The Influence of Lignin Derivatives on the Thermal Properties and Flammability of PLA+PET Blends
Abstract
1. Introduction
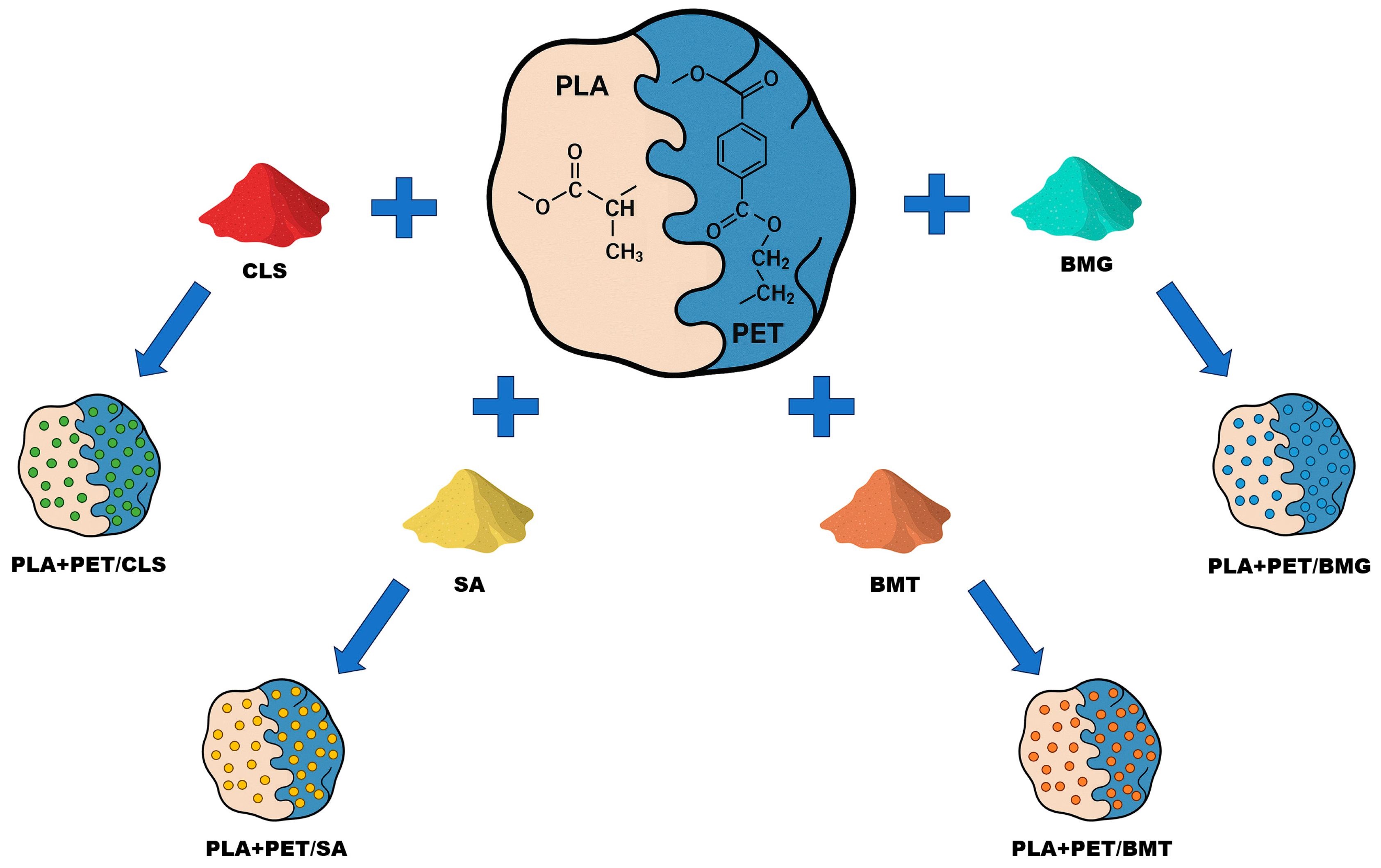
2. Materials
3. Sample Preparation
4. Methods
5. Results and Discussion
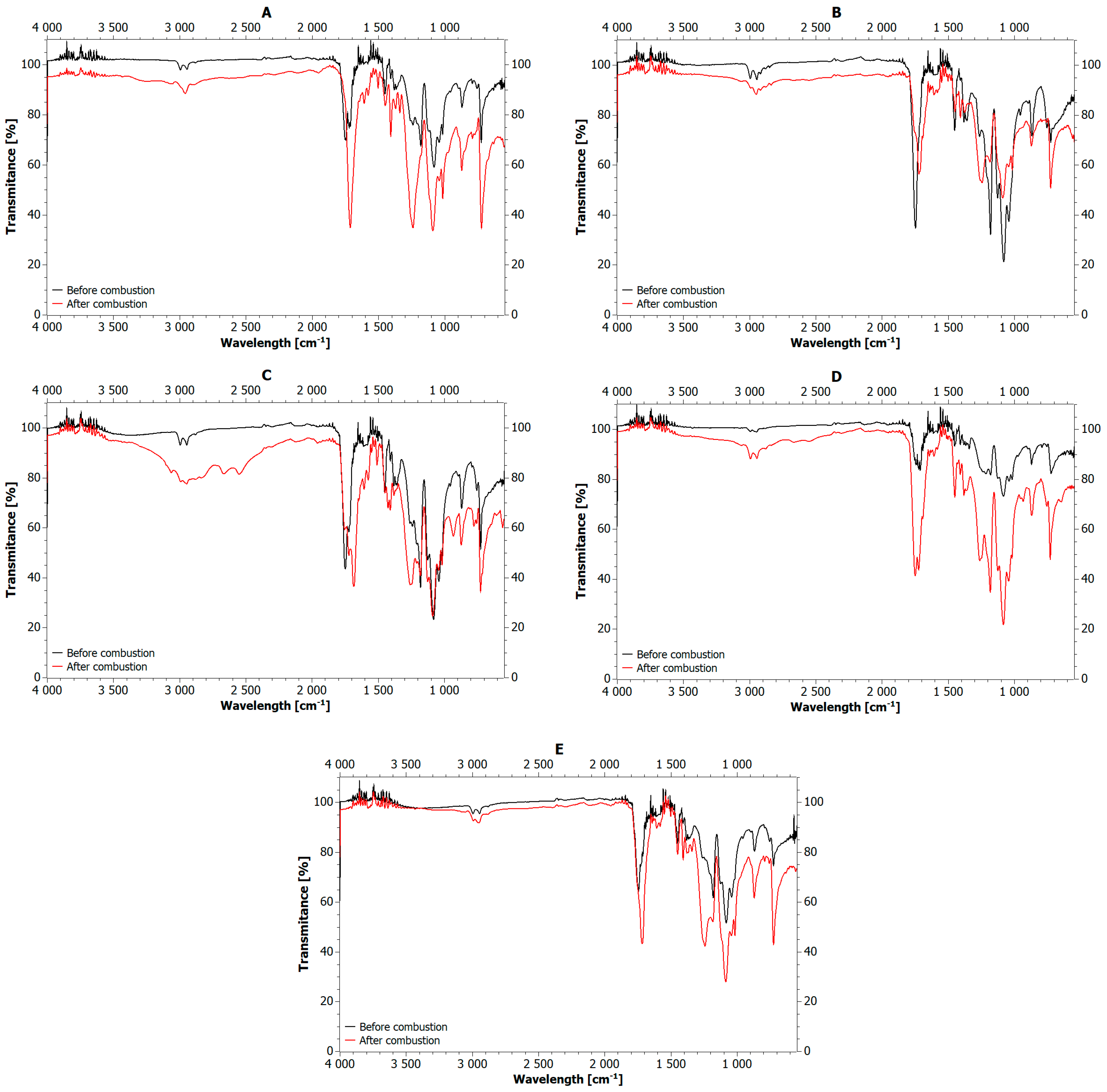
5.1. Analysis of Biofillers
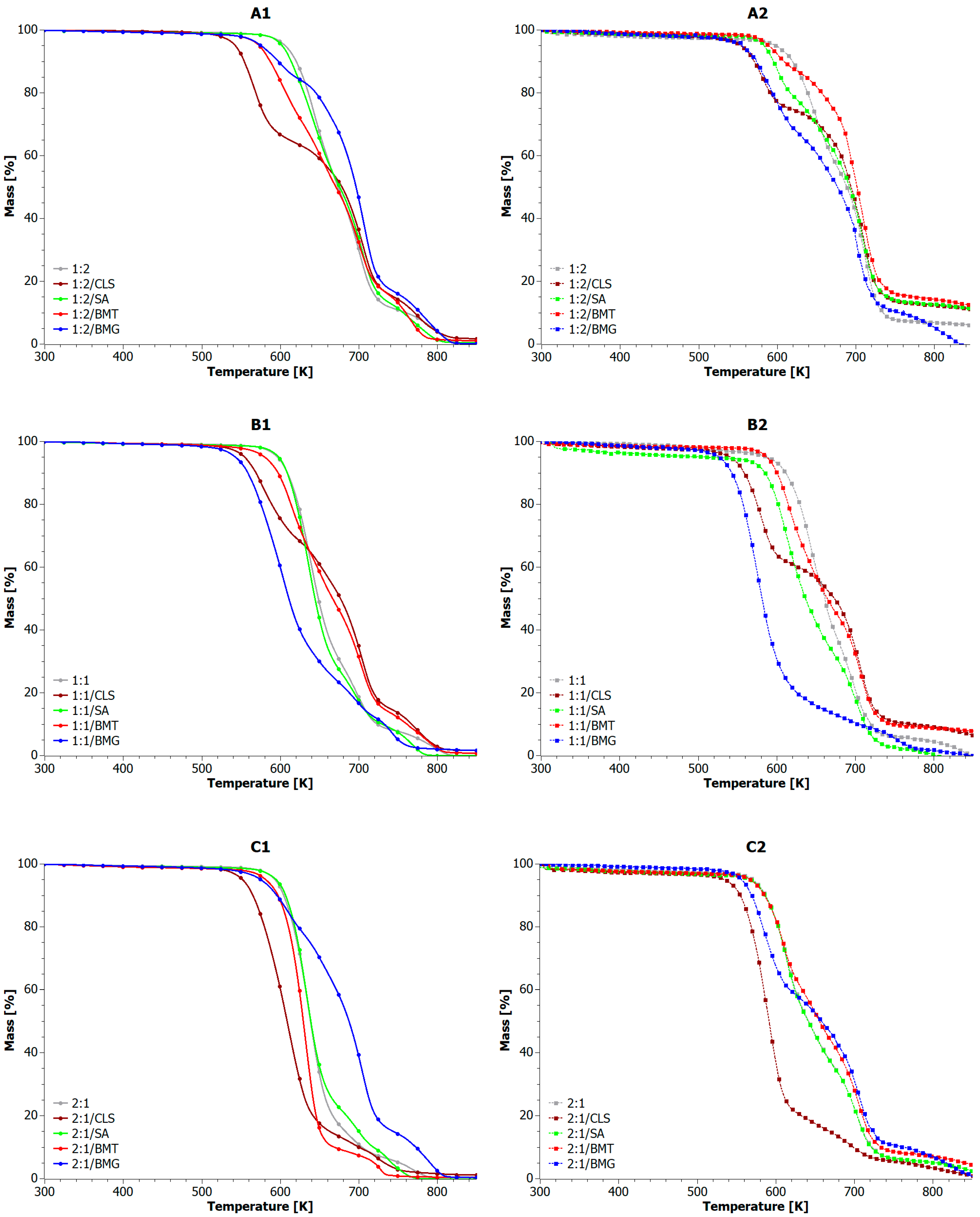
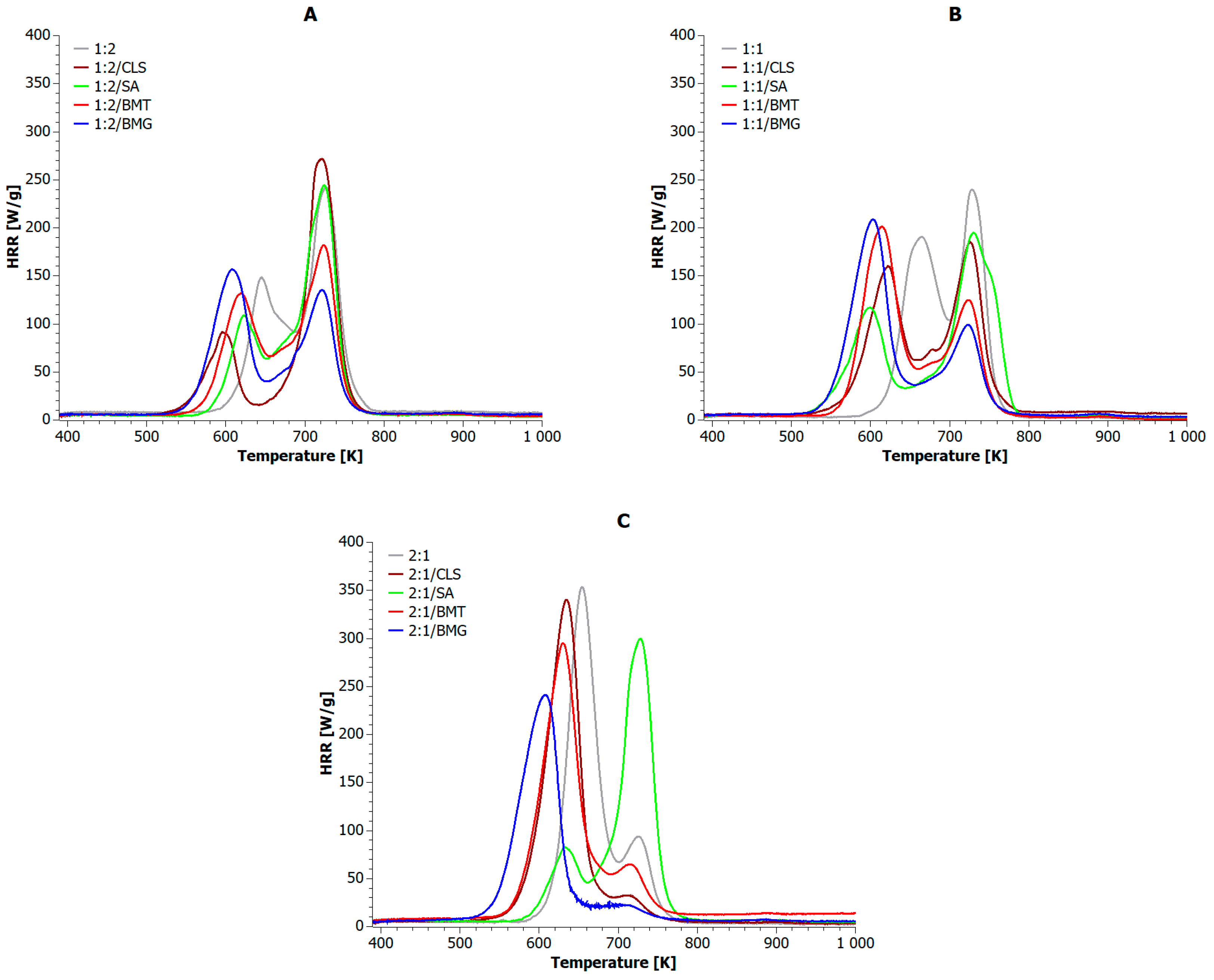
5.2. Analysis of Composite Blends
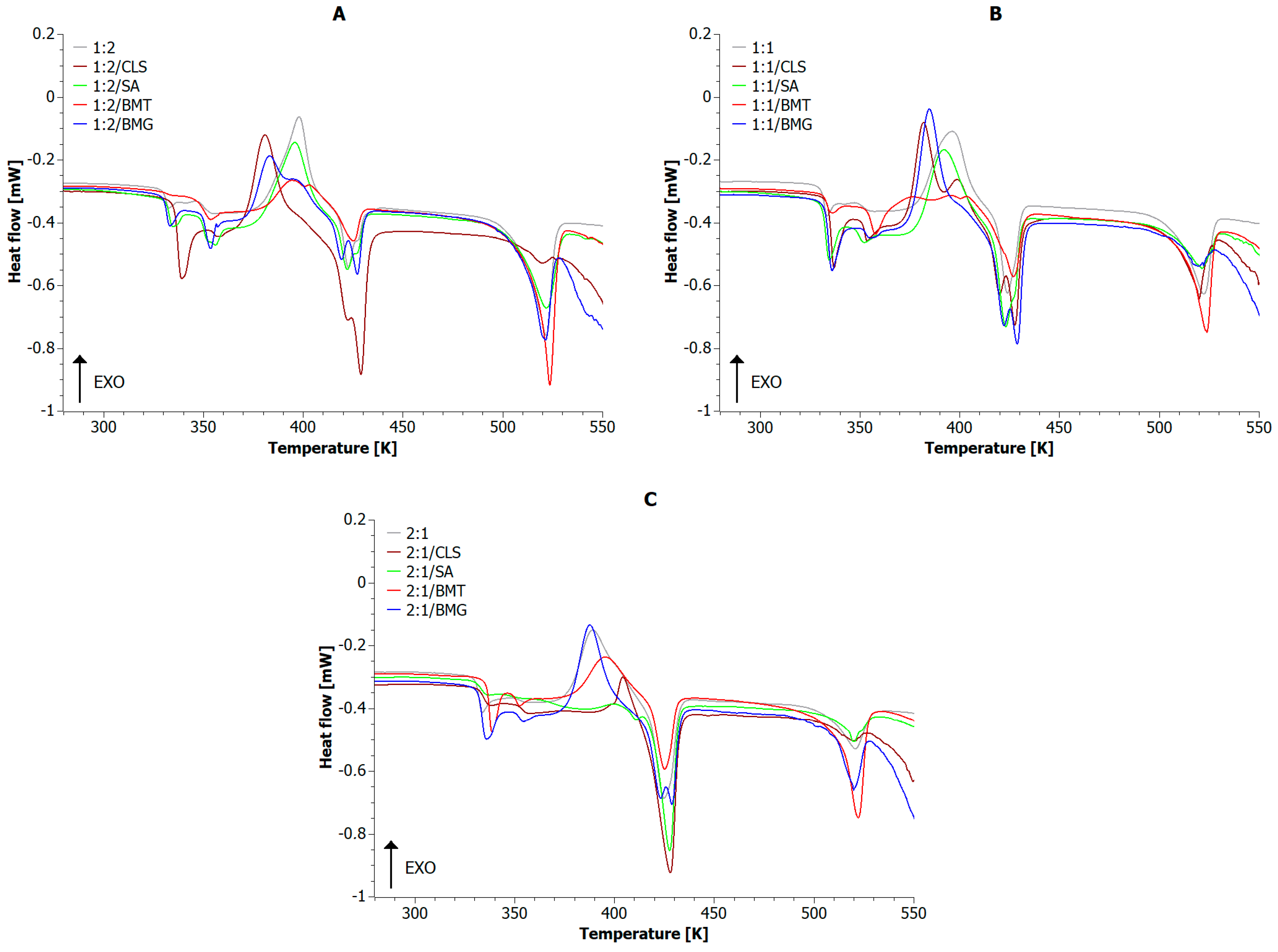
| Sample | Tg_PLA [K] | Tg_PET [K] | Tcc [K] | Tm_PLA [K] | Tm_PET [K] | ∆Hm_PLA [J/g] | ∆Hm_PET [J/g] | Xc_PLA [%] | Xc_PET [%] | Xc [%] |
|---|---|---|---|---|---|---|---|---|---|---|
| 1:2 | 330.19 | 350.55 | 397.97 | 422.54 | 521.79 | 8.92 | 28.12 | 13.10 | 27.44 | 40.55 |
| 1:2/CLS | 335.81 | 355.25 | 380.79 | 428.95 | 519.33 | 31.08 | 1.89 | 20.87 | 14.48 | 35.35 |
| 1:2/SA | 331.80 | 350.08 | 395.62 | 422.19 | 521.61 | 9.41 | 22.65 | 12.77 | 24.37 | 37.14 |
| 1:2/BMT | 331.60 | 350.43 | 394.27 | 425.22 | 523.57 | 4.97 | 32.94 | 6.13 | 22.49 | 28.62 |
| 1:2/BMG | 330.45 | 351.24 | 383.11 | 427.17 | 521.39 | 11.55 | 18.21 | 12.80 | 21.14 | 33.94 |
| 1:1 | 330.97 | 351.55 | 396.13 | 423.84 | 522.29 | 14.07 | 20.01 | 22.33 | 17.59 | 39.92 |
| 1:1/CLS | 333.88 | 352.41 | 381.97 | 427.65 | 519.96 | 21.48 | 12.85 | 28.68 | 16.51 | 45.19 |
| 1:1/SA | 331.34 | 349.41 | 391.95 | 423.15 | 521.32 | 18.58 | 10.16 | 25.32 | 14.28 | 39.60 |
| 1:1/BMT | 332.61 | 355.58 | --- | 426.84 | 523.74 | 16.48 | 22.06 | 9.00 | 8.29 | 17.29 |
| 1:1/BMG | 332.73 | 353.07 | 384.81 | 428.8 | 518.82 | 26.89 | 4.52 | 17.37 | 3.56 | 20.93 |
| 2:1 | 331.41 | 352.08 | 388.95 | 424.81 | 520.48 | 19.04 | 11.06 | 32.18 | 9.13 | 41.31 |
| 2:1/CLS | 333.71 | 354.12 | 404.10 | 427.97 | 519.19 | 31.81 | 2.25 | 26.60 | 1.77 | 28.37 |
| 2:1/SA | 332.59 | 368.55 | 399.25 | 427.65 | 519.52 | 24.38 | 5.67 | 18.23 | 1.60 | 19.82 |
| 2:1/BMT | 335.57 | 350.26 | 273.15 | 395.43 | 425.18 | 11.45 | 23.46 | 19.44 | 9.60 | 29.04 |
| 2:1/BMG | 332.29 | 351.58 | 387.62 | 428.65 | 519.79 | 22.18 | 9.17 | 34.22 | 8.45 | 42.67 |
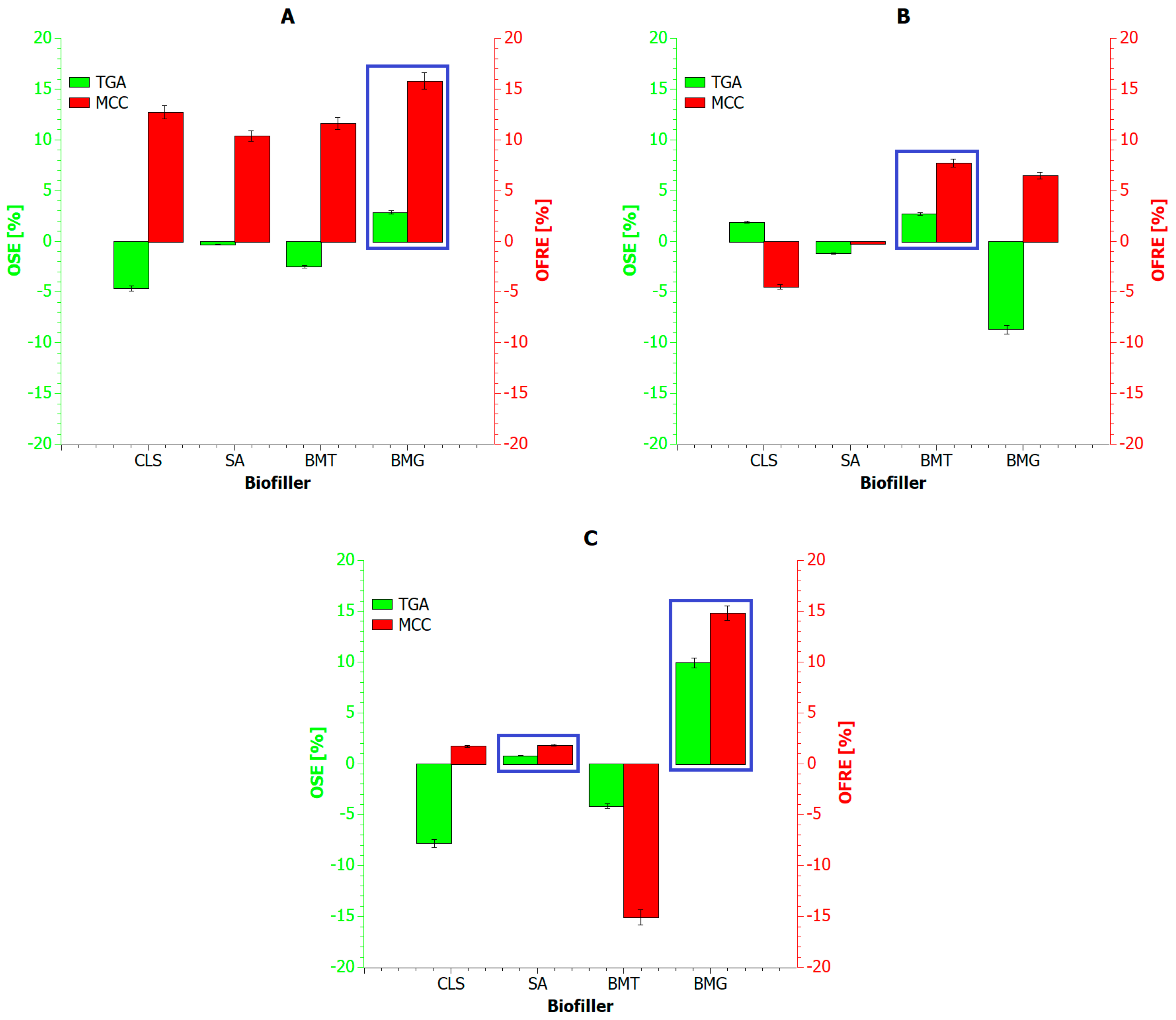
5.3. Potential Upcycling and Recycling Paths
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Korhonen, J.; Honkasalo, A.; Seppälä, J. Circular Economy: The Concept and Its Limitations. Ecol. Econ. 2018, 143, 37–46. [Google Scholar] [CrossRef]
- Geissdoerfer, M.; Savaget, P.; Bocken, N.M.P.; Hultink, E.J. The Circular Economy—A New Sustainability Paradigm? J. Clean. Prod. 2017, 143, 757–768. [Google Scholar] [CrossRef]
- Geisendorf, S.; Pietrulla, F. The Circular Economy and Circular Economic Concepts—A Literature Analysis and Redefinition. Thunderbird Int. Bus. Rev. 2018, 60, 771–782. [Google Scholar] [CrossRef]
- Gardetti, M.A. Introduction and the Concept of Circular Economy. In Circular Economy in Textiles and Apparel: Processing, Manufacturing, and Design; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–11. [Google Scholar] [CrossRef]
- Valerio, O.; Muthuraj, R.; Codou, A. Strategies for Polymer to Polymer Recycling from Waste: Current Trends and Opportunities for Improving the Circular Economy of Polymers in South America. Curr. Opin. Green Sustain. Chem. 2020, 25, 100381. [Google Scholar] [CrossRef]
- von Vacano, B.; Mangold, H.; Vandermeulen, G.W.M.; Battagliarin, G.; Hofmann, M.; Bean, J.; Künkel, A. Sustainable Design of Structural and Functional Polymers for a Circular Economy. Angew. Chem. Int. Ed. 2023, 62, e202210823. [Google Scholar] [CrossRef]
- Shanmugam, V.; Das, O.; Neisiany, R.E.; Babu, K.; Singh, S.; Hedenqvist, M.S.; Berto, F.; Ramakrishna, S. Polymer Recycling in Additive Manufacturing: An Opportunity for the Circular Economy. Mater. Circ. Econ. 2020, 2, 11. [Google Scholar] [CrossRef]
- Torres-Giner, S. Sustainable Polymer Technologies for a Circular Economy. Appl. Sci. 2023, 13, 5864. [Google Scholar] [CrossRef]
- Jacobsen, S.; Degée, P.; Fritz, H.G.; Dubois, P.; Jérôme, R. Polylactide (PLA)—A New Way of Production. Polym. Eng. Sci. 1999, 39, 1311–1319. [Google Scholar] [CrossRef]
- Ray, S.S. Polylactide-Based Bionanocomposites: A Promising Class of Hybrid Materials. Acc. Chem. Res. 2012, 45, 1710–1720. [Google Scholar] [CrossRef]
- Slomkowski, S.; Penczek, S.; Duda, A. Polylactides—An Overview. Polym. Adv. Technol. 2014, 25, 436–447. [Google Scholar] [CrossRef]
- Raquez, J.M.; Habibi, Y.; Murariu, M.; Dubois, P. Polylactide (PLA)-Based Nanocomposites. Prog. Polym. Sci. 2013, 38, 1504–1542. [Google Scholar] [CrossRef]
- Majka, T.M. Purification Effect of Pyrolyzed Filler on the Flammability of Polylactide Matrix. Iran. Polym. J. 2025, 34, 667–687. [Google Scholar] [CrossRef]
