Carrier Mobility, Electrical Conductivity, and Photovoltaic Properties of Ordered Nanostructures Assembled from Semiconducting Polymers
Abstract
1. Introduction
2. The Structure-Processing-Property Relationship and Charge Transport Mechanisms in Semiconducting Polymers
3. Polymeric Semiconducting Nanostructures Displaying High Charge Carrier Mobilities
3.1. Carrier Mobility in Diketopyrrolopyrrole-Based Nanostructures

3.2. Carrier Mobility in Thiophene-Based Nanostructures
3.3. Carrier Mobility in Naphthalenediimide-Based Nanostructures
3.4. Carrier Mobility in Isoindigo-Based Nanostructures
3.5. Carrier Mobility in Nanostructures Based on Other Semiconducting Polymeric Systems
3.6. Improved Carrier Mobility in Semiconducting Polymers Blended with Other Polymers
3.7. Enhancing Carrier Mobility of Semiconducting Polymers by Chemical Doping
| Polymer System (* = Full Name) | Molecular Weight (kg/mol) | Nanostructure Type | Processing Strategy | π–π Spacing (nm) | Measurement Technique | Charge Mobility (cm2V−1s−1) | Current On/Off Ratio | Threshold Voltage (V) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| DPP-based polymers | |||||||||
| PDTTDPP | - | nanowires | thermal protocols and drop casting | 0.370 | OFET | µ = 7 | - | −15 | [111] |
| DPPBTSPE | Mn = 8 | nanowires | thermal protocols and drop casting | 0.346 | OFET | µh = 24 | 104 | −4 | [29] |
| DPPBTSPE | Mn = 68 | nanowires | thermal protocols and drop casting | 0.372 | OFET | µ = 4.15 | 108 | 0 | [29] |
| DPP-2T | Mn = 74 | strongly packed aggregates | TA | 0.380 | OFET | µh = 1.33 | 104 | −3 | [113] |
| PDPPT3 | Mn = 88 | high continuity structures with tie chains | TA | 0.372 | OFET | µh = 4.9 | 104–105 | −7 to −15 | [114] |
| PDPPy-Se | Mn = 409.1 | large-scale crystallites | TA | 0.362 | OFET | µe = 2.22 | - | − | [112] |
| P(gDPP-TT) P(gDPP-T2) P(gDPP-MeOT2) | - - - | (highly) ordered and crystalline edge-on oriented surfaces | spin coating | 0.358 0.354 0.352 | OECT | µ = 0.57 µ = 1.55 µ = 0.28 | 105 105 104 | −0.54 −0.52 −0.26 | [117] |
| P(TDPP-BT) P(TDPP-TQ) P(TDPP-BBT) | Mn = 42 Mn = 16.1 Mn = 21 | fiber-like domains with face-on packing | TA | 0.357 0.369 0.369 | OFET | µe/h = 3.83/2.77 µe/h = 7.76/6.16 µe/h = 0.35/0.25 | - - - | − − − | [118] |
| TDPP-Se | Mn = 21.2 Mn = 40.7 Mn = 61.3 Mn = 73.3 Mn = 135.3 | 1D rod-like nanostructures | bar-coating | 0.369 0.370 0.370 0.369 0.370 | OFET | µh = 6.47 µh = 13.77 µh = 12.05 µh = 6.81 µh = 7.42 | 103 ÷106 105 ÷107 105 ÷107 104 ÷107 105 ÷107 | −11 ÷ −6 −8 ÷ −3 −7 ÷ 0 −10 ÷ −2 −12 ÷ −6 | [119] |
| NH-FDPP-TT NH-FDPP-BT | Mw = 8.69 Mw = 5.61 | smooth surfaces with nanopinholes | TA | 0.390 - | OFET | µh = 5 × 10−3 µh = 2.2 × 10−3 | 105 105 | −17.5 −25.4 | [120] |
| PTNDP-IDT PNDP-2T | Mw = 41.8 Mw = 29.2 | coplanar π–π stacked structures | TA | 0.386 0.366 | OFET | µe = 0.99 µe = 0.82 | 6 6 | 17−21 15−18 | [121] |
| IDTz-DPP-based | Mn = 48.5 | amorphous structures without long-range order | TA | - | OFET | µe = 1.3 | - | 2.6 | [116] |
| PDPP-TT PDPP-TT-PDMS-1k PDPP-TT-PDMS-2.5k PDPP-TT-PDMS-25k | Mn = 17.7 Mn = 14 Mn = 14.6 Mn = 37.5 | interconnected fiber-like nanostructures | solution shearing and TA | 0.369 0.363 0.370 0.365 | OFET | µ = 0.7 µ = 0.21 µ = 0.15 µ = 0.1 | ~105 ~106 ~106 ~106 | 22.69 ± 8 29.06± 4 25.76 ± 2 17.08 ± 1 | [122] |
| P(g2T-T) P(gT2) | Mn = 67.5 Mn = 71 | aligned crystalline structures with tie-chains | TA | 0.356 0.374 | OECT | µ = 0.93 µ = 0.38 | - - | - - | [123] |
| 3-octyl and 3-hexyl-thiophene (3-O/H-T)-based polymers | |||||||||
| P3OT | Mw = 120 | single crystal | - | - | OFET | µ = 1.54 × 10−4 | 37 | 7.3 | [127] |
| P3OT | Mw = 51.2 | single crystal | - | - | OFET | µ = 0.62 | - | - | [126] |
| P3HT | Mw = 39.6 | single crystal | - | - | OFET | µ = 1.57 × 10−3 | - | - | [126] |
| P3HT | Mn = 1.332 | single crystal | - | - | C-AFM | µ = 0.5 | - | 8 | [90] |
| P3HT P3HT-b-PDL | Mn = 15 Mn = 29.6 | nanofibers | TA | 0.384 0.385 | OFET | µ = 0.116 µ = 0.088 | 3.9 × 105 4.5 × 105 | −2.6 −2.6 | [130] |
| RR-P3HT | - | unidirectionally aligned domains | unidirectional floating-film transfer method | - | OFET | µ = 0.12 | 104 | - | [131] |
| Bithiophene, IDT, CDT-based polymers | |||||||||
| CDT-BTZ | Mn = 50 | nanofibers | - | - | OFET | µ = 5.5 | 106 | −60 | [135] |
| s-BTI2-FT f-BTI2-FT | Mn = 19.5 Mn = 13.8 | crystalline edge-on aggregates | TA | 0.360 0.360 | OFET | µe = 0.82 µe = 1.13 | 106 106 | 19 18 | [170] |
| f-BTI2TEG-T f-BTI2TEG-FT | Mn = 6.7 Mn = 6.3 | fibrillar nanostructure | TA | 0.353 0.353 | OECT | µe = 0.044 µe = 0.034 | - | 0.68 0.53 | [171] |
| OFET | µe = 6.34 × 10−4 µe = 3.67 × 10−4 | 16.8 1.3 × 103 | - - | ||||||
| P(g2T-TT) PgBTTT | Mn = 7 Mn = 10 | 2D islands of elongated parallel backbones | drop-casting and electrospray deposition | 0.358 0.354 | OECT | µh = 0.41 µh = 3.44 | - - | - - | [172] |
| PBDP-F2 | Mn = 40.9 | continuous donor and acceptor nanostructures | TA | 0.369 | OFET | µh = 1.93 | - | - | [136] |
| F8T2 | - | fibrillar structures | TA | - | OFET | µh = 5.40 | >105 | -3 | [137] |
| BDT-based | Mn = 12.8 | nanostructures comprising large crystallite grains | on-center vs. off-center and TA | 0.386 0.382 - - | PMMA-gated PMMA-gated pristine OFET functionalized | µh = 0.44 × 10−2 µh = 1.90 × 10−2 µh = 5.56 µh = 8.03 | >102 >102 >105 ~106 | −16.90 −26.64 −1.25 −1.47 | [138] |
| C16-IDT-BT | Mw = 108 | - | TA | 0.410 | OFET | µh = 1.2 | - | − | [173] |
| IDT-BT TTIF-BT | Mn = 58 Mn = 51 | surfaces of enhanced crystalline order | TA | 0.410 0.414 | OFET | µh = 1.5 µh = 1.1 | - 106 | 0 −12 | [174] |
| PCDTPT | Mw = 76 | nanowires | liquid-bridge-mediated nano- transfer molding | 0.442 | OFET | µ = 92.64 | 1.8 × 104 | 1.77 | [96] |
| IID-based polymers | |||||||||
| Polyisoindigo Poly(ethynylisoindigo) Poly(bisisoindigo) | Mn = 21.9 Mn = 15.4 Mn = 15.5 | surfaces with different molecular order | TA | 0.370 0.350 0.380 | OFET | µe = 2.85 × 10−4 µe = 4.15 × 10−4 µe = 7.67 × 10−5 | 104 104 104 | 59 57 51 | [175] |
| P(TzII-dTh-dTh) P(TzII-dTh-dTz) | Mn = 236 Mn = 138 | poorly crystalline structures | TA | - - | OFET | µh/e = 1.43/0.55 µh/e = 0.38/0.56 | 103 ~3 | 15.96 79.78 | [150] |
| P2FIID-2FBT | Mn = 59 | fiber-like poly-crystalline grains | TA | 0.350 | OFET | µe = 9.7 | 103–104 | 57 | [149] |
| PTIIG-Np | Mn = 21 | nanofibers | TA | 0.361 | OFET | µh = 14.4 | - | −45÷ −48 | [95] |
| NDI-based polymers | |||||||||
| P(NDI2OD-Se-Th 1.0) | Mn = 70 | surfaces comprising face-on packed crystalline structures | TA | - | OFET SCLC | µ = 0.138 µe = 2.5 × 10−4 | >103 - | 1.3 - | [145] |
| P(NDI2OD-T2) | Mn = 76.6 | microwire | OFET | µ = 2.56 | - | - | [142] | ||
| SNTandNDI-based | Mw = 54.9 | nanofiber-like crystalline structures | TA | 0.340 | OFET | μe = 7.37 | 106–107 | 1-5 | [144] |
| TFE-based polymers | |||||||||
| PNBDO-FDTE100 PNBDO-FDTE90 PNBDO-FDTE80 PNBDO-FDTE70 PNBDO-FDTE60 PNBDO-FDTE0 | Mw = 164.4 Mw = 117.6 Mw = 115.5 Mw = 108.1 Mw = 105.5 Mw = 76.9 | crystalline structures comprising molecular self-assemblies | spin coating and TA | 0.350 0.350 0.353 0.356 0.357 0.329 | OFET | µe = 7.43 µe = 7.25 µe = 6 µe = 1.75 µe = 062 µe = 0.182 | 102–103 102–103 102–103 ~102 ~102 102–103 | 19.18 18.76 17.5 18.4 14.75 17.73 | [151] |
| Other types of polymers | |||||||||
| PFO Y80F8:20F5 S50F8:50F5 | - | aligned nematic structures | spin coated from heated solutions | - | time-of-flight | µ = 2.7 × 10−4 µ = 3.7 × 10−2 µ = 2.7 × 10−2 | - | - | [105] |
| TA-PPE | Mw = 51.328 | nanowire | OFET | µ = 0.1 | - | −40 | [31] | ||
| F4BDOPV-2T | Mn = 60.4 | microwire | OFET | µ = 5.58 | 103-104 | 2 | [142] | ||
| F4BDOPV-2T | Mn = 38 | surfaces of edge-on packed crystallites | TA | 0.352 | OFET | µe = 14.9 | 103-104 | −17 | [153] |
| QA based | Mw = 38.5 | block- and fiber-shaped structures | TA | 0.356 | OFET | µh = 1.02 | 5-6 | −17 | [152] |
| Doped polymers | |||||||||
| P3HT/CPE | Mw = 80 | ordered crystallites | dopant induced | - | OFET | µh = 0.135 | ~103 | −22.4 ± 2.1 | [168] |
| P3HT/DDB | Mn = 50–70 | lamellar crystallites | spin coating | - | AC-Hall effect | µ = 0.095 | - | - | [167] |
| PEDOT/FeCl3 | - | - | oCVD | - | AC-Hall effect | µ = 33.6 | - | - | [164] |
| Blended polymers | |||||||||
| PCDTPT/PS | Mw = 50 | nanofibers | slow-drying | - | OFET | µh = 23.7 | - | - | [157] |
| P3HT PTB7-th P3HT/PTB7-th | - - - | nanopillars nanopillars nanopillars | self-assembly under nano confinement | 0.373 0.391 0.378:0.387 | conductive scanning force microscopy | µvertical = 0.96 µvertical = 0.036 µvertical = 0.73 | - - - | - - - | [158] |
| P2TDPP2TFT4/SEBS | Mn = 97 | nanofibers | solution shearing | 0.366 | OFET | µ = 2 | 104–105 | - | [162] |
| PU(DPP)35 PU(DPP)35/PDPPT325% | Mn = 20 - | tens to hundred nm large phase- separated domains | TA | - 0.364 | OFET | µh = 0.19 µh = 1.28 | - - | - - | [161] |
| P(DPP-T)/BPE | Mw = 146.3 | phase-separated granular nanostructures | slot-die coating | - | OFET | µh = 1.37 × 10−2 | 102 | 14.6 | [160] |
| direct-ink writing | - | µh = 7 × 10−4 | 101 | −13.3 | |||||
| DPPF-NTz/SEBS | Mn = 34 | fibrillar nanostructures and nanoclusters | TA | 0.390 | OFET | µe = 0.0024 | 105 | 4.61 | [159] |
| P3HT/PEGDA/HOMPP based | MwP3HT = 87 | nanofibers | electrospinning | - | OFET | µ = 23 | 105 | −3÷ −2.7 | [156] |
4. The Enhancement of Electrical Conductivity in Nanostructures of Semiconducting Polymers
| Polymer System/Dopant (If the Case) | Molecular Weight (kg/mol) | Nanostructure Type | Processing Strategy | Measurement Configuration | Electrical Conductivity (S/cm) | Ref. |
|---|---|---|---|---|---|---|
| PEDOT | - | scale-like morphology | chemical oxidative method and TA | four-probe measurements | 0.0002–0.001 | [178] |
| PEDOT | - | nanofibers | evaporative vapor-phase polymerization | four-probe measurements | 130 | [179] |
| PEDOT | - | microfibers | surfactant-free interfacial polymerization | four-probe measurements | 375 | [180] |
| PEDOT | - | face-on fibrillar domains | oCVD and acid treatment | four-probe measurements | 6259 | [182] |
| PEDOT:PSS | - | well-defined (elongated) nanofibers | solution shearing, TA, treated with methanol | four-probe measurements | 4600 | [187] |
| PEDOT:PSS/ additives | - | fibrous morphology | blending with additives and light oxygen plasma treatment, + soaking in solvents and TA | four-probe measurements | 5012 | [188] |
| PEDOT:PSS/PEGDA/PEG | - | phase-separated PEDOT and PSS domains | triple-network strategy: incorporating PEGDA in PEDOT:PSS, diffusion of PEG | four-probe measurements | 0.3 | [189] |
| Selenium substituted DPP | - | ordered edge-on oriented crystalline structures | drop casting followed by immersion in dopant solution | four-probe measurements | 977 | [194] |
| PDFSe | Mn = 95.5 | near-amorphous featureless surfaces | spin coating and TA | four-probe measurements | 62.6 | [200] |
| PTz-5-DPP/N-DMBI | Mn = 22 | mixed face-on and edge-on orientated structures | spin coating and TA | four-probe measurements | 8.31 | [196] |
| P(PzDPP-2FT) | - | structures of pyridine counterions docked near the polymer backbone | spin coating followed by immersion in dopant solution | four-probe measurements | 218 | [201] |
| P3HT/FeCl3 | Mn = 24.2 | π-stacked ordered domains of highly aligned polymer chains intercalated with dopants | doctor blading of hot polymer solutions followed by high-temperature film rubbing and TA | four-probe measurements | 570 | [205] |
| PBTTT derivative/FeCl3 | Mn = 26 | π-stacked ordered domains of highly aligned polymer chains intercalated with dopants | doctor blading of hot polymer solutions followed by high-temperature film rubbing and TA | four-probe measurements | 2.2 × 105 | [205] |
| PDTzSI-Se/N-DMBI | Mn = 145.7 | crystalline structures exhibiting high backbone coplanarity | spin coating and TA | - | 164 | [211] |
| PO12/N-DMBI | Mn = 23.8 | large highly crystalline domains intercalated with small dopant domains | spin coating and TA | - | 92 | [208] |
| n-PT4/N-DMBI | Mn = 137 | crystalline fibril-textured morphology | spin coating and TA | four-probe measurements | 133.3 | [207] |
| PCNI2-BTI/N-DMBI | Mn = 70.9 | oriented-fiber-like aggregates | solution shearing and TA | - | 150.2 | [209] |
| FBDPPV-OEG/TAM | Mn = 65.63 | disconnected regions of pore structures | spin coating followed by vapor doping and TA | four-probe measurements | 39 | [214] |
| PFClTVT/N-DMBI | Mn = 39.4 | nanofiber-like aggregates | spin coating and TA | four-probe measurements | 38.3 | [215] |
| TANI | - | single crystals | grown from solution on guiding substrates | C-AFM | 12.3 | [216] |
| P3HT/DDB | Mn = 50–70 | lamellar crystallites | spin casting | AC-Hall effect | 12.9 | [167] |
| PANI/TSA | - | self-assembled nanotubes | template-free and interfacial polymerization method | four-probe measurements | 320 | [220] |
5. Latest Photovoltaic Properties of Polymer-Based Semiconducting Nanostructures
5.1. Nanostructures with Enhanced Photovoltaic Properties Found in Fullerene-Based OSCs
