Click-Ready Gold Nanoparticles from Aqueous Mechanochemistry: 2-Propynylamine as a Reducing Agent and Surface Ligand
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
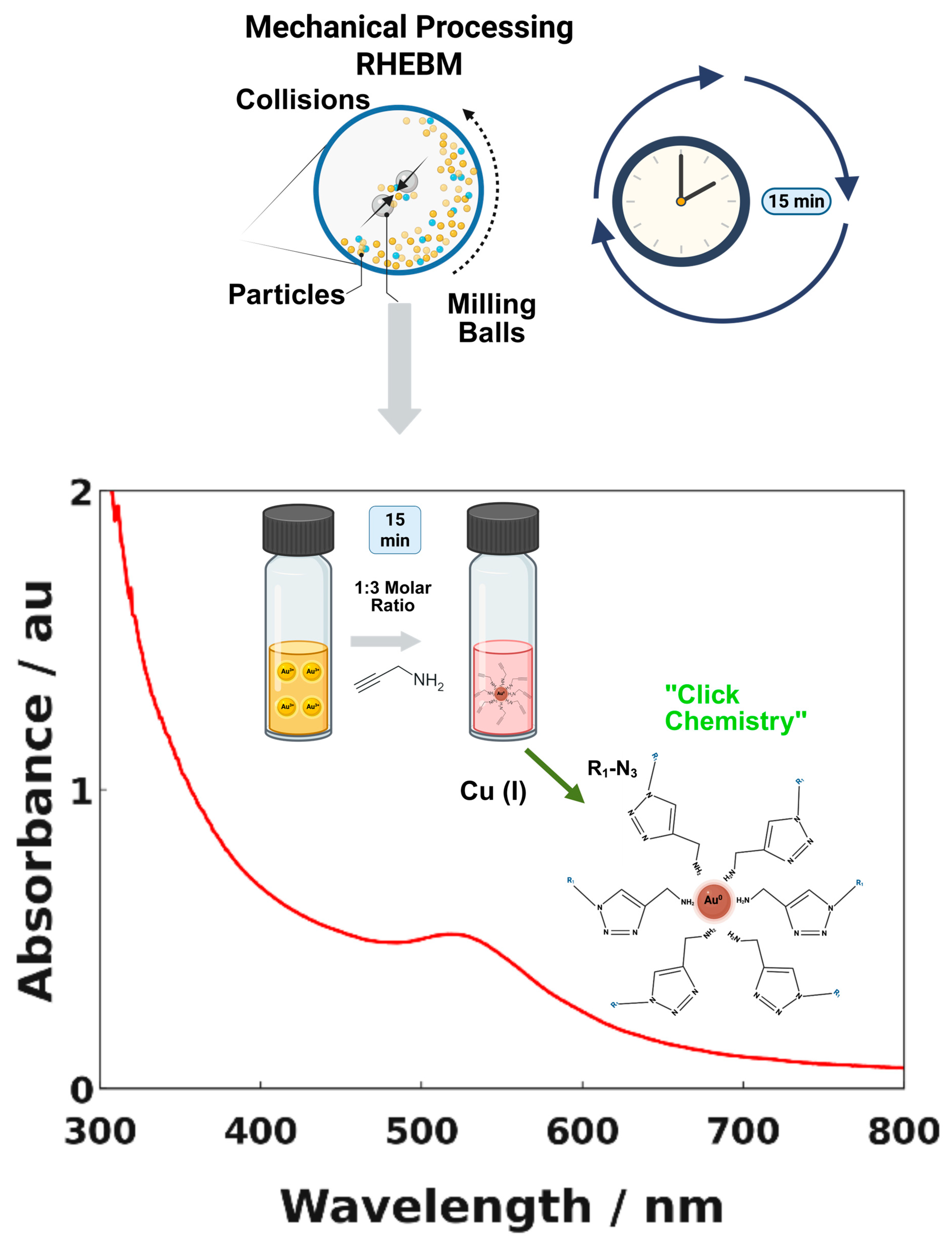
2.2.1. Synthesis of AuNPs Passivated with 2-Propynylamine
2.2.2. Control Experiments: The Role of RHEBM and Reflux in Nanoparticle Formation
- Zirconia milling vial + stirring (Vial F);
- Zirconia milling vial + iron flakes + stirring (Vial A);
- Zirconia milling vial + ball milling (Vial H);
- Steel milling vial + iron flakes + stirring (Vial E);
- Steel milling vial + ball milling (Vial G);
- Methacrylate milling vial + stirring (Vial C);
- Glass storage vial + stirring (Vial B);
- Glass storage vial + iron flakes + stirring (Vial D);
- Reflux: The same reaction conditions as RHEMB, evaluating the time it takes for the solution to change from yellow to burgundy.
2.2.3. Time-Control Experiments
2.2.4. Characterization of Gold Nanoparticles and Related Materials
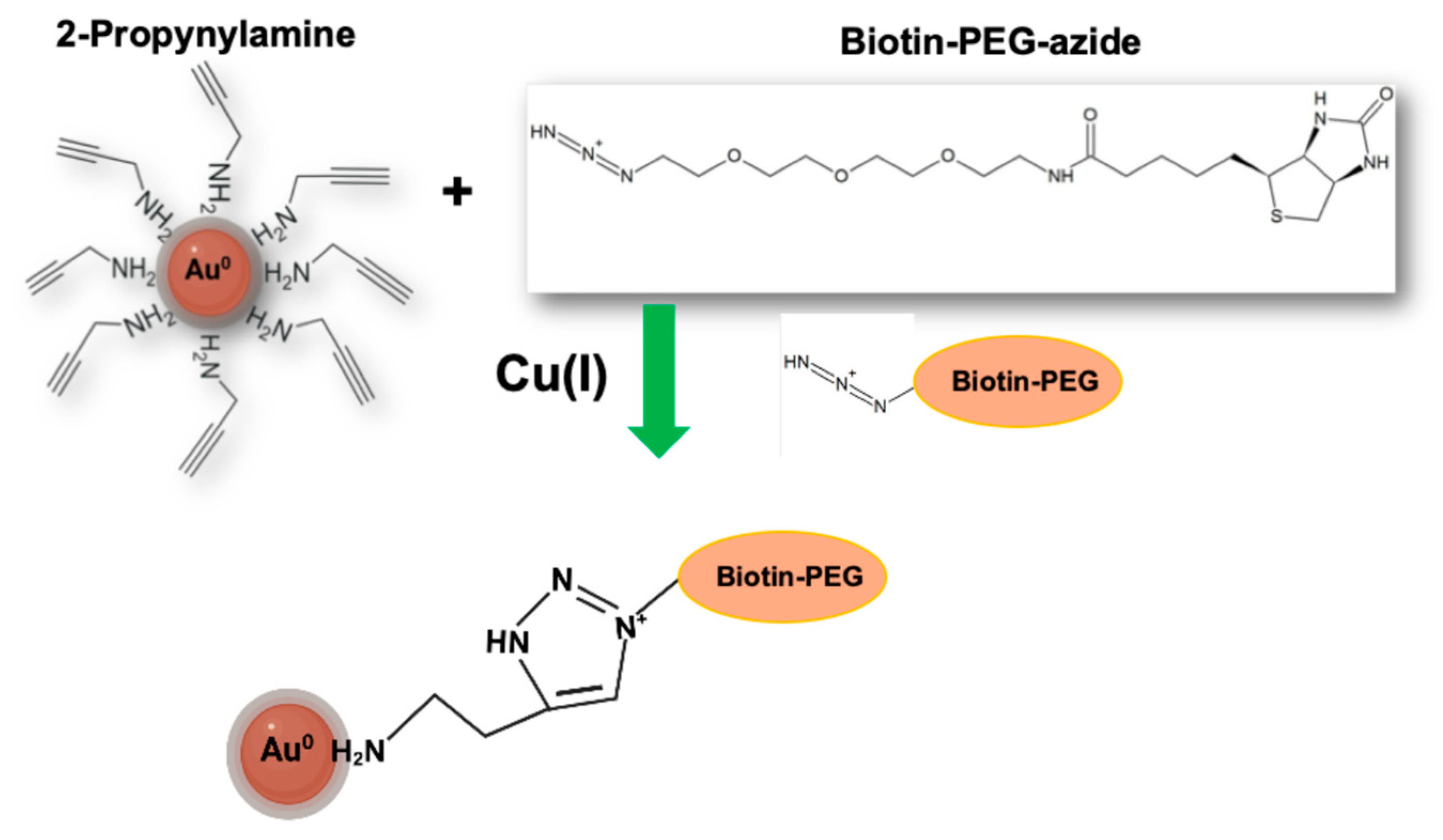
2.2.5. Surface Alkyne Post-Synthetic Click Functionalization with Biotin-PEG4-Azide and Controls
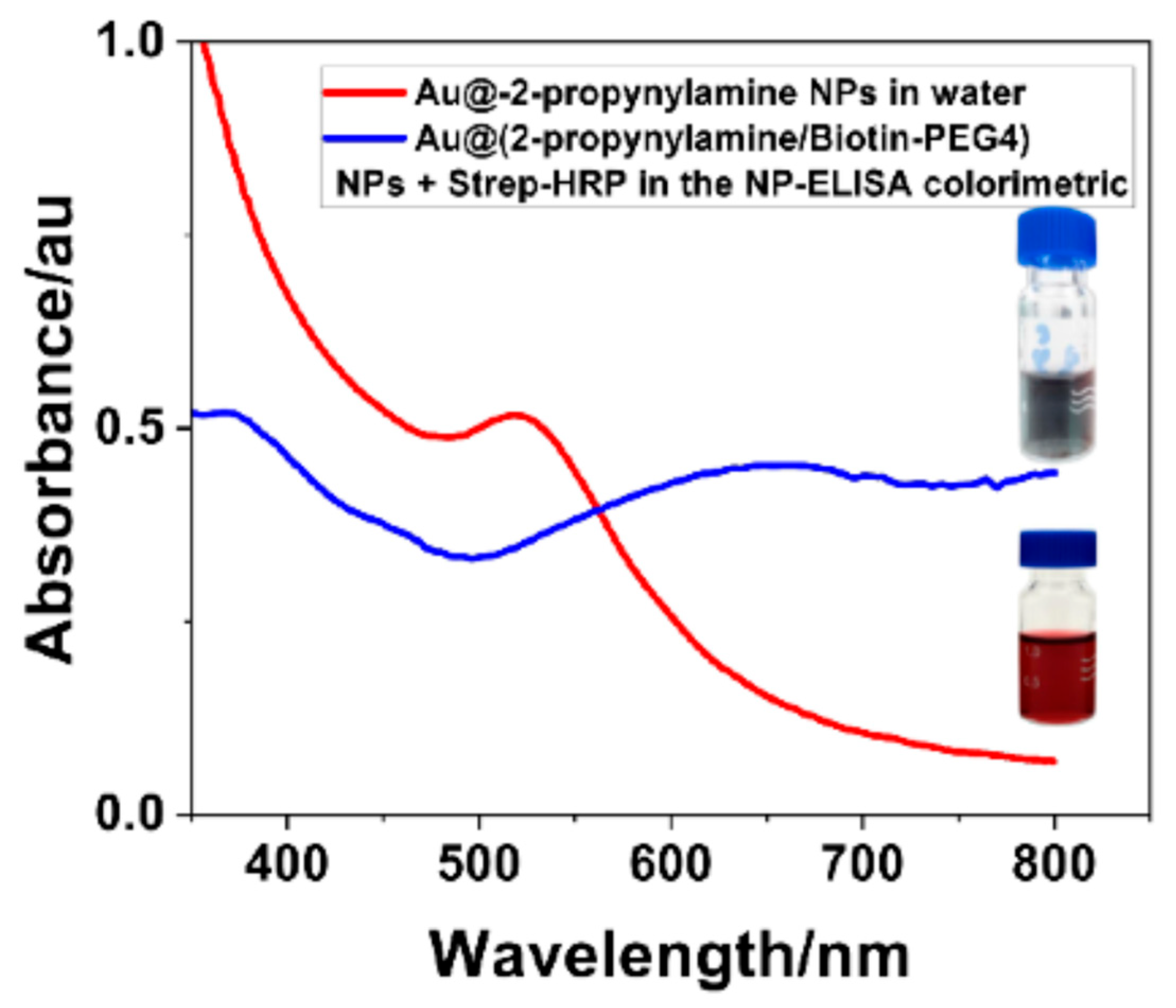
2.2.6. Nanoparticle-ELISA Validation with Streptavidin-HRP and TMB
3. Results and Discussion
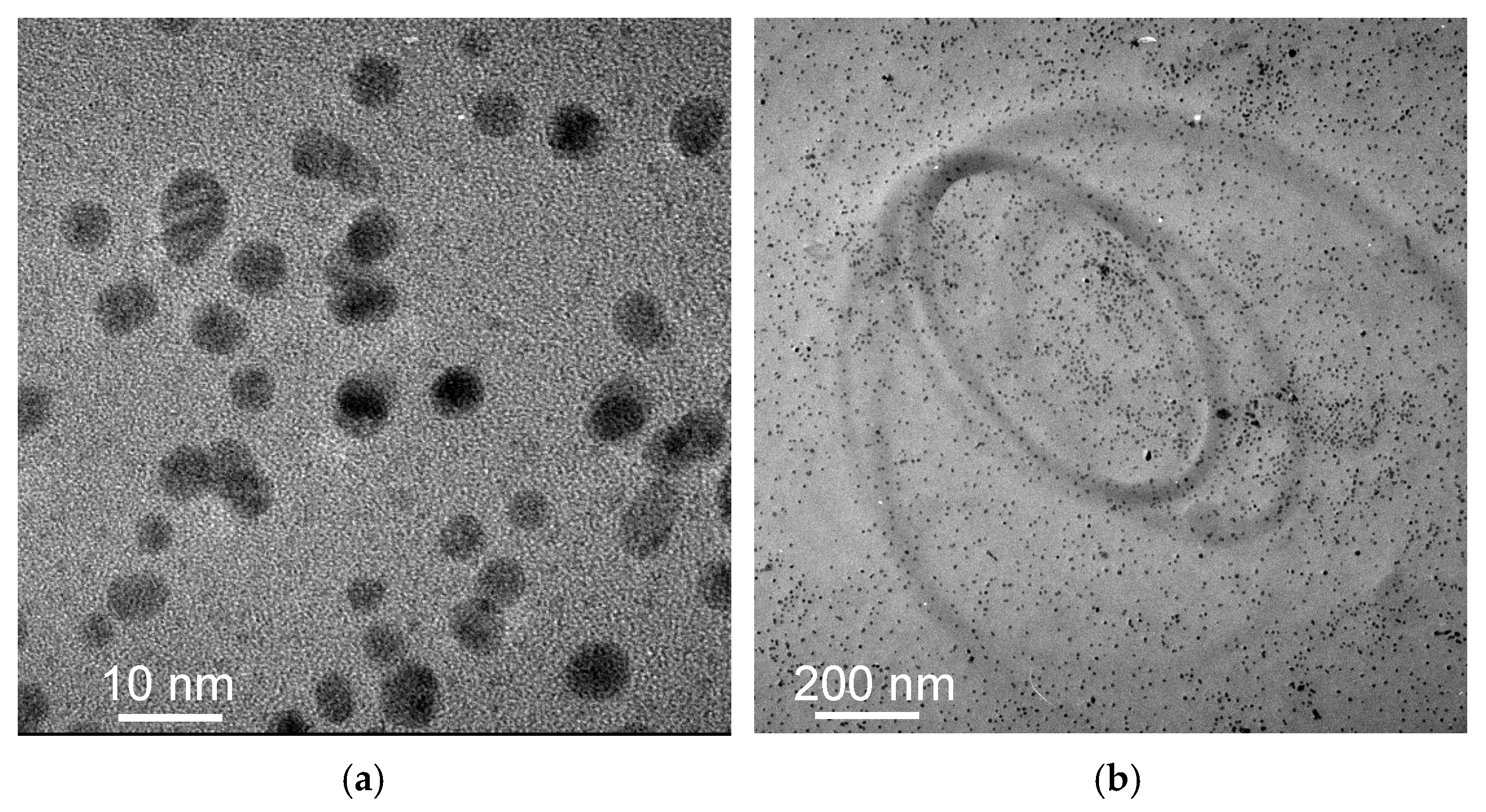
3.1. Transmission Electron Microscopy
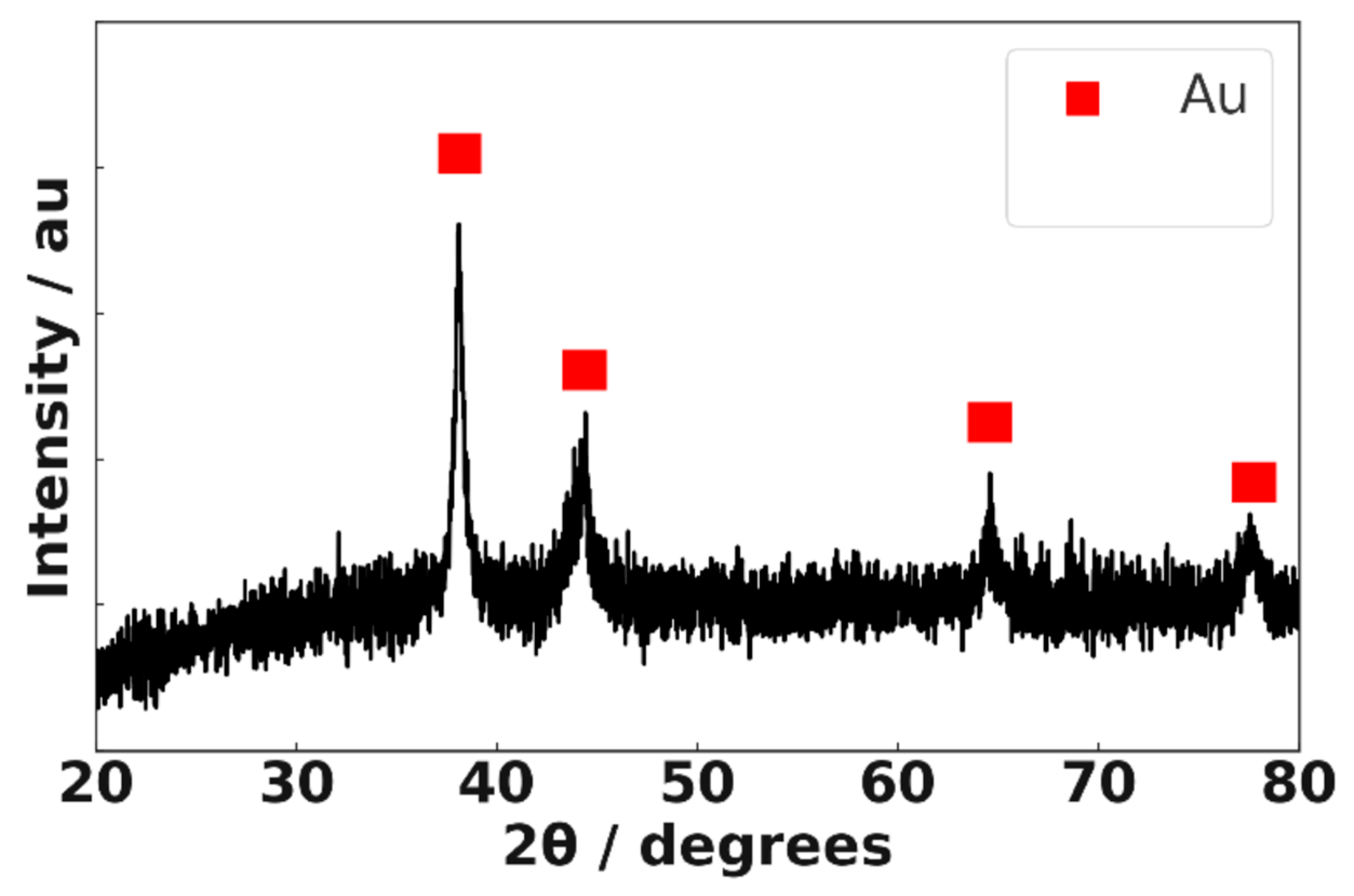
3.2. X-Ray Diffraction
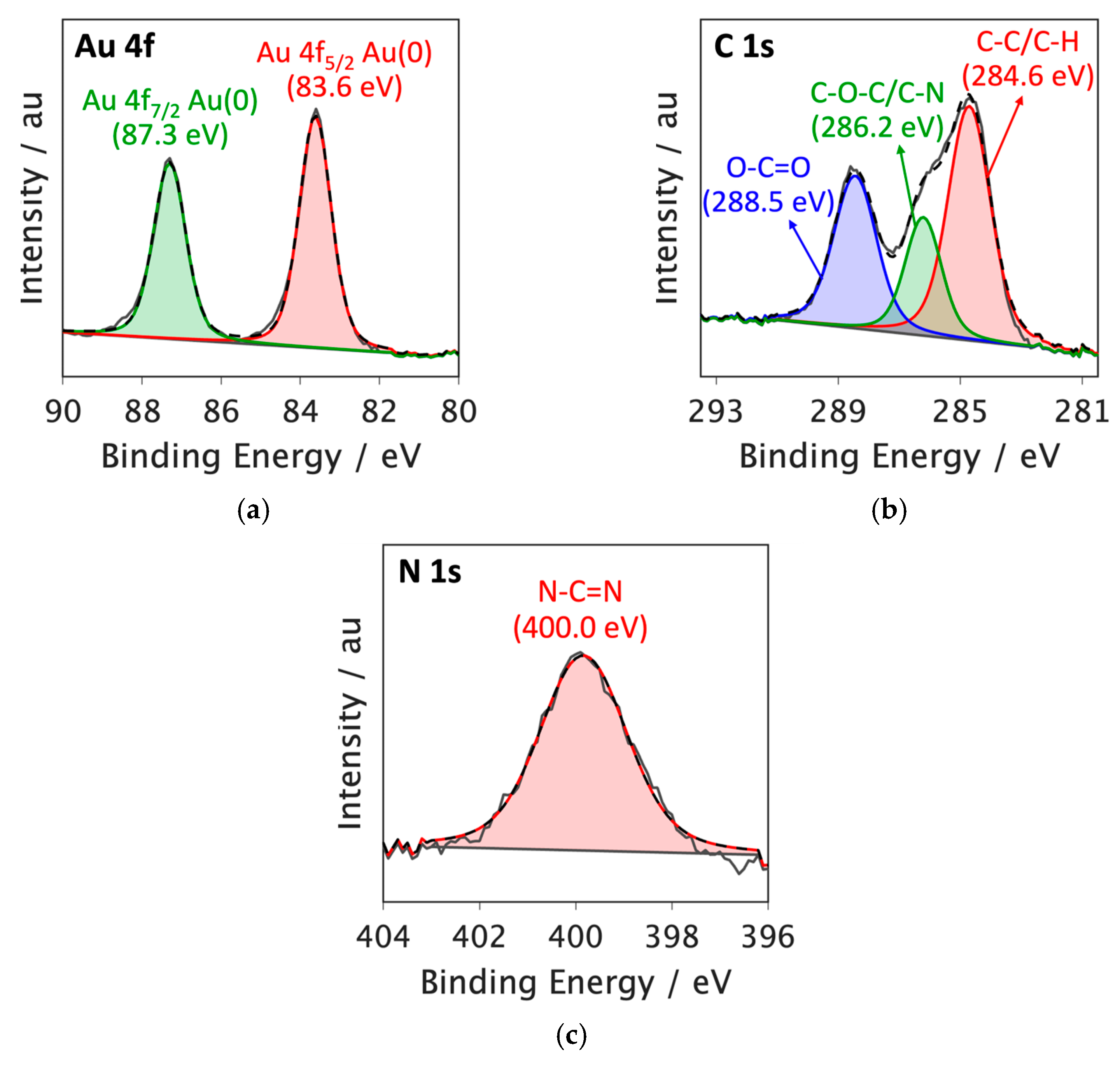
3.3. X-Ray Photoelectron Spectroscopy
3.4. Ligand Structure at the NP Interface
3.5. Surface Click Modification of Alkyne-Functionalized AuNPs Using Biotin-PEG4-Azide
3.6. Colorimetric Assay (NP-ELISA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AuNPs | Gold nanoparticles |
| RHEBM | Reactive high-energy ball milling |
| PBS | Phosphate-buffered saline |
| SPR | Surface plasmon resonance |
| CuAAC | Copper(I)-catalyzed azide-alkyne cycloaddition |
| NP-ELISA | Nanoparticle-based enzyme-linked immunosorbent assay |
| TMB | 3,3′,5,5′-Tetramethylbenzidine |
References
- Liu, K.; He, Z.; Curtin, J.F.; Byrne, H.J.; Tian, F. A novel, rapid, seedless, in situ synthesis method of shape and size controllable gold nanoparticles using phosphates. Sci. Rep. 2019, 9, 7421. [Google Scholar] [CrossRef]
- Chen, R.; Wu, J.; Li, H.; Cheng, G.; Lu, Z.; Che, C. Fabrication of gold nanoparticles with different morphologies in HEPES buffer. Rare Met. 2010, 29, 180–186. [Google Scholar] [CrossRef]
- Gutiérrez-Wing, C.; Esparza, R.; Vargas-Hernández, C.; García, M.E.F.; José-Yacamán, M. Microwave-assisted synthesis of gold nanoparticles self-assembled into self-supported superstructures. Nanoscale 2012, 4, 2281. [Google Scholar] [CrossRef]
- Tyagi, H.; Kushwaha, A.; Kumar, A.; Aslam, M. A facile PH controlled Citrate-Based reduction method for gold nanoparticle synthesis at room temperature. Nanoscale Res. Lett. 2016, 11, 362. [Google Scholar] [CrossRef]