- Cheng, K.C.; Lin, Y.H.; Guo, W.; Chuang, T.H.; Chang, S.C.; Wang, S.F.; Don, T.M. Flammability and Tensile Properties of Polylactide Nanocomposites with Short Carbon Fibers. J. Mater. Sci. 2015, 50, 1605–1612. [Google Scholar] [CrossRef]
- Fontaine, G.; Bourbigot, S. Intumescent Polylactide: A Nonflammable Material. J. Appl. Polym. Sci. 2009, 113, 3860–3865. [Google Scholar] [CrossRef]
- Zhao, X.; Guerrero, F.R.; Llorca, J.; Wang, D.Y. New Superefficiently Flame-Retardant Bioplastic Poly(Lactic Acid): Flammability, Thermal Decomposition Behavior, and Tensile Properties. ACS Sustain. Chem. Eng. 2016, 4, 202–209. [Google Scholar] [CrossRef]
- Cheng, K.C.; Yu, C.B.; Guo, W.; Wang, S.F.; Chuang, T.H.; Lin, Y.H. Thermal Properties and Flammability of Polylactide Nanocomposites with Aluminum Trihydrate and Organoclay. Carbohydr. Polym. 2012, 87, 1119–1123. [Google Scholar] [CrossRef]
- Yin, W.; Chen, L.; Lu, F.; Song, P.; Dai, J.; Meng, L. Mechanically Robust, Flame-Retardant Poly(Lactic Acid) Biocomposites via Combining Cellulose Nanofibers and Ammonium Polyphosphate. ACS Omega 2018, 3, 5615–5626. [Google Scholar] [CrossRef]
- Gere, D.; Czigany, T. Future Trends of Plastic Bottle Recycling: Compatibilization of PET and PLA. Polym. Test. 2020, 81, 106160. [Google Scholar] [CrossRef]
- Belioka, M.P.; Markozanne, G.; Chrissopoulou, K.; Achilias, D.S. Advanced Plastic Waste Recycling—The Effect of Clay on the Morphological and Thermal Behavior of Recycled PET/PLA Sustainable Blends. Polymers 2023, 15, 3145. [Google Scholar] [CrossRef]
- Girija, B.G.; Sailaja, R.R.N.; Madras, G. Thermal Degradation and Mechanical Properties of PET Blends. Polym. Degrad. Stab. 2005, 90, 147–153. [Google Scholar] [CrossRef]
- Ostrowski, K.A.; Spyrowski, M.; Romańska, P.; Majka, T.M.; Piech, R.; Zawadzka, Z.; Furtak, K.; Bednarowski, D. PET Composites and Their Applications in the Face of Green Chemistry Challenges—An Overview. Polimery 2025, 70, 227–239. [Google Scholar] [CrossRef]
- McLauchlin, A.R.; Ghita, O.R. Studies on the Thermal and Mechanical Behavior of PLA-PET Blends. J. Appl. Polym. Sci. 2016, 133, 44147. [Google Scholar] [CrossRef]
- Chen, R.S.; Ahmad, S.; Gan, S.; Salleh, M.N.; Ab Ghani, M.H.; Tarawneh, M.A. Effect of Polymer Blend Matrix Compatibility and Fibre Reinforcement Content on Thermal Stability and Flammability of Ecocomposites Made from Waste Materials. Thermochim. Acta 2016, 640, 52–61. [Google Scholar] [CrossRef]
- Habib, U.; Mohsin, M.E.A.; Khan, Z.I.; Mohamad, Z.; Othman, N.; Mousa, S.; Hossain, S.S.; Ali, S.S. Mechanical, Thermal, and Flammability Properties of Eco-Friendly Nanocomposites from Recycled PET/PA-11 Blends Reinforced with Graphene Nanoplatelets. Polymers 2025, 17, 1038. [Google Scholar] [CrossRef]
- Liu, B.W.; Zhao, H.B.; Wang, Y.Z. Advanced Flame-Retardant Methods for Polymeric Materials. Adv. Mater. 2022, 34, 2107905. [Google Scholar] [CrossRef]
- Innes, A.; Innes, J. Flame Retardants. In Applied Plastics Engineering Handbook: Processing and Materials; Elsevier: Amsterdam, The Netherlands, 2011; pp. 469–485. [Google Scholar] [CrossRef]
- Velencoso, M.M.; Battig, A.; Markwart, J.C.; Schartel, B.; Wurm, F.R. Molecular Firefighting—How Modern Phosphorus Chemistry Can Help Solve the Challenge of Flame Retardancy. Angew. Chem. Int. Ed. 2018, 57, 10450–10467. [Google Scholar] [CrossRef]
- Iqbal, M.; Syed, J.H.; Katsoyiannis, A.; Malik, R.N.; Farooqi, A.; Butt, A.; Li, J.; Zhang, G.; Cincinelli, A.; Jones, K.C. Legacy and Emerging Flame Retardants (FRs) in the Freshwater Ecosystem: A Review. Environ. Res. 2017, 152, 26–42. [Google Scholar] [CrossRef]
- Morgan, A.B.; Gilman, J.W. An Overview of Flame Retardancy of Polymeric Materials: Application, Technology, and Future Directions. Fire Mater. 2013, 37, 259–279. [Google Scholar] [CrossRef]
- Jeong, S.H.; Park, C.H.; Song, H.; Heo, J.H.; Lee, J.H. Biomolecules as Green Flame Retardants: Recent Progress, Challenges, and Opportunities. J. Clean. Prod. 2022, 368, 133241. [Google Scholar] [CrossRef]
- Costes, L.; Laoutid, F.; Brohez, S.; Dubois, P. Bio-Based Flame Retardants: When Nature Meets Fire Protection. Mater. Sci. Eng. R Rep. 2017, 117, 1–25. [Google Scholar] [CrossRef]
- Basak, S.; Raja, A.S.M.; Saxena, S.; Patil, P.G. Tannin Based Polyphenolic Bio-Macromolecules: Creating a New Era towards Sustainable Flame Retardancy of Polymers. Polym. Degrad. Stab. 2021, 189, 109603. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, P.; Liu, Y.; Zhu, P. Green Flame-Retardant Flexible Polyurethane Foam Based on Polyphenol-Iron-Phytic Acid Network to Improve the Fire Safety. Compos. B Eng. 2022, 239, 109958. [Google Scholar] [CrossRef]
- Yao, F.; Zhai, C.; Wang, H.; Tao, J. Characterization of Tea Polyphenols as Potential Environment-Friendly Fire Retardants. IOP Conf. Ser. Earth Environ. Sci. 2018, 121, 22016. [Google Scholar] [CrossRef]