5.2. Nanostructures Employed in Efficient OSCs Based on Non-Fullerene Acceptors
5.3. Nanostructures Leading to High Power Conversion Efficiencies in All-Polymer OSCs
| Donor:acceptor | Polymer Molecular Weight (kg/mol) | Nanostructure Type | Processing Method | JSC (mA/cm2) | FF (%) | VOC (V) | PCE (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Fullerene-based OSCs | ||||||||
| P3HT:PC71BM | Mn = 7.15 | single crystal | solution grown crystals/nanofibers were mixed with PC71BM and spin coated to fabricate thin films | 7.69 | 54 | 0.59 | 2.45 | [232] |
| P3HT:PC71BM | Mn = 7.15 | nanofiber | 5.93 | 45 | 0.57 | 1.52 | [232] | |
| P3HT:PC71BM | Mn = 48.8 | single crystal | 9.18 | 55 | 0.58 | 2.93 | [232] | |
| P3HT:PC71BM | Mn = 48.8 | nanofiber | 7.10 | 47 | 0.59 | 1.97 | [232] | |
| P3HT-b-PEG:PC71BM | Mn = 49.55 | hairy single crystal | 9.26 | 54 | 0.58 | 2.90 | [232] | |
| P3HT-b-PEG:PC71BM | Mn = 49.55 | nanofiber | 7.11 | 53 | 0.58 | 2.18 | [232] | |
| P3HT-b-PS:PC71BM | Mn = 7.669 | hairy single crystal | 7.74 | 51 | 0.58 | 2.29 | [232] | |
| P3HT-b-PMMA:PC71BM | Mn = 7.647 | hairy nanofiber | 5.47 | 47 | 0.58 | 1.49 | [232] | |
| PffBT4T-C9C13:PC71BM | - | 38 nm polymer-rich domains comprising molecules adopting face-on orientations | spin coating from warm solutions | 19.8 | 73 | 0.784 | 11.7 | [93] |
| PM6:BTP-eC9:PC71BM multicomponent | - | ~35 nm long nanofiber-like structures | spin coating solutions containing DQ | 26.93 | 79.4 | 0.856 | 18.3 | [234] |
| OSCs based on non-fullerene acceptors | ||||||||
| PBDB-T-SF:IT-4F | Mn = 20.9 | nanophase-separated morphology | spin coating | 20.88 | 71.3 | 0.88 | 13.1 | [236] |
| PM6:Y6 | - | morphology comprising homogeneous nanophase | processed with BDT additive, TA | 27.61 | 77.11 | 0.841 | 17.91 | [84] |
| D18:Y6 D18-Cl:Y6 | - | small and homogeneous aggregation domains | processed with DBTF and DBOF additives, TA | 27.65 | 77.99 | 0.890 | 19.19 | [238] |
| D18:Y6 | - | nanophase-separated morphology comprising nanofibril networks | synergistically dual-phases morphology control | 28.6 | 80.84 | 0.836 | 19.35 | [239] |
| D18:L8-BO | - | 20 nm large nanofibers | spin coating sequential deposition | 26.86 | 77.25 | 0.918 | 19.05 | [240] |
| PM6:L8-BO | - | morphology comprising homogeneous nanophase | processed with BDT additive, TA | 26.59 | 80.03 | 0.893 | 19.01 | [84] |
| PMQ-Si605 | Mn = 51.1 | phase-separated fibrous structures | spin coating and TA | 26.16 | 77.40 | 0.893 | 18.08 | [241] |
| D18:L8-BO | Mn = 84.5 | highly crystalline nanofibers | high-pressure fabrication in a pressure-tight vial | 26.48 | 80.65 | 0.910 | 19.65 | [243] |
| MIX-D18:L8-BO | Mn ≥ 40/<40 | multiscale interpenetrating fiber network structure | spin coating and TA | 26.75 | 81 | 0.920 | 20.0 | [85] |
| D18:L8-BO | - | morphology comprising nanofibril aggregates with dominant face-on orientation | processed with alkoxythiophene additives, TA | 26.5 | 81.2 | 0.906 | 20.1 | [94] |
| PM6:L8-BO | - | phase-separated nanofibrillar morphology | processed with alkoxythiophene additives, TA | 26.8 | 80.20 | 0.902 | 19.8 | [94] |
| PM6:L8-BO-X | - | nanophase-aggregated honeycomb resembling nanostructures | reversed TA | 28.12 | 79.46 | 0.891 | 19.91 | [94] |
| D18:Z19 | - | phase-separated fibrous morphology with preferential face-on orientation | spin coating and TA | 24.6 | 77.60 | 1.002 | 19.2 | [92] |
| PBQx-TF:BTP-eC9-2Cl | - | phase-separated fibrillar network with face-on orientation | processed with additives, TA | 27.2 | 80.40 | 0.879 | 19.2 | [245] |
| PM6:BTP-eC9 PM6:BTP-eC9:QD-1 | Mn = 45 | interpenetrating phase-separated fiber-like network structures | spin coating and TA | 28.51 28.98 | 79.30 79.82 | 0.864 0.873 | 19.53 20.19 | [246] |
| All polymer OSCs | ||||||||
| f-BTI2-FT:PTB7-Th | Mn = 13.8/- | phase-separated bicontinuous network | TA | 11.55 | 57.04 | 1.04 | 6.85 | [170] |
| PTzBI-Si:P(NDI2OD-T2) | Mn = 38.4/75.1 | phase-separated crystalline morphology with preferential face-orientation | spin coating and TA | 15.57 | 73.39 | - | 10.1 | [251] |
| PBDB-T:PF5-Y5 | Mw = -/33.3 | fibral microstructures | spin coating and TA | 20.65 | 74 | 0.946 | 14.45 | [255] |
| PBDB-T:PJ1 | Mn = -/23.3 | finely nanoscale phase-separated interpenetrating network | spin coating and TA | 22.3 | 70 | 0.9 | 14.4 | [256] |
| PBDB-T:PJ1 | Mn = 38/11.4 | fibril-like nanostructures with preferential face-on orientation | spin coating and TA | 22.7 | 75.3 | 0.9 | 15.4 | [257] |
| PBDB-T:PZT-γ | Mn = -/7.8 | phase-separated fibrous interpenetrating nanostructures | solvent optimization, spin coating and TA | 24.7 | 71.3 | 0.896 | 15.8 | [258] |
| PBBTz-Cl:PY-IT | Mn = 61.3/- | nanofibrillar D-A structures | spin coating and TA | 24.56 | 73.73 | 0.947 | 17.15 | [264] |
| PBQx-TCl:BTPICγ-BDD | Mn = -/12.38 | nanofibrillar D-A structures | spin coating and TA | 24.1 | 76.92 | 0.944 | 17.5 | [263] |
| PM6:PY-IT | - | interconnected nanofibrillar D-A networks | spin coating combined with SVA, TA, use of additives and topologically modified surfaces | 26.37 | 76.48 | 0.945 | 19.06 | [262] |
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2DCP-MPc | 2D phthalocyanine-based poly(benzimidazobenzophenanthroline); |
| 2DCPs | two-dimensional conjugated polymers; |
| BDD | 1,3-bis(thiophen-2-yl)-5,7-bis(2-ethylhexyl)benzo-[1,2-c:4,5-c′]dithiophene-4,8-dione; |
| BDT | benzo [1,2-b:4,5-b′]dithiophene; |
| BFDO | benzodifurandione; |
| BHJ | bulk heterojunction; |
| BPE | branched polyethylene; |
| BTBT | (di)thiophene, [1]benzothieno[3,2-b][1]benzothiophene; |
| BTP-eC9 | 2,2′- [[12,13-Bis(2-butyloctyl)-12,13-dihydro-3,9 dinonylbisthieno[2″,3″:4′,5′]thieno[2′,3′:4,5] pyrrolo[3,2-e:2′,3′-g][2,1,3]benzothiadiazole-2,10-diyl]bis[methylidyne(5,6-chloro-3-oxo-1H-indene-2,1(3H)-diylidene)]]bis [propanedinitrile]; |
| BTP-eC9-2Cl | 2,2′- [[12,13-Bis(2-butyloctyl)-12,13-dihydro-3,9-dinonylbisthieno[2″,3″:4′,5′] thieno[2′,3′:4,5]pyrrolo[3,2-e:2′,3′-g][2,1,3]benzothiadiazole-2,10-diyl]bis[methylidyne(2 or 3-chloro-3-oxo-1H-indene-2,1(3H)-diylidene) ]]bis [propanedinitrile]; |
| C-AFM | conductive atomic force microscopy; |
| CDT | cyclopentadithiophene; |
| CDT-BTZ | polymer based on ecosyl-substituted cyclopentadithiophene-benzothiadiazole; |
| CN6-CP | hexacyano-trimethylene-cyclopropane; |
| CoCp2 | bis(cyclopentadienyl)cobalt; |
| CPE | conjugated polyelectrolyte; |
| D-ODCB | deuterated 1,2 dichlorobenzene; |
| D-A | donor-acceptor; |
| D18 | poly[(2,6-(4,8-bis(5-(2-ethylhexyl)-4-fluoro)thiophen-2-yl)-benzo[1,2-b:4,5-b′]dithiophene)-alt-5,5′-(5,8-bis(4-(2-butyloctyl)thiophen-2-yl)dithieno [3′,2′:3,4;2″,3″:5,6] benzo [1,2-c][1,2,5]thiadiazole)]; |
| D18-Cl | poly[(2,6-(4,8-bis(5-(2-ethylhexyl)-4-chlorothiophen-2-yl)-benzo[1,2-b:4,5-b′]dithiophene))-alt-5,5′-(5,8-bis(4-(2-butyloctyl)thiophen-2-yl)dithieno[3′,2′:3,4;2″,3″:5,6]benzo[1,2-c][1,2,5]thiadiazole)]; |
| DBOF | 3,5-dibromotriffluoromethoxybenzene; |
| DBTF | 3,5-dibromobenzotrifluoride; |
| DBTTT | dibenzothiopheno[6,5-b:6′,5′-f]thieno[3,2-b]thiophene; |
| DDB | dodecaborane; |
| DFSe | diketopyrrolopyrrole-difluorobenzoselenadiazole-diketopyrrolopyrrole; |
| DIO | 1,8-diiodooctane; |
| DMSO | dimethyl sulfoxide; |
| DNTT | dinaphtho[2,3-b:2′,3′-f]thieno[3,2-b]thiophene; |
| DPP | diketopyrrolopyrrole; |
| DPP-2T | a polymer based on DPP and containing bithiophene; |
| DPPBTSPE | is based on DPP and 1,2-bis(5-(thiophen-2-yl)selenophen-2-yl)ethene (BTSPE); |
| DPPF-NTz | a polymer made of a furan-flanked diketopyrrolopyrrole (DPPF) as a monomer and napthobisthiadiazole (NTz) as comonomer units; |
| DTQx | dithieno[3,2-f:2′,3′-h]quinoxaline; |
| EMIM TFSI | 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl) imide; |
| F4BDOPV-2T | a polymer composed of four-fluorinated benzodifurandione-based oligo(p-phenylene vinylene) (F4BDOPV) and 2,2′-bithiophene; |
| F4TCNQ | 2,3,5,6-tetrafluoro-7,7,8,8-tetracyanoquinodimethane; |
| F8T2 | poly(9,9-dioctylfuorene-co-bithiophene); |
| FBDPPV | a polymer system derived from fluorinated benzodifurandione (FBD); |
| FBDPPV-OEG | a polymer which incorporates oligo(ethylene glycol) (OEG)-substituted FBD; |
| Fe(OTf)3 | iron(III) trifluoromethanesulfonate; |
| f-BSeI2TEG-FT | a polymer system obtained by selenium substitution in bithiophene imide; |
| f-BTI2-FT | a polymer based on a fused bithiophene imide (BTI); |
| f-BTI2TEG-T and f-BTI2TEG-FT | polymers comprising f-BTI2 bearing 3-(2-(2-methoxyethoxy)ethoxy)-2-((2-(2-methoxyethoxy)ethoxy)methyl)propan-1-yl side chain as the acceptor unit and thiophene/difluorothiophene as the donor co-unit (fluorine F atoms were introduced to further tune polymers′ frontier molecular orbital energy levels and crystallinity; f-fused); |
| GIWAXS | grazing incidence wide-angle X-ray scattering; |
| H2SO4 | sulfuric acid; |
| HDPE | high-density polyethylene; |
| HOMO | highest occupied molecular orbital; |
| HOMPP | 2-hydroxy-2-methyl propiophenone; |
| ID | drain current; |
| IDT | indacenodithiophene; |
| IDTT | indacenodithieno[3,2-b]thiophene; |
| IDTz | indacenodithiazole; |
| IID | isoindigo; |
| IT-4F | 3,9-bis(2-methylene-((3-(1,1-dicyanomethylene)-6,7-difluoro)-indanone))-5,5,11,11-tetrakis(4-hexyl phenyl)-dithieno[2,3-d:2′,3′-d′]-s-indaceno[1,2-b:5,6-b′]dithiophene; |
| ITIC | 3,9-bis(2-methylene-(3-(1,1-dicyanomethylene)-indanone))-5,5,11,11-tetrakis(4-hexylphenyl)-dithieno[2,3-d:2′,3′-d′]-s-indaceno[1,2-b:5,6-b′]dithiophene; |
| J71 | poly[[5,6-difluoro-2-(2-hexyldecyl)-2H-benzotriazole-4,7-diyl]-2,5-thiophenediyl[4,8-bis[5-(tripropylsilyl)-2-thienyl]benzo[1,2-b:4,5-b′]dithiophene-2,6-diyl]-2,5-thiophenediyl]; |
| L8-BO (also known as L8-BO-2F) | 2,2′-((2Z,2′Z)-((12,13-bis(2-ethylhexyl)-3,9-(2-butyloctyl)-12,13-dihydro-[1,2,5] thiadiazolo[3,4-e]thieno[2″,3″:4′,5′]thieno[2′,3′:4,5]pyrrolo[3,2-g]thieno[2′,3′:4,5]thieno[3,2-b]indole-2,10-diyl)bis (methanylylidene))bis(5,6-difluoro-3-oxo-2,3-dihydro-1H-indene-2,1-diylidene))dimalononitrile; |
| LUMO | lowest unoccupied molecular orbital; |
| MEH-PPV | poly[2-methoxy-5-(2-ethylhexyloxy)-1,4-phenylenevinylene]; |
| N-DMBI | 4-(1,3-dimethyl-2,3-dihydro-1H-benzoimidazol-2-yl)-N,N-dimethylaniline; |
| n-PT4 | a polymer system created by functionalizing each thiophene unit of a non-fused-ring polythiophene backbone with imide or cyano groups; |
| NFA | non-fullerene acceptor; |
| NDI | naphthalenediimide; |
| NH-FDPP-BT | fluorene-flanked diketopyrrolopyrrole (FDPP)-based semiconducting polymers containing bithiophene (BT) as the donor comonomers; |
| NH-FDPP-TT | fluorene-flanked diketopyrrolopyrrole (FDPP)-based semiconducting polymers containing thieno[3,2-b]thiophene (TT) as the donor comonomers; |
| NTz | napthobisthiadiazole; |
| oCVD | oxidative chemical vapor deposition; |
| OECTs | organic electrochemical transistors; |
| OFETs | organic field-effect transistors; |
| OLEDs | organic light-emitting diodes; |
| OSCs | organic solar cells; |
| P(g2T-T) | poly(2-(3,3′-bis(2-(2-(2-methoxyethoxy)ethoxy)ethoxy)-[2,2′-bithiophen]-5)yl thiophene); |
| P(g2T-TT) | poly(2--(3,3′--bis(2--(2--(2--methoxyethoxy)ethoxy)ethoxy)--[2,2′--bithiophen]--5--yl)thieno 3,2--b] thiophene); |
| P(gDPP-MeOT2), P(gDPP-T2) and P(gDPP-TT) | D-A copolymers based on 3,6-bis(5-bromothiophen-2-yl)-2,5-bis(2-(2-(2-methoxyethoxy)ethoxy) ethyl)-2,5dihydropyrrolo [3,4-c]pyrrole-1,4-dione; |
| P(gT2) | poly-[3,3′-bis(2-(2-(2-methoxyethoxy)ethoxy)ethoxy)-2,2′-bithiophene]; |
| P(DPP-CNPz) | a polymer based on 3,6-dibromopyrazine-2-carbonitrile (CNPz); |
| P(DPP-DCNPz) | a polymer based on 3,6-dibromopyrazine-2,5-di-carbonitrile (DCNPz); |
| P(DPP-T) | poly(diketopyrrolopyrrole-co-thiophene); |
| P(NDI2OD-Se-Th 1.0) | poly[4-methyl-9-(5-(5-(9-(5-methylselenopheno[3,2-b]thiophen-2-yl)-2,7-bis(2-octyldodecyl)-1,3,6,8-tetraoxo-1,2,3,6,7,8-hexahydrobenzo[lmn][3,8]-phenanthrolin-4-yl)selenophen-2-yl)thiophen-2-yl)-2,7-bis(2-octyldodecyl)benzo[lmn][3,8]phenanthroline -1,3,6,8(2H,7H)-tetraone]; |
| P(NDI2OD-T2) or N2200 | poly{[N,N′-bis(2-octyldodecyl)-1,4,5,8-naphthalene diimide-2,6-diyl]-alt-5,5′-(2,2′-bithiophene)}; |
| P(NDI2SiC6-T2) | a polymer composed of naphthalenediimide and bithiophene (T2) repeating units; |
| P(PzDPP-2FT) | a polymer featuring pyrido[3,4-b]pyrazine-diketopyrrolopyrrole (PzDPP) units and fluorinated bithiophene (2FT) blocks; |
| P(TDPP-BBT) | a D-A polymer based on thiophene-flanked diketopyrrolopyrrole and benzobisthiadiazole (BBT); |
| P(TDPP-BT) | a D-A polymer based on thiophene-flanked diketopyrrolopyrrole and benzothiadiazole (BT); |
| P(TDPP-TQ) | a D-A polymer (see its chemical structure in the inset) based on thiophene-flanked DPP and thymoquinone (TQ); |
| P(TzII-dTh-dTh) | a polymer synthesized by copolymerizing thiophene-flanked thiazoloisoindigo (TzII) with bithiophene; |
| P(TzII-dTh-dTz) | a polymer synthesized by copolymerizing thiophene-flanked TzII with bithiazole; |
| P2TDPP2TFT4 | poly[((2,6--bis(thiophen--2--yl)--3,7--bis(9--octylnonadecyl)thieno[3,2--b]thieno[2′,3′:4,5] thieno[2,3--d]thiophene)--5,5′--diyl)(2,5--bis(8--octyl octadecyl)--3,6--di(thiophen--2--yl)pyrrolo[3,4--c]pyrrole--1,4--dione)--5,5′--diyl]]; |