- Dykman, L.; Khlebtsov, N. Gold nanoparticles in biomedical applications: Recent advances and perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282. [Google Scholar] [CrossRef]
- Wang, Y.; Black, K.C.L.; Luehmann, H.; Li, W.; Zhang, Y.; Cai, X.; Wan, D.; Liu, S.-Y.; Li, M.; Kim, P.; et al. Comparison study of gold nanohexapods, nanorods, and nanocages for photothermal cancer treatment. ACS Nano 2013, 7, 2068–2077. [Google Scholar] [CrossRef]
- Xavier, I.P.L.; Lemos, L.L.; De Melo, E.C.; Campos, E.T.; De Souza, B.L.; Faustino, L.A.; Galante, D.; De Oliveira, P.F.M. Mechanochemical hydroquinone regeneration promotes gold salt reduction in sub-stoichiometric conditions of the reducing agent. Phys. Chem. Chem. Phys. 2024, 26, 11436–11444. [Google Scholar] [CrossRef]
- Rak, M.J.; Saadé, N.K.; Friščić, T.; Moores, A. Mechanosynthesis of ultra-small monodisperse amine-stabilized gold nanoparticles with controllable size. Green Chem. 2013, 16, 86–89. [Google Scholar] [CrossRef]
- Nailwal, Y.; Zhang, Q.; Brown, N.; Alsudairy, Z.; Harrod, C.; Uddin, M.H.; Akram, F.; Li, J.; Liu, Y.; Li, X. A rapid, sustainable, one-step mechanochemical strategy for synthesizing gold Nanoparticle-Doped covalent organic frameworks. Chem. Eur. J. 2025, 31, e202500339. [Google Scholar] [CrossRef]
- Dong, J.; Carpinone, P.L.; Pyrgiotakis, G.; Demokritou, P.; Moudgil, B.M. Synthesis of precision gold nanoparticles using Turkevich method. KONA Powder Part. J. 2019, 37, 224–232. [Google Scholar] [CrossRef]
- De Oliveira, P.F.M.; Michalchuk Aa, L.; Marquardt, J.; Feiler, T.; Prinz, C.; Torresi, R.M.; Camargo, P.H.C.; Emmerling, F. Investigating the role of reducing agents on mechanosynthesis of Au nanoparticles. CrystEngComm 2020, 22, 6261–6267. [Google Scholar] [CrossRef]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friščić, T.; Grepioni, F.; Harris, K.D.M.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2011, 41, 413–447. [Google Scholar] [CrossRef]
- Howard, J.L.; Cao, Q.; Browne, D.L. Mechanochemistry as an emerging tool for molecular synthesis: What can it offer? Chem. Sci. 2018, 9, 3080–3094. [Google Scholar] [CrossRef]
- Ma, T.; Li, J.; Li, Y.; Zhang, Y.; Wang, L. Amazing, highly active catalyst for the synthesis of primary amines via reductive amination over a single cobalt atom catalyst. Mater. Today Chem. 2023, 30, 101759. [Google Scholar] [CrossRef]
- Lin, C.; Tao, K.; Hua, D.; Ma, Z.; Zhou, S. Size Effect of Gold Nanoparticles in Catalytic Reduction of p-Nitrophenol with NaBH4. Molecules 2013, 18, 12609–12620. [Google Scholar] [CrossRef]
- Liuzzi, F.; Ventimiglia, A.; Allegri, A.; Rodríguez-Aguado, E.; Cecilia, J.A.; Rivalta, I.; Dimitratos, N.; Albonetti, S. Effect of capping ligands for the synthesis of gold nanoparticles and on the catalytic performance for the oxidation of 5-Hydroxymethyl-2-furfural. Catalysts 2023, 13, 990. [Google Scholar] [CrossRef]
