The Impact of Molecular Weight Distribution on the Crystalline Texture of Polymers—Illustrated by Lamellar Crystal, Shish–Kebab and Nested Spherulites
Abstract
1. Introduction
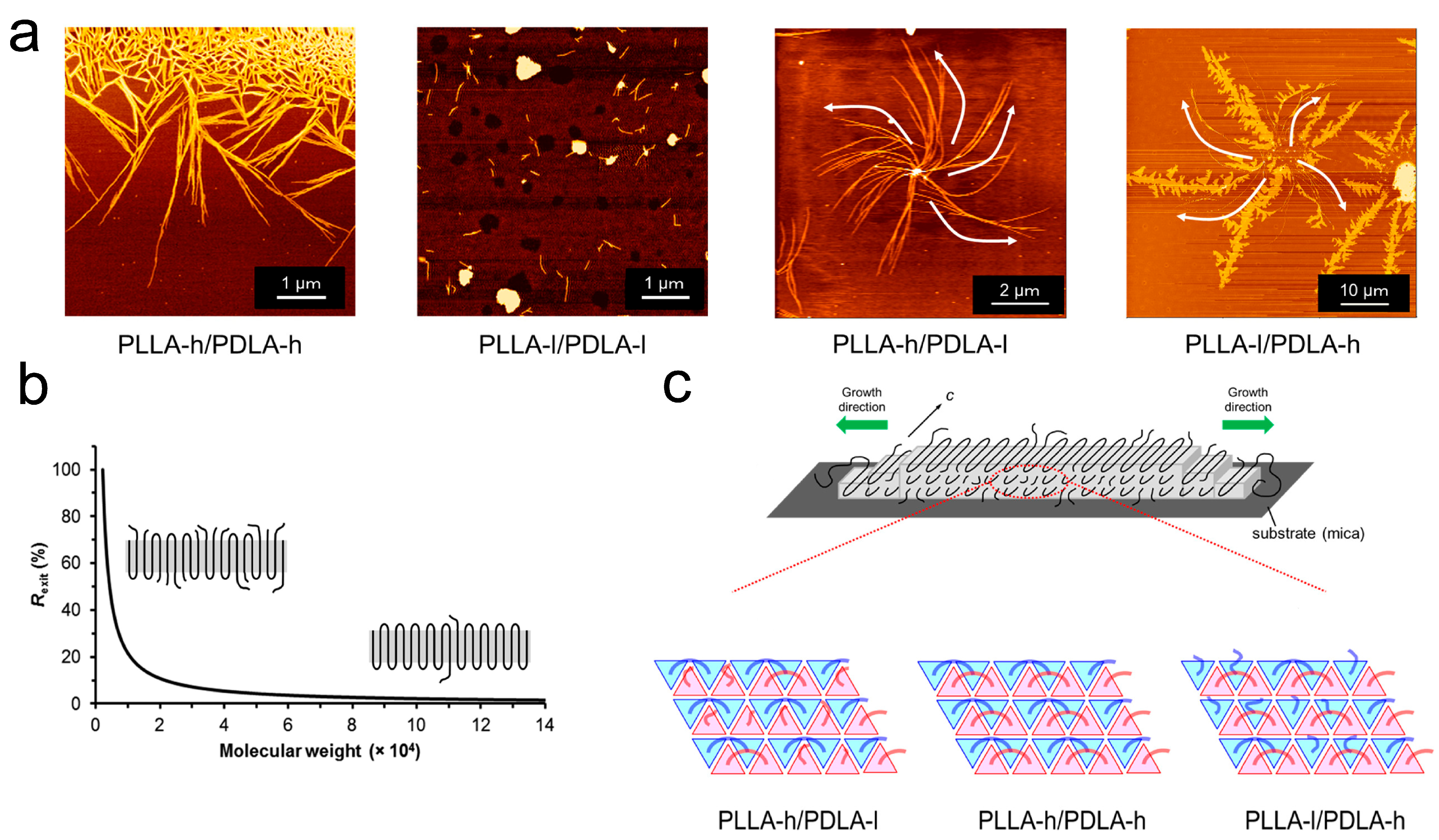
2. Impact on Crystalline Structures Rooted in Molecular Segregation
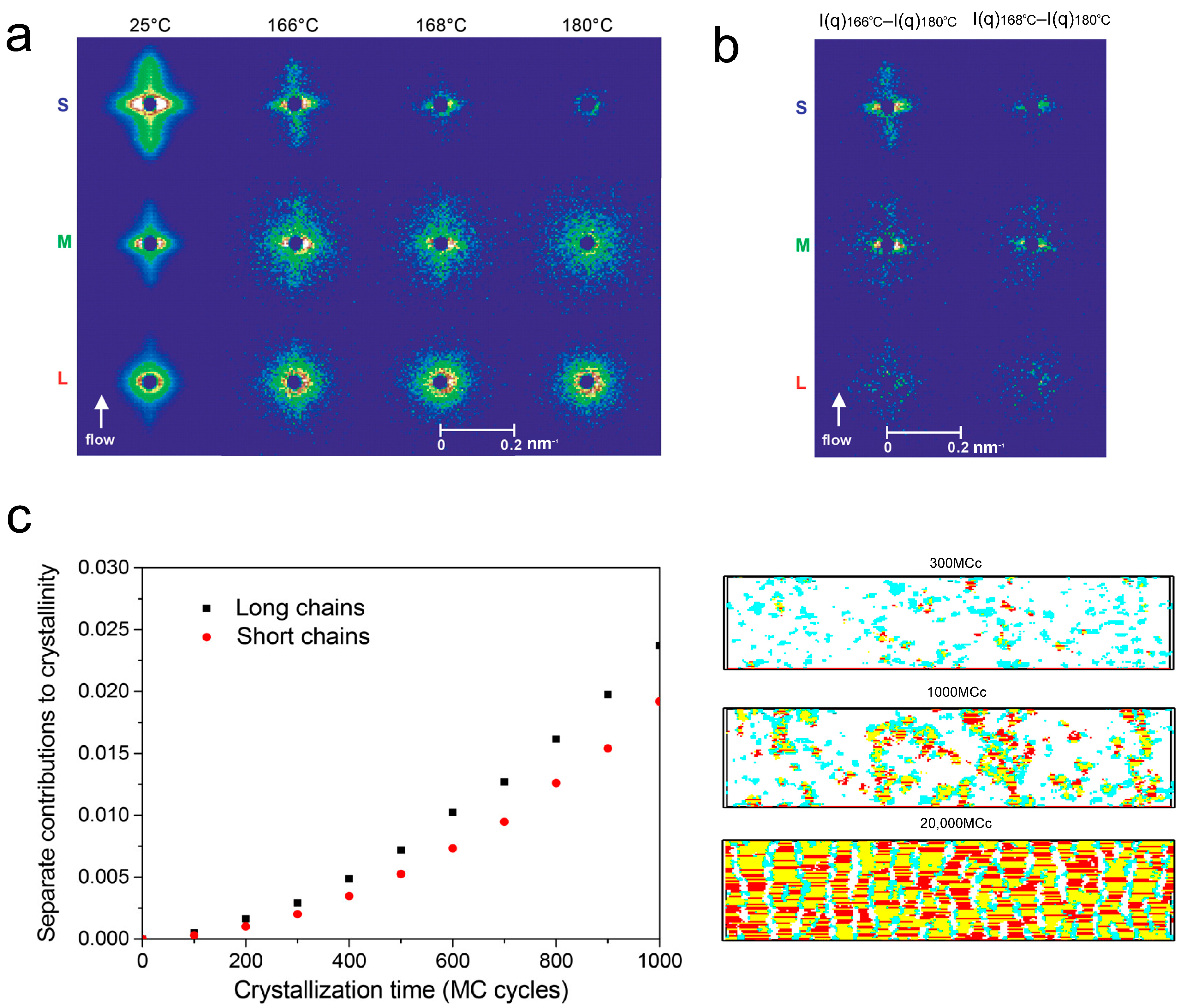
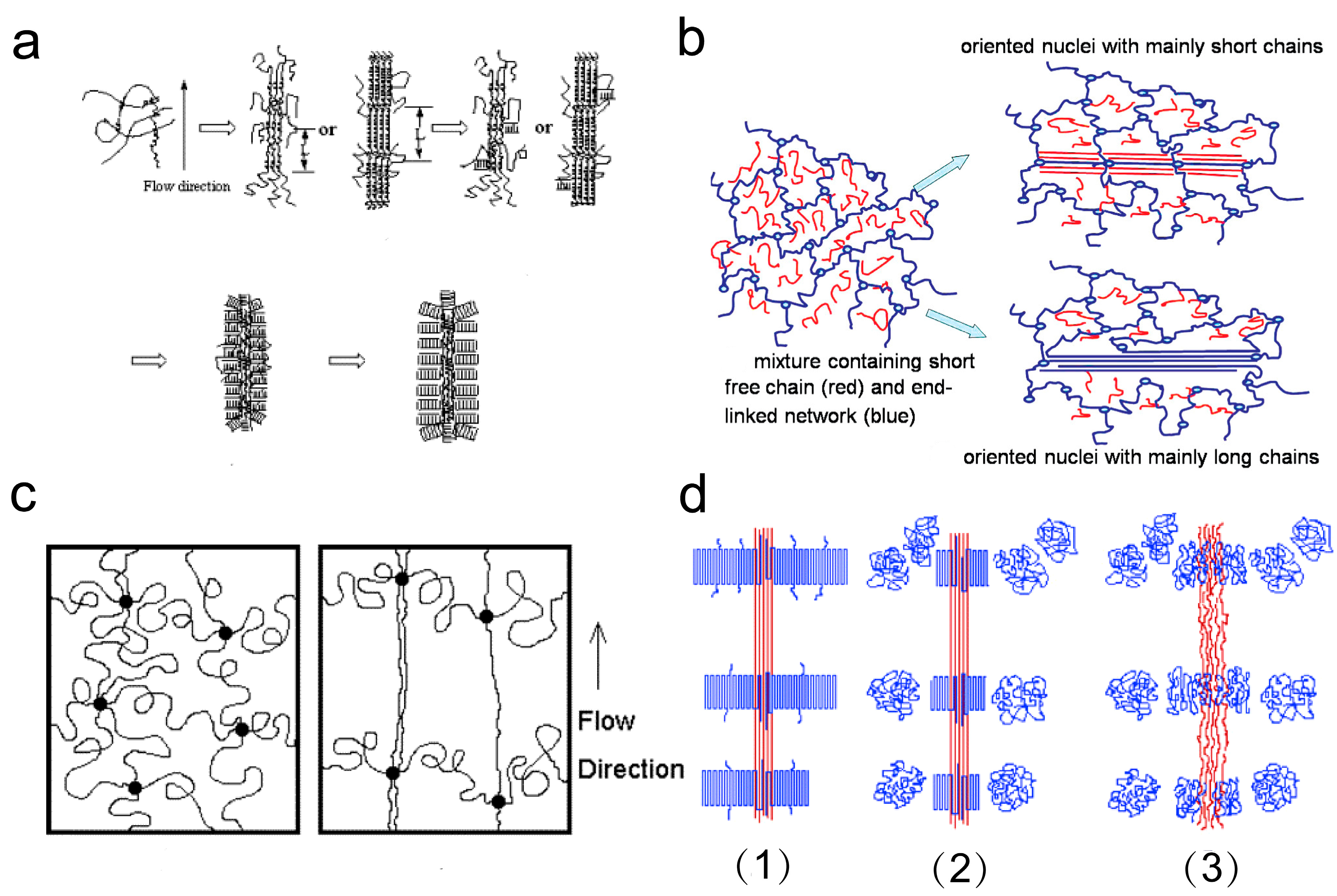
3. Molecular Weight Distribution Effect on Shish Kebab Structures Under Flow Fields
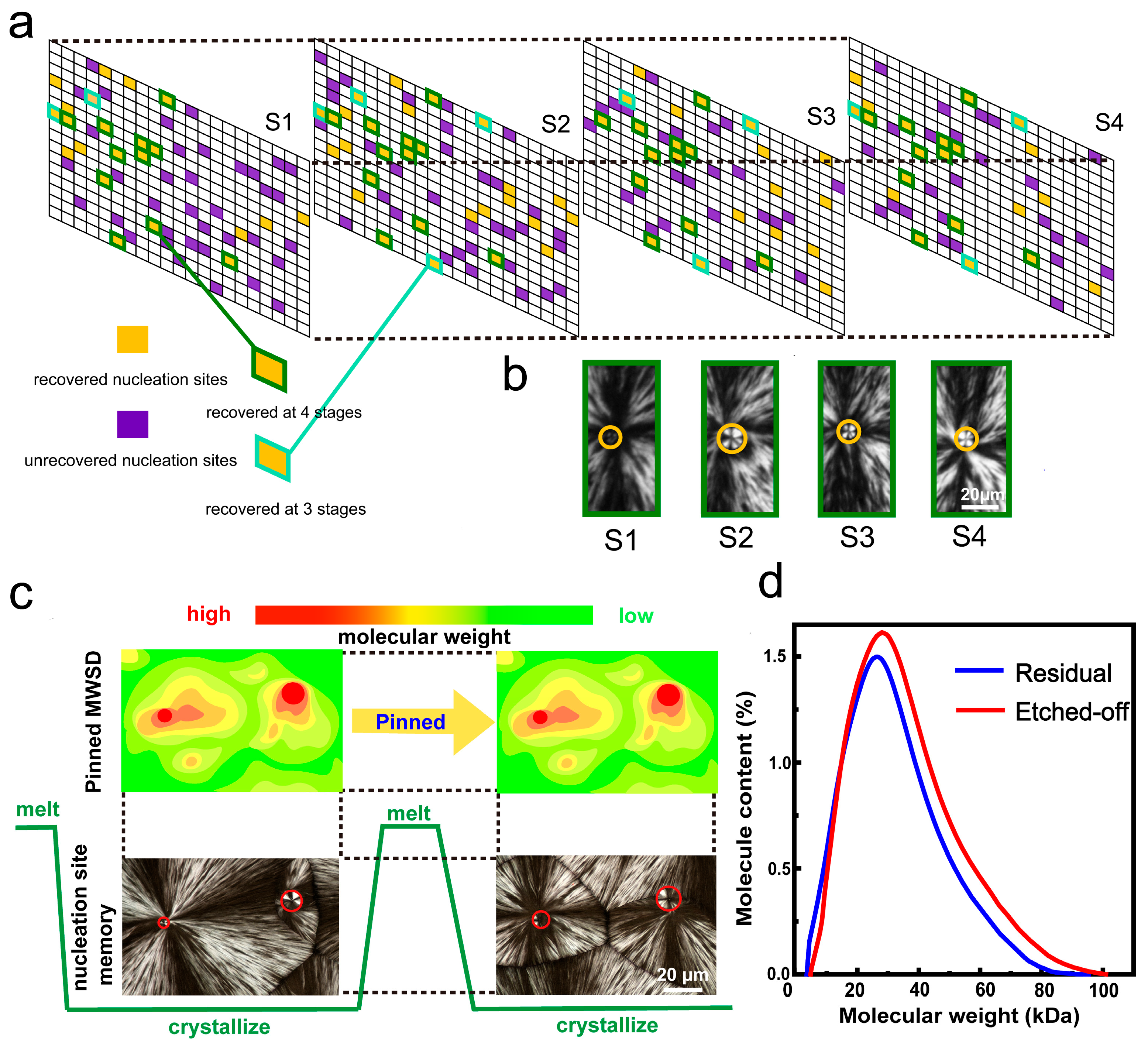
4. Role of Molecular Weight Distribution in Solution-Processed Polymer Spherulite Films
5. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bates, F.S.; Hillmyer, M.A.; Lodge, T.P.; Bates, C.M.; Delaney, K.T.; Fredrickson, G.H. Multiblock Polymers: Panacea or Pandora’s Box? Science 2012, 336, 434–440. [Google Scholar] [CrossRef]
- Nichetti, D.; Manas-Zloczower, I. Influence of Molecular Parameters on Material Processability in Extrusion Processes. Polym. Eng. Sci. 1999, 39, 887–895. [Google Scholar] [CrossRef]
- Lynd, N.A.; Meuler, A.J.; Hillmyer, M.A. Polydispersity and Block Copolymer Self-Assembly. Prog. Polym. Sci. 2008, 33, 875–893. [Google Scholar] [CrossRef]
- Sides, S.W.; Fredrickson, G.H. Continuous Polydispersity in a Self-Consistent Field Theory for Diblock Copolymers. J. Chem. Phys. 2004, 121, 4974–4986. [Google Scholar] [CrossRef]
- Collis, M.W.; Mackley, M.R. The Melt Processing of Monodisperse and Polydisperse Polystyrene Melts within a Slit Entry and Exit Flow. J. Non-Newton. Fluid Mech. 2005, 128, 29–41. [Google Scholar] [CrossRef]
- Long, C.; Dong, Z.; Yu, F.; Wang, K.; He, C.; Chen, Z.-R. Molecular Weight Distribution Shape Dependence of the Crystallization Kinetics of Semicrystalline Polymers Based on Linear Unimodal and Bimodal Polyethylenes. ACS Appl. Polym. Mater. 2023, 5, 2654–2663. [Google Scholar] [CrossRef]
- Chen, H.-L.; Li, L.-J.; Lin, T.-L. Formation of Segregation Morphology in Crystalline/Amorphous Polymer Blends: Molecular Weight Effect. Macromolecules 1998, 31, 2255–2264. [Google Scholar] [CrossRef]
- Zhai, Z.; Morthomas, J.; Fusco, C.; Perez, M.; Lame, O. Crystallization and Molecular Topology of Linear Semicrystalline Polymers: Simulation of Uni- and Bimodal Molecular Weight Distribution Systems. Macromolecules 2019, 52, 4196–4208. [Google Scholar] [CrossRef]
- Schulz, M.; Schäfer, M.; Saalwächter, K.; Thurn-Albrecht, T. Competition between Crystal Growth and Intracrystalline Chain Diffusion Determines the Lamellar Thickness in Semicrystalline Polymers. Nat. Commun. 2022, 13, 119. [Google Scholar] [CrossRef]