- Solihat, N.N.; Hidayat, A.F.; Taib, M.N.A.M.; Hussin, M.H.; Lee, S.H.; Ghani, M.A.A.; Al Edrus, S.S.O.; Vahabi, H.; Fatriasari, W. Recent Developments in Flame-Retardant Lignin-Based Biocomposite: Manufacturing, and Characterization. J. Polym. Environ. 2022, 30, 4517–4537. [Google Scholar] [CrossRef]
- De Chirico, A.; Armanini, M.; Chini, P.; Cioccolo, G.; Provasoli, F.; Audisio, G. Flame Retardants for Polypropylene Based on Lignin. Polym. Degrad. Stab. 2003, 79, 139–145. [Google Scholar] [CrossRef]
- Podkościelna, B.; Gargol, M.; Goliszek, M.; Klepka, T.; Sevastyanova, O. Degradation and Flammability of Bioplastics Based on PLA and Lignin. Polym. Test. 2022, 111, 107622. [Google Scholar] [CrossRef]
- Réti, C.; Casetta, M.; Duquesne, S.; Bourbigot, S.; Delobel, R. Flammability Properties of Intumescent PLA Including Starch and Lignin. Polym. Adv. Technol. 2008, 19, 628–635. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, A.; Cheng, Y.; Li, M.; Cui, Y.; Li, Z. Recent Advances in Biomass Phytic Acid Flame Retardants. Polym. Test. 2023, 124, 108100. [Google Scholar] [CrossRef]
- Mokhena, T.C.; Sadiku, E.R.; Ray, S.S.; Mochane, M.J.; Matabola, K.P.; Motloung, M. Flame Retardancy Efficacy of Phytic Acid: An Overview. J. Appl. Polym. Sci. 2022, 139, e52495. [Google Scholar] [CrossRef]
- Sykam, K.; Försth, M.; Sas, G.; Restás, Á.; Das, O. Phytic Acid: A Bio-Based Flame Retardant for Cotton and Wool Fabrics. Ind. Crops Prod. 2021, 164, 113349. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Li, T.; Zhang, S.; Ma, P.; Shi, D.; Chen, M.; Dong, W. A Bio-Based Flame-Retardant Starch Based on Phytic Acid. ACS Sustain. Chem. Eng. 2020, 8, 10265–10274. [Google Scholar] [CrossRef]
- Chen, M.; Guo, Q.; Yuan, Y.; Li, A.; Lin, B.; Xiao, Y.; Xu, L.; Wang, W. Recent Advancements of Bio-Derived Flame Retardants for Polymeric Materials. Polymers 2025, 17, 249. [Google Scholar] [CrossRef] [PubMed]
- Vidal, R.; Moliner, E.; Martin, P.P.; Fita, S.; Wonneberger, M.; Verdejo, E.; Vanfleteren, F.; Lapeña, N.; González, A. Life Cycle Assessment of Novel Aircraft Interior Panels Made from Renewable or Recyclable Polymers with Natural Fiber Reinforcements and Non-Halogenated Flame Retardants. J. Ind. Ecol. 2018, 22, 132–144. [Google Scholar] [CrossRef]
- Morão, A.; de Bie, F. Life Cycle Impact Assessment of Polylactic Acid (PLA) Produced from Sugarcane in Thailand. J. Polym. Environ. 2019, 27, 2523–2539. [Google Scholar] [CrossRef]
- Majka, T.M.; Pimentel, A.C.; Fernandes, S.; de Almeida, H.V.; Borges, J.P.; Martins, R. Experimental Consideration of the Effects of Calcium Lignosulfonate and Tannic Acid on the Flammability and Thermal Properties of Polylactide Composites. Thermochim. Acta 2024, 737, 179769. [Google Scholar] [CrossRef]
- Majka, T.M.; Zawadzka, Z.; Piech, R. Lignosulfonamides as a New Group Halogen Free Flame Retardant for PLA. Polimery 2024, 69, 681–693. [Google Scholar] [CrossRef]
- Majka, T.M. The Influence of Amino Chain Length and Calcium Lignosulfonate Modification on Lignosulfonamides Flammability and Thermal Stability. Polimery 2023, 68, 544–554. [Google Scholar] [CrossRef]
- Dawy, M.; Shabaka, A.A.; Nada, A.M.A. Molecular Structure and Dielectric Properties of Some Treated Lignins. Polym. Degrad. Stab. 1998, 62, 455–462. [Google Scholar] [CrossRef]
- Telysheva, G.; Dizhbite, T.; Paegle, E.; Shapatin, A.; Demidov, I. Surface-Active Properties of Hydrophobized Derivatives of Lignosulfonates: Effect of Structure of Organosilicon Modifier. J. Appl. Polym. Sci. 2001, 82, 1013–1020. [Google Scholar] [CrossRef]
- Han, H.; Li, J.; Wang, H.; Han, Y.; Chen, Y.; Li, J.; Zhang, Y.; Wang, Y.; Wang, B. One-Step Valorization of Calcium Lignosulfonate to Produce Phenolics with the Addition of Solid Base Oxides in the Hydrothermal Reaction System. Energy Fuels 2019, 33, 4302–4309. [Google Scholar] [CrossRef]
- Fernández-Pérez, M.; Flores-Céspedes, F.; Daza-Fernández, I.; Vidal-Peña, F.; Villafranca-Sánchez, M. Lignin and Lignosulfonate-Based Formulations To Protect Pyrethrins against Photodegradation and Volatilization. Ind. Eng. Chem. Res. 2014, 53, 13557–13564. [Google Scholar] [CrossRef]
- Lewis, R.J. Sax’s Dangerous Properties of Industrial Materials. In Sax’s Dangerous Properties of Industrial Materials; Wiley: Hoboken, NJ, USA, 2004. [Google Scholar] [CrossRef]
- Robles, H. Tannic Acid. In Encyclopedia of Toxicology: Third Edition; Elsevier: Amsterdam, The Netherlands, 2014; pp. 474–475. [Google Scholar] [CrossRef]
- Chung, K.T.; Wong, T.Y.; Wei, C.I.; Huang, Y.W.; Lin, Y. Tannins and Human Health: A Review. Crit. Rev. Food Sci. Nutr. 1998, 38, 421–464. [Google Scholar] [CrossRef] [PubMed]
