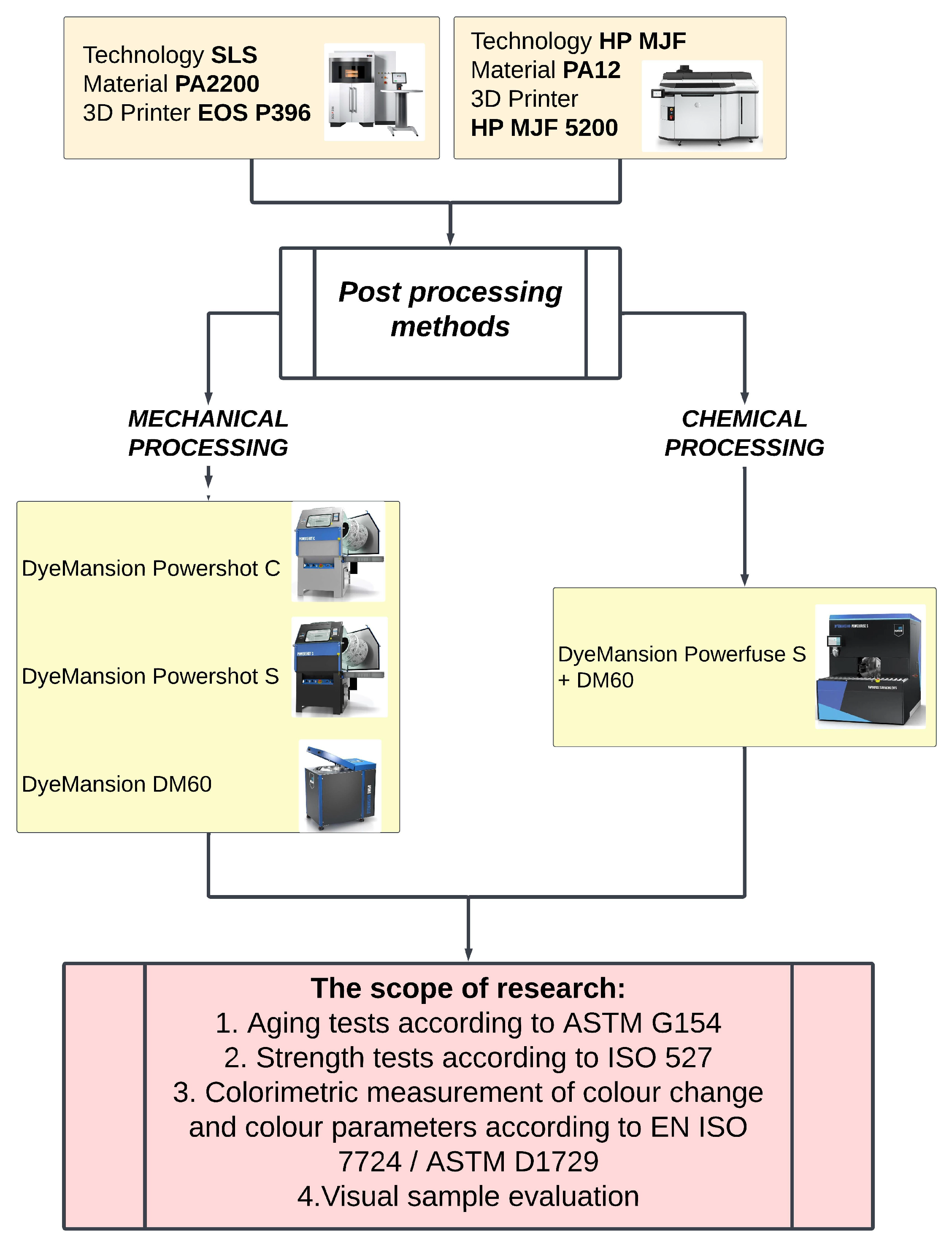
1. Introduction
Three-dimensional printing, also known as additive manufacturing (AM), is a rapidly developing technology that has revolutionized many sectors, including the biomedical industry [
1,
2,
3,
4,
5,
6,
7,
8]. In medical applications, 3D printing offers several unique advantages, including the ability to produce customized parts quickly and cost-effectively, as well as the integration of different functions and materials into a single structure [
9,
10,
11,
12].
In 3D printing, biocompatible products are produced from various polymers, including polymethyl methacrylate (PMMA), thermoplastic polyurethane (TPU) [
13] processed using Fused Filament Fabrication (FFF) technology [
14], silicone using Liquid 3D Printing technology [
15], Bio-Med Clear 3D resins [
16] in stereolithography (SLA) [
17], and polyamides PA11 and PA12 and TPU [
18] in selective laser sintering (SLS) [
19] and Multi Jet Fusion (MJF) [
20] technologies. Key application areas include implants and prostheses, dentistry (crowns, bridges, diagnostic models, surgical guides, orthodontic trays, and retainers), the production of surgical instruments and orthoses [
21,
22,
23,
24,
25,
26], cranioplasty implants [
27], and wearable biosensors [
28] and smart wristbands [
29,
30].
The particular value of 3D printing in medicine is the ability to customize the device to the patient’s anatomy, which significantly affects the effectiveness of therapy, comfort of use, and recovery time. The technology also enables the design and production of complex geometries while maintaining cost-effectiveness for low-volume production and custom devices [
31,
32,
33,
34,
35,
36].