- Ghiassian, S.; Gobbo, P.; Workentin, M.S. Water-Soluble Maleimide-Modified Gold Nanoparticles (AUNPS) as a platform for cycloaddition reactions. Eur. J. Org. Chem. 2015, 2015, 5438–5447. [Google Scholar] [CrossRef]
- Elliott, E.W.; Ginzburg, A.L.; Kennedy, Z.C.; Feng, Z.; Hutchison, J.E. Single-Step synthesis of small, Azide-Functionalized gold nanoparticles: Versatile, Water-Dispersible reagents for click chemistry. Langmuir 2017, 33, 5796–5802. [Google Scholar] [CrossRef]
- Boisselier, E.; Salmon, L.; Ruiz, J.; Astruc, D. How to very efficiently functionalize gold nanoparticles by “click” chemistry. Chem. Commun. 2008, 44, 5788. [Google Scholar] [CrossRef]
- Sander, F.; Fluch, U.; Hermes, J.P.; Mayor, M. Dumbbells, Trikes and Quads: Organic–Inorganic hybrid nanoarchitectures based on “Clicked” gold nanoparticles. Small 2013, 10, 349–359. [Google Scholar] [CrossRef]
- Van Der Meer, S.B.; Loza, K.; Wey, K.; Heggen, M.; Beuck, C.; Bayer, P.; Epple, M. Click Chemistry on the Surface of Ultrasmall Gold Nanoparticles (2 nm) for Covalent Ligand Attachment Followed by NMR Spectroscopy. Langmuir 2019, 35, 7191–7204. [Google Scholar] [CrossRef]
- Thomsett-Scott, B. Origin 7.5. J. Chem. Inf. Model. 2004, 45, 209–210. [Google Scholar] [CrossRef]
- Willcott, M.R. MestRe nova. J. Am. Chem. Soc. 2009, 131, 13180. [Google Scholar] [CrossRef]
- Fairley, N.; Fernandez, V.; Richard-Plouet, M.; Guillot-Deudon, C.; Walton, J.; Smith, E.; Flahaut, D.; Greiner, M.; Biesinger, M.; Tougaard, S.; et al. Systematic and collaborative approach to problem solving using X-ray photoelectron spectroscopy. Appl. Surf. Sci. Adv. 2021, 5, 100112. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Lallana, E.; Riguera, R.; Fernandez-Megia, E. Reliable and Efficient Procedures for the Conjugation of Biomolecules through Huisgen Azide–Alkyne Cycloadditions. Angew. Chem. Int. Ed. 2011, 50, 8794–8804. [Google Scholar] [CrossRef]
- Basso, C.R.; Cruz, T.F.; Vieira, L.B.; De Albuquerque Pedrosa, V.; Possebon, F.S.; Araujo, J.P., Jr. Development of a gold Nanoparticle-Based ELISA for detection of PCV2. Pathogens 2024, 13, 108. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Sun, M.; Zhang, X.; Song, H.; Yan, Y.; Sun, J.; Li, X.; Fang, W. A magnetic nanoparticle based Enzyme-Linked immunosorbent assay for sensitive quantification of zearalenone in cereal and feed samples. Toxins 2015, 7, 4216–4231. [Google Scholar] [CrossRef]
- Chen, Y.; Xianyu, Y.; Wu, J.; Yin, B.; Jiang, X. Click Chemistry-Mediated Nanosensors for biochemical assays. Theranostics 2016, 6, 969–985. [Google Scholar] [CrossRef]
- Lee, M.; Harrison, B.A.; Lewis, G.E. A rapid sporozoite Elisa using 3,3′,5,5′-Tetramethylbenzidine as the substrate chromogen. Am. J. Trop. Med. Hyg. 1990, 42, 314–319. [Google Scholar] [CrossRef]
- Khramtsov, P.; Novokshonova, A.; Galaeva, Z.; Morozova, M.; Bezukladnikova, T.; Rayev, M. A systematic investigation of TMB substrate composition for signal enhancement in ELISA. Anal. Biochem. 2025, 704, 115908. [Google Scholar] [CrossRef]
