Mechanism of Activation and Microstructural Evolution in Calcium Carbide Slag-Activated GGBS-CG Composite Cementitious Materials
Highlights
- Waste-derived activator (calcium carbide slag) enables 100% solid-waste geopolymer from slag and coal gangue.
- Optimal 10 wt% activator maximizes compressive strength (10.13 MPa) by balancing alkalinity.
- Hydrotalcite and silicate gels synergistically densify microstructure via pore filling.
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Mix Proportion Design
2.3. Sample Preparation and Curing
2.4. Testing Methods
3. Results and Discussion
3.1. Compressive Strength Test
3.2. XRD Analysis
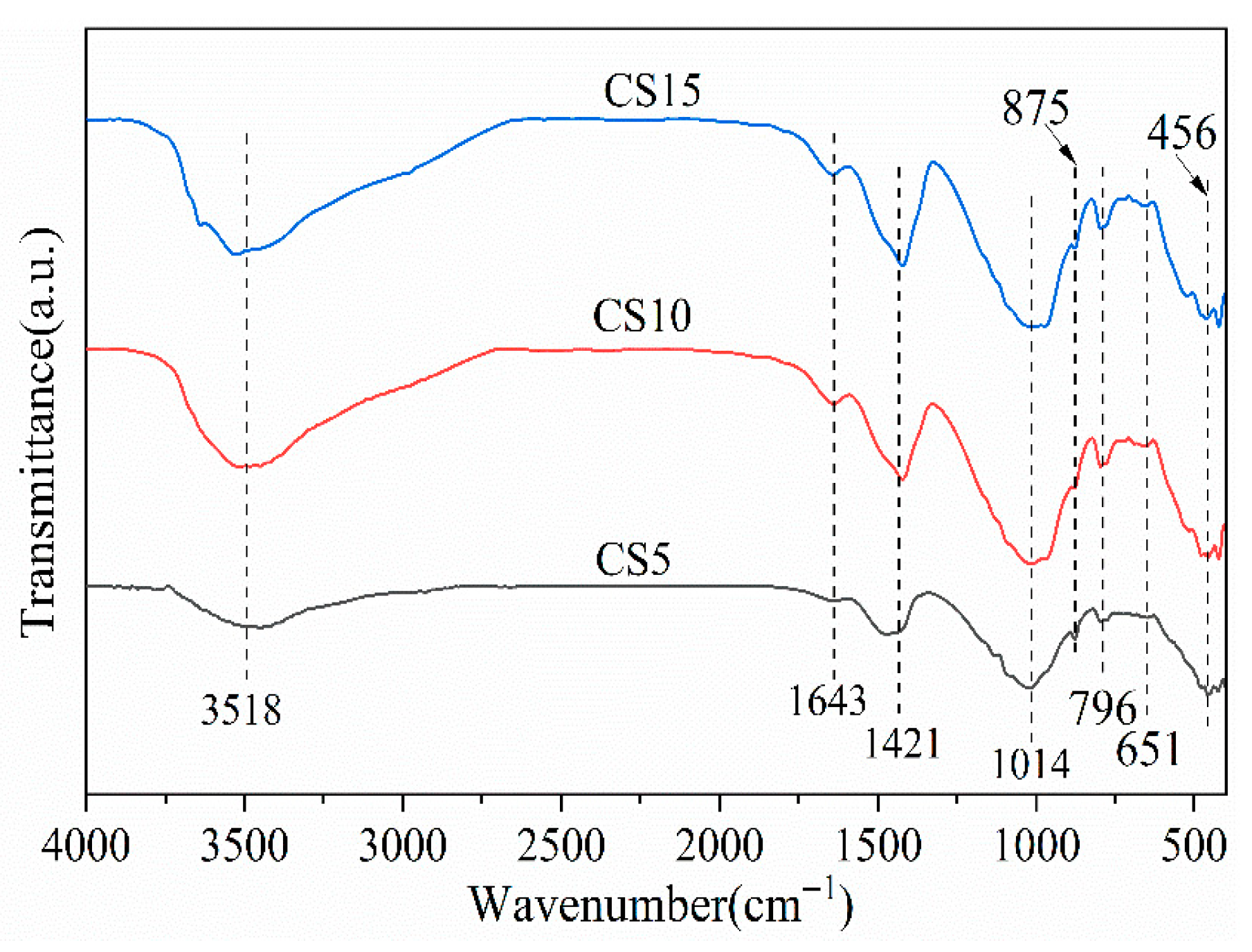
3.3. FTIR Analysis
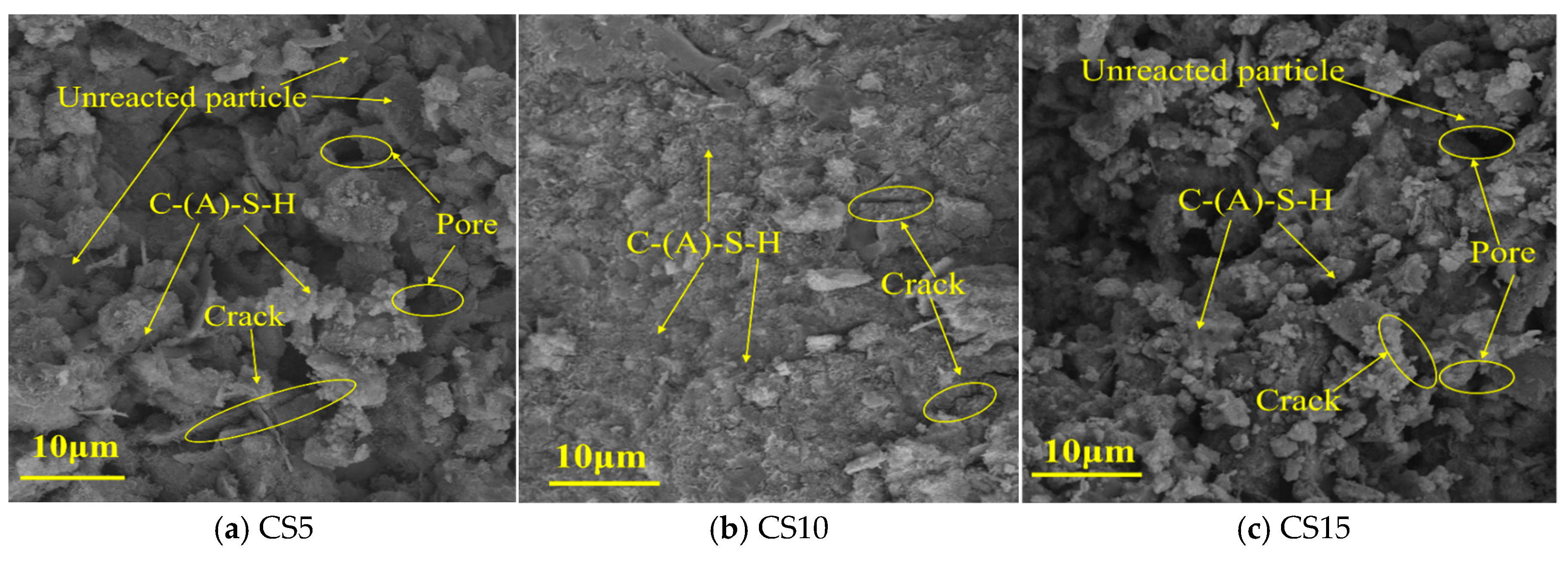
3.4. SEM Analysis
3.5. Mechanism of Strength Formation in the Cementitious System
- Raw Material Dissolution: Calcium carbide slag establishes a highly alkaline environment. Initially, the Ca–O and Mg–O bonds, which have relatively weaker bond energies in the slag glassy structure, break first, releasing Ca2+ and Mg2+ ions. Subsequently, the stronger Al–O and Si–O bonds in the glassy structures of both the slag and coal gangue begin to break down under the attack of OH− ions, forming silicate and aluminate ionic species ([H3SiO4]−, [H3AlO4]2−, [Al(OH)6]3−). The octahedral [Al(OH)6]3− is primarily formed from the dissolution of slag. Concurrently, part of the calcium carbide slag dissolves, releasing OH−, Ca2+, and other ions.
- Formation of Hydration Products: The [H3SiO4]− ions in the solution combine with Ca2+ to form initial C-S-H gel, while also interacting with [H3AlO4]2− to form C-A-S-H gel. A small amount of [Al(OH)6]3− combines with CO32− and Mg2+ to form hydrotalcite (HT, 6MgO·Al2O3·CO2·12H2O). As the reaction progresses, the formation of C-(A)-S-H gel and HT continuously consumes [H3SiO4]−, [H3AlO4]2−, [Al(OH)6]3−, OH−, and Ca2+, which in turn promotes further dissolution of the raw materials. Furthermore, as water is consumed, a higher CO2 content in the environment can lead to the carbonation of Ca(OH)2 in the system, forming calcite crystals.