One of the most commonly used materials in medical 3D printing is polyamide 12 (PA12) [
37], which is particularly suitable for Selective Laser Sintering (SLS) [
38,
39,
40,
41,
42] and HP Multi Jet Fusion (MJF) [
43,
44] technologies. Its popularity is due to its favorable mechanical properties, low density, good dimensional stability, low moisture absorption (for a polyamide), and easy availability [
45,
46]. The leading manufacturers of 3D printers that use SLS technology include EOS [
47], Sinterit [
48], Formlabs [
49], and 3D Systems [
50]. HP is the leader in MJF technology [
51]. PA12 is the most commonly used powder in both technologies due to its ease of processing, relatively low price, and high mechanical strength [
52]. The PA12 material-designated PA2200, which is used in EOS devices, is certified for biocompatibility according to EN ISO 10993 [
53]. In contrast, the PA12 used by HP is certified according to ISO 10993, as well as FDA and USP Class I-VI [
54].
A rough and matte surface characterizes parts made of PA12 printed by SLS or MJF, which have a porous structure [
43]. Although they already exhibit good mechanical properties in their raw state, many medical applications require additional smoothing and refinement of the surface. Smoothness, uniformity, and surface integrity are critical due to the need to maintain hygiene, minimize the risk of irritation, and reduce bacterial colonization [
55]. Post-processing also enhances aesthetics (e.g., achieving uniform color, gloss) and functional properties (e.g., UV and moisture resistance, and tightness). The most commonly used methods include staining, chemical or mechanical smoothing, and coating. Companies such as DyeMansion [
56] and AMT [
57] specialize in automating these processes, offering industrial solutions with ISO 10993 biocompatibility certification [
53] for PA12 materials used in EOS and HP printers.
Polyamide 12, like most polyamide plastics, is susceptible to ageing due to environmental factors. In the context of medical devices, it is essential to understand how prolonged exposure to UV light, elevated temperatures, and humidity affects the mechanical properties and durability of 3D-printed products made from PA12 [
58,
59]. An additional consideration is the issue of reusing finished medical devices. Although many are designed to be disposable, such as personalized surgical guides, reuse is considered, especially when they are expensive or used by the same patient. In this case, it is essential to determine the impact of repeated use and sterilization on their durability and safety. According to ISO 14971: 2019 [
60], the manufacturer is required to analyze risks at each stage of the device’s life cycle, including those associated with reuse, especially if the device was initially designed as a single-use device. In the context of Europe’s ageing population, a significant increase in osteoporotic fractures is anticipated, resulting in a greater demand for customized orthopedic solutions [
61,
62,
63]. Due to its manufacturing flexibility and ability to tailor devices to individual patients, 3D printing can play a crucial role in delivering personalized care. In this context, it is essential to incorporate the principles of sustainability—Reduce, Reuse, Recycle [
64]—in the assessment of material sustainability and the potential reuse of medical components. Medical devices printed from PA12 can be exposed to cyclical changes in humidity, temperature, UV radiation, or repeated heating, even if they are not continuously exposed to external conditions. In the case of devices not subjected to high mechanical stress, such as orthoses used outdoors, they can be expected to be maintained for several years [
65,
66]. Meanwhile, the current state of knowledge focuses mainly on the ageing of PA12 powder used in the 3D printing process, including its repeated use, heating in the working chamber, and deterioration of the properties of subsequent prints [
67]. This phenomenon manifests itself as an increase in melt viscosity, a decrease in sintering ability, and a reduction in the mechanical quality of the products. However, the effects of environmental ageing on the properties of finished parts have been insufficiently analyzed, especially considering post-processing such as coloring, mechanical smoothing, or chemical smoothing, including their impact on mechanical properties (e.g., tensile strength). Meanwhile, a complete understanding of the production chain—from design, 3D printing, and post-processing to clinical use—is crucial to ensure the quality and safety of medical devices in accordance with current standards and regulations. The existing literature on polyamide PA12 in SLS and MJF technologies mainly concentrates on powders and process parameters; comparisons between SLS and MJF, e.g., Cai [
68] and Sillani [
69], primarily address the influence of powder ageing and the properties of freshly printed parts, neglecting the long-term ageing of finished, post-processed products. A few exceptions, such as D’Andrea et al. [
70], examine the physical ageing of completed MJF parts after 90 days, noting decreases in yield strength and fatigue life, as well as greater variability in results. It is also understood that powder ageing in SLS diminishes the mechanical properties of finished parts (∼15% reduction in tensile strength, ∼20% in bending strength) due to increased viscosity/molecular weight and poorer sintering [
71]. The work of Gruber [
72] suggests that additional powder processing (e.g., spheroidization) may alter the functionality of the final component. Simultaneously, there is a lack of data on the long-term effects of mechanical processing combined with coloring and chemical smoothing on the mechanical and color stability (ΔE) of PA12 parts. Aside from reports on annealing [
73], no studies explore the actual service life cycles of medical components.
Despite the increasing use of additive manufacturing technologies in medicine, there is still a lack of comprehensive data on the impact of ageing on the efficacy and safety of PA12 components [
74]. Another challenge is the development of a consistent process for the design of a personalized medical device, taking into account the diagnosis of the clinical problem, the selection of the 3D printing material and technology, the choice of finishing method, design taking biomechanics into account, validation through strength analysis and digital twin, and finally, market implementation. This publication presents the results of a study on the mechanical properties of PA12 manufactured by the SLS and HP MJF methods, including DyeMansion post-processing techniques. This research aims to determine the durability of components intended for use in a medical environment over up to 12 months. Additionally, an integrated workflow for producing a medical device is presented, utilizing the Industry 4.0 concept. The developed research results can be applied to evaluate the durability of a medical device.
3. Results and Discussion
3.1. Ageing and Tensile Test Results and Discussion
In this study, the effect of the ageing process on the mechanical properties of polyamide PA12 printed using SLS (EOS PA2200) and MJF (HP High Reusability PA12) technologies was assessed.