| P3HT | poly(3-hexylthiophene); |
| P3HT-b-PDL | poly(3-hexylthiophene)-block-poly(δ-decanolactone); |
| P3OT | poly(3-octylthiophene); |
| PA | polyacetylene; |
| PANI and TANI | polyaniline and tetraaniline; |
| PB | polybutadiene; |
| PBDB-T | poly[(2,6-(4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)-benzo[1,2-b:4,5-b′]dithiophene))-alt-(5,5-(1′,3′-di-2-thienyl-5′,7′-bis(2-ethylhexyl)benzo[1′,2′-c:4′,5′-c′]dithiophene-4,8-dione))]; |
| PBDB-T-SF | poly[(2,6-(4,8-bis(5-(2-ethylhexylthio)-4-fluorothiophen-2-yl)-benzo[1,2-b:4,5-b′]dithiophene))-alt-(5,5-(1′,3′-di-2-thienyl-5′,7′-bis(2-ethylhexyl)benzo[1′,2′-c:4′,5′-c′]dithiophene-4,8-dione)]; |
| PBDP-F2 | a polymer based on 3-dodecylthiophene-flanked (BDP) as the electron-acceptor moiety and thiophene derivatives containing fluorine atoms as electron-donor moieties; |
| PBDTTT-EF-T | poly[[4,8-bis[5-(2-ethylhexyl)-2-thienyl]benzo[1,2-b:4,5-b′]dithiophene-2,6-diyl][2-[[(2-ethylhexyl)oxy] carbonyl]-3-fluorothieno[3,4-b]thiophenediyl]]; |
| PBFDO | poly(benzodifurandione); |
| PBQx-TF | a polymer that results from the copolymerization of a fluorinated benzo[1,2-b:4,5-b′]dithiophene (BDT-T) unit with dithieno[3,2-f:2′,3′-h]quinoxaline (DTQx); |
| PBTTT | poly(2,5-bis(3-alkylthiophen-2-yl)thieno[3,2-b]thiophene); |
| PCBM | phenyl-C61-butyric acid methyl ester; |
| PCE | power conversion efficiency; |
| PCDTPT | poly[4-(4,4-dihexadecyl-4H-cyclopenta [1,2-b:5,4-b′]dithiophen-2-yl)-alt-[1,2,5]thiadiazolo[3,4-c]pyridine]; |
| PCDTPT-ODD | poly[4-(4,4-bis(2-octyldodecyl)-4H-cyclopenta[1,2-b:5,4-b′]dithiophen-2-yl)-alt-[1,2,5]-thiadiazolo [3,4-c]pyridine]; |
| PCNI2-BTI | a polymer based on a cyano-functionalized fused bithiophene imide dimer; |
| PDFDSe | poly(4-(4,4-bis(2-ethylhexyl)-4H-silolo[3,2-b:4,5-b′]dithiophene-2-yl)-7-(4,4-bis(2-ethylhexyl)-6-(selenophene-2-yl)-4H-silolo[3,2-b:4,5-b′]dithiophene-2-yl)-5,6-difluorobenzo[c][1,2,5]thiadiazole; |
| PDMS | poly(dimethylsiloxane); |
| PDPP-DTT | polydiketopyrrolopyrrole-dithienylthieno[3,2-b]thiophene; |
| PDPP-TT-PDMS | poly-diketopyrrolopyrrole-thienothiophene-poly(dimethylsiloxane); |
| PDPP-TVT | poly[2,5-bis(2-decyltetradecyl)pyrrolo[3,4-c]pyrrole-1,4-(2H,5H)-dione-(E)-1,2-di(2,20-bithiophen-5-yl) ethene]; |
| PDPP3T | poly(diketopyrrolopyrroleterthiophene); |
| PDPIN | a polymer based on the 2,2′-((2E,2′E)-(((2,5-bis(2-octyldodecyl)-3,6-dioxo-2,3,5,6-tetrahydropyrrolo[3,4-c] pyrrole-1,4-diyl)bis(thiophene-5,2-diyl))bis(methanylylidene))bis(3-oxo-2,3-dihydro-1H-indene-2,1-diylidene))dimalononitrile repeat unit; |
| PDPPT3 | poly{2,2′-[(2,5-bis(2-hexyldecyl)-3,6-dioxo-2,3,5,6- tetrahydropyrrolo[3,4-c]pyrrole-1,4-diyl)dithiophene]- 5,5′-diyl-alt-thiophen-2,5-diyl}; |
| PDPPT325% | poly(2,5-bis (4-hexyldodecyl)-2,5-dihydro-3,6-di-2-thienyl-pyrrolo[3,4-c] pyrrole-1,4-dionealt-thiophene); |
| PDPPy-Se | a polymer based on pyridine and selenophene; |
| PDTTDPP | a copolymer composed of DPP and dithieno[3,2-b:20,30-d]thiophene (DTT); |
| PDTzSI-Se | a polymer which is based on thiazole imide with a high content of selenophene; |
| PEDOT | poly(3,4-ethylenedioxythiophene); |
| PE | polyethylene; |
| PEGDA | poly(ethylene glycol) diacrylate; |
| PF5-Y5 | a polymer synthesized by coupling a (2,2′-((2Z,2′Z)-((12,13-bis(2-ethylhexyl)-3,9-diundecyl-12,13-dihydro[1,2,5]thiadiazolo[3,4e]thieno[2″,3″:4′,5′] thieno[2′,3′:4,5]pyrrolo[3,2-g] thieno[2′,3′:4,5]thieno[3,2-b]indole-2,10-diyl)bis(methanylylidene))bis(3-oxo-2,3-dihydro1H-indene-2,1-diylidene))dimalononitrile) (Y5) acceptor unit with a thienyl-benzodithiophene (BDT-T) donor unit; |
| PFClTVT | a polymer based on dichlorodithienylethene (ClTVT); |
| PffBT4T-C9C13 | poly[(5,6-difluoro-2,1,3-benzothiadiazol-4,7-diyl)-alt-(3,3″′- di(2-nonyltridecyl)-2,2′;5′,2″;5″,2″′-quaterthiophen-5,5″′-diyl)]; |
| PFO | poly(9,9-dioctylfluorene); |
| PgBTTT | a polymer obtained by relocating the glycol side chains position on the bithiophene unit of poly(2--(3,3′--bis(2--(2--(2--methoxyethoxy)ethoxy)ethoxy)--[2,2′--bithiophen]--5--yl)thieno 3,2--b] thiophene); |
| PJ1 | poly[[12,13-bis(2-decyltetradecyl)-12,13-dihydro-3,9 diundecylbisthieno[2″,3″:4′,5′]thieno[2′,3′:4,5]pyrrolo[3,2-e:2′,3′-g][2,1,3]benzothiadiazole-2,10-diyl]methylidyne[1-(dicyanomethylene)-1,3-dihydro-3-oxo-2H-inden-yl-2-ylidene]-2,5-thiophenediyl[1-(dicyanomethylene)-1,3-dihydro-3-oxo-2H-inden-yl-2-ylidene]methylidyne]; |
| PM6 (also known as PBDB-T-2F) | poly[(2,6-(4,8-bis(5-(2-ethylhexyl)-4-fluorothiophen-2-yl)-benzo[1,2-b:4,5-b′]dithiophene))-alt-(5,5-(1′,3′-di-2-thienyl-5′,7′-bis(2-ethylhexyl)benzo[1′,2′-c:4′,5′-c′]dithiophene-4,8-dione))]; |
| PMMA | poly(methyl methacrylate); |
| PMQ-Si605 | a polymer based on 6,7-difluoro-3-methylquinoxaline-thiophene backbone and containing 5% siloxane; |
| PNBDO-FDTE | poly((3E,7E)-3,7-bis(2-oxo-1Hpyrrolo[2,3-b]pyridin-3(2H)-ylidene)benzo[1,2-b:4,5-b′]-difuran-2,6 (3H,7H)-dione)-(E)-1,2-bis(3-fluorothiophen-2-yl)ethene; |
| PNDI-RO | a polymer consisting of p-conjugated backbones adjacent to alkyl chains; |
| PNDI2TEG-2Tz | a polymer system synthesized using bithiazole/2Tz donor units and NDI carrying triethylene glycol acceptors; |
| PNDIF-T2 | poly[2,7-bis(11,11,12,12,13,13,14,14,15,15,16,16,17,17,18,18,18-heptadecafluoro octadecyl)-4-methyl-9-(5′-methyl-[2,2′-bithiophen]-5-yl)benzo[lmn][3,8]phenanthroline-1,3,6,8 (2H,7H)-tetraone]; |
| PNDIF-TVT | poly[(E)-2,7-bis(11,11,12,12,13,13,14,14,15,15,16,16,17,17, 18,18,18-heptadecafluorooctadecyl)-4-methyl-9-(5-(2-(5-methylthiophen-2-yl)vinyl)thiophen-2-yl)benzo[lmn][3,8]phenanthroline-1,3,6,8(2H,7H)-tetraone]; |
| PO12 | a fused bithiophene imide dimer-based polymer incorporating distinct oligo(ethylene glycol) side chains; |
| PPE | poly(para-phenylene ethynylene); |
| PPV | poly(p-phenylene vinylene); |
| PPy | polypyrrole; |
| PQT | poly[5,50-bis(3-dodecyl-2-thienyl)-2,20-bithiophene)]; |
| PS | polystyrene; |
| PSpF | poly(4-styrenesulfonyl fluoride); |
| PSS | polystyrene sulfonate; |
| PTB7 | poly{[4,8-bis[(2-ethylhexyl)oxy]benzo[1,2-b:4,5-b′]dithiophene-2,6-diyl][3-fluoro-2-[(2-ethylhexyl)carbonyl] thieno[3,4-b]thiophenediyl]}; |
| PTzBI-Si | poly{(4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)benzo[1,2-b:4,5-b′]dithiophene-co-4,8-di(thien-2-yl)-2-(6-(1,1,1, 3,5,5,5-heptamethyltrisiloxan-3-yl)hexyl)-6-octyl[1,2,3]triazolo[4,5-f]isoindole-5,7(2H,6H)-dione}; |
| PTB7-th | poly([2,6′-4,8-di(5-ethylhexylthienyl)benzo[1,2-b;3,3-b]dithiophene]{3-fluoro-2[(2-ethylhexyl)carbonyl] thieno[3,4-b]thiophenediyl}); |
| PTIIG-Np | poly(thienoisoindigo-alt-naphthalene); |
| PTQ10 | poly[[6,7-difluoro[(2-hexyldecyl)oxy]-5,8-quinoxalinediyl]-2,5-thiophenediyl]]; |
| PTz-5-DPP | a polymer based on 3,6-di(thiazol-5-yl)-diketopyrrolopyrrole; |
| PU | polyurethane; |
| PU(DPP)35 | a polyurethane multiblock copolymer containing a diketopyrrolopyrrole-based rigid block and a hydrogenated polybutadiene flexible block linked via urethane bonds; |
| PY-IT | poly[[12,13-bis(2-octyldodecyl)-12,13-dihydro-3,9-diundecylbisthieno[2″,3″:4′,5′]thieno[2′,3′:4,5] pyrrolo[3,2-e:2′,3′-g][2,1,3]benzothiadiazole-2,10-diyl]methylidyne[1-(dicyanomethylene)-1,3-dihydro-3-oxo-2H-inden-yl-2-ylidene]-2,5-thiophenediyl[1-(dicyanomethylene)-1,3-dihydro-3-oxo-2H-inden-yl-2-ylidene]methylidyne]; |
| SBS | poly(styrene-b-butadiene-b-styrene); |
| SCLC | space charge-limited current; |
| SEBS | polystyrene-block-poly(ethyleneran-butylene)-block-polystyrene; |
| SNT | dithiophene-thiadiazolebenzotriazole; |
| SVA | solvent vapor annealing; |
| TA | thermal annealing; |
| T-2OBO | 3,4-bis((2-butyloctyl)oxy)thiophene; |
| T-2OHD | 3,4-bis((2-hexyldecyl)oxy)thiophene; |
| T-2OEH | 3,4-bis((2-ethylhexyl)oxy)thiophene; |
| T-2OOD | 3,4-bis((2-octyldodecyl)oxy)thiophene; |
| TAM | triaminomethane; |
| TDAE | tetrakis(dimethylamino)ethylene; |
| TDPP-Se | a copolymer composed of thiophene-flanked diketopyrrolopyrrole; |
| TFE | tetrafluoroethylene; |
| TfOH | trifluoromethanesulfonic acid; |
| ThDPP-CNBTz | a polymer which comprises a thiophene-flanked diketopyrrolopyrrole and a cyano functionalized benzothiadiazole; |
| TMB-PN | 1,2,4-trimethylbenzene containing 1-phenylnaphthalene; |
| TNDP | thienyl naphthodipyrrolopyrrole; |
| TSA | p-toluene sulfonic acid; |
| TsOH | para-toluenesulfonic acid; |
| TTIF-BT | a donor-acceptor fused ring π-polymer featuring a dithioeno[3,2-b] thioenoindeno[1,2-b] fluorene (TTIF) backbone as the donor component; |
| TzII | thiophene-flanked thiazoloisoindigo; |
| VD | drain voltage; |
| VG | gate voltage; |
| XRD | X-ray diffraction; |
| Y12 (also known as BTP-4F-12 or Y6-BO) | 2,2′-((2Z,2′Z)-((12,13-bis(2-butyloctyl)-3,9-diundecyl-12,13-dihydro-[1,2,5]thiadiazolo[3,4-e]thieno[2″,3″:4′,5′]thieno[2′,3′:4,5]pyrrolo[3,2-g]thieno[2′,3′:4,5]thieno[3,2-b]indole-2,10-diyl)bis (methanylylidene))bis(5,6-difluoro-3-oxo-2,3-dihydro-1H-indene-2,1-diylidene))dimalononitrile; |
| Y6 (also known as BTP-4F) | 2,2′-((2Z,2′Z)-((12,13-bis(2-ethylhexyl)-3,9-diundecyl-12,13-dihydro-[1,2,5]thiadiazolo[3,4-e]thieno[2″,3″:4′,5′]thieno[2′,3′:4,5]pyrrolo[3,2-g]thieno[2′,3′:4,5]thieno[3,2-b]indole-2,10-diyl)bis(methanylylidene)) bis(5,6-difluoro-3-oxo-2,3-dihydro-1H-indene-2,1-diylidene))dimalononitrile; |
| Z19 | a polymer based on a backbone containing alkoxy and phenyl-substituted alkyl chains. |
References
- Acikgoz, C.; Hempenius, M.A.; Huskens, J.; Vancso, G.J. Polymers in Conventional and Alternative Lithography for the Fabrication of Nanostructures. Eur. Polym. J. 2011, 47, 2033–2052. [Google Scholar] [CrossRef]
- Wang, W.; Qi, L. Light Management with Patterned Micro- and Nanostructure Arrays for Photocatalysis, Photovoltaics, and Optoelectronic and Optical Devices. Adv. Funct. Mater. 2019, 29, 1807275. [Google Scholar] [CrossRef]
- Tatsi, E.; Griffini, G. Polymeric Materials for Photon Management in Photovoltaics. Sol. Energy Mater. Sol. Cells 2019, 196, 43–56. [Google Scholar] [CrossRef]
- Handrea-Dragan, M.; Botiz, I. Multifunctional Structured Platforms: From Patterning of Polymer-Based Films to Their Subsequent Filling with Various Nanomaterials. Polymers 2021, 13, 445. [Google Scholar] [CrossRef]
- Handrea-Dragan, I.M.; Botiz, I.; Tatar, A.-S.; Boca, S. Patterning at the Micro/Nano-Scale: Polymeric Scaffolds for Medical Diagnostic and Cell-Surface Interaction Applications. Colloids Surf. B Biointerfaces 2022, 218, 112730. [Google Scholar] [CrossRef]
- Xiao, S.; Yang, X.; Lee, K.; Hwu, J.; Wago, K.; Kuo, D. Directed Self-Assembly for High-Density Bit-Patterned Media Fabrication Using Spherical Block Copolymers. J. Micro/Nanolithography MEMS MOEMS 2013, 12, 031110. [Google Scholar] [CrossRef]
- Di Mauro, A.E.; Striccoli, M.; Depalo, N.; Fanizza, E.; Cano, L.; Ingrosso, C.; Agostiano, A.; Curri, M.L.; Tercjak, A. Selective Confinement of Oleylamine Capped Au Nanoparticles in Self-Assembled PS-b-PEO Diblock Copolymer Templates. Soft Matter 2014, 10, 1676–1684. [Google Scholar] [CrossRef]
- Sun, W.; Shen, J.; Zhao, Z.; Arellano, N.; Rettner, C.; Tang, J.; Cao, T.; Zhou, Z.; Ta, T.; Streit, J.K.; et al. Precise Pitch-Scaling of Carbon Nanotube Arrays within Three-Dimensional DNA Nanotrenches. Science 2020, 368, 874–877. [Google Scholar] [CrossRef]
- Leordean, C.; Marta, B.; Gabudean, A.-M.; Focsan, M.; Botiz, I.; Astilean, S. Fabrication of Highly Active and Cost Effective SERS Plasmonic Substrates by Electrophoretic Deposition of Gold Nanoparticles on a DVD Template. Appl. Surf. Sci. 2015, 349, 190–195. [Google Scholar] [CrossRef]
- Chen, S.; Haehnle, B.; der Laan, X.V.; Kuehne, A.J.C.; Botiz, I.; Stavrinou, P.N.; Stingelin, N. Understanding Hierarchical Spheres-in-Grating Assembly for Bio-Inspired Colouration. Mater. Horiz. 2021, 8, 2230–2237. [Google Scholar] [CrossRef]
- Albalak, R.J.; Thomas, E.L. Microphase Separation of Block Copolymer Solutions in a Flow Field. J. Polym. Sci. B Polym. Phys. 1993, 31, 37–46. [Google Scholar] [CrossRef]
- Hong, K.M.; Noolandi, J. The Effect of Polydispersity on the Microphase Separation of a Block Copolymer System. Polym. Commun. 1984, 25, 265–268. [Google Scholar]
- Todor-Boer, O.; Petrovai, I.; Tarcan, R.; Vulpoi, A.; David, L.; Astilean, S.; Botiz, I. Enhancing Photoluminescence Quenching in Donor–Acceptor PCE11:PPCBMB Films through the Optimization of Film Microstructure. Nanomaterials 2019, 9, 1757. [Google Scholar] [CrossRef]
- Kikuchi, M.; Binder, K. Microphase Separation in Thin Films of the Symmetric Diblock-Copolymer Melt. J. Chem. Phys. 1994, 101, 3367–3377. [Google Scholar] [CrossRef]
- Nickmans, K.; Schenning, A.P.H.J. Directed Self-Assembly of Liquid-Crystalline Molecular Building Blocks for Sub-5 Nm Nanopatterning. Adv. Mater. 2018, 30, 1703713. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Shin, W.H.; Park, T.W.; Choi, Y.J.; Yoon, Y.J.; Park, S.H.; Lim, J.-H.; Kwon, J.-D.; Lee, J.W.; Kwon, S.-H.; et al. Hierarchical Multi-Level Block Copolymer Patterns by Multiple Self-Assembly. Nanoscale 2019, 11, 8433–8441. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Link, J.M.; Hu, J.C.Y.; Athanasiou, K.A. The Self-Assembling Process and Applications in Tissue Engineering. Cold Spring Harb. Perspect. Med. 2017, 7, a025668. [Google Scholar] [CrossRef]