- Zhang, D.; Li, W.; Ma, Z.; Han, H. Improved ELISA for tumor marker detection using electro-readout-mode based on label triggered degradation of methylene blue. Biosens. Bioelectron. 2018, 126, 800–805. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, M.A. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef]
- Vanegas, J.P.; Scaiano, J.C.; Lanterna, A.E. Thiol-stabilized gold nanoparticles: New ways to displace thiol layers using yttrium or lanthanide chlorides. Langmuir 2017, 33, 12149–12154. [Google Scholar] [CrossRef]
- Vanegas, J.P.; Peisino, L.E.; Pocoví-Martínez, S.; Zaragozá, R.J.; Zaballos-García, E.; Pérez-Prieto, J. Unzipping nucleoside channels by means of alcohol disassembly. Chem. Eur. J. 2013, 19, 16248–16255. [Google Scholar] [CrossRef]
- Zhang, M. Insight into the implication of high-loading mechanical force on the mechanical bond. Innov. Mater. 2024, 2, 100066. [Google Scholar] [CrossRef]
- Kubota, K.; Pang, Y.; Miura, A.; Ito, H. Redox reactions of small organic molecules using ball milling and piezoelectric materials. Science 2019, 366, 1500–1504. [Google Scholar] [CrossRef]
- Wei, L.; Rahim, S.A.; Abdullah, M.A.B.; Yin, A.; Ghazali, M.; Omar, M.; Nemeș, O.; Sandu, A.; Vizureanu, P.; Abdellah, A. Producing metal powder from machining chips using ball milling process: A review. Materials 2023, 16, 4635. [Google Scholar] [CrossRef]
- Huang, P.; Ding, Y.; Wu, L.; Fu, S.; Jia, M. A novel approach of evaluating crushing energy in ball mills using regional total energy. Powder Technol. 2019, 355, 289–299. [Google Scholar] [CrossRef]
- Mateti, S.; Mathesh, M.; Liu, Z.; Tao, T.; Ramireddy, T.; Glushenkov, A.M.; Yang, W.; Chen, Y.I. Mechanochemistry: A force in disguise and conditional effects towards chemical reactions. Chem. Commun. 2020, 57, 1080–1092. [Google Scholar] [CrossRef]
- Venkataraman, K.; Narayanan, K. Energetics of collision between grinding media in ball mills and mechanochemical effects. Powder Technol. 1998, 96, 190–201. [Google Scholar] [CrossRef]
- Sneha, K.; Sathishkumar, M.; Kim, S.; Yun, Y. Counter ions and temperature incorporated tailoring of biogenic gold nanoparticles. Process Biochem. 2010, 45, 1450–1458. [Google Scholar] [CrossRef]
- Singh, M.; Kalaivani, R.; Manikandan, S.; Sangeetha, N.; Kumaraguru, A.K. Facile green synthesis of variable metallic gold nanoparticle using Padina gymnospora, a brown marine macroalga. Appl. Nanosci. 2012, 3, 145–151. [Google Scholar] [CrossRef]
- Behera, M.; Ram, S. Spectroscopy-based study on the interaction between gold nanoparticle and poly(vinylpyrrolidone) molecules in a non-hydrocolloid. Int. Nano Lett. 2013, 3, 17. [Google Scholar] [CrossRef]