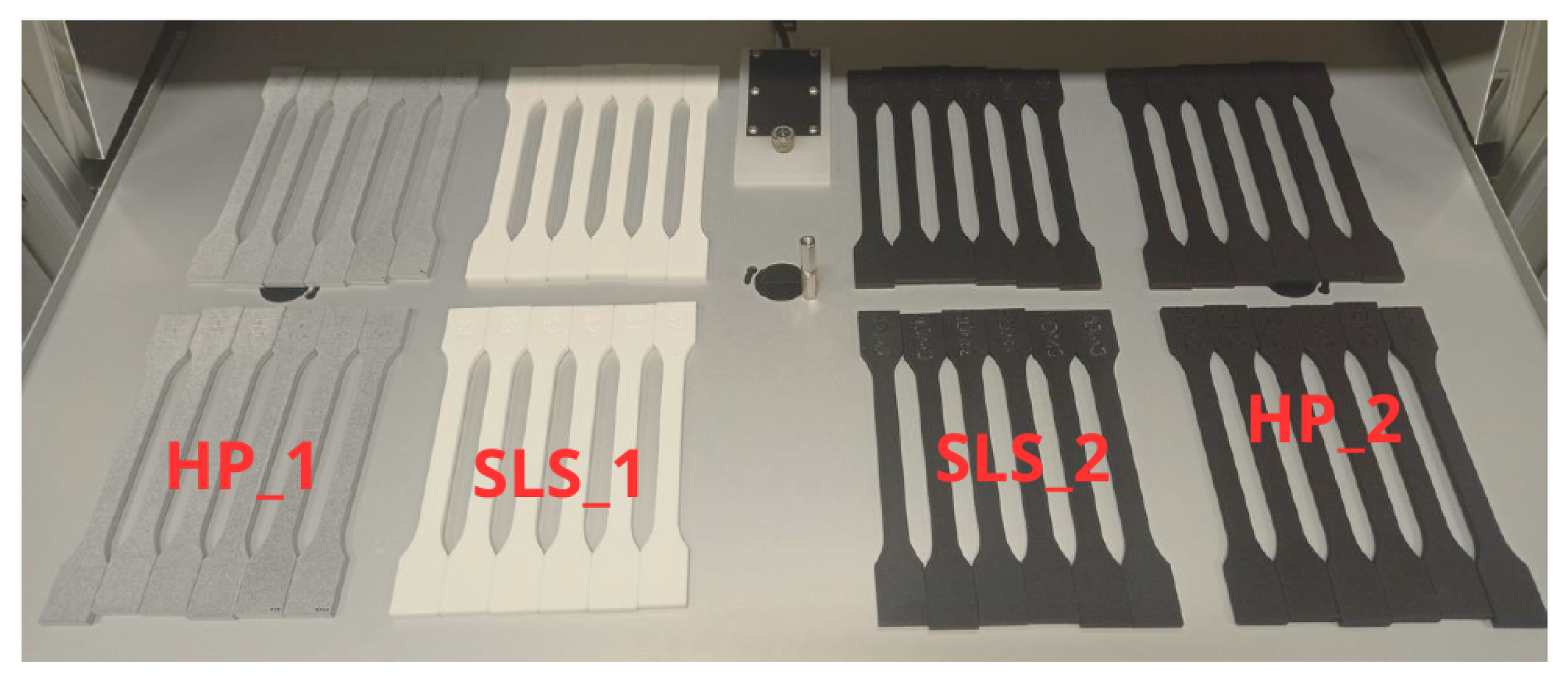
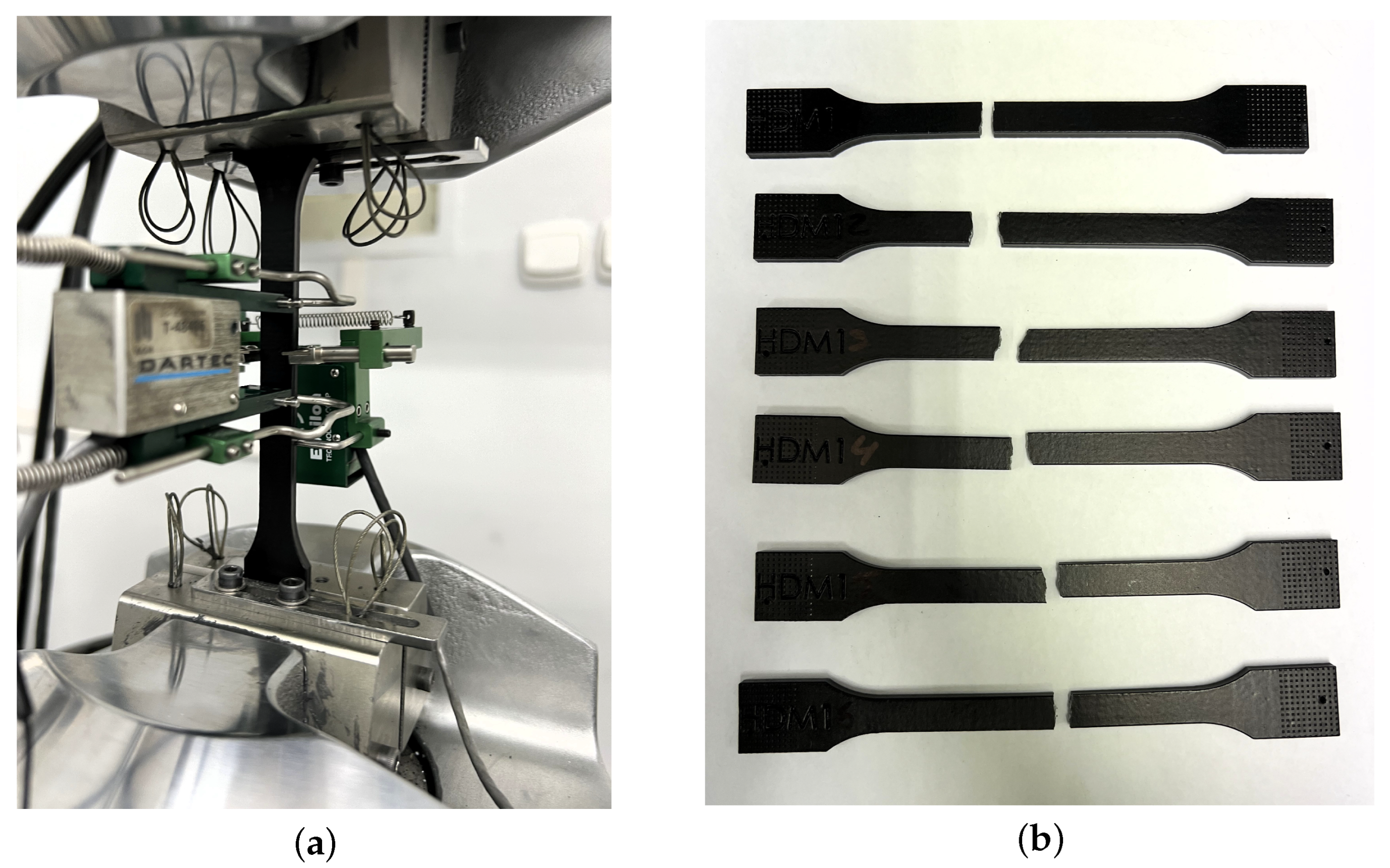
Figure 5 shows the tensile test performed and the specimens from the Powerfuse S chemical post-processing. The specimens were tested at three time points: 0, 6, and 12 months of service. Three surface post-processing options were considered: (1) Powershot C + Powershot S, (2) Powershot C + DM60 + Powershot S, and (3) Powershot C + DM60 + Powershot S + Powerfuse S. Tensile strength (σ), Young’s modulus (E), and elongation at break (ε) were analyzed. The results of the tensile strength parameters for the aged specimens from the tensile tests are shown in
Table 6,
Table 7 and
Table 8.