- Flory, P.J.; Yoon, D.Y. Molecular Morphology in Semicrystalline Polymers. Nature 1978, 272, 226–229. [Google Scholar] [CrossRef]
- Hu, W. The Physics of Polymer Chain-Folding. Phys. Rep. 2018, 747, 1–50. [Google Scholar] [CrossRef]
- Hu, W. Molecular Segregation in Polymer Melt Crystallization: Simulation Evidence and Unified-Scheme Interpretation. Macromolecules 2005, 38, 8712–8718. [Google Scholar] [CrossRef]
- Bukowski, C.; Zhang, T.; Riggleman, R.A.; Crosby, A.J. Load-Bearing Entanglements in Polymer Glasses. Sci. Adv. 2021, 7, eabg9763. [Google Scholar] [CrossRef] [PubMed]
- Schuster, G.B.; Cafferty, B.J.; Karunakaran, S.C.; Hud, N.V. Water-Soluble Supramolecular Polymers of Paired and Stacked Heterocycles: Assembly, Structure, Properties, and a Possible Path to Pre-RNA. J. Am. Chem. Soc. 2021, 143, 9279–9296. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Wang, T.; Wu, Y.; Sun, R.; Wang, W.; Guo, J.; Wu, Q.; Yang, W.; Min, J. The Intrinsic Role of Molecular Mass and Polydispersity Index in High-Performance Non-Fullerene Polymer Solar Cells. Adv. Energy Mater. 2021, 11, 2002709. [Google Scholar] [CrossRef]
- Kardos, J.L.; Li, H.-M.; Huckshold, K.A. Fractionation of Linear Polyethylene during Bulk Crystallization under High Pressure. J. Polym. Sci. A-2 Polym. Phys. 1971, 9, 2061–2080. [Google Scholar] [CrossRef]
- Jin, L.; Zhang, G.; Zhai, X.; Ma, Z.; Zheng, P.; Wang, W. Macromolecular Effect on Crystal Pattern Formation in Ultra-Thin Films: Molecular Segregation in a Binary Blend of PEO Fractions. Polymer 2009, 50, 6157–6165. [Google Scholar] [CrossRef]
- Cheng, S.Z.D.; Wu, S.S.; Chen, J.; Zhuo, Q.; Quirk, R.P.; Von Meerwall, E.D.; Hsiao, B.S.; Habenschuss, A.; Zschack, P.R. Isothermal Thickening and Thinning Processes in Low-Molecular-Weight Poly(Ethylene Oxide) Fractions Crystallized from the Melt. 4. End-Group Dependence. Macromolecules 1993, 26, 5105–5117. [Google Scholar] [CrossRef]
- Meille, S.V.; Romita, V.; Caronna, T.; Lovinger, A.J.; Catellani, M.; Belobrzeckaja, L. Influence of Molecular Weight and Regioregularity on the Polymorphic Behavior of Poly(3-Decylthiophenes). Macromolecules 1997, 30, 7898–7905. [Google Scholar] [CrossRef]
- Xiang, S.; Jun, S.; Li, G.; Bian, X.; Feng, L.; Chen, X.; Liu, F.; Huang, S. Effects of Molecular Weight on the Crystallization and Melting Behaviors of Poly(L-Lactide). Chin. J. Polym. Sci. 2016, 34, 69–76. [Google Scholar] [CrossRef]
