Microstructure and Properties of Cu-Fe Immiscible Coatings Fabricated via Combined Mechanical Alloying and Laser Cladding
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Laser Cladding Process
2.3. Characterization and Test
3. Results and Discussion
3.1. Morphologies of Cu–Fe Composite Powders
3.2. Microstructure of Immiscible Composite Coating
3.3. Hardness
3.4. Electrical Resistivity
3.5. Electrochemical Corrosion
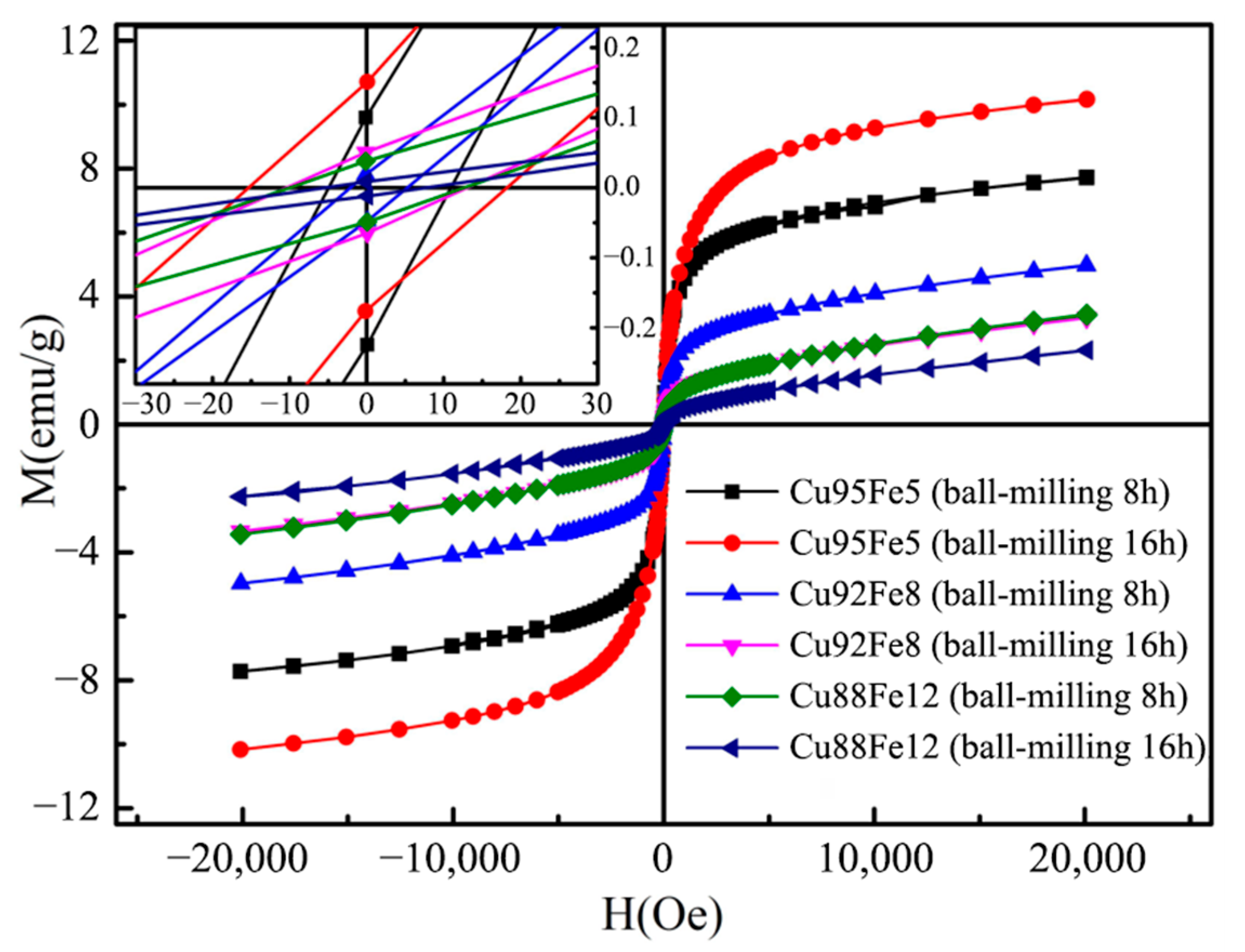
3.6. Magnetic Hysteresis Loop Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, Y.; Wang, J.; Su, J.; Zhang, L.; Gao, Z.; Gao, Z.; Hou, Z.; Du, M.; Duan, J. Evolution kinetics and morphology selections of familiar and anomalous core-shell macrosegregation in undercooled Cu75Fe25 immiscible alloy. Mater. Today Commun. 2025, 48, 113352. [Google Scholar] [CrossRef]
- Roostaei, M.; Uggowitzer, P.J.; Pippan, R.; Renk, O. Severe plastic deformation close to the melting point enables Mg-Fe nanocomposites with exceptional strength. Scr. Mater. 2023, 230, 115428. [Google Scholar] [CrossRef]
- Roostaei, M.; Uggowitzer, P.J.; Pippan, R.; Renk, O. Assessment of different processing strategies to fabricate bulk Mg-Fe nanocomposites. J. Alloys Metall. Syst. 2023, 4, 100034. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Liu, J.; Liang, C.; Zhang, H.; Li, Z.; Qiu, Y. Synergistically enhancing electrical conductivity, magnetic properties, and mechanical properties for Cu–5wt% Fe alloy by multi-stage thermomechanical processing. Mater. Charact. 2025, 227, 115308. [Google Scholar] [CrossRef]