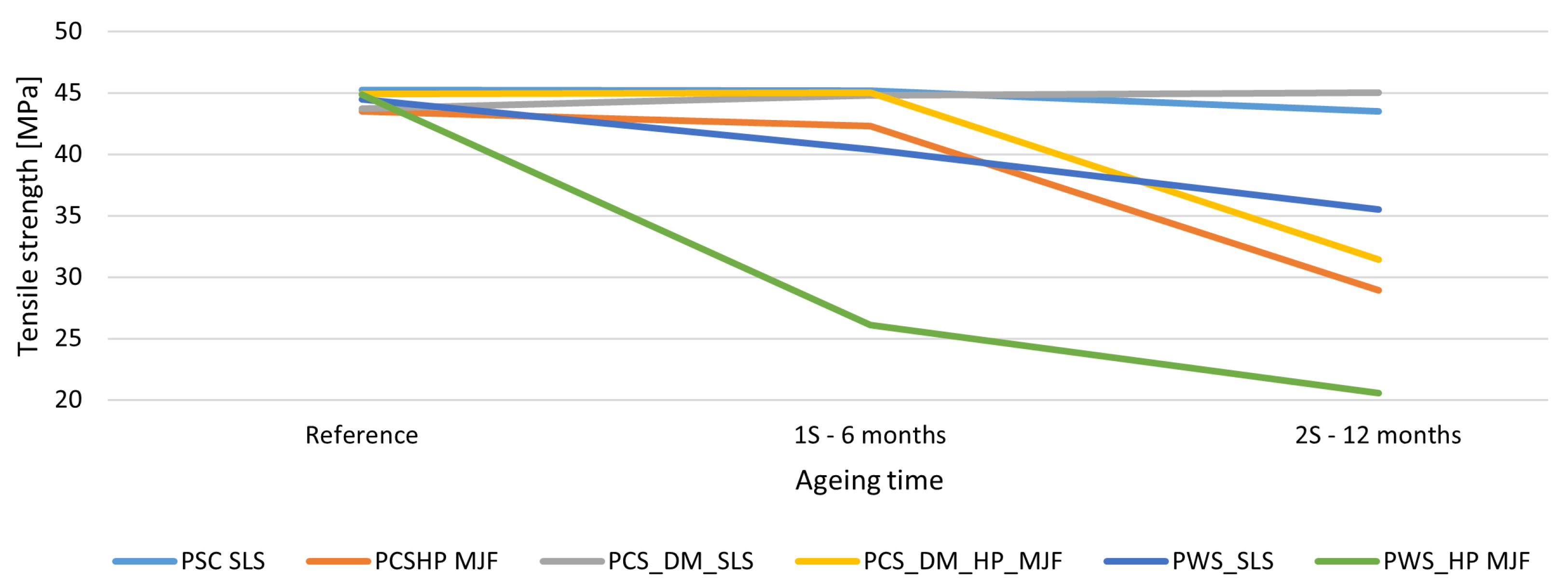
The biggest differences between the technologies were noted in terms of the loss of mechanical properties due to the ageing process. In the SLS technology for PA2200, the tensile strength remained stable over time for the dyed variant (DM60), with a slight increase in tensile strength to 45 MPa after 12 months. In contrast, for the MJF samples with HP High Reusability PA12, a substantial decrease in tensile strength was observed, as much as 54% for the variant with chemical smoothing in Powerfuse S. These trends are consistent with the results of Puttonen [
101], who reported a ∼40% decrease in strength after accelerated ageing of PA12 under UV conditions.