- Casaletto, M.P.; Longo, A.; Martorana, A.; Prestianni, A.; Venezia, A.M. XPS study of supported gold catalysts: The role of Au0 and Au+δ species as active sites. Surf. Interface Anal. 2006, 38, 215–218. [Google Scholar] [CrossRef]
- Wang, Y.; Engelhard, M.H.; Baer, D.R.; Castner, D.G. Quantifying the impact of nanoparticle coatings and nonuniformities on XPS analysis: Gold/Silver Core–Shell nanoparticles. Anal. Chem. 2016, 88, 3917–3925. [Google Scholar] [CrossRef]
- Yuan, H.; Sun, G.; Peng, W.; Ji, W.; Chu, S.; Liu, Q.; Liang, Y. Thymine-Functionalized Gold Nanoparticles (AUNPS) for a highly sensitive Fiber-Optic surface plasmon resonance Mercury ion nanosensor. Nanomaterials 2021, 11, 397. [Google Scholar] [CrossRef]
- Han, G.; Li, F.; Chen, Z.; Coppex, C.; Kim, S.; Noh, H.; Fu, Z.; Lu, Y.; Singh, C.V.; Siahrostami, S.; et al. Mechanochemistry for ammonia synthesis under mild conditions. Nat. Nanotechnol. 2020, 16, 325–330. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Hamada, Y.; Tsuboi, M.; Nakata, M.; Tasumi, M. Infrared spectrum of propargylamine. J. Mol. Spectrosc. 1984, 107, 269–283. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Z.; Guo, X. 2-Propynylamine as a Vapor Corrosion Inhibitor for Carbon Steel under Adsorbed Thin Electrolyte Layers. J. Electrochem. Soc. 2014, 161, C151–C155. [Google Scholar] [CrossRef]
- Sheng, X.; Chen, K.; Shi, C.; Huang, D. Recent advances in reactions of propargylamines. Synthesis 2019, 52, 1–20. [Google Scholar] [CrossRef]
- Yadav, M.; Akita, T.; Tsumori, N.; Xu, Q. Strong metal–molecular support interaction (SMMSI): Amine-functionalized gold nanoparticles encapsulated in silica nanospheres highly active for catalytic decomposition of formic acid. J. Mater. Chem. 2012, 22, 12582. [Google Scholar] [CrossRef]
- Patil, T.; Gambhir, R.; Vibhute, A.; Tiwari, A.P. Gold nanoparticles: Synthesis methods, functionalization and biological applications. J. Clust. Sci. 2022, 34, 705–725. [Google Scholar] [CrossRef]
- Diehl, B. Principles in NMR Spectroscopy; Elsevier eBooks; Elsevier: Amsterdam, The Netherlands, 2008; pp. 1–41. [Google Scholar]
- Bhogadia, M.; Edgar, M.; Hunwin, K.; Page, G.; Grootveld, M. Detection and Quantification of Ammonia as the Ammonium Cation in Human Saliva by 1H NMR: A Promising Probe for Health Status Monitoring, with Special Reference to Cancer. Metabolites 2023, 13, 792. [Google Scholar] [CrossRef]
- Kolen, M.; Smith, W.A.; Mulder, F.M. Accelerating 1H NMR detection of aqueous ammonia. ACS Omega 2021, 6, 5698–5704. [Google Scholar] [CrossRef]
- Simpson, J.H. Organic Structure Determination Using 2-D NMR Spectroscopy; Academic Press: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Claridge, T.D.W. High-Resolution NMR Techniques in Organic Chemistry; Elsevier: Oxford, UK, 2016. [Google Scholar]
- Noey, E.L.; Luo, Y.; Zhang, L.; Houk, K.N. Mechanism of Gold(I)-Catalyzed rearrangements of acetylenic Amine-N-Oxides: Computational investigations lead to a new mechanism confirmed by experiment. J. Am. Chem. Soc. 2011, 134, 1078–1084. [Google Scholar] [CrossRef]
