BaTiO3-Based Electrocaloric Materials—Recent Progresses and Perspective
Highlights
- Thermodynamics of ECE were introduced; EC strength was proposed to illustrate the ECE in a smaller electric field.
- Direct and indirect ECE measurement methods were revealed and evaluated.
- Advantages and disadvantages of BaTiO3-based ceramics were presented.
- Strategies to enhance the ECE for BaTiO3-based materials were summarized.
Abstract
1. Introduction
2. Indirect Calculation Method
2.1. Maxwell Relations
2.2. Landau Phenomenological Theory
2.3. Electrocaloric Strength ()
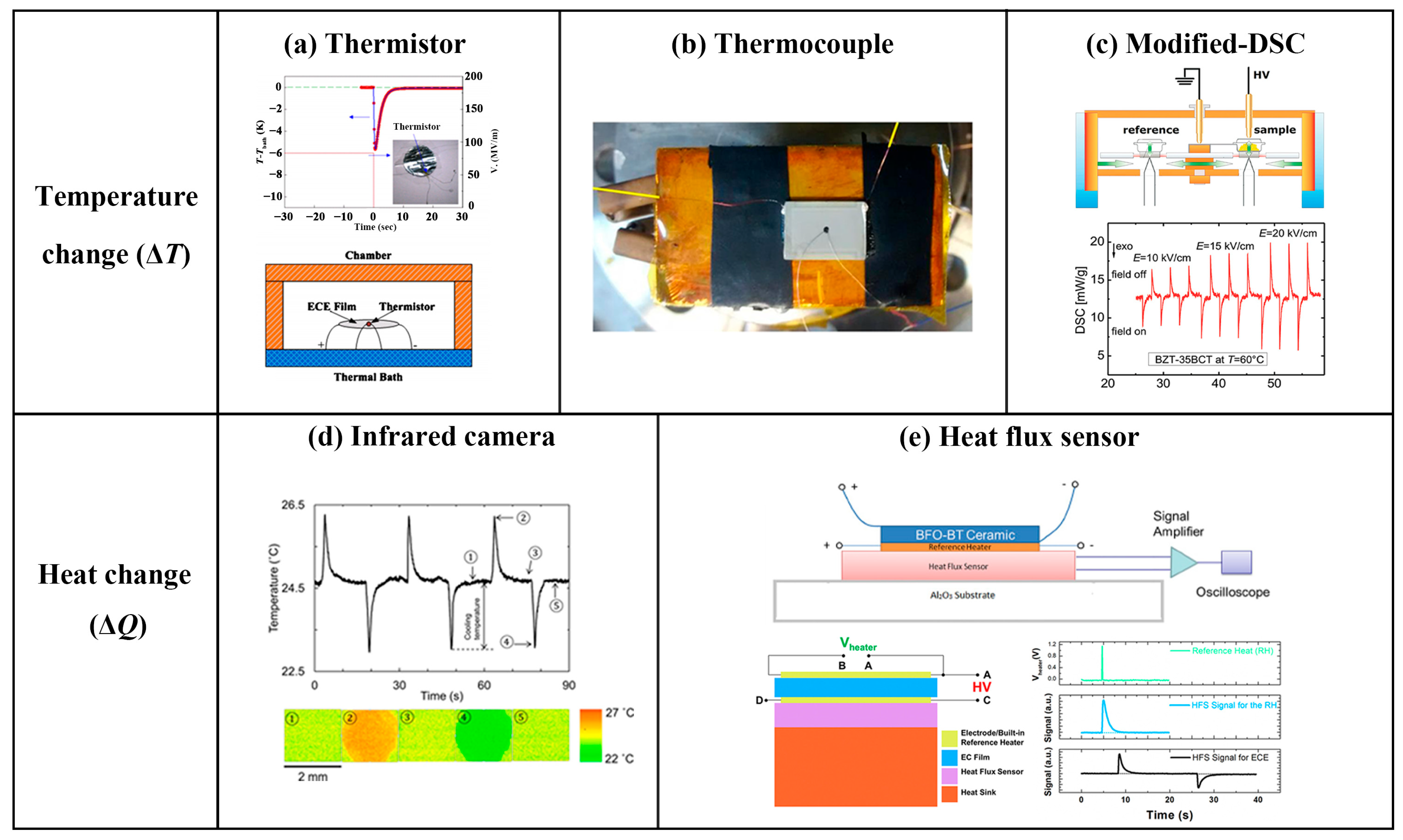
3. Direct Measurement Methods
4. Why Choose BT as an Electrocaloric Material?
- Large spontaneous polarization. Pristine BT exhibits a relatively large spontaneous polarization, which is crucial for achieving a large ECE. For instance, a pure BT single crystal exhibits a saturation polarization of ~26 μC/cm2 in the tetragonal phase at room temperature [92], providing a strong basis for polarization entropy change under an applied electric field.
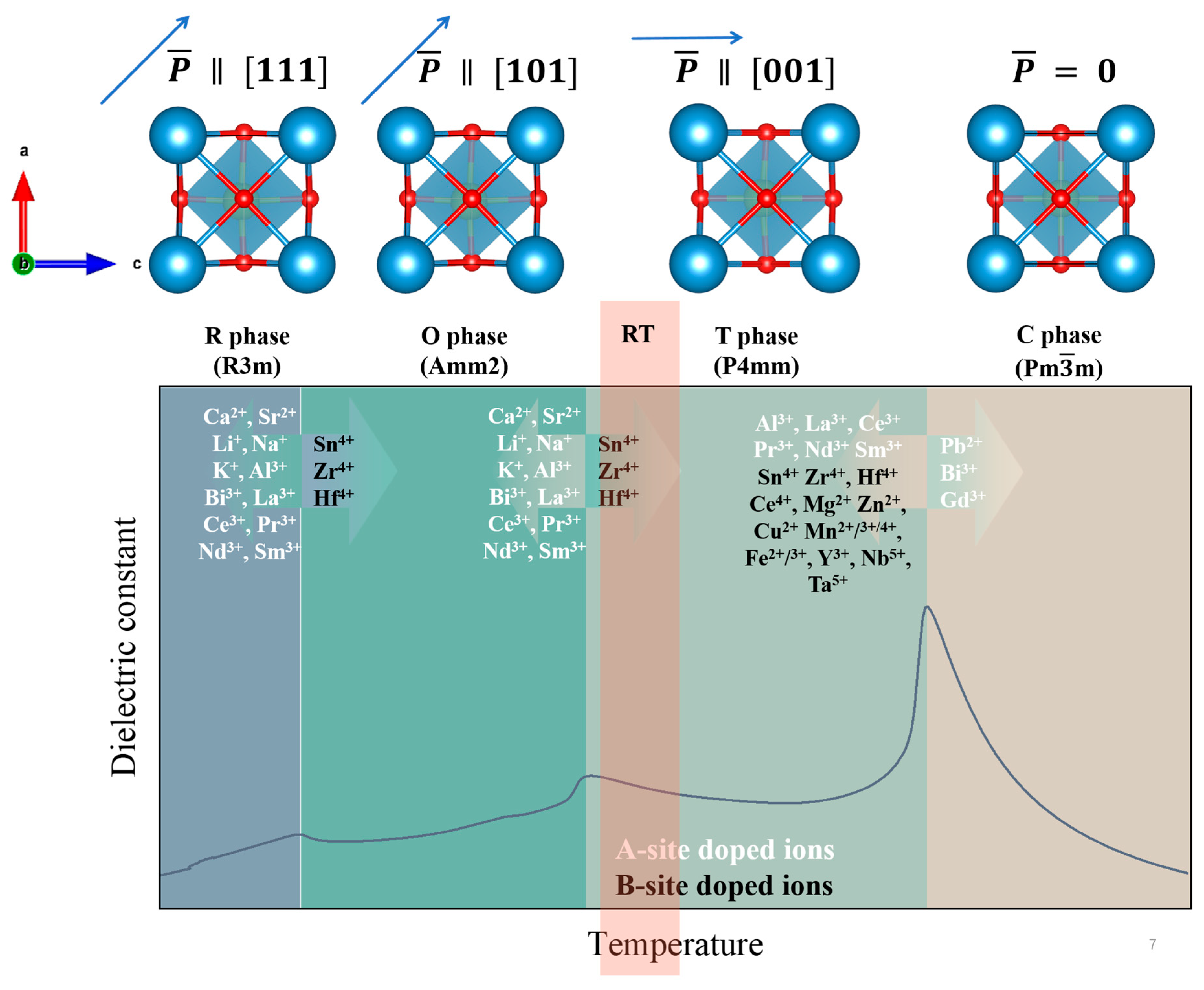
- Rich and well-defined phase transitions. The pristine BT unit cell (Figure 3) belongs to a perovskite ABO3 structure, where Ba2+ occupies the A sites, Ti4+ occupies the B sites, and O2− occupies the six face centers. Due to the temperature-induced symmetry’s variation, pristine BT undergoes a series of well-characterized phase transitions with the increasing temperature, i.e., rhombohedral (R, R3m space group, spontaneous polarization direction along [1,1,1]), orthorhombic (O, Amm2 space group, spontaneous polarization direction along [1,0,1]), tetragonal (T, P4mm space group, spontaneous polarization direction along [0,0,1]), and finally cubic (C, Pmm space group, paraelectric). These transitions can be clearly observed in the dielectric constant versus temperature spectra, with peaks corresponding to R–O (~−90 °C, ), O–T (~0 °C, ), and T–C (~120 °C, Curie point, ) transitions, as shown in Figure 3. Generally, TC is especially important for the ECE due to the largest pyroelectric coefficient (dP/dT) near TC. Based on these temperature-induced phase transitions, one can obtain the enhanced dielectric and ferroelectric properties by adjusting the phase transitions and merging the various phase transitions. The fundamental mechanisms and effects of specific dopants are discussed below in detail.