- Polymerization of Hydration Products: As the dissolution of raw materials and the formation of hydration products proceed continuously, the C-(A)-S-H gel grows steadily, adhering to the surfaces of unreacted particles and binding the dispersed particles into an integrated whole. The primary crystalline product, HT, along with other crystals such as calcite and a small amount of unreacted quartz, are distributed on the gel surface and within existing microcracks, serving to bridge and fill these microcracks. The gel and crystalline hydration products together form the hardened paste skeleton, thereby developing strength.SiO2 + OH− + H2O = [H3SiO4]−AlO2− + OH− + H2O = [H3AlO4]2−AlO2−+OH−+H2O = [Al(OH)6]3−[H3SiO4]− + ([H3AlO4]2−) + Ca2+ = C-(A)-S-H4OH− + CO32− + 6Mg2+ + 2[Al(OH)6]3+ 4H2O = 6MgO·Al2O3·CO3·12H2O
3.6. Discussion
4. Conclusions
- CS content critically regulates the alkaline activation process. A dosage of 10 wt% was identified as the most effective among the tested formulations, providing a chemical environment that maximizes aluminosilicate dissolution and promotes the synergistic formation of C-(A)-S-H gel and hydrotalcite, leading to effective pore filling and matrix densification.
- Both mechanical performance and microstructural evolution are governed by the balance between activation and precursor availability. Excessive CS content disrupts this balance through passivation effects and reduction of reactive components, resulting in strength decline and microstructural degradation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McLellan, B.C.; Williams, R.P.; Lay, J.; van Riessen, A.; Corder, G.D. Costs and carbon emissions for geopolymer pastes in comparison to ordinary portland cement. J. Clean. Prod. 2011, 19, 1080–1090. [Google Scholar] [CrossRef]
- Zhao, J.; Tong, L.; Li, B.; Chen, T.; Wang, C.; Yang, G.; Zheng, Y. Eco-friendly geopolymer materials: A review of performance improvement, potential application and sustainability assessment. J. Clean. Prod. 2021, 307, 127085. [Google Scholar] [CrossRef]
- Song, L.; Liu, S.; Li, W. Quantitative Inversion of Fixed Carbon Content in Coal Gangue by Thermal Infrared Spectral Data. Energies 2019, 12, 1659. [Google Scholar] [CrossRef]
- Guan, J.; Lu, M.; Yao, X.; Wang, Q.; Wang, D.; Yang, B.; Liu, H. An Experimental Study of the Road Performance of Cement Stabilized Coal Gangue. Crystals 2021, 11, 993. [Google Scholar] [CrossRef]
- Meng, G.H.; Zhang, J.X.; Li, M.; Zhu, C.L.; Zhang, Q. Prediction of compression and deformation behaviours of gangue backfill materials under multi-factor coupling effects for strata control and pollution reduction. Environ. Sci. Pollut. Res. 2020, 27, 36528–36540. [Google Scholar] [CrossRef]
- Yi, C.; Ma, H.; Zhu, H.; Li, W.; Xin, M.; Liu, Y.; Guo, Y. Study on chloride binding capability of coal gangue based cementitious materials. Constr. Build. Mater. 2018, 167, 649–656. [Google Scholar] [CrossRef]
- Duan, P.; Yan, C.; Zhou, W. Compressive strength and microstructure of fly ash based geopolymer blended with silica fume under thermal cycle. Cem. Concr. Compos. 2017, 78, 108–119. [Google Scholar] [CrossRef]
- Qiu, J.; Zhu, M.; Zhou, Y.; Guan, X. Effect and mechanism of coal gangue concrete modification by fly ash. Constr. Build. Mater. 2021, 294, 123563. [Google Scholar] [CrossRef]
- Ma, H.; Zhu, H.; Wu, C.; Chen, H.; Sun, J.; Liu, J. Study on compressive strength and durability of alkali-activated coal gangue-slag concrete and its mechanism. Powder Technol. 2020, 368, 112–124. [Google Scholar] [CrossRef]
- Öz, A.; Dursun, F.M.; Benli, A.; Kaplan, G. Optimization of sustainable high-performance alkali-activated composites using industrial and agricultural wastes: A comprehensive performance evaluation. J. Build. Eng. 2025, 111, 113319. [Google Scholar] [CrossRef]