- Tributsch, H.; Fiechter, S. The Material Strategy Of Fire-Resistant Barks. High Perform. Struct. Mater. IV 2008, 97, 43–52. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Santos, N.A.; Cordeiro, A.M.T.M.; Damasceno, S.S.; Aguiar, R.T.; Rosenhaim, R.; Carvalho Filho, J.R.; Santos, I.M.G.; Maia, A.S.; Souza, A.G. Commercial Antioxidants and Thermal Stability Evaluations. Fuel 2012, 97, 638–643. [Google Scholar] [CrossRef]
- Garro Galvez, J.M.; Fechtal, M.; Riedl, B. Gallic Acid as a Model of Tannins in Condensation with Formaldehyde. Thermochim. Acta 1996, 274, 149–163. [Google Scholar] [CrossRef]
- Boles, J.S.; Crerar, D.A.; Grissom, G.; Key, T.C. Aqueous Thermal Degradation of Gallic Acid. Geochim. Cosmochim. Acta 1988, 52, 341–344. [Google Scholar] [CrossRef]
- Volf, I.; Ignat, I.; Neamtu, M.; Popa, V.I. Thermal Stability, Antioxidant Activity, and Photo-Oxidation of Natural Polyphenols. Chem. Pap. 2014, 68, 121–129. [Google Scholar] [CrossRef]
- Lokhande, K.D.; Bhakare, M.A.; Bondarde, M.P.; Dhumal, P.S.; Some, S. Bio-Derived Efficient Flame-Retardants for Cotton Fabric. Cellulose 2022, 29, 3583–3593. [Google Scholar] [CrossRef]
- Qian, B.; Wang, W.; Zhu, H.; Zhang, J.; Wu, M.; Liu, J.; Wu, Q.; Yang, J. Synthesis, Characterization and Performance Evaluation of a Flame Retardant Plasticizer for Poly(Vinyl Chloride) Derived from Biobased Vanillic Acid. Chem. Eng. J. 2023, 476, 146859. [Google Scholar] [CrossRef]
- Qian, B.; Zhu, H.; Wang, P.; Peng, P.; Zhang, J.; Wu, M.; Liu, J.; Wu, Q.; Yang, J. Synthesis, Characterization and Performance Evaluation of Different Alkyl Chain Lengths Flame-Retardant Plasticizers for Poly(Vinyl Chloride) Derived from Sustainable Vanillic Acid. Eur. Polym. J. 2024, 214, 113154. [Google Scholar] [CrossRef]
- Wang, S.; Ma, S.; Xu, C.; Liu, Y.; Dai, J.; Wang, Z.; Liu, X.; Chen, J.; Shen, X.; Wei, J.; et al. Vanillin-Derived High-Performance Flame Retardant Epoxy Resins: Facile Synthesis and Properties. Macromolecules 2017, 50, 1892–1901. [Google Scholar] [CrossRef]
- Silva, M.J.; Hilton, D.; Furr, J.; Gray, L.E.; Preau, J.L.; Calafat, A.M.; Ye, X. Quantification of Tetrabromo Benzoic Acid and Tetrabromo Phthalic Acid in Rats Exposed to the Flame Retardant Uniplex FPR-45. Arch. Toxicol. 2016, 90, 551–557. [Google Scholar] [CrossRef]
- Wensing, M.; Uhde, E.; Salthammer, T. Plastics Additives in the Indoor Environment—Flame Retardants and Plasticizers. Sci. Total Environ. 2005, 339, 19–40. [Google Scholar] [CrossRef] [PubMed]
- Karaseva, V.; Bergeret, A.; Lacoste, C.; Fulcrand, H.; Ferry, L. New Biosourced Flame Retardant Agents Based on Gallic and Ellagic Acids for Epoxy Resins. Molecules 2019, 24, 4305. [Google Scholar] [CrossRef] [PubMed]
- Nabipour, H.; Qiu, S.; Wang, X.; Song, L.; Hu, Y. Phosphorus-Free Ellagic Acid-Derived Epoxy Thermosets with Intrinsic Antiflammability and High Glass Transition Temperature. ACS Sustain. Chem. Eng. 2021, 9, 10799–10808. [Google Scholar] [CrossRef]
- Roca, M.; Althaus, R.L.; Molina, M.P. Thermodynamic Analysis of the Thermal Stability of Sulphonamides in Milk Using Liquid Chromatography Tandem Mass Spectrometry Detection. Food Chem. 2013, 136, 376–383. [Google Scholar] [CrossRef]
- Wesołowski, M.; Kosecka, E.; Erecińska, J.; Kobyłczyk, K. The Influence of Chemical Structure of Sulfonamides on the Course of Their Thermal Decomposition. J. Therm. Anal. Calorim. 2003, 74, 465–476. [Google Scholar] [CrossRef]
- Tian, L.; Khalil, S.; Bayen, S. Effect of Thermal Treatments on the Degradation of Antibiotic Residues in Food. Crit. Rev. Food Sci. Nutr. 2017, 57, 3760–3770. [Google Scholar] [CrossRef]
- Bisanda, E.T.N.; Ogola, W.O.; Tesha, J.V. Characterisation of Tannin Resin Blends for Particle Board Applications. Cem. Concr. Compos. 2003, 25, 593–598. [Google Scholar] [CrossRef]
- Ledesma, E.B.; Marsh, N.D.; Sandrowitz, A.K.; Wornat, M.J. An Experimental Study on the Thermal Decomposition of Catechol. Proc. Combust. Inst. 2002, 29, 2299–2306. [Google Scholar] [CrossRef]
- Xia, Z.; Kiratitanavit, W.; Facendola, P.; Yu, S.; Kumar, J.; Mosurkal, R.; Nagarajan, R. A Bio-Derived Char Forming Flame Retardant Additive for Nylon 6 Based on Crosslinked Tannic Acid. Thermochim. Acta 2020, 693, 178750. [Google Scholar] [CrossRef]
- He, Y.Z.; Mallard, W.G.; Tsang, W. Kinetics of Hydrogen and Hydroxyl Radical Attack on Phenol at High Temperatures. J. Phys. Chem. 1988, 92, 2196–2201. [Google Scholar] [CrossRef]
- Coleman, D.J.; Pilcher, G. Heats of Combustion of Biphenyl, Bibenzyl, Naphthalene, Anthracene and Phenanthrene. Trans. Faraday Soc. 1966, 62, 821–827. [Google Scholar] [CrossRef]
- Rao, C.R.M.; Reddy, V.B.; Mehrotra, P.N. Derivatographic Studies of Gallic Acid. Thermochim. Acta 1981, 46, 65–69. [Google Scholar] [CrossRef]