- Stephanopoulos, N.; Ortony, J.H.; Stupp, S.I. Self-Assembly for the Synthesis of Functional Biomaterials. Acta Materialia 2013, 61, 912–930. [Google Scholar] [CrossRef]
- Babutan, I.; Todor-Boer, O.; Atanase, L.I.; Vulpoi, A.; Simon, S.; Botiz, I. Self-Assembly of Block Copolymers on Surfaces Exposed to Space-Confined Solvent Vapor Annealing. Polymer 2023, 273, 125881. [Google Scholar] [CrossRef]
- Babutan, I.; Todor-Boer, O.; Atanase, L.I.; Vulpoi, A.; Botiz, I. Self-Assembly of Block Copolymers in Thin Films Swollen-Rich in Solvent Vapors. Polymers 2023, 15, 1900. [Google Scholar] [CrossRef]
- Le, T.P.; Smith, B.H.; Lee, Y.; Litofsky, J.H.; Aplan, M.P.; Kuei, B.; Zhu, C.; Wang, C.; Hexemer, A.; Gomez, E.D. Enhancing Optoelectronic Properties of Conjugated Block Copolymers through Crystallization of Both Blocks. Macromolecules 2020, 53, 1967–1976. [Google Scholar] [CrossRef]
- Yu, L.; Davidson, E.; Sharma, A.; Andersson, M.R.; Segalman, R.; Müller, C. Isothermal Crystallization Kinetics and Time–Temperature–Transformation of the Conjugated Polymer: Poly(3-(2′-Ethyl)Hexylthiophene). Chem. Mater. 2017, 29, 5654–5662. [Google Scholar] [CrossRef]
- Marsh, H.S.; Reid, O.G.; Barnes, G.; Heeney, M.; Stingelin, N.; Rumbles, G. Control of Polythiophene Film Microstructure and Charge Carrier Dynamics through Crystallization Temperature. J. Polym. Sci. B Polym. Phys. 2014, 52, 700–707. [Google Scholar] [CrossRef]
- Darko, C.; Botiz, I.; Reiter, G.; Breiby, D.W.; Andreasen, J.W.; Roth, S.V.; Smilgies, D.M.; Metwalli, E.; Papadakis, C.M. Crystallization in Diblock Copolymer Thin Films at Different Degrees of Supercooling. Phys. Rev. E 2009, 79, 041802. [Google Scholar] [CrossRef] [PubMed]
- Jahanshahi, K.; Botiz, I.; Reiter, R.; Thomann, R.; Heck, B.; Shokri, R.; Stille, W.; Reiter, G. Crystallization of Poly(γ-Benzyl L-Glutamate) in Thin Film Solutions: Structure and Pattern Formation. Macromolecules 2013, 46, 1470–1476. [Google Scholar] [CrossRef]
- Babutan, I.; Todor-Boer, O.; Atanase, L.I.; Vulpoi, A.; Botiz, I. Crystallization of Poly(Ethylene Oxide)-Based Triblock Copolymers in Films Swollen-Rich in Solvent Vapors. Coatings 2023, 13, 918. [Google Scholar] [CrossRef]
- Hill, J.D.; Millett, P.C. Directed Self-Assembly in Diblock Copolymer Thin Films for Uniform Hemisphere Pattern Formation. Macromolecules 2019, 52, 9495–9503. [Google Scholar] [CrossRef]
- Rahimi, K.; Botiz, I.; Stingelin, N.; Kayunkid, N.; Sommer, M.; Koch, F.P.V.; Nguyen, H.; Coulembier, O.; Dubois, P.; Brinkmann, M.; et al. Controllable Processes for Generating Large Single Crystals of Poly(3-Hexylthiophene). Angew. Chem. Int. Ed. 2012, 51, 11131–11135. [Google Scholar] [CrossRef]
- Um, H.A.; Lee, D.H.; Heo, D.U.; Yang, D.S.; Shin, J.; Baik, H.; Cho, M.J.; Choi, D.H. High Aspect Ratio Conjugated Polymer Nanowires for High Performance Field-Effect Transistors and Phototransistors. ACS Nano 2015, 9, 5264–5274. [Google Scholar] [CrossRef]
- Cao, X.; Zhao, K.; Chen, L.; Liu, J.; Han, Y. Conjugated Polymer Single Crystals and Nanowires. Polym. Cryst. 2019, 2, e10064. [Google Scholar] [CrossRef]
- Dong, H.; Jiang, S.; Jiang, L.; Liu, Y.; Li, H.; Hu, W.; Wang, E.; Yan, S.; Wei, Z.; Xu, W.; et al. Nanowire Crystals of a Rigid Rod Conjugated Polymer. J. Am. Chem. Soc. 2009, 131, 17315–17320. [Google Scholar] [CrossRef]
- Garcia-Cruz, A.; Lee, M.; Marote, P.; Zine, N.; Sigaud, M.; Bonhomme, A.; Pruna, R.; Lopez, M.; Bausells, J.; Jaffrezic, N.; et al. Large Area in Situ Fabrication of Poly(Pyrrole)-Nanowires on Flexible Thermoplastic Films Using Nanocontact Printing. Mater. Res. Express 2016, 3, 085018. [Google Scholar] [CrossRef]
- Baghgar, M.; Labastide, J.; Bokel, F.; Dujovne, I.; McKenna, A.; Barnes, A.M.; Pentzer, E.; Emrick, T.; Hayward, R.; Barnes, M.D. Probing Inter- and Intrachain Exciton Coupling in Isolated Poly(3-Hexylthiophene) Nanofibers: Effect of Solvation and Regioregularity. J. Phys. Chem. Lett. 2012, 3, 1674–1679. [Google Scholar] [CrossRef] [PubMed]
- Baghgar, M.; Pentzer, E.; Wise, A.J.; Labastide, J.A.; Emrick, T.; Barnes, M.D. Cross-Linked Functionalized Poly (3-Hexylthiophene) Nanofibers with Tunable Excitonic Coupling. ACS Nano 2013, 7, 8917–8923. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.-H.; Price, M.B.; Finnegan, J.R.; Boott, C.E.; Richter, J.M.; Rao, A.; Menke, S.M.; Friend, R.H.; Whittell, G.R.; Manners, I. Long-Range Exciton Transport in Conjugated Polymer Nanofibers Prepared by Seeded Growth. Science 2018, 360, 897–900. [Google Scholar] [CrossRef]
- Fasano, V.; Polini, A.; Morello, G.; Moffa, M.; Camposeo, A.; Pisignano, D. Bright Light Emission and Waveguiding in Conjugated Polymer Nanofibers Electrospun from Organic Salt Added Solutions. Macromolecules 2013, 46, 5935–5942. [Google Scholar] [CrossRef]
- Moohan, J.; Stewart, S.A.; Espinosa, E.; Rosal, A.; Rodríguez, A.; Larrañeta, E.; Donnelly, R.F.; Domínguez-Robles, J. Cellulose Nanofibers and Other Biopolymers for Biomedical Applications. A Review. Appl. Sci. 2020, 10, 65. [Google Scholar] [CrossRef]
- Zhu, R.-M.; Chang, Z.-X.; Zhang, W.-J.; Hong, C.-Y. Polymerization-Induced Self-Assembly with Assistance of Aromatic Interactions Facilitates the Formation of Polymeric Nanotubes. Macromolecules 2023, 56, 3296–3303. [Google Scholar] [CrossRef]
- Chapman, R.; Jolliffe, K.A.; Perrier, S. Modular Design for the Controlled Production of Polymeric Nanotubes from Polymer/Peptide Conjugates. Polym. Chem. 2011, 2, 1956–1963. [Google Scholar] [CrossRef]
- Botiz, I. Single Crystals of Established Semiconducting Polymers. Polymers 2024, 16, 761. [Google Scholar] [CrossRef]
- Xu, J.; Ma, Y.; Hu, W.; Rehahn, M.; Reiter, G. Cloning Polymer Single Crystals through Self-Seeding. Nat. Mater. 2009, 8, 348–353. [Google Scholar] [CrossRef]
- Verduzco, R.; Botiz, I.; Pickel, D.L.; Kilbey, S.M.; Hong, K.; Dimasi, E.; Darling, S.B. Polythiophene-Block-Polyfluorene and Polythiophene-Block-Poly(Fluorene-Co-Benzothiadiazole): Insights into the Self-Assembly of All-Conjugated Block Copolymers. Macromolecules 2011, 44, 530–539. [Google Scholar] [CrossRef]
- Wu, L.; Lodge, T.P.; Bates, F.S. Bridge to Loop Transition in a Shear Aligned Lamellae Forming Heptablock Copolymer. Macromolecules 2004, 37, 8184–8187. [Google Scholar] [CrossRef]
- Lane, A.P.; Yang, X.; Maher, M.J.; Blachut, G.; Asano, Y.; Someya, Y.; Mallavarapu, A.; Sirard, S.M.; Ellison, C.J.; Willson, C.G. Directed Self-Assembly and Pattern Transfer of Five Nanometer Block Copolymer Lamellae. ACS Nano 2017, 11, 7656–7665. [Google Scholar] [CrossRef] [PubMed]
- Amundson, K.; Helfand, E.; Quan, X.; Smith, S.D. Alignment of Lamellar Block Copolymer Microstructure in an Electric Field. 1. Alignment Kinetics. Macromolecules 1993, 26, 2698–2703. [Google Scholar] [CrossRef]
- Babutan, I.; Atanase, L.I.; Botiz, I. Self-Assembly of Lamellar/Micellar Block Copolymers Induced Through Their Rich Exposure to Various Solvent Vapors: An AFM Study. Materials 2025, 18, 1759. [Google Scholar] [CrossRef]
- Bates, F.S.; Fredrickson, G.H. Block Copolymers—Designer Soft Materials. Physics Today 1999, 52, 32–38. [Google Scholar] [CrossRef]
- Hu, D.; Yu, J.; Wong, K.; Bagchi, B.; Rossky, P.J.; Barbara, P.F. Collapse of Stiff Conjugated Polymers with Chemical Defects into Ordered, Cylindrical Conformations. Nature 2000, 405, 1030–1033. [Google Scholar] [CrossRef]
- Liao, Y.; Liu, K.; Chen, W.-C.; Wei, B.; Borsali, R. Robust Sub-10 Nm Pattern of Standing Sugar Cylinders via Rapid “Microwave Cooking”. Macromolecules 2019, 52, 8751–8758. [Google Scholar] [CrossRef]
- Angelescu, D.E.; Waller, J.H.; Adamson, D.H.; Deshpande, P.; Chou, S.Y.; Register, R.A.; Chaikin, P.M. Macroscopic Orientation of Block Copolymer Cylinders in Single-Layer Films by Shearing. Adv. Mater. 2004, 16, 1736–1740. [Google Scholar] [CrossRef]
- Komura, M.; Yoshitake, A.; Komiyama, H.; Iyoda, T. Control of Air-Interface-Induced Perpendicular Nanocylinder Orientation in Liquid Crystal Block Copolymer Films by a Surface-Covering Method. Macromolecules 2015, 48, 672–678. [Google Scholar] [CrossRef]
- Nagy-Simon, T.; Diaconu, O.; Focsan, M.; Vulpoi, A.; Botiz, I.; Craciun, A.-M. Pluronic Stabilized Conjugated Polymer Nanoparticles for NIR Fluorescence Imaging and Dual Phototherapy Applications. J. Mol. Struct. 2021, 1243, 130931. [Google Scholar] [CrossRef]
- Băbuțan, M.; Botiz, I. Morphological Characteristics of Biopolymer Thin Films Swollen-Rich in Solvent Vapors. Biomimetics 2024, 9, 396. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.J.; Wong, E.H.H.; Boyer, C.; Qiao, G.G. Antimicrobial Polymeric Nanoparticles. Prog. Polym. Sci. 2018, 76, 40–64. [Google Scholar] [CrossRef]
- MacFarlane, L.R.; Shaikh, H.; Garcia-Hernandez, J.D.; Vespa, M.; Fukui, T.; Manners, I. Functional Nanoparticles through π-Conjugated Polymer Self-Assembly. Nat. Rev. Mater. 2021, 6, 7–26. [Google Scholar] [CrossRef]
- Artar, M.; Huerta, E.; Meijer, E.W.; Palmans, A.R.A. Dynamic Single Chain Polymeric Nanoparticles: From Structure to Function. In Sequence-Controlled Polymers: Synthesis, Self-Assembly, and Properties; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2014; Volume 1170, pp. 313–325. ISBN 978-0-8412-3001-9. [Google Scholar]
- Zhang, C.; Zhu, Y.; Zhou, C.; Yuan, W.; Du, J. Antibacterial Vesicles by Direct Dissolution of a Block Copolymer in Water. Polym. Chem. 2013, 4, 255–259. [Google Scholar] [CrossRef]
- Zhu, H.; Geng, Q.; Chen, W.; Zhu, Y.; Chen, J.; Du, J. Antibacterial High-Genus Polymer Vesicle as an “Armed” Drug Carrier. J. Mater. Chem. B 2013, 1, 5496–5504. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, B.; Chen, S.; Du, J. Polymer Vesicles: Mechanism, Preparation, Application, and Responsive Behavior. Prog. Polym. Sci. 2017, 64, 1–22. [Google Scholar] [CrossRef]
- Zhu, Y.; Fan, L.; Yang, B.; Du, J. Multifunctional Homopolymer Vesicles for Facile Immobilization of Gold Nanoparticles and Effective Water Remediation. ACS Nano 2014, 8, 5022–5031. [Google Scholar] [CrossRef]
- Ahmad, Z.; Shah, A.; Siddiq, M.; Kraatz, H.-B. Polymeric Micelles as Drug Delivery Vehicles. Rsc Adv. 2014, 4, 17028–17038. [Google Scholar] [CrossRef]
- He, Y.; Eloi, J.-C.; Harniman, R.L.; Richardson, R.M.; Whittell, G.R.; Mathers, R.T.; Dove, A.P.; O’Reilly, R.K.; Manners, I. Uniform Biodegradable Fiber-Like Micelles and Block Comicelles via “Living” Crystallization-Driven Self-Assembly of Poly(l-Lactide) Block Copolymers: The Importance of Reducing Unimer Self-Nucleation via Hydrogen Bond Disruption. J. Am. Chem. Soc. 2019, 141, 19088–19098. [Google Scholar] [CrossRef]
- Xu, W.; Ling, P.; Zhang, T. Polymeric Micelles, a Promising Drug Delivery System to Enhance Bioavailability of Poorly Water-soluble Drugs. J. Drug Deliv. 2013, 2013, 340315. [Google Scholar] [CrossRef] [PubMed]
- Hisey, B.; Ragogna, P.J.; Gillies, E.R. Phosphonium-Functionalized Polymer Micelles with Intrinsic Antibacterial Activity. Biomacromolecules 2017, 18, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Tong, L.; Kuwabara, J.; Kanbara, T.; Saeki, A.; Seki, S.; Yamamoto, Y. Spherical Assemblies from π-Conjugated Alternating Copolymers: Toward Optoelectronic Colloidal Crystals. J. Am. Chem. Soc. 2013, 135, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Chuang, V.P.; Cheng, J.Y.; Savas, T.A.; Ross, C.A. Three-Dimensional Self-Assembly of Spherical Block Copolymer Domains into V-Shaped Grooves. Nano Lett. 2006, 6, 2332–2337. [Google Scholar] [CrossRef]
- Cho, H.K.; Cheong, I.W.; Lee, J.M.; Kim, J.H. Polymeric Nanoparticles, Micelles and Polymersomes from Amphiphilic Block Copolymer. Korean J. Chem. Eng. 2010, 27, 731–740. [Google Scholar] [CrossRef]
- Valsesia, A.; Colpo, P.; Meziani, T.; Bretagnol, F.; Lejeune, M.; Rossi, F.; Bouma, A.; Garcia-Parajo, M. Selective Immobilization of Protein Clusters on Polymeric Nanocraters. Adv. Funct. Mater. 2006, 16, 1242–1246. [Google Scholar] [CrossRef]
- Botiz, I.; Codescu, M.-A.; Farcau, C.; Leordean, C.; Astilean, S.; Silva, C.; Stingelin, N. Convective Self-Assembly of π-Conjugated Oligomers and Polymers. J. Mater. Chem. C 2017, 5, 2513–2518. [Google Scholar] [CrossRef]
- Yao, Z.-F.; Wang, J.-Y.; Pei, J. Controlling Morphology and Microstructure of Conjugated Polymers via Solution-State Aggregation. Prog. Polym. Sci. 2023, 136, 101626. [Google Scholar] [CrossRef]
- Ma, J.; He, Q.; Xue, Z.; Sou, H.L.; Han, Y.; Zhong, H.; Pietrangelo, A.; Heeney, M.; Fei, Z. Regulation of Microstructure and Charge Transport Properties of Cyclopentadiene-Based Conjugated Polymers via Side-Chain Engineering. J. Mater. Chem. C 2024, 12, 3549–3556. [Google Scholar] [CrossRef]
- Noriega, R.; Rivnay, J.; Vandewal, K.; Koch, F.P.; Stingelin, N.; Smith, P.; Toney, M.F.; Salleo, A. A General Relationship between Disorder, Aggregation and Charge Transport in Conjugated Polymers. Nat. Mater. 2013, 12, 1038–1044. [Google Scholar] [CrossRef]