- Large phase transition enthalpy. Pristine BaTiO3 exhibits a phase transition enthalpy of approximately 900 J/kg, characterized by a first-order phase transition feature [93]. Under the influence of the electric field and the temperature, this enthalpy can be modulated. Although doping may slightly alter the phase transition enthalpy, when the doping concentration is low, the combined effect of phase transition enthalpy and the ECE can lead to an enhanced isothermal entropy change and adiabatic temperature change.
- Stability, environmental friendliness, and low cost. As a commonly used composition in industrialized multilayer ceramic capacitors (MLCCs), BT exhibits excellent chemical stability without volatile constituents. In addition, as a lead-free material, it is environmentally friendly, and its raw materials are significantly less expensive compared to lead-based counterparts.
- High Curie temperature. Ferroelectrics exhibit a large pyroelectric coefficient near their Curie temperatures. However, the of pristine BT is ~120 °C, which is far above room temperature. As a result, a pronounced ECE can only be achieved in the vicinity of 120 °C.
- Sharp first-order phase transition. Pristine BT exhibits a typical first-order ferroelectric–paraelectric phase transition, leading to a large but narrow EC response localized near 120 °C. This is unfavorable for applications requiring a broad operating temperature range.
- A-site doping. Generally, monovalent (Li+ [94], Na+ [95], K+ [96], etc.), divalent (Ca2+ [97], Pb2+ [98], Sr2+ [84], etc.), and trivalent (Bi3+ [99], La3+ [100], Ce3+ [101], Sm3+ [57,72], etc.) ions are often introduced at the A-sites. Doping ions introduces compositional and structural fluctuations, which lead to reductions in the , , and transition temperatures while also enhancing their relaxor ferroelectric behaviors, as shown in Figure 3. Notably, only Pb2+, Gd3+, and Bi3+ are capable of shifting toward higher temperatures [102,103,104,105]. In addition, Ca2+ can effectively stabilize the ferroelectric phase, resulting in negligible changes to . However, both and decrease progressively with increasing Ca2+ content.
- B-site doping. Ions such as Zr4+, Sn4+, and Hf4+ [18,37,82,106]—with similar radii and valences to Ti4+ (0.605 Å)—are commonly substituted at the B sites. These dopants typically reduce but enhance the lower phase transition temperatures (, ) without significantly breaking the long-range ferroelectric ordering. Other dopants, such as Ce4+, Mg2+, Mn2+/3+/4+, Y3+, Nb5+, and Ta5+ [101,107,108], have also been used, though many tend to degrade the ferroelectricities and broaden the phase transitions, resulting in diffuse EC responses.
5. Strategies
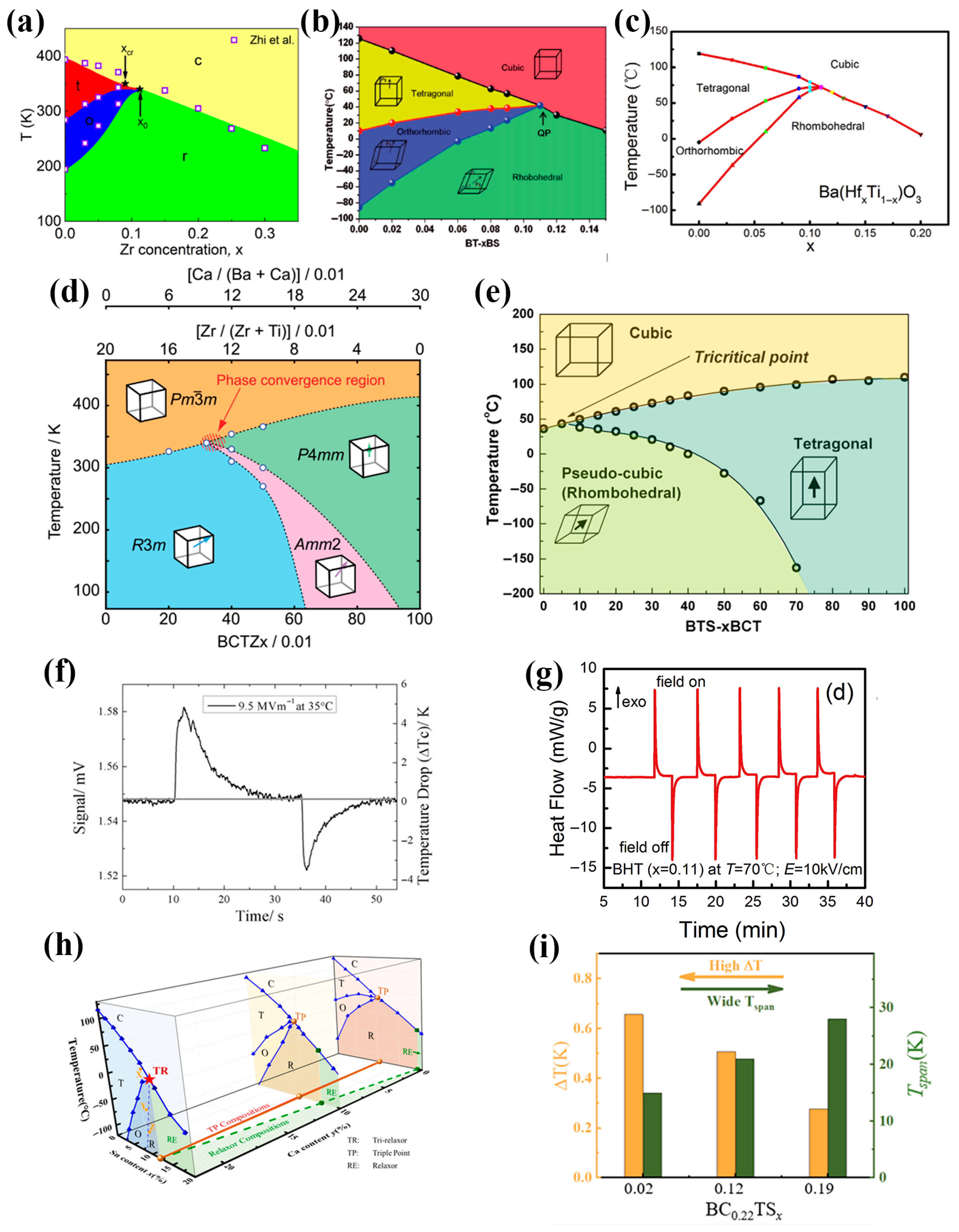
5.1. Constructing Relaxor Ferroelectrics (RFEs)
5.2. Multi-Phase Coexistence
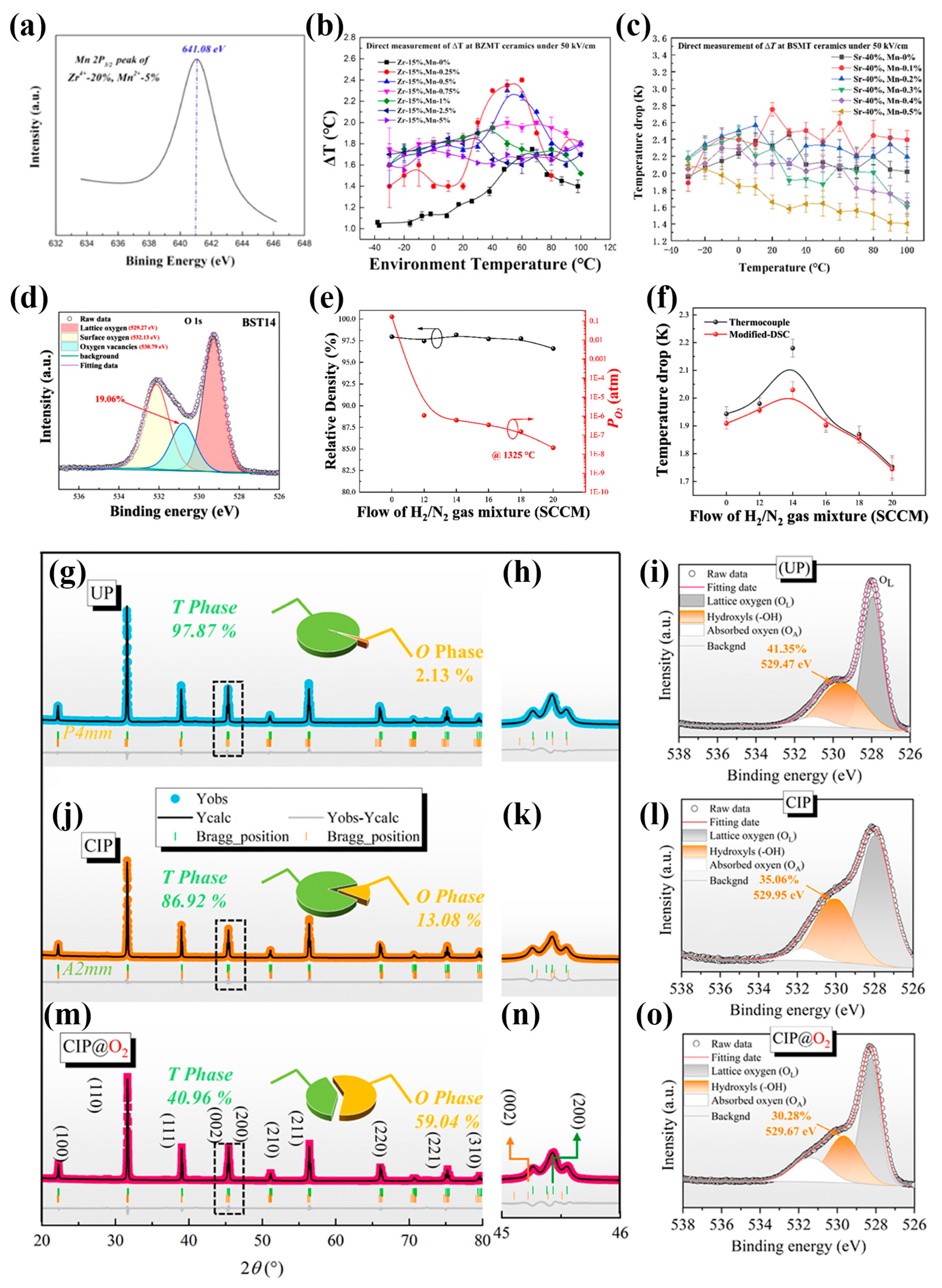
5.3. Control of Oxygen Vacancies
5.3.1. Compensation of Oxygen Vacancies via Doping with Multivalent Ions with a High Valence
5.3.2. Regulation of Oxygen Partial Pressure During Sintering
5.3.3. Post-Sintering Treatment to Optimize the Oxygen Vacancy Concentration and Microstructure
5.4. High-Entropy Design
5.5. Improving the Dielectric Breakdown Strength (DBS)
5.6. Polarization Flip
- The high remanent polarization of NFEs can be fully used for polarization flip to obtain an over four-fold enhancement in ECE and .
