Compressive Strength and CO2 Mineralization Mechanism of Copper Slag-GGBS Alkali-Activated Geopolymer Composites Enhanced by MgO and Biochar
Highlights
- The enhanced compressive strength and CO2 mineralization of geopolymer composites has been experimentally evaluated by incorporating MgO and DBS.
- Mesoporous DSB outperforms microporous biochar in CO2 capture.
- The CO2 capture of the geopolymer composite has been explored through real-time monitoring.
- Geopolymer composites with 5% MgO and 1.5% DSB maximize both compressive strength and CO2 capture.
- CS-GGBS geopolymer composites mineralize 40.2 kg t−1 of CO2 at least.
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mixing Design and Procedure
2.3. Test Methods
3. Results and Discussion
3.1. Compressive Strength Analysis
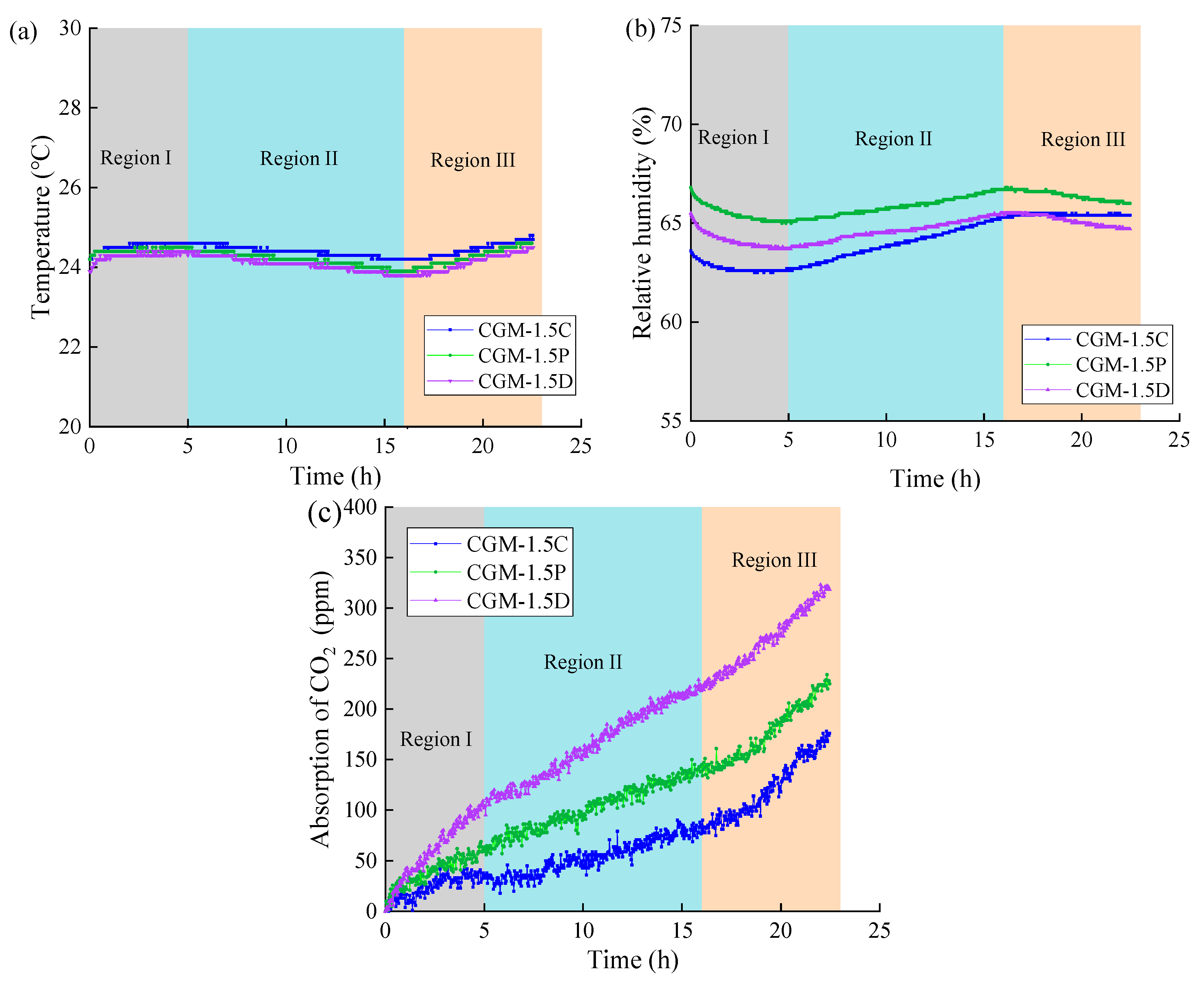
3.2. Analysis of CO2 Real-Time Monitoring
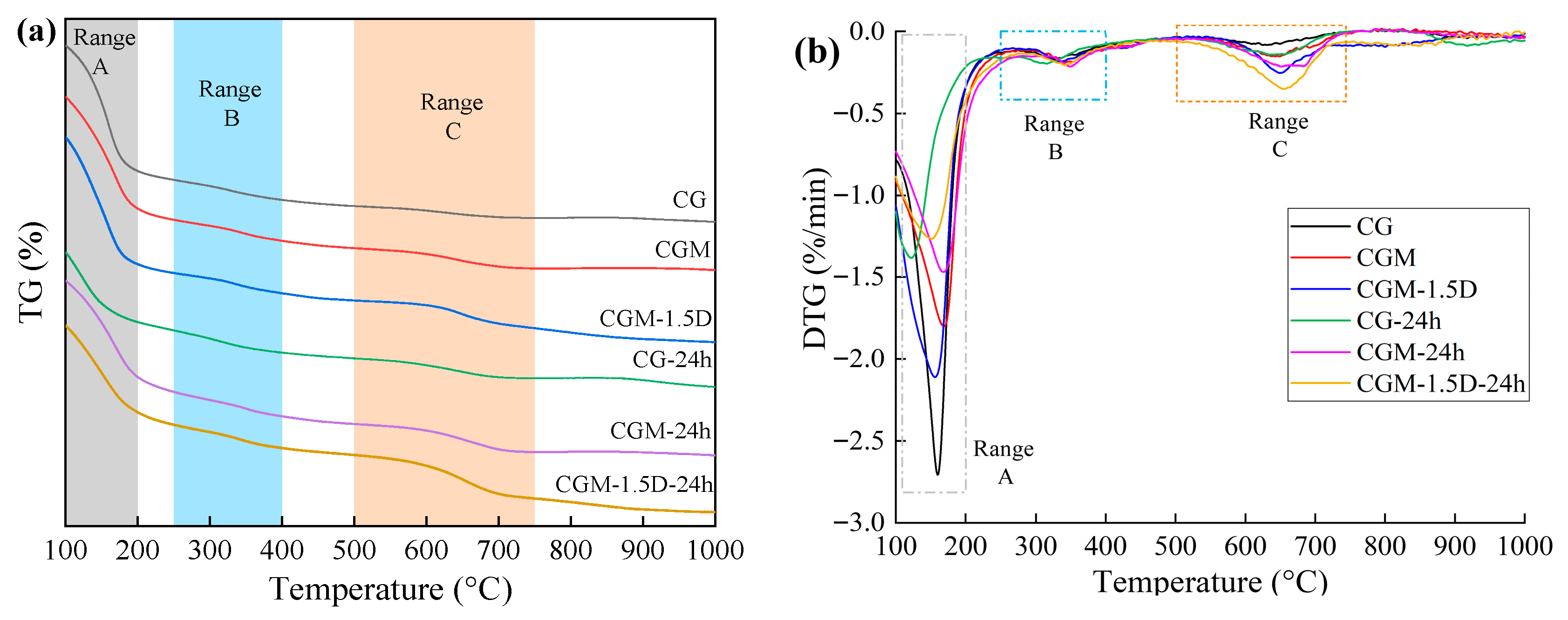
3.3. TGA
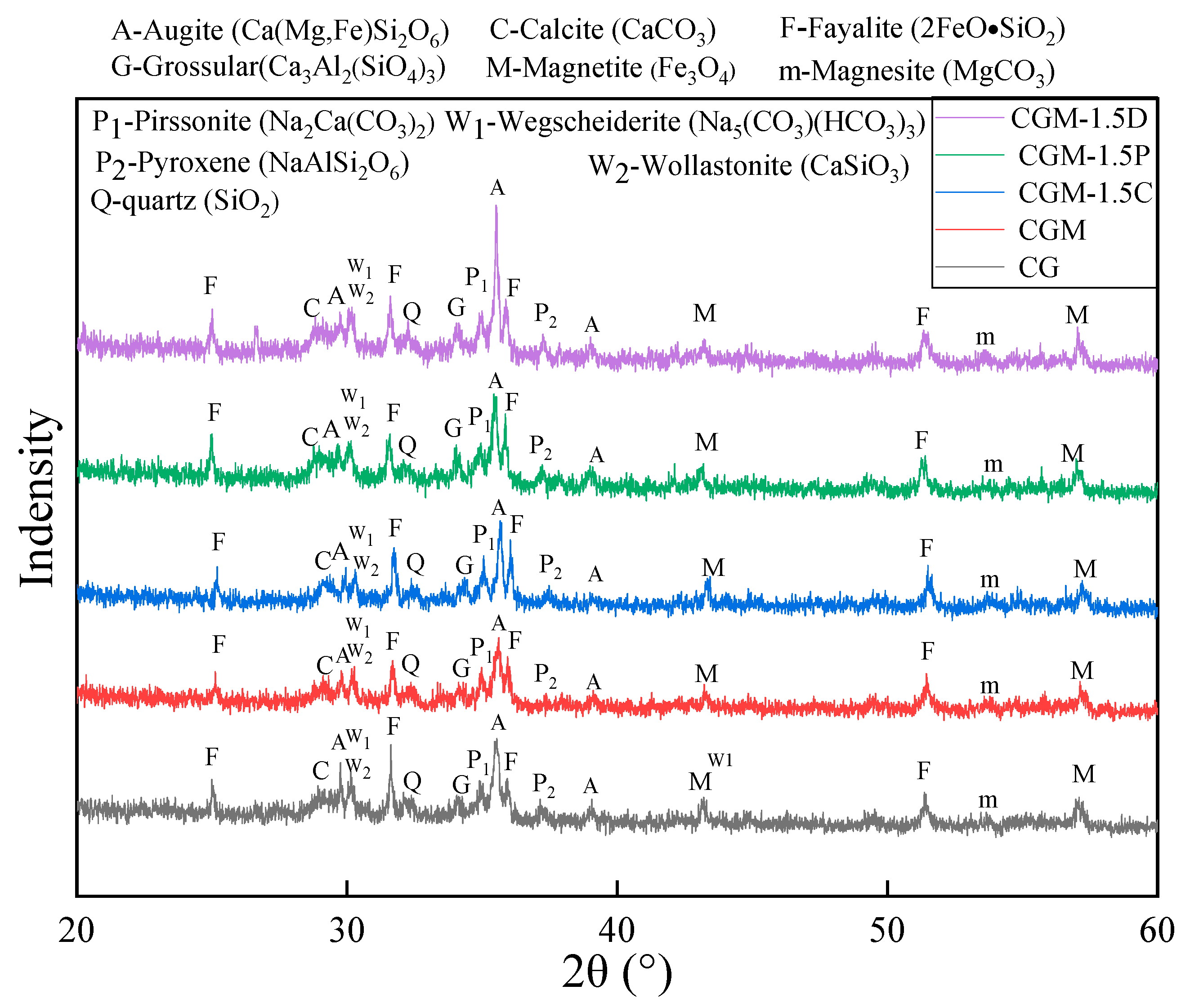
3.4. XRD Analysis