- Kurtay-Yıldız, M.; Emiroğlu, M. Synergistic effect of steel and PVA fibers on the mechanical and microstructural performance of ceramic waste-GGBS-based green composites. Constr. Build. Mater. 2025, 491, 142653. [Google Scholar] [CrossRef]
- Yin, H.; Zhang, Y.; Wang, Q.; Zhang, J.; Liu, X. Study of the hydration characteristics of alkali-activated materials with spontaneous combustion coal gangue, waste ceramic powder and slag. Mater. Today Commun. 2025, 43, 111597. [Google Scholar] [CrossRef]
- Qiao, W.; Yue, B.; Luo, Z.; Zhu, S.; Li, L.; Yang, H.; Luo, B. Mechanical Properties and Microstructures of Solid Waste Composite-Modified Lateritic Clay via NaOH/Na2CO3 Activation: A Sustainable Recycling Solution of Steel Slag, Fly Ash, and Granulated Blast Furnace Slag. Materials 2025, 18, 3307. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Lin, C. Compressive and Flexural Properties of Ultra-Fine Coal Gangue-Based Geopolymer Gels and Microscopic Mechanism Analysis. Gels 2022, 8, 145. [Google Scholar] [CrossRef]
- Zhang, C.S.; Fang, L.M. Hardening mechanisms of alkali activated burned gangue cementitious material. Mater. Sci. Technol. 2004, 12, 597–601. [Google Scholar] [CrossRef]
- Han, J.Y.; Song, X.Y.; Gao, Z.H. Excitation Effect of Soluble Glass on Composite System with Calcined Coal Gangue and Slag. Appl. Mech. Mater. 2012, 174–177, 30–34. [Google Scholar] [CrossRef]
- Ma, H.; Zhu, H.; Yi, C.; Fan, J.; Chen, H.; Xu, X.; Wang, T. Preparation and Reaction Mechanism Characterization of Alkali-activated Coal Gangue–Slag Materials. Materials 2019, 12, 2250. [Google Scholar] [CrossRef]
- Geng, J.; Zhou, M.; Li, Y.; Chen, Y.; Han, Y.; Wan, S.; Zhou, X.; Hou, H. Comparison of red mud and coal gangue blended geopolymers synthesized through thermal activation and mechanical grinding preactivation. Constr. Build. Mater. 2017, 153, 185–192. [Google Scholar] [CrossRef]
- Horpibulsuk, S.; Phetchuay, C.; Chinkulkijniwat, A. Soil Stabilization by Calcium Carbide Residue and Fly Ash. J. Mater. Civ. Eng. 2012, 24, 184–193. [Google Scholar] [CrossRef]
- Yang, J.; Bai, H.; He, X.; Zeng, J.; Su, Y.; Wang, X.; Zhao, H.; Mao, C. Performances and microstructure of one-part fly ash geopolymer activated by calcium carbide slag and sodium metasilicate powder. Constr. Build. Mater. 2023, 367, 130303. [Google Scholar] [CrossRef]
- Shi, Y.; Zhao, Q.; Xue, C.; Jia, Y.; Guo, W.; Zhang, Y.; Qiu, Y. Preparation and curing method of red mud-calcium carbide slag synergistically activated fly ash-ground granulated blast furnace slag based eco-friendly geopolymer. Cem. Concr. Compos. 2023, 139, 104999. [Google Scholar] [CrossRef]
- Hanjitsuwan, S.; Phoo-ngernkham, T.; Li, L.-Y.; Damrongwiriyanupap, N.; Chindaprasirt, P. Strength development and durability of alkali-activated fly ash mortar with calcium carbide residue as additive. Constr. Build. Mater. 2018, 162, 714–723. [Google Scholar] [CrossRef]
- Li, Z.; Xu, K.; Sun, N.; Wang, J.; Xue, K.; Xu, L.; Ren, Y.; Yan, Z.; Sima, T. Compressive Strength and Microstructure of Carbide Slag and Alkali-Activated Blast Furnace Slag Pastes in China. Buildings 2024, 14, 1681. [Google Scholar] [CrossRef]
- Zhao, Y.; Gu, X.; Xu, X.; Zhang, Z. Using calcium carbide residue to prepare ecological alkali activated slag composites: Effect of anion type. Ceram. Int. 2023, 49, 25092–25104. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Cui, J.; Shan, Y.; Niu, Y. Experimental study on calcium carbide residue as a combined activator for coal gangue geopolymer and feasibility for soil stabilization. Constr. Build. Mater. 2021, 312, 125465. [Google Scholar] [CrossRef]
- Zhang, W.; Dong, C.; Huang, P.; Sun, Q.; Li, M.; Chai, J. Experimental Study on the Characteristics of Activated Coal Gangue and Coal Gangue-Based Geopolymer. Energies 2020, 13, 2504. [Google Scholar] [CrossRef]
- GB/T 18046-2017; Ground Granulated Blast Furnace Slag Used for Cement, Mortar and Concrete. Standards Press of China: Beijing, China, 2017.