- Müller, C.; Zhigadlo, N.D.; Kumar, A.; Baklar, M.A.; Karpinski, J.; Smith, P.; Kreouzis, T.; Stingelin, N. Enhanced Charge-Carrier Mobility in High-Pressure-Crystallized Poly(3-Hexylthiophene). Macromolecules 2011, 44, 1221–1225. [Google Scholar] [CrossRef]
- Hagler, T.W.; Pakbaz, K.; Voss, K.F.; Heeger, A.J. Enhanced Order and Electronic Delocalization in Conjugated Polymers Oriented by Gel Processing in Polyethylene. Phys. Rev. B 1991, 44, 8652–8666. [Google Scholar] [CrossRef]
- Panzer, F.; Bässler, H.; Köhler, A. Temperature Induced Order–Disorder Transition in Solutions of Conjugated Polymers Probed by Optical Spectroscopy. J. Phys. Chem. Lett. 2016, 8, 114–125. [Google Scholar] [CrossRef]
- Panzer, F.; Sommer, M.; Bässler, H.; Thelakkat, M.; Köhler, A. Spectroscopic Signature of Two Distinct H-Aggregate Species in Poly(3-Hexylthiophene). Macromolecules 2015, 48, 1543–1553. [Google Scholar] [CrossRef]
- Todor-Boer, O.; Petrovai, I.; Tarcan, R.; David, L.; Astilean, S.; Botiz, I. Control of Microstructure in Polymer: Fullerene Active Films by Convective Self-Assembly. Thin Solid Films 2020, 697, 137780. [Google Scholar] [CrossRef]
- Rahimi, K.; Botiz, I.; Agumba, J.O.; Motamen, S.; Stingelin, N.; Reiter, G. Light Absorption of Poly(3-Hexylthiophene) Single Crystals. RSC Adv. 2014, 4, 11121–11123. [Google Scholar] [CrossRef]
- Chang, R.; Hsu, J.H.; Fann, W.S.; Liang, K.K.; Chang, C.H.; Hayashi, M.; Yu, J.; Lin, S.H.; Chang, E.C.; Chuang, K.R.; et al. Experimental and Theoretical Investigations of Absorption and Emission Spectra of the Light Emitting Polymer MEH-PPV in Solution. Chem. Phys. Lett. 2000, 317, 142–152. [Google Scholar] [CrossRef]
- Todor-Boer, O.; Farcău, C.; Botiz, I. Large Enhancement of Photoluminescence Obtained in Thin Polyfluorene Films of Optimized Microstructure. Polymers 2024, 16, 2278. [Google Scholar] [CrossRef]
- Wan, L.; Shi, X.; Wade, J.; Campbell, A.J.; Fuchter, M.J. Strongly Circularly Polarized Crystalline and β-Phase Emission from Poly(9,9-Dioctylfluorene)-Based Deep-Blue Light-Emitting Diodes. Adv. Opt. Mater. 2021, 9, 2100066. [Google Scholar] [CrossRef]
- Krotkus, S.; Kasemann, D.; Lenk, S.; Leo, K.; Reineke, S. Adjustable White-Light Emission from a Photo-Structured Micro-OLED Array. Light Sci. Appl. 2016, 5, e16121. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Xiao, B.; Sun, R.; Yang, X.; Zhang, M.; Gao, Y.; Xiao, B.; Papkovskaya, E.D.; Luponosov, Y.; Brabec, C.J.; et al. 19.46%-Efficiency All-Polymer Organic Solar Cells with Excellent Outdoor Operating Stability Enabled by Active Layer Reconstruction. Energy Environ. Sci. 2025, 18, 1812–1823. [Google Scholar] [CrossRef]
- Dong, M.; Chen, S.; Hong, L.; Jing, J.; Bai, Y.; Liang, Y.; Zhu, C.; Shi, T.; Zhong, W.; Ying, L.; et al. 19.0% Efficiency Binary Organic Solar Cells Enabled by Using a Building Block as Solid Additive. Nano Energy 2024, 119, 109097. [Google Scholar] [CrossRef]
- Wei, N.; Chen, J.; Cheng, Y.; Bian, Z.; Liu, W.; Song, H.; Guo, Y.; Zhang, W.; Liu, Y.; Lu, H.; et al. Constructing Multiscale Fibrous Morphology to Achieve 20% Efficiency Organic Solar Cells by Mixing High and Low Molecular Weight D18. Adv. Mater. 2024, 36, 2408934. [Google Scholar] [CrossRef]
- Heydari Gharahcheshmeh, M.; Gleason, K.K. Texture and Nanostructural Engineering of Conjugated Conducting and Semiconducting Polymers. Mater. Today Adv. 2020, 8, 100086. [Google Scholar] [CrossRef]
- Li, H.; Yang, H.; Zhang, L.; Wang, S.; Chen, Y.; Zhang, Q.; Zhang, J.; Tian, H.; Han, Y. Optimizing the Crystallization Behavior and Film Morphology of Donor–Acceptor Conjugated Semiconducting Polymers by Side-Chain–Solvent Interaction in Nonpolar Solvents. Macromolecules 2021, 54, 10557–10573. [Google Scholar] [CrossRef]
- Chew, A.R.; Ghosh, R.; Pakhnyuk, V.; Onorato, J.; Davidson, E.C.; Segalman, R.A.; Luscombe, C.K.; Spano, F.C.; Salleo, A. Unraveling the Effect of Conformational and Electronic Disorder in the Charge Transport Processes of Semiconducting Polymers. Adv. Funct. Mater. 2018, 28, 1804142. [Google Scholar] [CrossRef]
- Peng, Z.; Ye, L.; Ade, H. Understanding, Quantifying, and Controlling the Molecular Ordering of Semiconducting Polymers: From Novices to Experts and Amorphous to Perfect Crystals. Mater. Horiz. 2022, 9, 577–606. [Google Scholar] [CrossRef]
- Hourani, W.; Rahimi, K.; Botiz, I.; Koch, F.; Reiter, G.; Lienerth, P.; Heiser, T.; Bubendorff, J.-L.; Simon, L. Anisotropic Charge Transport in Large Single Crystals of Pi-Conjugated Organic Molecules. Nanoscale 2014, 6, 4774–4780. [Google Scholar] [CrossRef]
- Bredas, J.L.; Marder, S.R. The WSPC Reference on Organic Electronics: Organic Semiconductors. In Volume 1: Basic Concepts; Volume 2: Fundamental Aspects of Materials and Applications; World Scientific Publishing Co. Pte Ltd.: Singapore, 2016; pp. 1–896. [Google Scholar]
- Xu, R.; Jiang, Y.; Liu, F.; Ran, G.; Liu, K.; Zhang, W.; Zhu, X. High Open-Circuit Voltage Organic Solar Cells with 19.2% Efficiency Enabled by Synergistic Side-Chain Engineering. Adv. Mater. 2024, 36, 2312101. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y.; Yang, G.; Jiang, K.; Lin, H.; Ade, H.; Ma, W.; Yan, H. Efficient Organic Solar Cells Processed from Hydrocarbon Solvents. Nat. Energy 2016, 1, 15027. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, L.; Liu, C.; Guo, C.; Chen, C.; Sun, Y.; Yang, Y.; Cheng, J.; Gan, Z.; Chen, Z.; et al. Tuning of the Polymeric Nanofibril Geometry via Side-Chain Interaction toward 20.1% Efficiency of Organic Solar Cells. J. Am. Chem. Soc. 2024, 146, 34998–35006. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Kang, S.-J.; Dutta, G.K.; Han, Y.-K.; Shin, T.J.; Noh, Y.-Y.; Yang, C. A Thienoisoindigo-Naphthalene Polymer with Ultrahigh Mobility of 14.4 Cm2/V·s That Substantially Exceeds Benchmark Values for Amorphous Silicon Semiconductors. J. Am. Chem. Soc. 2014, 136, 9477–9483. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Jung, J.W.; Kang, H.; Seth, J.; Kang, Y.; Sung, M.M. Single-Crystal Poly [4-(4,4-Dihexadecyl-4H-Cyclopenta[1,2-b:5,4-B′]Dithiophen-2-Yl)-Alt-[1,2,5]Thiadiazolo[3,4-c]Pyridine] Nanowires with Ultrahigh Mobility. Nano Lett. 2019, 19, 1028–1032. [Google Scholar] [CrossRef]
- Janasz, L.; Borkowski, M.; Blom, P.W.M.; Marszalek, T.; Pisula, W. Organic Semiconductor/Insulator Blends for Elastic Field-Effect Transistors and Sensors. Adv. Funct. Mater. 2022, 32, 2105456. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, P.; Xiong, M.; Zhang, L.; Chen, J.; Lei, X.; Pan, X.; Wang, X.; Deng, X.-Y.; Shen, W.; et al. Continuous Production of Ultratough Semiconducting Polymer Fibers with High Electronic Performance. Sci. Adv. 2024, 10, eadk0647. [Google Scholar] [CrossRef]
- Ding, L.; Yu, Z.-D.; Wang, X.-Y.; Yao, Z.-F.; Lu, Y.; Yang, C.-Y.; Wang, J.-Y.; Pei, J. Polymer Semiconductors: Synthesis, Processing, and Applications. Chem. Rev. 2023, 123, 7421–7497. [Google Scholar] [CrossRef]
- Botiz, I. Prominent Processing Techniques to Manipulate Semiconducting Polymer Microstructures. J. Mater. Chem. C 2023, 11, 364–405. [Google Scholar] [CrossRef]
- Tripathi, A.S.M.; Pandey, M.; Sadakata, S.; Nagamatsu, S.; Takashima, W.; Hayase, S.; Pandey, S.S. Anisotropic Charge Transport in Highly Oriented Films of Semiconducting Polymer Prepared by Ribbon-Shaped Floating Film. Appl. Phys. Lett. 2018, 112, 123301. [Google Scholar] [CrossRef]
- O’Connor, B.; Kline, R.J.; Conrad, B.R.; Richter, L.J.; Gundlach, D.; Toney, M.F.; DeLongchamp, D.M. Anisotropic Structure and Charge Transport in Highly Strain-Aligned Regioregular Poly (3-Hexylthiophene). Adv. Funct. Mater. 2011, 21, 3697–3705. [Google Scholar] [CrossRef]
- Pela, R.R.; Hsiao, C.-L.; Hultman, L.; Birch, J.; Gueorguiev, G.K. Electronic and Optical Properties of Core–Shell InAlN Nanorods: A Comparative Study via LDA, LDA-1/2, mBJ, HSE06, G0W0 and BSE Methods. Phys. Chem. Chem. Phys. 2024, 26, 7504–7514. [Google Scholar] [CrossRef]
- Utochnikova, V.V. Chapter 318—Lanthanide Complexes as OLED Emitters. In Handbook on the Physics and Chemistry of Rare Earths; Bünzli, J.-C.G., Pecharsky, V.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 59, pp. 1–91. ISBN 0168-1273. [Google Scholar]
- Yap, B.K.; Xia, R.; Campoy-Quiles, M.; Stavrinou, P.N.; Bradley, D.D.C. Simultaneous Optimization of Charge-Carrier Mobility and Optical Gain in Semiconducting Polymer Films. Nat. Mater. 2008, 7, 376–380. [Google Scholar] [CrossRef]
- Pasveer, W.F.; Cottaar, J.; Tanase, C.; Coehoorn, R.; Bobbert, P.A.; Blom, P.W.M.; de Leeuw, D.M.; Michels, M.A.J. Unified Description of Charge-Carrier Mobilities in Disordered Semiconducting Polymers. Phys. Rev. Lett. 2005, 94, 206601. [Google Scholar] [CrossRef] [PubMed]
- Fratini, S.; Nikolka, M.; Salleo, A.; Schweicher, G.; Sirringhaus, H. Charge Transport in High-Mobility Conjugated Polymers and Molecular Semiconductors. Nat. Mater. 2020, 19, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Guo, X.; Facchetti, A. High-Performance n-Type Polymer Semiconductors: Applications, Recent Development, and Challenges. Chem 2020, 6, 1310–1326. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Z.; Zhang, G.; Zhang, X.; Zhang, D. The Effects of Side Chains on the Charge Mobilities and Functionalities of Semiconducting Conjugated Polymers beyond Solubilities. Adv. Mater 2019, 31, 1903104. [Google Scholar] [CrossRef]
- Kim, M.; Ryu, S.U.; Park, S.A.; Choi, K.; Kim, T.; Chung, D.; Park, T. Donor–Acceptor-Conjugated Polymer for High-Performance Organic Field-Effect Transistors: A Progress Report. Adv. Funct. Mater. 2020, 30, 1904545. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, D.H.; Yang, D.S.; Heo, D.U.; Kim, K.H.; Shin, J.; Kim, H.-J.; Baek, K.-Y.; Lee, K.; Baik, H.; et al. Novel Polymer Nanowire Crystals of Diketopyrrolopyrrole-Based Copolymer with Excellent Charge Transport Properties. Adv. Mater. 2013, 25, 4102–4106. [Google Scholar] [CrossRef]
- Liu, Q.; Kumagai, S.; Manzhos, S.; Chen, Y.; Angunawela, I.; Nahid, M.M.; Feron, K.; Bottle, S.E.; Bell, J.; Ade, H.; et al. Synergistic Use of Pyridine and Selenophene in a Diketopyrrolopyrrole-Based Conjugated Polymer Enhances the Electron Mobility in Organic Transistors. Adv. Funct. Mater. 2020, 30, 2000489. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Huang, Y.-W.; Hung, C.-C.; Chiang, Y.-C.; Chen, C.-K.; Hsu, L.-C.; Chueh, C.-C.; Chen, W.-C. Backbone Engineering of Diketopyrrolopyrrole-Based Conjugated Polymers through Random Terpolymerization for Improved Mobility–Stretchability Property. ACS Appl. Mater. Interfaces 2020, 12, 50648–50659. [Google Scholar] [CrossRef]
- Pei, D.; Wang, Z.; Peng, Z.; Zhang, J.; Deng, Y.; Han, Y.; Ye, L.; Geng, Y. Impact of Molecular Weight on the Mechanical and Electrical Properties of a High-Mobility Diketopyrrolopyrrole-Based Conjugated Polymer. Macromolecules 2020, 53, 4490–4500. [Google Scholar] [CrossRef]
- Back, J.Y.; Yu, H.; Song, I.; Kang, I.; Ahn, H.; Shin, T.J.; Kwon, S.-K.; Oh, J.H.; Kim, Y.-H. Investigation of Structure–Property Relationships in Diketopyrrolopyrrole-Based Polymer Semiconductors via Side-Chain Engineering. Chem. Mater. 2015, 27, 1732–1739. [Google Scholar] [CrossRef]
- Makala, M.; Barłóg, M.; Dremann, D.; Attar, S.; Fernández, E.G.; Al-Hashimi, M.; Jurchescu, O.D. High-Performance n-Type Polymer Field-Effect Transistors with Exceptional Stability. J. Mater. Chem. C 2024, 12, 17089–17098. [Google Scholar] [CrossRef]
- Moser, M.; Savva, A.; Thorley, K.; Paulsen, B.D.; Hidalgo, T.C.; Ohayon, D.; Chen, H.; Giovannitti, A.; Marks, A.; Gasparini, N.; et al. Polaron Delocalization in Donor–Acceptor Polymers and Its Impact on Organic Electrochemical Transistor Performance. Angew. Chem. Int. Ed. 2021, 60, 7777–7785. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-X.; Li, J.-T.; Fang, Y.-H.; Deng, X.-Y.; Wang, X.-Q.; Liu, G.; Wang, Y.; Gu, X.; Jiang, S.-D.; Lei, T. High-Mobility Semiconducting Polymers with Different Spin Ground States. Nat. Commun. 2022, 13, 2258. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gao, M.; He, C.; Shi, W.; Deng, Y.; Han, Y.; Ye, L.; Geng, Y. Unraveling the Molar Mass Dependence of Shearing-Induced Aggregation Structure of a High-Mobility Polymer Semiconductor. Adv. Mater. 2022, 34, 2108255. [Google Scholar] [CrossRef]
- Singh, R.; Venkateswarlu, S.; Zhong, Y.; Li, Y. Synthesis of Fluorene-Flanked Diketopyrrolopyrrole-Based Semiconducting Polymers with Thermocleavable Side Chains and Their Application in Organic Field Effect Transistors. Can. J. Chem. Eng. 2024, 102, 4137–4151. [Google Scholar] [CrossRef]
- Ji, Z.; Dai, Z.; Wang, C.; Wu, X.; Guo, J.; Zhang, H. Thienyl Naphthodipyrrolopyrrole (TNDP)-Based Polymers Semiconductor for n-Type OFETs. Dye. Pigment. 2024, 227, 112160. [Google Scholar] [CrossRef]
- Ditte, K.; Perez, J.; Chae, S.; Hambsch, M.; Al-Hussein, M.; Komber, H.; Formanek, P.; Mannsfeld, S.C.B.; Fery, A.; Kiriy, A.; et al. Ultrasoft and High-Mobility Block Copolymers for Skin-Compatible Electronics. Adv. Mater. 2021, 33, 2005416. [Google Scholar] [CrossRef]
