Evaluation of the Impact of Hydrogen Peroxide on ANFO-Based Materials’ Morphology
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
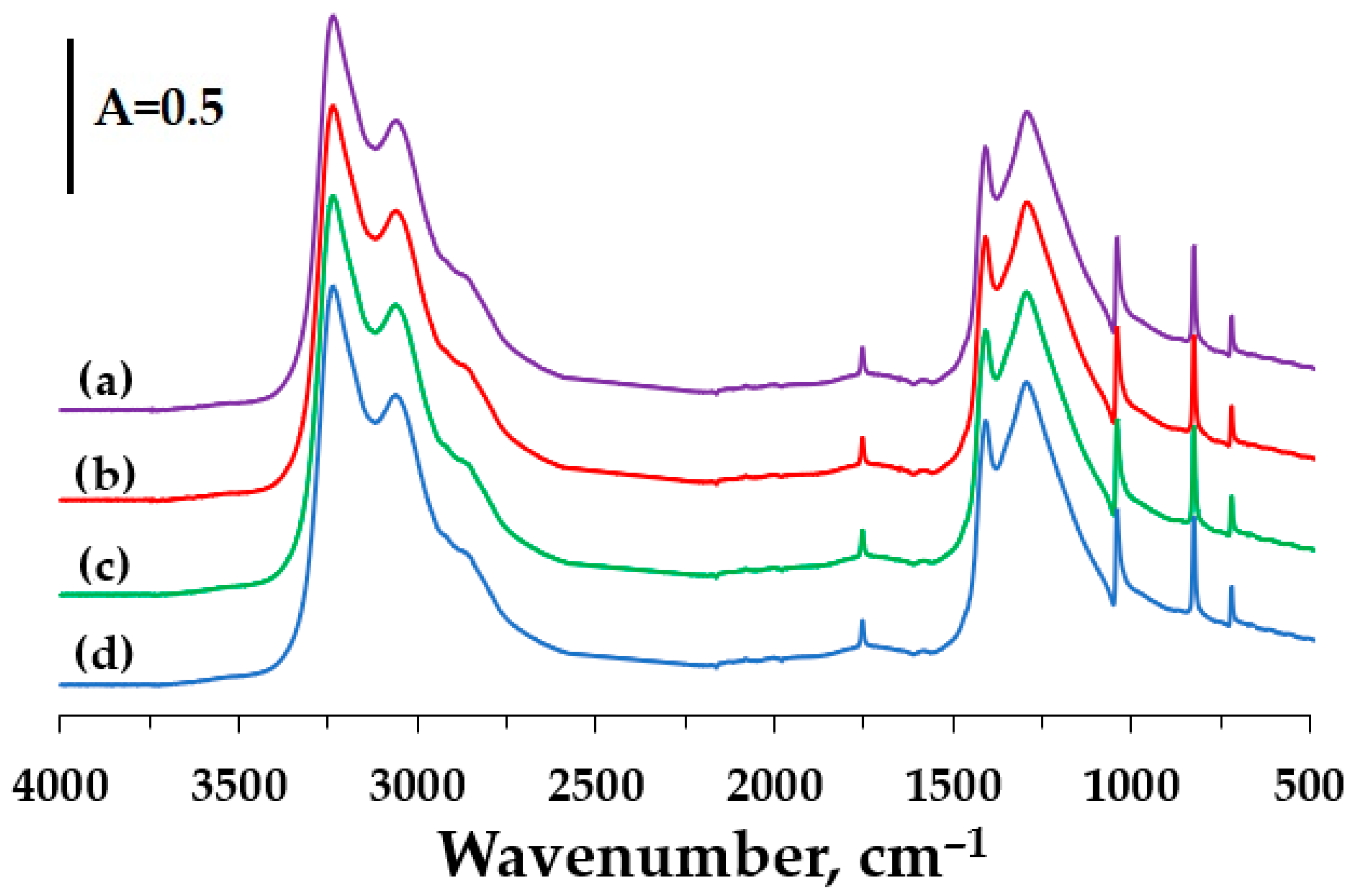
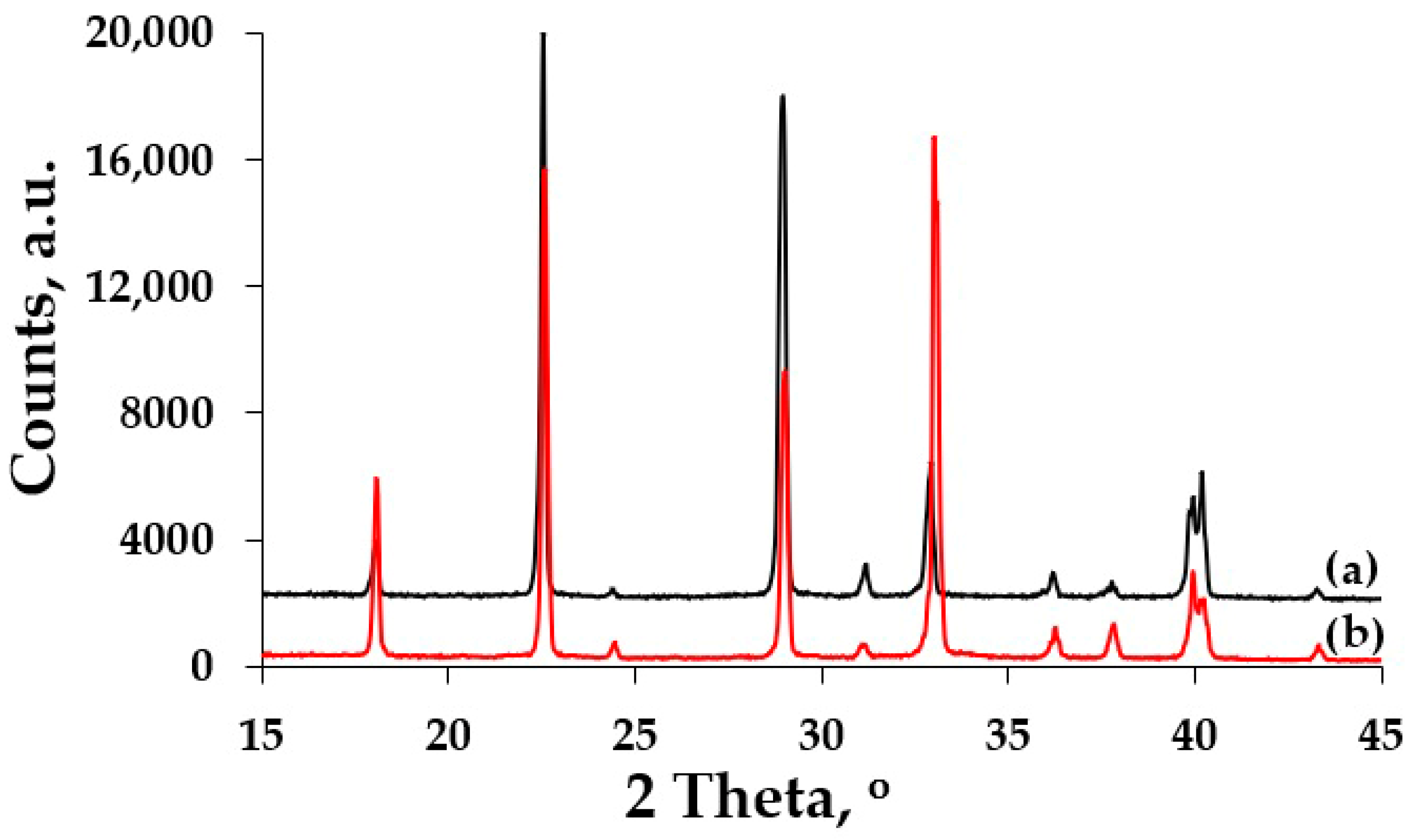
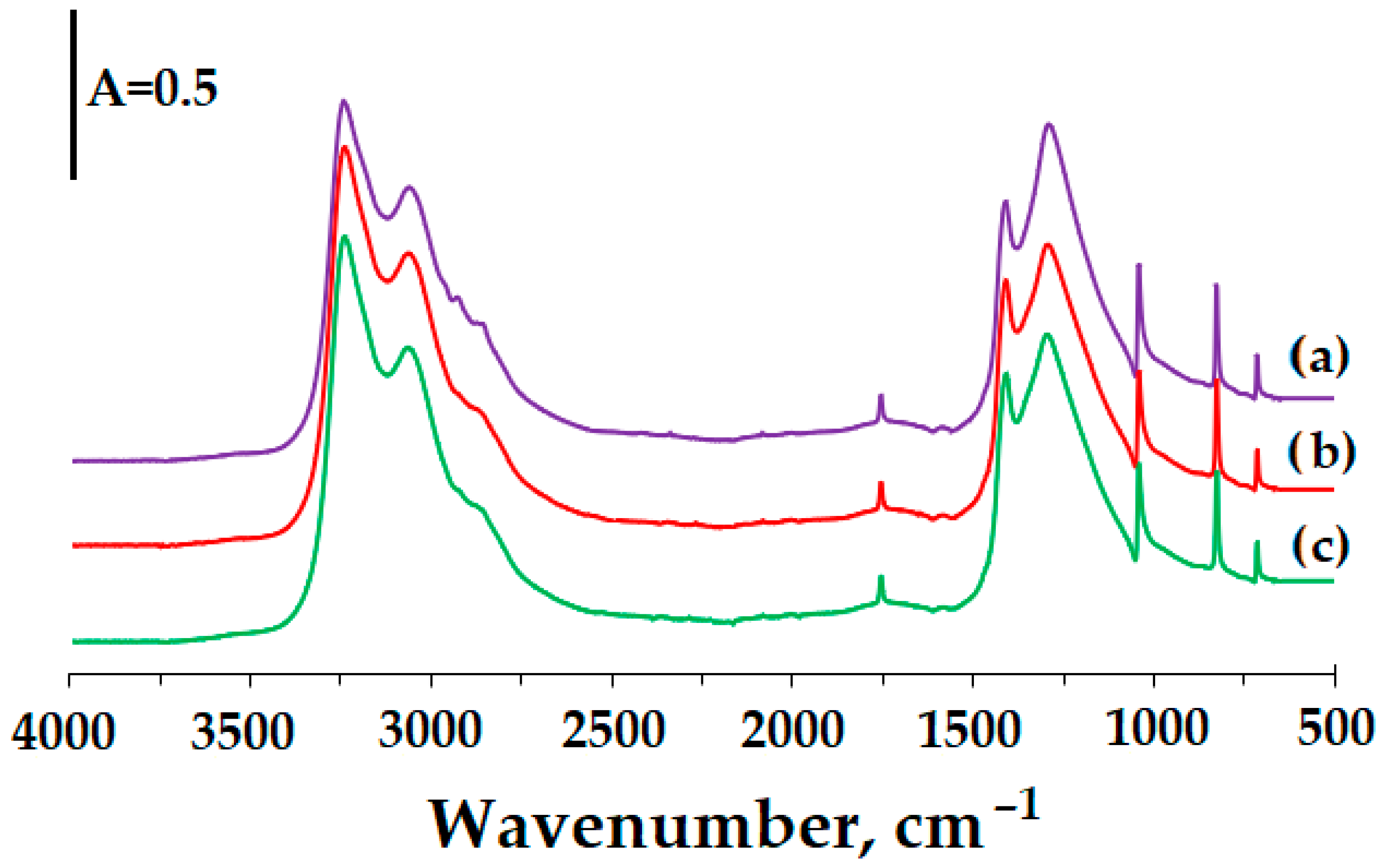
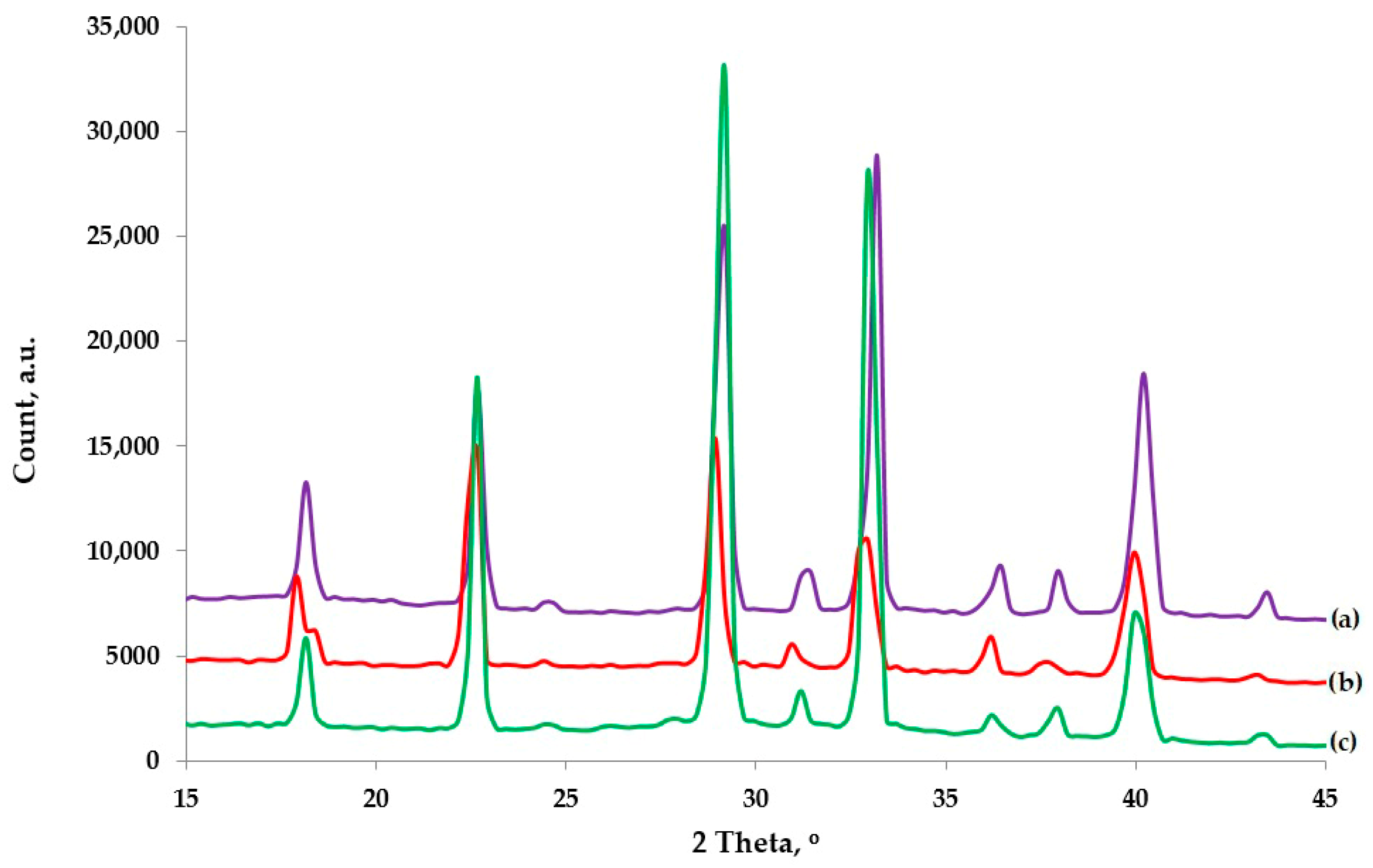
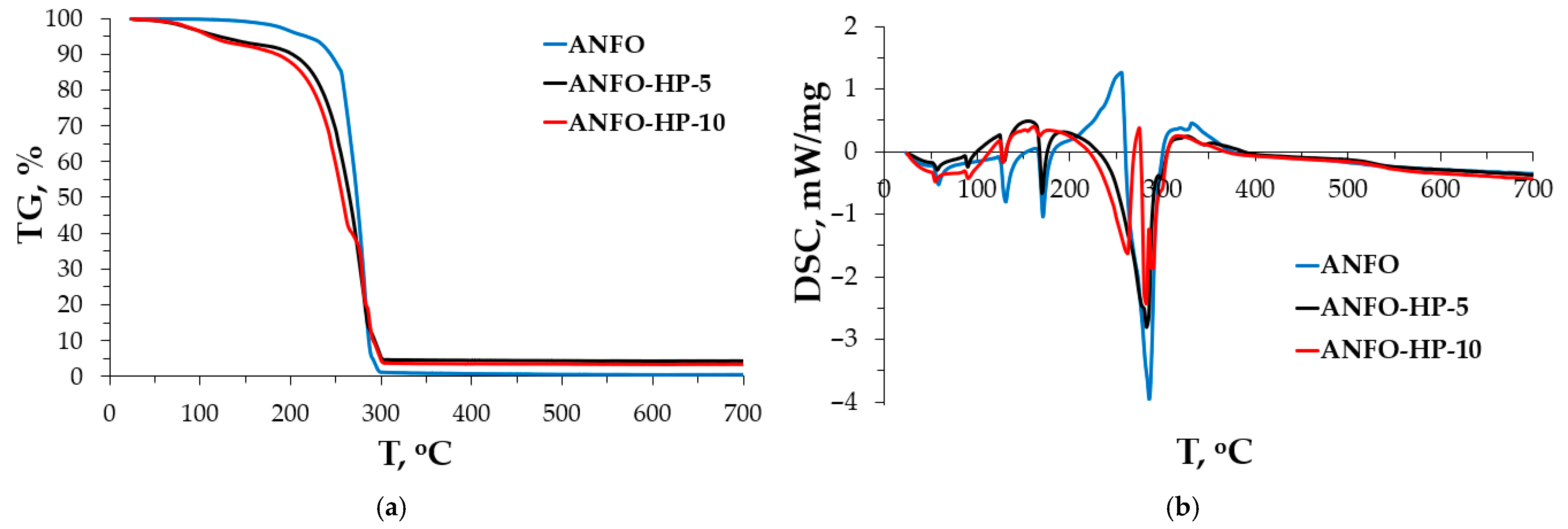
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Biessikirski, A.; Dworzak, M.; Pytlik, M.; Nachlik, S. Impact of the Type of Energetic Material on the Fume Emission in Open-Pit Mining. Sustainability 2025, 17, 2075. [Google Scholar] [CrossRef]
- Suceska, M.; Tumara, S.; Skrlec, V.; Stankovic, S. Prediction of concentration of toxic gases produced by detonation of commercial explosives by thermochemical equilibrium calculations. Def. Technol. 2022, 18, 2181–2189. [Google Scholar] [CrossRef]
- Suppajariyawat, P.; Elie, M.; Baron, M.; Gonzalez-Rodriguez, J. Classification of ANFO samples based on their fuel composition by GC–MS and FTIR combined with chemometric. Forensic Sci. Int. 2019, 301, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Onederra, I.; Bailey, V.; Cavanough, G.; Torrance, A. Understanding main causes of nitrogen oxide fumes in surface blasting. Min. Technol. 2012, 121, 151–159. [Google Scholar] [CrossRef]
- Oluwoye, I.; Dlugogorski, B.Z.; Gore, J.; Oskierski, H.; Altarawneh, M. Atmospheric emission of NOx from mining explosives: A critical review. Atmos. Environ. 2017, 167, 81–96. [Google Scholar] [CrossRef]
- Rawicki, Z.; Maj, A. Bezpieczeństwo robót strzałowych w aspekcie niebezpiecznych zdarzeń zaistniałych w zakłądach górniczych w latach 2020–2022. In Proceedings of the Bezpieczeństwo Robót Strzałowych, Ustroń, Poland, 4–6 October 2022. [Google Scholar]
- Kuterasiński, Ł.; Sadowska, M.; Żeliszewska, P.; Napruszewska, B.D.; Ruggiero-Mikołajczyk, M.; Pytlik, M.; Biessikirski, A. Cu-Containing Faujasite-Type Zeolite as an Additive in Eco-Friendly Energetic Materials. Molecules 2024, 29, 3184. [Google Scholar] [CrossRef]
- Biessikirski, A.; Gotovac Atlagić, S.; Pytlik, M.; Kuterasiński, Ł.; Dworzak, M.; Twardosz, M.; Nowak-Senderowska, D.; Napruszewska, B.D. The Influence of Microstructured Charcoal Additive on ANFO’s Properties. Energies 2021, 14, 4354. [Google Scholar] [CrossRef]