- Torres-Huerta, A.M.; Del Angel-López, D.; Domínguez-Crespo, M.A.; Palma-Ramírez, D.; Perales-Castro, M.E.; Flores-Vela, A. Morphological and Mechanical Properties Dependence of PLA Amount in PET Matrix Processed by Single-Screw Extrusion. Polym. Plast. Technol. Eng. 2016, 55, 672–683. [Google Scholar] [CrossRef]
- Palma-Ramírez, D.; Torres-Huerta, A.M.; Domínguez-Crespo, M.A.; Del Angel-López, D.; Flores-Vela, A.I.; de la Fuente, D. Data Supporting the Morphological/Topographical Properties and the Degradability on PET/PLA and PET/Chitosan Blends. Data Brief. 2019, 25, 104012. [Google Scholar] [CrossRef]
- You, X.; Snowdon, M.R.; Misra, M.; Mohanty, A.K. Biobased Poly(Ethylene Terephthalate)/Poly(Lactic Acid) Blends Tailored with Epoxide Compatibilizers. ACS Omega 2018, 3, 11759–11769. [Google Scholar] [CrossRef]
- Kheirandish, M.; Mohaddes Mojtahedi, M.R.; Nazockdast, H. Assessing Compatibility, Tansesterification, and Disintegration of PET/PLA Fiber Blend in Composting Conditions. Front. Mater. 2023, 10, 1225200. [Google Scholar] [CrossRef]
- Andrzejewski, J.; Das, S.; Lipik, V.; Mohanty, A.K.; Misra, M.; You, X.; Tan, L.P.; Chang, B.P. The Development of Poly(Lactic Acid) (PLA)-Based Blends and Modification Strategies: Methods of Improving Key Properties towards Technical Applications—Review. Materials 2024, 17, 4556. [Google Scholar] [CrossRef]
- Jafari, S.M.A.; Khajavi, R.; Goodarzi, V.; Kalaee, M.R.; Khonakdar, H.A. Nonisothermal Crystallization Kinetic Studies on Melt Processed Poly(Ethylene Terephthalate)/Polylactic Acid Blends Containing Graphene Oxide and Exfoliated Graphite Nanoplatelets. J. Appl. Polym. Sci. 2019, 136, 47569. [Google Scholar] [CrossRef]
- Tripathi, N.; Misra, M.; Mohanty, A.K. Durable Polylactic Acid (PLA)-Based Sustainable Engineered Blends and Biocomposites: Recent Developments, Challenges, and Opportunities. ACS Eng. Au 2021, 1, 7–38. [Google Scholar] [CrossRef]
- Janczak, K.; Dąbrowska, G.B.; Raszkowska-Kaczor, A.; Kaczor, D.; Hrynkiewicz, K.; Richert, A. Biodegradation of the Plastics PLA and PET in Cultivated Soil with the Participation of Microorganisms and Plants. Int. Biodeterior. Biodegrad. 2020, 155, 105087. [Google Scholar] [CrossRef]
- Polymer Blends Handbook, Volumes 1−2 Edited by L. A. Utracki (National Research Council Canada). Kluwer Academic Publishers: Dordrecht. 2002. xxxvi + 1442 pp. $583.50. ISBN 1-4020-1114-8 (Set). J. Am. Chem. Soc. 2003, 125, 10145. [CrossRef]
- Kong, Y.; Hay, J.N. The Measurement of the Crystallinity of Polymers by DSC. Polymer 2002, 43, 3873–3878. [Google Scholar] [CrossRef]
- Saxena, P.; Shukla, P.; Gaur, M.S. Thermal Analysis of Polymer Blends and Double Layer by DSC. Polym. Polym. Compos. 2021, 29, S11–S18. [Google Scholar] [CrossRef]
- Zhang, L.; Xiong, C.; Deng, X. Miscibility, Crystallization and Morphology of Poly(β-Hydroxybutyrate)/Poly(d,l-Lactide) Blends. Polymer 1996, 37, 235–241. [Google Scholar] [CrossRef]
- Topkanlo, H.A.; Ahmadi, Z.; Taromi, F.A. An In-Depth Study on Crystallization Kinetics of PET/PLA Blends. Iran. Polym. J. 2018, 27, 13–22. [Google Scholar] [CrossRef]
- Azizi Topkanlo, H.; Ahamadi, Z.; Afshar Taromi, F. PET/PLA Blends Crystallization Kinetics. In Eco-Friendly and Smart Polymer Systems; Springer: Cham, Switzerland, 2020; pp. 682–685. [Google Scholar] [CrossRef]
- Tábi, T.; Hajba, S.; Kovács, J.G. Effect of Crystalline Forms (A′ and α) of Poly(Lactic Acid) on Its Mechanical, Thermo-Mechanical, Heat Deflection Temperature and Creep Properties. Eur. Polym. J. 2016, 82, 232–243. [Google Scholar] [CrossRef]
- Slonov, A.L.; Musov, I.V.; Zhansitov, A.A.; Khakulova, D.M.; Borukaev, T.A.; Khashirova, S.Y.; Mikitaev, A.K. Investigation of the Structure and Thermal Properties of Composites Based on Polyethylene Terephthalate and Organoclay. Int. Polym. Sci. Technol. 2016, 43, 43–47. [Google Scholar] [CrossRef]
- Kuru, Z.; Kaya, M.A. Poly(Lactic Acid)/Polyester Blends: Review of Current and Future Applications. Eur. J. Res. Dev. 2023, 3, 175–199. [Google Scholar] [CrossRef]
- Torres-Huerta, A.M.; Palma-Ramírez, D.; Domínguez-Crespo, M.A.; Del Angel-López, D.; De La Fuente, D. Comparative Assessment of Miscibility and Degradability on PET/PLA and PET/Chitosan Blends. Eur. Polym. J. 2014, 61, 285–299. [Google Scholar] [CrossRef]
- Lu, W.; Ye, J.; Zhu, L.; Jin, Z.; Matsumoto, Y. Intumescent Flame Retardant Mechanism of Lignosulfonate as a Char Forming Agent in Rigid Polyurethane Foam. Polymers 2021, 13, 1585. [Google Scholar] [CrossRef]
- Pantaleoni, A.; Sarasini, F.; Russo, P.; Passaro, J.; Giorgini, L.; Bavasso, I.; Santarelli, M.L.; Petrucci, E.; Valentini, F.; Bracciale, M.P.; et al. Facile and Bioinspired Approach from Gallic Acid for the Synthesis of Biobased Flame Retardant Coatings of Basalt Fibers. ACS Omega 2024, 9, 19099–19107. [Google Scholar] [CrossRef]
