Effect of CaO in Alkali-Activated Fly Ash Mortar Under Different Curing Temperatures
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mix Proportions, Preparation Process, and Curing Conditions
- Room temperature (RT): Samples were placed directly into a climatic chamber at 21 °C and 65% relative humidity, without additional heating. They were unmoulded after 24 h and cured at room temperature until the designated testing ages (7, 14, and 28 days). Reference samples (M0) were too fresh to be unmoulded after 24 h, whereas M2 and M4 samples could be conveniently unmoulded.
- Curing temperature (CT): Samples were heated at 70 °C for 24 h, then unmoulded and subsequently cured at room temperature until the testing ages (7, 14, and 28 days).
2.2.1. XRD and XRF Analysis
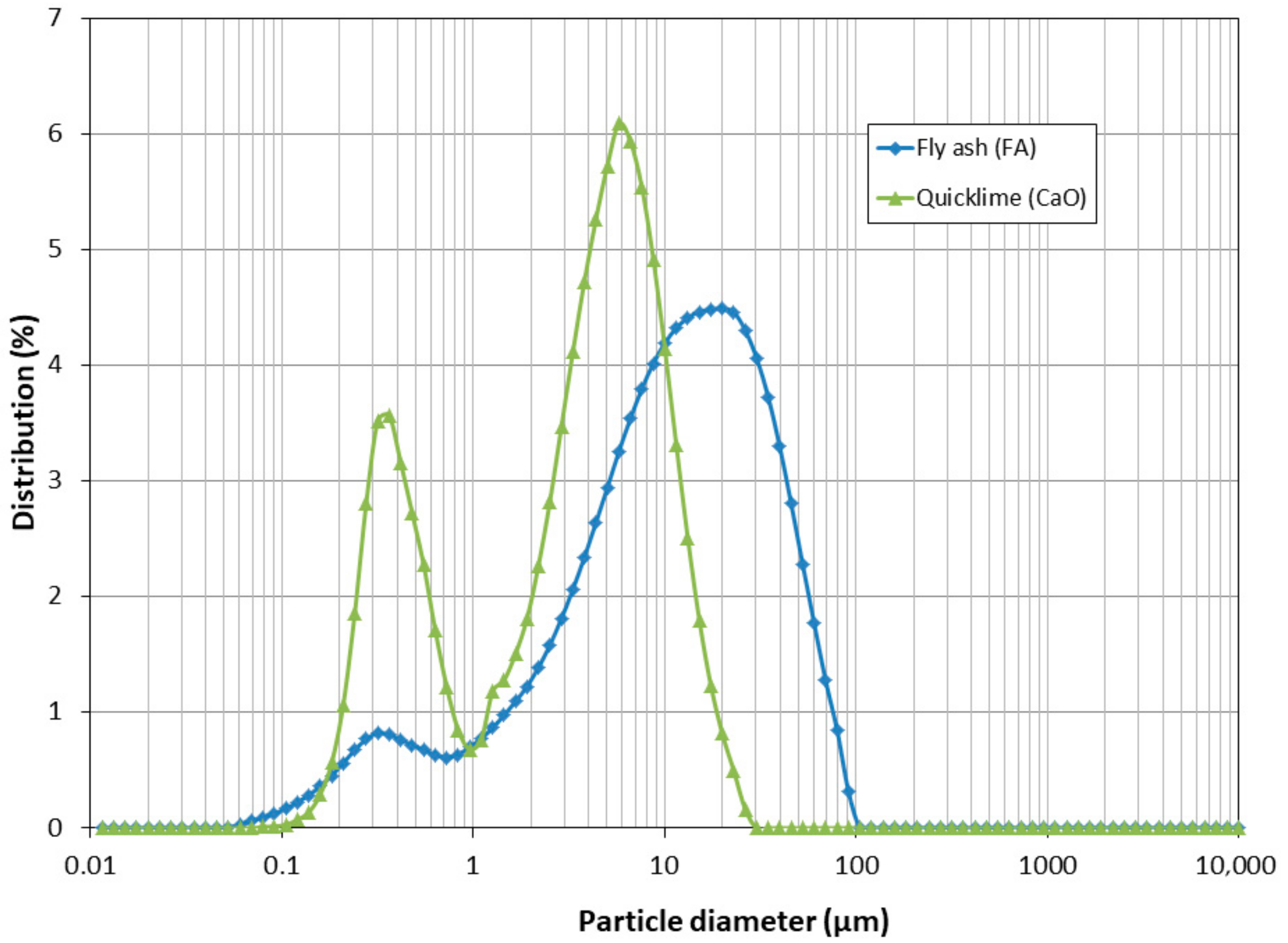
2.2.2. Particle Size Distribution
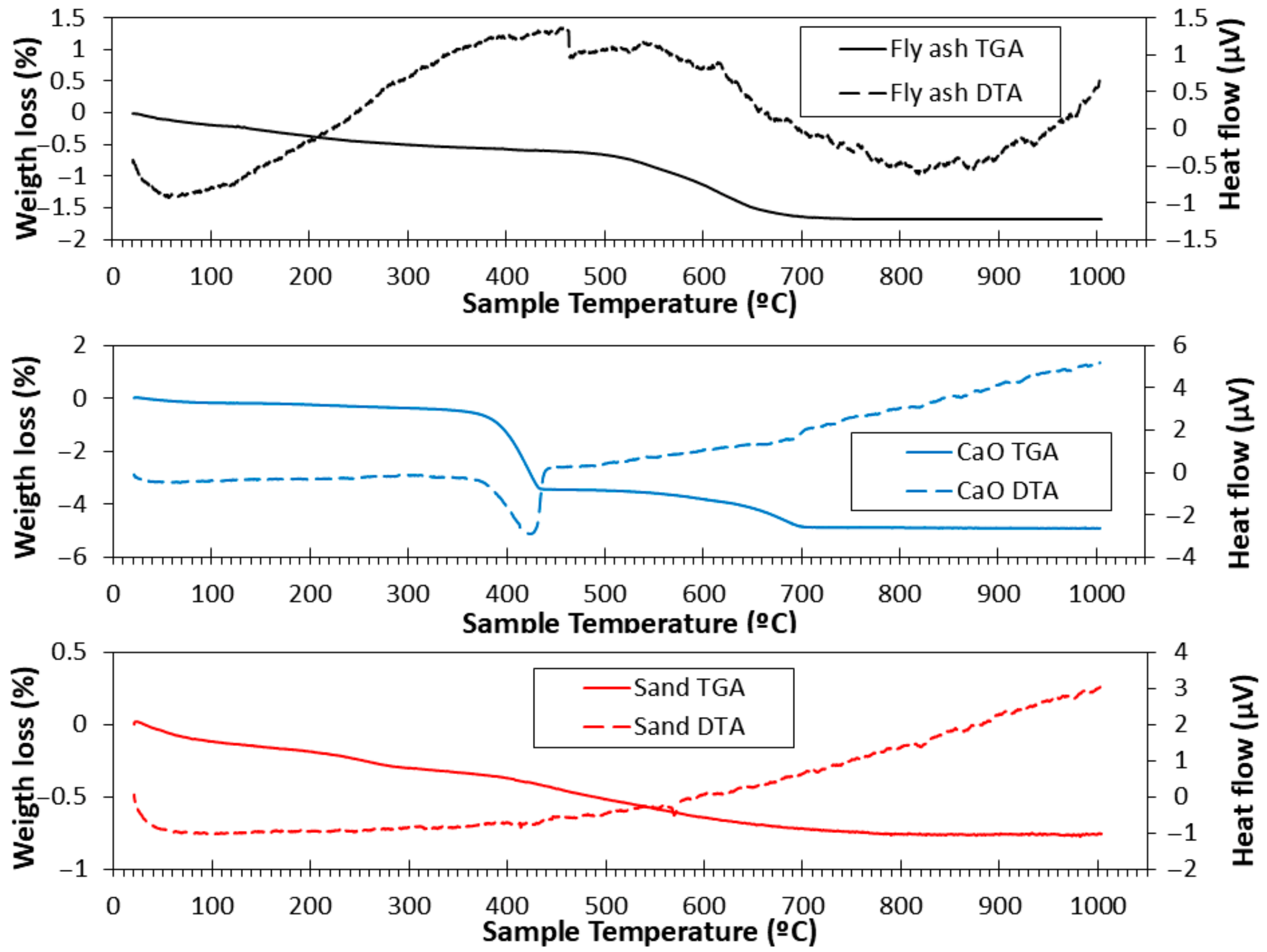
2.2.3. TGA and DTA
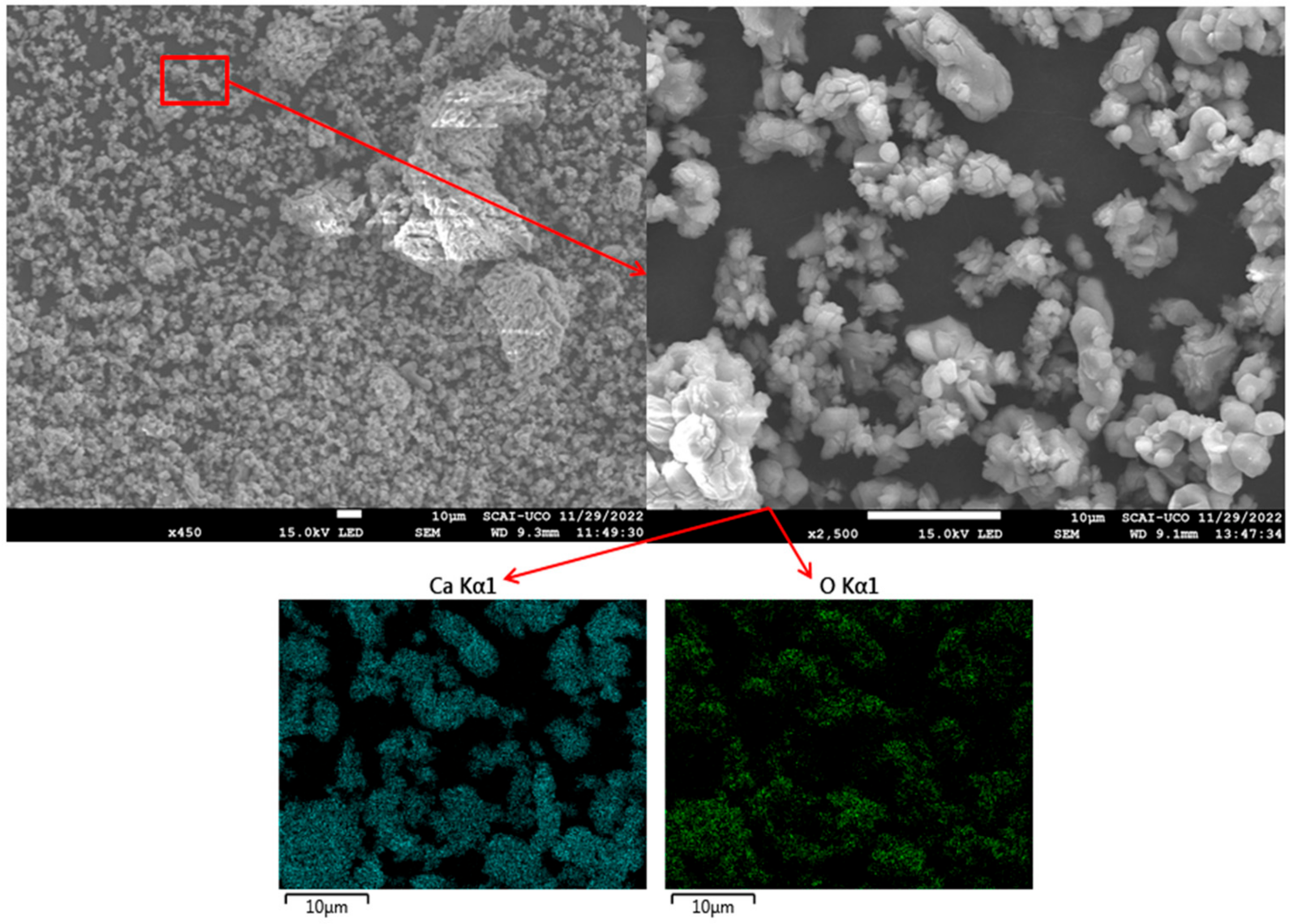
2.2.4. SEM
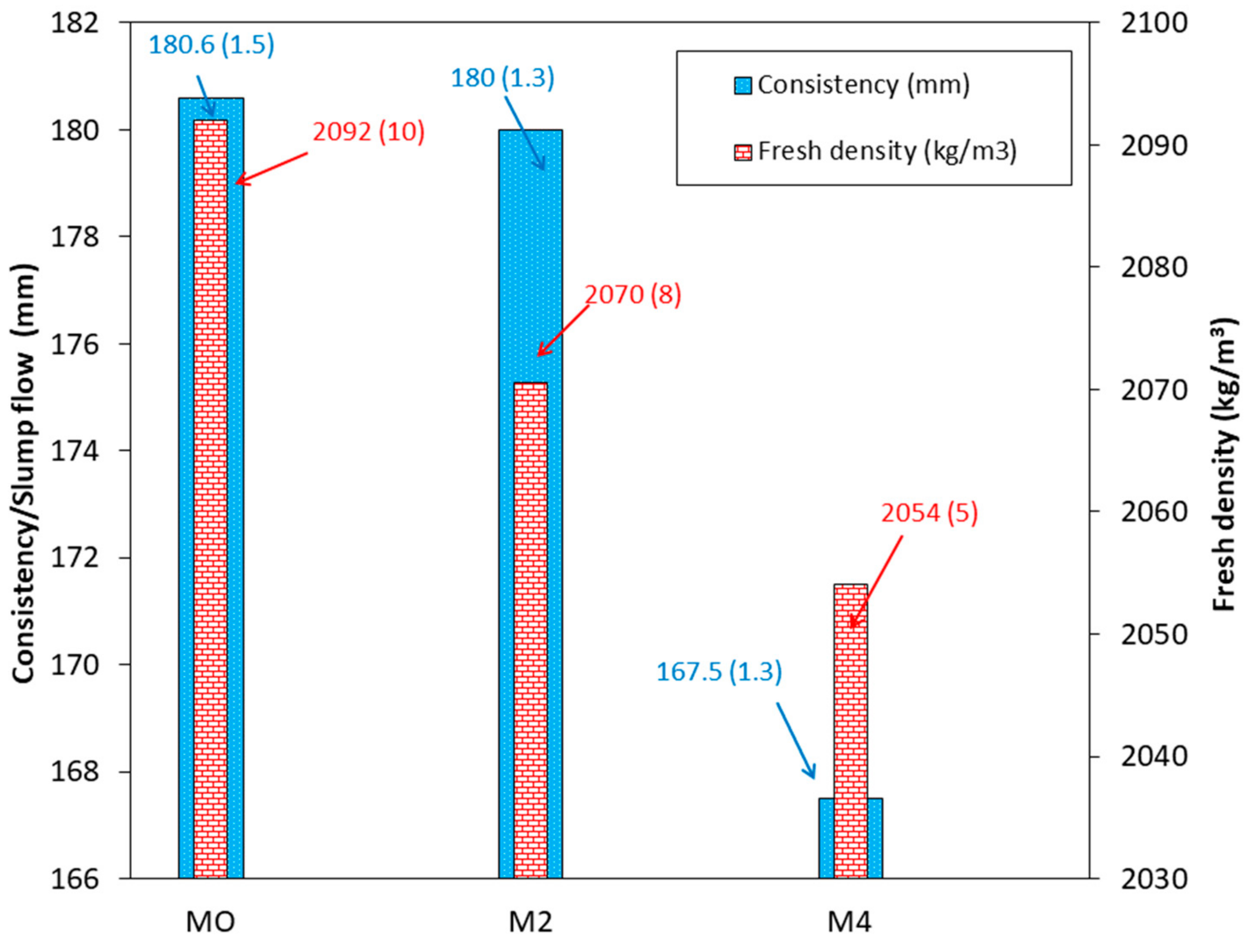