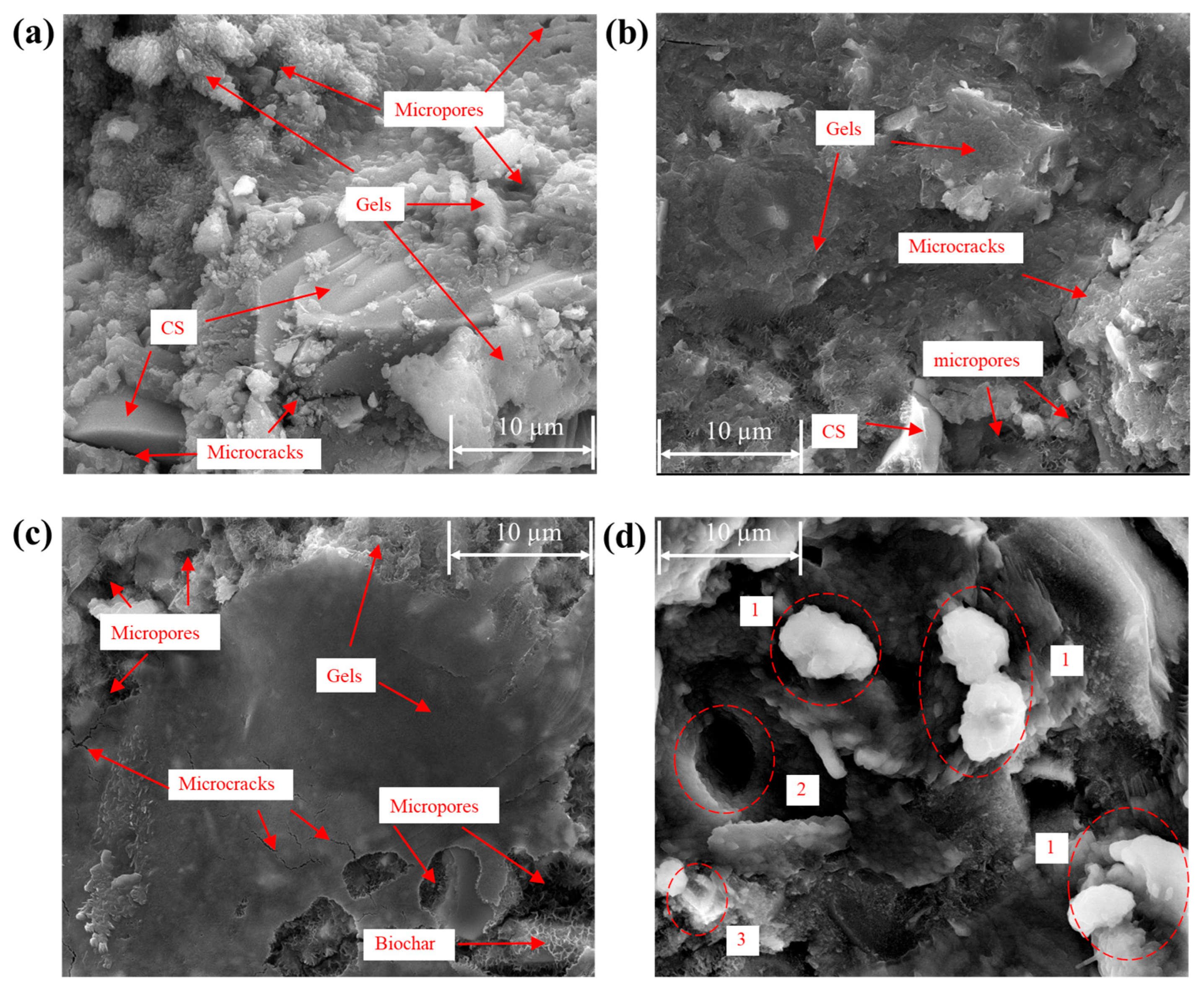
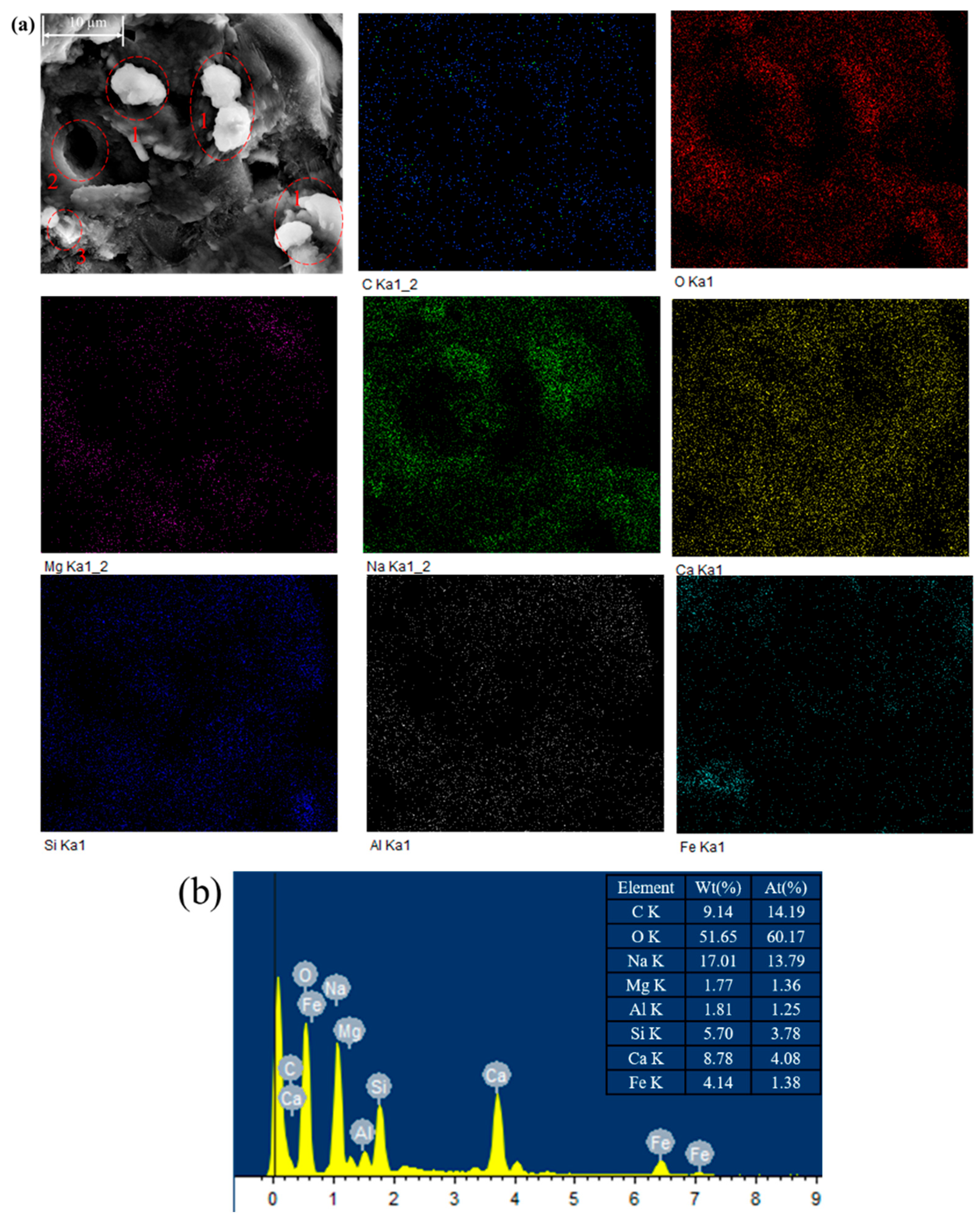
3.5. Microstructural Analysis
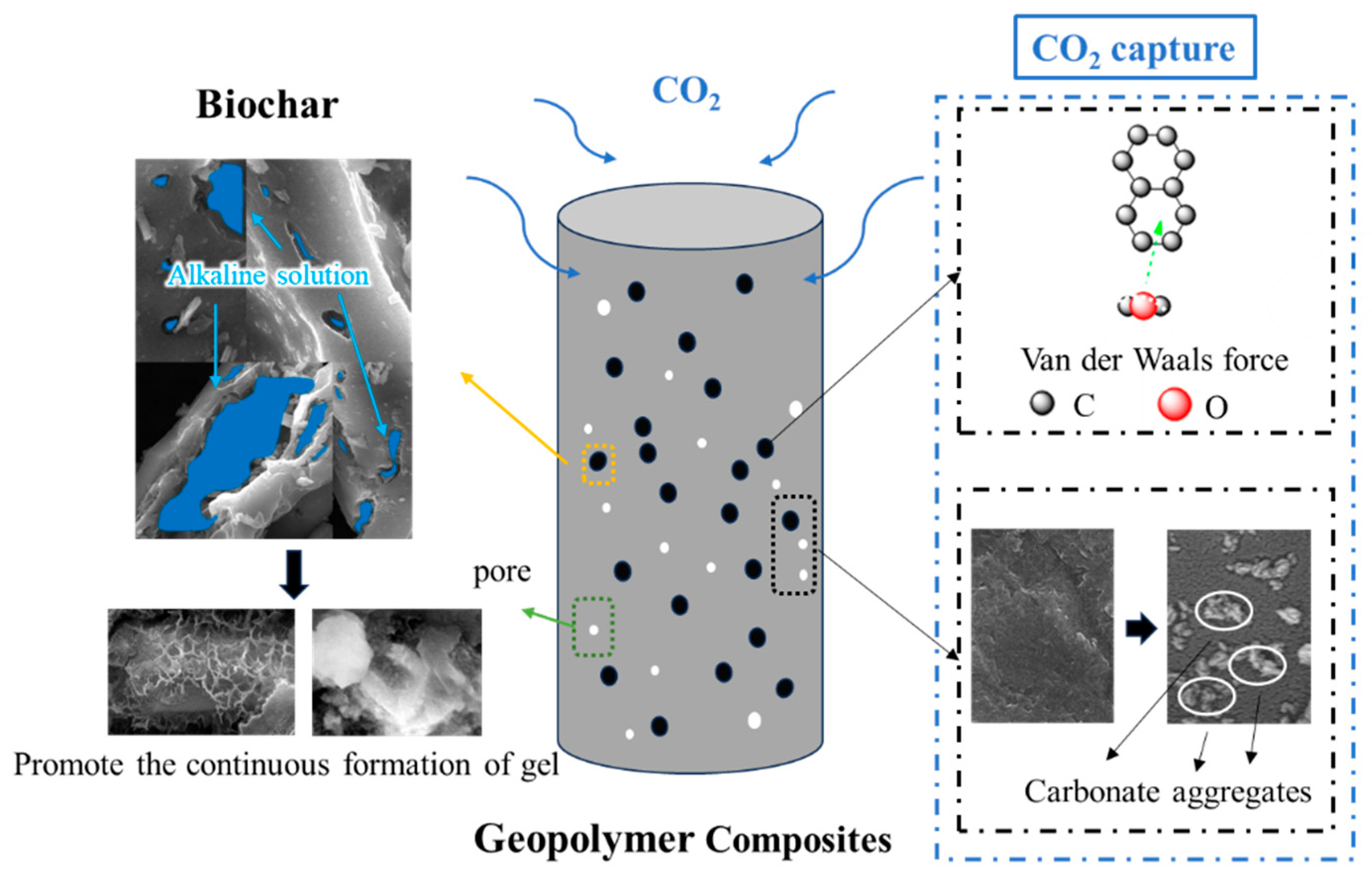
3.6. Discussion
4. Conclusions
- (1)
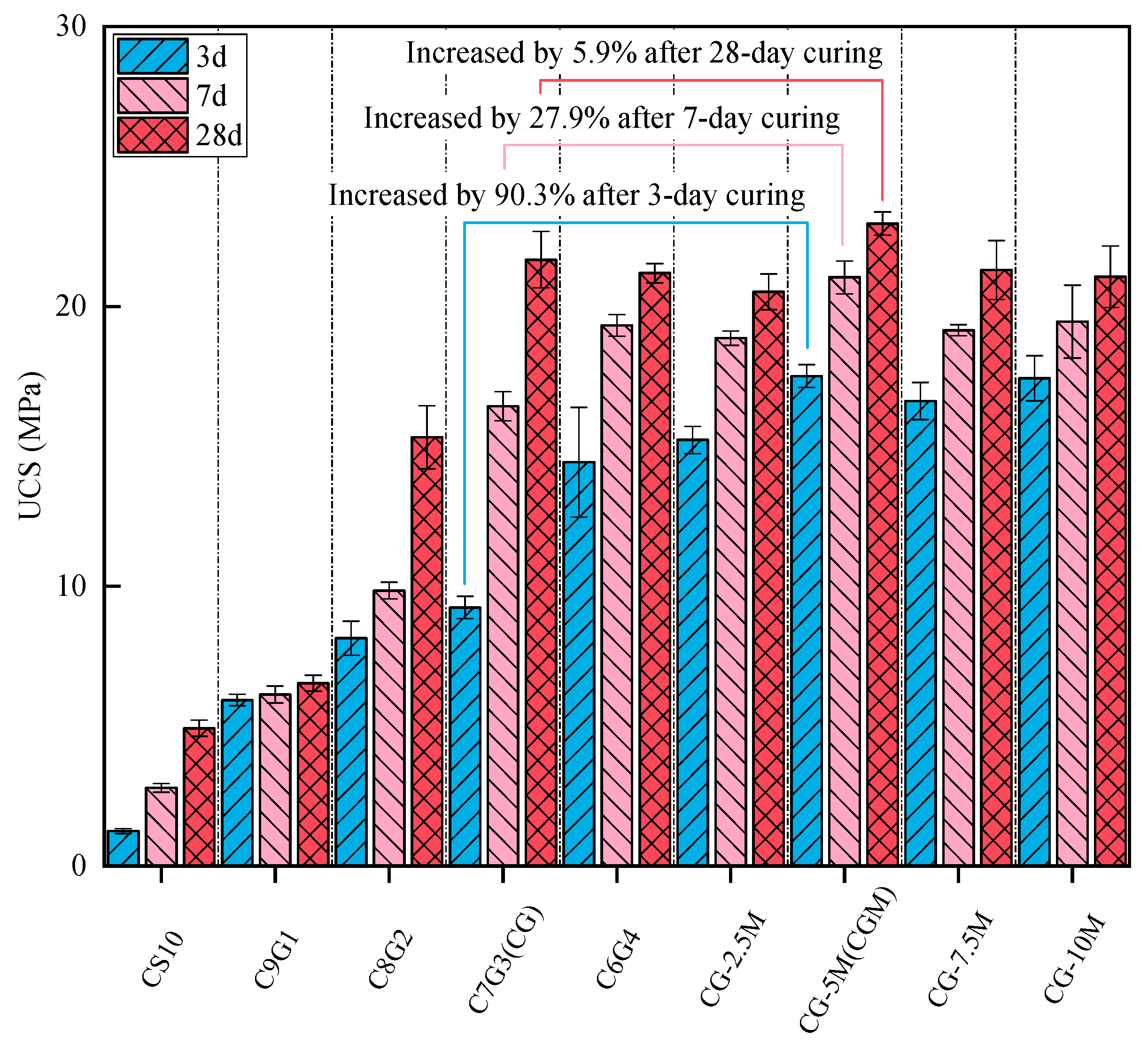
- CS plays a role not only as a cementitious material, but also as a fine aggregate in geopolymer composites produced from solid waste materials. The addition of MgO enhances the early strength of the specimens, while biochar contributes to the long-term strength development.