- The full ferroelectric phase region can be employed for polarization flip, not just the FE-PE phase transition region. Then, the working temperature can be extended to the whole ferroelectric phase region.
- High can be obtained due to the huge enhanced ECE . Here, in [110] PMN-PT single crystals, an over 2.50 K/(MV/m) was obtained from 290 K to 370 K, and the maximum ΔT/ΔE was 2.75 K/(MV/m).
6. Perspective
- Enhancing ECE via local structural engineering
- 2.
- Exploring the underlying mechanism of polarization flip
- 3.
- Bridging material design and device integration.
- 4.
- Employing machine learning (ML) tools to predict ECE.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mardini-Bovea, J.; Torres-Díaz, G.; Sabau, M.; De-la-Hoz-Franco, E.; Niño-Moreno, J.; Pacheco-Torres, P.J. A review to refrigeration with thermoelectric energy based on the Peltier effect. Dyna 2019, 86, 9–18. [Google Scholar] [CrossRef]
- Franco, V.; Blázquez, J.S.; Ipus, J.J.; Law, J.Y.; Moreno-Ramírez, L.; Conde, A. Magnetocaloric effect: From materials research to refrigeration devices. Prog. Mater. Sci. 2018, 93, 112–232. [Google Scholar] [CrossRef]
- Moya, X.; Mathur, N.D. Caloric materials for cooling and heating. Science 2020, 370, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Geng, Y.; Wang, Y.; Ling, J.; Hwang, Y.; Radermacher, R.; Takeuchi, I.; Cui, J. A review of elastocaloric cooling: Materials, cycles and system integrations. Int. J. Refrig. 2016, 64, 1–19. [Google Scholar] [CrossRef]
- Cirillo, L.; Greco, A.; Masselli, C. Cooling through barocaloric effect: A review of the state of the art up to 2022. Therm. Sci. Eng. Prog. 2022, 33, 101380. [Google Scholar] [CrossRef]
- Zhang, F.; Miao, X.; van Dijk, N.; Brück, E.; Ren, Y. Advanced Magnetocaloric Materials for Energy Conversion: Recent Progress, Opportunities, and Perspective. Adv. Energy Mater. 2024, 14, 2400369. [Google Scholar] [CrossRef]
- Kabirifar, P.; Žerovnik, A.; Ahčin, Ž.; Porenta, L.; Brojan, M.; Tušek, J. Elastocaloric cooling: State-of-the-art and future challenges in designing regenerative elastocaloric devices. J. Mech. Eng. Stroj. Vestn. 2019, 65, 615–630. [Google Scholar] [CrossRef]
- Lu, B.; Liu, J. Mechanocaloric materials for solid-state cooling. Sci. Bull. 2015, 60, 1638–1643. [Google Scholar] [CrossRef]
- Valant, M. Electrocaloric materials for future solid-state refrigeration technologies. Prog. Mater. Sci. 2012, 57, 980–1009. [Google Scholar] [CrossRef]
- Correia, T.; Zhang, Q. Electrocaloric Materials: New Generation of Coolers; Correia, T., Zhang, Q., Eds.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Thomson, W., II. On the thermoelastic, thermomagnetic, and pyroelectric properties of matter. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1878, 5, 4–27. [Google Scholar] [CrossRef]
- Lang, S.B. Pyroelectricity: From Ancient Curiosity to Modern Imaging Tool. Physics Today 2005, 58, 31–36. [Google Scholar] [CrossRef]
- Kobeco, P.; Kurtschatov, I. Dielectric properties of Rochelle salt crystal. Z. Phys. 1930, 66, 192–205. [Google Scholar] [CrossRef]
- Wiseman, G.G.; Kuebler, J.K. Electrocaloric Effect in Ferroelectric Rochelle Salt. Phys. Rev 1963, 131, 2023–2027. [Google Scholar] [CrossRef]
- Mischenko, A.S.; Zhang, Q.; Scott, J.F.; Whatmore, R.W.; Mathur, N.D. Giant Electrocaloric Effect in Thin-Film PbZr0.95Ti0.05O3. Science 2006, 311, 1270–1271. [Google Scholar] [CrossRef]
- Neese, B.; Chu, B.; Lu, S.-G.; Wang, Y.; Furman, E.; Zhang, Q.M. Large Electrocaloric Effect in Ferroelectric Polymers Near Room Temperature. Science 2008, 321, 821–823. [Google Scholar] [CrossRef]
- Lu, S.G.; Rožič, B.; Zhang, Q.M.; Kutnjak, Z.; Pirc, R.; Lin, M.; Li, X.; Gorny, L. Comparison of directly and indirectly measured electrocaloric effect in relaxor ferroelectric polymers. Appl. Phys. Lett. 2010, 97, 202901. [Google Scholar] [CrossRef]
- Niu, X.; Jian, X.; Gong, W.; Liang, W.; Gong, X.; Zhang, G.; Jiang, S.; Yu, K.; Zhao, X.; Yao, Y.; et al. Field-driven merging of polarizations and enhanced electrocaloric effect in BaTiO3-based lead-free ceramics. J. Adv. Ceram. 2022, 11, 1777–1788. [Google Scholar] [CrossRef]
- Cao, W.P.; Li, W.L.; Xu, D.; Hou, Y.F.; Wang, W.; Fei, W.D. Enhanced electrocaloric effect in lead-free NBT-based ceramics. Ceram. Int. 2014, 40, 9273–9278. [Google Scholar] [CrossRef]
- Liu, N.; Liang, R.; Zhang, G.; Zhou, Z.; Yan, S.; Li, X.; Dong, X. Colossal negative electrocaloric effects in lead-free bismuth ferrite-based bulk ferroelectric perovskite for solid-state refrigeration. J. Mater. Chem. C 2018, 6, 10415–10421. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, C.; Zheng, T.; Wu, J. Large electrocaloric response with superior temperature stability in NaNbO3-based relaxor ferroelectrics benefiting from the crossover region. J. Mater. Chem. A 2021, 9, 2806–2814. [Google Scholar] [CrossRef]
- Yang, J.; Hao, X. Electrocaloric effect and pyroelectric performance in (K,Na)NbO3-based lead-free ceramics. J. Am. Ceram. Soc. 2019, 102, 6817–6826. [Google Scholar] [CrossRef]
- Lu, S.-G.; Zhang, Q. Large Electrocaloric Effect in Relaxor Ferroelectrics. J. Adv. Dielectr. 2012, 2, 230011. [Google Scholar] [CrossRef]
- Lu, S.G.; Rozic, B.; Zhang, Q.M.; Kutnjak, Z.; Pirc, R. Electrocaloric effect in ferroelectric polymers. Appl. Phys. A 2012, 107, 559–566. [Google Scholar] [CrossRef]
- Lu, S.G.; Cai, Z.H.; Ouyang, Y.X.; Deng, Y.M.; Zhang, S.J.; Zhang, Q.M. Electrical field dependence of electrocaloric effect in relaxor ferroelectrics. Ceram. Int. 2015, 41, S15–S18. [Google Scholar] [CrossRef]
- Sheng-Guo, L.; Dan-Dan, L.; Xiong-Wei, L.; Xiao-Dong, J.; Xiao-Bo, Z.; Ying-Bang, Y.; Tao, T.; Bo, L. Influence of electric field on the phenomenological coefficient and electrocaloric strength in ferroelectrics. Acta Phys. Sin. 2020, 69, 127701. [Google Scholar] [CrossRef]
- Wang, J.; Yang, T.; Chen, S.; Li, G.; Zhang, Q.; Yao, X. Nonadiabatic direct measurement electrocaloric effect in lead-free Ba,Ca(Zr,Ti)O3 ceramics. J. Alloys Compd. 2013, 550, 561–563. [Google Scholar] [CrossRef]
- Greiner, A.; Molin, C.; Neubert, H.; Gebhardt, S.E.; Neumeister, P. Direct Measurement of the Electrocaloric Temperature Change in Multilayer Ceramic Components using Resistance-Welded Thermocouple Wires. Energy Technol. 2018, 6, 1535–1542. [Google Scholar] [CrossRef]