Studies by Machotova [
102], Wahab [
103], and Weinmann [
71] indicate that PA12 parts manufactured using SLS technology exhibit a tensile strength of 43–46 MPa from virgin powder without post-processing. However, ageing and repeated recycling of the powder lead to significant decreases–even below 10 MPa after repeated use. In the case of MJF technology, the initial values are comparable or higher, at approximately 49 MPa (without finishing). More recent studies show that, here as well, powder ageing and physical ageing processes can reduce strength to around 30 MPa, emphasizing the importance of material quality control and the material’s life cycle.
For SLS technology, the loss of strength was minimal across all variants. In the case of the DM60 + S variant, an increase in strength was even noted from 44.0 to 45.0 MPa. The largest reduction among SLS samples was observed in the Powershot C + DM60 + S + Powerfuse S variant from 44.5 to 35.5 MPa, although it remained lower than that in MJF technology. MJF samples exhibited a significant decrease in strength after just 6 months, especially following chemical smoothing from 44.9 to 20.5 MPa after 12 months. The Powershot C+S variant also experienced a notable decline from 44.9 to 28.9 MPa. Components produced using SLS technology demonstrate higher mechanical stability over time, whereas MJF appears highly susceptible to degradation, particularly after chemical smoothing. The effect of the ageing process on tensile strength is shown in
Figure 6.
Young’s modulus (
E) remained relatively stable. Changes did not exceed 20% and were in line with the observations of Ghimouz [
46], who showed little dependence of Young’s modulus on ageing conditions. The increase in stiffness in some samples (e.g., MJF+DM60) may be a result of the material’s co-crystallization, as also noted in the work of Gazzerro [
104] in the context of regranulated PA12 powder. Regarding SLS technology (without post-processing), Young’s modulus of PA12 parts reaches 1600–2000 MPa, but with ageing and powder recycling, a notable decrease occurs, limiting long-term mechanical stability. For MJF technology (without post-processing), Young’s modulus values are comparable or higher (around 1700 MPa), but studies indicate that physical ageing and secondary crystallization can lower the modulus to approximately 1450 MPa, emphasizing the importance of controlling processing and usage. The effect of the ageing process on Young’s modulus is shown in
Figure 7.
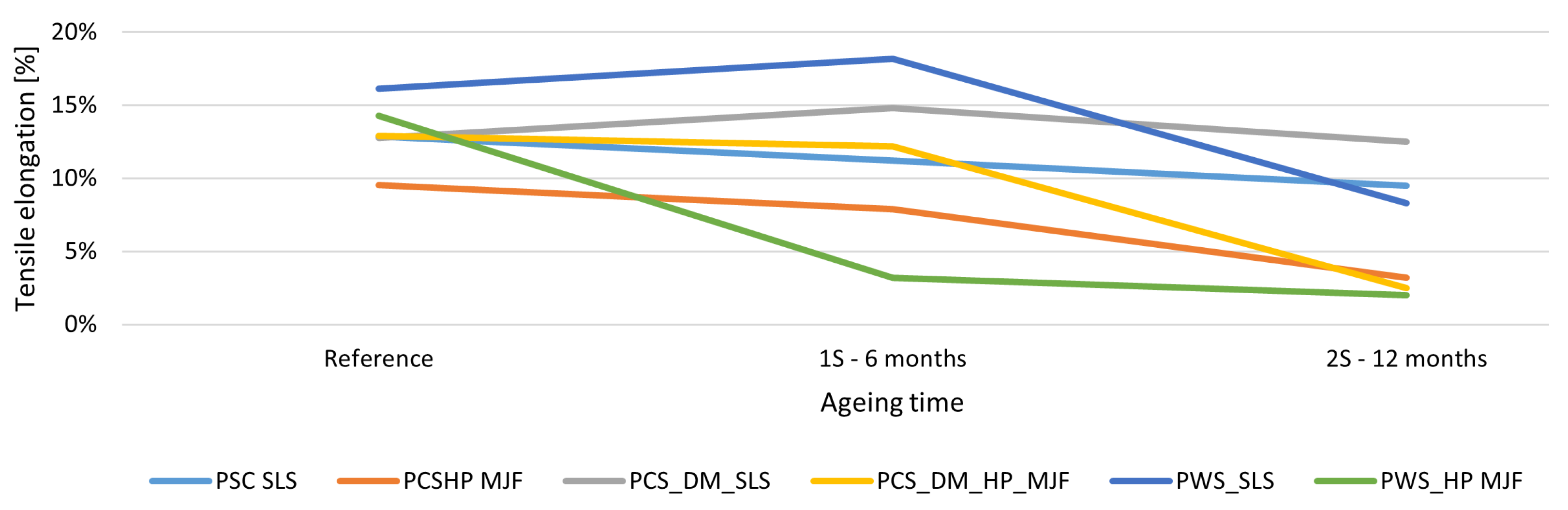
The elongation at break showed the most incredible sensitivity to ageing. MJF samples only achieved
elongation after 12 months, implying a loss of up to 85% ductility. SLS samples, on the other hand, retained high ductility—especially the colored variants, for which the maximum elongation value remained at
. Puttonen [
101] and Ghimouz [
46] confirmed that elongation was the most severely degraded parameter due to environmental ageing. The surface finishes used had a significant effect on mechanical durability. DM60 staining had a stabilizing effect, particularly with SLS technology, probably forming a barrier against UV and oxygen. In contrast, chemical smoothing improved initial aesthetics and ductility, but led to faster degradation—especially in MJF technology. Rosso [
105], Fiorillo [
106], and Zakręcki [
52] showed that MJF is more sensitive to property degradation, which explains the observations of a significant decrease in maximum elongation at break and maximum tensile strength. Furthermore, PA12 parts produced using SLS technology (without post-processing) exhibit relatively low elongation at break (5–9%), which decreases further due to powder ageing, although an unusual increase in this parameter has been observed under certain thermal conditions. In MJF technology (without post-processing), the elongation at break is considerably higher (15–20%) and may even increase during physical ageing (to over 30%), indicating better interlayer bonding and greater flexibility of the material compared with SLS. The effect of the ageing process on elongation is shown in
Figure 8.