- Androsch, R.; Di Lorenzo, M.L. Effect of Molar Mass on the α′/α-Transition in Poly (l-Lactic Acid). Polymer 2017, 114, 144–148. [Google Scholar] [CrossRef]
- De Lima, B.M.; Hayes, P.L.; Wood-Adams, P.M. Influence of Polymer Molecular Weight on Chain Conformation at the Polystyrene/Silver Interface. Langmuir 2021, 37, 10036–10045. [Google Scholar] [CrossRef]
- Harada, J.; Takahashi, H.; Notsuka, R.; Takehisa, M.; Takahashi, Y.; Usui, T.; Taniguchi, H. Ferroelectric Ionic Molecular Crystals with Significant Plasticity and a Low Melting Point: High Performance in Hot-Pressed Polycrystalline Plates and Melt-Grown Crystalline Sheets. Angew. Chem. Int. Ed. 2023, 62, e202215286. [Google Scholar] [CrossRef] [PubMed]
- Marubayashi, H.; Nobuoka, T.; Iwamoto, S.; Takemura, A.; Iwata, T. Atomic Force Microscopy Observation of Polylactide Stereocomplex Edge-On Crystals in Thin Films: Effects of Molecular Weight on Lamellar Curvature. ACS Macro Lett. 2013, 2, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Kimata, S.; Sakurai, T.; Nozue, Y.; Kasahara, T.; Yamaguchi, N.; Karino, T.; Shibayama, M.; Kornfield, J.A. Molecular Basis of the Shish-Kebab Morphology in Polymer Crystallization. Science 2007, 316, 1014–1017. [Google Scholar] [CrossRef] [PubMed]
- Somani, R.H.; Yang, L.; Zhu, L.; Hsiao, B.S. Flow-Induced Shish-Kebab Precursor Structures in Entangled Polymer Melts. Polymer 2005, 46, 8587–8623. [Google Scholar] [CrossRef]
- Hu, D.P.; Chen, M.; Yang, Y.H.; Li, H.Y. Texture Induced by Molecular Weight Dispersity: Polymorphism within Poly(l-Lactic Acid) Spherulites. Chin. J. Polym. Sci. 2020, 38, 1365–1373. [Google Scholar] [CrossRef]
- Hu, D.; Chen, M.; Lu, S.; Li, H. Polymorphism Texture Induced by Fractional Precipitation of Poly(l-Lactic Acid). Macromolecules 2022, 55, 8195–8202. [Google Scholar] [CrossRef]
- Lu, S.; Chen, M.; Li, H. Nucleation Site Memory in the Spherulite Films of Polydisperse Poly(l-Lactic Acid). Macromolecules 2024, 57, 10219–10226. [Google Scholar] [CrossRef]
- Zhang, G.; Zhai, X.; Ma, Z.; Jin, L.; Zheng, P.; Wang, W.; Cheng, S.Z.D.; Lotz, B. Morphology Diagram of Single-Layer Crystal Patterns in Supercooled Poly(Ethylene Oxide) Ultrathin Films: Understanding Macromolecular Effect of Crystal Pattern Formation and Selection. ACS Macro Lett. 2012, 1, 217–221. [Google Scholar] [CrossRef]