- Li, H.; Yang, H.; Wang, X.; Cheng, J.; Fu, G.; Xu, L.; Chai, Q. Microstructural evolution and property correlation of Cu–Fe powders prepared by magnetic field fluidized bed electrodes. Powder Technol. 2025, 465, 121379. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, C.; Cui, H.; Jiang, J.; Wang, Y.; Wu, S.; Wei, S.; Liu, J. Phase-field study of the evolution of Cu–rich phase precipitation in Fe–Cu–based quinary alloys. Mater. Today Commun. 2025, 47, 113164. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, B.; Du, H.; Xia, Z.; Su, J.; Gao, Z. Metastable phase separation kinetics controlled by superheating and undercooling of liquid Fe–Cu peritectic alloys. J. Alloys Compd. 2022, 913, 165268. [Google Scholar] [CrossRef]
- Li, Y.; Xia, W.; Wang, X.; Ju, Y.; Liu, T.; Zhao, D.; Zuo, M. Effects of cooling rate on the microstructure control and liquid–liquid phase separation behavior of Cu–Fe–P immiscible alloys. Mater. Today Commun. 2022, 33, 104300. [Google Scholar] [CrossRef]
- Liu, S.; Jie, J.; Dong, B.; Guo, Z.; Wang, T.; Li, T. Novel insight into evolution mechanism of second liquid-liquid phase separation in metastable immiscible Cu–Fe alloy. Mater. Des. 2018, 156, 71–81. [Google Scholar] [CrossRef]
- Liu, N.; Liu, F.; Chen, Z.; Yang, G.; Yang, C.; Zhou, Y. Liquid-phase separation in rapid solidification of undercooled Fe–Co–Cu melts. J. Mater. Sci. Technol. 2012, 28, 622–625. [Google Scholar] [CrossRef]