- Dai, Y.; Dai, S.; Li, N.; Li, Y.; Moser, M.; Strzalka, J.; Prominski, A.; Liu, Y.; Zhang, Q.; Li, S.; et al. Stretchable Redox-Active Semiconducting Polymers for High-Performance Organic Electrochemical Transistors. Adv. Mater. 2022, 34, 2201178. [Google Scholar] [CrossRef]
- Griggs, S.; Marks, A.; Bristow, H.; McCulloch, I. N-Type Organic Semiconducting Polymers: Stability Limitations, Design Considerations and Applications. J. Mater. Chem. C 2021, 9, 8099–8128. [Google Scholar] [CrossRef]
- Tsumura, A.; Koezuka, H.; Ando, T. Macromolecular Electronic Device: Field-Effect Transistor with a Polythiophene Thin Film. Appl. Phys. Lett. 1986, 49, 1210–1212. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, Z.; Hu, Z.; He, T. Single Crystals of Polythiophene with Different Molecular Conformations Obtained by Tetrahydrofuran Vapor Annealing and Controlling Solvent Evaporation. J. Phys. Chem. B 2010, 114, 7452–7460. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Hu, Z.; Wang, Z.; He, T. Study on the Single Crystals of Poly(3-Octylthiophene) Induced by Solvent-Vapor Annealing. J. Phys. Chem. B 2009, 113, 14604–14610. [Google Scholar] [CrossRef] [PubMed]
- Son, S.Y.; Kim, Y.; Lee, J.; Lee, G.-Y.; Park, W.-T.; Noh, Y.-Y.; Park, C.E.; Park, T. High-Field-Effect Mobility of Low-Crystallinity Conjugated Polymers with Localized Aggregates. J. Am. Chem. Soc. 2016, 138, 8096–8103. [Google Scholar] [CrossRef] [PubMed]
- Fei, Z.; Pattanasattayavong, P.; Han, Y.; Schroeder, B.C.; Yan, F.; Kline, R.J.; Anthopoulos, T.D.; Heeney, M. Influence of Side-Chain Regiochemistry on the Transistor Performance of High-Mobility, All-Donor Polymers. J. Am. Chem. Soc. 2014, 136, 15154–15157. [Google Scholar] [CrossRef]
- Hsu, L.-C.; Kobayashi, S.; Isono, T.; Chiang, Y.-C.; Ree, B.J.; Satoh, T.; Chen, W.-C. Highly Stretchable Semiconducting Polymers for Field-Effect Transistors through Branched Soft–Hard–Soft Type Triblock Copolymers. Macromolecules 2020, 53, 7496–7510. [Google Scholar] [CrossRef]
- Sharma, S.; Gaurav, K.V.; Nagamatsu, S.; Pandey, S.S. The Influence of a Microstructural Conformation of Oriented Floating Films of Semiconducting Polymers on Organic Device Performance. Polymers 2024, 16, 710. [Google Scholar] [CrossRef]
- Yamashita, Y.; Hinkel, F.; Marszalek, T.; Zajaczkowski, W.; Pisula, W.; Baumgarten, M.; Matsui, H.; Müllen, K.; Takeya, J. Mobility Exceeding 10 Cm2/(V·s) in Donor–Acceptor Polymer Transistors with Band-like Charge Transport. Chem. Mater. 2016, 28, 420–424. [Google Scholar] [CrossRef]
- Nketia-Yawson, B.; Jung, A.-R.; Nguyen, H.D.; Lee, K.-K.; Kim, B.; Noh, Y.-Y. Difluorobenzothiadiazole and Selenophene-Based Conjugated Polymer Demonstrating an Effective Hole Mobility Exceeding 5 Cm2 V–1 s–1 with Solid-State Electrolyte Dielectric. ACS Appl. Mater. Interfaces 2018, 10, 32492–32500. [Google Scholar] [CrossRef]
- Feng, K.; Guo, H.; Sun, H.; Guo, X. N-Type Organic and Polymeric Semiconductors Based on Bithiophene Imide Derivatives. Acc. Chem. Res. 2021, 54, 3804–3817. [Google Scholar] [CrossRef]
- Wang, S.; Kappl, M.; Liebewirth, I.; Müller, M.; Kirchhoff, K.; Pisula, W.; Müllen, K. Organic Field-Effect Transistors Based on Highly Ordered Single Polymer Fibers. Adv. Mater. 2012, 24, 417–420. [Google Scholar] [CrossRef]
- Wang, X.; He, Z.; Ma, Z.; Zhao, L.; Xie, C.; Chen, X. Benzodipyrrolidone-Based Donor-Acceptor Semiconducting Polymers with High Hole Mobility and Noncovalent Conformational Locks. Dyes Pigm. 2024, 228, 112225. [Google Scholar] [CrossRef]
- Nketia-Yawson, B.; Nketia-Yawson, V.; Buer, A.B.; Jo, J.W. Interfacial Charge Transport Enhancement of Liquid-Crystalline Polymer Transistors Enabled by Ionic Polyurethane Dielectric. Macromol. Rapid Commun. 2024, 45, 2400265. [Google Scholar] [CrossRef] [PubMed]
- Nketia-Yawson, V.; Buer, A.B.; Ahn, H.; Nketia-Yawson, B.; Jo, J.W. Hole Mobility Enhancement in Benzo[1,2-b:4,5-b’]Dithiophene-Based Conjugated Polymer Transistors through Directional Alignment, Perovskite Functionalization and Solid-State Electrolyte Gating. Macromol. Rapid Commun. 2024, 45, 2300634. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Long, D.X.; Lee, J.; Kim, G.; Shin, T.J.; Nam, K.-W.; Noh, Y.-Y.; Yang, C. A Balanced Face-On to Edge-On Texture Ratio in Naphthalene Diimide-Based Polymers with Hybrid Siloxane Chains Directs Highly Efficient Electron Transport. Macromolecules 2015, 48, 5179–5187. [Google Scholar] [CrossRef]
- Kim, R.; Kang, B.; Sin, D.H.; Choi, H.H.; Kwon, S.-K.; Kim, Y.-H.; Cho, K. Oligo(Ethylene Glycol)-Incorporated Hybrid Linear Alkyl Side Chains for n-Channel Polymer Semiconductors and Their Effect on the Thin-Film Crystalline Structure. Chem. Commun. 2015, 51, 1524–1527. [Google Scholar] [CrossRef]
- Bucella, S.G.; Luzio, A.; Gann, E.; Thomsen, L.; McNeill, C.R.; Pace, G.; Perinot, A.; Chen, Z.; Facchetti, A.; Caironi, M. Macroscopic and High-Throughput Printing of Aligned Nanostructured Polymer Semiconductors for MHz Large-Area Electronics. Nat. Commun. 2015, 6, 8394. [Google Scholar] [CrossRef]
- Yao, Z.-F.; Zheng, Y.-Q.; Dou, J.-H.; Lu, Y.; Ding, Y.-F.; Ding, L.; Wang, J.-Y.; Pei, J. Approaching Crystal Structure and High Electron Mobility in Conjugated Polymer Crystals. Adv. Mater. 2021, 33, 2006794. [Google Scholar] [CrossRef]
- Kang, B.; Kim, R.; Lee, S.B.; Kwon, S.-K.; Kim, Y.-H.; Cho, K. Side-Chain-Induced Rigid Backbone Organization of Polymer Semiconductors through Semifluoroalkyl Side Chains. J. Am. Chem. Soc. 2016, 138, 3679–3686. [Google Scholar] [CrossRef]
- Wang, Y.; Hasegawa, T.; Matsumoto, H.; Michinobu, T. Significant Improvement of Unipolar N-Type Transistor Performances by Manipulating the Coplanar Backbone Conformation of Electron-Deficient Polymers via Hydrogen Bonding. J. Am. Chem. Soc. 2019, 141, 3566–3575. [Google Scholar] [CrossRef]
- Lee, J.-W.; Sung, M.J.; Kim, D.; Lee, S.; You, H.; Kim, F.S.; Kim, Y.-H.; Kim, B.J.; Kwon, S.-K. Naphthalene Diimide-Based Terpolymers with Controlled Crystalline Properties for Producing High Electron Mobility and Optimal Blend Morphology in All-Polymer Solar Cells. Chem. Mater. 2020, 32, 2572–2582. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, W.; Yu, G. Semiconducting Polymers Based on Isoindigo and Its Derivatives: Synthetic Tactics, Structural Modifications, and Applications. Adv. Funct. Mater. 2021, 31, 2010979. [Google Scholar] [CrossRef]
- Kim, Y.; Hwang, H.; Kim, N.-K.; Hwang, K.; Park, J.-J.; Shin, G.-I.; Kim, D.-Y. π-Conjugated Polymers Incorporating a Novel Planar Quinoid Building Block with Extended Delocalization and High Charge Carrier Mobility. Adv. Mater. 2018, 30, 1706557. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-C.; Hung, C.-C.; Hong, C.-W.; Sun, H.-S.; Wang, J.-T.; Yamashita, G.; Higashihara, T.; Chen, W.-C. Isoindigo-Based Semiconducting Polymers Using Carbosilane Side Chains for High Performance Stretchable Field-Effect Transistors. Macromolecules 2016, 49, 8540–8548. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, Z.; Geng, H.; Cheng, C.; Chen, J.; Sun, Y.; Shi, L.; Yi, Y.; Shuai, Z.; Guo, Y.; et al. Isoindigo-Based Polymers with Small Effective Masses for High-Mobility Ambipolar Field-Effect Transistors. Adv. Mater. 2017, 29, 1702115. [Google Scholar] [CrossRef]
- Li, B.-W.; Xiong, M.; Liu, M.-H.; Li, Z.-G.; Sang, L.; Xiong, Z.-H.; Xiao, B.; Pei, J.; Wan, X.-B. Thiazoloisoindigo-Based Polymer Semiconductors: Synthesis, Structure-Property Relationship, Charge Carrier Polarity, and Field-Effect Transistor Performance. Chin. J. Polym. Sci. 2024, 42, 24–31. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, W.; Wei, C.; Zhou, Y.; Pan, Y.; Wei, X.; Huang, J.; Wang, L.; Yu, G. High-Electron Mobility Tetrafluoroethylene-Containing Semiconducting Polymers. Chem. Mater. 2020, 32, 2330–2340. [Google Scholar] [CrossRef]
- Yu, X.; Guo, J.; Wang, C.; Zhang, K.; Wang, H.; Ma, S.; Zhang, H. Self-Assembled Quinacridone (QA) Based Polymers with Strong Hydrogen Bonding for OFETs. Dye. Pigment. 2024, 227, 112157. [Google Scholar] [CrossRef]
- Zheng, Y.-Q.; Lei, T.; Dou, J.-H.; Xia, X.; Wang, J.-Y.; Liu, C.-J.; Pei, J. Strong Electron-Deficient Polymers Lead to High Electron Mobility in Air and Their Morphology-Dependent Transport Behaviors. Adv. Mater. 2016, 28, 7213–7219. [Google Scholar] [CrossRef]
- Choi, D.; Kim, H.; Persson, N.; Chu, P.-H.; Chang, M.; Kang, J.-H.; Graham, S.; Reichmanis, E. Elastomer–Polymer Semiconductor Blends for High-Performance Stretchable Charge Transport Networks. Chem. Mater. 2016, 28, 1196–1204. [Google Scholar] [CrossRef]
- Shin, M.; Oh, J.Y.; Byun, K.-E.; Lee, Y.-J.; Kim, B.; Baik, H.-K.; Park, J.-J.; Jeong, U. Polythiophene Nanofibril Bundles Surface-Embedded in Elastomer: A Route to a Highly Stretchable Active Channel Layer. Adv. Mater. 2015, 27, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Song, J.H.; Lim, G.-H.; Lim, B.; Park, J.-J.; Jeong, U. Highly Stretchable Polymer Transistors Consisting Entirely of Stretchable Device Components. Adv. Mater. 2014, 26, 3706–3711. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.-R.; Phan, H.; Luo, C.; Wang, M.; Perez, L.A.; Patel, S.N.; Ying, L.; Kramer, E.J.; Nguyen, T.-Q.; Bazan, G.C. High-Mobility Field-Effect Transistors Fabricated with Macroscopic Aligned Semiconducting Polymers. Adv. Mater 2014, 26, 2993–2998. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Kim, Y.; Kang, J.S.; Berger, R.; Yoon, H.; Char, K. Enhanced Vertical Charge Transport of Homo- and Blended Semiconducting Polymers by Nanoconfinement. Adv. Mater. 2020, 32, 1908087. [Google Scholar] [CrossRef]
- Kranthiraja, K.; Sethumadhavan, V.; Kumagai, S.; Xu, Y.; Erhardt, A.; McNeill, C.R.; Manzhos, S.; Takeya, J.; Sonar, P. Low Band Gap Furan-Flanked Diketopyrrolopyrrole-Naphthobisthiadiazole Based Conjugated Polymer/Stretchable Blend for Organic Field Effect Transistors. Adv. Electron. Mater. 2025, 11, 2400614. [Google Scholar] [CrossRef]
- Mason, G.T.; Skaf, D.; Roy, A.L.; Hussein, R.N.; Gomes, T.C.; Landry, E.; Xiang, P.; Walus, K.; Carmichael, T.B.; Rondeau-Gagné, S. Printing Organic-Field Effect Transistors from Semiconducting Polymers and Branched Polyethylene. Can. J. Chem. Eng. 2024, 102, 4166–4174. [Google Scholar] [CrossRef]
- Pei, D.; An, C.; Zhao, B.; Ge, M.; Wang, Z.; Dong, W.; Wang, C.; Deng, Y.; Song, D.; Ma, Z.; et al. Polyurethane-Based Stretchable Semiconductor Nanofilms with High Intrinsic Recovery Similar to Conventional Elastomers. ACS Appl. Mater. Interfaces 2022, 14, 33806–33816. [Google Scholar] [CrossRef]
- Nikzad, S.; Wu, H.-C.; Kim, J.; Mahoney, C.M.; Matthews, J.R.; Niu, W.; Li, Y.; Wang, H.; Chen, W.-C.; Toney, M.F.; et al. Inducing Molecular Aggregation of Polymer Semiconductors in a Secondary Insulating Polymer Matrix to Enhance Charge Transport. Chem. Mater. 2020, 32, 897–905. [Google Scholar] [CrossRef]
- Schmode, P.; Hochgesang, A.; Goel, M.; Meichsner, F.; Mohanraj, J.; Fried, M.; Thelakkat, M. A Solution-Processable Pristine PEDOT Exhibiting Excellent Conductivity, Charge Carrier Mobility, and Thermal Stability in the Doped State. Macromol. Chem. Phys. 2021, 222, 2100123. [Google Scholar] [CrossRef]
- Lee, S.; Paine, D.C.; Gleason, K.K. Heavily Doped Poly(3,4-Ethylenedioxythiophene) Thin Films with High Carrier Mobility Deposited Using Oxidative CVD: Conductivity Stability and Carrier Transport. Adv. Funct. Mater. 2014, 24, 7187–7196. [Google Scholar] [CrossRef]
- Dimov, I.B.; Moser, M.; Malliaras, G.G.; McCulloch, I. Semiconducting Polymers for Neural Applications. Chem. Rev. 2022, 122, 4356–4396. [Google Scholar] [CrossRef] [PubMed]
- Stanfield, D.A.; Wu, Y.; Tolbert, S.H.; Schwartz, B.J. Controlling the Formation of Charge Transfer Complexes in Chemically Doped Semiconducting Polymers. Chem. Mater. 2021, 33, 2343–2356. [Google Scholar] [CrossRef]
- Aubry, T.J.; Winchell, K.J.; Salamat, C.Z.; Basile, V.M.; Lindemuth, J.R.; Stauber, J.M.; Axtell, J.C.; Kubena, R.M.; Phan, M.D.; Bird, M.J.; et al. Tunable Dopants with Intrinsic Counterion Separation Reveal the Effects of Electron Affinity on Dopant Intercalation and Free Carrier Production in Sequentially Doped Conjugated Polymer Films. Adv. Funct. Mater. 2020, 30, 2001800. [Google Scholar] [CrossRef]
- Lee, J.H.; Shim, E.S.; Nketia-Yawson, B.; Opoku, H.; Ahn, H.; Bae, S.; Jo, J.W. Suppressed Surface Aggregation and Homogeneous Integration of π-Bridged Polyelectrolyte for Boosting Charge Transport in Conjugated Polymer Semiconductors. Appl. Surf. Sci. 2024, 665, 160347. [Google Scholar] [CrossRef]
- Wang, M.; Fu, S.; Petkov, P.; Fu, Y.; Zhang, Z.; Liu, Y.; Ma, J.; Chen, G.; Gali, S.M.; Gao, L.; et al. Exceptionally High Charge Mobility in Phthalocyanine-Based Poly(Benzimidazobenzophenanthroline)-Ladder-Type Two-Dimensional Conjugated Polymers. Nat. Mater. 2023, 22, 880–887. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, Z.; Guo, H.; Uddin, M.A.; Ling, S.; Zhou, X.; Su, H.; Dai, J.; Woo, H.Y.; Guo, X. Effects of Bithiophene Imide Fusion on the Device Performance of Organic Thin-Film Transistors and All-Polymer Solar Cells. Angew. Chem. Int. Ed. 2017, 56, 15304–15308. [Google Scholar] [CrossRef]