- Wu, J.; Feng, M.; Chen, Z.; Mao, X.; Han, G.; Wang, Y. Particle Size Distribution Effects on the Strength Characteristic of Cemented Paste Backfill. Minerals 2018, 8, 322. [Google Scholar] [CrossRef]
- Wu, J.; Feng, M.; Xu, J.; Qiu, P.; Wang, Y.; Han, G. Particle Size Distribution of Cemented Rockfill Effects on Strata Stability in Filling Mining. Minerals 2018, 8, 407. [Google Scholar] [CrossRef]
- ASTM C39/C39M; Standard Test Method for Compressive Strength of Cylindrical Concrete Specimens. ASTM International: West Conshohocken, PA, USA, 2021.
- Li, W.; Yi, Y. Use of carbide slag from acetylene industry for activation of ground granulated blast-furnace slag. Constr. Build. Mater. 2020, 238, 117713. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Castro-Gomes, J.P.; Jalali, S. Investigations on mix design of tungsten mine waste geopolymeric binder. Constr. Build. Mater. 2008, 22, 1939–1949. [Google Scholar] [CrossRef]
- Salih, M.A.; Farzadnia, N.; Abang Ali, A.A.; Demirboga, R. Effect of different curing temperatures on alkali activated palm oil fuel ash paste. Constr. Build. Mater. 2015, 94, 116–125. [Google Scholar] [CrossRef]
- Xu, D.Q.; Guo, L.Y.; Lin, Y.H.; Yang, Y.Y.; Li, P.J. Mechanical property of alkaliactivated cementitious material based soda residue and slag. New Chem. Mater. 2018, 46, 64–68. [Google Scholar]
- Walkley, B.; San Nicolas, R.; Sani, M.-A.; Bernal, S.A.; van Deventer, J.S.J.; Provis, J.L. Structural evolution of synthetic alkali-activated CaO-MgO-Na2O-Al2O3-SiO2 materials is influenced by Mg content. Cem. Concr. Res. 2017, 99, 155–171. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, Z.; Zhao, Q.; Song, R.; Liu, J. Mechanical properties and microstructure of binding material using slag-fly ash synergistically activated by wet-basis soda residue-carbide slag. Constr. Build. Mater. 2021, 269, 121301. [Google Scholar] [CrossRef]