In summary, PA12 printed with SLS technology demonstrates greater resistance to ageing, and the surface finish, achieved through industrial coloring, helps maintain mechanical properties. The MJF technology, although beneficial for production, necessitates additional protective measures for long-term use.
3.2. Color Measurement Results
In the experiments carried out, the color changes of the samples were measured in the CIE L*a*b* system as a difference in color change,
, from the baseline (0 h). The resulting
values for the individual samples (after 375 h and 750 h of exposure) are shown in
Figure 9. Minimal color changes (
) were observed in samples subjected solely to cleaning post-processing (Powershot C). For both the white SLS print and the grey HP MJF print, there was a slight color change (
–
) after 750 h of exposure. This indicates that the base material without additional dyeing maintains good color stability over the studied period. Such small
color changes suggest that the differences are nearly invisible to the eye. Therefore, the cleaning of the Powershot C did not introduce any factors that accelerate color degradation, and the materials themselves showed no significant tendency to discolor over approximately 750 h (roughly 12 months of simulated exposure).
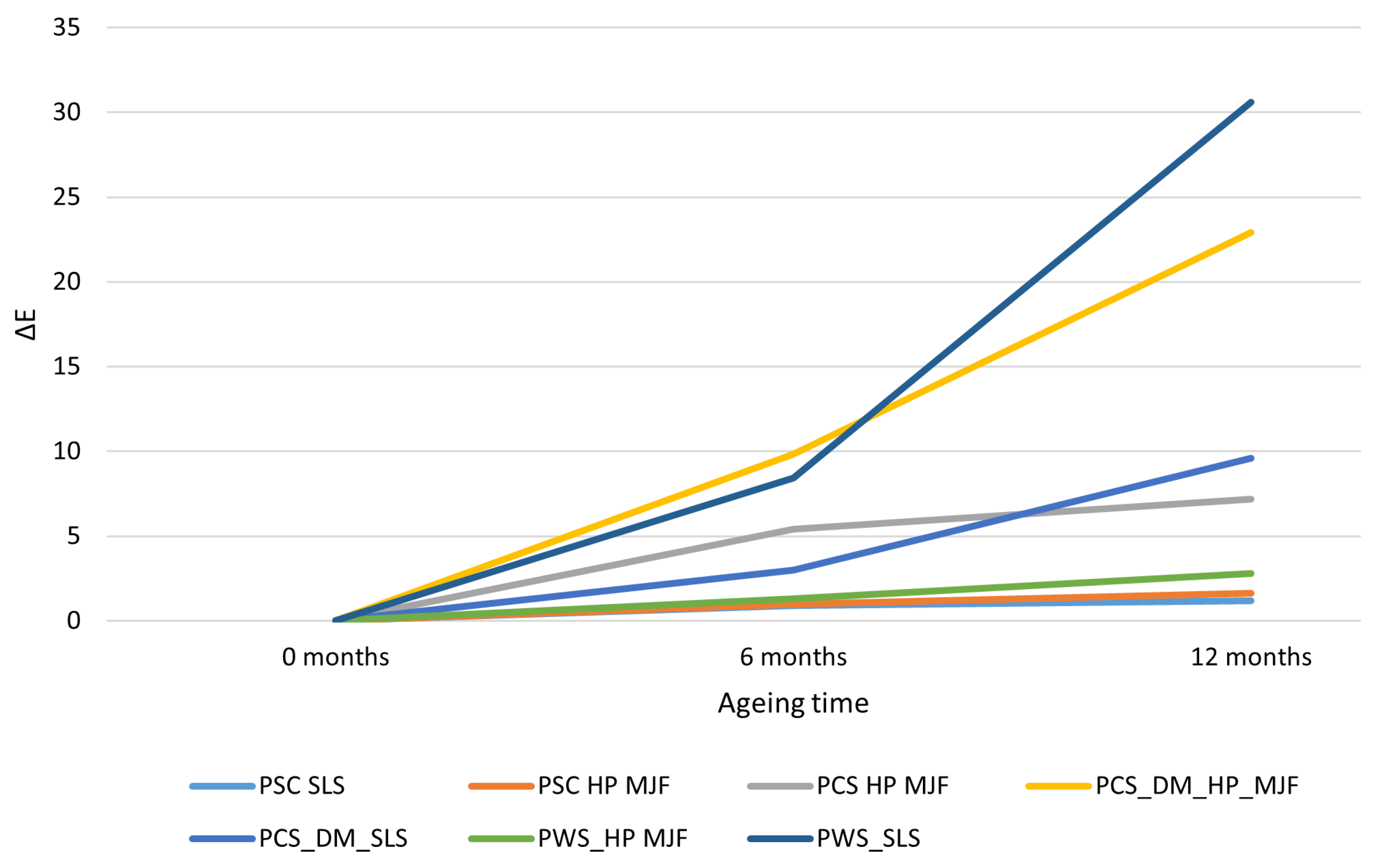
The graph shown in
Figure 9 illustrates the impact of ageing over a 6-month and 12-month reference period, depending on the production method based on 3D printing technology and the finishing method employed, in accordance with the variants presented in
Table 5.