- Pei, D.; Wang, Z.; Peng, Z.; Zhang, J.; Deng, Y.; Han, Y.; Ye, L.; Geng, Y. Impact of Molecular Weight on the Mechanical and Electrical Properties of a High-Mobility Diketopyrrolopyrrole-Based Conjugated Polymer. Macromolecules 2020, 53, 4490–4500. [Google Scholar] [CrossRef]
- Boyer, R.F. Mechanical Motions in Amorphous and Semi-Crystalline Polymers. Polymer 1976, 17, 996–1008. [Google Scholar] [CrossRef]
- Martin, J.R.; Johnson, J.F.; Cooper, A.R. Mechanical Properties of Polymers: The Influence of Molecular Weight and Molecular Weight Distribution. J. Macromol. Sci. C 1972, 8, 57–199. [Google Scholar] [CrossRef]
- Zuo, F.; Keum, J.K.; Yang, L.; Somani, R.H.; Hsiao, B.S. Thermal Stability of Shear-Induced Shish-Kebab Precursor Structure from High Molecular Weight Polyethylene Chains. Macromolecules 2006, 39, 2209–2218. [Google Scholar] [CrossRef]
- Gregorio, R.; Ueno, E.M. Effect of Crystalline Phase, Orientation and Temperature on the Dielectric Properties of Poly (Vinylidene Fluoride) (PVDF). J. Mater. Sci. 1999, 34, 4489–4500. [Google Scholar] [CrossRef]
- Lv, F.; Lin, J.; Zhou, Z.; Hong, Z.; Wu, Y.; Ren, Z.; Zhang, Q.; Dong, S.; Luo, J.; Shi, J.; et al. In-Situ Electrostatic Field Regulating the Recrystallization Behavior of P(VDF-TrFE) Films with High β-Phase Content and Enhanced Piezoelectric Properties towards Flexible Wireless Biosensing Device Applications. Nano Energy 2022, 100, 107507. [Google Scholar] [CrossRef]
- Zhang, Z.; Litt, M.H.; Zhu, L. Unified Understanding of Ferroelectricity in N-Nylons: Is the Polar Crystalline Structure a Prerequisite? Macromolecules 2016, 49, 3070–3082. [Google Scholar] [CrossRef]
- Harrisson, S. The Downside of Dispersity: Why the Standard Deviation Is a Better Measure of Dispersion in Precision Polymerization. Polym. Chem. 2018, 9, 1366–1370. [Google Scholar] [CrossRef]
- Rane, S.S.; Choi, P. Polydispersity Index: How Accurately Does It Measure the Breadth of the Molecular Weight Distribution? Chem. Mater. 2005, 17, 926. [Google Scholar] [CrossRef]
- Moyassari, A.; Gkourmpis, T.; Hedenqvist, M.S.; Gedde, U.W. Molecular Dynamics Simulations of Short-Chain Branched Bimodal Polyethylene: Topological Characteristics and Mechanical Behavior. Macromolecules 2019, 52, 807–818. [Google Scholar] [CrossRef]
- Moyassari, A.; Gkourmpis, T.; Hedenqvist, M.S.; Gedde, U.W. Molecular Dynamics Simulation of Linear Polyethylene Blends: Effect of Molar Mass Bimodality on Topological Characteristics and Mechanical Behavior. Polymer 2019, 161, 139–150. [Google Scholar] [CrossRef]