- Kim, J.K.; Shadangi, Y.; Min, H.G.; Shivam, V.; Park, E.S. Alloy design strategies for Fe–rich particle manipulation via unveiling the solidification behavior of Cu–Fe–Si alloys. Mater. Des. 2025, 256, 114296. [Google Scholar] [CrossRef]
- Mishra, D.; Dhal, A.; Li, X.; Guo, S.; Narayan, R.L.; Mishra, R.S.; Singh, J.; Nene, S.S. Phase separation induced heterostructure promotes strength-ductility synergy in Cu–rich compositionally complex alloy in as-cast state. Mater. Today Commun. 2025, 44, 112020. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Wu, T.; Wang, Y.; Fang, F.; Wang, G. Effects of single/double stage cold rolling and aging treatment on the α–Fe phase, microstructure, and properties of Cu–12Fe–0.42Si–0.008B alloy. J. Mater. Res. Technol. 2024, 33, 9552–9565. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, L.; Peng, Y.L.; Yao, W.J. Composition-dependence of core-shell microstructure formation in monotectic alloys under reduced gravity conditions. J. Alloys Compd. 2016, 663, 379–386. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, N. Effect of phase-separated patterns on the formation of core-shell structure. J. Mater. Sci. Technol. 2020, 38, 64–72. [Google Scholar] [CrossRef]
- Liu, M.; Cai, Y.; Duan, C.; Li, G. Key techniques in parts repair and remanufacturing based on laser cladding: A review. J. Manuf. Process. 2024, 132, 994–1014. [Google Scholar] [CrossRef]