In contrast, notable color changes occurred in samples stained with DyeMansion DM60 following mechanical processing in Powershot S. After just 375 h, significant differences were apparent—particularly for the HP-derived MJF sample (
)—while the SLS sample showed a
of approximately 3. By 750 h, both materials had notably faded as the dye degraded under UV light. The MJF sample experienced a greater color change (
) compared with the SLS sample (
), indicating that initial color intensity and material composition influence the rate of fading. The initially darker or colored MJF component likely lost more color, while the SLS sample benefited from slightly better dye penetration, which slowed the overall discoloration. Despite the manufacturer’s claims of high UV resistance for the DeepDye dyes, results demonstrate that, after long-term exposure (12 months), a noticeable loss of color still occurs, as shown in
Figure 10.
The most significant changes were observed in samples subjected to chemical smoothing using the Powerfuse S method, especially those derived from SLS technology. The SLS sample reached after 750 ageing hours, indicating significant darkening of the entire surface. The chemical smoothing process may have further influenced the progression of this phenomenon. The black MJF material is less susceptible to noticeable discoloration—probably owing to the pigment content acting as a UV stabilizer and masking any yellowing with the dark base color. In other words, the dark shade of the HP MJF material “hides” minor color changes, while the white SLS clearly reveals even slight discoloration.
The effect of exposure time is clearly observable in all cases, as indicated by the increases in between 375 h and 750 h, which suggest ongoing ageing processes. For samples that initially showed only minor changes, further exposure did not produce a significant visual difference. Conversely, samples with moderate changes after 375 h experienced rapid color deterioration up to 750 h. For instance, the dyed MJF more than doubled (from approximately 10 to 23), and the chemically smoothed SLS increased about 3.5 times (from roughly 8.4 to 30.6). This illustrates that color degradation can accelerate—initially, the material loses some color, but over time, photochemical mechanisms such as further dye decomposition or polymer oxidation lead to progressively greater changes. Practically, this means that assessing color fastness after a shorter period (e.g., 6 months of ageing) may not fully reveal the more severe deteriorations observed after longer exposure.
3.3. Three-Factor ANOVA Analysis
A three-factor ANOVA confirmed the significant influence of all factors considered—3D printing technology, post-processing variant, and ageing time—on the mechanical properties of PA12. The F-statistic values were very high, with corresponding significance levels of
for the main effects of each variable (technology, post-processing, ageing time) and most interactions. Partial
indicated a strong practical effect of printing technology, type of post-processing, and ageing time on tensile strength. Similarly, for Young’s modulus (
E), the post-processing factor was the most significant contributor to explaining the variation, while for elongation at break, printing technology and ageing time dominated. The assumptions of ANOVA were met—the distributions within the groups did not deviate significantly from normality, and the variances could be considered homogeneous, which legitimizes the use of a linear ANOVA model.
Table 9 and
Table 10 show the result of the statistical ANOVA analysis.
Interactions between factors were also found to be significant, indicating that the effect of one factor depended on the level of another. From an engineering research perspective, the most important relationships are the following:
- 1.
Technology × Ageing process—properties deteriorated significantly more over time for HP MJF samples than for SLS.
- 2.
Technology × Post-processing method—the effectiveness of individual treatments in maintaining mechanical parameters differed between SLS and MJF.
- 3.
Post-processing × Ageing process—the rate of property degradation depended on the type of surface finish.
The three-factor interaction was also statistically significant (), indicating the complex, synergistic nature of the influence of the printing process, treatment, and ageing. This makes it possible to answer the following key research questions:
- 1.
Does complete chemical smoothing (Powerfuse S) slow down the degradation of MJF samples more than SLS?
The results indicate that this is not the case; on the contrary, after 6 and 12 months, MJF samples subjected to smoothing showed a greater decrease in strength and ductility than the corresponding SLS samples (technology × time interaction for the Powerfuse S variant).
- 2.
Does chemical smoothing reduce the mechanical properties of MJF more than SLS?
Yes, the adverse effect of smoothing (reduction in strength and deformation) was stronger for MJF, especially over a long ageing period.
- 3.
Does mechanical treatment alone (Powershot C + S) protect MJF more effectively than SLS?
No, after 12 months, the mechanically finished MJF samples lost a significant amount of strength (from 43.5 to 28.9 MPa on average, a decrease of ∼34%), while the corresponding SLS samples remained almost at the same level (45.2→43.5 MPa, a decrease of only ∼4%).
- 4.
Does the addition of dyeing (DM60) improve the ageing resistance of MJF more than SLS?
No, although staining slightly reduced the degradation rate of MJF (e.g., MJF with staining dropped to 31.4 MPa instead of 28.9 MPa as for unstained samples), SLS samples with dyeing practically did not lose strength during the study period (43.7→45.0 MPa, no decrease).
3.4. Multivariate Analysis of Variance
The MANOVA analysis, applied to the correlated response variables (
,
E,
), confirmed significant changes in mechanical property profiles under all studied factors. Considering covariances between strength, stiffness, and elongation, the effects of printing technology, post-processing, and ageing time remained significant. The ageing factor was highly significant, confirming overall degradation with time. Interactions involving technology, treatment, and ageing were also significant in multivariate terms, while the technology × post-processing interaction showed a near-significant trend. The use of MANOVA reduced the risk of Type I error associated with multiple testing of separate ANOVAs and confirmed that the observed differences between SLS and MJF systems as a function of treatment and time relate to the overall mechanical property profile and not just to individual parameters, as shown in
Table 11.