- (2)
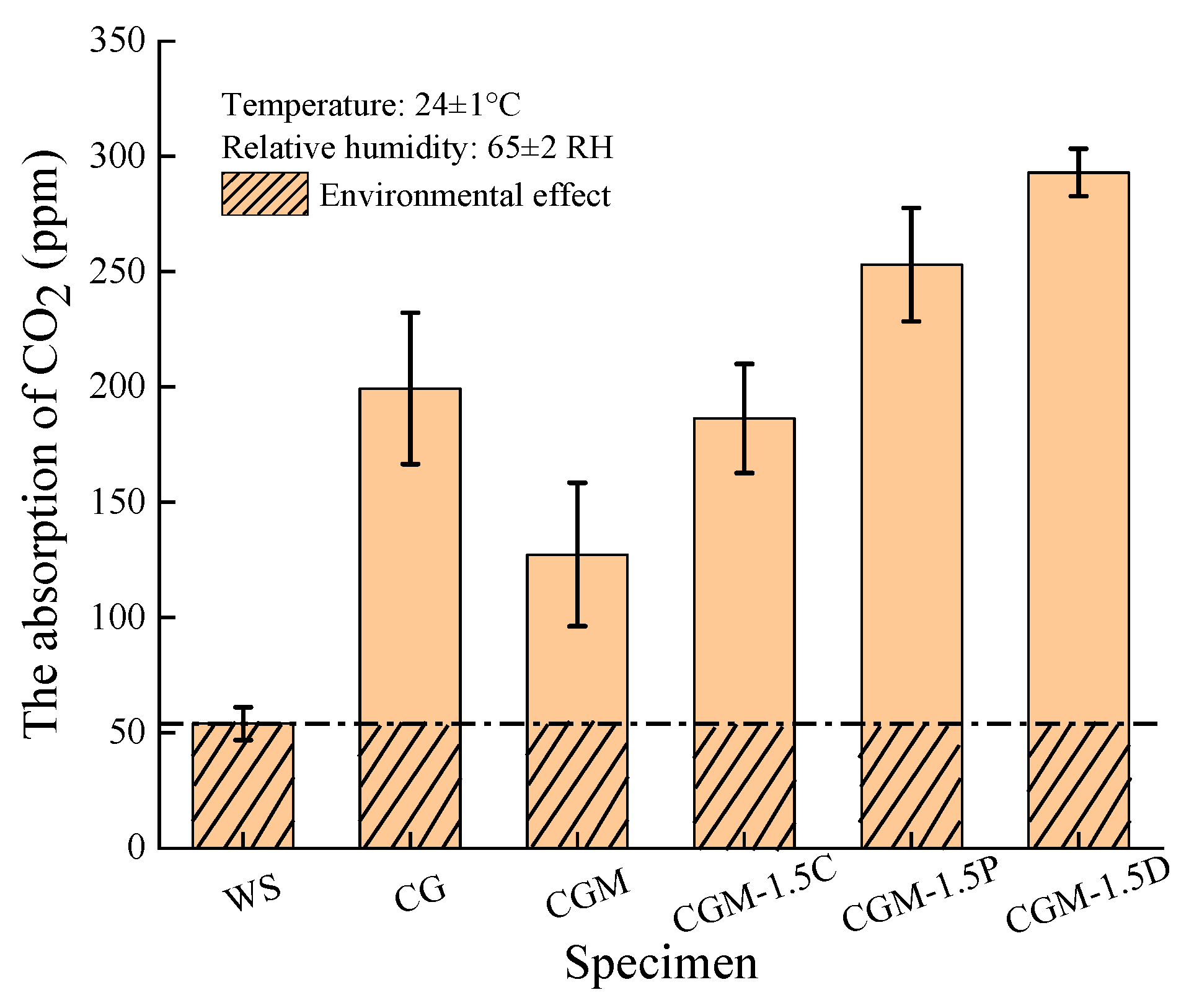
- As well as significant improvements in early strength (89.5%), 5% dosage of MgO added into CG can also improve the mineralization performance of CO2 in the preparation process. Although the CGM specimen exhibited diminished CO2 capture capacity during the monitoring stage, it achieved more efficient CO2 mineralization than the CG specimen.
- (3)
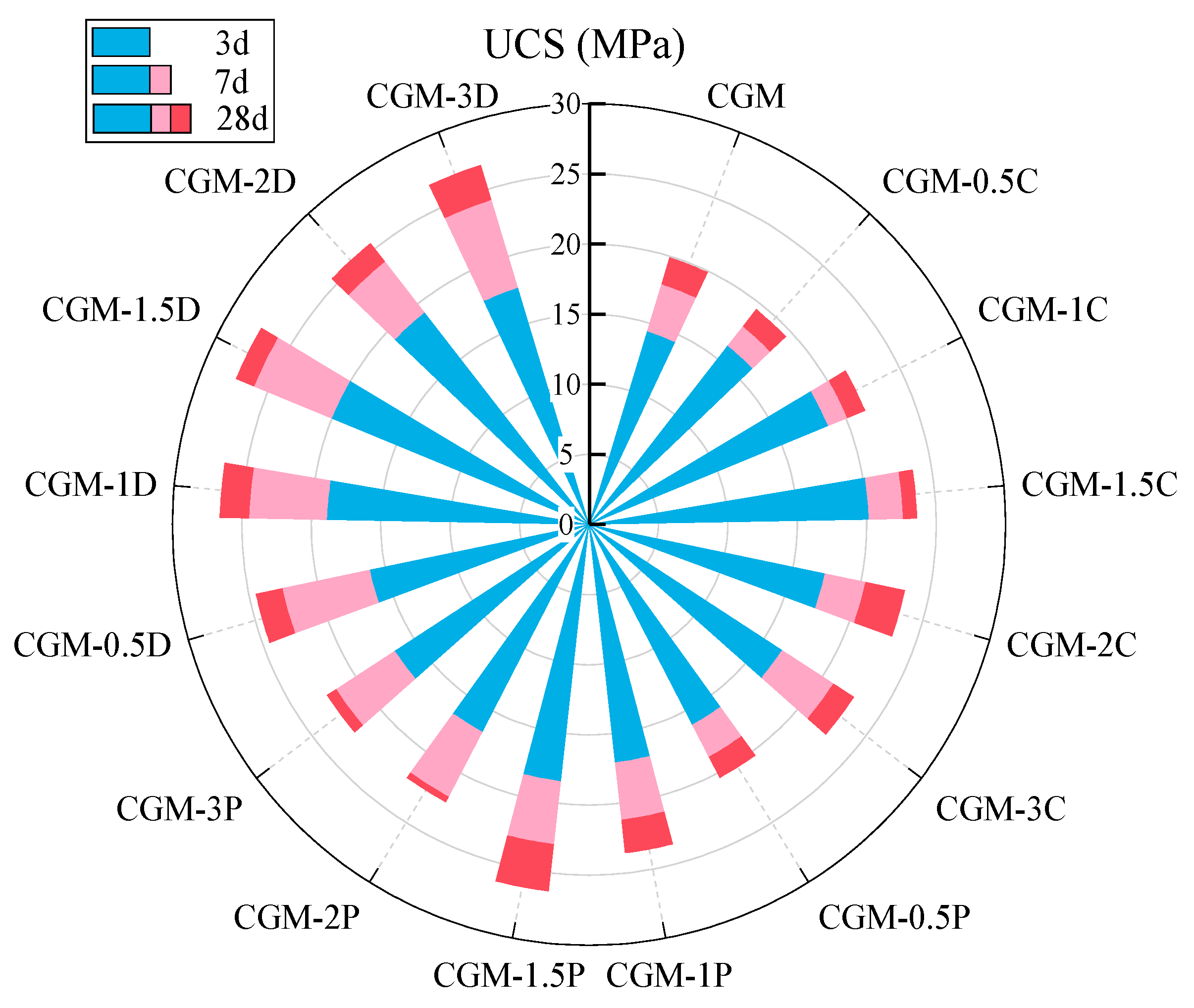
- The compressive strength of the geopolymer composite could be further increased by a 1.5% dosage of DSB through improving the pore structure and producing more gels (27.5 MPa at 28 days). In addition, the incorporation of an appropriate amount of porous biochar could not only enhance the CO2 capture capacity of the geopolymer composite but also improve the efficiency of CO2 mineralization.
- (4)
- DSB with an average pore size of 8.98 nm is more conducive to strength growth and CO2 mineralization than CSB and PSB with average pore sizes of around 1.99 and 2.17 nm, respectively.
- (5)
- The geopolymer composite formulated with solid waste materials (CS/GGBS, 7/3), 5% MgO, and 1.5% DSB represents the optimal mix ratio, demonstrating a compressive strength of 27.5 MPa, a CO2 mineralization weight ratio of 4.28%, and a CO2 capture capacity within a 24 h real-time monitoring test of 0.06318 g/kg. This optimized material formulation not only satisfies specific compressive strength requirements but also achieves a minimum CO2 mineralization capacity of 40.2 kg per ton of solid waste (CS/GGBS = 7/3), operating effectively without energy-intensive conditions (high temperature, pressure, humidity, or CO2 concentration).