- Zhao, S.; Zhou, S.; Xie, M.; Dai, X.; Chen, D.; Zhang, L.-C. Phase separation and enhanced wear resistance of Cu88Fe12 immiscible coating prepared by laser cladding. J. Mater. Res. Technol. 2019, 8, 2001–2010. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y.; Liu, J.; Wang, C.; Liu, W.; Zhang, C. Research on the elements diffusion behavior and properties in Cu/W composite coatings by high-speed laser cladding/laser remelting. J. Alloys Compd. 2025, 1036, 181746. [Google Scholar] [CrossRef]
- Jiang, Y.; Xiao, L.; Peng, Z.; Li, M.; Li, J.; Wang, X.; Deng, G.; Fang, J.; Cai, Z.; Zhao, X.; et al. Laser cladding of heterogeneous structured Cu–Cr–W–SiC coatings with balanced electrical conductivity and wear resistance. Mater. Des. 2025, 254, 114009. [Google Scholar] [CrossRef]
- Ning, H.; Liu, Z.; Kong, Y.; Li, J.; Shen, Y. Study on the dealloying of binary Cu–Fe laser cladding layer with Fe content less than 20 at.%. Acta Mater. 2025, 297, 121342. [Google Scholar] [CrossRef]
- Dai, X.; Xie, M.; Zhou, S.; Wang, C.; Yang, J.; Li, Z. Formation and properties of a self-assembled Cu–Fe–Ni–Cr–Si immiscible composite by laser induction hybrid cladding. J. Alloys Compd. 2018, 742, 910–917. [Google Scholar] [CrossRef]