- Majka, T.M. Flammability Analysis of Poly(Ethylene Terephthalate) and Recycled PET with Pyrolyzed Filler. J. Polym. Res. 2023, 30, 357. [Google Scholar] [CrossRef]
- Majka, T.M.; Bukowczan, A.; Pielichowski, K. Flame Retardation of Polyamide 6 by Electro-Sprayed Phosphorus–Copper Complex Supported by Layered Silicate. J. Mater. Eng. Perform. 2024, 33, 13637–13655. [Google Scholar] [CrossRef]
- Majka, T.M. Effect of LbL Deposited Chitosan-Nanosilica Bilayers on Flammability and Thermal Properties of Polylactide Materials. Int. J. Heat Technol. 2024, 42, 1257–1269. [Google Scholar] [CrossRef]
- Majka, T.M. Influence of Elettaria cardamomum L. and Myristica fragrans Houtt. Seeds on the Thermal Properties and Flammability of Poly(Lactic Acid). BioResources 2025, 20, 1655–1675. [Google Scholar] [CrossRef]
- Garlotta, D. A Literature Review of Poly(Lactic Acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Prasad, S.G.; De, A.; De, U.; Berg, R.W. Structural and Optical Investigations of Radiation Damage in Transparent PET Polymer Films. Int. J. Spectrosc. 2011, 2011, 810936. [Google Scholar] [CrossRef]
- Meaurio, E.; López-Rodríguez, N.; Sarasua, J.R. Infrared Spectrum of Poly(L-Lactide): Application to Crystallinity Studies. Macromolecules 2006, 39, 9291–9301. [Google Scholar] [CrossRef]
- Kister, G.; Cassanas, G.; Vert, M. Effects of Morphology, Conformation and Configuration on the IR and Raman Spectra of Various Poly(Lactic Acid)s. Polymer 1998, 39, 267–273. [Google Scholar] [CrossRef]
- Miyake, A. The Infrared Spectrum of Polyethylene Terephthalate. I The Effect of Crystallization. J. Polym. Sci. 1959, 38, 479–495. [Google Scholar] [CrossRef]
- Gardette, M.; Thérias, S.; Gardette, J.L.; Murariu, M.; Dubois, P. Photooxidation of Polylactide/Calcium Sulphate Composites. Polym. Degrad. Stab. 2011, 96, 616–623. [Google Scholar] [CrossRef]
- Holland, B.J.; Hay, J.N. The Thermal Degradation of PET and Analogous Polyesters Measured by Thermal Analysis–Fourier Transform Infrared Spectroscopy. Polymer 2002, 43, 1835–1847. [Google Scholar] [CrossRef]
- Pires, M.; Murariu, M.; Cardoso, A.M.; Bonnaud, L.; Dubois, P. Thermal Degradation of Poly(Lactic Acid)–Zeolite Composites Produced by Melt-Blending. Polym. Bull. 2020, 77, 2111–2137. [Google Scholar] [CrossRef]
- Ozdemir, E.; Hacaloglu, J. Thermal Degradation of Polylactide and Its Electrospun Fiber. Fibers Polym. 2016, 17, 66–73. [Google Scholar] [CrossRef]
- Bocchini, S.; Frache, A. Comparative Study of Filler Influence on Polylactide Photooxidation. Express Polym. Lett. 2013, 7, 431–442. [Google Scholar] [CrossRef]
- Kervran, M.; Vagner, C.; Cochez, M.; Ponçot, M.; Saeb, M.R.; Vahabi, H. Thermal Degradation of Polylactic Acid (PLA)/Polyhydroxybutyrate (PHB) Blends: A Systematic Review. Polym. Degrad. Stab. 2022, 201, 109995. [Google Scholar] [CrossRef]
- Rasselet, D.; Ruellan, A.; Guinault, A.; Miquelard-Garnier, G.; Sollogoub, C.; Fayolle, B. Oxidative Degradation of Polylactide (PLA) and Its Effects on Physical and Mechanical Properties. Eur. Polym. J. 2014, 50, 109–116. [Google Scholar] [CrossRef]
- Arrigo, R.; Morici, E.; Dintcheva, N.T. Biopolyester-Based Systems Containing Naturally Occurring Compounds with Enhanced Thermooxidative Stability. J. Appl. Biomater. Funct. Mater. 2016, 14, e455–e462. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Macêdo, E.N.; Nascimento, M.H.R.; de Freitas, C.A.O.; Junior, J.d.A.B. Fuzzy Method for in Control Acetaldehyde Generation in Resin Pet in the Process of Packaging Pre-Forms of Plastic Injection. Int. J. Adv. Eng. Res. Sci. 2018, 5, 237–248. [Google Scholar] [CrossRef]
- Romão, W.; Franco, M.F.; Corilo, Y.E.; Eberlin, M.N.; Spinacé, M.A.S.; De Paoli, M.A. Poly (Ethylene Terephthalate) Thermo-Mechanical and Thermo-Oxidative Degradation Mechanisms. Polym. Degrad. Stab. 2009, 94, 1849–1859. [Google Scholar] [CrossRef]
- Ma, T.; Wang, R.; Wang, W.; Gu, W.; Yuan, Y.; Zhang, A.; Wei, J. Studies on the Thermal Degradation Mechanism of Polyethylene Terephthalate and Its 2-Carboxy Ethyl (Phenyl) Phosphinic Acid Copolymers. Polym. Degrad. Stab. 2022, 206, 110185. [Google Scholar] [CrossRef]
- Čolnik, M.; Pečar, D.; Knez, Ž.; Goršek, A.; Škerget, M. Kinetics Study of Hydrothermal Degradation of PET Waste into Useful Products. Processes 2021, 10, 24. [Google Scholar] [CrossRef]


| Extrusion | |||||||
|---|---|---|---|---|---|---|---|
| Temperature [K] | Heating Zones | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | Die | |
| 493 | 498 | 503 | 508 | 513 | 513 | 513 | |
| Degassing | - | - | - | - | Yes | - | - |
| Screws Speed [rpm] | 150 | ||||||