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CO2 | carbon dioxide |
| CS | copper slag |
| GGBS | ground-granulated blast furnace slag |
| MgO | magnesium oxide |
| NaOH | sodium hydroxide |
| CSB | coconut shell biochar |
| PSB | peanut shell biochar |
| DSB | durian shell biochar |
| CC | Curvature coefficient |
| Cu | uniformity coefficient |
| 12 M | 12 mol/L |
| TGA | The thermogravimetric analysis |
| XRD | X-ray diffractometer |
| FESEM | Field Emission Scanning Electron Microscopy |
| EDS | Energy Dispersive Spectrometer |
| C-S-H | Calcium Silicate Hydrate |
| C-A-S-H | calcium alumina silicate hydrate |
| N-A-S-H | sodium alumina silicate hydrate |
| C7G3 | the weight ratio of CS and GGBS in solid waste raw materials is 7/3 |
| CG | the further simplification of C7G3 |
| CG-5M | the addition of MgO accounting for 5% by weight of solid waste raw materials in CG |
| CGM | the further simplification of CG-5M |
| CGM-1.5D | the addition of DSB, which accounted for 1.5% by weight of the solid waste raw materials in CG-5M |
References
- Freire, A.L.; Jose, H.J.; Moreira, R.D.F.P.M. Potential applications for geopolymers in carbon capture and storage. Int. J. Greenh. Gas Control 2022, 118, 103687. [Google Scholar] [CrossRef]
- Dunn, R.J.H.; Stanitski, D.M.; Gobron, N.; Willett, K.M. Global climate in “state of the climate in 2018”. Bull. Am. Meteorol. Soc. 2019, 100, 269. [Google Scholar]
- Li, M.S.; Wang, L.; Chang, S.; Liu, S.H. Comparative study on preparation and hydration mechanism of composite cementitious materials containing copper slag. Constr. Build. Mater. 2024, 446, 137977. [Google Scholar] [CrossRef]
- Wang, Y.B.; Li, X.H.; Miao, W.J.; Su, Y.; He, X.Y.; Strnadel, B. The carbon mineralization behavior of copper slag and its impact on pozzolanic reactivity. Cem. Concr. Compos. 2025, 157, 105899. [Google Scholar] [CrossRef]
- Miller, S.A.; Monteiro, P.J.M.; Ostertag, C.P.; Horvath, A. Comparison indices for design and proportioning of concrete mixtures taking environmental impacts into account. Cem. Concr. Compos. 2016, 70, 131–143. [Google Scholar] [CrossRef]
- Liang, X.; Ji, Y. Experimental study on durability of red mud-blast furnace slag geopolymer mortar. Constr. Build. Mater. 2020, 267, 120942. [Google Scholar] [CrossRef]
- Gupta, S.; Kua, H.W.; Koh, H.J. Application of biochar from food and wood waste as green admixture for cement mortar. Sci. Total Environ. 2018, 619, 419–435. [Google Scholar] [CrossRef]
- Tripathi, N.; Hills, C.D.; Singh, R.S.; Atkinson, C.J. Biomass waste utilisation in low-carbon products: Harnessing a major potential resource. NPJ Clim. Atmos. Sci. 2019, 2, 35. [Google Scholar] [CrossRef]
- Schlesinger, M.E.; Sole, K.C.; Davenport, W.G.; Flores, G.R.F.A. Extractive Metallurgy of Copper, 6th ed.; Elsevier: Cambridge, UK, 2021. [Google Scholar]
- Yan, Z.; Sun, Z.; Yang, J.; Yang, H.; Hu, K. Mechanical performance and reaction mechanism of copper slag activated with sodium silicate or sodium hydroxide. Constr. Build. Mater. 2021, 266, 120900. [Google Scholar] [CrossRef]
- Mirhosseini, S.R.; Fadaee, M.; Tabatabaei, R.; Fadaee, M.J. Mechanical properties of concrete with Sarcheshmeh mineral complex copper slag as a part of cementitious materials. Constr. Build. Mater. 2017, 134, 44–49. [Google Scholar] [CrossRef]
- Singh, J.; Singh, S. Development of alkali-activated cementitious material using copper slag. Constr. Build. Mater. 2019, 211, 73–79. [Google Scholar] [CrossRef]
- Spa, B.; Ps, A.; Rd, C. Abrasion resistance and slake durability of copper slag aggregate concrete. J. Build. Eng. 2020, 32, 101987. [Google Scholar] [CrossRef]
- Kranti, U.J.; Sai, A.N.; Krishna, A.R.; Srinivasu, K. An experimental investigation on effect of durability on strength properties of M40 grade concrete with partial replacement of sand with copper slag. Mater. Today Proc. 2020, 43, 2160–2165. [Google Scholar] [CrossRef]
- Sivasakthi, M.; Jeyalakshmi, R.; Rajamane, N.P. Fly ash geopolymer mortar: Impact of the substitution of river sand by copper slag as a fine aggregate on its thermal resistance properties. J. Clean. Prod. 2020, 279, 123766. [Google Scholar] [CrossRef]
- Chun, T.; Ning, C.; Long, H.; Li, J.; Yang, J. Mineralogical characterization of copper slag from tongling nonferrous metals group china. JOM 2016, 68, 2332–2340. [Google Scholar] [CrossRef]
- Edwin, R.S.; De Schepper, M.; Gruyaert, E.; De Belie, N. Effect of secondary copper slag as cementitious material in ultra-high performance mortar. Constr. Build. Mater. 2016, 119, 31–44. [Google Scholar] [CrossRef]
- Dhir, R.K.; Brito, J.; Mangabhai, R.; Lye, C.Q. Sustainable Construction Materials: Copper Slag, 1st ed.; Elsevier: Cambridge, UK, 2017. [Google Scholar]
- Moura, W.A.; Goncalves, J.P.; Lima, M.B.L. Copper slag waste as a supplementary cementing material to concrete. J. Mater. Sci. 2007, 42, 2226–2230. [Google Scholar] [CrossRef]
- Tixier, R.; Devaguptapu, R.; Mobasher, B. The effect of copper slag on the hydration and mechanical properties of cementitious mixtures. Cem. Concr. Res. 1997, 27, 1569–1580. [Google Scholar] [CrossRef]
- Sharma, R.; Khan, R.A. Sustainable use of copper slag in self compacting concrete containing supplementary cementitious materials. J. Clean. Prod. 2017, 151, 179–192. [Google Scholar] [CrossRef]
- Yaswanth, K.K.; Revathy, J.; Gajalakshmi, P. Influence of copper slag on Mechanical, durability and microstructural properties of GGBS and RHA blended strain hardening geopolymer composites. Constr. Build. Mater. 2022, 342, 128042. [Google Scholar] [CrossRef]
- Manjarrez, L.; Nikvar-Hassani, A.; Shadnia, R.; Zhang, L. Experimental Study of Geopolymer Binder Synthesized with Copper Mine Tailings and Low-Calcium Copper Slag. J. Mater. Civ. Eng. 2019, 31, 04019156. [Google Scholar] [CrossRef]
- Freire, A.L.; Moura-Nickel, C.D.; Scaratti, G.; De Rossi, A.; Araújo, M.H.; From Noni, A.D.N.; Rodrigues, A.E.; Castellón, E.R.; Moreira, R.D.P.M. Geopolymers produced with fly ash and rice husk ash applied to CO2 capture. J. Clean. Prod. 2020, 446, 122917. [Google Scholar] [CrossRef]
- Chen, Z.; Cang, Z.; Yang, F.; Zhang, J.; Zhang, L. Carbonation of steelmaking slag presents an opportunity for carbon neutral: A review. J. CO2 Util. 2021, 54, 101738. [Google Scholar] [CrossRef]
- Wang, J.; Chian, S.C.; Ding, M.J. Stabilization of dredged clays with ternary alkali-activated materials: Towards sustainable solid wastes recycling. J. Clean. Prod. 2023, 426, 139086. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, Q.; Xia, H.; Lv, J.; Feng, Z.; Yin, C.; Du, Y.; Li, Y.; Wang, Y. Highly efficient uranium (vi) capture by magnesium oxide loaded lotus seedpod-derived biochar via a hydrothermal and pyrolytic coupling process. Chem. Eng. Res. Des. Trans. Inst. Chem. Eng. Part A 2024, 206, 11. [Google Scholar] [CrossRef]
- Akinyemi, B.A.; Adesina, A. Recent advancements in the use of biochar for cementitious applications: A review. J. Build. Eng. 2020, 32, 101705. [Google Scholar] [CrossRef]
- Ruan, S.; Unluer, C. Comparative life cycle assessment of reactive MgO and Portland cement production. J. Clean. Prod. 2016, 137, 258–273. [Google Scholar] [CrossRef]
- Unluer, C.; Al-Tabbaa, A. Enhancing the carbonation of MgO cement porous blocks through improved curing conditions. Cem. Concr. Res. 2014, 59, 55–65. [Google Scholar] [CrossRef]
- Shanks, B.; Howe, C.; Draper, S.; Hong, W.; Cheeseman, C. Production of low-carbon amorphous SiO2 for use as a supplementary cementitious material and nesquehonite from olivine. Mater. Lett. 2024, 361, 136133. [Google Scholar] [CrossRef]
- Chen, Y.X.; Li, S.; Mezari, B.; Hensen, E.; Yu, R.; Schollbach, K.; Brouwers, H.; Yu, Q. Effect of highly dispersed colloidal olivine nano-silica on early age properties of ultra-high performance concrete. Cem. Concr. Compos. 2022, 131, 104564. [Google Scholar] [CrossRef]
- Jin, F.; Gu, K.; Al-Tabbaa, A. Strength and drying shrinkage of reactive mgo modified alkali-activated slag paste. Constr. Build. Mater. 2014, 51, 395–404. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, W.; Wang, R.; Chen, F.; Cong, P. Effects of reactive MgO on the reaction process of geopolymer. Materials 2019, 12, 526. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Tsang, D.C.W.; Guo, B.; Chi, S.P. Biochar as green additives in cement-based composites with carbon dioxide curing. J. Clean. Prod. 2020, 258, 120678. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Poon, C.S.; Wang, C.H.; Tsang, D.C.W. Roles of biochar and CO2 curing in sustainable magnesia cement-based composites. ACS Sustain. Chem. Eng. 2021, 9, 8603–8610. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Cheng, L.; Jin, H.; Xing, F. Sustainable upcycling of artificial lightweight cold-bonded aggregates (ALCBAs) designed by biochar and concrete slurry waste (CSW) into porous carbons materials for CO2 sequestration. Constr. Build. Mater. 2024, 412, 134736. [Google Scholar] [CrossRef]
- Yan, B.; Hu, Y.; Huan, Q.; Liu, Z.; Lai, J.; Wang, S.; Song, M. Optimal regulation of modified biochar in solid waste-derived lightweight aggregates: Enhancing carbonation mechanisms and negative carbon emissions. Chem. Eng. J. 2025, 508, 160920. [Google Scholar] [CrossRef]
- Mhasde, C.; Kurabayashi, K.; Gupta, N.; Behera, R.; Rahman, S.A. Beyond traditional solid adsorbents: A recent trend in carbon capture with geopolymer matrix composite. J. Environ. Chem. Eng. 2025, 13, 117311. [Google Scholar] [CrossRef]
- ASTM C618-23e1; Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D2166/D2166M-24; Standard Test Method for Unconfined Compressive Strength of Cohesive Soil. ASTM International: West Conshohocken, PA, USA, 2024.