- Biessikirski, A.; Gotovac Atlagić, S.; Pytlik, M.; Kuterasiński, Ł.; Dworzak, M.; Twardosz, M.; Cała, M.; Jakóbczyk, J.; Sukur, S.; Stopkowicz, A.; et al. Influence of the Nano-Iron Oxide Adsorption-Enhanced Microstructured Charcoal Additives on the ANFO’s Properties. Energies 2024, 17, 461. [Google Scholar] [CrossRef]
- Lissianski, V.V.; Zamansky, V.M.; Maly, P.M. Effect of metal-containing additives on nox reduction in combustion and reburning. Combust. Flame 2001, 125, 1118–1127. [Google Scholar] [CrossRef]
- Škrlec, V.; Sućeska, M.; Dobrilović, M.; Vincek, J. Detonability of Ammonium Nitrate Mixtures with the Addition of Organic Materials. Appl. Sci. 2024, 14, 1580. [Google Scholar] [CrossRef]
- Yi, H.; Zhang, X.; Yang, H.; Li, L.; Wang, Y.; Zhan, S. Controlling toxic and harmful gas in blasting with an inhibitor. PLoS ONE 2023, 18, e0291731. [Google Scholar] [CrossRef]
- Barański, K. Analysis of the Possibility of Using “Green Pyrotechnics” in the Detonators MW. Ph.D. Thesis, AGH University of Science and Technology, Cracow, Poland, 27 September 2019. [Google Scholar]
- Kalombo, L.; Del Fabbro, O.; Conradie, C.; Focke, W.W. Sb6O13 and Bi2O3 as oxidants for Si in pyrotechnic time delay compositions. Propellants Explos. Pyrotech. 2007, 32, 454–460. [Google Scholar] [CrossRef]
- Gerlich, M.; Trzciński, W.A.; Hara, M. Combustion Characteristics and Thermochemistry of Selected Silicon-Based Compositions for Time-Delay Detonators. Materials 2025, 18, 1456. [Google Scholar] [CrossRef] [PubMed]
- Zakusylo, R.; Pavlenko, O.; Jarosz, T.; Maranda, A.; Zakusylo, D.; Stolarczyk, A. Study of the Thermal Decomposition Process of Explosive Mixtures Based on Hydrogen Peroxide. Molecules 2024, 29, 5616. [Google Scholar] [CrossRef] [PubMed]
- Rarata, G.; Smętek, J. Explosives based on hydrogen peroxide—A historical review and novel applications. High Energy Mater. 2016, 8, 56–62. [Google Scholar]
- SAND2015-0133R. Sandia National Laboratories. U.S. Department of Energy. Available online: https://www.osti.gov/servlets/purl/1177376 (accessed on 15 May 2025).
- Novak, I. Electronic structure of peroxide-based explosives. J. Electron Spectrosc. Relat. Phenom. 2024, 275, 147475. [Google Scholar] [CrossRef]
- Michel, P.; Boudenne, J.C.; Peillard, F.R.; Coulomb, R. Analysis of home-made peroxide-based explosives in water: A review. TrAC Trends Anal. Chem. 2023, 258, 116884. [Google Scholar] [CrossRef]