- Hashmi, A.S.K.; Jaimes, M.C.B.; Schuster, A.M.; Rominger, F. From propargylic amides to functionalized oxazoles: Domino Gold Catalysis/Oxidation by dioxygen. J. Org. Chem. 2012, 77, 6394–6408. [Google Scholar] [CrossRef]
- Sasane, A.V.; Raj, A.S.K.; Chao, T.; Chen, M.; Liu, R. Gold-Catalyzed Oxidative Aminocyclizations of Propargyl Alcohols and Propargyl Amines to Form Two Distinct Azacyclic Products: Carbene Formation versus a 3,3-Sigmatropic Shift of an Initial Intermediate. Chem. Eur. J. 2020, 26, 16932–16938. [Google Scholar] [CrossRef]
- Wijaya, A.; Hamad-Schifferli, K. Ligand customization and DNA functionalization of gold nanorods via Round-Trip Phase Transfer Ligand Exchange. Langmuir 2008, 24, 9966–9969. [Google Scholar] [CrossRef]
- Wu, M.; Vartanian, A.M.; Chong, G.; Pandiakumar, A.K.; Hamers, R.J.; Hernandez, R.; Murphy, C.J. Solution NMR analysis of ligand environment in quaternary Ammonium-Terminated Self-Assembled monolayers on gold nanoparticles: The effect of surface curvature and ligand structure. J. Am. Chem. Soc. 2019, 141, 4316–4327. [Google Scholar] [CrossRef]
- Huang, W.; Chen, S.; Liu, Y.; Fu, H.; Wu, G. The controlled synthesis of stable gold nanoparticles in quaternary ammonium ionic liquids by simple heating. Nanotechnology 2010, 22, 025602. [Google Scholar] [CrossRef]
- Parmar, S.V.; Avasare, V. Syn-Aminoauration versus Anti-Aminoauration of Alkynes in Au(I)/Au(III) Catalysis: Understanding the Origin of Selectivity. J. Org. Chem. 2024, 89, 2951–2963. [Google Scholar] [CrossRef]
- Kegnæs, S.; Mielby, J.; Mentzel, U.V.; Christensen, C.H.; Riisager, A. Formation of imines by selective gold-catalysed aerobic oxidative coupling of alcohols and amines under ambient conditions. Green Chem. 2010, 12, 1437. [Google Scholar] [CrossRef]
- Sperling, R.A.; Rivera Gil, P.; Zhang, F.; Zanella, M.; Parak, W.J. Biological applications of gold nanoparticles. Chem. Soc. Rev. 2008, 37, 1896–1908. [Google Scholar] [CrossRef]
- Wang, S.; Hossain, M.Z.; Han, T.; Shinozuka, K.; Suzuki, T.; Kuwana, A.; Kobayashi, H. Avidin–Biotin Technology in Gold Nanoparticle-Decorated Graphene Field Effect Transistors for Detection of Biotinylated Macromolecules with Ultrahigh Sensitivity and Specificity. ACS Omega 2020, 5, 30037–30046. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Lutz, J. 1,3-Dipolar cycloadditions of azides and alkynes: A universal ligation tool in polymer and materials science. Angew. Chem. Int. Ed. 2007, 46, 1018–1025. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J.; Bryce, D.L. Spectrometric Identification of Organic Compounds, 8th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2014; p. 90. [Google Scholar]
- Chatterley, A.S.; Laity, P.; Holland, C.; Weidner, T.; Woutersen, S.; Giubertoni, G. Broadband multidimensional spectroscopy identifies the amide II vibrations in silkworm films. Molecules 2022, 27, 6275. [Google Scholar] [CrossRef]