2.2.5. Fresh and Hardened Properties
3. Results
3.1. XRF
3.2. XRD Analysis
3.3. TGA and DTA
3.4. Particle Size Distribution
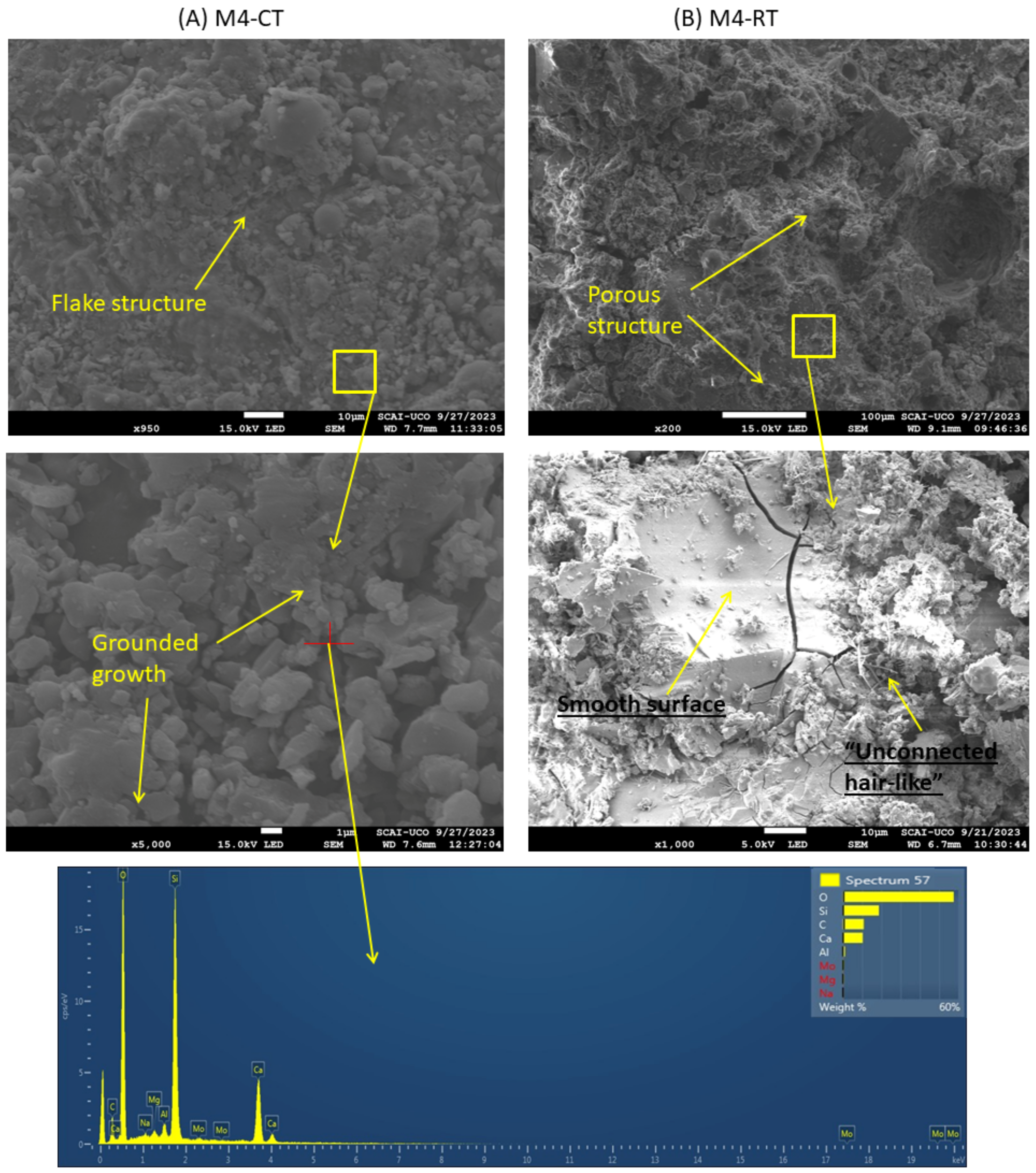
3.5. Morphological Characterisation by SEM
3.6. Bulk Density and Consistency of Fresh Mortar
3.7. Strength Development
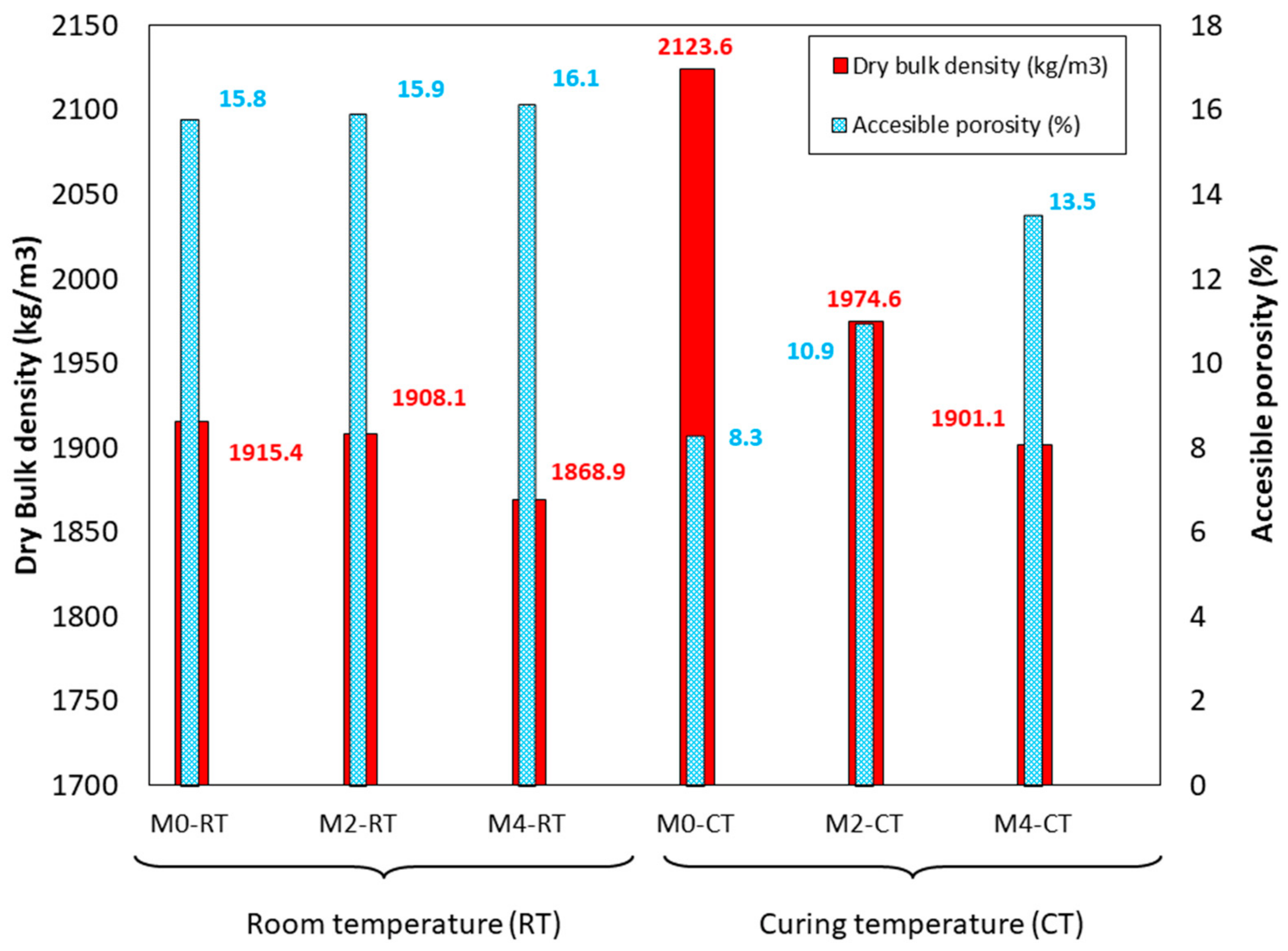
3.8. Dry Bulk Density and Accessible Porosity
3.9. Hydration Products
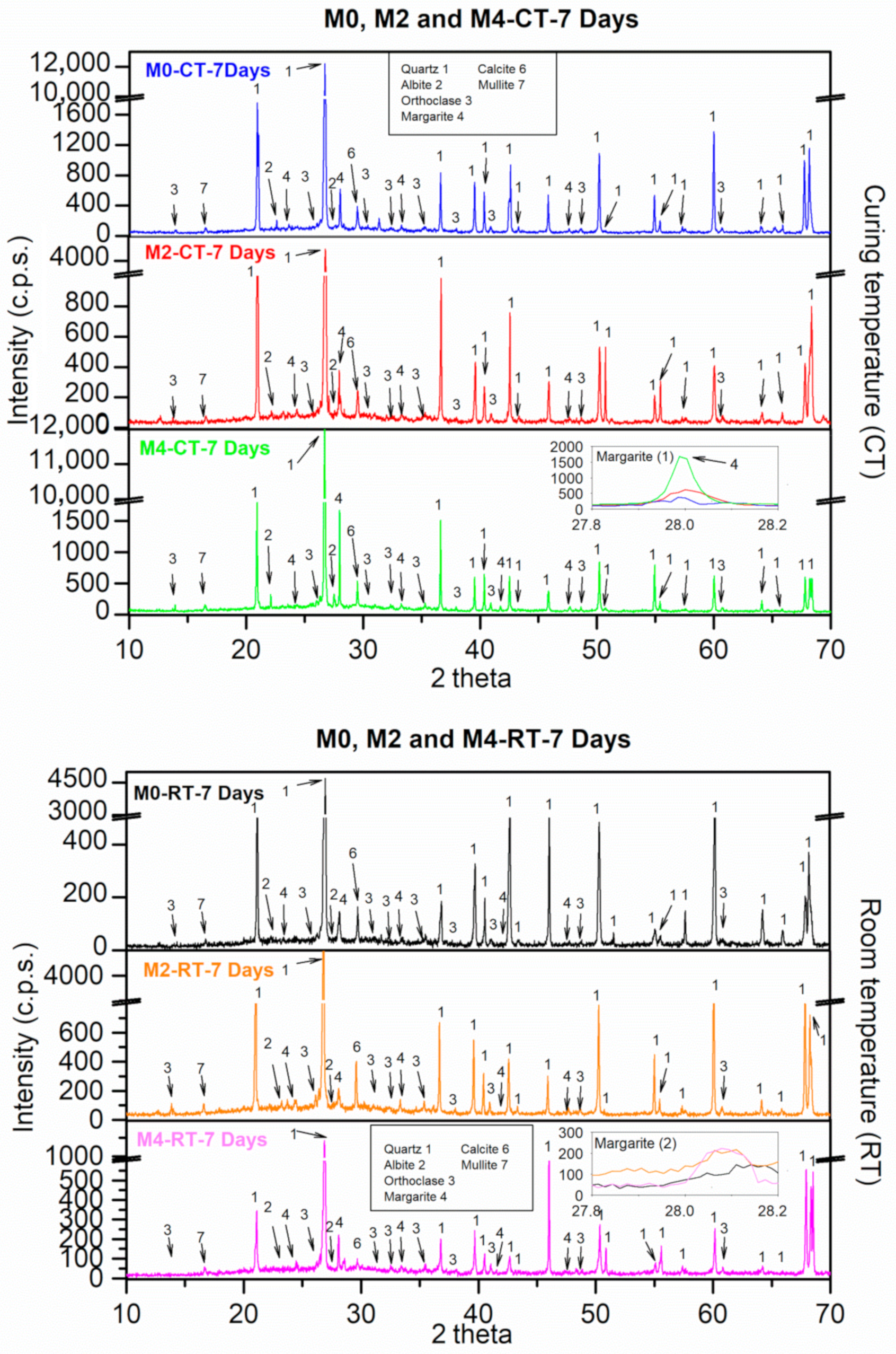
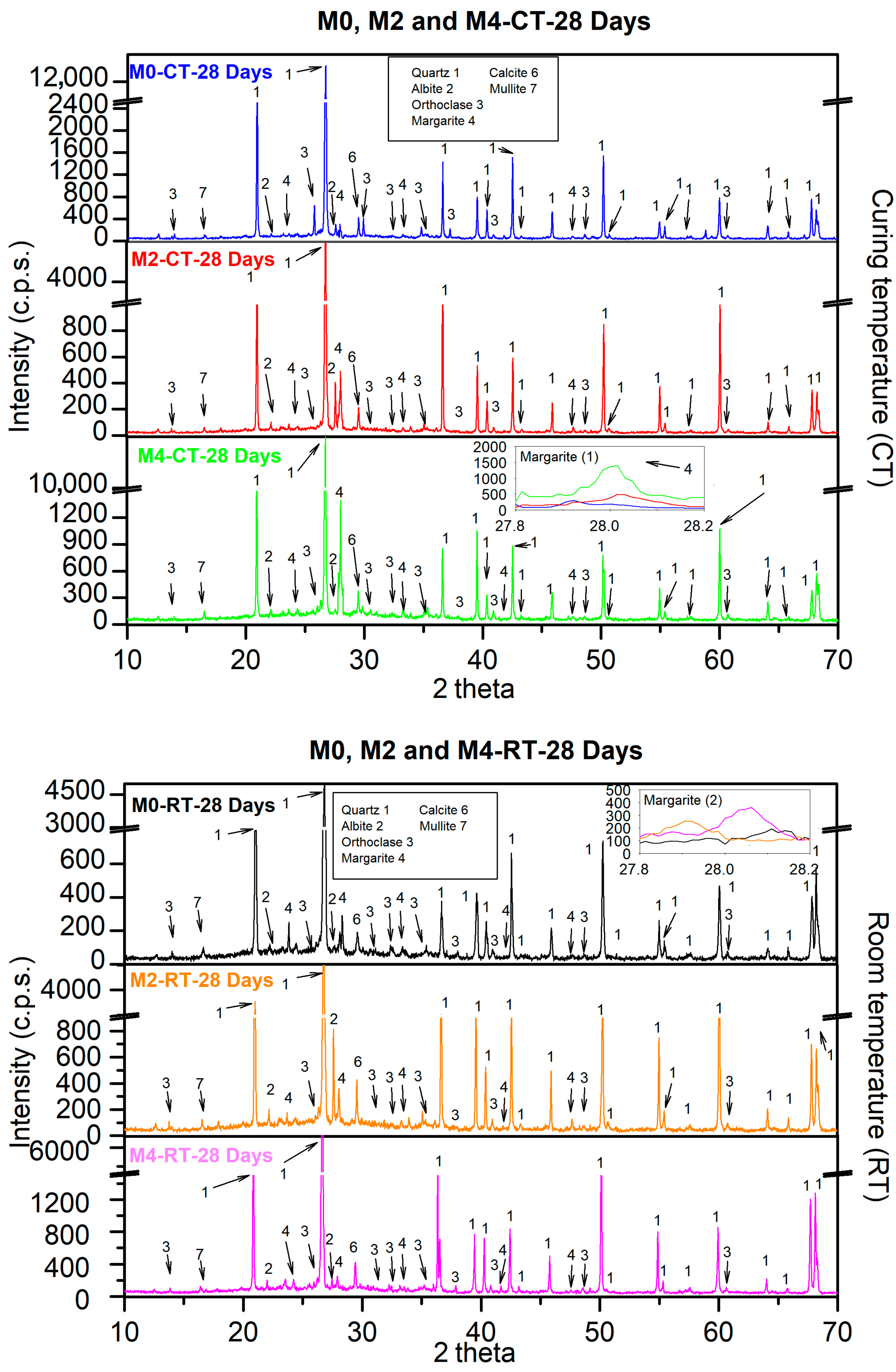
3.9.1. XRD
3.9.2. TGA