- Granizo, M.L.; Alonso, S.; Blanc-Varela, M.T.; Palomo, A. Alkaline Activation of Metakaolin: Effect of Calcium Hydroxide in the Products of Reaction. J. Am. Ceram. Soc. 2004, 85, 225–231. [Google Scholar] [CrossRef]
- Phoo-ngernkham, T.; Phiangphimai, C.; Intarabut, D.; Hanjitsuwan, S.; Damrongwiriyanupap, N.; Li, L.-Y.; Chindaprasirt, P. Low cost and sustainable repair material made from alkali-activated high-calcium fly ash with calcium carbide residue. Constr. Build. Mater. 2020, 247, 118543. [Google Scholar] [CrossRef]
- Ren, P.; Ling, T.-C. Roles of chlorine and sulphate in MSWIFA in GGBFS binder: Hydration, mechanical properties and stabilization considerations. Environ. Pollut. 2021, 284, 117175. [Google Scholar] [CrossRef]
- Ping, Y.; Kirkpatrick, R.J.; Poe, B.; McMillan, P.F.; Cong, X. Structure of calcium silicate hydrate (C-S-H): Near-, mid-, and far-infrared spectroscopy. J. Am. Ceram. Soc. 2010, 82, 742–748. [Google Scholar] [CrossRef]
- Mollah, M.Y.A.; Yu, W.; Schennach, R.; Cocke, D.L. A Fourier transform infrared spectroscopic investigation of the early hydration of Portland cement and the influence of sodium lignosulfonate. Cem. Concr. Res. 2000, 30, 267–273. [Google Scholar] [CrossRef]
- Yue, Y.; Wang, J.J.; Basheer, P.A.M.; Bai, Y. Raman spectroscopic investigation of Friedel’s salt. Cem. Concr. Compos. 2018, 86, 306–314. [Google Scholar] [CrossRef]