- Brockmann, M.; Glotz, G.; von Glasenapp, J.S.; Unterriker, L.; Neshchadin, D.; Gescheidt, G.; Herges, R. Highly Sensitive, Easy-to-Use, One-Step Detection of Peroxide-, Nitrate- and Chlorate-Based Explosives with Electron-Rich Ni Porphyrins. J. Am. Chem. Soc. 2024, 146, 13010–13024. [Google Scholar] [CrossRef]
- Papliński, A. Explosive potential of energetic mixtures based on hydrogen peroxide Potencjał wybuchowy mieszanin energetycznych zawierających nadtlenek wodoru. High Energy Mater. 2019, 11, 14–20. [Google Scholar] [CrossRef]
- Elzaki, B.I.; Zhang, Y.J. Anti-hygroscopic surface modification of ammonium nitrate (NH4NO3) coated by surfactants. Arab. J. Chem. 2020, 13, 3460–3473. [Google Scholar] [CrossRef]
- Lotspeich, E.; Petr, V. The characterization of ammonium nitrate mini-prills. In Dynamic Behavior of Materials; Song, B., Casem, D., Kimberley, J., Eds.; Conference Proceedings of the Society for Experimental Mechanics Series; Springer: Cham, Switzerland, 2015; Volume 1, pp. 319–325. [Google Scholar]
- Léonard, F.; Zhang, Z.; Krebs, H.; Bruno, G. Structural and Morphological Quantitative 3D Characterisation of Ammonium Nitrate Prills by X-Ray Computed Tomography. Materials 2020, 13, 1230. [Google Scholar] [CrossRef]
- Biessikirski, A.; Kaczmarczyk, G.P.; Kuterasiński, Ł.; Kolano, M.; Pytlik, M. Evaluation of Ammonium Nitrate(V) Morphology and Porosity Obtained by SEM and Tomography Imaging. Materials 2024, 17, 3156. [Google Scholar] [CrossRef]
- Biessikirski, A.; Kaczmarczyk, G.P.; Kuterasiński, Ł.; Machowski, G.; Stopkowicz, A.; Ruggiero-Mikołajczyk, M. Analysis of Wall Thickness and Absorption Characteristics of Ammonium Nitrate(V) from Various Sources. Materials 2024, 17, 4618. [Google Scholar] [CrossRef]
- Viktorov, S.D.; Frantov, A.E.; Lapikov, I.N.; Andreev, V.V.; Starshinov, A.V. Effect of the microstructure of ammonium nitrate granules on the detonability of composite propellants based on it. Combust. Explos. Shock Waves 2016, 52, 727–731. [Google Scholar] [CrossRef]
- Polis, M.; Stolarczyk, A.; Szydło, K.; Fabin, M.; Pytlik, M.; Lisiecka, B.; Jarosz, T. Comprehensive study on impact of hydrogen peroxide decomposition on the crucial parameters of OSM-type energetic materials. Sci. Rep. 2024, 14, 14093. [Google Scholar] [CrossRef]