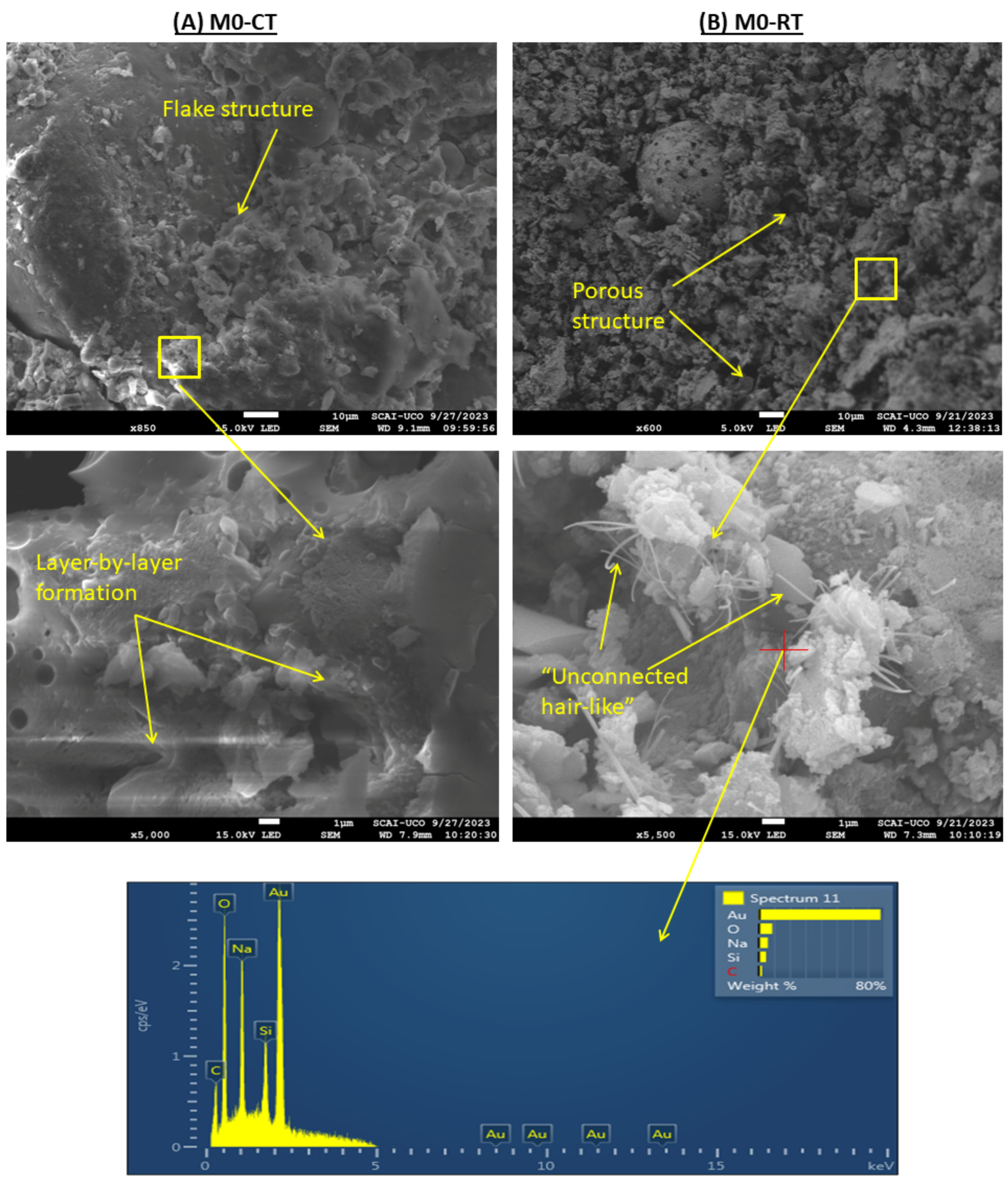
3.9.3. SEM
4. Conclusions
- Substituting FA with CaO improved compressive and flexural strength in both RT and CT environments, with the best results obtained at 4% CaO. Although CT still provided higher strength than RT, CaO addition enhanced mechanical properties even under ambient curing, showing partial effectiveness of the proposed strategy.
- Despite a reduction in precursor content, CaO substitution led to strength improvements. This indicates that CaO can serve as a partial substitute or reducer of thermal curing, enhancing early-age reactivity and mechanical performance.
- At the studied levels, increasing CaO content beyond 4% was not feasible due to fast setting and poor workability, which hindered practical application without the use of retarders.
- XRD and TGA/DTA confirmed that the enhanced performance was associated with the formation of C-S-H and C-A-S-H gels (notably margarite), which accelerated setting and enabled earlier demoulding (≈30 min for 4% CaO).