- Yu, H.; Luo, Z.; Feng, Y.; Liu, Z.; Xie, G.; Misra, R.D.K. Effect of Cu additions on the microstructure, wear resistance and corrosion resistance of Fe–based/B4C composite coating by vacuum cladding. Surf. Coat. Technol. 2023, 454, 129191. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, Z.; Wang, X.; Liang, B.; Zhang, Y. Microstructure and mechanical behavior of Cu–9Al–4Ni–3.5Fe–0.5Mn alloy fabricated by laser melting deposition. Mater. Sci. Eng. A 2021, 826, 142006. [Google Scholar] [CrossRef]
- Makarenko, K.I.; Konev, S.D.; Dubinin, O.N.; Shishkovsky, I.V. Mechanical characteristics of laser-deposited sandwich structures and quasi-homogeneous alloys of Fe–Cu system. Mater. Des. 2022, 224, 111313. [Google Scholar] [CrossRef]
- He, L.; Zhang, M.; Ye, X.; Ruan, D.; Zhang, W. CoCrMoNbTi refractory high-entropy alloys prepared by mechanical alloying combined with laser cladding. Powder Metall. Technol. 2023, 41, 500–507. [Google Scholar]
- Dai, X.Q.; Xie, M.; Zhou, S.F.; Wang, C.X.; Gu, M.H.; Yang, J.X.; Li, Z.Y. Formation mechanism and improved properties of Cu95Fe5 homogeneous immiscible composite coating by the combination of mechanical alloying and laser cladding. J. Alloys Compd. 2018, 740, 194–202. [Google Scholar] [CrossRef]
- Trapp, J.; Kieback, B. Solid-state reactions during high-energy milling of mixed powder. Acta Mater. 2013, 61, 310–320. [Google Scholar] [CrossRef]
- Dwivedi, S.P.; Saxena, A.; Sharma, S.; Singh, G.; Singh, J.; Mia, M.; Chattopadhyaya, S.; Pramanil, A. Effect of ball-milling process parameters on mechanical properties of Al/Al2O3/collagen powder composite using statistical approach. J. Mater. Res. Technol. 2021, 15, 2918–2932. [Google Scholar] [CrossRef]