- Paszula, J.; Maranda, A.; Nikolczuk, K.; Giercuszkiewicz, A. Modification of the Detonation Parameters of Mining Explosives Containing Hydrogen Peroxide and Aluminium Powder. Cent. Eur. J. Energ. Mater. 2021, 18, 477–491. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, P.; Shi, W.; Yu, X.; Li, D.; Li, Y.; Wang, T.; Zhao, Z.; Gong, T. Effects of hydrogen peroxide on the combustion and emissions of an ethanol/gasoline combined injection engine under different excess air ratios. Effects of hydrogen peroxide addition on combustion characteristics of n-decane/air mixtures. Fuel 2018, 223, 324–333. [Google Scholar] [CrossRef]
- Wu, B.H.; Chan, M.N.; Chan, C.K. FTIR characterization of polymorphic transformation of ammonium nitrate. Aerosol. Sci. Technol. 2007, 41, 581. [Google Scholar] [CrossRef]
- Theoret, A.; Sandorey, C. Infrared spectra and crystalline phase transitions of ammonium nitrate. Can. J. Chem. 1964, 42, 57–62. [Google Scholar] [CrossRef]
- Pretsch, E.; Buhlmann, P.; Badertscher, M. Structure Determination of Organic Compounds. In Tables of Spectral Data, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Minakshi, M.; Barmi, M.; Mitchell, D.R.G.; Barlow, A.J.; Fichtner, M. Effect of oxidizer in the synthesis of NiO anchored nanostructure nickel molybdate for sodium-ion battery. Mater. Today Energy 2018, 10, 1–14. [Google Scholar] [CrossRef]
- Biessikirski, A.; Kuterasiński, Ł.; Dworzak, M.; Twardosz, M.; Tatko, M.; Napruszewska, B.D. On the Influence of the Ammonium Nitrate(V) Provenance on Its Usefulness for the Manufacture of ANFO Type Explosives. Energies 2020, 13, 4942. [Google Scholar] [CrossRef]
- Hendricks, S.B.; Posnjak, E.; Kracek, F.C. Molecular rotation in the solid state. The variation of the crystal structure of ammonium nitrate with temperature. J. Am. Chem. Soc. 1932, 54, 2766–2787. [Google Scholar] [CrossRef]
- Biessikirski, A.; Kuterasiński, Ł. Research on Morphology and Topology of ANFO Based on Various Types of Oxygen Component; Wydawnictwa AGH: Kraków, Poland, 2018. [Google Scholar]
- Vargeese, A.A.; Joshi, S.S.; Krishnamurthy, V.N. Effect of method of crystallization on the IV-III and IV-II polymorphic transitions of ammonium nitrate. J. Hazard. Mater. 2009, 161, 373–379. [Google Scholar] [CrossRef]
- Boeyens, J.C.A.; Ferg, E.; Levendis, D.C.; Schoning, F.R.L. X-ray diffraction analysis of the ammonium nitrate IV–III–II and IV–II phase changes under controlled humidity conditions. S.-Afr. Tydskr. Chem. 1991, 44, 42–46. [Google Scholar]
