Study on Electrochemical Corrosion Behavior of Plasma Sprayed Al2O3-3%TiO2 Coatings Doped with CeO2 for Long-Term Immersion
Abstract
1. Introduction
2. Experimental
Electrochemical Test
3. Results and Discussion
3.1. SEM
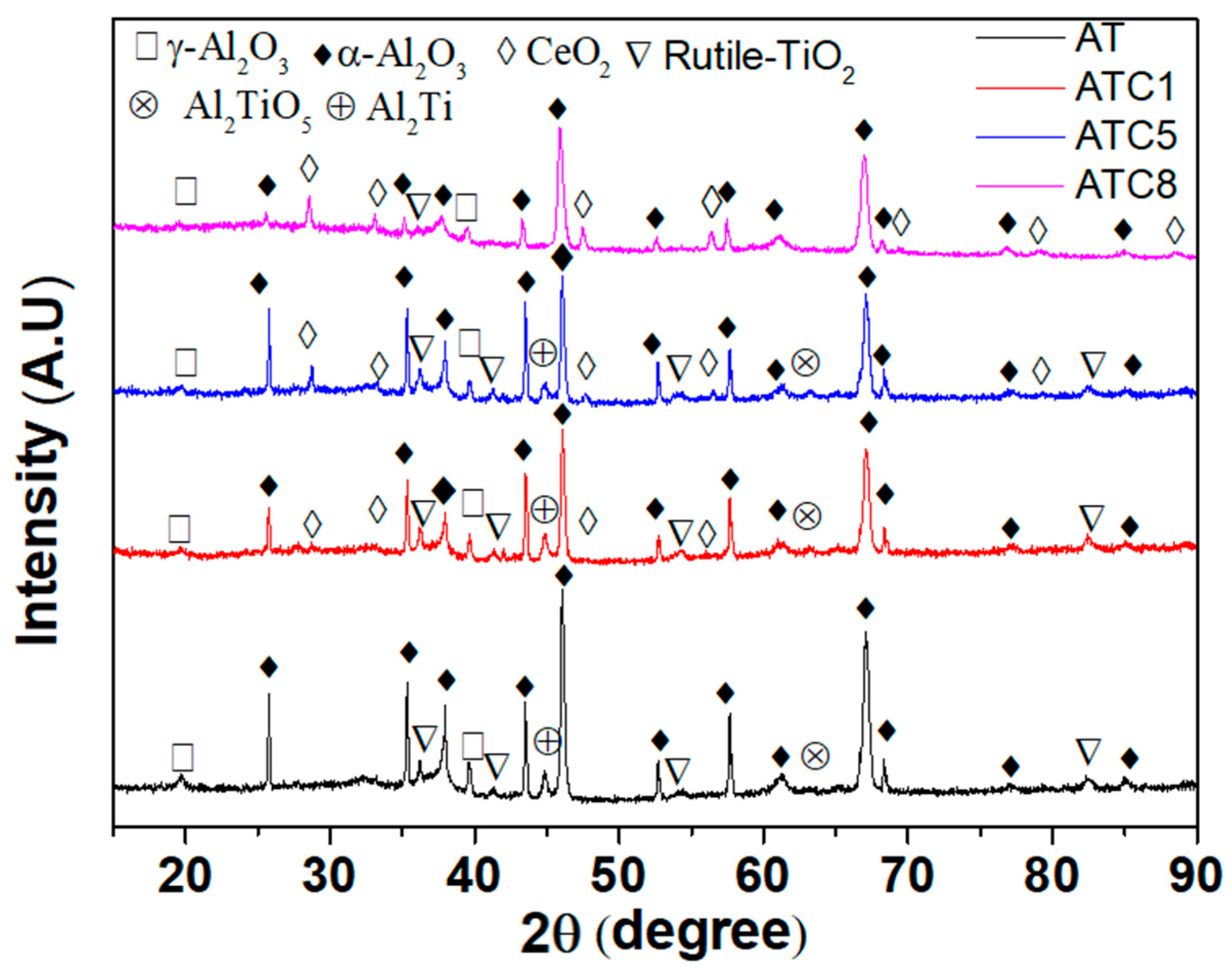
3.2. XRD Patterns
3.3. XPS
3.4. Open Circuit Potential Curve of Long-Term Immersion
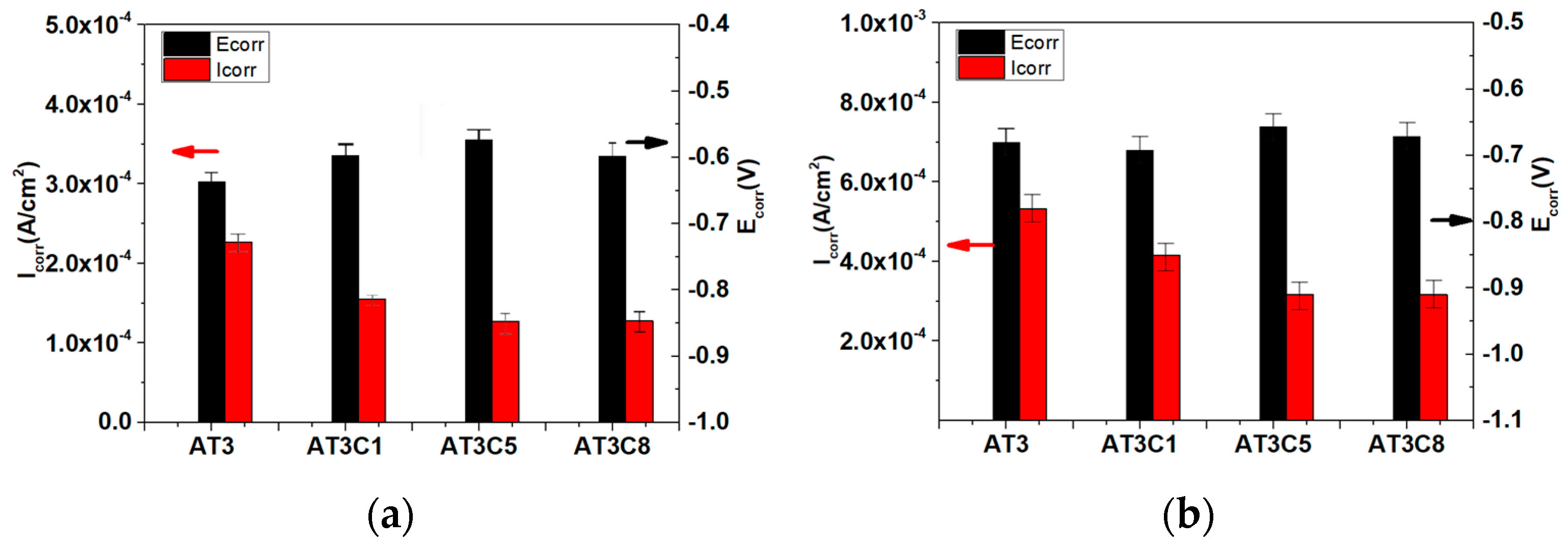
3.5. Potentiodynamic Polarization
3.6. EIS
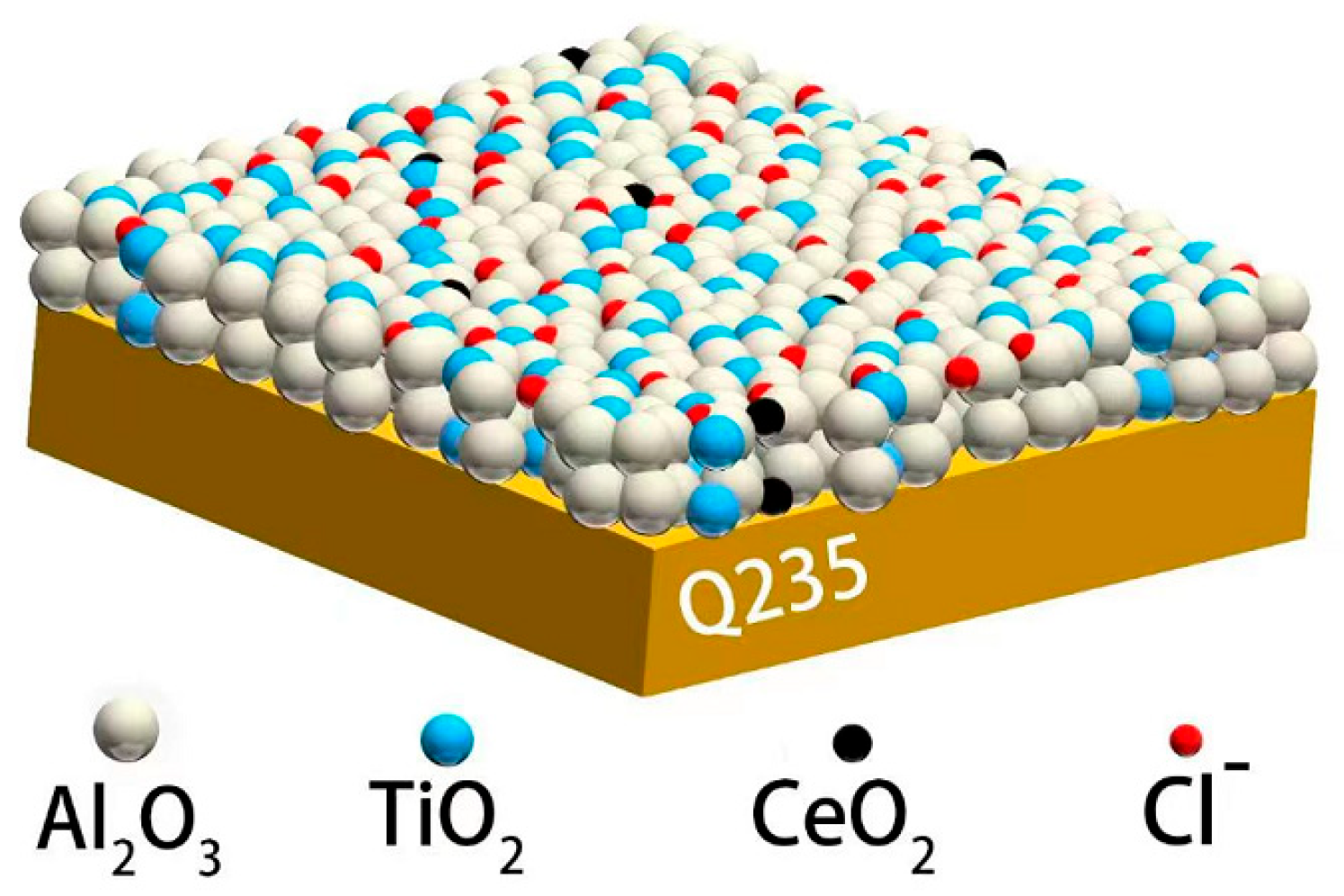
3.7. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tian, W.; Wang, Y.; Zhang, T.; Yang, Y. Sliding wear and electrochemical corrosion behavior of plasma sprayed nanocomposite Al2O3-13%TiO2 Coatings. Mater. Chem. Phys. 2009, 118, 37–45. [Google Scholar] [CrossRef]
- Fervel, V.; Normand, B.; Coddet, C. Tribological behavior of plasma sprayed Al2O3-based cermet coatings. Wear 1999, 230, 70–77. [Google Scholar] [CrossRef]
- Lin, X.H.; Zeng, Y.; Lee, S.W.; Ding, C. Charatcrization of alumina-3wt% titania coating prepared by plasma spraying of nanostructured powder. J. Eur. Ceram. Soc. 2014, 24, 627–634. [Google Scholar] [CrossRef]
- Zavareh, M.A.; Sarhan, A.A.D.M.; Karimzadeh, R.; Singh, R.S.A. Analysis of corrosion protection behavior of Al2O3-TiO2 oxide ceramic coating on carbon steel pipes for petroleum industry. Ceram. Int. 2018, 44, 5967–5975. [Google Scholar] [CrossRef]
- Martucci, A.; Innocenzi, P.; Traversa, E. Crystallization of Al2O3-TiO2 sol-gel systems. J. Ceram. Soc. Jpn. 1999, 107, 891–894. [Google Scholar] [CrossRef]