3.5. Principal Component Analysis
To better understand the patterns of change of the three correlated characteristics, a principal component analysis was conducted. The first two principal components, together, explain approximately 95% of the total variance in the data, enabling the mapping of sample states in 2D space with high accuracy.
As expected, PC1 mainly combines strength and ductility, with high positive loadings for tensile strength and strain and a much smaller contribution from Young’s modulus (
E). This indicates that PC1 reflects the overall mechanical condition of the material in terms of strength and ductility—specimens with high tensile strength also tended to have high elongation (making them more ’tough’ than brittle), whereas a notable decrease in ductility accompanied a reduction in strength. PC2, on the other hand, primarily correlates with Young’s modulus (
E) under high loading conditions, with little influence from other variables—it can be seen as a measure of stiffness. Importantly, these components were nearly orthogonal, confirming that, by and large, changes in stiffness occurred independently of simultaneous changes in strength and elongation (for example, specimens could lose ductility and strength without a proportional change in Young’s modulus), as demonstrated in
Table 12.
The coordinates of each group of specimens (for each combination of technology and post-processing, at baseline and after 6 and 12 months) were plotted on a graph in the system. PC1 corresponds to strength and ductility, while PC2 corresponds to stiffness and Young’s modulus (
E), resulting in a map of the property degradation trajectories shown in
Figure 11.
Key observations are that SLS samples have short trajectories and minimally degrade, while MJF samples with long trajectories exhibit strong degradation, especially after chemical smoothing. PCA confirms the greater stability of SLS samples, which retain their original properties even after 12 months. MJF trajectories are a clear indicator of degradation—the material loses plastic and strength properties and, in some cases, becomes brittle and stiff.
3.6. Post Hoc Tests
Once the significance of the factors’ effect (ANOVA) was established, post hoc procedures were performed to identify significant differences between pairs of groups. The Tukey HSD test, which controls for type I error accumulation, was used. The results revealed all pairs of samples that were statistically different in terms of tensile strength, Young’s modulus, or strain. For example, already after 6 months, the differences in elongation at break between the MJF and SLS samples were significant across all variants (
), and after 12 months, the strength of the MJF samples also fell significantly below the values recorded for the corresponding SLS samples (
for each pair of SLS vs. MJF with the same post-processing variant). Importantly, alongside reporting
p-values, measures of effect size were also presented—partial
for overall effects and Cohen’s
d for significant differences between groups. The inclusion of effect sizes enables the practical significance of the results to be assessed. Substantial effect sizes were observed in all key comparisons. For example, the difference in strength between chemically smoothed MJF and SLS samples after 12 months is
, while the difference in elongation at break exceeds
(indicating an extremely large property divergence). Additionally, the effect of ageing itself was quantified: for the MJF samples (black variant), the decrease in ductility after 1 year reached
relative to the initial condition, whereas for the SLS samples,
(moderate effect).
Table 13 displays the results of the post hoc tests conducted.
4. Conclusions
To the authors’ knowledge, this is the first systematic experimental study directly comparing SLS and MJF PA12 components for medical devices under controlled long-term ageing conditions.
The aim of this study was to evaluate the impact of 3D printing technologies (SLS and HP MJF), post-processing methods (mechanical cleaning and smoothing—Powershot C/S, dyeing—DM60, chemical smoothing—Powerfuse S), and ageing time (0, 6, and 12 months of exposure in a climate chamber) on the mechanical and aesthetic properties of PA12 components used in medical devices. The results showed that SLS (PA2200 prints) has significantly better resistance to physical degradation over time than HP MJF (PA12 HP 3D High Reusability), particularly in terms of tensile strength and elongation at break. After 12 months, the mechanical properties of SLS remained at a level similar to the initial values (, 8–13%), while HP MJF exhibited significant deterioration (reduction in strength and increased brittleness of the material).
In terms of aesthetics, it was found that exposure time and post-processing significantly influence color stability. The greatest color fastness was observed in uncolored samples, especially dark MJF parts, which retained their color almost unchanged even after 750 h of exposure. Conversely, dyed and white samples produced via SLS showed noticeable color changes, indicating the need to use UV stabilizers or choose darker colors when designing medical devices.
Based on this, the following conclusions were drawn:
The manufacturing technology is vital for the long-term durability of PA12 components—SLS-printed parts retained most of their mechanical properties after 12 months of exposure, whereas MJF parts exhibited significant degradation and loss of plasticity.
Post-processing clearly affected the properties—Powershot C+DM60+Powershot S post-processing variant in SLS stabilized strength after ageing, while chemical smoothing (Powerfuse S) caused a short-term increase in ductility at the expense of long-term stability, especially in MJF technology.
Color stability depended on the technology and post-processing method—dark, uncolored MJF parts demonstrated the highest color fastness, while light and colored SLS parts were more vulnerable to discoloration.
The results highlight the need for UV stabilizers or darker shades in designs requiring aesthetic durability, especially in medical applications.
The proposed medical product life cycle (up to 12 months of use with a review after 6 months) ensures safety and functionality while reflecting the actual ageing resistance of the materials.
The research presented offers valuable data to support the design and selection of technologies for 3D-printed medical devices, combining mechanical and aesthetic analysis in relation to ageing processes.
The results offer practical guidance for medical device designers, emphasizing the importance of carefully choosing manufacturing and finishing technologies within the context of expected mechanical and aesthetic durability. The suggested product life cycle aligns with the test findings and ensures safety and reliability in clinical applications. Direct applications include orthoses, prostheses, and medical equipment components where it is vital to preserve functionality and aesthetics during use. Future research will explore the roughness, hardness, and wear of PA12 components and extend to other biocompatible materials such as PA11 and TPU to assess their long-term suitability for medical devices.