- Shen, H.; Xie, B.; Yang, W.; Yang, M. Non-Isothermal Crystallization of Polyethylene Blends with Bimodal Molecular Weight Distribution. Polym. Test. 2013, 32, 1385–1391. [Google Scholar] [CrossRef]
- Cai, T.; Ma, Y.; Yin, P.; Hu, W. Understanding the Growth Rates of Polymer Cocrystallization in the Binary Mixtures of Different Chain Lengths. J. Phys. Chem. B 2008, 112, 7370–7376. [Google Scholar] [CrossRef] [PubMed]
- Krumme, A.; Lehtinen, A.; Viikna, A. Crystallisation Behaviour of High Density Polyethylene Blends with Bimodal Molar Mass Distribution 1. Basic Characteristics and Isothermal Crystallisation. Eur. Polym. J. 2004, 40, 359–369. [Google Scholar] [CrossRef]
- Krumme, A.; Lehtinen, A.; Viikna, A. Crystallisation Behaviour of High Density Polyethylene Blends with Bimodal Molar Mass Distribution: 2. Non-Isothermal Crystallisation. Eur. Polym. J. 2004, 40, 371–378. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Q.; Tian, H.; Liu, J.; Geng, Y.; Yan, D. Insight into Lamellar Crystals of Monodisperse Polyfluorenes—Fractionated Crystallization and the Crystal’s Stability. Polymer 2013, 54, 1251–1258. [Google Scholar] [CrossRef]
- Huang, G.; Wu, N.; Wang, X.; Zhang, G.; Qiu, L. Role of Molecular Weight in the Mechanical Properties and Charge Transport of Conjugated Polymers Containing Siloxane Side Chains. Macromol. Rapid Commun. 2022, 43, 2200149. [Google Scholar] [CrossRef]
- Li, W.; Yang, L.; Tumbleston, J.R.; Yan, L.; Ade, H.; You, W. Controlling Molecular Weight of a High Efficiency Donor-Acceptor Conjugated Polymer and Understanding Its Significant Impact on Photovoltaic Properties. Adv. Mater. 2014, 26, 4456–4462. [Google Scholar] [CrossRef]
- Son, S.Y.; Park, T.; You, W. Understanding of Face-On Crystallites Transitioning to Edge-On Crystallites in Thiophene-Based Conjugated Polymers. Chem. Mater. 2021, 33, 4541–4550. [Google Scholar] [CrossRef]
- Saito, M.; Koganezawa, T.; Osaka, I. Understanding Comparable Charge Transport Between Edge-on and Face-on Polymers in a Thiazolothiazole Polymer System. ACS Appl. Polym. Mater. 2019, 1, 1257–1262. [Google Scholar] [CrossRef]
- Tong, M.; Cho, S.; Rogers, J.T.; Schmidt, K.; Hsu, B.B.Y.; Moses, D.; Coffin, R.C.; Kramer, E.J.; Bazan, G.C.; Heeger, A.J. Higher Molecular Weight Leads to Improved Photoresponsivity, Charge Transport and Interfacial Ordering in a Narrow Bandgap Semiconducting Polymer. Adv. Funct. Mater. 2010, 20, 3959–3965. [Google Scholar] [CrossRef]