- Huang, J.; Cho, Y.; Zhang, Z.; Jan, A.; Wong, K.T.; Nemani, S.D.; Yieh, E.; Kummel, A.C. Selective pulsed chemical vapor deposition of water-free TiO2/Al2O3 and HfO2/Al2O3 nanolaminates on Si and SiO2 in preference to SiCOH. ACS Appl. Mater. Interfaces 2022, 14, 15716–15727. [Google Scholar] [CrossRef] [PubMed]
- Tomaszek, R.; Pawlowski, L.; Zdanowski, J.; Grimblot, J.; Laureyns, J. Microstructural transformations of TiO2, Al2O3 + 13TiO2 and Al2O3 + 40TiO2 at plasma spraying and laser engraving. Surf. Coat. Technol. 2004, 185, 137–149. [Google Scholar] [CrossRef]
- Bannier, E.; Vicent, M.; Rayón, E.; Benavente, R.; Salvador, M.D.; Sánchez, E. Effect of TiO2 addition on the microstructure and nanomechanical properties of Al2O3 Suspension Plasma Sprayed coatings. Appl. Surf. Sci. 2014, 316, 141–146. [Google Scholar] [CrossRef]
- Song, R.G. Hydrogen permeation resistance of plasma-sprayed Al2O3 and Al2O3-13 wt% TiO2 ceramic coatings on austenitic stainless steel. Surf. Coat. Technol. 2003, 168, 191–194. [Google Scholar] [CrossRef]
- Singh, H.; Sidhu, B.S.; Puri, D.; Prakash, S. Use of plasma spray technology for deposition of high temperature oxidation/corrosion resistant coatings—A review. Mater. Corros. 2007, 58, 92–102. [Google Scholar] [CrossRef]
- Pan, X.; Niu, Y.; Liu, T.; Zhong, X.; Li, C.; Shi, M.; Zheng, X.; Ding, C. Ablation behaviors of ZrC-TiC coatings prepared by vacuum plasma spray: Above 2000 °C. J. Eur. Ceram. Soc. 2019, 39, 3292–3300. [Google Scholar] [CrossRef]
- Celik, E.; Ozdemir, I.; Avci, E.; Tsunekawa, Y. Corrosion behaviour of plasma sprayed coatings. Surf. Coat. Technol. 2005, 193, 297–302. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, W.; Zhang, T.; Yang, Y. Microstructure, spallation and corrosion of plasma sprayed Al2O3–13%TiO2 coatings. Corros. Sci. 2009, 51, 2924–2931. [Google Scholar] [CrossRef]