- Brogly, M.; Bistac, S.; Bindel, D. Adsorption and structuration of PEG thin films: Influence of the substrate chemistry. Polymers 2024, 16, 1244. [Google Scholar] [CrossRef]
- Dulkeith, E.; Morteani, A.C.; Niedereichholz, T.; Klar, T.A.; Feldmann, J.; Levi, S.A.; Van Veggel, F.C.J.M.; Reinhoudt, D.N.; Möller, M.; Gittins, D.I. Fluorescence Quenching of Dye Molecules near Gold Nanoparticles: Radiative and Nonradiative Effects. Phys. Rev. Lett. 2002, 89, 203002. [Google Scholar] [CrossRef]
- Chen, Y.; Munechika, K.; Ginger, D.S. Dependence of Fluorescence Intensity on the Spectral Overlap between Fluorophores and Plasmon Resonant Single Silver Nanoparticles. Nano Lett. 2007, 7, 690–696. [Google Scholar] [CrossRef]
- Lim, S.I.; Zhong, C. Molecularly mediated processing and assembly of nanoparticles: Exploring the interparticle interactions and structures. Acc. Chem. Res. 2009, 42, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Fort, E.; Grésillon, S. Surface enhanced fluorescence. J. Phys. D Appl. Phys. 2007, 41, 013001. [Google Scholar] [CrossRef]
- Zhou, X.; Li, H.; Xie, S.; Fu, S.; Xu, H.; Liu, Z. Effects of dielectric core and embedding medium on plasmonic coupling of gold nanoshell arrays. Solid State Commun. 2011, 151, 1049–1052. [Google Scholar] [CrossRef]
- Wu, Z.; Jin, R. On the Ligand’s Role in the Fluorescence of Gold Nanoclusters. Nano Lett. 2010, 10, 2568–2573. [Google Scholar] [CrossRef]
- Borse, S.; Murthy, Z.V.P.; Park, T.J.; Kailasa, S.K. The influence of surface ligand chemistry for the synthesis of blue, fluorescent gold nanoclusters for the detection of serotonin in biofluids. New J. Chem. 2023, 47, 3075–3083. [Google Scholar] [CrossRef]
- Hermanson, G.T. Bioconjugate Techniques, 3rd ed.; Academic Press: Amsterdam, The Netherlands, 2013; pp. 473–476. [Google Scholar]
- Linstrom, P.J.; Mallard, W.G. (Eds.) NIST Chemistry WebBook, NIST Standard Reference Database Number 69; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2024. [Google Scholar]
- Flon, V.; Bénard, M.; Schapman, D.; Galas, L.; Renard, P.; Sabot, C. Fluorophore-Assisted Click Chemistry through Copper(I) Complexation. Biomolecules 2020, 10, 619. [Google Scholar] [CrossRef]
- Pyo, K.; Thanthirige, V.D.; Kwak, K.; Pandurangan, P.; Ramakrishna, G.; Lee, D. Ultrabright Luminescence from Gold Nanoclusters: Rigidifying the Au(I)–Thiolate Shell. J. Am. Chem. Soc. 2015, 137, 8244–8250. [Google Scholar] [CrossRef]
- Chinen, A.B.; Guan, C.M.; Ferrer, J.R.; Barnaby, S.N.; Merkel, T.J.; Mirkin, C.A. Nanoparticle probes for the detection of cancer biomarkers, cells, and tissues by fluorescence. Chem. Rev. 2015, 115, 10530–10574. [Google Scholar] [CrossRef]
- Astruc, D.; Liang, L.; Rapakousiou, A.; Ruiz, J. Click Dendrimers and Triazole-Related Aspects: Catalysts, Mechanism, Synthesis, and Functions. A Bridge between Dendritic Architectures and Nanomaterials. Acc. Chem. Res. 2011, 45, 630–640. [Google Scholar] [CrossRef]