- Romero-Hermida, I.; Santos, A.; Pérez-López, R.; García-Tenorio, R.; Esquivias, L.; Morales-Flórez, V. New method for carbon dioxide mineralization based on phosphogypsum and aluminium-rich industrial wastes resulting in valuable carbonated by-products. J. CO2 Util. 2017, 18, 15–22. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, Q.; Ma, S.; Huang, X.; Gan, M.; Ji, Z.; Chen, X.; Fan, X. Steel slag for carbon fixation and synthesis of alkali-activated material. Constr. Build. Mater. 2022, 351, 128959. [Google Scholar] [CrossRef]
- Wang, B.; Pan, Z.; Cheng, H.; Zhang, Z.; Cheng, F. A review of carbon dioxide sequestration by mineral carbonation of industrial byproduct gypsum. J. Clean. Prod. 2021, 310, 126930. [Google Scholar] [CrossRef]
- Chen, Z.; You, N.; Chen, C.; Gao, Z. Properties of dredged sludge solidified with alkali-activated slag-based materials and blended with copper slag as fine aggregates of mortars. Constr. Build. Mater. 2021, 312, 125459. [Google Scholar] [CrossRef]
- Jin, Q.B.; Liu, Z.B.; Lu, L.L.; Zhang, Y.; Luo, T.Y.; Tang, Y.S. Experimental study on the properties of CMTs-incorporated geopolymers prepared at low temperatures. J. Southeast Univ. (Engl. Ed.) 2024, 40, 295–303. [Google Scholar] [CrossRef]
- You, N.; Chen, Z.; Gao, Z.; Song, X. The effect of copper slag as a precursor on the mechanical properties, shrinkage and pore structure of alkali-activated slag-copper slag mortar. J. Build. Eng. 2024, 98, 111151. [Google Scholar] [CrossRef]
- Ali, H.A.; Xuan, D.X.; Lu, J.X.; Poon, C.S. Enhancing the resistance to microbial induced corrosion of alkali-activated glass powder/GGBS mortars by calcium aluminate cement. Constr. Build. Mater. 2022, 341, 127912. [Google Scholar] [CrossRef]
- Bajpai, R.; Shrivastava, A.; Singh, M. Properties of fly ash geopolymer modified with red mud and silica fume: A comparative study. SN Appl. Sci. 2020, 2, 1846. [Google Scholar] [CrossRef]
- Kaya, M.; Koksal, F.; Gencel, O.; Munir, M.; Kazmi, S. Influence of micro Fe2O3 and MgO on the physical and mechanical properties of the zeolite and kaolin based geopolymer mortar. J. Build. Eng. 2022, 52, 104443. [Google Scholar] [CrossRef]
- Hwang, C.L.; Vo, D.H.; Tran, V.A.; Yehualaw, M.D. Effect of high MgO content on the performance of alkali-activated fine slag under water and air curing conditions. Constr. Build. Mater. 2018, 186, 503–513. [Google Scholar] [CrossRef]
- Jin, F.; Gu, K.; Abdollahzadeh, A.; Al-Tabbaa, A. Effects of Different Reactive MgOs on the Hydration of MgO-Activated GGBS Paste. J. Mater. Civ. Eng. 2015, 27, B4014001. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Y.Y.; Wang, L.; Ruan, S.Q.; Chen, J.F.; Li, H.Y.; Yang, J.; Mechtcherine, V.; Tsang, D.C.W. Biochar-augmented carbon-negative concrete. Chem. Eng. J. 2022, 431, 133946. [Google Scholar] [CrossRef]
- GB/T 21144-2023; Solid Concrete Brick. National Standards of the People’s Republic of China: Beijing, China, 2023.
- JC/T 422-2007; Non-Fired Brick with Solid Waste or Tailings. People’s Republic of China Building Material Industry Standard: Shanghai, China, 2007.