- Harju, M.E.E.; Minkkinen, P.; Valkonen, J. Transition paths between phases IV, III and II of ammonium nitrate predicted from X-ray powder diffractometer and differential scanning calorimeter data by partial least-squares regression. Chemom. Intell. Lab. Syst. 1994, 23, 341–350. [Google Scholar] [CrossRef]
- Golovina, N.; Nechiporenko, G.; Nemtsev, G.; Zyuzin, I.; Manelis, G.B.; Lempert, D. Ammonium nitrate phase state stabilization with small amounts of some organic compounds. Cent. Eur. J. Energ. Mater. 2009, 6, 45–56. [Google Scholar]
- van Driel, C.A.; van der Heijden, A.E.D.M.; de Boer, S.; van Rosmalen, G.M. The III–IV phase transition in ammonium nitrate: Mechanisms. J. Cryst. Growth 1994, 141, 404–418. [Google Scholar] [CrossRef]
- Yu, H.; Duan, D.; Liu, H.; Yang, T.; Tian, F.; Bao, K.; Li, D.; Zhao, Z.; Liu, B.; Cui, T. Ab initio molecular dynamic study of solid-state transitions of ammonium nitrate. Sci. Rep. 2016, 6, 18918. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, A.G.; Mel’nik, E.A.; Mikhaylov, A.A.; Mayorov, N.S.; Tripol’skaya, T.A.; Lev, O.; Prikhodchenko, P.V.; Churakov, A.V. Hydrogen bonding of H2O2 molecules in crystal structures of alkali metal and tetraethylammonium nitrate peroxosolvates. CrystEngComm 2025, 13, 1896–1903. [Google Scholar] [CrossRef]
- Wagaman, K.L. Inorganic Nitrate-Hydrogen Peroxide Adducts and Methods for Their Preparation. Patent US20080190525A1, 20 August 2007. [Google Scholar]
- Engel, W.; Eisenreich, N. Thermal analysis of the system NH4NO3/CsNO3 by means of X-ray diffraction. Thermochim. Acta 1985, 85, 35–38. [Google Scholar] [CrossRef]
- Oxley, J.C.; Smith, J.L.; Rogers, E.; Yu, M. Ammonium nitrate: Thermal stability and explosivity modifiers. Thermochim. Acta 2002, 384, 23–45. [Google Scholar] [CrossRef]
- Babrauskas, V.; Leggett, D. Thermal decomposition of ammonium nitrate. FAM Fire Mater. 2020, 44, 250–268. [Google Scholar] [CrossRef]
- Boast, C.W.; Simmons, F.W. Encyclopedia of Soils in the Environment. In Evaporation of Water from Base Soil; Hillel, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 494–502. ISBN 9780123485304. [Google Scholar]
- Klemm, E.; Mathivanan, G.; Schwarz, T.; Schirrmeister, S. Evaporation of hydrogen peroxide with a microstructured falling film. Chem. Eng. Process. Process Intensif. 2011, 50, 1010–1016. [Google Scholar] [CrossRef]