- Zhang, J.; He, J.; Dong, Y.; Li, X.; Yan, D. Microstructurecharacteristics of Al2O3-13wt% TiO2 coating plasma spray deposited with nanocrystalline powders. J. Mater. Process. Tech. 2008, 197, 31–35. [Google Scholar] [CrossRef]
- Kim, H.-J.; Odoul, S.; Lee, C.-H.; Kweon, Y.-G. The electrical insulation behavior and sealing effects of plasma-sprayed alumina–titania coatings. Surf. Coat. Technol. 2001, 140, 293–301. [Google Scholar] [CrossRef]
- Zeng, L.; Li, L.M.; Li, Q. Effect of CeO2 on microstructure and properties of plasma sprayed Al2O3-13wt% TiO2 coating. J. Fujian Univ. Technol. 2011, 6, 521–525. [Google Scholar]
- Liu, Y.; Gou, G.Q.; Wang, X.M.; Jia, Q.; Chen, H.; Tu, M.J. Effects of rare earth elements on the microstructure and mechanical properties of HVOF-sprayed WC-Co coatings. J. Therm. Spray Technol. 2014, 23, 1225–1237. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, S.; Zhao, L.; Yang, C.; Wu, L.; Guo, Y.; Ang, A.S.M. Corrosion behavior of plasma-sprayed Al2O3-3%TiO2 coatings doped with CeO2. J. Therm. Spray Technol. 2022, 32, 290–305. [Google Scholar] [CrossRef]
- Çelik, E.; Şengil, I.; Avcı, E. Effects of some parameters on corrosion behaviour of plasma-sprayed coatings. Surf. Coat. Technol. 1997, 97, 355–360. [Google Scholar] [CrossRef]
- Harju, M.; Järn, M.; Dahlsten, P.; Rosenholm, J.B.; Mäntylä, T. Influence of long-term aqueous exposure on surface properties of plasma sprayed oxides Al2O3, TiO2 and their mixture Al2O3–13TiO2. Appl. Surf. Sci. 2008, 254, 7272–7279. [Google Scholar] [CrossRef]