- Nair, B.; Usui, T.; Crossley, S.; Kurdi, S.; Guzmán-Verri, G.G.; Moya, X.; Hirose, S.; Mathur, N.D. Large electrocaloric effects in oxide multilayer capacitors over a wide temperature range. Nature 2019, 575, 468–472. [Google Scholar] [CrossRef]
- Jian, X.-D.; Lu, B.; Li, D.-D.; Yao, Y.-B.; Tao, T.; Liang, B.; Guo, J.-H.; Zeng, Y.-J.; Chen, J.-L.; Lu, S.-G. Direct Measurement of Large Electrocaloric Effect in Ba(ZrxTi1–x)O3 Ceramics. ACS Appl. Mater. Interfaces 2018, 10, 4801–4807. [Google Scholar] [CrossRef]
- Sanlialp, M.; Shvartsman, V.V.; Acosta, M.; Dkhil, B.; Lupascu, D.C. Strong electrocaloric effect in lead-free 0.65Ba(Zr0.2Ti0.8)O3-0.35(Ba0.7Ca0.3)TiO3 ceramics obtained by direct measurements. Appl. Phys. Lett. 2015, 106, 062901. [Google Scholar] [CrossRef]
- Guo, D.; Gao, J.; Yu, Y.-J.; Santhanam, S.; Fedder, G.K.; McGaughey, A.J.H.; Yao, S.C. Electrocaloric characterization of a poly(vinylidene fluoride-trifluoroethylene-chlorofluoroethylene) terpolymer by infrared imaging. Appl. Phys. Lett. 2014, 105, 031906. [Google Scholar] [CrossRef]
- Crossley, S.; Usui, T.; Nair, B.; Kar-Narayan, S.; Moya, X.; Hirose, S.; Ando, A.; Mathur, N.D. Direct electrocaloric measurement of 0.9Pb(Mg1/3Nb2/3)O3-0.1PbTiO3 films using scanning thermal microscopy. Appl. Phys. Lett. 2016, 108, 032902. [Google Scholar] [CrossRef]
- Shi, J.; Han, D.; Li, Z.; Yang, L.; Lu, S.-G.; Zhong, Z.; Chen, J.; Zhang, Q.M.; Qian, X. Electrocaloric Cooling Materials and Devices for Zero-Global-Warming-Potential, High-Efficiency Refrigeration. Joule 2019, 3, 1200–1225. [Google Scholar] [CrossRef]
- Cheng, L.-Q.; Liu, W.; Li, Q.; Wang, S.; Dong, X.; Xu, H.; Jiang, G.; Chen, K. Enhancing electrocaloric performance by compositionally graded laminated structure design in lead-free BZT-BST ceramics. Ceram. Int. 2025, 51, 34789–34794. [Google Scholar] [CrossRef]
- Hao, M.; Wu, G.; Zheng, Y.; Liang, Y.; Li, H.; Yan, Y.; Li, Q.; Yu, K.; Hai, W.; Hou, J.; et al. Enhanced electrocaloric effect in BaTiO3-based ceramics over a wide working temperature range by domain engineering. J. Am. Ceram. Soc. 2025, 108, e20545. [Google Scholar] [CrossRef]
- Lin, M.; Luo, Z.; Sun, H.; Zhang, B.; Han, F.; Niu, X.; Wang, D.; Bai, Y.; Chen, X.; Peng, B.; et al. Enhanced room-temperature electrocaloric performance by both multiphase coexistence and diffused phase transition in (Ba0.65Sr0.3Ca0.05)(SnxTi1−x)O3 ferroelectric ceramics. J. Mater. Chem. C 2025, 13, 1713–1723. [Google Scholar] [CrossRef]
- Tang, S.; Huan, C.; Zhou, X.; Lu, Z.; Meng, Y.; Liu, L.; Ke, Q. Selective forming process for microstructures engineering of BST-based ceramics to achieve promoted electrocaloric performance. Ceram. Int. 2025, 51, 10174–10183. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Li, Y.; Liu, X.; Yang, S.; Wu, J.; Hou, D.; Zhang, J.; Wu, H.; Zhang, Y.; et al. High performance relaxor ferroelectric textured ceramics for electrocaloric refrigeration. Nat. Commun. 2025, 16, 4613. [Google Scholar] [CrossRef]
- Temel, H.; Avci, T.; Okatan, M.B.; Alkoy, S.; Misirlioglu, İ.B.; Mensur, E. Effect of the synthesis method and particle size on BCZT electrocaloric properties. J. Phys. Chem. Solids 2025, 199, 112512. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, X.; Shi, W.; Yang, Y.; Shur, V.; Laletin, V.; Jin, L. Electrocaloric response enhancement over a broad temperature range in lead-free BT-based ceramics. Ceram. Int. 2024, 50, 28159–28167. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, X.; Shi, W.; Zhang, H.; Tran, N.-M.; Laletin, V.; Shur, V.; Lu, S.; Jin, L. Electrocaloric effect in chemically modified barium titanate ferroelectric ceramics. Ceram. Int. 2024, 50, 50098–50106. [Google Scholar] [CrossRef]
- Chen, K.; Ma, J.; Wu, B.; Zhao, P.; Chen, J.; Yang, C.; Tang, B. Optimization of an indirect method for electrocaloric effect in BT-based ceramics validated through the Rayleigh relationship and direct method. J. Mater. Chem. C 2024, 12, 14395–14403. [Google Scholar] [CrossRef]
- Li, Z.; Molin, C.; Gebhardt, S.E. Influence of Grain-Growth Inhibitors on Modified (Ba,Sr)(Sn,Ti)O3 for Electrocaloric Application. Materials 2024, 17, 1036. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Huang, Y.; Wu, X.; Gao, M.; Lin, T.; Lin, C. Evolution behavior of electrocaloric effect in BaTiO3-based ceramics with different phase structures and phase transitions. J. Am. Ceram. Soc. 2024, 107, 195–204. [Google Scholar] [CrossRef]
- Chen, K.; Zhao, P.; Chen, J.; Yang, C.; Tang, B. Broad temperature span and large electrocaloric effect in lead-free ceramics via multi-strategy synergistic optimization. Chem. Eng. J. 2024, 502, 157863. [Google Scholar] [CrossRef]
- Tang, S.; Meng, Y.; Cai, Y.; Peng, B.; Liu, L.; Ke, Q. Achieving improved electrocaloric effect and broad operation temperature by tailoring phase fraction in BaTiO3-based ceramics. Ceram. Int. 2024, 50, 10825–10834. [Google Scholar] [CrossRef]
- Chitra; Chatterjee, S.; Barman, S.; Rawat, S.; Singh, K.C.; Mukherjee, D. Large electrocaloric effect across the non-hysteretic electrostructural phase transition in lead-free (Ba0.88Ca0.12)(Ti0.94Sn0.06)O3 ceramics. Mater. Res. Bull. 2024, 178, 112894. [Google Scholar] [CrossRef]
- Kang, R.; Qiao, W.; Xiong, J.; Hu, H.; Wu, M.; Li, F.; Gao, Y.; Zhang, L.; Zhao, Y.; Lou, X. Large room-temperature electrocaloric response achieved by tailoring the first-order orthorhombic-tetragonal phase transition in BaTiO3-based ferroelectric ceramics. Chem. Eng. J. 2024, 488, 150736. [Google Scholar] [CrossRef]
- Karakaya, M.; Gürbüz, I.; Fulanović, L.; Adem, U. Stabilization of the first-order phase transition character and enhancement of the electrocaloric effect by Na0.5Bi0.5TiO3 substitution in BaTiO3 ceramics. J. Mater. Chem. C 2024, 12, 19612–19619. [Google Scholar] [CrossRef]
- Sharma, S.; Nandan, R.; Malhotra, P.; Kumar, S.; Kumar, R.; Negi, N.S. Morphotropic phase boundary evolution with synergistic effect of sintering temperature to improve electrocaloric and energy storage performances of lead-free Ba0.95Ca0.05Sn0.09Ti0.91O3 (BCST) ceramic. J. Energy Storage 2024, 99, 113295. [Google Scholar] [CrossRef]