- SEM analysis revealed that CT samples exhibited denser, flake- and layer-like microstructures, while RT samples remained more porous and weakly connected. The addition of CaO slightly improved RT microstructures, though not to the level of CT.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Q.F.; Cai, Y.; Peng, H.; Meng, Z.; Mundra, S.; Castel, A. A numerical study on chloride transport in alkali-activated fly ash/slag concretes. Cem. Concr. Res. 2023, 166, 107094. [Google Scholar] [CrossRef]
- Gattuso, J.P.; Magnan, A.; Billé, R.; Cheung, W.W.L.; Howes, E.L.; Joos, F.; Allemand, D.; Bopp, L.; Cooley, S.R.; Eakin, C.M.; et al. Contrasting futures for ocean and society from different anthropogenic CO2 emissions scenarios. Science 2015, 349, aac4722. [Google Scholar] [CrossRef] [PubMed]
- Suescum-Morales, D.; Jiménez, J.R.; Fernández-Rodríguez, J.M. Review of the Application of Hydrotalcite as CO2 Sinks for Climate Change Mitigation. ChemEngineering 2022, 6, 50. [Google Scholar] [CrossRef]
- Reis, L.A.; Tavoni, M. Glasgow to Paris—The impact of the Glasgow commitments for the Paris climate agreement. iScience 2023, 26, 105933. [Google Scholar] [CrossRef]
- Huang, L.; Krigsvoll, G.; Johansen, F.; Liu, Y.; Zhang, X. Carbon emission of global construction sector. Renew. Sustain. Energy Rev. 2018, 81, 1906–1916. [Google Scholar] [CrossRef]
- He, Z.; Zhu, X.; Wang, J.; Mu, M.; Wang, Y. Comparison of CO2 emissions from OPC and recycled cement production. Constr. Build. Mater. 2019, 211, 965–973. [Google Scholar] [CrossRef]
- Benhelal, E.; Zahedi, G.; Shamsaei, E.; Bahadori, A. Global strategies and potentials to curb CO2 emissions in cement industry. J. Clean. Prod. 2013, 51, 142–161. [Google Scholar] [CrossRef]
- Nehdi, M.L.; Marani, A.; Zhang, L. Is net-zero feasible: Systematic review of cement and concrete decarbonization technologies. Renew. Sustain. Energy Rev. 2024, 191, 114169. [Google Scholar] [CrossRef]
- Wang, W.; Wang, B.; Zhang, S. Dispersion, properties, and mechanisms of nanotechnology-modified alkali-activated materials: A review. Renew. Sustain. Energy Rev. 2024, 192, 114215. [Google Scholar] [CrossRef]
- Ouellet-Plamondon, C.; Habert, G. 25—Life cycle assessment (LCA) of alkali-activated cements and concrete. In Handbook of Alkali-Activated Cements, Mortars and Concretes; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Runci, A.; Provis, J.; Serdar, M. Microstructure as a key parameter for understanding chloride ingress in alkali-activated mortars. Cem. Concr. Compos. 2022, 134, 104818. [Google Scholar] [CrossRef]
- Yang, K.-H.; Song, J.-K.; Song, K.-I. Assessment of CO2 reduction of alkali-activated concrete. J. Clean. Prod. 2013, 39, 265–272. [Google Scholar] [CrossRef]
- Candeloro, M.; Carlin, S.; Shapiro, M.J.; Douketis, D. Estimating the impacts of a new power system on electricity prices under dual carbon targets. Res. Pract. Thromb. Haemost. 2023, 438, 100137. [Google Scholar] [CrossRef] [PubMed]
- Eurostat. Coal Production and Consumption Statistics. 2023. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Coal_production_and_consumption_statistics#Consumption_and_production_of_hard_coal (accessed on 24 December 2023).
- Bijańska, J.; Wodarski, K. Hard coal production in Poland in the aspect of climate and energy policy of the European Union and the war in Ukraine. Investment case study. Resour. Policy 2024, 88, 104390. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Liu, F.; Su, J.; Zhao, Q.; Liu, L. Effect of replacement of limestone mineral powder with fly ash and direct coal liquefaction residue on the rheological properties of asphalt mastic. Constr. Build. Mater. 2024, 412, 134803. [Google Scholar] [CrossRef]
- Yao, Z.T.; Ji, X.S.; Sarker, P.K.; Tang, J.H.; Ge, L.Q.; Xia, M.S.; Xi, Y.Q. A comprehensive review on the applications of coal fly ash. Earth Sci. Rev. 2015, 141, 105–121. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers: Inorganic polymeric new materials. J. Therm. Anal. Calorim. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Whiting, J. Manufacture of Cement. U.S. Patent 544,706, 20 August 1895. [Google Scholar]
- Kühl, H. Slag Cement and Process of Making the Same. U.S. Patent 900,989, 13 October 1908. [Google Scholar]
- Purdon, A.O. The action of alkalis on blast furnace slag. J. Soc. Chem. Ind. 1940, 12, 1475–1499. [Google Scholar]
- Krivenko, P. Why alkaline activation—60 years of the theory and practice of alkali-activated materials. J. Ceram. Sci. Technol. 2017, 8, 323–333. [Google Scholar] [CrossRef]
- Nasr, D.; Pakshir, A.H.; Ghayour, H. The influence of curing conditions and alkaline activator concentration on elevated temperature behavior of alkali activated slag (AAS) mortars. Constr. Build. Mater. 2018, 190, 108–119. [Google Scholar] [CrossRef]
- Suescum-Morales, D.; Silva, R.V.; Bravo, M.; Jiménez, J.R.; Fernández-Rodríguez, J.M.; de Brito, J. Effect of incorporating municipal solid waste incinerated bottom ash in alkali-activated fly ash concrete subjected to accelerated CO2 curing. J. Clean. Prod. 2022, 370, 133533. [Google Scholar] [CrossRef]
- Suescum-Morales, D.; Bravo, M.; Silva, R.V.; Jiménez, J.R.; Fernandez-Rodriguez, J.M.; de Brito, J. Effect of reactive magnesium oxide in alkali-activated fly ash mortars exposed to accelerated CO2 curing. Constr. Build. Mater. 2022, 342, 127999. [Google Scholar] [CrossRef]
- Athira, V.S.; Bahurudeen, A.; Saljas, M.; Jayachandran, K. Influence of different curing methods on mechanical and durability properties of alkali activated binders. Constr. Build. Mater. 2021, 299, 123963. [Google Scholar] [CrossRef]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. One-part alkali-activated materials: A review. Cem. Concr. Res. 2018, 103, 21–34. [Google Scholar] [CrossRef]
- Nodehi, M.; Ozbakkaloglu, T.; Gholampour, A.; Mohammed, T.; Shi, X. The effect of curing regimes on physico-mechanical, microstructural and durability properties of alkali-activated materials: A review. Constr. Build. Mater. 2022, 321, 126335. [Google Scholar] [CrossRef]
- Gao, X.; Yu, Q.L.; Brouwers, H.J.H. Reaction kinetics, gel character and strength of ambient temperature cured alkali activated slag-fly ash blends. Constr. Build. Mater. 2015, 80, 105–115. [Google Scholar] [CrossRef]
- Wei, X.; Li, D.; Ming, F.; Yang, C.; Chen, L.; Liu, Y. Influence of low-temperature curing on the mechanical strength, hydration process, and microstructure of alkali-activated fly ash and ground granulated blast furnace slag mortar. Constr. Build. Mater. 2021, 269, 121811. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A.; Criado, M. Microstructure development of alkali-activated fly ash cement: A descriptive model. Cem. Concr. Res. 2005, 35, 1204–1209. [Google Scholar] [CrossRef]
- Pangdaeng, S.; Phoo-ngernkham, T.; Sata, V.; Chindaprasirt, P. Influence of curing conditions on properties of high calcium fly ash geopolymer containing Portland cement as additive. Mater. Des. 2014, 53, 269–274. [Google Scholar] [CrossRef]
- Elzeadani, M.; Bompa, D.V.; Elghazouli, A.Y. One part alkali activated materials: A state-of-the-art review. J. Build. Eng. 2022, 57, 104871. [Google Scholar] [CrossRef]
- Nayak, D.K.; Abhilash, P.P.; Singh, R.; Kumar, R.; Kumar, V. Fly ash for sustainable construction: A review of fly ash concrete and its beneficial use case studies. Clean. Mater. 2022, 6, 100143. [Google Scholar] [CrossRef]
- Nurruddin, E.A. Methods of curing geopolymer concrete: A review. Int. J. Adv. Appl. Sci. 2018, 5, 31–36. [Google Scholar] [CrossRef]
- Saludung, A.; Azeyanagi, T.; Ogawa, Y.; Kawai, K. Mechanical and microstructural evolutions of fly ash/slag-based geopolymer at high temperatures: Effect of curing conditions. Ceram. Int. 2023, 49, 2091–2101. [Google Scholar] [CrossRef]
- Ma, C.K.; Awang, A.Z.; Omar, W. Structural and material performance of geopolymer concrete: A review. Constr. Build. Mater. 2018, 186, 90–102. [Google Scholar] [CrossRef]
- Samantasinghar, S.; Singh, S. Effects of curing environment on strength and microstructure of alkali-activated fly ash-slag binder. Constr. Build. Mater. 2020, 235, 117481. [Google Scholar] [CrossRef]
- Rafeet, A.; Vinai, R.; Soutsos, M.; Sha, W. Effects of slag substitution on physical and mechanical properties of fly ash-based alkali activated binders (AABs). Cem. Concr. Res. 2019, 122, 118–135. [Google Scholar] [CrossRef]
- Perumal, P.; Piekkari, K.; Sreenivasan, H.; Kinnunen, P.; Illikainen, M. One-part geopolymers from mining residues—Effect of thermal treatment on three different tailings. Min. Eng. 2019, 144, 106026. [Google Scholar] [CrossRef]
- Luukkonen, T.; Sreenivasan, H.; Abdollahnejad, Z.; Yliniemi, J.; Kantola, A.; Telkki, V.V.; Kinnunen, P.; Illikainen, M. Influence of sodium silicate powder silica modulus for mechanical and chemical properties of dry-mix alkali-activated slag mortar. Constr. Build. Mater. 2020, 233, 117354. [Google Scholar] [CrossRef]
- Qing, L.; Shaokang, S.; Zhen, J.; Junxiang, W.; Xianjun, L. Effect of CaO on hydration properties of one-part alkali-activated material prepared from tailings through alkaline hydrothermal activation. Constr. Build. Mater. 2021, 308, 124931. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Phoo-ngernkham, T.; Hanjitsuwan, S.; Horpibulsuk, S.; Poowancum, A.; Injorhor, B. Effect of calcium-rich compounds on setting time and strength development of alkali-activated fly ash cured at ambient temperature. Case Stud. Constr. Mater. 2018, 9, e00198. [Google Scholar] [CrossRef]
- ASTM-C618; Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete. ASTM International: West Conshohocken, PA, USA, 2019.