- Hoffman, J.D.; Lauritzen, J.I. Crystallization of Bulk Polymers with Chain Folding: Theory of Growth of Lamellar Spherulites. J. Res. Natl. Bur. Stand. A Phys. Chem. 1961, 65A, 297–336. [Google Scholar] [CrossRef] [PubMed]
- Keller, A. The Spherulitic Structure of Crystalline Polymers. Part I. Investigations with the Polarizing Microscope. J. Polym. Sci. 1955, 17, 291–308. [Google Scholar] [CrossRef]
- Zhai, X.; Wang, W.; Zhang, G.; He, B. Crystal Pattern Formation and Transitions of PEO Monolayers on Solid Substrates from Nonequilibrium to near Equilibrium. Macromolecules 2006, 39, 324–329. [Google Scholar] [CrossRef]
- Honjo, H.; Ohta, S.; Matsushita, M. Phase Diagram of a Growing Succinonitrile Crystal in Supercooling-Anisotropy Phase Space. Phys. Rev. A 1987, 36, 4555–4558. [Google Scholar] [CrossRef]
- Liang, S.; Wang, K.; Tang, C.; Zhang, Q.; Du, R.; Fu, Q. Unexpected Molecular Weight Dependence of Shish-Kebab Structure in the Oriented Linear Low Density Polyethylene/High Density Polyethylene Blends. J. Chem. Phys. 2008, 128, 174902. [Google Scholar] [CrossRef]
- Androsch, R.; Schick, C.; Di Lorenzo, M.L. Melting of Conformationally Disordered Crystals (α′-Phase) of Poly(l-Lactic Acid). Macromol. Chem. Phys. 2014, 215, 1134–1139. [Google Scholar] [CrossRef]
- Fillon, B.; Thierry, A.; Wittmann, J.C.; Lotz, B. Self-nucleation and recrystallization of polymers. Isotactic polypropylene, β phase: β-α conversion and β-α growth transitions. J. Polym. Sci. B Polym. Phys. 1993, 31, 1407–1424. [Google Scholar] [CrossRef]
- Hill, M.J.; Barham, P.J.; Keller, A. Phase Segregation in Blends of Linear with Branched Polyethylene: The Effect of Varying the Molecular Weight of the Linear Polymer. Polymer 1992, 33, 2530–2541. [Google Scholar] [CrossRef]
- Hoffman, J.D.; Miller, R.L. Kinetic of Crystallization from the Melt and Chain Folding in Polyethylene Fractions Revisited: Theory and Experiment. Polymer 1997, 38, 3151–3212. [Google Scholar] [CrossRef]
- Lauritzen, J.I.; Hoffman, J.D. Theory of Formation of Polymer Crystals with Folded Chains in Dilute Solution. J. Res. Natl. Bur. Stand. A Phys. Chem. 1960, 64A, 73–102. [Google Scholar] [CrossRef]
- Mandelkern, L.; Price, J.M.; Gopalan, M.; Fatou, J.G. Sizes and Interfacial Free Energies of Crystallites Formed from Fractionated Linear Polyethylene. J. Polym. Sci. A-2 Polym. Phys. 1966, 4, 385–400. [Google Scholar] [CrossRef]
- Mandelkern, L. The Effect of Molecular Weight on the Crystallization, Melting, and Morphology of Long-Chain Molecules. J. Polym. Sci. C Polym. Symp. 1967, 15, 129–162. [Google Scholar] [CrossRef]
