, X.; Sun, Z.; Yu, Z. Thermal phase transition of poly(N-vinyl caprolactam)-based copolymers: The distribution of hydrophilic units within polymeric chains. Colloid. Polym. Sci. 2019, 297, 1255–1264. [Google Scholar] [CrossRef]
- Akar, I.; Keogh, R.; Blackman, L.D.; Foster, J.C.; Mathers, R.T.; O’Reilly, R.K. Grafting density governs the thermoresponsive behavior of P(OEGMA-co-RMA) statistical copolymers. ACS Macro Lett. 2020, 9, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, L.; Chen, F.; Constantinou, A.P.; Georgiou, T.K. Thermoresponsive oligo(ethylene glycol) methyl ether methacrylate based copolymers: Composition and comonomer effect. Polym. Chem. 2022, 13, 2506–2518. [Google Scholar] [CrossRef]
- Chen, J.; Rizvi, A.; Patterson, J.P.; Hawker, C.J. Discrete libraries of amphiphilic poly(ethylene glycol) graft copolymers: Synthesis, assembly, and bioactivity. J. Am. Chem. Soc. 2022, 144, 19466–19474. [Google Scholar] [CrossRef] [PubMed]
- Chroni, A.; Forys, A.; Sentoukas, T.; Trzebicka, B.; Pispas, S. Poly [(vinyl benzyl trimethylammonium chloride)]-based nanoparticulate copolymer structures encapsulating insulin. Eur. Polym. J. 2022, 169, 111158. [Google Scholar] [CrossRef]
- Xu, J.; Abetz, V. Double thermoresponsive graft copolymers with different chain ends: Feasible precursors for covalently crosslinked hydrogels. Soft Matter 2022, 18, 2082–2091. [Google Scholar] [CrossRef]
- Sung, T.C.; Yang, J.S.; Yeh, C.C.; Liu, Y.C.; Jiang, Y.P.; Lu, M.W.; Ling, Q.D.; Kumar, S.S.; Chang, Y.; Umezawa, A.; et al. The design of a thermoresponsive surface for the continuous culture of human pluripotent stem cells. Biomaterials 2019, 221, 119411. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Lei, J.; Wu, L.; Guo, C.; Fang, J.; Bai, R.; Wyman, I. Poly(imidazoled glycidyl methacrylate-co-diethyleneglycol methyl ether methacrylate)–A new copolymer with tunable LCST and UCST behavior in water. Polymer 2018, 157, 79–86. [Google Scholar] [CrossRef]
- da Silva, J.B.; Haddow, P.; Bruschi, M.L.; Cook, M.T. Thermoresponsive poly(di (ethylene glycol) methyl ether methacrylate)-ran-(poly(ethylene glycol) methacrylate) graft copolymers exhibiting temperature-dependent rheology and self-assembly. J. Mol. Liq. 2022, 346, 117906. [Google Scholar] [CrossRef]
- Porsch, C.; Hansson, S.; Nordgren, N.; Malmström, E. Thermo-responsive cellulose-based architectures: Tailoring LCST using poly(ethylene glycol) methacrylates. Polym. Chem. 2011, 2, 1114–1123. [Google Scholar] [CrossRef]
- Kitano, H.; Hirabayashi, T.; Gemmei-Ide, M.; Kyogoku, M. Effect of Macrocycles on the Temperature-Responsiveness of Poly [(methoxy diethylene glycol methacrylate)-graft-PEG]. Macromol. Chem. Phys. 2004, 205, 1651–1659. [Google Scholar] [CrossRef]
- Kim, Y.C.; Kil, D.S.; Kim, J.C. Synthesis and phase separation of poly(N-isopropyl acrylamide-co-methoxy polyethyleneglycol monomethacrylate). J. Appl. Polym. Sci. 2006, 101, 1833–1841. [Google Scholar] [CrossRef]
- Di, C.; Jiang, X.; Yin, J. Synthesis of stimuli-responsive star-like copolymer H20-PNIPAm-r-PEGMA via the ATRP copolymerization technique and its micellization in aqueous solution. J. Appl. Polym. Sci. 2010, 115, 1831–1840. [Google Scholar] [CrossRef]
- Osváth, Z.; Iván, B. The dependence of the cloud point, clearing point, and hysteresis of poly(N-isopropylacrylamide) on experimental conditions: The need for standardization of thermoresponsive transition determinations. Macromol. Chem. Phys. 2017, 218, 1600470. [Google Scholar] [CrossRef]
- Osváth, Z.; Tóth, T.; Iván, B. Sustained drug release by thermoresponsive sol-gel hybrid hydrogels of poly(N-Isopropylacrylamide-co-3-(Trimethoxysilyl)propyl methacrylate) copolymers. Macromol. Rapid Commun. 2017, 38, 1600724. [Google Scholar] [CrossRef] [PubMed]
- Halperin, A.; Kröger, M.; Winnik, F.M. Poly(N-isopropylacrylamide) phase diagrams: Fifty years of research. Angew. Chem. Int. Edit. 2015, 54, 15342–15367. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Trujillo, F.J.; Xu, J.; Boyer, C.; Corrigan, N. Influence of Molecular Weight Distribution on the Thermoresponsive Transition of Poly(N-isopropylacrylamide). Macromol. Rapid Commun. 2021, 42, 2100212. [Google Scholar] [CrossRef]
- Gurnani, P.; Williams, G.R. Investigating oligo(ethylene glycol) methacrylate thermoresponsive copolymers. Eur. Polym. J. 2024, 216, 113266. [Google Scholar] [CrossRef]