- García Lodeiro, I.; Macphee, D.E.; Palomo, A.; Fernández-Jiménez, A. Effect of alkalis on fresh C–S–H gels. FTIR analysis. Cem. Concr. Res. 2009, 39, 147–153. [Google Scholar] [CrossRef]
- Fernandez, L.; Alonso, C.; Hidalgo, A.; Andrade, C. The role of magnesium during the hydration of C3S and CSH formation. Scanning electron microscopy and mid-infrared studies. Adv. Cem. Res. 2005, 17, 9–21. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, Z.; Bai, Y.; Zhao, G.; Sang, Z.; Zhao, Q. Development and characterization of a new multi-strength level binder system using soda residue-carbide slag as composite activator. Constr. Build. Mater. 2021, 291, 123367. [Google Scholar] [CrossRef]
- Li, W.; Yi, Y.; Puppala, A.J. Comparing carbide sludge-ground granulated blastfurnace slag and ordinary Portland cement: Different findings from binder paste and stabilized clay slurry. Constr. Build. Mater. 2022, 321, 126382. [Google Scholar] [CrossRef]
- Cong, P.; Mei, L. Using silica fume for improvement of fly ash/slag based geopolymer activated with calcium carbide residue and gypsum. Constr. Build. Mater. 2021, 275, 122171. [Google Scholar] [CrossRef]
- Guo, W.; Wang, S.; Xu, Z.; Zhang, Z.; Zhang, C.; Bai, Y.; Zhao, Q. Mechanical performance and microstructure improvement of soda residue–carbide slag–ground granulated blast furnace slag binder by optimizing its preparation process and curing method. Constr. Build. Mater. 2021, 302, 124403. [Google Scholar] [CrossRef]

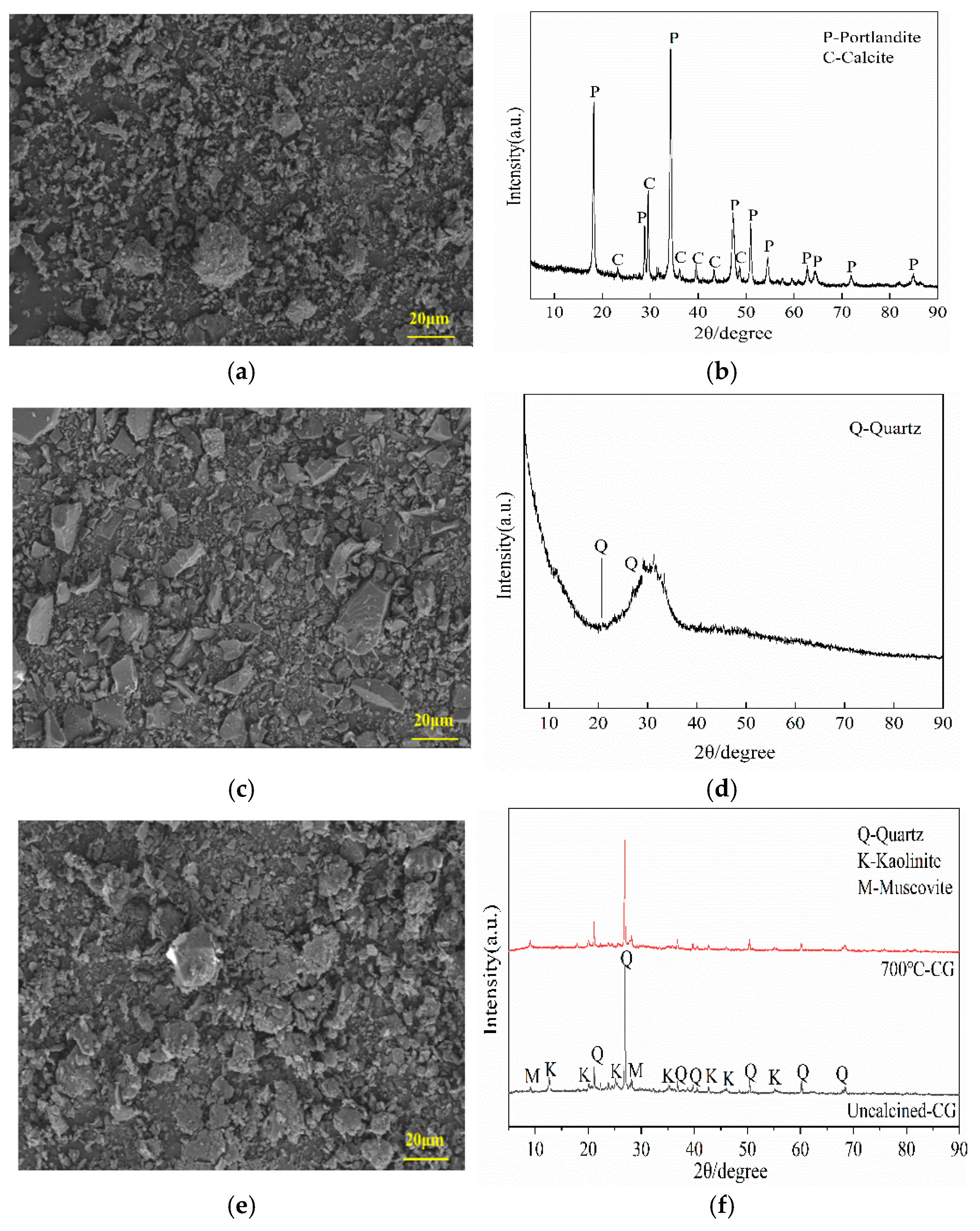
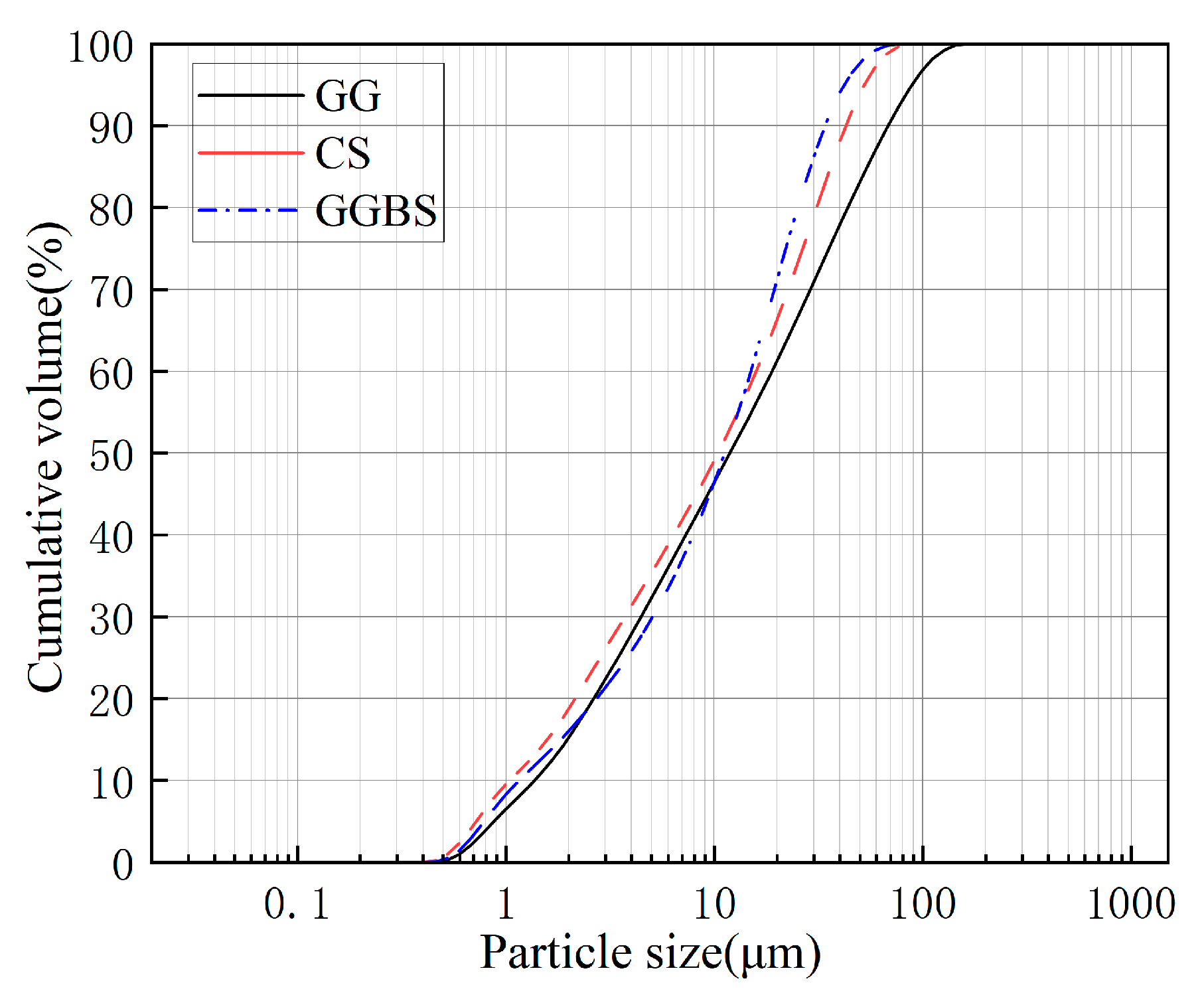


| Material | Chemical Composition by Weight (wt.%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | CaO | MgO | Fe2O3 | Na2O | K2O | MnO | SO3 | |
| CS | 2.56 | 1.68 | 67.20 | 0.22 | 0.09 | 0.35 | 0.03 | / | 0.68 |