- Xiao, W.; Zhang, C.; Gong, X.; Qiu, S.; Wang, J.; Zhang, H.; Luo, W.; Jiang, S.; Li, K.; Zhang, G. Significant Enhancement of Electrocaloric Effect in Ferroelectric Polycrystalline Ceramics Through Grain Boundary Barrier Engineering. Adv. Funct. Mater. 2024, 34, 2405241. [Google Scholar] [CrossRef]
- Ma, X.; Huang, Y.; Jing, R.; Alikin, D.; Wei, X.; Yan, Y.; Jin, L. Rare-earth element doped barium titanate-based ceramics exhibiting ultra-wide temperature span electrocaloric effect. Ceram. Int. 2024, 50, 12341–12350. [Google Scholar] [CrossRef]
- Feng, H.; Hao, M.; Wu, G.; Zheng, Y.; Yang, L.; Liu, X.; Zhao, Y.; Liu, X.; Zhang, H.; Liu, G. Large electrocaloric effect and wide working area in the transition from ferroelectric to nanodomains. J. Am. Ceram. Soc. 2024, 107, 4777–4788. [Google Scholar] [CrossRef]
- Wu, G.; He, M.; Hao, M.; Zheng, Y.; Feng, H.; Tian, Y.; Fan, B.; Yan, Y.; Jin, L.; Liu, G. Wide working temperature range and large electrocaloric effect in BaTiO3 based ceramics achieved by regulating phase boundaries through a compensatory ion co-doping strategy. Ceram. Int. 2024, 50, 32147–32155. [Google Scholar] [CrossRef]
- Tasneem, M.; Kamakshi, K. Dysprosium doping induced effects on structural, dielectric, energy storage density, and electro-caloric response of lead-free ferroelectric barium titanate ceramics. J. Mater. Sci. 2024, 59, 1472–1485. [Google Scholar] [CrossRef]
- Feng, H.; Wen, H.; Huang, Y.; Wu, B.; Wu, W.; Wu, X.; Gao, M.; Lin, T.; Lin, C.; Zhao, C. Low-electric-field-induced high electrocaloric properties via defect regulation in samarium-doped BaTiO3 ceramics. J. Alloys Compd. 2024, 989, 174362. [Google Scholar] [CrossRef]
- Ben Abdessalem, M.; Kriaa, I.; Sassi, Z.; Aydi, A.; Abdelmoula, N. Structure, dielectric and electrocaloric properties of (Ba0.87Ca0.13(Ti0.9Zr0.1)1−x (Zn1/3Nb2/3)xO3 ferroelectric ceramics. J. Mater. Sci. Mater. Electron. 2024, 35, 1215. [Google Scholar] [CrossRef]
- Ma, X.; Shi, W.; Yang, Y.; Alikin, D.; Shur, Y.Y.; Gao, J.; Wei, X.; Liu, G.; Du, H.; Jin, L. Giant electrocaloric effect and high-field electrostrictive properties in Ba(Ti1−xSnx)O3 ceramics. Ceram. Int. 2023, 49, 18517–18524. [Google Scholar] [CrossRef]
- Liu, G.; Yu, W.; Wang, Y.; Feng, H.; Hao, M.; Wu, G.; Li, Q.; Yu, K.; Fan, B.; Jin, L.; et al. Electrocaloric effect of (Ba1−xSrx)(HfxTi1−x)O3 lead-free ferroelectric ceramics with phase structure regulation. Ceram. Int. 2023, 49, 34387–34396. [Google Scholar] [CrossRef]
- Nandan, R.; Syal, R.; Kumar, S.; Negi, N.S. Enhanced room-temperature electrocaloric and pyroelectric responses around morphotropic phase boundary and energy storage performance of lead-free Ba0.85Ca0.15Hf0.10Ti0.90O3 ceramics sintered at various temperatures. J. Alloys Compd. 2023, 968, 171856. [Google Scholar] [CrossRef]
- Zhang, C.; Dou, Z.; Zeng, S.; Li, K.; Zeng, F.; Xiao, W.; Qiu, S.; Fan, G.; Jiang, S.; Luo, W.; et al. Substantially Enhanced Electrocaloric Effect in Ba(Zr0.2Ti0.8)O3 Lead-Free Ferroelectric Ceramics via Lattice Stress Engineering. ACS Appl. Mater. Interfaces 2023, 15, 18065–18073. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, J.; Chen, Y. Improved electrocaloric effect of Ba0.7Sr0.3TiO3 ceramics doped with B and Mn. J. Sol-Gel Sci. Technol. 2023, 107, 483–489. [Google Scholar] [CrossRef]
- Ray, A.; Nayak, B.; Elorika, P.; Barman, R.; Sharmistha, A.; Badapanda, T.; Anwar, S. Investigation of thermal energy potential, pyroelectric, and electrocaloric performance of Ba0.7Ca0.3TiO3 ceramic. J. Korean Ceram. Soc. 2023, 60, 781–789. [Google Scholar] [CrossRef]
- Du, F.; Song, Z.; Xu, Y.; Han, D.; Li, Q.; Zheng, S.; Shen, J.; Qian, X. Multi-element B-site substituted perovskite ferroelectrics exhibit enhanced electrocaloric effect. Sci. China Technol. Sci. 2023, 66, 1119–1128. [Google Scholar] [CrossRef]
- Li, Z.; Molin, C.; Michaelis, A.; Gebhardt, S.E. Modified (Ba,Sr)(Sn,Ti)O3 via hydrothermal synthesis for electrocaloric application. Open Ceram. 2023, 16, 100502. [Google Scholar] [CrossRef]
- Yuan, R.; Liu, Z.; Xu, Y.; Yin, R.; He, J.; Bai, Y.; Zhou, Y.; Li, J.; Xue, D.; Lookman, T. Optimizing Electrocaloric Effect in Barium Titanate-based Room Temperature Ferroelectrics: Combining Landau Theory, Machine Learning and Synthesis. Acta Mater. 2022, 235, 118054. [Google Scholar] [CrossRef]
- Yin, R.; Li, J.; Su, X.; Qin, S.; Yu, C.; Hou, Y.; Liu, C.; Su, Y.; Qiao, L.; Lookman, T.; et al. Emergent Enhanced Electrocaloric Effect within Wide Temperature Span in Laminated Composite Ceramics. Adv. Funct. Mater. 2022, 32, 2108182. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; He, J.; Hou, Y.; Su, Y.; Qiao, L.; Bai, Y. Near-Room-Temperature Large Electrocaloric Effect in Barium Titanate Single Crystal Based on the Electric Field–Temperature Phase Diagram. Phys. Status Solidi RRL 2021, 15, 2100251. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, B.; Shi, P.; Guo, M.; Zhu, X.; Qiao, W.; Kang, R.; Ke, Q.; Zhao, J.; Lou, X. Aging-induced negative electrocaloric effect in Mn-doped Ba0.8Sr0.2TiO3 ceramics. J. Alloys Compd. 2025, 1027, 180540. [Google Scholar] [CrossRef]
- Li, J.; Lv, J.; Zhang, D.; Zhang, L.; Hao, X.; Wu, M.; Xu, B.-X.; Otonicar, M.; Lookman, T.; Dkhil, B.; et al. Doping-induced Polar Defects Improve the Electrocaloric Performance of Ba0.9Sr0.1Hf0.1Ti0.9O3. Phys. Rev Appl. 2021, 16, 014033. [Google Scholar] [CrossRef]
- Zhang, B.; Lou, X.; Zheng, K.; Xie, X.; Shi, P.; Guo, M.; Zhu, X.; Gao, Y.; Liu, Q.; Kang, R. Enhanced electrocaloric effect in the Sm and Hf co-doped BaTiO3 ceramics. Ceram. Int. 2021, 47, 1101–1108. [Google Scholar] [CrossRef]
- Shirsath, S.E.; Cazorla, C.; Lu, T.; Zhang, L.; Tay, Y.Y.; Lou, X.; Liu, Y.; Li, S.; Wang, D. Interface-Charge Induced Giant Electrocaloric Effect in Lead Free Ferroelectric Thin-Film Bilayers. Nano Lett. 2020, 20, 1262–1271. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Bhaumik, I.; Ganesamoorthy, S.; Bhatt, R.; Karnal, A.K.; Tiwari, V.S.; Gupta, P.K. Electro-caloric effect in 0.45BaZr0.2Ti0.8O3-0.55Ba0.7Ca0.3TiO3 single crystal. Appl. Phys. Lett. 2013, 102, 082902. [Google Scholar] [CrossRef]