- Feng, K.; Shan, W.; Ma, S.; Wu, Z.; Chen, J.; Guo, H.; Liu, B.; Wang, J.; Li, B.; Woo, H.Y.; et al. Fused Bithiophene Imide Dimer-Based n-Type Polymers for High-Performance Organic Electrochemical Transistors. Angew. Chem. Int. Ed. 2021, 60, 24198–24205. [Google Scholar] [CrossRef]
- Hallani, R.K.; Paulsen, B.D.; Petty, A.J.I.; Sheelamanthula, R.; Moser, M.; Thorley, K.J.; Sohn, W.; Rashid, R.B.; Savva, A.; Moro, S.; et al. Regiochemistry-Driven Organic Electrochemical Transistor Performance Enhancement in Ethylene Glycol-Functionalized Polythiophenes. J. Am. Chem. Soc. 2021, 143, 11007–11018. [Google Scholar] [CrossRef]
- Wadsworth, A.; Chen, H.; Thorley, K.J.; Cendra, C.; Nikolka, M.; Bristow, H.; Moser, M.; Salleo, A.; Anthopoulos, T.D.; Sirringhaus, H.; et al. Modification of Indacenodithiophene-Based Polymers and Its Impact on Charge Carrier Mobility in Organic Thin-Film Transistors. J. Am. Chem. Soc. 2020, 142, 652–664. [Google Scholar] [CrossRef]
- Wang, M.; Xia, G.; Yang, C.; Zhang, L.; Nikolka, M.; Zhang, W.; Cendra, C.; Liu, W.; Zhao, S.; Zeng, J.; et al. An Amorphous Donor-Acceptor Conjugated Polymer with Both High Charge Carrier Mobility and Luminescence Quantum Efficiency. Angew. Chem. Int. Ed. 2025, 64, e202421199. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, A.; He, K.; Hendsbee, A.D.; Abdelsamie, M.; Bennett, R.N.; Li, Y.; Toney, M.F.; Kelly, T.L. Synthesis of Poly(Bisisoindigo) Using a Metal-Free Aldol Polymerization for Thin-Film Transistor Applications. ACS Appl. Mater. Interfaces 2020, 12, 14265–14271. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Peng, L.; Ding, Y.; Zhao, Y.; Yu, G. Nanostructured Conductive Polymers for Advanced Energy Storage. Chem. Soc. Rev. 2015, 44, 6684–6696. [Google Scholar] [CrossRef] [PubMed]
- Pooja; Kumar, A.; Prasher, P.; Mudila, H. Factors Affecting the Electrical Conductivity of Conducting Polymers. Carbon Lett. 2023, 33, 307–324. [Google Scholar] [CrossRef]
- Jiang, C.; Chen, G.; Wang, X. High-Conversion Synthesis of Poly(3,4-Ethylenedioxythiophene) by Chemical Oxidative Polymerization. Synth. Met. 2012, 162, 1968–1971. [Google Scholar] [CrossRef]
- D’Arcy, J.M.; El-Kady, M.F.; Khine, P.P.; Zhang, L.; Lee, S.H.; Davis, N.R.; Liu, D.S.; Yeung, M.T.; Kim, S.Y.; Turner, C.L.; et al. Vapor-Phase Polymerization of Nanofibrillar Poly(3,4-Ethylenedioxythiophene) for Supercapacitors. ACS Nano 2014, 8, 1500–1510. [Google Scholar] [CrossRef]
- Anothumakkool, B.; Soni, R.; Bhange, S.N.; Kurungot, S. Novel Scalable Synthesis of Highly Conducting and Robust PEDOT Paper for a High Performance Flexible Solid Supercapacitor. Energy Environ. Sci. 2015, 8, 1339–1347. [Google Scholar] [CrossRef]
- Massonnet, N.; Carella, A.; de Geyer, A.; Faure-Vincent, J.; Simonato, J.-P. Metallic Behaviour of Acid Doped Highly Conductive Polymers. Chem. Sci. 2015, 6, 412–417. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Sun, L.; Lee, D.; Lee, S.; Wang, M.; Zhao, J.; Shao-Horn, Y.; Dincă, M.; Palacios, T.; et al. High Electrical Conductivity and Carrier Mobility in oCVD PEDOT Thin Films by Engineered Crystallization and Acid Treatment. Sci. Adv. 2018, 4, eaat5780. [Google Scholar] [CrossRef]
- Guo, B.; Yin, Q.; Zhou, J.; Li, W.; Zhang, K.; Li, Y. Semiconductive Polymer-Doped PEDOT with High Work Function, Conductivity, Reversible Dispersion, and Application in Organic Solar Cells. ACS Sustain. Chem. Eng. 2019, 7, 8206–8214. [Google Scholar] [CrossRef]
- Yu, Z.; Xia, Y.; Du, D.; Ouyang, J. PEDOT:PSS Films with Metallic Conductivity through a Treatment with Common Organic Solutions of Organic Salts and Their Application as a Transparent Electrode of Polymer Solar Cells. ACS Appl. Mater. Interfaces 2016, 8, 11629–11638. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Liu, C.; Jiang, Q.; Xu, J. Effective Approaches to Improve the Electrical Conductivity of PEDOT:PSS: A Review. Adv. Electron. Mater. 2015, 1, 1500017. [Google Scholar] [CrossRef]
- Fan, X.; Nie, W.; Tsai, H.; Wang, N.; Huang, H.; Cheng, Y.; Wen, R.; Ma, L.; Yan, F.; Xia, Y. PEDOT:PSS for Flexible and Stretchable Electronics: Modifications, Strategies, and Applications. Adv. Sci. 2019, 6, 1900813. [Google Scholar] [CrossRef] [PubMed]
- Worfolk, B.J.; Andrews, S.C.; Park, S.; Reinspach, J.; Liu, N.; Toney, M.F.; Mannsfeld, S.C.B.; Bao, Z. Ultrahigh Electrical Conductivity in Solution-Sheared Polymeric Transparent Films. Proc. Natl. Acad. Sci. USA 2015, 112, 14138–14143. [Google Scholar] [CrossRef]
- Vaagensmith, B.; Reza, K.M.; Hasan, M.N.; Elbohy, H.; Adhikari, N.; Dubey, A.; Kantack, N.; Gaml, E.; Qiao, Q. Environmentally Friendly Plasma-Treated PEDOT:PSS as Electrodes for ITO-Free Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 35861–35870. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, L.; Geng, B.; Chen, F.; Yuan, Y.; Li, D.; Wang, Y.-X.; Jia, W.; Hu, W. Triple-Network-Based Conductive Polymer Hydrogel for Soft and Elastic Bioelectronic Interfaces. SmartMat 2024, 5, e1229. [Google Scholar] [CrossRef]
- Karpov, Y.; Erdmann, T.; Raguzin, I.; Al-Hussein, M.; Binner, M.; Lappan, U.; Stamm, M.; Gerasimov, K.L.; Beryozkina, T.; Bakulev, V.; et al. High Conductivity in Molecularly P-Doped Diketopyrrolopyrrole-Based Polymer: The Impact of a High Dopant Strength and Good Structural Order. Adv. Mater. 2016, 28, 6003–6010. [Google Scholar] [CrossRef]
- Kim, M.J.; Ryu, H.S.; Choi, Y.Y.; Ho, D.H.; Lee, Y.; Tripathi, A.; Son, J.H.; Lee, Y.; Kim, S.; Kang, M.S.; et al. Completely Foldable Electronics Based on Homojunction Polymer Transistors and Logics. Sci. Adv. 2021, 7, eabg8169. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, M.; Ma, C.; Wang, Y.; Li, X.; Yu, G. A Conductive Self-Healing Hybrid Gel Enabled by Metal–Ligand Supramolecule and Nanostructured Conductive Polymer. Nano Lett. 2015, 15, 6276–6281. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, Y.; Souri, M.; Luo, X.; Boehm, A.M.; Li, R.; Zhang, Y.; Wang, T.; Kim, D.-Y.; Mei, J.; et al. Influence of Dopant Size and Electron Affinity on the Electrical Conductivity and Thermoelectric Properties of a Series of Conjugated Polymers. J. Mater. Chem. A 2018, 6, 16495–16505. [Google Scholar] [CrossRef]
- Ding, J.; Liu, Z.; Zhao, W.; Jin, W.; Xiang, L.; Wang, Z.; Zeng, Y.; Zou, Y.; Zhang, F.; Yi, Y.; et al. Selenium-Substituted Diketopyrrolopyrrole Polymer for High-Performance p-Type Organic Thermoelectric Materials. Angew. Chem. Int. Ed. 2019, 58, 18994–18999. [Google Scholar] [CrossRef]
- Han, J.; Jiang, Y.; Tiernan, E.; Ganley, C.; Song, Y.; Lee, T.; Chiu, A.; McGuiggan, P.; Adams, N.; Clancy, P.; et al. Blended Conjugated Host and Unconjugated Dopant Polymers Towards N-Type All-Polymer Conductors and High-ZT Thermoelectrics. Angew. Chem. Int. Ed. 2023, 62, e202219313. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, X.; Du, T.; Han, Y.; Deng, Y.; Geng, Y. A High-Performance n-Type Thermoelectric Polymer from C−H/C−H Oxidative Direct Arylation Polycondensation. Angew. Chem. Int. Ed. 2023, 62, e202219262. [Google Scholar] [CrossRef]
- Tu, L.; Wang, J.; Wu, Z.; Li, J.; Yang, W.; Liu, B.; Wu, S.; Xia, X.; Wang, Y.; Woo, H.Y.; et al. Cyano-Functionalized Pyrazine: A Structurally Simple and Easily Accessible Electron-Deficient Building Block for n-Type Organic Thermoelectric Polymers. Angew. Chem. Int. Ed. 2024, 63, e202319658. [Google Scholar] [CrossRef]
- Gao, Y.; Ke, Y.; Wang, T.; Shi, Y.; Wang, C.; Ding, S.; Wang, Y.; Deng, Y.; Hu, W.; Geng, Y. An N-Type Conjugated Polymer with Low Crystallinity for High-Performance Organic Thermoelectrics. Angew. Chem. Int. Ed. 2024, 63, e202402642. [Google Scholar] [CrossRef]
- Cai, H.; Tang, H.; Wang, T.; Xu, C.; Xie, J.; Fu, M.; Luo, X.; Hu, Z.; Zhang, Y.; Deng, Y.; et al. An N-Type Open-Shell Conjugated Polymer with High-Spin Ground-State and High Intrinsic Electrical Conductivity. Angew. Chem. Int. Ed. 2024, 63, e202402375. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Liu, D.; Zhang, J.; Wei, Z.; Wang, Y. A High-Mobility n-Type Noncovalently-Fused-Ring Polymer for High-Performance Organic Thermoelectrics. Angew. Chem. Int. Ed. 2024, 63, e202409018. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Deng, X.-Y.; Tian, S.-Y.; Liu, K.-K.; Fang, Y.-H.; Wang, J.-R.; Wang, Y.; Liu, G.; Chen, J.; Villalva, D.R.; et al. Counterion Docking: A General Approach to Reducing Energetic Disorder in Doped Polymeric Semiconductors. Nat. Commun. 2024, 15, 4972. [Google Scholar] [CrossRef] [PubMed]
- Aziz, E.F.; Vollmer, A.; Eisebitt, S.; Eberhardt, W.; Pingel, P.; Neher, D.; Koch, N. Localized Charge Transfer in a Molecularly Doped Conducting Polymer. Adv. Mater. 2007, 19, 3257–3260. [Google Scholar] [CrossRef]
- Cochran, J.E.; Junk, M.J.N.; Glaudell, A.M.; Miller, P.L.; Cowart, J.S.; Toney, M.F.; Hawker, C.J.; Chmelka, B.F.; Chabinyc, M.L. Molecular Interactions and Ordering in Electrically Doped Polymers: Blends of PBTTT and F4TCNQ. Macromolecules 2014, 47, 6836–6846. [Google Scholar] [CrossRef]
- Scholes, D.T.; Hawks, S.A.; Yee, P.Y.; Wu, H.; Lindemuth, J.R.; Tolbert, S.H.; Schwartz, B.J. Overcoming Film Quality Issues for Conjugated Polymers Doped with F4TCNQ by Solution Sequential Processing: Hall Effect, Structural, and Optical Measurements. J. Phys. Chem. Lett. 2015, 6, 4786–4793. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, V.; Zhong, Y.; Untilova, V.; Bahri, M.; Herrmann, L.; Biniek, L.; Leclerc, N.; Brinkmann, M. Bringing Conducting Polymers to High Order: Toward Conductivities beyond 105 S Cm−1 and Thermoelectric Power Factors of 2 mW M−1 K−2. Adv. Eng. Mater. 2019, 9, 1900266. [Google Scholar] [CrossRef]
- Zhu, M.; He, B.; Zhang, K.; Hussain, S.; Li, T. Recent Progress of Poly(3-Hexylthiophene)-Based Materials for Thermoelectric Applications. Mater. Chem. Front. 2024, 8, 2454–2492. [Google Scholar] [CrossRef]
- Deng, S.; Kuang, Y.; Liu, L.; Liu, X.; Liu, J.; Li, J.; Meng, B.; Di, C.; Hu, J.; Liu, J. High-Performance and Ecofriendly Organic Thermoelectrics Enabled by N-Type Polythiophene Derivatives with Doping-Induced Molecular Order. Adv. Mater. 2024, 36, 2309679. [Google Scholar] [CrossRef]
- Feng, K.; Wang, J.; Jeong, S.Y.; Yang, W.; Li, J.; Woo, H.Y.; Guo, X. High-Performance n-Type Organic Thermoelectrics Enabled by Synergistically Achieving High Electron Mobility and Doping Efficiency. Adv. Sci. 2023, 10, 2302629. [Google Scholar] [CrossRef]
- Feng, K.; Yang, W.; Jeong, S.Y.; Ma, S.; Li, Y.; Wang, J.; Wang, Y.; Woo, H.Y.; Chan, P.K.L.; Wang, G.; et al. Cyano-Functionalized Fused Bithiophene Imide Dimer-Based n-Type Polymers for High-Performance Organic Thermoelectrics. Adv. Mater. 2023, 35, 2210847. [Google Scholar] [CrossRef]
- Li, J.; Liu, M.; Yang, K.; Wang, Y.; Wang, J.; Chen, Z.; Feng, K.; Wang, D.; Zhang, J.; Li, Y.; et al. Selenium Substitution in Bithiophene Imide Polymer Semiconductors Enables High-Performance n-Type Organic Thermoelectric. Adv. Funct. Mater. 2023, 33, 2213911. [Google Scholar] [CrossRef]
- Li, Y.; Wu, W.; Wang, Y.; Huang, E.; Jeong, S.Y.; Woo, H.Y.; Guo, X.; Feng, K. Multi-Selenophene Incorporated Thiazole Imide-Based n-Type Polymers for High-Performance Organic Thermoelectrics. Angew. Chem. Int. Ed. 2024, 63, e202316214. [Google Scholar] [CrossRef]
- Lu, Y.; Yu, Z.-D.; Liu, Y.; Ding, Y.-F.; Yang, C.-Y.; Yao, Z.-F.; Wang, Z.-Y.; You, H.-Y.; Cheng, X.-F.; Tang, B.; et al. The Critical Role of Dopant Cations in Electrical Conductivity and Thermoelectric Performance of N-Doped Polymers. J. Am. Chem. Soc. 2020, 142, 15340–15348. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Ding, Y.-F.; Huang, D.; Wang, J.; Yao, Z.-F.; Huang, C.-X.; Lu, Y.; Un, H.-I.; Zhuang, F.-D.; Dou, J.-H.; et al. A Thermally Activated and Highly Miscible Dopant for N-Type Organic Thermoelectrics. Nat. Commun. 2020, 11, 3292. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Yu, Z.-D.; Lu, Y.; Yao, Z.-F.; Zhou, Y.-Y.; Pan, C.-K.; Liu, Y.; Wang, Z.-Y.; Ding, Y.-F.; Wang, J.-Y.; et al. Density of States Engineering of N-Doped Conjugated Polymers for High Charge Transport Performances. Adv. Mater. 2023, 35, 2300634. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Fan, H.; Zhang, Q.; Hu, Q.; Russell, T.P.; Katz, H.E. Dichlorinated Dithienylethene-Based Copolymers for Air-Stable n-Type Conductivity and Thermoelectricity. Adv. Funct. Mater. 2021, 31, 2005901. [Google Scholar] [CrossRef]
- Wang, Y.; Torres, J.A.; Stieg, A.Z.; Jiang, S.; Yeung, M.T.; Rubin, Y.; Chaudhuri, S.; Duan, X.; Kaner, R.B. Graphene-Assisted Solution Growth of Vertically Oriented Organic Semiconducting Single Crystals. ACS Nano 2015, 9, 9486–9496. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tran, H.D.; Liao, L.; Duan, X.; Kaner, R.B. Nanoscale Morphology, Dimensional Control, and Electrical Properties of Oligoanilines. J. Am. Chem. Soc. 2010, 132, 10365–10373. [Google Scholar] [CrossRef]