- Chen, X.; Xi, X.; Xiao, G.; Zhang, L.; Wang, Z.; Long, W. Effect of ambient temperature and water content on emulsified heavy fuel oil droplets evaporation: Evaporation enhancement by droplet puffing and micro-explosion. Fuel 2023, 334, 126614. [Google Scholar] [CrossRef]
- Fauzy, A.; Chen, G.B.; Lin, T.H. Applications of the addition of hydrogen peroxide solution on methane premixed combustion. Case Stud. Therm. Eng. 2025, 73, 106403. [Google Scholar] [CrossRef]
- Kaniewski, M.; Huculak-Mączka, M.; Zieliński, J.; Biegun, M.; Hoffmann, K.; Hoffmann, J. Crystalline phase transitions and reactivity of ammonium nitrate in systems containing selected carbonate salts. Crystals 2021, 11, 1250. [Google Scholar] [CrossRef]
- Oxley, J.C.; Kaushik, S.M.; Gilson, N.S. Thermal decomposition of ammonium nitrate-based composites. Thermochim. Acta 1989, 153, 269–286. [Google Scholar] [CrossRef]
- Wolny, P.; Tuśnio, N.; Lewandowski, A.; Mikołajczyk, F.; Kuberski, S. Formation of an ammonium nitrate fuel oil similar type of explosive under fire conditions: Materials based on selected polymers (PUR). Energies 2022, 15, 1674. [Google Scholar] [CrossRef]
- Dave, P.N.; Sirach, R. Comparative study of the thermal decomposition of ammonium nitrate in the presence of nanocrystalline copper ferrite. Mater. Adv. 2023, 4, 6665–6672. [Google Scholar] [CrossRef]
- Bohn, M.A.; Heil, M.; Pontius, H.; Koch, E.-C. Insensitive high explosives: VI. Experimental determination of the chemical compatibility of nitroguanidine with seven high explosives. Propellants Explos. Pyrotech. 2024, 49, e202300055. [Google Scholar] [CrossRef]
- Scholl, J.; Werling, L.; Freudenmann, D.; Schlechtriem, S. Activation energy determination of high-test peroxide decomposition in Inconel 718 crucibles. In Proceedings of the Aerospace Europe Conference 2023, 10th EUCASS 9th CEA, Lausanne, Switzerland, 9–13 July 2023. [Google Scholar] [CrossRef]
- Fabin, M.; Stolarczyk, A.; Zakusylo, R.; Jarosz, T. Following the decomposition of hydrogen peroxide in on-site mixture explosives: Study of the effect of the auxiliary oxidising agent and binder. Molecules 2023, 28, 5957. [Google Scholar] [CrossRef]
- Siddiqui, S.; Keswani, M.; Brooks, B.; Fuerst, A.; Raghavan, S. A study of hydrogen peroxide decomposition in ammonia–peroxide mixtures (APM). Microelectron. Eng. 2013, 102, 68–73. [Google Scholar] [CrossRef]