| GGBS | 32.80 | 14.30 | 39.50 | 9.20 | 0.88 | 0.20 | 0.63 | 0.07 | 1.32 |
| CG | 46.55 | 44.46 | 2.98 | 0.52 | 2.71 | 0.12 | 0.68 | 0.12 | 0.31 |
| Groups | GG (wt%) | GGBS (wt%) | CS% (wt%) | Water-to-Binder Ratio (W/B) | Si/Al |
|---|---|---|---|---|---|
| CS0 | 60% | 40% | 0% | 0.5 | 1.08 |
| CS5 | 57% | 38% | 5% | 0.5 | 1.08 |
| CS10 | 54% | 36% | 10% | 0.5 | 1.08 |
| CS15 | 51% | 34% | 15% | 0.5 | 1.08 |
| CS20 | 48% | 32% | 20% | 0.5 | 1.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Ju, F.; Xiao, M.; Wang, D.; Yin, L.; Si, L.; Wang, Y.; Xu, M.; Yang, D. Mechanism of Activation and Microstructural Evolution in Calcium Carbide Slag-Activated GGBS-CG Composite Cementitious Materials. Materials 2025, 18, 4189. https://doi.org/10.3390/ma18174189
Wang T, Ju F, Xiao M, Wang D, Yin L, Si L, Wang Y, Xu M, Yang D. Mechanism of Activation and Microstructural Evolution in Calcium Carbide Slag-Activated GGBS-CG Composite Cementitious Materials. Materials. 2025; 18(17):4189. https://doi.org/10.3390/ma18174189
Chicago/Turabian StyleWang, Tengfei, Feng Ju, Meng Xiao, Dong Wang, Lidong Yin, Lu Si, Yingbo Wang, Mengxin Xu, and Dongming Yang. 2025. "Mechanism of Activation and Microstructural Evolution in Calcium Carbide Slag-Activated GGBS-CG Composite Cementitious Materials" Materials 18, no. 17: 4189. https://doi.org/10.3390/ma18174189
APA StyleWang, T., Ju, F., Xiao, M., Wang, D., Yin, L., Si, L., Wang, Y., Xu, M., & Yang, D. (2025). Mechanism of Activation and Microstructural Evolution in Calcium Carbide Slag-Activated GGBS-CG Composite Cementitious Materials. Materials, 18(17), 4189. https://doi.org/10.3390/ma18174189