- Moya, X.; Stern-Taulats, E.; Crossley, S.; González-Alonso, D.; Kar-Narayan, S.; Planes, A.; Mañosa, L.; Mathur, N.D. Giant Electrocaloric Strength in Single-Crystal BaTiO3. Adv. Mater. 2013, 25, 1360–1365. [Google Scholar] [CrossRef]
- Li, J.; Torelló, A.; Nouchokgwe, Y.; Granzow, T.; Kovacova, V.; Hirose, S.; Defay, E. Electrocaloric effect in BaTiO3 multilayer capacitors with first-order phase transitions. J. Phys. Energy 2023, 5, 024017. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, L.; Gao, S.; Liu, J.Q.; Xu, B.; Xia, Y.D.; Yin, J.; Liu, Z.G. Large electrocaloric effect in Ba(Ti1−xSnx)O3 ceramics over a broad temperature region. AIP Adv. 2015, 5, 047134. [Google Scholar] [CrossRef]
- Jian, X.-D.; Lu, B.; Li, D.-D.; Yao, Y.-B.; Tao, T.; Liang, B.; Lin, X.-W.; Guo, J.-H.; Zeng, Y.-J.; Lu, S.-G. Enhanced Electrocaloric Effect in Sr2+-Modified Lead-Free BaZrxTi1–xO3 Ceramics. ACS Appl. Mater. Interfaces 2019, 11, 20167–20173. [Google Scholar] [CrossRef]
- Qian, X.-S.; Ye, H.-J.; Zhang, Y.-T.; Gu, H.; Li, X.; Randall, C.A.; Zhang, Q.M. Giant Electrocaloric Response Over A Broad Temperature Range in Modified BaTiO3 Ceramics. Adv. Funct. Mater. 2014, 24, 1300–1305. [Google Scholar] [CrossRef]
- Li, J.; Zhang, D.; Qin, S.; Li, T.; Wu, M.; Wang, D.; Bai, Y.; Lou, X. Large room-temperature electrocaloric effect in lead-free BaHfxTi1−xO3 ceramics under low electric field. Acta Mater. 2016, 115, 58–67. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Xu, J.; Zhong, L.; Gao, J.; Xiao, A.; Wu, M.; He, Z.; Yao, R.; Li, S.; et al. Trirelaxor Ferroelectric Material with Giant Dielectric Permittivity over a Wide Temperature Range. ACS Appl. Mater. Interfaces 2021, 13, 33272–33281. [Google Scholar] [CrossRef]
- Li, H.-X.; Jian, X.-D.; Niu, X.; Yao, Y.-B.; Tao, T.; Liang, B.; Lu, S.-G. Direct measurement of enhanced electrocaloric effect in Mn2+ doped lead-free Ba(ZrTi)O3 ceramics. Scr. Mater. 2020, 176, 67–72. [Google Scholar] [CrossRef]
- Niu, X.; Jian, X.; Chen, X.; Li, H.; Liang, W.; Yao, Y.; Tao, T.; Liang, B.; Lu, S.-G. Enhanced electrocaloric effect at room temperature in Mn2+ doped lead-free (BaSr)TiO3 ceramics via a direct measurement. J. Adv. Ceram. 2021, 10, 482–492. [Google Scholar] [CrossRef]
- Liang, W.; Niu, X.; Jian, X.; Zeng, Z.; Lai, J.; Wang, T.; Gong, W.; Zeng, W.; Yao, Y.; Tao, T.; et al. The impact of oxygen partial pressure during sintering on the electrocaloric effect of Ba0.7Sr0.3TiO3 ceramics. J. Mater. Chem. C 2022, 10, 16847–16856. [Google Scholar] [CrossRef]
- Pu, Y.; Zhang, Q.; Li, R.; Chen, M.; Du, X.; Zhou, S. Dielectric properties and electrocaloric effect of high-entropy (Na0.2Bi0.2Ba0.2Sr0.2Ca0.2)TiO3 ceramic. Appl. Phys. Lett. 2019, 115, 223901. [Google Scholar] [CrossRef]
- Liu, W.; Li, F.; Chen, G.; Li, G.; Shi, H.; Li, L.; Guo, Y.; Zhai, J.; Wang, C. Comparative study of phase structure, dielectric properties and electrocaloric effect in novel high-entropy ceramics. J. Mater. Sci. 2021, 56, 18417–18429. [Google Scholar] [CrossRef]
- Wang, Z.; Meng, Y.; Tang, S.; Niu, X.; Zhang, H.; Wang, D.; Bai, Y.; Peng, B.; Chen, X.; Ke, Q.; et al. Simultaneous achievement of large electrocaloric effect and ultra-wide operating temperature range in BaTiO3-based lead-free ceramic. J. Adv. Ceram. 2024, 13, 1234–1241. [Google Scholar] [CrossRef]
- Wang, S.-B.; Dai, G.-Z.; Yao, Y.-B.; Zhao, X.-B.; Tao, T.; Liang, B.; Lu, S.-G. Direct and indirect measurement of large electrocaloric effect in B2O3-ZnO glass modified Ba0.65Sr0.35TiO3 bulk ceramics. Scr. Mater. 2021, 193, 59–63. [Google Scholar] [CrossRef]
- Ye, H.-J.; Qian, X.-S.; Jeong, D.-Y.; Zhang, S.; Zhou, Y.; Shao, W.-Z.; Zhen, L.; Zhang, Q.M. Giant electrocaloric effect in BaZr0.2Ti0.8O3 thick film. Appl. Phys. Lett. 2014, 105, 152908. [Google Scholar] [CrossRef]
- Hui, T.; Xiang, N.; Zhi-Peng, Y.; Xiao-Cao, P.; Xiao-Bo, Z.; Ying-Bang, Y.; Tao, T.; Bo, L.; Xin-Gui, T.; Sheng-Guo, L. Giant electrocaloric effect enhancement due to the polarization flip and influence of Mn4+ doping on the dielectric, ferroelectric properties in 0.7 BiFeO3-0.3 BaTiO3 ceramics. ACTA Phys. Sin. 2022, 71, 286–295. [Google Scholar] [CrossRef]
- Su, M.; Grimes, R.; Garg, S.; Xue, D.; Balachandran, P.V. Machine-Learning-Enabled Prediction of Adiabatic Temperature Change in Lead-Free BaTiO3-Based Electrocaloric Ceramics. ACS Appl. Mater. Interfaces 2021, 13, 53475–53484. [Google Scholar] [CrossRef]
- Merz, W.J. Double Hysteresis Loop of BaTiO3 at the Curie Point. Phys. Rev. 1953, 91, 513–517. [Google Scholar] [CrossRef]
- Samara, G.A. Pressure and temperature dependence of the dielectric properties and phase transitions of the ferroelectric perovskites: PbTiO3 and BaTiO3. Ferroelectrics 1971, 2, 277–289. [Google Scholar] [CrossRef]
- Guvenc, C.M.; Adem, U. Influence of aging on electrocaloric effect in Li+ doped BaTiO3 ceramics. J. Alloys Compd. 2019, 791, 674–680. [Google Scholar] [CrossRef]
- Liu, X.; Tan, X. Crystal Structure and Electrical Properties of Lead-Free (1 − x)BaTiO3–x(Bi1/2A1/2)TiO3 (A = Ag, Li, Na, K, Rb, Cs) Ceramics. J. Am. Ceram. Soc. 2013, 96, 3425–3429. [Google Scholar] [CrossRef][Green Version]
- Ren, P.; Fan, H.; Wang, X. Impedance spectroscopy studies of bulk electrical conduction in A-site acceptor (K)-doped BaTiO3. J. Mater. Sci. 2013, 48, 7028–7035. [Google Scholar] [CrossRef]
- Durst, G.; Grotenhuis, M.; Barkow, A.G. Solid Solubility Study of Barium, Strontium, and Calcium Titanates. J. Am. Ceram. Soc. 1950, 33, 133–139. [Google Scholar] [CrossRef]
- Shirane, G.; Takeda, A. On the Phase Transition in Barium-Lead Titanate (II). J. Phys. Soc. Jpn. 1951, 6, 329–332. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, J.; Luo, H.; Yao, Y.; Wang, N.; Chen, L.; Li, T.; Hu, C.; Qi, H.; Deng, S.; et al. Superior Capacitive Energy-Storage Performance in Pb-Free Relaxors with a Simple Chemical Composition. J. Am. Chem. Soc. 2023, 145, 6194–6202. [Google Scholar] [CrossRef]
- Han, F.; Bai, Y.; Qiao, L.-J.; Guo, D. A systematic modification of the large electrocaloric effect within a broad temperature range in rare-earth doped BaTiO3 ceramics. J. Mater. Chem. C 2016, 4, 1842–1849. [Google Scholar] [CrossRef]
- Xie, S.; Bai, Y.; Han, F.; Qin, S.; Li, J.; Qiao, L.; Guo, D. Distinct effects of Ce doping in A or B sites on the electrocaloric effect of BaTiO3 ceramics. J. Alloys Compd. 2017, 724, 163–168. [Google Scholar] [CrossRef]