- Ergoz, E.; Fatou, J.G.; Mandelkern, L. Molecular Weight Dependence of the Crystallization Kinetics of Linear Polyethylene. I. Experimental Results. Macromol. 1972, 5, 147–157. [Google Scholar] [CrossRef]
- Mandelkern, L.; Alamo, R.G.; Kennedy, M.A. The Interphase Thickness of Linear Polyethylene. Macromolecules 1990, 23, 4721–4723. [Google Scholar] [CrossRef]
- Fatou, J.G.; Marco, C.; Mandelkern, L. The Influence of Molecular Weight on the Regime Crystallization of Linear Polyethylene. Polymer 1990, 31, 1685–1693. [Google Scholar] [CrossRef]
- Voigt-Martin, I.G.; Fischer, E.W.; Mandelkern, L. Morphology of Melt-Crystallized Linear Polyethylene Fractions and Its Dependence on Molecular Weight and Crystallization Temperature. J. Polym. Sci. Polym. Phys. Ed. 1980, 18, 2347–2367. [Google Scholar] [CrossRef]
- Voigt-Martin, I.G.; Mandelkern, L. A Quantitative Electron Microscopic Study of the Crystallite Structure of Molecular Weight Fractions of Linear Polyethylene. J. Polym. Sci. Polym. Phys. 1984, 22, 1901–1917. [Google Scholar] [CrossRef]
- Richards, R.B. The Phase Equilibria between a Crystalline Polymer and Solvents. Trans. Faraday Soc. 1946, 42, 10. [Google Scholar] [CrossRef]
- Pennings, A.J. Fractionation of Polymers by Crystallization from Solutions. II J. Polym. Sci. C Polym. Symp. 1967, 16, 1799–1812. [Google Scholar] [CrossRef]
- Sadler, D.M. Fractionation during Crystallization. J. Polym. Sci. A-2 Polym. Phys. 1971, 9, 779–799. [Google Scholar] [CrossRef]
- Prime, R.B.; Wunderlich, B.; Melillo, L. Extended-Chain Crystals. V. Thermal Analysis and Electron Microscopy of the Melting Process in Polyethylene. J. Polym. Sci. A-2: Polym. Phys. 1969, 7, 2091–2097. [Google Scholar] [CrossRef]
- Mehta, A.; Wunderlich, B. A Study of Molecular Fractionation during the Crystallization of Polymers. Colloid Polym. Sci. 1975, 253, 193–205. [Google Scholar] [CrossRef]
- Hoffman, J.D. Theoretical Aspects of Polymer Crystallization with Chain Folds: Bulk Polymers. Polym. Eng. Sci. 1964, 4, 315–362. [Google Scholar] [CrossRef]
- Lindenmeyer, P.H.; Peterson, J.M. Chain-Folding and Molecular-Species Segregation in the Crystallization of Linear High Polymers. J. Appl. Phys. 1968, 39, 4929–4931. [Google Scholar] [CrossRef]
- Wunderlich, B.; Mehta, A. Macromolecular Nucleation. J. Polym. Sci. Polym. Phys. Ed. 1974, 12, 255–263. [Google Scholar] [CrossRef]