- Pędziwiatr, P.; Mikołajczyk, F.; Zawadzki, D.; Mikołajczyk, K.; Bedka, A. Decomposition of hydrogen peroxide—Kinetics and review of chosen catalysts. Acta Innov. 2018, 26, 45. [Google Scholar] [CrossRef]
- Araos, M.; Onederra, I. Detonation characteristics of a NOx-free mining explosive based on sensitised mixtures of low concentration hydrogen peroxide and fuel. Cent. Eur. J. Energ. Mater. 2017, 14, 759–774. [Google Scholar] [CrossRef]
- Fullelove, I.; Araos, M.; Onederra, I. Detonation performance of novel hydrogen peroxide and nitrate-based hybrid explosives. In Stockholm Conference Proceedings 2017; Holmberg, R., Ed.; European Federation of Explosives Engineers: Stockholm, Sweden, 2017; ISBN 978-0-9550290-5-9. [Google Scholar]
- Wintenberger, E.; Shepherd, J.E. Detonation Waves and Pulse Detonation Engines; Explosion Dynamics Laboratory, Graduate Aeronautical Laboratories, California Institute of Technology: Pasadena, CA, USA, 2004. [Google Scholar]
- Bailey, V. Characterisation of Hydrogen Peroxide Based Explosives and Ventilation Modelling to Quantify Re-Entry Times in Underground Development Blasting. Master’s Thesis, The University of Queensland, Brisbane, Australia, 2017. [Google Scholar]
- Zong-Xian, Z. Rock Fracture and Blasting Theory and Applications Book; Butterworth-Heinemann: Oxford, UK, 2016. [Google Scholar]
- Meyers, A. Encyclopedia of Physical Science and Technology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Glassman, I.; Yetter, R.A. Combustion Book, 4th ed.; Academic Press: Cambridge, MA, USA, 2007. [Google Scholar]
- Sarin, V.K. Comprehensive Hard Materials. Hardmetals Ceramics Super Hard Materials. Volume 1–3; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Miyake, A.; Takahara, K.; Ogawa, T.; Ogata, Y.; Wada, Y.; Arai, H. Influence of physical properties of ammonium nitrate on the detonation behavior of ANFO. J. Loss Prev. Process. Ind. 2001, 14, 533–538. [Google Scholar] [CrossRef]
- Cummock, N.R.; Mares, J.O., Jr.; Gunduz, I.E.; Son, S.F. Relating a small-scale shock sensitivity experiment to large-scale failure diameter in an aluminized ammonium nitrate non-ideal explosive. Combust. Flame 2018, 194, 271–277. [Google Scholar] [CrossRef]
- Paszula, J.; Kępisty, A.; Maranda, A.; Kukfisz, B. Study on Detonation Characteristics of Ammonium Nitrate-Polyhydroxy Alcohol Mixtures. Energies 2022, 15, 6843. [Google Scholar] [CrossRef]