- Riabkina-Fishman, M.; Rabkin, E.; Levin, P.; Frage, N.; Dariel, M.P.; Weisheit, A.; Galun, R.; Mordike, B.L. Laser produced functionally graded tungsten carbide coatings on M2 high-speed tool steel. Mater. Sci. Eng. A 2001, 302, 106–114. [Google Scholar] [CrossRef]
- He, J.; Zhao, J.Z.; Ratke, L. Solidification microstructure and dynamics of metastable phase transformation in undercooled liquid Cu–Fe alloys. Acta Mater. 2006, 54, 1749–1757. [Google Scholar] [CrossRef]
- Liu, N.; Liu, F.; Yang, W.; Chen, Z.; Yang, G.C. Movement of minor phase in undercooled immiscible Fe–Co–Cu alloys. J. Alloys Compd. 2013, 551, 323–326. [Google Scholar] [CrossRef]
- Kaban, I.; Köhler, M.; Ratke, L.; Nowak, R.; Sobczak, N.; Mattern, N. Phase separation in monotectic alloys as a route for liquid state fabrication of composite materials. J. Mater. Sci. 2012, 47, 8360–8366. [Google Scholar] [CrossRef]
- Wang, C.P.; Liu, X.J.; Ohnuma, I.; Kainuma, R.; Ishida, K. Formation of Immiscible Alloy Powders with Egg-Type Microstructure. Science 2002, 297, 990–993. [Google Scholar] [CrossRef]
- Hong, S.I.; Hill, M.A. Microstructure and conductivity of Cu-Nb microcomposites fabricated by the bundling and drawing process. Scr. Mater. 2001, 44, 2509–2515. [Google Scholar] [CrossRef]