- Amin, R.; Khatun, N.; Sen, S. Optimization of Pb content in enhancing ferroelectricity and shifting the Tc of BaTiO3 to a higher temperature. J. Appl. Phys. 2019, 126, 174105. [Google Scholar] [CrossRef]
- Sareecha, N.; Shah, W.A.; Mirza, M.L.; Maqsood, A.; Awan, M.S. Electrical investigations of Bi-doped BaTiO3 ceramics as a function of temperature. Phys. B Condens. Matter 2018, 530, 283–289. [Google Scholar] [CrossRef]
- Lu, B.; Yao, Y.; Jian, X.; Tao, T.; Liang, B.; Zhang, Q.; Lu, S.-G. Enhancement of the electrocaloric effect over a wide temperature range in PLZT ceramics by doping with Gd3+ and Sn4+ ions. J. Eur. Ceram. Soc. 2019, 39, 1093–1102. [Google Scholar] [CrossRef]
- Issa, M.A.A.; Molokhia, N.M.; Nasser, S.A. Factors affecting the permittivity of Gd-doped BaTiO3. J. Phys. D Appl. Phys. 1984, 17, 571. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, H.; Xiong, J.; Wu, J. Composition-driven phase boundary and electrical properties in (Ba0.94Ca0.06)(Ti1–xMx)O3 (M = Sn, Hf, Zr) lead-free ceramics. Dalton Trans. 2016, 45, 6466–6480. [Google Scholar] [CrossRef]
- Zhao, C.; Huang, Y.; Wu, J. Multifunctional barium titanate ceramics via chemical modification tuning phase structure. InfoMat 2020, 2, 1163–1190. [Google Scholar] [CrossRef]
- Shukla, A.; Choudhary, R.N.P.; Thakur, A.K. Thermal, structural and complex impedance analysis of Mn4+ modified BaTiO3 electroceramic. J. Phys. Chem. Solids 2009, 70, 1401–1407. [Google Scholar] [CrossRef]
- Liu, Y.; Withers, R.L. Structural Disorder, Polarisation and the Normal to Relaxor Ferroelectric Transition in BaTiO3 Based Perovskites. Ferroelectrics 2010, 402, 3–9. [Google Scholar] [CrossRef]
- Liu, Y.; Withers, R.L.; Wei, X.; Fitz Gerald, J.D. Structured diffuse scattering and polar nano-regions in the Ba(Ti1−xSnx)O3 relaxor ferroelectric system. J. Solid State Chem. 2007, 180, 858–865. [Google Scholar] [CrossRef]
- Pirc, R.; Kutnjak, Z.; Blinc, R.; Zhang, Q.M. Upper bounds on the electrocaloric effect in polar solids. Appl. Phys. Lett. 2011, 98, 021909. [Google Scholar] [CrossRef]
- Jaffe, B.; Roth, R.S.; Marzullo, S. Piezoelectric Properties of Lead Zirconate-Lead Titanate Solid-Solution Ceramics. J. Appl. Phys. 1954, 25, 809–810. [Google Scholar] [CrossRef]
- Singh, A.K.; Pandey, D. Evidence for MB and MC phases in the morphotropic phase boundary region of (1−x)[ Pb(Mg1/3Nb2/3)O3] -xPbTiO3 A Rietveld study. PhRvB 2003, 67, 064102. [Google Scholar] [CrossRef]
- Takenaka, T.; Maruyama, K.I.M.K.I.; Sakata, K.S. (Bi1/2Na1/2)TiO3-BaTiO3 System for Lead-Free Piezoelectric Ceramics. Jpn. J. Appl. Phys. 1991, 30, 2236. [Google Scholar] [CrossRef]
- Ma, C.; Guo, H.; Beckman, S.P.; Tan, X. Creation and Destruction of Morphotropic Phase Boundaries through Electrical Poling: A Case Study of Lead-Free (Bi1/2Na1/2)TiO3-BaTiO3 Piezoelectrics. Phys. Rev. Lett. 2012, 109, 107602. [Google Scholar] [CrossRef] [PubMed]
- Novak, N.; Weyland, F.; Khakpash, N.; Rossetti, G.A. Electrocaloric effect at special points in the composition-temperature-electric field phase diagram of barium zirconate-titanate (BZT). J. Eur. Ceram. Soc. 2023, 43, 1952–1963. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, C.; Lv, D.; Wang, D.; Wu, H.; Yang, Y.; Ren, X. Large piezoelectricity and dielectric permittivity in BaTiO3-xBaSnO3 system: The role of phase coexisting. Europhys. Lett. 2012, 98, 27008. [Google Scholar] [CrossRef]
- Liu, W.; Ren, X. Large Piezoelectric Effect in Pb-Free Ceramics. Phys. Rev Lett. 2009, 103, 257602. [Google Scholar] [CrossRef]
- Xue, D.; Zhou, Y.; Bao, H.; Gao, J.; Zhou, C.; Ren, X. Large piezoelectric effect in Pb-free Ba(Ti,Sn)O3-x(Ba,Ca)TiO3 ceramics. Appl. Phys. Lett. 2011, 99, 122901. [Google Scholar] [CrossRef]
- Liu, Z.K.; Li, X.; Zhang, Q.M. Maximizing the number of coexisting phases near invariant critical points for giant electrocaloric and electromechanical responses in ferroelectrics. Appl. Phys. Lett. 2012, 101, 082904. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, J.; Yao, W.; Liu, D.; He, G. Remarkably strong piezoelectricity, rhombohedral-orthorhombic-tetragonal phase coexistence and domain structure of (K,Na)(Nb,Sb)O3–(Bi,Na)ZrO3–BaZrO3 ceramics. J. Alloys Compd. 2020, 820, 153411. [Google Scholar] [CrossRef]
- Yeh, J.-W.; Chen, S.K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Song, Y.; Yu, J.; Tian, Y.; Robson, M.J.; Wang, J.; Zhang, Z.; Lin, X.; Zhou, G.; et al. High-Entropy Perovskites for Energy Conversion and Storage: Design, Synthesis, and Potential Applications. Small Methods 2023, 7, 2201138. [Google Scholar] [CrossRef] [PubMed]
- Akrami, S.; Edalati, P.; Fuji, M.; Edalati, K. High-entropy ceramics: Review of principles, production and applications. Mater. Sci. Eng. R Rep. 2021, 146, 100644. [Google Scholar] [CrossRef]
- Xu, W.; Huang, X.; Li, H.; Lin, C.; Wu, X.; Gao, M.; Lin, T.; Zhao, C. Calcination temperature-associated discontinuous grain size effect on electrocaloric properties in fine-/large-grain BaTiO3-based ferroelectric ceramics. J. Eur. Ceram. Soc. 2024, 44, 7071–7080. [Google Scholar] [CrossRef]
- Niu, X.; Liang, W.; Jian, X.; Tang, H.; Wang, T.; Gong, W.; Shi, H.; Li, F.; Zhao, X.; Yao, Y.-B.; et al. Superior energy storage properties in lead-free Na0.5Bi0.5TiO3-based relaxor ferroelectric ceramics via compositional tailoring and bandgap engineering. Scr. Mater. 2023, 230, 115387. [Google Scholar] [CrossRef]
- Lu, B.; Jian, X.; Lin, X.; Yao, Y.; Tao, T.; Liang, B.; Luo, H.; Lu, S.-G. Enhanced Electrocaloric Effect in 0.73Pb(Mg1/3Nb2/3)O3-0.27PbTiO3 Single Crystals via Direct Measurement. Crystals 2020, 10, 451. [Google Scholar] [CrossRef]
- Weyland, F.; Eisele, T.; Steiner, S.; Frömling, T.; Rossetti, G.A., Jr.; Rödel, J.; Novak, N. Long term stability of electrocaloric response in barium zirconate titanate. J. Eur. Ceram. Soc. 2018, 38, 551–556. [Google Scholar] [CrossRef]
- Jiang, C.; Du, J.; Sun, Y.; Huang, Y.-C.; Wang, D. Strong electrocaloric response with ultrawide working temperature span in lead-free BaTiO3-based ceramics by composition design. Ceram. Int. 2024, 50, 11609–11616. [Google Scholar] [CrossRef]