- Swift, J.L.; Cramb, D.T. Nanoparticles as Fluorescence Labels: Is Size All that Matters? Biophys. J. 2008, 95, 865–876. [Google Scholar] [CrossRef] [PubMed]
- McVey, C.; Logan, N.; Thanh, N.T.K.; Elliott, C.; Cao, C. Unusual switchable peroxidase-mimicking nanozyme for the determination of proteolytic biomarker. Nano Res. 2018, 12, 509–516. [Google Scholar] [CrossRef]
- Gebremedhin, K.H.; Kahsay, M.H.; Wegahita, N.K.; Teklu, T.; Berhe, B.A.; Gebru, A.G.; Tesfay, A.H.; Asgedom, A.G. Nanomaterial-based optical colorimetric sensors for rapid monitoring of inorganic arsenic species: A review. Discov. Nano 2024, 19, 38. [Google Scholar] [CrossRef] [PubMed]


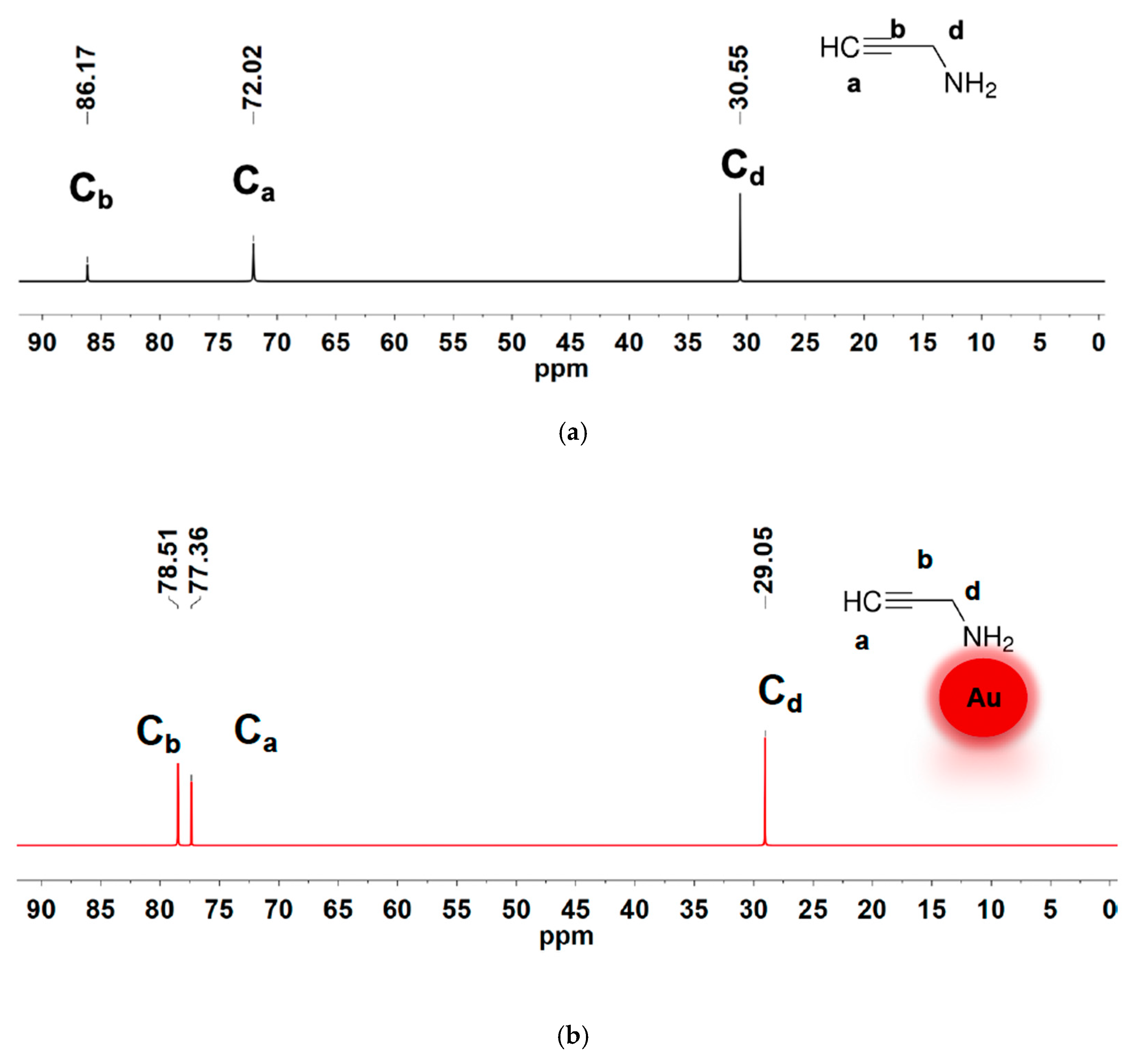

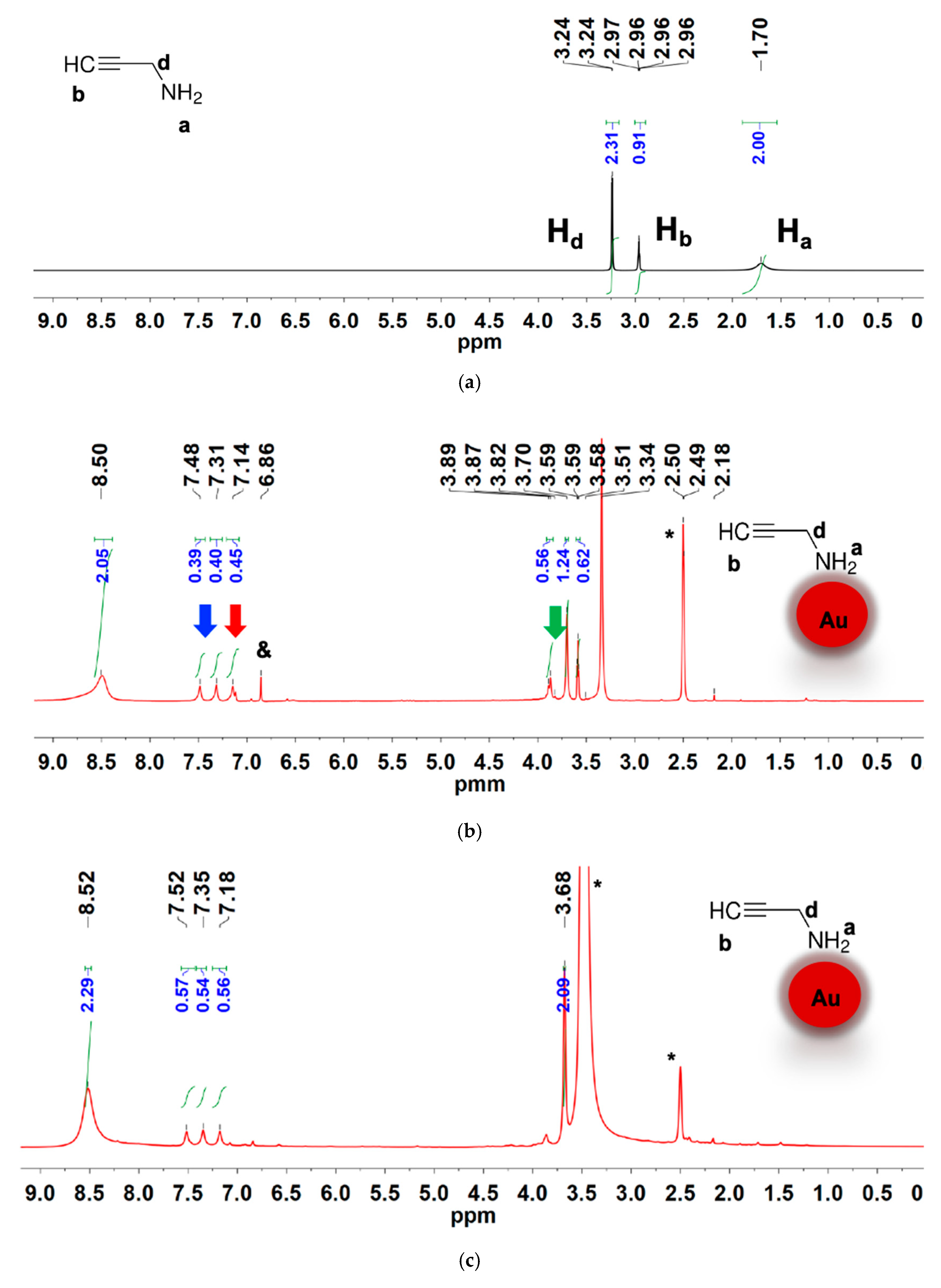
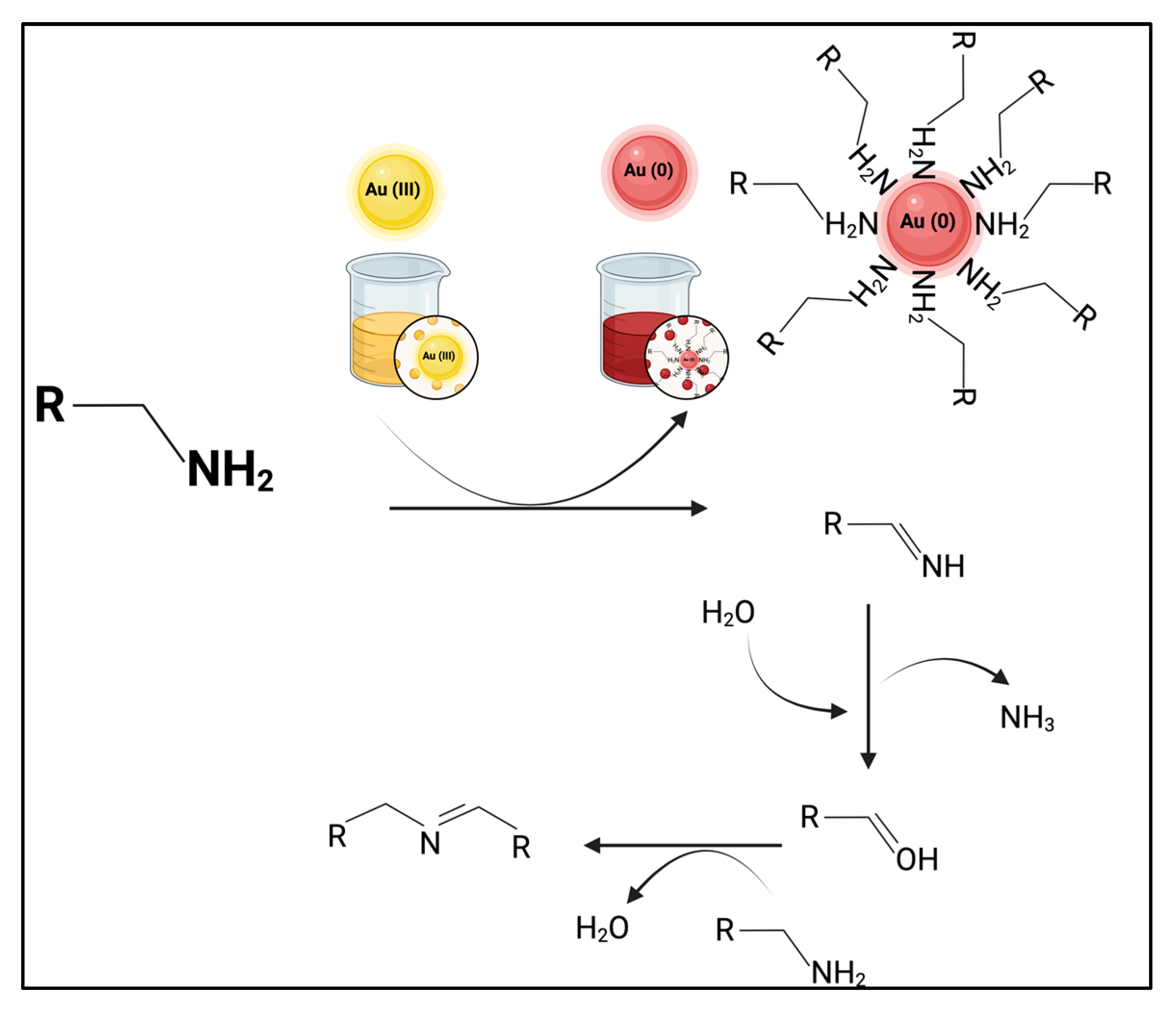
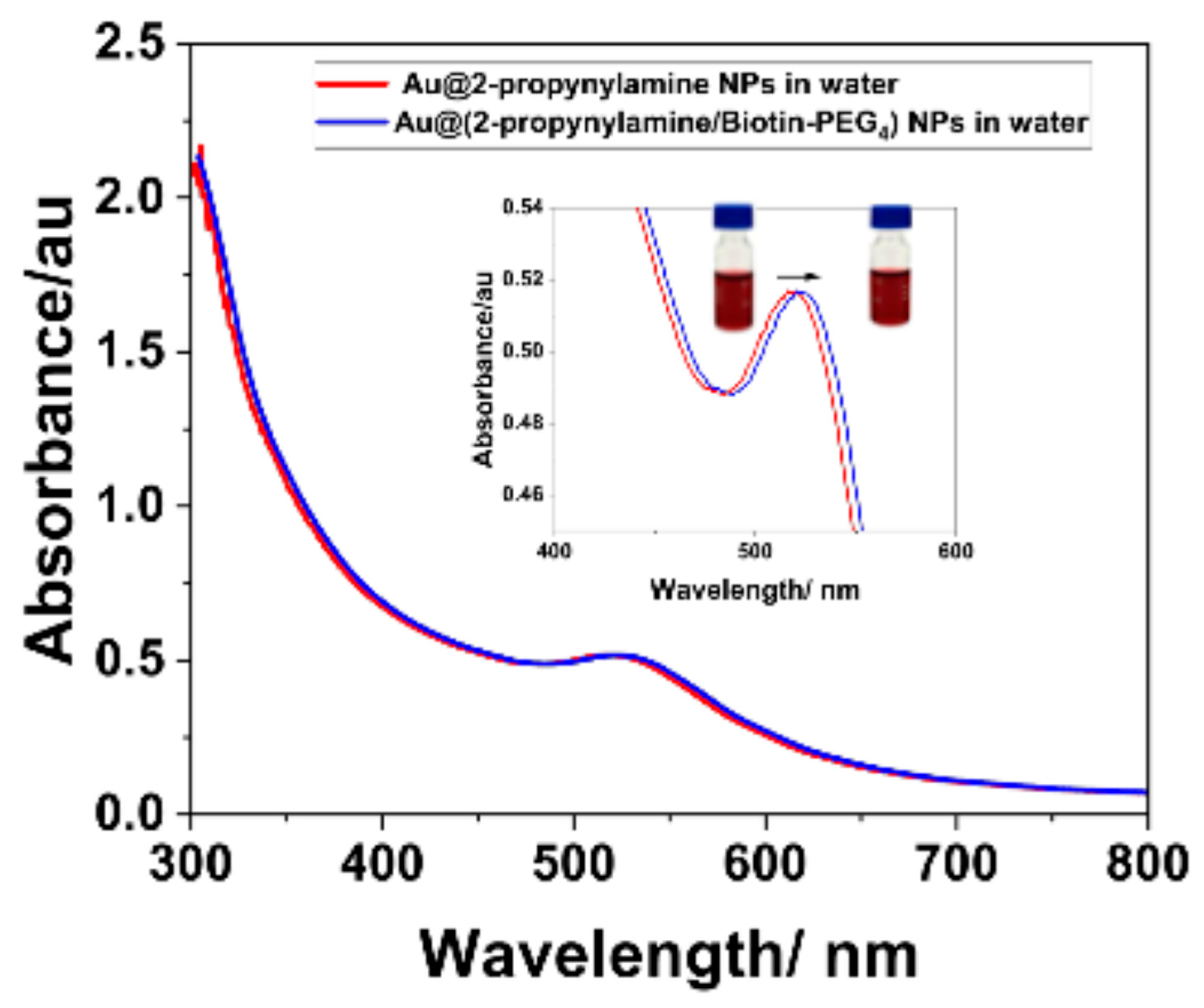
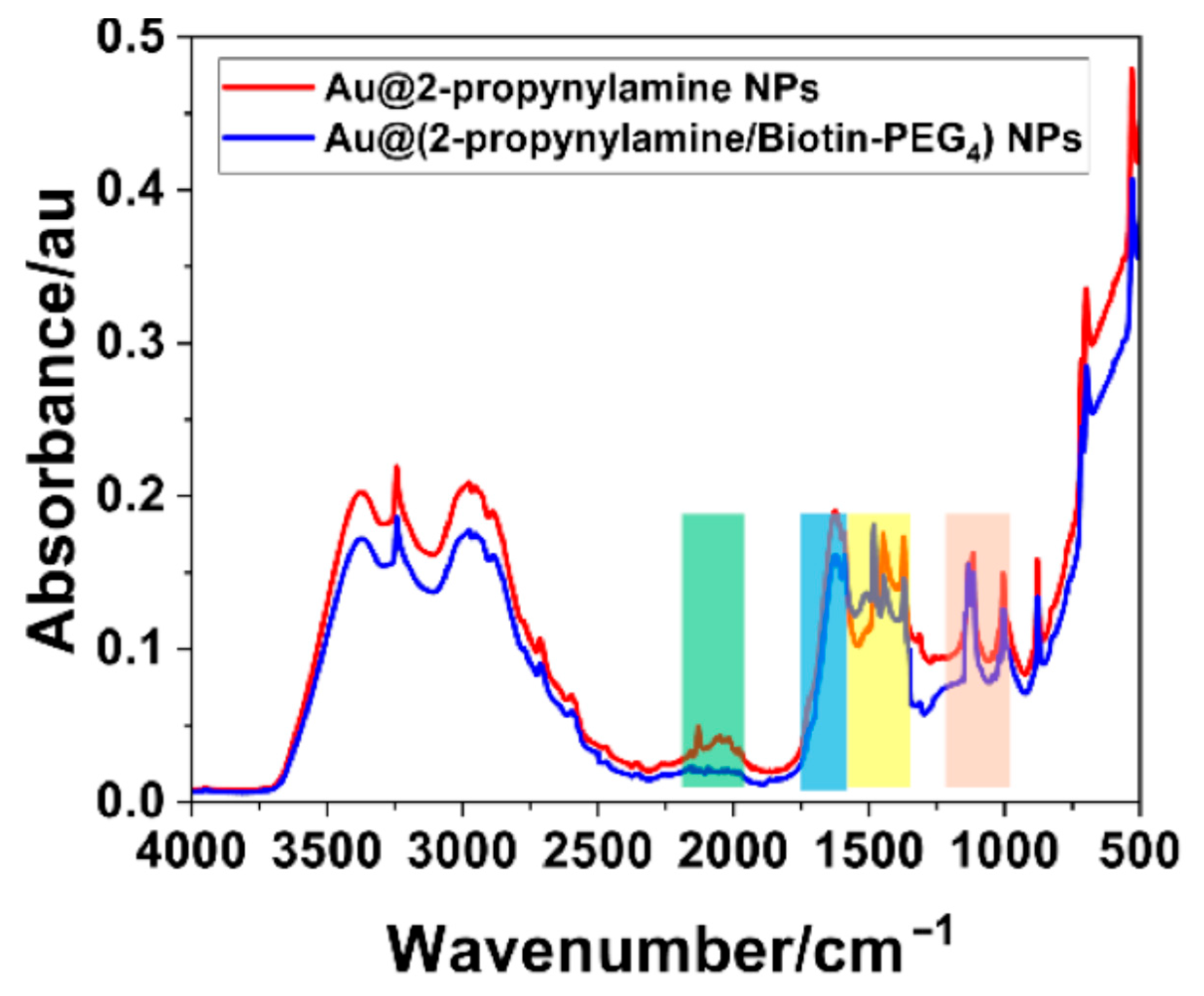

| 2-propynylamine (cm−1) | Au@2-propynylamine NPs (cm−1) | Assignment |
|---|---|---|
| 3370 (m) | 3375 (br) | ≡C-H stretch |
| 3286 (m) | 3238 (m) | N-H stretch |
| 2853 (m) | – | C-H stretch |
| 2933 (m) | 2956 (m) | C-H stretch |
| 2112 (m) | 2130 (m) | C≡C stretch |
| 1670 (m) | 1622 (m) | N–H bend |
| – | 1470 and 1448 (w) | (primary amines) |
| NH4+, bending vibrations. | ||
| 1381 (m) | 1373 (m) | C-H, Alkane |
| 1330 (w) | – | C-H, Alkane |
| 1104 (m) | – | |
| 967 (m) 877 (m) 636 (s) | 1117 (m) 1006 (m) 880 (m) 700 (s) | C-N, aliphatic amines |
| C-H, bending. | ||
| N–H wag | ||
| C-H deformation | ||
| –C≡C–H: C–H | ||
| 541 (s) | 530 (s) | Hydrocarbons C-H and C-C stretching and bending vibrations |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, A.L.; Mitchell, B.S.; Reusch, A.; Fink, M.J.; Hinestroza, J.P.; Ko, Y.; Vanegas, J.P. Click-Ready Gold Nanoparticles from Aqueous Mechanochemistry: 2-Propynylamine as a Reducing Agent and Surface Ligand. Materials 2025, 18, 4470. https://doi.org/10.3390/ma18194470
Garcia AL, Mitchell BS, Reusch A, Fink MJ, Hinestroza JP, Ko Y, Vanegas JP. Click-Ready Gold Nanoparticles from Aqueous Mechanochemistry: 2-Propynylamine as a Reducing Agent and Surface Ligand. Materials. 2025; 18(19):4470. https://doi.org/10.3390/ma18194470
Chicago/Turabian StyleGarcia, Amber L., Brian S. Mitchell, Amanda Reusch, Mark J. Fink, Juan P. Hinestroza, Yelin Ko, and Julie P. Vanegas. 2025. "Click-Ready Gold Nanoparticles from Aqueous Mechanochemistry: 2-Propynylamine as a Reducing Agent and Surface Ligand" Materials 18, no. 19: 4470. https://doi.org/10.3390/ma18194470
APA StyleGarcia, A. L., Mitchell, B. S., Reusch, A., Fink, M. J., Hinestroza, J. P., Ko, Y., & Vanegas, J. P. (2025). Click-Ready Gold Nanoparticles from Aqueous Mechanochemistry: 2-Propynylamine as a Reducing Agent and Surface Ligand. Materials, 18(19), 4470. https://doi.org/10.3390/ma18194470