- Liu, S.C.; Jie, J.C.; Guo, Z.K.; Yue, S.P.; Li, T.J. A comprehensive investigation on microstructure and magnetic properties of immiscible Cu-Fe alloys with variation of Fe content. Mater. Chem. Phys. 2019, 238, 121909. [Google Scholar] [CrossRef]
- Wen, C.Y.; Qiu, Y.T.; Zhang, Z.G.; Li, K.M.; Deng, C.; Hu, L.X.; Chen, D.C.; Lu, Y.; Zhou, S.F. Deformation behavior of heterogeneous lamellar Cu-Fe-P immiscible alloys with enhanced strength and ductility produced by laser powder bed fusion. J. Alloys Compd. 2024, 971, 172675–172687. [Google Scholar] [CrossRef]
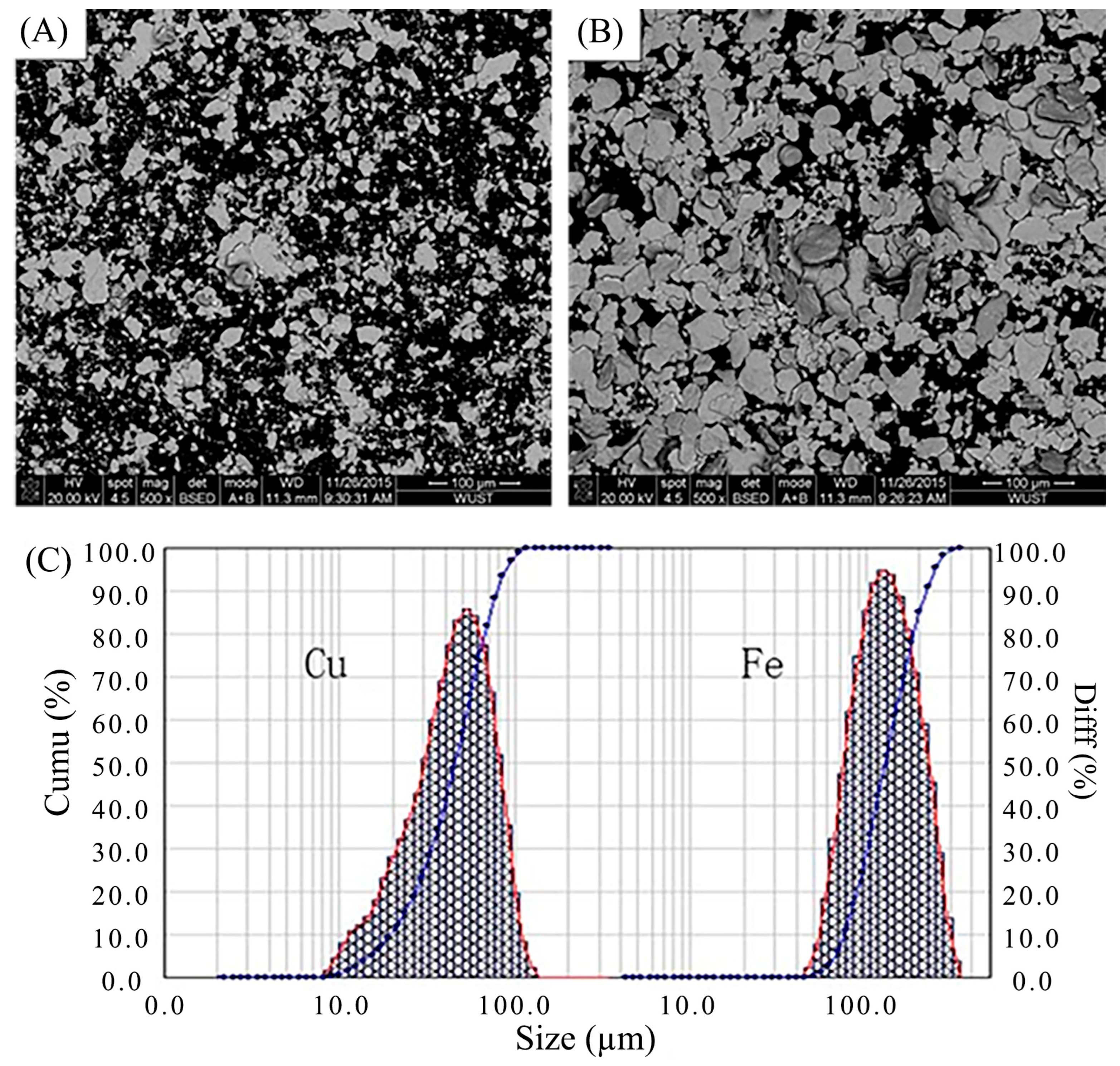

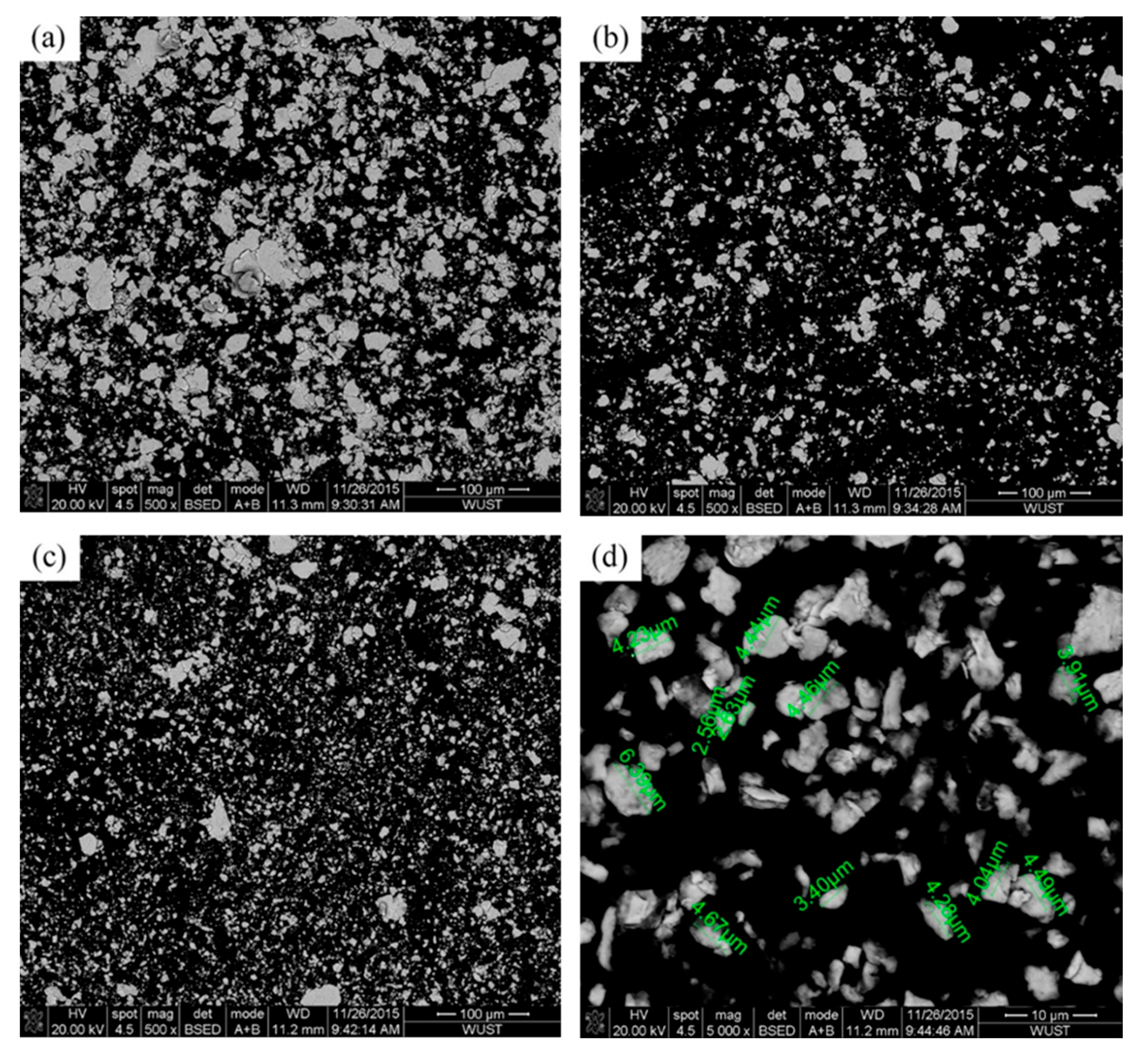



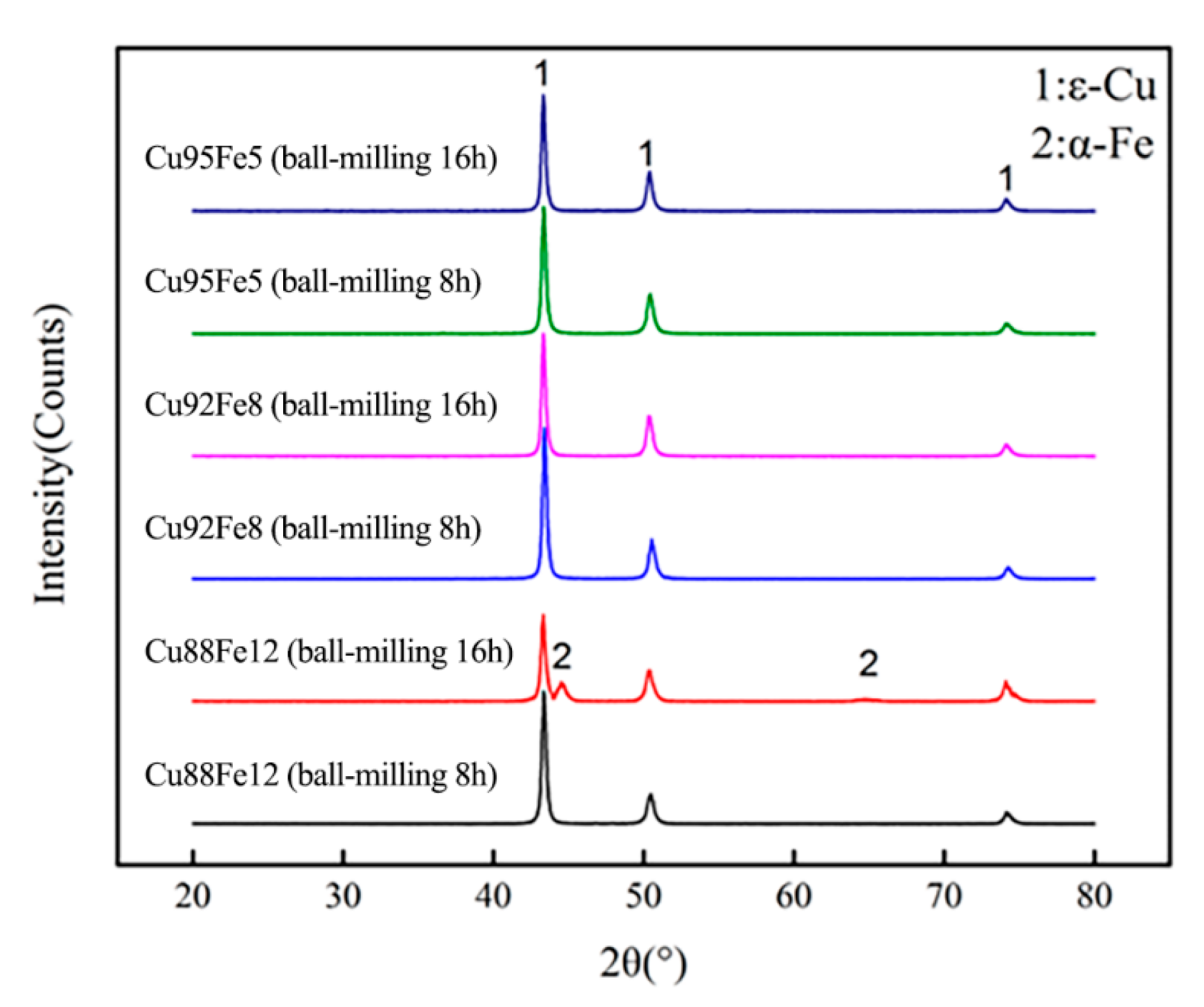
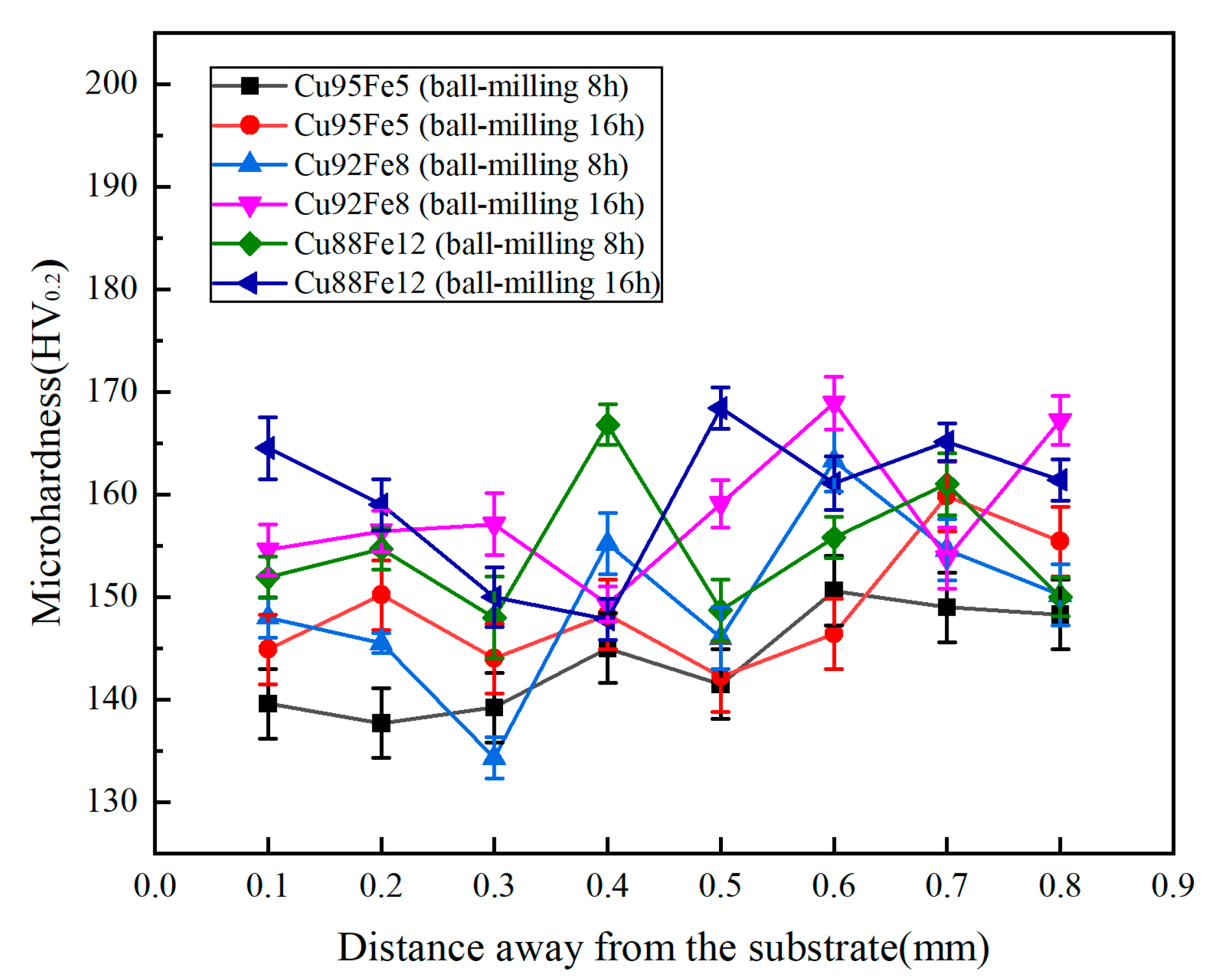


| Element | C | Mn | Si | S | P | Fe |
|---|---|---|---|---|---|---|
| Composition (wt.%) | 0.22 | 0.14 | 0.35 | 0.05 | 0.045 | Bal. |
| Location | Composition (wt.%) | |||||
|---|---|---|---|---|---|---|
| P | Cr | Fe | Ni | Cu | Zn | |
| Spot 1 | 0 | 6.15 | 60.02 | 17.95 | 15.88 | 0 |
| Spot 2 | 2.59 | 7.55 | 53.63 | 20.98 | 15.25 | 0 |
| Spot 3 | 0 | 7.10 | 58.57 | 19.64 | 14.69 | 0 |
| Sample | Ball-Milling Time (h) | Ecorr (V) | Icorr (A/cm2) |
|---|---|---|---|
| Cu95Fe5 | 8 | −0.444 | 1.906 × 10−6 |
| Cu92Fe8 | 8 | −0.473 | 4.818 × 10−6 |
| Cu88Fe12 | 8 | −0.581 | 5.895 × 10−6 |
| Cu95Fe5 | 16 | −0.585 | 0.936 × 10−6 |
| Cu92Fe8 | 16 | −0.413 | 3.908 × 10−6 |
| Cu88Fe12 | 16 | −0.471 | 5.849 × 10−6 |
| Sample | Ball Milling Time (h) | Saturated Magnetization (emu/g) | Remnant Magnetization (emu/g) | Coercive Force (Oe) |