- Zhao, F.; Shi, Y.; Pan, L.; Yu, G. Multifunctional Nanostructured Conductive Polymer Gels: Synthesis, Properties, and Applications. Acc. Chem. Res. 2017, 50, 1734–1743. [Google Scholar] [CrossRef]
- Grancarić, A.M.; Jerković, I.; Koncar, V.; Cochrane, C.; Kelly, F.M.; Soulat, D.; Legrand, X. Conductive Polymers for Smart Textile Applications. J. Ind. Text. 2018, 48, 612–642. [Google Scholar] [CrossRef]
- Khalid, M.; Tumelero, M.A.; Brandt, I.S.; Zoldan, V.C.; Acuña, J.J.S.; Pasa, A.A. Electrical Conductivity Studies of Polyaniline Nanotubes Doped with Different Sulfonic Acids. Indian J. Eng. Mater. Sci. 2013, 2013, 718304. [Google Scholar] [CrossRef]
- Yuan, D.; Liu, W.; Zhu, X. Efficient and Air-Stable n-Type Doping in Organic Semiconductors. Chem. Soc. Rev. 2023, 52, 3842–3872. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Chen, Z.; Wang, L.; Yu, G. Recent Developments in Polymer Semiconductors with Excellent Electron Transport Performances. Chem. Soc. Rev. 2025, 54, 2483–2519. [Google Scholar] [CrossRef]
- Feig, V.R.; Tran, H.; Bao, Z. Biodegradable Polymeric Materials in Degradable Electronic Devices. ACS Cent. Sci. 2018, 4, 337–348. [Google Scholar] [CrossRef]
- Matsuhisa, N.; Kaltenbrunner, M.; Yokota, T.; Jinno, H.; Kuribara, K.; Sekitani, T.; Someya, T. Printable Elastic Conductors with a High Conductivity for Electronic Textile Applications. Nat. Commun. 2015, 6, 7461. [Google Scholar] [CrossRef]
- Tang, H.; Liang, Y.; Liu, C.; Hu, Z.; Deng, Y.; Guo, H.; Yu, Z.; Song, A.; Zhao, H.; Zhao, D.; et al. A Solution-Processed n-Type Conducting Polymer with Ultrahigh Conductivity. Nature 2022, 611, 271–277. [Google Scholar] [CrossRef]
- Botiz, I.; Durbin, M.M.; Stingelin, N. Providing a Window into the Phase Behavior of Semiconducting Polymers. Macromolecules 2021, 54, 5304–5320. [Google Scholar] [CrossRef]
- Botiz, I.; Stingelin, N. Influence of Molecular Conformations and Microstructure on the Optoelectronic Properties of Conjugated Polymers. Materials 2014, 7, 2273–2300. [Google Scholar] [CrossRef]
- Tong, Y.; Xiao, Z.; Du, X.; Zuo, C.; Li, Y.; Lv, M.; Yuan, Y.; Yi, C.; Hao, F.; Hua, Y.; et al. Progress of the Key Materials for Organic Solar Cells. Sci. China Chem. 2020, 63, 758–765. [Google Scholar] [CrossRef]
- Yi, J.; Zhang, G.; Yu, H.; Yan, H. Advantages, Challenges and Molecular Design of Different Material Types Used in Organic Solar Cells. Nat. Rev. Mater. 2024, 9, 46–62. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, L.; Wei, Z. Toward Over 15% Power Conversion Efficiency for Organic Solar Cells: Current Status and Perspectives. Small Methods 2017, 1, 1700258. [Google Scholar] [CrossRef]
- Yuan, X.; Zhao, Y.; Xie, D.; Pan, L.; Liu, X.; Duan, C.; Huang, F.; Cao, Y. Polythiophenes for Organic Solar Cells with Efficiency Surpassing 17%. Joule 2022, 6, 647–661. [Google Scholar] [CrossRef]
- Zenoozi, S.; Agbolaghi, S.; Poormahdi, E.; Hashemzadeh-Gargari, M.; Mahmoudi, M. Verification of Scherrer Formula for Well-Shaped Poly(3-Hexylthiophene)-Based Conductive Single Crystals and Nanofibers and Fabrication of Photovoltaic Devices from Thin Film Coating. Macromol. Res. 2017, 25, 826–840. [Google Scholar] [CrossRef]
- Saito, M.; Fukuhara, T.; Kamimura, S.; Ichikawa, H.; Yoshida, H.; Koganezawa, T.; Ie, Y.; Tamai, Y.; Kim, H.D.; Ohkita, H.; et al. Impact of Noncovalent Sulfur–Fluorine Interaction Position on Properties, Structures, and Photovoltaic Performance in Naphthobisthiadiazole-Based Semiconducting Polymers. Adv. Eng. Mater. 2020, 10, 1903278. [Google Scholar] [CrossRef]
- Lin, Y.; Nugraha, M.I.; Firdaus, Y.; Scaccabarozzi, A.D.; Aniés, F.; Emwas, A.-H.; Yengel, E.; Zheng, X.; Liu, J.; Wahyudi, W.; et al. A Simple N-Dopant Derived from Diquat Boosts the Efficiency of Organic Solar Cells to 18.3%. ACS Energy Lett. 2020, 5, 3663–3671. [Google Scholar] [CrossRef]
- Zhao, W.; Qian, D.; Zhang, S.; Li, S.; Inganäs, O.; Gao, F.; Hou, J. Fullerene-Free Polymer Solar Cells with over 11% Efficiency and Excellent Thermal Stability. Adv. Mater. 2016, 28, 4734–4739. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, S.; Yao, H.; Zhang, S.; Zhang, Y.; Yang, B.; Hou, J. Molecular Optimization Enables over 13% Efficiency in Organic Solar Cells. J. Am. Chem. Soc. 2017, 139, 7148–7151. [Google Scholar] [CrossRef] [PubMed]
- Cotoarbă, Y.; Todor-Boer, O.; Botiz, I. Crystals of Nonfullerene Acceptors Generated by Rich Exposure to Solvent Vapors. Cryst. Growth Des. 2025, 25, 2337–2356. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, H.; Du, X.; Lin, H.; Zheng, C.; Yang, G.; Deng, M.; Xu, X.; Tao, S.; Peng, Q. Highly Electronegative Additives Suppress Energetic Disorder to Realize 19.19 % Efficiency Binary Organic Solar Cells. Nano Energy 2024, 129, 110016. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, W.; Qin, X.; Li, A.; Guo, W.; Lv, M.; He, Z.; Hua, Y.; Tang, D.; Zhang, B. Achieving 19.3%-Efficiency Binary Organic Solar Cells via Synergistically Dual-Phases Morphology Control. Chem. Eng. J. 2025, 506, 160133. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, Z.; Lu, G.; Yu, N.; Li, C.; Gao, J.; Gu, X.; Hao, X.; Lu, G.; Tang, Z.; et al. Binary Organic Solar Cells Breaking 19% via Manipulating the Vertical Component Distribution. Adv. Mater. 2022, 34, 2204718. [Google Scholar] [CrossRef]
- Chen, X.; Chen, M.; Liang, J.; Liu, H.; Xie, X.; Zhang, L.; Ma, D.; Chen, J. Polymer Donor with a Simple Skeleton and Minor Siloxane Decoration Enables 19% Efficiency of Organic Solar Cells. Adv. Mater. 2024, 36, 2313074. [Google Scholar] [CrossRef]
- Huang, T.; Zhang, Y.; Wang, J.; Cao, Z.; Geng, S.; Guan, H.; Wang, D.; Zhang, Z.; Liao, Q.; Zhang, J. Dual-Donor Organic Solar Cells with 19.13% Efficiency through Optimized Active Layer Crystallization Behavior. Nano Energy 2024, 121, 109226. [Google Scholar] [CrossRef]
- Lu, H.; Liu, W.; Ran, G.; Liang, Z.; Li, H.; Wei, N.; Wu, H.; Ma, Z.; Liu, Y.; Zhang, W.; et al. High-Pressure Fabrication of Binary Organic Solar Cells with High Molecular Weight D18 Yields Record 19.65 % Efficiency. Angew. Chem. Int. Ed. 2023, 62, e202314420. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, K.; Li, C.; Zhao, C.; Gao, C.; Zhu, L.; Bai, Q.; Xie, C.; You, P.; Lv, J.; et al. A Novel Upside-Down Thermal Annealing Method Toward High-Quality Active Layers Enables Organic Solar Cells with Efficiency Approaching 20%. Adv. Mater. 2024, 36, 2411957. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Bi, P.; Chen, Z.; Qiao, J.; Li, J.; Wang, W.; Zheng, Z.; Zhang, S.; Hao, X.; et al. Binary Organic Solar Cells with 19.2% Efficiency Enabled by Solid Additive. Adv. Mater. 2023, 35, 2301583. [Google Scholar] [CrossRef]
- Shi, W.; Han, Q.; Zhao, W.; Wang, R.; Li, L.; Song, G.; Chen, X.; Long, G.; Yao, Z.; Lu, Y.; et al. A Large Conjugated Rigid Dimer Acceptor Enables 20.19% Efficiency in Organic Solar Cells. Energy Environ. Sci. 2025, 18, 5356–5364. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Z.; Bi, P.; Chen, Z.; Wang, Y.; Liu, X.; Zhang, S.; Hao, X.; Zhang, M.; Li, Y.; et al. Tandem Organic Solar Cells with 20.6% Efficiency Enabled by Reduced Voltage Losses. Natl. Sci. Rev. 2023, 10, nwad085. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Heeger, A.J. Charge Separation and Photovoltaic Conversion in Polymer Composites with Internal Donor/Acceptor Heterojunctions. J. Appl. Phys. 1995, 78, 4510–4515. [Google Scholar] [CrossRef]
- Tang, Y.; McNeill, C.R. All-Polymer Solar Cells Utilizing Low Band Gap Polymers as Donor and Acceptor. J. Polym. Sci. B Polym. Phys. 2013, 51, 403–409. [Google Scholar] [CrossRef]
- Mori, D.; Benten, H.; Okada, I.; Ohkita, H.; Ito, S. Highly Efficient Charge-Carrier Generation and Collection in Polymer/Polymer Blend Solar Cells with a Power Conversion Efficiency of 5.7%. Energy Environ. Sci. 2014, 7, 2939–2943. [Google Scholar] [CrossRef]
- Fan, B.; Ying, L.; Zhu, P.; Pan, F.; Liu, F.; Chen, J.; Huang, F.; Cao, Y. All-Polymer Solar Cells Based on a Conjugated Polymer Containing Siloxane-Functionalized Side Chains with Efficiency over 10%. Adv. Mater. 2017, 29, 1703906. [Google Scholar] [CrossRef]
- Li, Z.; Ying, L.; Zhu, P.; Zhong, W.; Li, N.; Liu, F.; Huang, F.; Cao, Y. A Generic Green Solvent Concept Boosting the Power Conversion Efficiency of All-Polymer Solar Cells to 11%. Energy Environ. Sci. 2019, 12, 157–163. [Google Scholar] [CrossRef]
- Wang, G.; Melkonyan, F.S.; Facchetti, A.; Marks, T.J. All-Polymer Solar Cells: Recent Progress, Challenges, and Prospects. Angew. Chem. Int. Ed. 2019, 58, 4129–4142. [Google Scholar] [CrossRef]
- Zhao, R.; Liu, J.; Wang, L. Polymer Acceptors Containing B←N Units for Organic Photovoltaics. Acc. Chem. Res. 2020, 53, 1557–1567. [Google Scholar] [CrossRef]
- Fan, Q.; An, Q.; Lin, Y.; Xia, Y.; Li, Q.; Zhang, M.; Su, W.; Peng, W.; Zhang, C.; Liu, F.; et al. Over 14% Efficiency All-Polymer Solar Cells Enabled by a Low Bandgap Polymer Acceptor with Low Energy Loss and Efficient Charge Separation. Energy Environ. Sci. 2020, 13, 5017–5027. [Google Scholar] [CrossRef]
- Jia, T.; Zhang, J.; Zhong, W.; Liang, Y.; Zhang, K.; Dong, S.; Ying, L.; Liu, F.; Wang, X.; Huang, F.; et al. 14.4% Efficiency All-Polymer Solar Cell with Broad Absorption and Low Energy Loss Enabled by a Novel Polymer Acceptor. Nano Energy 2020, 72, 104718. [Google Scholar] [CrossRef]
- Zhang, L.; Jia, T.; Pan, L.; Wu, B.; Wang, Z.; Gao, K.; Liu, F.; Duan, C.; Huang, F.; Cao, Y. 15.4% Efficiency All-Polymer Solar Cells. Sci. China Chem. 2021, 64, 408–412. [Google Scholar] [CrossRef]
- Fu, H.; Li, Y.; Yu, J.; Wu, Z.; Fan, Q.; Lin, F.; Woo, H.Y.; Gao, F.; Zhu, Z.; Jen, A.K.-Y. High Efficiency (15.8%) All-Polymer Solar Cells Enabled by a Regioregular Narrow Bandgap Polymer Acceptor. J. Am. Chem. Soc. 2021, 143, 2665–2670. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Liu, B.; Ma, Y.; Lee, J.-W.; Yang, J.; Wang, J.; Li, Y.; Li, B.; Feng, K.; Shi, Y.; et al. Regioregular Narrow-Bandgap n-Type Polymers with High Electron Mobility Enabling Highly Efficient All-Polymer Solar Cells. Adv. Mater. 2021, 33, 2102635. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, C.; Angunawela, I.; Meng, L.; Qin, S.; Zhou, L.; Li, S.; Zhuo, H.; Yang, G.; Zhang, Z.-G.; et al. 16.52% Efficiency All-Polymer Solar Cells with High Tolerance of the Photoactive Layer Thickness. Adv. Mater. 2022, 34, 2108749. [Google Scholar] [CrossRef]
- Ma, R.; Zhou, K.; Sun, Y.; Liu, T.; Kan, Y.; Xiao, Y.; Dela Peña, T.A.; Li, Y.; Zou, X.; Xing, Z.; et al. Achieving High Efficiency and Well-Kept Ductility in Ternary All-Polymer Organic Photovoltaic Blends Thanks to Two Well Miscible Donors. Matter 2022, 5, 725–734. [Google Scholar] [CrossRef]
- Zeng, R.; Zhu, L.; Zhang, M.; Zhong, W.; Zhou, G.; Zhuang, J.; Hao, T.; Zhou, Z.; Zhou, L.; Hartmann, N.; et al. All-Polymer Organic Solar Cells with Nano-to-Micron Hierarchical Morphology and Large Light Receiving Angle. Nat. Commun. 2023, 14, 4148. [Google Scholar] [CrossRef]
- Deng, M.; Xu, X.; Qiu, W.; Duan, Y.; Li, R.; Yu, L.; Peng, Q. Improving Miscibility of Polymer Donor and Polymer Acceptor by Reducing Chain Entanglement for Realizing 18.64 % Efficiency All Polymer Solar Cells. Angew. Chem. Int. Ed. 2024, 63, e202405243. [Google Scholar] [CrossRef]
- Wu, P.; Duan, Y.; Li, Y.; Xu, X.; Li, R.; Yu, L.; Peng, Q. 18.6% Efficiency All-Polymer Solar Cells Enabled by a Wide Bandgap Polymer Donor Based on Benzo[1,2-d:4,5-D′]Bisthiazole. Adv. Mater. 2024, 36, 2306990. [Google Scholar] [CrossRef]
- Wang, L.; Chen, C.; Gan, Z.; Liu, C.; Guo, C.; Xia, W.; Sun, W.; Cheng, J.; Sun, Y.; Zhou, J.; et al. Optimizing the Power Conversion Processes in Diluted Donor/Acceptor Heterojunctions towards 19.4% Efficiency All-Polymer Solar Cells. J. Energy Chem. 2024, 96, 345–350. [Google Scholar] [CrossRef]
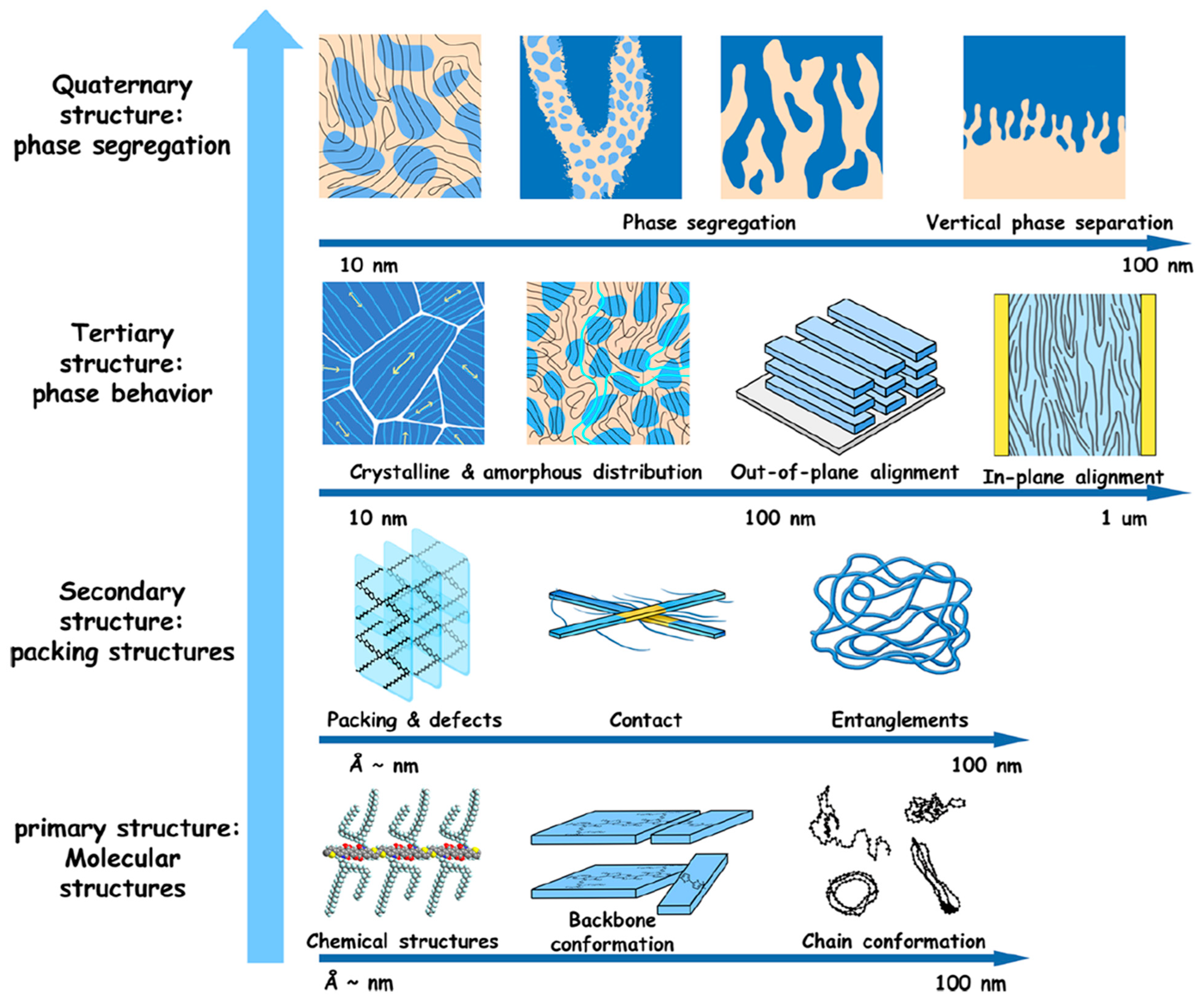
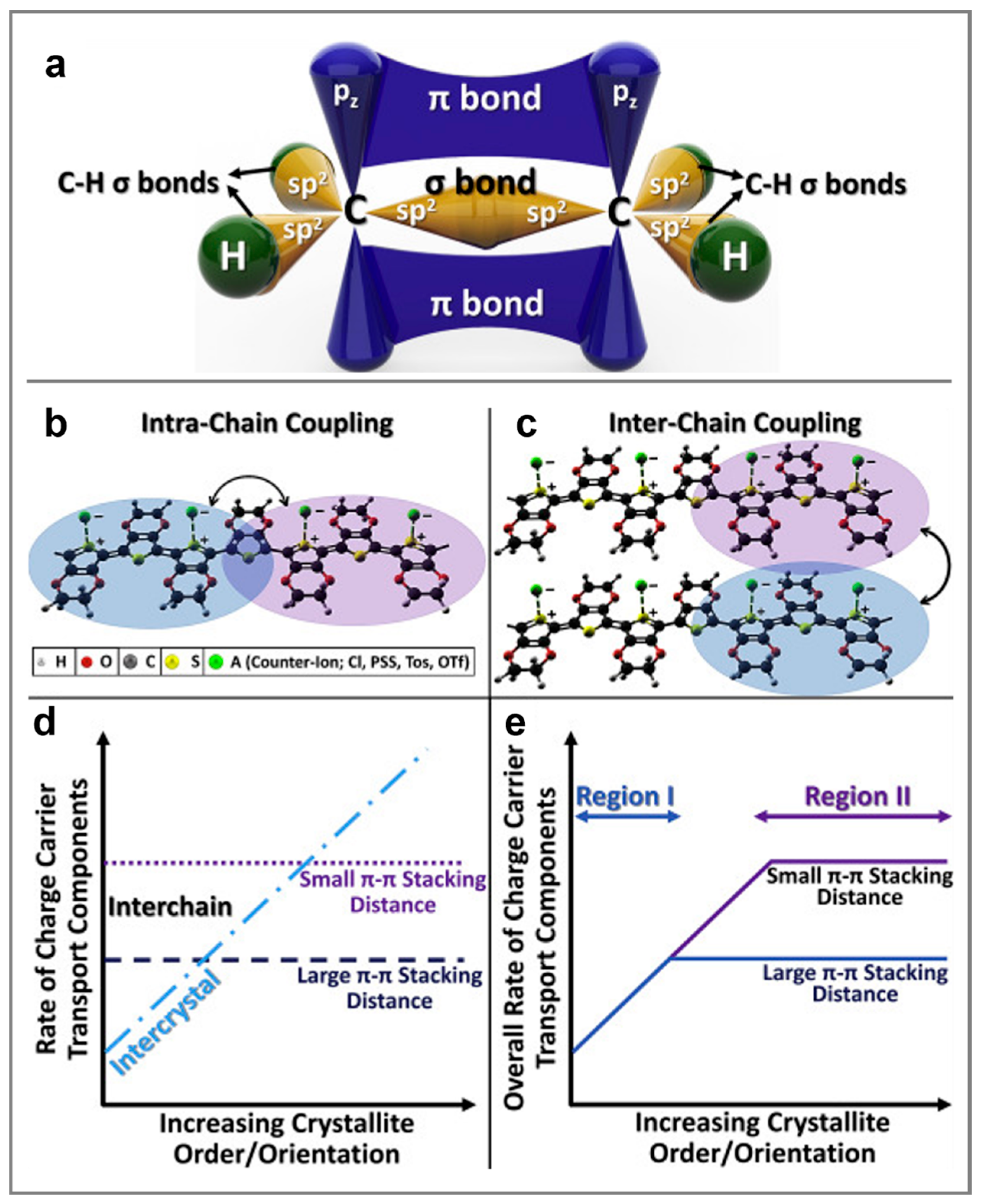

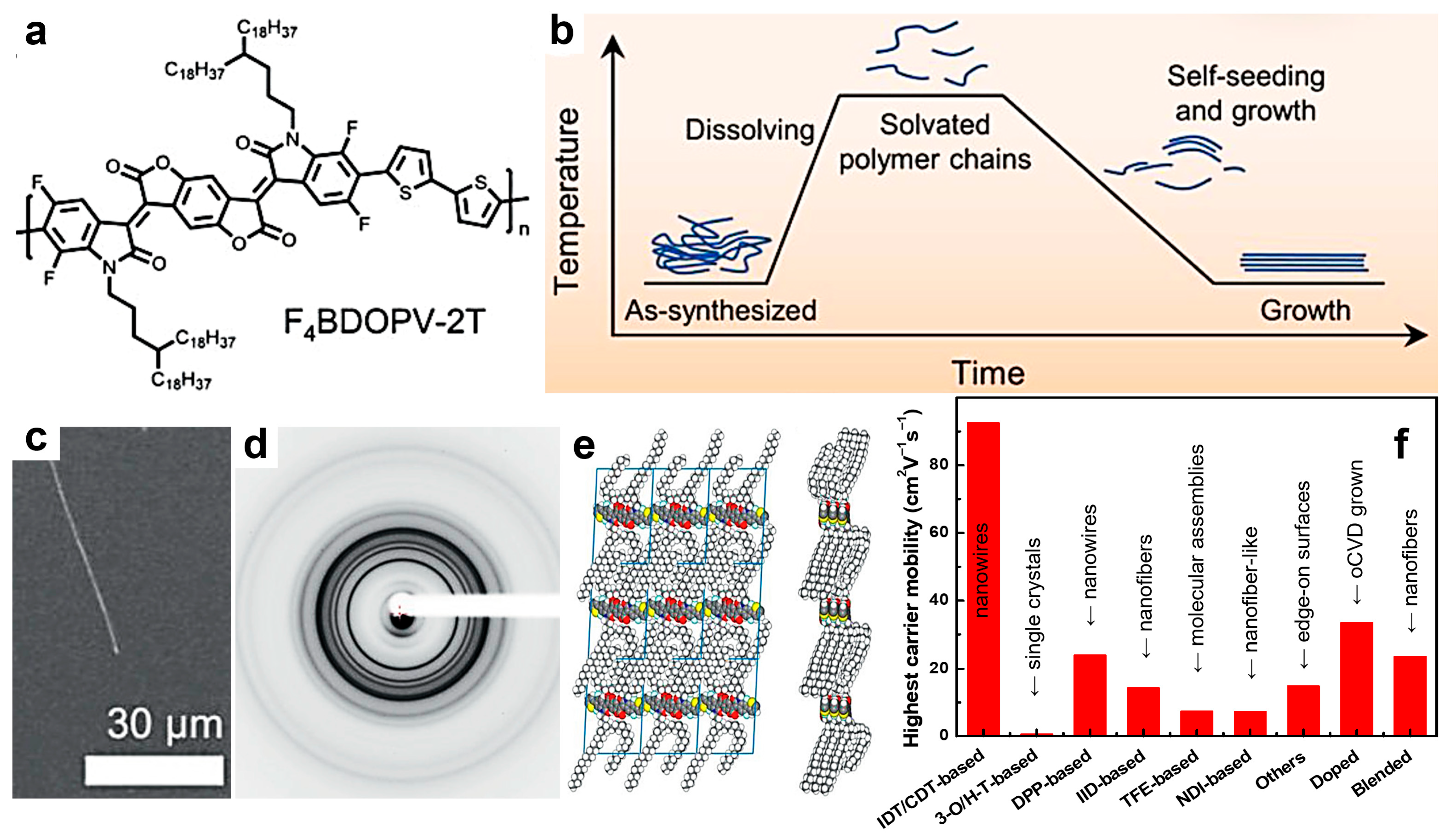

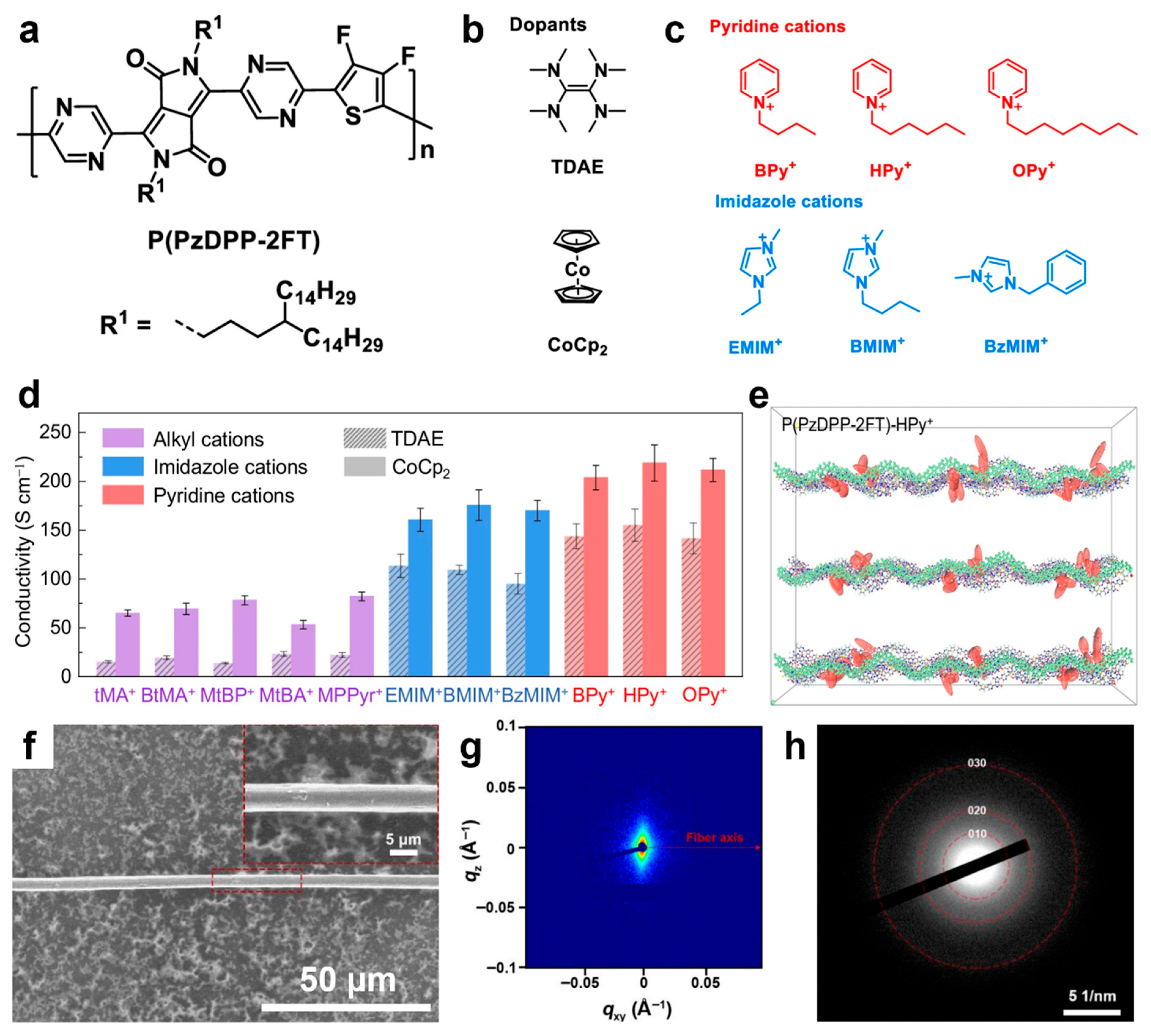
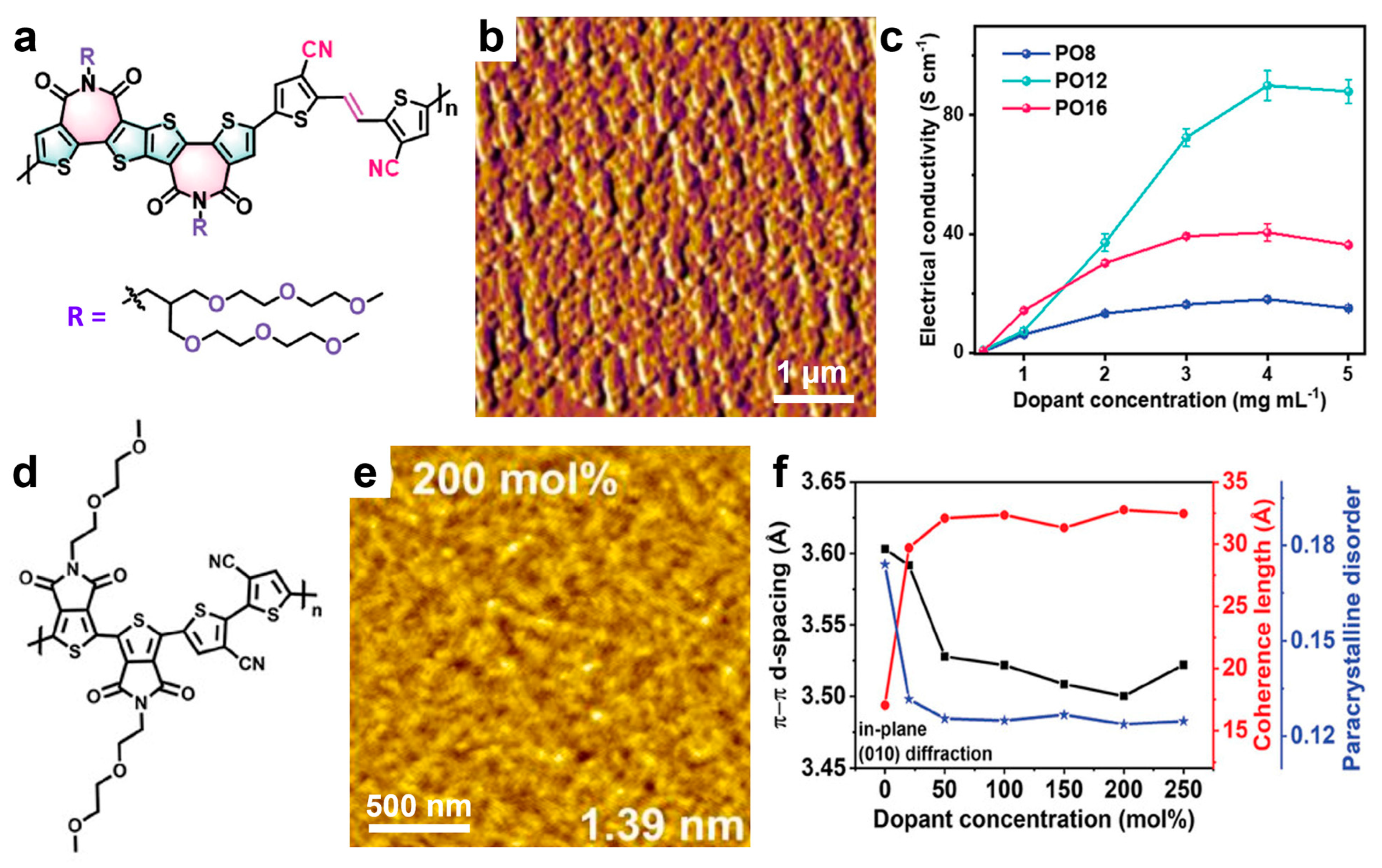

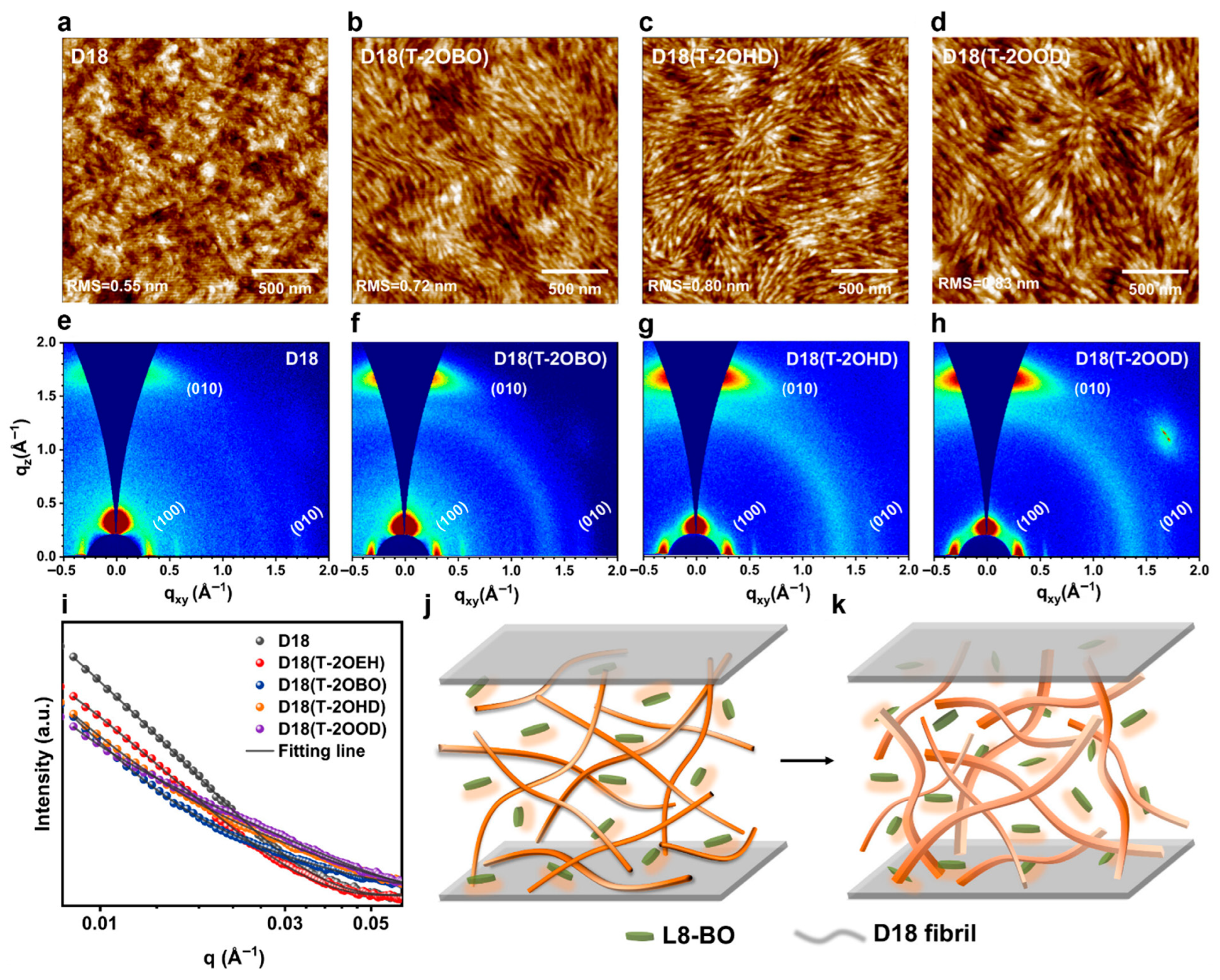
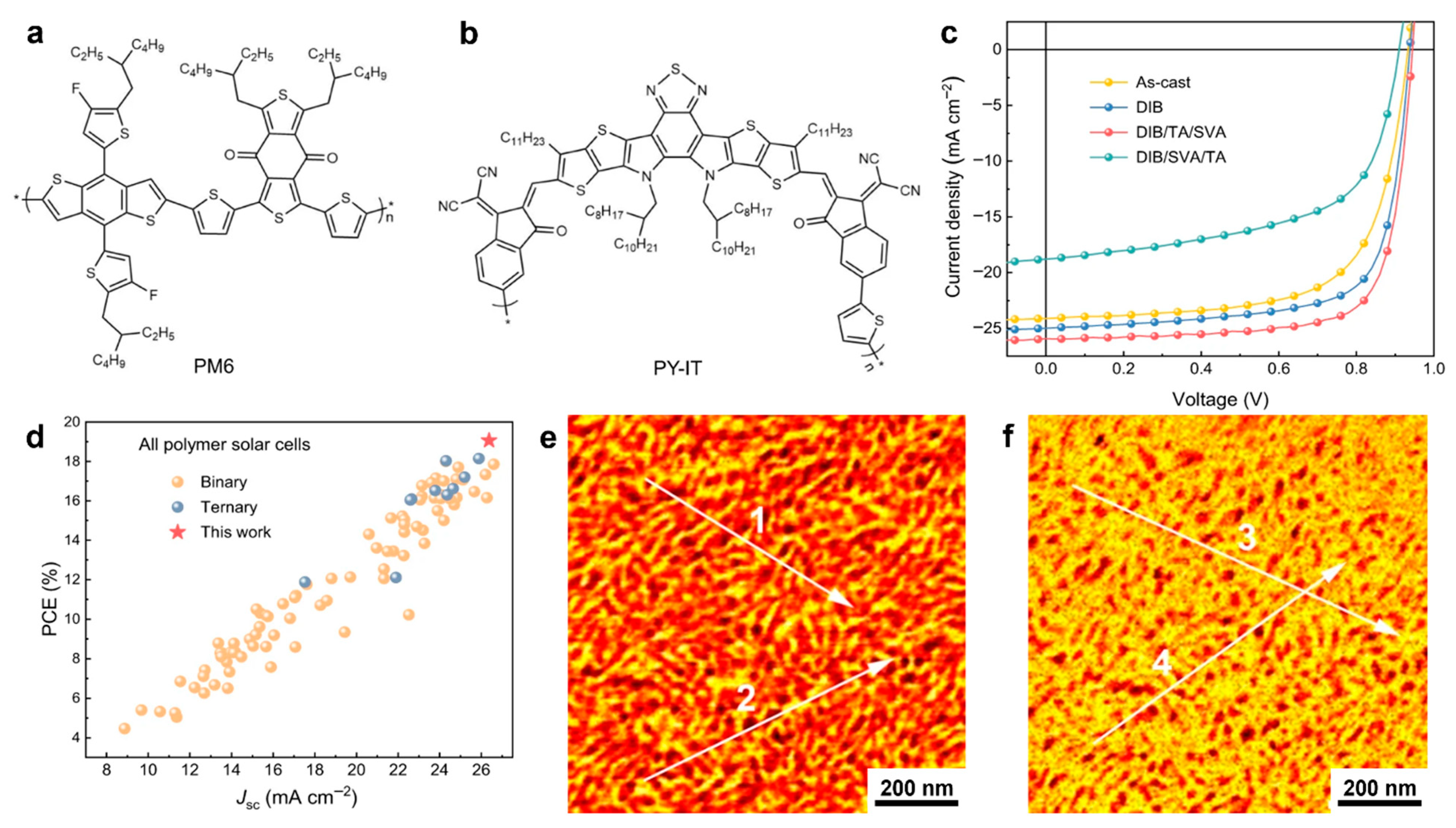
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pop, M.; Botiz, I. Carrier Mobility, Electrical Conductivity, and Photovoltaic Properties of Ordered Nanostructures Assembled from Semiconducting Polymers. Materials 2025, 18, 4580. https://doi.org/10.3390/ma18194580
Pop M, Botiz I. Carrier Mobility, Electrical Conductivity, and Photovoltaic Properties of Ordered Nanostructures Assembled from Semiconducting Polymers. Materials. 2025; 18(19):4580. https://doi.org/10.3390/ma18194580
Chicago/Turabian StylePop, Maria, and Ioan Botiz. 2025. "Carrier Mobility, Electrical Conductivity, and Photovoltaic Properties of Ordered Nanostructures Assembled from Semiconducting Polymers" Materials 18, no. 19: 4580. https://doi.org/10.3390/ma18194580
APA StylePop, M., & Botiz, I. (2025). Carrier Mobility, Electrical Conductivity, and Photovoltaic Properties of Ordered Nanostructures Assembled from Semiconducting Polymers. Materials, 18(19), 4580. https://doi.org/10.3390/ma18194580