- Maranda, A.; Szymański, R. Tests on critical diameter and detonation velocity of mixtures of ammonium nitrate (V) and selected organic substances. Chemik 2013, 67, 13–18. [Google Scholar]
- Cetner, Z.; Maranda, A. Selected parameters of heavy-ANFO explosive materials. High Energy Mater. 2014, 6, 31–37. [Google Scholar]
- Biessikirski, A.; Czerwonka, D.; Biegańska, J.; Kuterasiński, Ł.; Ziąbka, M.; Dworzak, M.; Twardosz, M. Research on the Possible Application of Polyolefin Waste-Derived Pyrolysis Oils for ANFO Manufacturing. Energies 2021, 14, 172. [Google Scholar] [CrossRef]


| Sample Name | Component, wt.% | ||
|---|---|---|---|
| AN | FO | HP | |
| ANFO | 94 | 6 | 0 |
| ANFO-HP-5 | 89 | 6 | 5 |
| ANFO-HP-10 | 83.5 | 6.5 | 10.0 |
| Parameter | ANFO | ANFO-HP-5 | ANFO-HP-10 |
|---|---|---|---|
| Energy of formation, kJ·kg−1 | −4162.35 | −4415.58 | −4694.17 |
| Detonation temperature, °C | 2620.45 | 2593.40 | 2551.843 |
| Detonation pressure, GPa | 5.15 | 5.16 | 5.10 |
| Compression energy, kJ·kg−1 | 853.83 | 856.93 | 841.21 |
| Velocity of detonation, m·s−1 | 4814.58 | 4807.68 | 4798.26 |
| Volume of gas products, dm3 | 1057 | 1059 | 1064 |
| Oxygen balance, % | 0 | −0.58 | −0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biessikirski, A.; Dworzak, M.; Ziąbka, M.; Polak, K.; Pytlik, M.; Napruszewska, B.D.; Kuterasiński, Ł. Evaluation of the Impact of Hydrogen Peroxide on ANFO-Based Materials’ Morphology. Materials 2025, 18, 4254. https://doi.org/10.3390/ma18184254
Biessikirski A, Dworzak M, Ziąbka M, Polak K, Pytlik M, Napruszewska BD, Kuterasiński Ł. Evaluation of the Impact of Hydrogen Peroxide on ANFO-Based Materials’ Morphology. Materials. 2025; 18(18):4254. https://doi.org/10.3390/ma18184254
Chicago/Turabian StyleBiessikirski, Andrzej, Michał Dworzak, Magdalena Ziąbka, Krzysztof Polak, Mateusz Pytlik, Bogna Daria Napruszewska, and Łukasz Kuterasiński. 2025. "Evaluation of the Impact of Hydrogen Peroxide on ANFO-Based Materials’ Morphology" Materials 18, no. 18: 4254. https://doi.org/10.3390/ma18184254
APA StyleBiessikirski, A., Dworzak, M., Ziąbka, M., Polak, K., Pytlik, M., Napruszewska, B. D., & Kuterasiński, Ł. (2025). Evaluation of the Impact of Hydrogen Peroxide on ANFO-Based Materials’ Morphology. Materials, 18(18), 4254. https://doi.org/10.3390/ma18184254