- JC/T 239-2014; Fly Ash Brick. People’s Republic of China Building Material Industry Standard: Shanghai, China, 2014.
- ASTM C216-24; Standard Specification for Facing Brick (Solid Masonry Units Made from Clay or Shale). ASTM International: West Conshohocken, PA, USA, 2024.
- ASTM C62-23; Standard Specification for Building Brick (Solid Masonry Units Made from Clay or Shale). ASTM International: West Conshohocken, PA, USA, 2023.
- ASTM C902-22; Standard Specification for Pedestrian and Light Traffic Paving Brick. ASTM International: West Conshohocken, PA, USA, 2023.
- Zeng, Q.; Fang, R.; Li, H.D.; Peng, Y.; Wang, J.Y. Tailoring the thermal and mechanical properties of lightweight cement-based composites by macro and micro fillers. Cem. Concr. Compos. 2019, 102, 169–184. [Google Scholar] [CrossRef]
- Amar, M.; Ladduri, B.; Alloul, A.; Benzerzour, M.; Abriak, N.E. Geopolymer synthesis and performance paving the way for greener building material: A comprehensive study. Case Stud. Constr. Mater. 2024, 20, e03280. [Google Scholar] [CrossRef]
- Sun, X.; Xu, B.; Yi, Y. Effects of accelerated carbonation on fine incineration bottom ash: CO2 uptake, strength improvement, densification, and heavy metal immobilization. J. Clean. Prod. 2024, 475, 143714. [Google Scholar] [CrossRef]
- Xu, R.; Liu, W.; Wang, A.; Zhang, Z.; Chen, J. Strength development, phase evolution, microstructure change and environmental leaching behavior of alkali-activated copper slag. Constr. Build. Mater. 2025, 473, 141037. [Google Scholar] [CrossRef]
- Guo, L.; Liu, J.; Chen, D.; An, S. Mechanical properties and microstructure evolution of alkali-activated GGBS-fly ash-steel slag ternary cements. Constr. Build. Mater. 2024, 444, 137727. [Google Scholar] [CrossRef]
- Shi, D.; Ye, J.; Zhang, W.; Shen, W. Properties, mineralogy and microstructure evolution of 4-year calcium silicate slag-based alkali-activated materials. Cem. Concr. Compos. 2023, 136, 104857. [Google Scholar] [CrossRef]
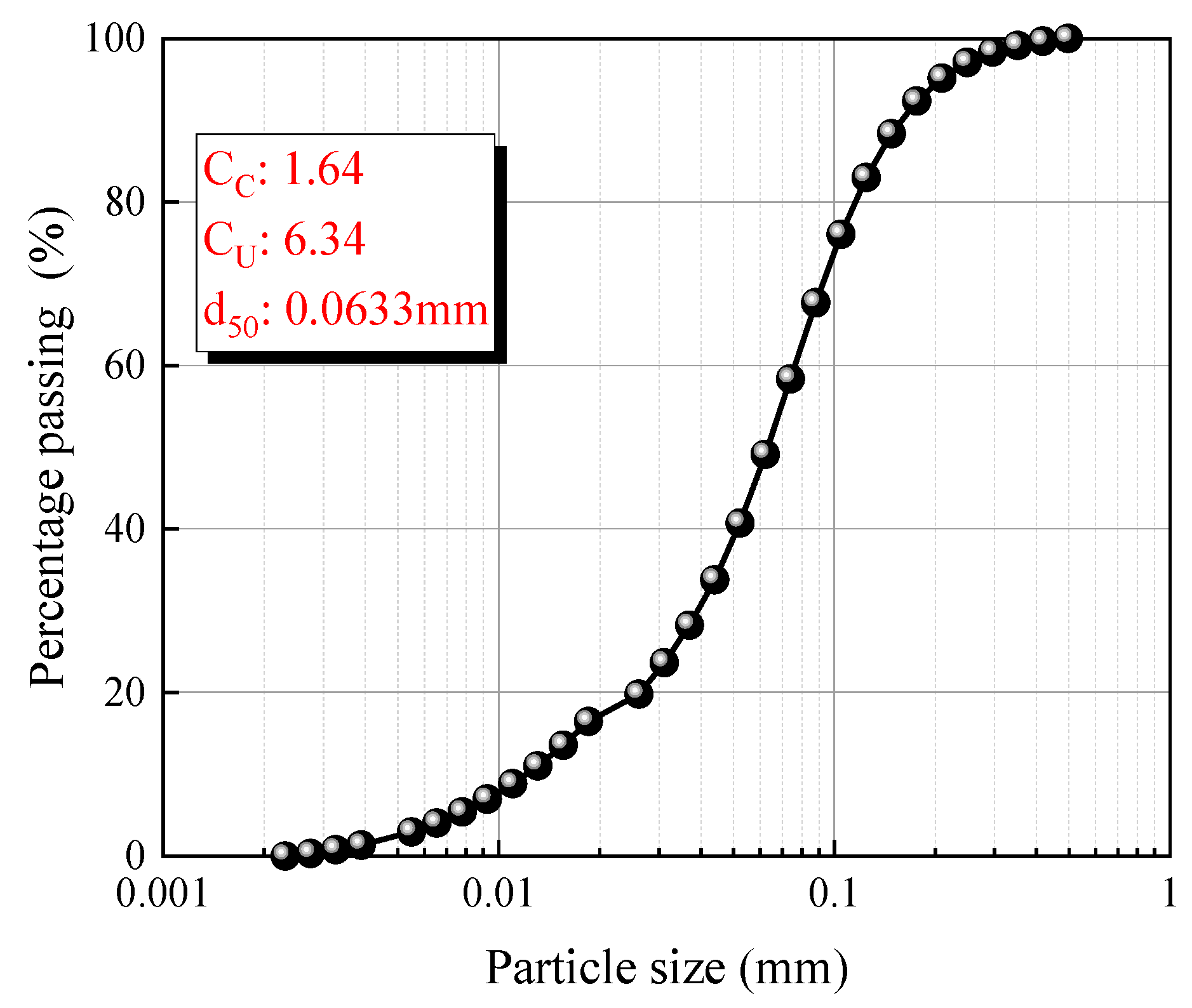
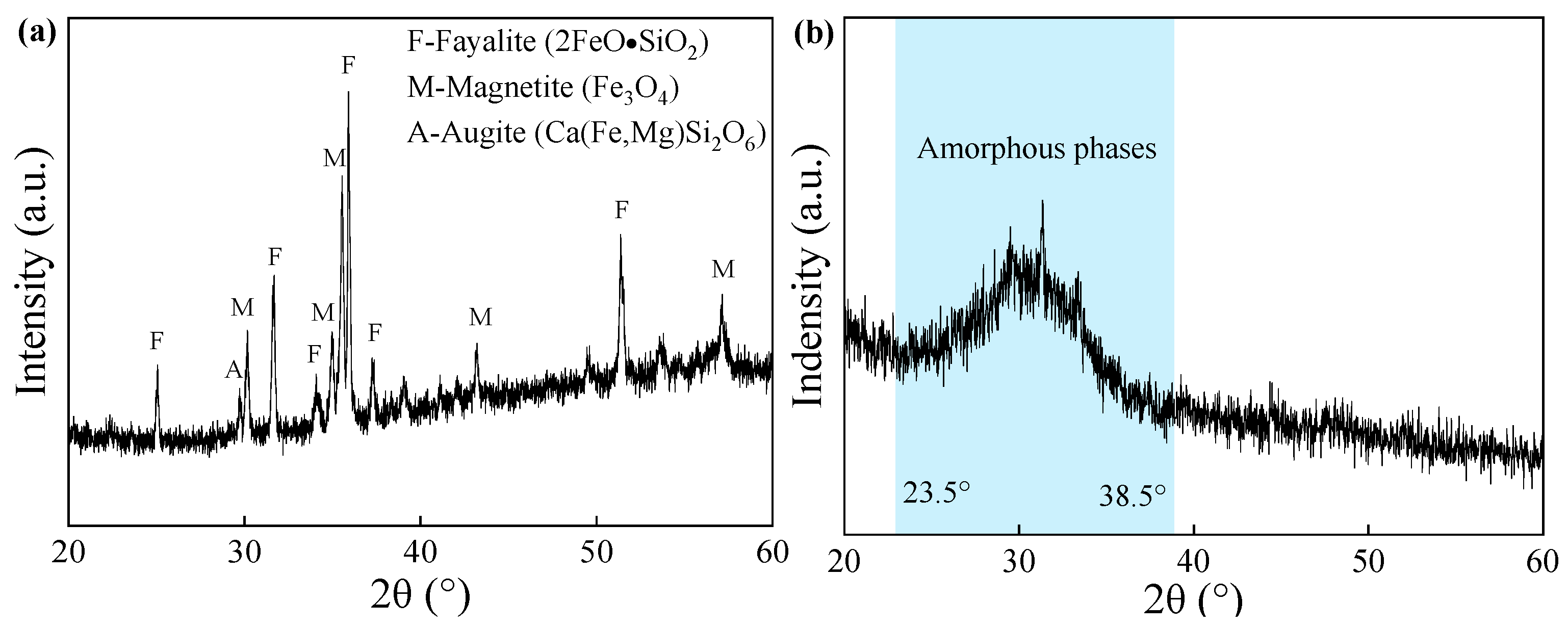

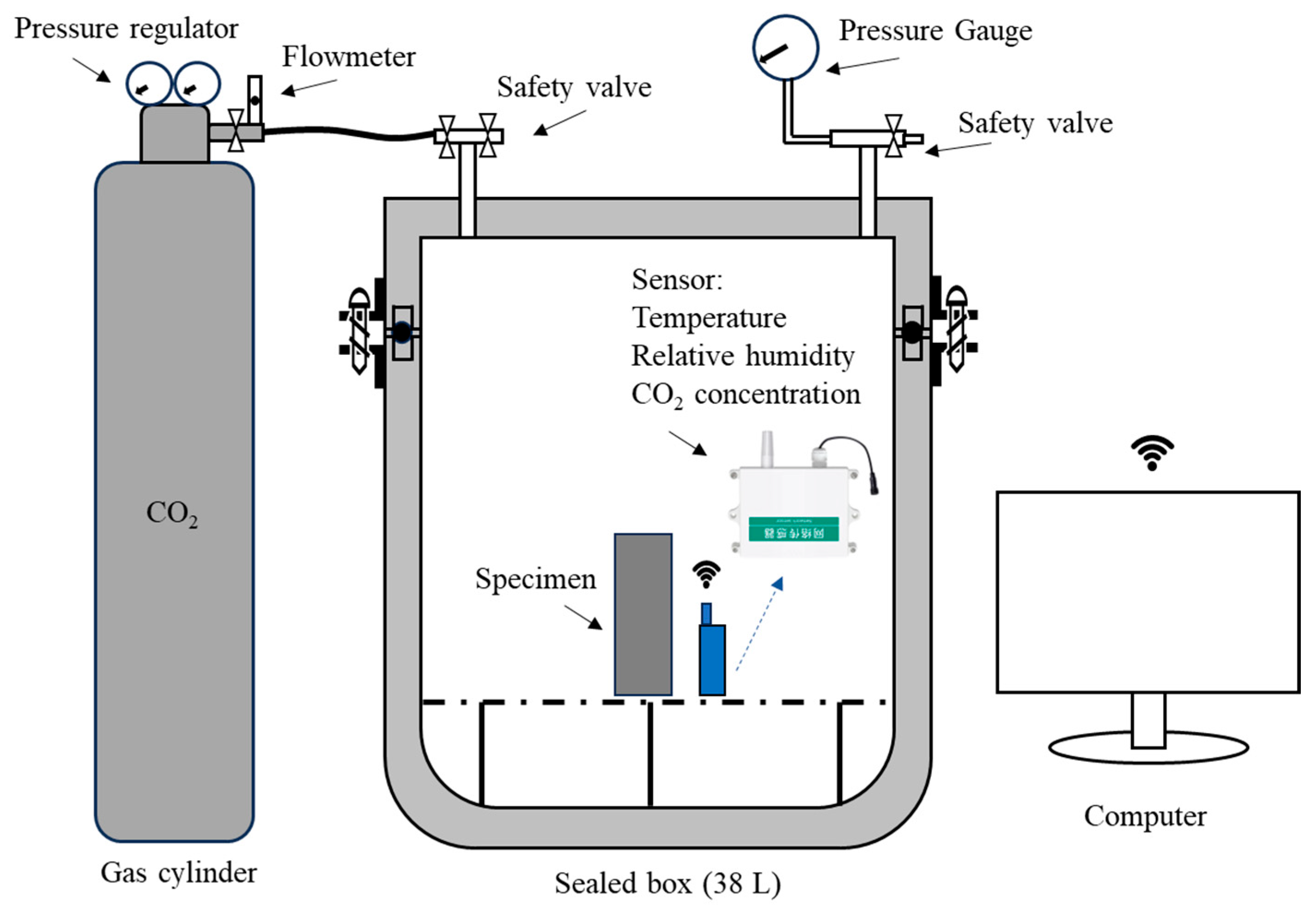
| Oxide Composition (wt.%) | CS | GGBS |
|---|---|---|
| Fe2O3 | 52.90 | 0.27 |
| SiO2 | 25.78 | 27.22 |
| Al2O3 | 6.05 | 15.86 |
| CaO | 4.60 | 37.74 |
| Na2O | 2.82 | 0.67 |
| ZnO | 1.94 | - |
| MgO | 1.72 | 7.63 |
| K2O | 1.16 | 0.34 |
| TiO2 | 0.35 | 1.14 |
| CuO | 0.27 | - |
| PbO | 0.25 | - |
| MnO | 0.21 | 0.26 |
| As2O3 | 0.19 | - |
| P2O5 | 0.13 | 0.02 |
| Cr2O3 | 0.05 | - |
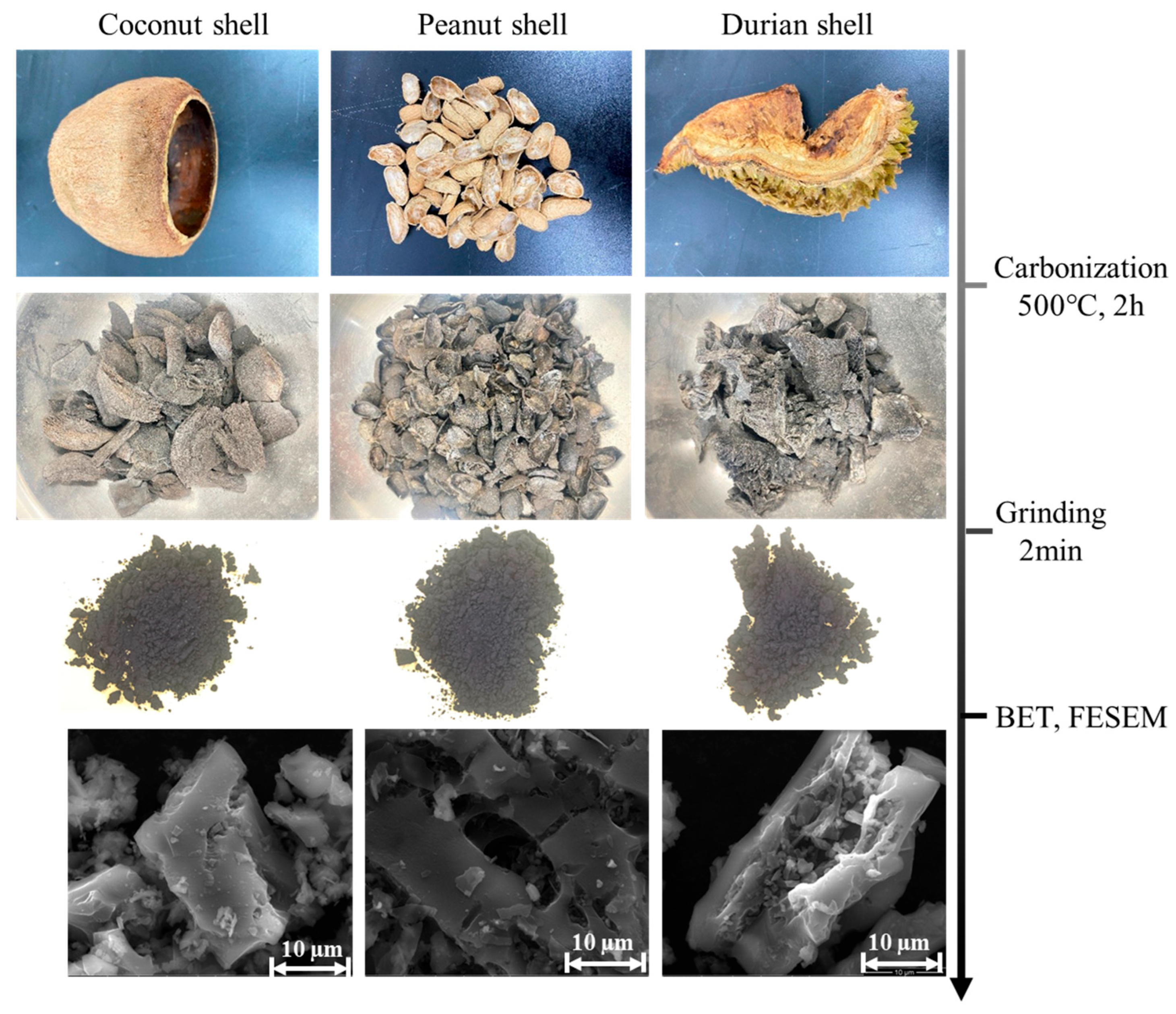
| Biochar | Specific Surface Area (m2/g) | Average Pore Size (nm) |
|---|---|---|
| Coconut shell biochar (CSB) | 108.54 | 1.99 |
| Peanut shell biochar (PSB) | 96.96 | 2.17 |
| Durian shell biochar (DSB) | 6.62 | 8.98 |
| Step | Mix ID | The Ratio of Solid Waste Raw Materials | MgO (%) | Biochar | NaOH (M) | W (%) | ||
|---|---|---|---|---|---|---|---|---|
| CS % | GGBS % | (%) | Type | |||||
| I | CS10 | 100 | 0 | - | - | - | 12.0 | 25.0 |
| C9G1 | 90 | 10 | - | - | - | |||
| C8G2 | 80 | 20 | - | - | - | |||
| C7G3 (CG) | 70 | 30 | - | - | - | |||
| C6G4 | 60 | 40 | - | - | - | |||