| Composition | E (MV/m) | Temperature (℃) | (K) | (K·m/MV) | Methods | Ref. |
|---|---|---|---|---|---|---|
| 0.5Ba(Sn0.11Ti0.89)O3-0.5Ba(Zr0.15Ti0.85)O3 three-layers | 3 | 70.6 | 0.52 | 0.173 | Indirect | [35] |
| (Ba0.98125La0.0125) (Sn0.05Nb0.01Ti0.9375)O3 | 5 | RT | 1.31 | 0.262 | Indirect | [36] |
| (Ba0.65Sr0.3Ca0.05)(Sn0.02Ti0.98)O3 | 9 | 20 | 2.19 | 0.243 | Direct | [37] |
| Ba0.75Sr0.25Zr0.05 (Ti0.999Mn0.001)0.95O3 | 3.5 | 70 | 1.3 | 0.371 | Direct | [38] |
| <111>c-texture 0.955BaTiO3-0.045KNbO3 | 10 | 50 | 3.9 | 0.390 | Direct | [39] |
| 0.5Ba(Zr0.2Ti0.8)O3-0.5(Ba0.7Ca0.3)TiO3 | 8 | 50 | 2.5 | 0.313 | Indirect | [40] |
| Ba0.9925La0.005Ti0.9Zr0.1O3 | 5 | 90 | 1.22 | 0.244 | Direct | [41] |
| 0.98Ba(Ti0.9Sn0.1)O3-0.02Bi(Mg0.5Ti0.5)O3 | 5 | 90 | 0.41 | 0.082 | Direct | [42] |
| Ba0.85Sr0.15Ti0.9Hf0.1O3 -Ba0.85Sr0.0725Ca0.0725Ti0.9Hf0.1O3- Ba0.85Ca0.15Ti0.9Hf0.1O3 three-layers | 5 | 80 | 1.2 | 0.240 | Indirect | [43] |
| Ba0.62Ca0.20Sr0.18Sn0.065Ti0.935O3 | 5 | 40 | 0.49 | 0.098 | Direct | [44] |
| (Ba0.85Ca0.15)(Ti0.94Hf0.06)O3 | 4 | 105 | 1.03 | 0.258 | Indirect | [45] |
| 0.3Ba0.72Sr0.28TiO3-0.5BaTi0.8Sn0.2O3-0.2Ba0.72Ca0.28TiO3 | 14 | 38 | 2.71 | 0.194 | Indirect | [46] |
| Ba0.8Sr0.2Zr0.15(Ti0.999Mn0.001)0.85O3 | 5 | 70 | 1.08 | 0.216 | Direct | [47] |
| (Ba0.88Ca0.12)(Ti0.94Sn0.06)O3 | 1.67 | 107 | 0.52 | 0.311 | Indirect | [48] |
| 0.6(Ba0.7Sr0.3)TiO3-0.4 Ba(Zr0.2Ti0.8)O3 | 3 | 69 | 0.53 | 0.177 | Indirect | [49] |
| 0.8BaTiO3–0.2Na0.5Bi0.5TiO3 | 4 | 184 | 1.65 | 0.413 | Direct | [50] |
| Ba0.95Ca0.05Sn0.09Ti0.91O3 | 3 | 60 | 1.03 | 0.343 | Indirect | [51] |
| Ba0.8Zr0.2TiO3 | 8 | 50 | 1.63 | 0.204 | Direct | [52] |
| (Ba0.9925Sm0.005)(Ti0⋅9Sn0.1)O3 | 9 | 100 | 0.9 | 0.100 | Indirect | [53] |
| Ba0.9Ca0.1Ti0.85Sn0.15O3 | 5 | 30 | 0.85 | 0.170 | Direct | [54] |
| (Ba0.985La0.01)[Nb0.028(Ti0.96Sn0.005)]O3 | 5 | 53 | 1.14 | 0.228 | Indirect | [55] |
| Ba0.99Dy0.01TiO3 | 5 | 120 | 1.92 | 0.384 | Indirect | [56] |
| (Ba0.97Sm0.02)TiO3 | 3 | 72 | 1.11 | 0.370 | Indirect | [57] |
| Ba0.87Ca0.13Ti0.8955Zr0.0005Zn0.005/3Nb0.01/3O3 | 3 | 65 | 0.701 | 0.234 | Indirect | [58] |
| Ba(Ti0.96Sn0.04)O3 | 20 | 48.4 | 6.36 | 0.318 | Indirect | [59] |
| (Ba0.9Sr0.1)(Hf0.1Ti0.9)O3 | 5 | 87 | 1.2 | 0.240 | Indirect | [60] |
| Ba0.85Ca0.15Hf0.10Ti0.90O3 | 2 | 87 | 1.01 | 0.505 | Indirect | [61] |
| BaZr0.2Ti0.8O3-5.7 mol % Li2CO3 | 15 | 60 | 2.26 | 0.151 | Direct | [62] |
| 1 mol% B3+ + 0.5 mol% Mn2+- Ba0.7Sr0.3TiO3 | 10 | RT | 3.08 | 0.308 | Indirect | [63] |
| Ba0.7Ca0.3TiO3 | 3 | 127 | 0.419 | 0.140 | Indirect | [64] |
| Ba(Hf0.05Sn0.05Zr0.07Ti0.83)O3 | 4 | RT | 1.7 | 0.425 | Direct | [65] |
| Ba0.82Sr0.18Sn0.065Ti0.935O3 | 5 | 30 | 0.59 | 0.118 | Indirect | [66] |
| (Ba0.82Ca0.05Sr0.13)(Ti0.89Zr0.01Sn0.10)O3 | 2 | 15 | 0.6 | 0.300 | Direct | [67] |
| BaTi0.89Sn0.11O3-BaTi0.85Zr0.15O3-BaTi0.89Hf0.11O3 three-layers | 2 | 84 | 0.64 | 0.320 | Direct | [68] |
| <011>-oriented BaTiO3 single crystal | 1.5 | 15 | 1.33 | 0.887 | Direct | [69] |
| Ba0.8Sr0.2TiO3-1mol. % Mn3+ | 2 | 80 | 0.61 | 0.305 | Indirect | [70] |
| Ba0.9Sr0.1Hf0.1Ti0.9O3-2 mol% CuO | 3 | RT | 0.368 | 0.123 | Direct | [71] |
| 0.30BaHf0.2Ti0.8O3-0.7Ba0.94Sm0.04TiO3 | 3 | 64 | 0.46 | 0.153 | Indirect | [72] |
| [0.94(Bi0.5Na0.5)TiO3–0.06BaTiO3]200/[0.5(Ba0.7Ca0.3)TiO3–0.5Ba(Zr0.2Ti0.8)O3]200 bilayers | 62 | 97 | 23 | 0.371 | Indirect | [73] |
| 0.45BaZr0.2Ti0.8O3-0.55Ba0.7Ca0.3TiO3 single crystal | 1.2 | 131 | 0.46 | 0.383 | Indirect | [74] |
| BaTiO3 Single crystal | 1.2 | 130 | 0.8 | 0.667 | Direct | [75] |
| BaTi0.998Mn0.002O3 | 17 | 126 | 2.4 | 0.141 | Direct | [76] |
| Ba(Ti0.88Sn0.12)O3 | 2 | 50 | 0.27 | 0.135 | Indirect | [77] |
| Ba(Zr0.2Ti0.8)O3 | 14.5 | 39 | 4.5 | 0.310 | Direct | [79] |
| Ba(Hf0.11Ti0.89)O3 | 5 | 65 | 1.35 | 0.270 | Indirect | [80] |
| (Ba0.78Ca0.22)(Ti0.88Sn0.12)O3 | 2.5 | 40 | 0.5 | 0.200 | Indirect | [81] |
| Ba(Sn0.014Ti0.186)O3-Ba(Hf0.08Ti0.72)O3 | 7 | 80 | 3.35 | 0.479 | Direct | [18] |
| Ba(Zr0.15Mn0.0025Ti0.8475)O3 | 5 | 62 | 2.35 | 0.470 | Direct | [82] |
| Ba0.6Sr0.4Mn0.001Ti0.999O3 | 5 | 21 | 2.75 | 0.550 | Direct | [83] |
| Ba0.7Sr0.3TiO3 | 4 | 40 | 2.18 | 0.545 | Direct | [84] |
| Na0.2Bi0.2Ba0.2Sr0.2Ca0.2TiO3 | 6 | 40 | 0.63 | 0.105 | Indirect | [85] |
| (Bi1/6Na1/6Sr1/6Ba1/6Pb1/6Ca1/6)TiO3 | 6 | 140 | 0.63 | 0.105 | Direct | [86] |
| (Bi1/6La1/6Na1/6K1/6Sr1/6Ba1/6)TiO3 | 6 | RT | 0.14 | 0.023 | Indirect | [86] |
| Ba0.85Ca0.15Ti0.85Zr0.15O3 | 14 | 40 | 1.78 | 0.127 | Indirect | [87] |
| Ba0.65Sr0.35TiO3-0.5 wt% B2O3-ZnO | 5 | 10 | 2.29 | 0.458 | Direct | [88] |
| Ba(Zr0.2Ti0.8)O3 | 19.5 | 40 | 7 | 0.359 | Indirect | [89] |
| 0.7BiFeO3-0.3BaTiO3-0.05 wt% MnO2 | 3 | 21.6 | 0.56 | 0.187 | Direct | [90] |
| BaTi0.88Hf0.12O3 | 4.8 | 67 | 1.284 | 0.268 | Indirect | [91] |
| Ion | CN | Radius (Å) |
|---|---|---|
| Ba2+ | 12 | 1.61 |
| Li+ | 1.18 | |
| Na+ | 1.39 | |
| K+ | 1.64 | |
| Ca2+ | 1.34 | |
| Sr2+ | 1.44 | |
| Pb2+ | 1.49 | |
| Bi3+ | 1.32 | |
| La3+ | 1.36 | |
| Ce3+ | 1.34 | |
| Sm3+ | 1.24 |
| Ion | CN | Radius (Å) |
|---|---|---|
| Ti4+ | 6 | 0.605 |
| Zr4+ | 0.72 | |
| Sn4+ | 0.69 | |
| Hf4+ | 0.71 | |
| Ce4+ | 0.87 | |
| Mg2+ | 0.72 | |
| Mn2+ | 0.83 | |
| Mn3+ | 0.645 | |
| Mn4+ | 0.53 | |
| Y3+ | 0.9 | |
| Ta5+ | 0.64 | |
| Nb5+ | 0.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Niu, X.; Jiang, Y.; Cao, J.; Lai, J.; He, H.; Chen, J.; Jian, X.; Lu, S.-G. BaTiO3-Based Electrocaloric Materials—Recent Progresses and Perspective. Materials 2025, 18, 4190. https://doi.org/10.3390/ma18174190
Tang Y, Niu X, Jiang Y, Cao J, Lai J, He H, Chen J, Jian X, Lu S-G. BaTiO3-Based Electrocaloric Materials—Recent Progresses and Perspective. Materials. 2025; 18(17):4190. https://doi.org/10.3390/ma18174190
Chicago/Turabian StyleTang, Yi, Xiang Niu, Yuleng Jiang, Junxi Cao, Junying Lai, Houzhu He, Jianpeng Chen, Xiaodong Jian, and Sheng-Guo Lu. 2025. "BaTiO3-Based Electrocaloric Materials—Recent Progresses and Perspective" Materials 18, no. 17: 4190. https://doi.org/10.3390/ma18174190
APA StyleTang, Y., Niu, X., Jiang, Y., Cao, J., Lai, J., He, H., Chen, J., Jian, X., & Lu, S.-G. (2025). BaTiO3-Based Electrocaloric Materials—Recent Progresses and Perspective. Materials, 18(17), 4190. https://doi.org/10.3390/ma18174190