- Ding, Q.J.; Zhao, G. Effects of CeO2 on the microstructure and tribological properties of atmospheric plasma-sprayed Cr2O3-TiO2 coatings. Ind. Lubr. Tribol. 2020, 72, 341–347. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, S.; Wang, M.; Wang, S.; Xiao, T.; Strutt, P.R. Abrasive wear characteristics of plasma sprayed nanostructured alumina/titania coatings. Wear 2000, 237, 176–185. [Google Scholar] [CrossRef]
- Tao, Z.; Xun, C.; Shunxing, W.; Shian, Z. Effect of CeO2 on microstructure and corrosive wear behavior of laser-cladded Ni/WC coating. Thin Solid Films 2000, 379, 128–132. [Google Scholar] [CrossRef]
- He, L.; Tan, Y.; Wang, X.; Xu, T.; Hong, X. Microstructure and wear properties of Al2O3-CeO2/Ni-base alloy composite coatings on aluminum alloys by plasma spray. Appl. Surf. Sci. 2014, 314, 760–767. [Google Scholar] [CrossRef]
- Holgado, J.P.; Alvarez, R.; Munuera, G. Study of CeO2 XPS spectra by factor analysis: Reduction of CeO2. Appl. Surf. Sci. 2000, 161, 301–315. [Google Scholar] [CrossRef]
- Bagus, P.; Nelin, C.; Ilton, E.; Baron, M.; Abbott, H.; Primorac, E.; Kuhlenbeck, H.; Shaikhutdinov, S.; Freund, H.-J. The complex core level spectra of CeO2: An analysis in terms of atomic and charge transfer effects. Chem. Phys. Lett. 2010, 487, 237–240. [Google Scholar] [CrossRef]
- Wenjuan, L.; Fahe, C.; Linrong, C.; Zhao, Z.; Jianqing, Z. Effect of rare earth element Ce and La on corrosion behavior of AM60 magnesium alloy. Corros. Sci. 2009, 51, 1334–1343. [Google Scholar] [CrossRef]