|---|---|---|---|---|
| Cu95Fe5 | 8 | 7.723 | 0.175 | 9.858 |
| Cu92Fe8 | 8 | 4.980 | 0.046 | 2.313 |
| Cu88Fe12 | 8 | 3.348 | 0.043 | 1.756 |
| Cu95Fe5 | 16 | 10.172 | 0.163 | 17.249 |
| Cu92Fe8 | 16 | 3.342 | 0.059 | 11.053 |
| Cu88Fe12 | 16 | 2.319 | 0.010 | 4.983 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, C.; Xie, T.; Wan, Z.; Feng, G.; Yang, Y.; Wu, Z.; Wang, X.; Zhou, S.; Chen, J. Microstructure and Properties of Cu-Fe Immiscible Coatings Fabricated via Combined Mechanical Alloying and Laser Cladding. Materials 2025, 18, 4436. https://doi.org/10.3390/ma18194436
Deng C, Xie T, Wan Z, Feng G, Yang Y, Wu Z, Wang X, Zhou S, Chen J. Microstructure and Properties of Cu-Fe Immiscible Coatings Fabricated via Combined Mechanical Alloying and Laser Cladding. Materials. 2025; 18(19):4436. https://doi.org/10.3390/ma18194436
Chicago/Turabian StyleDeng, Cheng, Tao Xie, Zihao Wan, Guangjian Feng, Yuanlun Yang, Zhaozhi Wu, Xinhua Wang, Shengfeng Zhou, and Jie Chen. 2025. "Microstructure and Properties of Cu-Fe Immiscible Coatings Fabricated via Combined Mechanical Alloying and Laser Cladding" Materials 18, no. 19: 4436. https://doi.org/10.3390/ma18194436
APA StyleDeng, C., Xie, T., Wan, Z., Feng, G., Yang, Y., Wu, Z., Wang, X., Zhou, S., & Chen, J. (2025). Microstructure and Properties of Cu-Fe Immiscible Coatings Fabricated via Combined Mechanical Alloying and Laser Cladding. Materials, 18(19), 4436. https://doi.org/10.3390/ma18194436