- Carvalho, R.; Silva, R.V.; de Brito, J.; Pereira, M.F.C. Alkali activation of bottom ash from municipal solid waste incineration: Optimization of NaOH- and Na2SiO3-based activators. J. Clean. Prod. 2021, 291, 125930. [Google Scholar] [CrossRef]
- Korniejenko, K.; Mikuła, J.; Brudny, K.; Aruova, L.; Zhakanov, A.; Jexembayeva, A.; Zhaksylykova, L. A Review of Industrial By-Product Utilization and Future Pathways of Circular Economy: Geopolymers as Modern Materials for Sustainable Building. Sustainability 2025, 17, 4536. [Google Scholar] [CrossRef]
- Cao, R.; Zhang, S.; Banthia, N.; Zhang, Y.; Zhang, Z. Interpreting the early-age reaction process of alkali-activated slag by using combined embedded ultrasonic measurement, thermal analysis, XRD, FTIR and SEM. Compos. B Eng. 2020, 186, 107840. [Google Scholar] [CrossRef]
- UNE-EN 196-1: 2018; Methods of Testing Cement—Part 1: Determination of Strength. European Standards: Brussels, Belgium, 2018.
- JCPDS. Joint Committee on Power Diffraction Standard-International Centre for Diffraction; JCPDS: Newtown Square, PA, USA, 2003. [Google Scholar]
- EN-1015-6; Methods of Test Mortar for Mansory—Part 6: Determination of Bulk Density of Fresh Mortar. European Standards: Brussels, Belgium, 1999.
- EN-1015-3; Methods of Test Mortart Masonry: Part 3: Determination of Consistency of Fresh Mortar (by Flow Table). European Standards: Brussels, Belgium, 2000.
- EN-1015-11; Methods of Test for Mortar for Mansory—Part 11: Determination of Flexural and Compressive Strenght of Hardened Mortar. European Standards: Brussels, Belgium, 2020.
- EN-1015-10; Methods of Test for Mortar for Mansory—Part 6: Determination of Bulk Density of Hardened Mortar. European Standards: Brussels, Belgium, 2000.
- UNE-EN 450-1; Fly Ash for Concrete—Pat 1: Definition, Specifications and Conformity Criteria. European Standards: Brussels, Belgium, 2013.
- Xiao, R.; Jiang, X.; Zhang, M.; Polaczyk, P.; Huang, B. Analytical investigation of phase assemblages of alkali-activated materials in CaO-SiO2-Al2O3 systems: The management of reaction products and designing of precursors. Mater. Des. 2020, 194, 108975. [Google Scholar] [CrossRef]
- Mishra, J.; Nanda, B.; Patro, S.K.; Krishna, R.S. Sustainable Fly Ash Based Geopolymer Binders: A Review on Compressive Strength and Microstructure Properties. Sustainability 2022, 14, 15062. [Google Scholar] [CrossRef]
- Suh, J.I.; Yum, W.S.; Jeong, Y.; Yoon, S.; Park, H.G.; Oh, J.E. Mechanical and microstructural properties of lightweight CaO-activated fly ash composites in the presence of magnesium nitrate. J. Build. Eng. 2023, 65, 105641. [Google Scholar] [CrossRef]
- Wang, Y.; Acarturk, B.C.; Burris, L.; Hooton, R.D.; Shearer, C.R.; Suraneni, P. Physicochemical characterization of unconventional fly ashes. Fuel 2022, 316, 123318. [Google Scholar] [CrossRef]
- Haq, E.U.; Padmanabhan, S.K.; Licciulli, A. Synthesis and characteristics of fly ash and bottom ash based geopolymers-A comparative study. Ceram. Int. 2014, 40, 2965–2971. [Google Scholar] [CrossRef]
- Ali, M.B.; Saidur, R.; Hossain, M.S. A review on emission analysis in cement industries. Renew. Sustain. Energy Rev. 2011, 15, 2252–2261. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, S. Quicklime and calcium sulfoaluminate cement used as mineral accelerators to improve the properties of cemented paste backfill with a high volume of fly ash. Materials 2020, 13, 4018. [Google Scholar] [CrossRef]
- Antiohos, S.; Tsimas, S. Activation of fly ash cementitious systems in the presence of quicklime: Part I. Compressive strength and pozzolanic reaction rate. Cem. Concr. Res. 2004, 34, 769–779. [Google Scholar] [CrossRef]
- Cabrera-Luna, K.; Perez-Cortes, P.; Garcia, J.I.E. Influence of quicklime and Portland cement, as alkaline activators, on the reaction products of supersulfated cements based on pumice. Cem. Concr. Compos. 2024, 146, 105379. [Google Scholar] [CrossRef]
- Hanjitsuwan, S.; Phoo-ngernkham, T.; Damrongwiriyanupap, N. Comparative study using Portland cement and calcium carbide residue as a promoter in bottom ash geopolymer mortar. Constr. Build. Mater. 2017, 133, 128–134. [Google Scholar] [CrossRef]
- Jiang, S.; Xu, J.; Song, Y.; Xu, Y. Alkali-activated fly ash foam concrete with Yellow River silt: Physico-mechanical and structural properties. Constr. Build. Mater. 2023, 373, 130879. [Google Scholar] [CrossRef]
- Suescum-Morales, D.; Kalinowska-wichrowska, K.; Fernández, J.M.; Jiménez, J.R. Accelerated carbonation of fresh cement-based products containing recycled masonry aggregates for CO2 sequestration. J. CO2 Util. 2021, 46, 101461. [Google Scholar] [CrossRef]
- Criado, M.; Fernández-Jiménez, A.; de la Torre, A.G.; Aranda, M.A.G.; Palomo, A. An XRD study of the effect of the SiO2/Na2O ratio on the alkali activation of fly ash. Cem. Concr. Res. 2007, 37, 671–679. [Google Scholar] [CrossRef]
- Tu, W.; Fang, G.; Dong, B.; Zhang, M. Multiscale study of microstructural evolution in alkali-activated fly ash-slag paste at elevated temperatures. Cem. Concr. Compos. 2023, 143, 105258. [Google Scholar] [CrossRef]
- Chi, M.; Huang, R. Binding mechanism and properties of alkali-activated fly ash/slag mortars. Constr. Build. Mater. 2013, 40, 291–298. [Google Scholar] [CrossRef]