- Arrabal, R.; Matykina, E.; Viejo, F.; Skeldon, P.; Thompson, G. Corrosion resistance of WE43 and AZ91D magnesium alloys with phosphate PEO coatings. Corros. Sci. 2008, 50, 1744–1752. [Google Scholar] [CrossRef]
- Duan, H.; Du, K.; Yan, C.; Wang, F. Electrochemical corrosion behavior of composite coatings of sealed MAO film on magnesium alloy AZ91D. Electrochim. Acta 2006, 51, 2898–2908. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, W.; Xue, Q. Study on lubricating mechanisms of La(OH)3 nanocluster modified by compound containing nitrogen in liquid paraffin. Wear 1998, 218, 139–144. [Google Scholar] [CrossRef]









| Specimens | AT3/wt% | CeO2/wt%(Add as Powder) | Ce/wt% |
|---|---|---|---|
| 1 | 100 | 0 | 0 |
| 2 | 99 | 1 | 0.81 |
| 3 | 95 | 5 | 3.87 |
| 4 | 92 | 8 | 6.03 |
| Voltage/V | Electric Current/A | Spray Distance/mm | Ar Flow Rate/(L·min−1) | H2 Flow Rate/(L·min−1) | Powder Feeding/(g·min−1) |
|---|---|---|---|---|---|
| 27 | 580 | 100 | 45 | 12 | 30 |
| As Prepare | After Immersion | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Peak | Binding Energy (eV) | Intensity (a.u) | FWHM (eV) | Valence State | Peak | Binding Energy (eV) | Intensity (a.u) | FWHM (eV) | Valence State | |
| AT3 + CeO2 1% Coating | 1 | 881.92 | 7109.97 | 3.75 | Ce4+ | 1 | 881.71 | 389.85 | 0.89 | Ce4+ |
| 2 | 899.74 | 3437.79 | 4.07 | Ce4+ | 2 | 885.46 | 817.29 | 2.83 | Ce3+ | |
| 3 | 904.14 | 2461.44 | 3.95 | Ce4+ | 3 | 904.01 | 84.32 | 1 | Ce4+ | |
| 4 | 916.65 | 2196.46 | 3.2 | Ce4+ | - | - | - | - | - | |
| AT3 + CeO2 5% Coating | 1 | 883.01 | 29,485.89 | 4.45 | Ce4+ | 1 | 881.71 | 3731.99 | 3.71 | Ce4+ |