| Feed Capacity [%] | 5 | ||||||
| Cooling Bath | |||||||
| Temperature [K] | 293 | ||||||
| Compression Press | |||||||
| Temperature of Stamps [K] | 513 | ||||||
| Pressure [Bar] | 240 | ||||||
| Pressing Time [s] | 420 | ||||||
| Sample No. | PLA: PET Mass Ratio | Biofiller | Sample Designation | |||
|---|---|---|---|---|---|---|
| CLS | SA | BMT | BMG | |||
| 1 | 1:2 | - | - | - | - | 1:2 |
| 2 | ✓ | - | - | - | 1:2/CLS | |
| 3 | - | ✓ | - | - | 1:2/SA | |
| 4 | - | - | ✓ | - | 1:2/BMT | |
| 5 | - | - | - | ✓ | 1:2/BMG | |
| 6 | 1:1 | - | - | - | - | 1:1 |
| 7 | ✓ | - | - | - | 1:1/CLS | |
| 8 | - | ✓ | - | - | 1:1/SA | |
| 9 | - | - | ✓ | - | 1:1/BMT | |
| 10 | - | - | - | ✓ | 1:1/BMG | |
| 11 | 2:1 | - | - | - | - | 2:1 |
| 12 | ✓ | - | - | - | 2:1/CLS | |
| 13 | - | ✓ | - | - | 2:1/SA | |
| 14 | - | - | ✓ | - | 2:1/BMT | |
| 15 | - | - | - | ✓ | 2:1/BMG | |
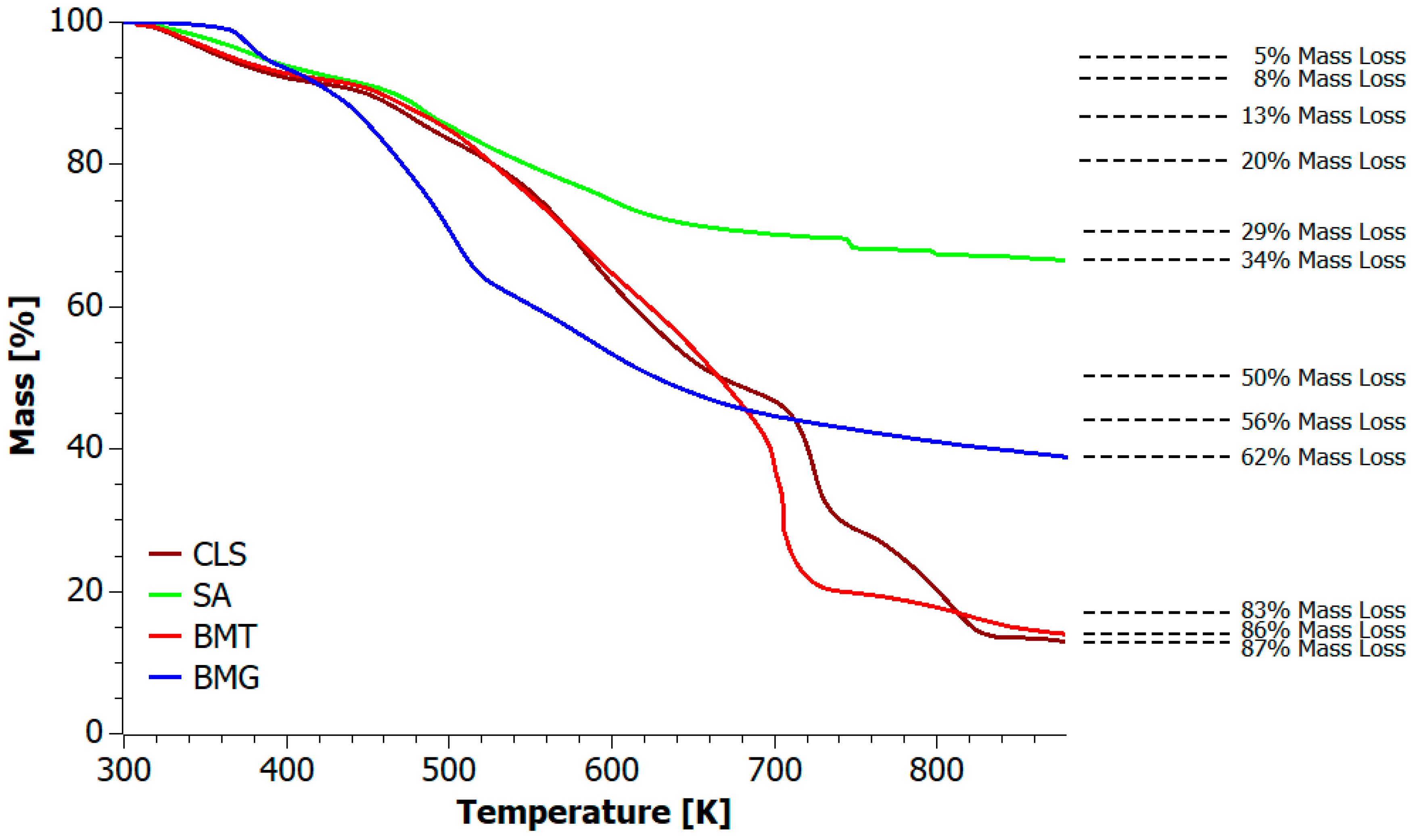
| Sample | T5% [K] | T10% [K] | T20% [K] | T50% [K] | TMAX [K] | Residue at 873 K [%] |
|---|---|---|---|---|---|---|
| CLS | 361 | 449 | 527 | 667 | 723 | 13.10 |
| SA | 384 | 465 | 548 | --- | 746 | 66.60 |
| BMT | 367 | 457 | 527 | 666 | 703 | 14.12 |
| BMG | 386 | 428 | 471 | 628 | 504 | 39.06 |
| Sample | PHRR [W·g−1] | HRRAV [W·g−1] | HOCAV [kJ·g−1] | THR600s [kJ·g−1] | TTI | TOF | Combustion Time [s] | ||
|---|---|---|---|---|---|---|---|---|---|
| [s] | [K] | [s] | [K] | ||||||
| CLS | 23 | 12 | 3.86 | 7.26 | 120 | 448 | 384 | 712 | 319 |
| SA | 35 | 8 | 2.73 | 4.60 | 115 | 423 | 558 | 863 | 443 |
| BMT | 26 | 12 | 3.81 | 6.85 | 154 | 468 | 425 | 742 | 271 |
| BMG | 39 | 11 | 4.13 | 6.31 | 3 | 382 | 411 | 727 | 408 |
| Sample | T5% [K] | T10% [K] | T20% [K] | T50% [K] | TMAX [K] | Residue at 873 K [%] | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OX | IN | OX | IN | OX | IN | OX | IN | OX | IN | OX | IN | |
| 1:2 | 606 | 598 | 620 | 619 | 635 | 638 | 673 | 688 | 701 | 711 | 0.00 | 5.18 |
| 1:2/CLS | 543 | 554 | 554 | 570 | 569 | 591 | 678 | 694 | 705 | 710 | 1.54 | 9.97 |
| 1:2/SA | 602 | 583 | 614 | 595 | 630 | 616 | 675 | 692 | 704 | 711 | 0.51 | 9.99 |
| 1:2/BMT | 573 | 589 | 587 | 608 | 607 | 656 | 671 | 701 | 701 | 710 | 0.95 | 10.51 |
| 1:2/BMG | 575 | 555 | 597 | 573 | 645 | 594 | 696 | 676 | 707 | 699 | 0.04 | 0.00 |
| 1:1 | 596 | 588 | 609 | 611 | 622 | 629 | 648 | 661 | 637 | 657 | 0.00 | 0.00 |
| 1:1/CLS | 555 | 541 | 569 | 558 | 589 | 575 | 676 | 673 | 704 | 707 | 1.51 | 4.89 |
| 1:1/SA | 598 | 512 | 608 | 585 | 620 | 602 | 644 | 636 | 636 | 609 | 0.00 | 0.00 |
| 1:1/BMT | 580 | 587 | 597 | 600 | 613 | 614 | 666 | 664 | 703 | 709 | 0.60 | 6.86 |
| 1:1/BMG | 543 | 527 | 558 | 543 | 575 | 557 | 611 | 581 | 604 | 576 | 1.45 | 0.00 |
| 2:1 | 593 | 571 | 604 | 587 | 617 | 603 | 638 | 642 | 636 | 613 | 0.00 | 0.56 |
| 2:1/CLS | 552 | 536 | 565 | 555 | 580 | 569 | 608 | 591 | 611 | 588 | 1.04 | 0.00 |
| 2:1/SA | 595 | 568 | 607 | 586 | 618 | 603 | 639 | 641 | 634 | 614 | 0.00 | 0.44 |
| 2:1/BMT | 581 | 568 | 597 | 586 | 610 | 603 | 629 | 655 | 632 | 609 | 0.46 | 2.57 |
| 2:1/BMG | 575 | 556 | 595 | 569 | 622 | 583 | 687 | 658 | 704 | 711 | 0.35 | 0.00 |
| Feature | OX Conditions | IN Conditions |
|---|---|---|
| TMAX range | 604–707 K | 576–711 K |
| Best sample up to T20% point | 1:2 | 1:2 |
| Worst sample up to T20% point | 1:2/CLS | 1:1/BMG |
| Best sample above T20% point | 1:2/BMG | 1:2/BMT |
| Worst sample above T20% point | 1:1/BMG | 1:1/BMG |
| Char residue | Very low (0–1.5%) | High (up to 10.5%) |
| Additive improving stability | --- | BMT, SA |
| Additive decreasing stability | CLS, BMG | CLS, BMG |
| Degradation character | Combustion (full degradation) | Pyrolysis, carbonization |
| Sample | PHRRMAX [W·g−1] | PHRRPLA [W·g−1] | PHRRPET [W·g−1] | HRRAV [W·g−1] | HOCAV [kJ·g−1] | THR600s [kJ·g−1] | TTI | TOF | Combustion Time [s] | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| [s] | [K] | [s] | [K] | ||||||||
| 1:2 | 242 | 148 (645 K) | 242 (727 K) | 32.81 | 10.59 | 22.39 | 263 | 574 | 479 | 796 | 216 |
| 1:2/CLS | 272 | 91 (595 K) | 272 (721 K) | 28.87 | 9.44 | 19.74 | 196 | 509 | 478 | 797 | 282 |
| 1:2/SA | 244 | 109 (623 K) | 244 (724 K) | 29.76 | 9.81 | 20.43 | 236 | 559 | 466 | 794 | 230 |
| 1:2/BMT | 182 | 132 (619 K) | 182 (724 K) | 29.38 | 10.07 | 20.11 | 213 | 538 | 468 | 798 | 255 |
| 1:2/BMG | 157 | 157 (608 K) | 135 (722 K) | 28.41 | 9.99 | 19.03 | 202 | 513 | 474 | 792 | 272 |
| 1:1 | 240 | 191 (665 K) | 240 (728 K) | 30.25 | 9.96 | 21.56 | 264 | 568 | 487 | 797 | 223 |
| 1:1/CLS | 185 | 160 (623 K) | 185 (726 K) | 32.22 | 11.02 | 22.00 | 200 | 517 | 477 | 802 | 277 |
| 1:1/SA | 195 | 117 (599 K) | 195 (730 K) | 30.57 | 10.33 | 21.45 | 197 | 503 | 490 | 803 | 293 |