- Kazantsev, O.A.; Orekhov, D.V.; Simagin, A.S.; Kamorin, D.M.; Sivokhin, A.P.; Savinova, M.V.; Arifullin, I.R.; Kavtrova, V.D.; Lobayev, A.N. Oligo(ethylene glycol) methacrylate-based molecular bottlebrushes: Correlations between composition and phase transition temperatures in aqueous solutions. Eur. Polym. J. 2024, 218, 113340. [Google Scholar] [CrossRef]
- Li, Q.; Constantinou, A.P.; Georgiou, T.K. A library of thermoresponsive PEG-based methacrylate homopolymers: How do the molar mass and number of ethylene glycol groups affect the cloud point? J. Polym. Sci. 2021, 59, 230–239. [Google Scholar] [CrossRef]
- Srinivasulu, B.; Raghunath Rao, P.; Sundaram, E.V. Synthesis and characterization of acrylamide-hexyl methacrylate copolymers. Polym. Int. 1992, 28, 129–132. [Google Scholar] [CrossRef]
- Xu, B.; Gu, G.; Feng, C.; Jiang, X.; Hu, J.; Lu, G.; Zhang, S.; Huang, X. (PAA-g-PS)-co-PPEGMEMA asymmetric polymer brushes: Synthesis, self-assembly, and encapsulating capacity for both hydrophobic and hydrophilic agents. Polym. Chem. 2016, 7, 613–624. [Google Scholar] [CrossRef]
- Hakim, M.; Verhoeven, V.; McManus, N.T.; Dubé, M.A.; Penlidis, A. High-temperature solution polymerization of butyl acrylate/methyl methacrylate: Reactivity ratio estimation. J. Appl. Polym. Sci. 2000, 77, 602–609. [Google Scholar] [CrossRef]
- Reddy, G.J.; Reddy, M.M.; Reddy, G.R.; Naidu, S.V.; Reddy, A.V.R. Synthesis and characterization of co-polymers of N-phenyl methacrylamide with glycidyl methacrylate. Des. Monomers Polym. 2008, 11, 581–591. [Google Scholar] [CrossRef]
- Kucharski, M.; Lubczak, R. Copolymerization of hydroxyalkyl methacrylates with acrylamide and methacrylamide I. Determination of reactivity ratios. J. Appl. Polym. Sci. 1997, 64, 1259–1265. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Xu, S.; Liu, H. Design and Synthesis of Multi-Responsive Copolymers for Drug Carrier. Acta Phys.-Chim. Sin. 2019, 35, 876–884. [Google Scholar] [CrossRef]
- Ramírez-Jiménez, A.; Montoya-Villegas, K.A.; Licea-Claverie, A.; Gónzalez-Ayón, M.A. Tunable thermo-responsive copolymers from DEGMA and OEGMA synthesized by RAFT polymerization and the effect of the concentration and saline phosphate buffer on its phase transition. Polymers 2019, 11, 1657. [Google Scholar] [CrossRef]
- Yamamoto, S.I.; Pietrasik, J.; Matyjaszewski, K. The effect of structure on the thermoresponsive nature of well-defined poly(oligo(ethylene oxide) methacrylates) synthesized by ATRP. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 194–202. [Google Scholar] [CrossRef]
- Constantinou, A.P.; Wang, L.; Wang, S.; Georgiou, T.K. Thermoresponsive block copolymers of increasing architecture complexity: A review on structure–property relationships. Polym. Chem. 2023, 14, 223–247. [Google Scholar] [CrossRef]
- Ganachaud, F.; Monteiro, M.J.; Gilbert, R.G.; Dourges, M.A.; Thang, S.H.; Rizzardo, E. Molecular weight characterization of poly(N-isopropylacrylamide) prepared by living free-radical polymerization. Macromolecules 2000, 33, 6738–6745. [Google Scholar] [CrossRef]
- Beitl, K.N.; Reimhult, E. Effect of Solvent Properties on the Critical Solution Temperature of Thermoresponsive Polymers. Int. J. Mol. Sci. 2024, 25, 7734. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasza, G.; Sármezey, B.; Fecske, D.; Verebélyi, K.; Iván, B. When a Small Amount of Comonomer Is Enough: Tailoring the Critical Solution Temperature of LCST-Type Thermoresponsive Random Copolymers by PEG Methyl Ether Methacrylate with 1100 g/mol Molecular Weight. Materials 2025, 18, 372. https://doi.org/10.3390/ma18020372
Kasza G, Sármezey B, Fecske D, Verebélyi K, Iván B. When a Small Amount of Comonomer Is Enough: Tailoring the Critical Solution Temperature of LCST-Type Thermoresponsive Random Copolymers by PEG Methyl Ether Methacrylate with 1100 g/mol Molecular Weight. Materials. 2025; 18(2):372. https://doi.org/10.3390/ma18020372
Chicago/Turabian StyleKasza, György, Bence Sármezey, Dóra Fecske, Klára Verebélyi, and Béla Iván. 2025. "When a Small Amount of Comonomer Is Enough: Tailoring the Critical Solution Temperature of LCST-Type Thermoresponsive Random Copolymers by PEG Methyl Ether Methacrylate with 1100 g/mol Molecular Weight" Materials 18, no. 2: 372. https://doi.org/10.3390/ma18020372
APA StyleKasza, G., Sármezey, B., Fecske, D., Verebélyi, K., & Iván, B. (2025). When a Small Amount of Comonomer Is Enough: Tailoring the Critical Solution Temperature of LCST-Type Thermoresponsive Random Copolymers by PEG Methyl Ether Methacrylate with 1100 g/mol Molecular Weight. Materials, 18(2), 372. https://doi.org/10.3390/ma18020372