| 2 | 898.26 | 9825.02 | 2 | Ce4+ | 2 | 885.23 | 5326.55 | 4.43 | Ce3+ | |
| 3 | 901.05 | 14,137.62 | 4.06 | Ce4+ | 3 | 900.07 | 4005.15 | 4.86 | Ce4+ | |
| 4 | 916.49 | 7491.32 | 1.88 | Ce4+ | 4 | 904.13 | 4776.94 | 6.29 | Ce4+/Ce3+ | |
| AT3 + CeO2 8% Coating | 1 | 883.03 | 29,914.54 | 4.52 | Ce4+ | 1 | 882.02 | 4514.66 | 1.69 | Ce4+ |
| 2 | 898.28 | 10,104.11 | 2.03 | Ce4+ | 2 | 885.44 | 10,634.82 | 2.92 | Ce3+ | |
| 3 | 901.08 | 13,584.21 | 3.95 | Ce4+ | 3 | 899.88 | 2177.49 | 2.99 | Ce4+ | |
| 4 | 916.51 | 7339.72 | 1.85 | Ce4+ | 4 | 904.34 | 33,682.54 | 9.41 | Ce4+/Ce3+ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.; Zhang, Y.; Dai, P.; Zhao, L.; Wang, X.; Yi, X. Study on Electrochemical Corrosion Behavior of Plasma Sprayed Al2O3-3%TiO2 Coatings Doped with CeO2 for Long-Term Immersion. Materials 2025, 18, 4532. https://doi.org/10.3390/ma18194532
Yan J, Zhang Y, Dai P, Zhao L, Wang X, Yi X. Study on Electrochemical Corrosion Behavior of Plasma Sprayed Al2O3-3%TiO2 Coatings Doped with CeO2 for Long-Term Immersion. Materials. 2025; 18(19):4532. https://doi.org/10.3390/ma18194532
Chicago/Turabian StyleYan, Jiahang, Yu Zhang, Pengyu Dai, Lin Zhao, Xin Wang, and Xiaohong Yi. 2025. "Study on Electrochemical Corrosion Behavior of Plasma Sprayed Al2O3-3%TiO2 Coatings Doped with CeO2 for Long-Term Immersion" Materials 18, no. 19: 4532. https://doi.org/10.3390/ma18194532
APA StyleYan, J., Zhang, Y., Dai, P., Zhao, L., Wang, X., & Yi, X. (2025). Study on Electrochemical Corrosion Behavior of Plasma Sprayed Al2O3-3%TiO2 Coatings Doped with CeO2 for Long-Term Immersion. Materials, 18(19), 4532. https://doi.org/10.3390/ma18194532