- Esquinas, A.R.; Álvarez, J.I.; Jiménez, J.R.; Fernández, J.M.; de Brito, J. Durability of self-compacting concrete made with recovery filler from hot-mix asphalt plants. Constr. Build. Mater. 2018, 161, 407–419. [Google Scholar] [CrossRef]
- Thi, K.D.T.; Liao, M.C.; Vo, D.H. The characteristics of alkali-activated slag-fly ash incorporating the high volume wood bottom ash: Mechanical properties and microstructures. Constr. Build Mater. 2023, 394, 132240. [Google Scholar] [CrossRef]
- de Moraes Pinheiro, S.M.; Font, A.; Soriano, L.; Tashima, M.M.; Monzó, J.; Borrachero, M.V.; Payá, J. Olive-stone biomass ash (OBA): An alternative alkaline source for the blast furnace slag activation. Constr. Build. Mater. 2018, 178, 327–338. [Google Scholar] [CrossRef]
- Merino-Lechuga, A.M.; González-Caro, Á.; Fernández-Ledesma, E.; Jiménez, J.R.; Fernández-Rodríguez, J.M.; Suescum-Morales, D. Accelerated Carbonation of Vibro-Compacted Porous Concrete for Eco-Friendly Precast Elements. Materials 2023, 16, 2995. [Google Scholar] [CrossRef]
- Suescum-morales, D.; Fernández-ledesma, E.; González-caro, Á.; Merino-lechuga, A.M.; Fernández-rodríguez, J.M.; Jiménez, J.R. Carbon Emission Evaluation of CO2 Curing in Vibro-Compacted Precast Concrete Made with Recycled Aggregates. Materials 2023, 16, 2436. [Google Scholar] [CrossRef]
- Unluer, C.; Al-Tabbaa, A. Impact of hydrated magnesium carbonate additives on the carbonation of reactive MgO cements. Cem. Concr. Res. 2013, 54, 87–97. [Google Scholar] [CrossRef]
- Li, Z.; Shi, X. Graphene oxide modified, clinker-free cementitious paste with principally alkali-activated fly ash. Fuel 2020, 269, 117418. [Google Scholar] [CrossRef]
- Shi, S.; Li, H.; Zhou, Q.; Zhang, H.; Basheer, P.A.M.; Bai, Y. Alkali-activated fly ash cured with pulsed microwave and thermal oven: A comparison of reaction products, microstructure and compressive strength. Cem. Concr. Res. 2023, 166, 107104. [Google Scholar] [CrossRef]
- Kaya, M. The effect of micro-SiO2 and micro-Al2O3 additive on the strength properties of ceramic powder-based geopolymer pastes. J. Mater. Cycles Waste Manag. 2022, 24, 333–350. [Google Scholar] [CrossRef]
- Kaya, M.; Karahan, O.; Atiş, C.D. Influence of Silica Fume Additive and Activator Ratio on Mechanical Properties in Slaked Lime-Based Alkali-Activated Mortars. Iran. J. Sci. Technol. Trans. Civ. Eng. 2023, 47, 873–889. [Google Scholar] [CrossRef]
- Lee, J.; Lee, T.; Jeong, J.; Jeong, J. Mix design optimization and environmental impact assessment of low-carbon materials containing alkali-activated slag and high CaO fly ash. Constr Build Mater 2021, 267, 120932. [Google Scholar] [CrossRef]






| Mortar Type | FA | CaO | Water Reducing Admixture | Water | Sand 0/4 | NaOH | Na2SiO3 |
|---|---|---|---|---|---|---|---|
| M0 | 450 | - | 0.88 | 879 | 1316 | 57 | 224 |
| M2 | 441 | 9 | 0.88 | 879 | 1316 | 57 | 224 |
| M4 | 432 | 18 | 0.88 | 879 | 1316 | 57 | 224 |
| Oxides | FA | CaO | Natural Sand (NS-0/4) |
|---|---|---|---|
| Na2O | 0.55 | - | 0.81 |
| MgO | 0.47 | 0.48 | 0.95 |
| Al2O3 | 15.77 | 0.06 | 13.36 |
| SiO2 | 74.46 | 0.14 | 77.07 |
| P2O5 | 0.20 | 0.22 | 0.13 |
| SO3 | 0.47 | 0.74 | 0.11 |
| Cl− | 0.08 | 0.05 | - |
| K2O | 0.97 | 0.15 | 3.47 |
| CaO | 1.27 | 97.83 | 0.71 |
| TiO2 | 1.47 | - | 0.82 |
| MnO2 | 0.05 | - | 0.08 |
| Fe2O3 | 4.15 | 0.03 | 3.98 |
| CuO | - | - | - |
| ZnO | - | - | - |
| SrO | 0.04 | 0.27 | 0.01 |
| ZrO2 | 0.04 | - | 0.02 |
| BaO | - | - | - |
| TOTAL | 100 | 100 | 100 |
| Mixes | RT-105 °C | 105–250 °C | 250–600 °C | 600–1000 °C |
|---|---|---|---|---|
| M0-RT-7 D | 3.279 | 0.741 | 0.734 | 1.031 |
| M2-RT-7 D | 3.979 | 1.106 | 1.267 | 1.417 |
| M4-RT-7 D | 5.711 | 1.212 | 1.413 | 1.549 |
| M0-CT-7 D | 2.288 | 0.910 | 0.746 | 1.131 |
| M2-CT-7 D | 3.508 | 1.305 | 1.289 | 1.549 |
| M4-CT-7 D | 4.032 | 1.74 | 1.456 | 1.608 |
| M0-RT-28 D | 3.277 | 1.284 | 1.332 | 1.461 |
| M2-RT-28 D | 3.576 | 1.435 | 1.499 | 1.488 |
| M4-RT-28 D | 4.576 | 2.040 | 1.583 | 1.598 |
| M0-CT-28 D | 3.110 | 1.445 | 1.491 | 1.502 |
| M2-CT-28 D | 3.456 | 1.706 | 1.654 | 1.546 |
| M4-CT-28 D | 4.126 | 2.069 | 1.702 | 1.604 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murillo-Silo, D.; Fernández-Ledesma, E.; Jiménez, J.R.; Fernández-Rodríguez, J.M.; Suescum-Morales, D. Effect of CaO in Alkali-Activated Fly Ash Mortar Under Different Curing Temperatures. Materials 2025, 18, 4250. https://doi.org/10.3390/ma18184250
Murillo-Silo D, Fernández-Ledesma E, Jiménez JR, Fernández-Rodríguez JM, Suescum-Morales D. Effect of CaO in Alkali-Activated Fly Ash Mortar Under Different Curing Temperatures. Materials. 2025; 18(18):4250. https://doi.org/10.3390/ma18184250
Chicago/Turabian StyleMurillo-Silo, David, Enrique Fernández-Ledesma, José Ramón Jiménez, José María Fernández-Rodríguez, and David Suescum-Morales. 2025. "Effect of CaO in Alkali-Activated Fly Ash Mortar Under Different Curing Temperatures" Materials 18, no. 18: 4250. https://doi.org/10.3390/ma18184250
APA StyleMurillo-Silo, D., Fernández-Ledesma, E., Jiménez, J. R., Fernández-Rodríguez, J. M., & Suescum-Morales, D. (2025). Effect of CaO in Alkali-Activated Fly Ash Mortar Under Different Curing Temperatures. Materials, 18(18), 4250. https://doi.org/10.3390/ma18184250