- Wunderlich, B. Molecular Nucleation and Segregation. Faraday Discuss. Chem. Soc. 1979, 68, 239. [Google Scholar] [CrossRef]
- Keller, A.; Cheng, S.Z.D. The Role of Metastability in Polymer Phase Transitions. Polymer 1998, 39, 4461–4487. [Google Scholar] [CrossRef]
- Dukovski, I.; Muthukumar, M. Langevin Dynamics Simulations of Early Stage Shish-Kebab Crystallization of Polymers in Extensional Flow. J. Chem. Phys. 2003, 118, 6648–6655. [Google Scholar] [CrossRef]
- Nie, Y.; Zhao, Y.; Matsuba, G.; Hu, W. Shish-Kebab Crystallites Initiated by Shear Fracture in Bulk Polymers. Macromolecules 2018, 51, 480–487. [Google Scholar] [CrossRef]
- Zhao, L.; Hu, Y.; Shao, Y.; Liu, Z.; Liu, B.; He, X. Molecular Dynamics Simulation of Shish-Kebab Crystallization of Polyethylene: Unraveling the Effects of Molecular Weight Distribution. J. Chem. Phys. 2019, 150, 184114. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, H.; Wang, D.; Yan, S.; Han, C.C. In Situ Optical Microscope Study of the Shear-Induced Crystallization of Isotactic Polypropylene. Polymer 2005, 46, 8157–8161. [Google Scholar] [CrossRef]
- Zhao, B.; Li, X.; Huang, Y.; Cong, Y.; Ma, Z.; Shao, C.; An, H.; Yan, T.; Li, L. Inducing Crystallization of Polymer through Stretched Network. Macromolecules 2009, 42, 1428–1432. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, M.; Li, H.; Li, H. The Degree of Crystallinity Exhibiting a Spatial Distribution in Polymer Films. Eur. Polym. J. 2018, 107, 303–307. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, S.; Chen, M.; Li, H. The Impact of Molecular Weight Distribution on the Crystalline Texture of Polymers—Illustrated by Lamellar Crystal, Shish–Kebab and Nested Spherulites. Materials 2025, 18, 4196. https://doi.org/10.3390/ma18174196
Lu S, Chen M, Li H. The Impact of Molecular Weight Distribution on the Crystalline Texture of Polymers—Illustrated by Lamellar Crystal, Shish–Kebab and Nested Spherulites. Materials. 2025; 18(17):4196. https://doi.org/10.3390/ma18174196
Chicago/Turabian StyleLu, Songyan, Min Chen, and Hanying Li. 2025. "The Impact of Molecular Weight Distribution on the Crystalline Texture of Polymers—Illustrated by Lamellar Crystal, Shish–Kebab and Nested Spherulites" Materials 18, no. 17: 4196. https://doi.org/10.3390/ma18174196
APA StyleLu, S., Chen, M., & Li, H. (2025). The Impact of Molecular Weight Distribution on the Crystalline Texture of Polymers—Illustrated by Lamellar Crystal, Shish–Kebab and Nested Spherulites. Materials, 18(17), 4196. https://doi.org/10.3390/ma18174196