| II | CG-2.5M | 70 | 30 | 2.5 | - | - | ||
| CG-5M (CGM) | 70 | 30 | 5.0 | - | - | |||
| CG-7.5M | 70 | 30 | 7.5 | - | - | |||
| CG-10M | 70 | 30 | 10.0 | - | - | |||
| III | CGM-0.5C | 70 | 30 | 5.0 | 0.5 | CSB | ||
| CGM-1C | 70 | 30 | 5.0 | 1.0 | ||||
| CGM-1.5C | 70 | 30 | 5.0 | 1.5 | ||||
| CGM-2C | 70 | 30 | 5.0 | 2.0 | ||||
| CGM-3C | 70 | 30 | 5.0 | 3.0 | ||||
| CGM-0.5P | 70 | 30 | 5.0 | 0.5 | PSB | |||
| CGM-1P | 70 | 30 | 5.0 | 1.0 | ||||
| CGM-1.5P | 70 | 30 | 5.0 | 1.5 | ||||
| CGM-2P | 70 | 30 | 5.0 | 2.0 | ||||
| CGM-3P | 70 | 30 | 5.0 | 3.0 | ||||
| CGM-0.5D | 70 | 30 | 5.0 | 0.5 | DSB | |||
| CGM-1D | 70 | 30 | 5.0 | 1.0 | ||||
| CGM-1.5D | 70 | 30 | 5.0 | 1.5 | ||||
| CGM-2D | 70 | 30 | 5.0 | 2.0 | ||||
| CGM-3D | 70 | 30 | 5.0 | 3.0 | ||||
| Standard Code | Strength Grade | Average Compressive Strength (MPa) | Application |
|---|---|---|---|
| GB/T 21144-2023 [54] | MU20 | 20.0 | Use for buildings or structures |
| MU15 | 15.0 | ||
| MU10 | 10.0 | ||
| JC/T 422-2007 [55] | MU20 | 20.0 | |
| MU15 | 15.0 | ||
| JC/T 239-2014 [56] | MU20 | 20.0 | Use for industrial and civil buildings |
| MU15 | 15.0 | ||
| ASTM C216-24 [57] | SW | 20.7 | Use for buildings or structures |
| MW | 17.2 | ||
| ASTM C62-23 [58] | SW | 20.7 | |
| MW | 17.2 | ||
| NW | 10.3 | ||
| ASTM C902-22 [59] | MX | 17.2 | Use as paving material |
| NX | 17.2 |
| Specimen | The Absorption of CO2 (g) | The Absorption of CO2 per Unit Weight (mg/g) |
|---|---|---|
| CG | 0.0099 | 0.03613 |
| CGM | 0.0050 | 0.01894 |
| CGM-1.5C | 0.0090 | 0.03371 |
| CGM-1.5P | 0.0136 | 0.05292 |
| CGM-1.5D | 0.0163 | 0.06318 |
| Specimens | TG | Proportion of CO2 Mineralization in 24 h Monitoring (%) | ||
|---|---|---|---|---|
| A (105–200 °C, %) | B (250–400 °C, %) | C (500–750 °C, %) | ||
| CG | 12.39 | 1.97 | 1.17 | - |
| CGM | 11.02 | 2.07 | 2.01 | - |
| CGM-1.5D | 12.55 | 2.00 | 2.74 | - |
| CG-24h | 6.90 | 2.19 | 1.96 | 0.80 |
| CGM-24h | 9.48 | 2.41 | 2.76 | 0.75 |
| CGM-1.5D-24h | 8.56 | 2.29 | 4.28 | 1.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Q.; Xiang, W.; Xu, C.; Tang, G.; Liu, Z. Compressive Strength and CO2 Mineralization Mechanism of Copper Slag-GGBS Alkali-Activated Geopolymer Composites Enhanced by MgO and Biochar. Materials 2025, 18, 4434. https://doi.org/10.3390/ma18194434
Jin Q, Xiang W, Xu C, Tang G, Liu Z. Compressive Strength and CO2 Mineralization Mechanism of Copper Slag-GGBS Alkali-Activated Geopolymer Composites Enhanced by MgO and Biochar. Materials. 2025; 18(19):4434. https://doi.org/10.3390/ma18194434
Chicago/Turabian StyleJin, Quanbin, Wei Xiang, Chenghua Xu, Guoyi Tang, and Zhibin Liu. 2025. "Compressive Strength and CO2 Mineralization Mechanism of Copper Slag-GGBS Alkali-Activated Geopolymer Composites Enhanced by MgO and Biochar" Materials 18, no. 19: 4434. https://doi.org/10.3390/ma18194434
APA StyleJin, Q., Xiang, W., Xu, C., Tang, G., & Liu, Z. (2025). Compressive Strength and CO2 Mineralization Mechanism of Copper Slag-GGBS Alkali-Activated Geopolymer Composites Enhanced by MgO and Biochar. Materials, 18(19), 4434. https://doi.org/10.3390/ma18194434