| 1:1/BMT | 201 | 201 (615 K) | 125 (723 K) | 28.76 | 10.51 | 19.91 | 214 | 536 | 474 | 803 | 260 |
| 1:1/BMG | 209 | 209 (603 K) | 99 (723 K) | 29.68 | 11.00 | 19.96 | 174 | 503 | 463 | 798 | 289 |
| 2:1 | 353 | 353 (654 K) | 94 (726 K) | 31.24 | 10.83 | 21.86 | 267 | 573 | 475 | 787 | 208 |
| 2:1/CLS | 340 | 340 (634 K) | 33 (711 K) | 31.02 | 11.61 | 21.43 | 203 | 514 | 456 | 773 | 253 |
| 2:1/SA | 300 | 83 (633 K) | 300 (728 K) | 31.20 | 9.88 | 21.32 | 239 | 564 | 467 | 797 | 228 |
| 2:1/BMT | 295 | 295 (630 K) | 65 (715 K) | 36.80 | 12.93 | 24.34 | 183 | 508 | 445 | 776 | 262 |
| 2:1/BMG | 241 | 241 (607 K) | 23 (702 K) | 26.85 | 10.46 | 18.29 | 139 | 455 | 459 | 776 | 320 |
| Sample | T5% | T95% | FGC [J/gK] | ηc [J/gK] | hc [kJ/g] | ||
|---|---|---|---|---|---|---|---|
| [s] | [K] | [s] | [K] | ||||
| 1:2 | 219 | 529 | 555 | 873 | 167.53 | 269.08 | 23.15 |
| 1:2/CLS | 233 | 546 | 517 | 836 | 151.14 | 302.61 | 20.21 |
| 1:2/SA | 264 | 588 | 396 | 723 | 225.19 | 266.94 | 20.74 |
| 1:2/BMT | 236 | 560 | 486 | 816 | 158.10 | 199.22 | 20.47 |
| 1:2/BMG | 216 | 544 | 525 | 860 | 140.94 | 170.18 | 19.49 |
| 1:1 | 312 | 618 | 442 | 752 | 229.25 | 271.33 | 21.65 |
| 1:1/CLS | 236 | 554 | 541 | 865 | 161.20 | 204.63 | 22.64 |
| 1:1/SA | 240 | 547 | 455 | 767 | 185.92 | 219.76 | 21.72 |
| 1:1/BMT | 244 | 566 | 421 | 748 | 184.40 | 220.93 | 19.99 |
| 1:1/BMG | 210 | 539 | 459 | 793 | 163.65 | 227.43 | 20.24 |
| 2:1 | 290 | 596 | 439 | 750 | 217.86 | 395.58 | 22.12 |
| 2:1/CLS | 246 | 558 | 444 | 760 | 191.33 | 380.82 | 21.75 |
| 2:1/SA | 249 | 574 | 493 | 823 | 165.86 | 330.27 | 21.71 |
| 2:1/BMT | 215 | 540 | 587 | 918 | 172.54 | 323.05 | 25.46 |
| 2:1/BMG | 209 | 521 | 540 | 858 | 140.36 | 268.21 | 18.84 |
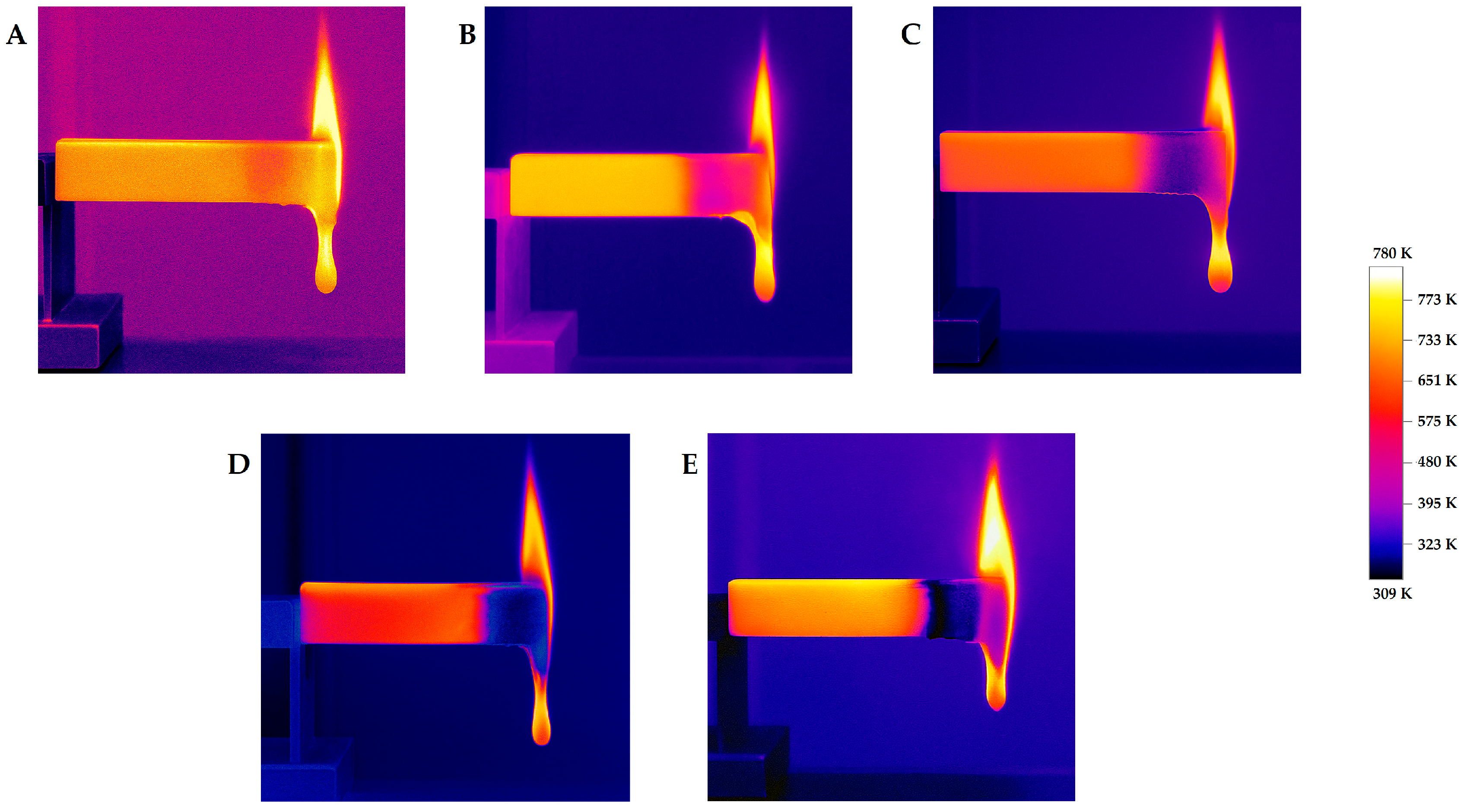
| Sample | Average Combustion Time [s] | Average Combustion Rate [mm/min] | Length of the Burned Section [mm] | Average Number of Drops | |
|---|---|---|---|---|---|
| 1 * | 2 * | ||||
| 1:2 | 92 | 61.1 | 48.9 | 75 | 64 |
| 1:2/CLS | 58 | 97.0 | 77.6 | 38 | |
| 1:2/SA | 78 | 72.1 | 57.7 | 25 | |
| 1:2/BMT | 111 | 50.7 | 40.5 | 19 | |
| 1:2/BMG | 114 | 49.3 | 39.5 | 18 | |
| 1:1 | 98 | 60.2 | 45.9 | 75 | 82 |
| 1:1/CLS | 62 | 95.1 | 72.6 | 49 | |
| 1:1/SA | 86 | 68.5 | 52.3 | 27 | |
| 1:1/BMT | 105 | 56.1 | 42.9 | 20 | |
| 1:1/BMG | 114 | 51.7 | 39.5 | 15 | |
| 2:1 | 105 | 57.0 | 42.9 | 75 | 96 |
| 2:1/CLS | 63 | 95.0 | 71.4 | 54 | |
| 2:1/SA | 90 | 66.5 | 50.0 | 32 | |
| 2:1/BMT | 98 | 61.1 | 45.9 | 25 | |
| 2:1/BMG | 118 | 50.7 | 38.1 | 14 | |
| Sample | Average Combustion Time [s] | Fabric Inflammation | Flame Progressed Up to the Holding Clamp | Average Number of Drops | Type of Standard Class |
|---|---|---|---|---|---|
| 1:2 | 40 | YES | YES | 20 | - |
| 1:2/CLS | 47 | YES | YES | 11 | - |
| 1:2/SA | 57 | YES | NO | 3 | FV-2 |
| 1:2/BMT | 64 | NO | NO | 4 | FV-1 |
| 1:2/BMG | 78 | NO | NO | 3 | FV-1 |
| 1:1 | 36 | YES | YES | 31 | - |
| 1:1/CLS | 45 | YES | YES | 12 | - |
| 1:1/SA | 52 | YES | NO | 5 | FV-2 |
| 1:1/BMT | 55 | NO | NO | 5 | FV-1 |
| 1:1/BMG | 77 | NO | NO | 4 | FV-1 |
| 2:1 | 30 | YES | YES | 37 | - |
| 2:1/CLS | 42 | YES | YES | 12 | - |
| 2:1/SA | 48 | YES | NO | 10 | FV-2 |
| 2:1/BMT | 53 | NO | NO | 6 | FV-1 |
| 2:1/BMG | 68 | NO | NO | 4 | FV-1 |
| Sample | LOI [% Oxygen] | ∆LOI [% Oxygen] |
|---|---|---|
| 1:2 | 22.8 ± 0.1 | --- |
| 1:2/CLS | 23.5 ± 0.1 | 0.7 |
| 1:2/SA | 25.5 ± 0.1 | 2.7 |
| 1:2/BMT | 26.4 ± 0.1 | 3.6 |
| 1:2/BMG | 27.5 ± 0.1 | 4.7 |
| 1:1 | 22.0 ± 0.1 | --- |
| 1:1/CLS | 23.4 ± 0.1 | 1.4 |
| 1:1/SA | 24.1 ± 0.1 | 2.1 |
| 1:1/BMT | 24.8 ± 0.1 | 2.8 |
| 1:1/BMG | 27.1 ± 0.1 | 5.1 |
| 2:1 | 21.0 ± 0.1 | --- |
| 2:1/CLS | 23.1 ± 0.1 | 2.1 |
| 2:1/SA | 24.4 ± 0.1 | 3.4 |
| 2:1/BMT | 24.8 ± 0.1 | 3.8 |
| 2:1/BMG | 24.9 ± 0.1 | 3.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majka, T.M.; Al Nakib, R.; Menceloglu, Y.Z.; Pielichowski, K. The Influence of Lignin Derivatives on the Thermal Properties and Flammability of PLA+PET Blends. Materials 2025, 18, 4181. https://doi.org/10.3390/ma18174181
Majka TM, Al Nakib R, Menceloglu YZ, Pielichowski K. The Influence of Lignin Derivatives on the Thermal Properties and Flammability of PLA+PET Blends. Materials. 2025; 18(17):4181. https://doi.org/10.3390/ma18174181
Chicago/Turabian StyleMajka, Tomasz M., Rana Al Nakib, Yusuf Z. Menceloglu, and Krzysztof Pielichowski. 2025. "The Influence of Lignin Derivatives on the Thermal Properties and Flammability of PLA+PET Blends" Materials 18, no. 17: 4181. https://doi.org/10.3390/ma18174181
APA StyleMajka, T. M., Al Nakib, R., Menceloglu, Y. Z., & Pielichowski, K. (2025). The Influence of Lignin Derivatives on the Thermal Properties and Flammability of PLA+PET Blends. Materials, 18(17), 4181. https://doi.org/10.3390/ma18174181








