Carbonation Treatments for Durable Low-Carbon Recycled Aggregate Concrete
Abstract
1. Introduction
2. Materials
2.1. Cement and Admixtures
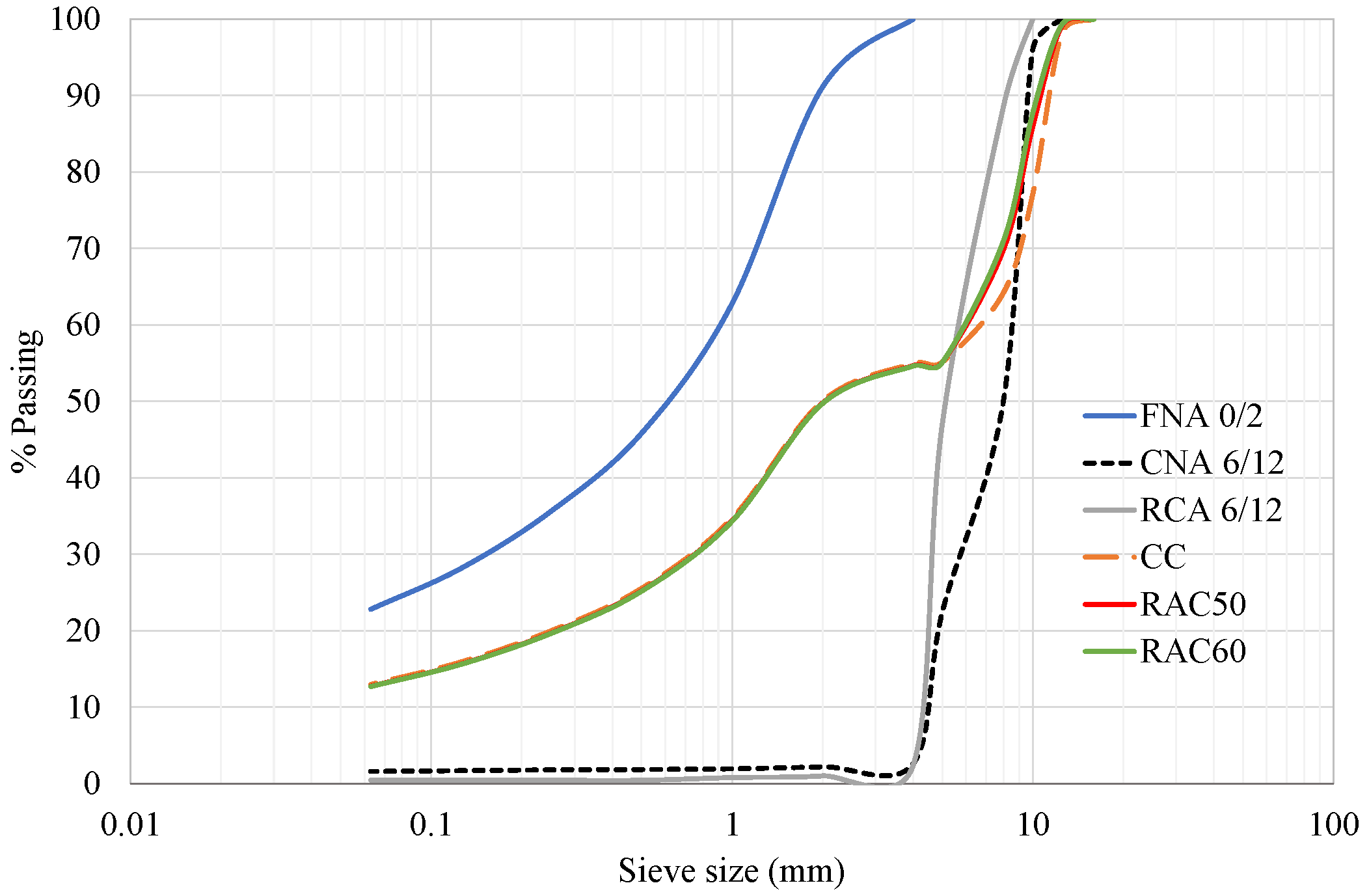
2.2. Natural Aggregates (NAs)
2.3. Recycled Concrete Aggregates (RCAs)
2.4. Carbonated Recycled Concrete Aggregates (cRCAs)
3. Concrete Production and Test Procedure
3.1. Concrete Production
| Mix | Cement (kg) | Total Water (kg) | NFA (kg) | NCA (kg) | RCA (kg) | CRCA (kg) | SP (%) | W/C ef. | Slump Flow (mm) | Viscosity t500 (s) |
|---|---|---|---|---|---|---|---|---|---|---|
| Series 1 | ||||||||||
| CC-I | 340 | 185.1 | 1055 | 891 | 2.5 | 0.49 | 730 | 5 | ||
| RAC50-I | 340 | 208.7 | 1055 | 446 | 389 | 2.5 | 0.49 | 760 | 3 | |
| RAC60-I | 340 | 214.8 | 1055 | 356 | 466 | 2.5 | 0.49 | 750 | 4 | |
| RAC-C50-I | 340 | 205.3 | 1055 | 446 | 394 | 2.5 | 0.49 | 730 | 5 | |
| RAC-C60-I | 340 | 209.9 | 1055 | 356 | 476 | 2.5 | 0.49 | 750 | 4 | |
| Series 2 | ||||||||||
| CC-IIB | 365 | 184.8 | 1046 | 884 | 2.6 | 0.45 | 720 | 6 | ||
| RAC50-IIB | 365 | 206.4 | 1046 | 442 | 385 | 2.6 | 0.45 | 690 | 8 | |
| RAC60-IIB | 365 | 210.0 | 1046 | 354 | 463 | 2.6 | 0.45 | 720 | 8 | |
| RAC-C50-IIB | 365 | 202.9 | 1046 | 442 | 390 | 2.6 | 0.45 | 720 | 6 | |
| RAC-C60-IIB | 365 | 205.9 | 1046 | 354 | 473 | 2.3 | 0.45 | 730 | 9 | |
| EN-12350-8 [48] | 660–750 | ≥2.0 s | ||||||||
3.2. Curing Processes
Optimisation of the CO2 Curing Process: Duration 1 and Duration 2
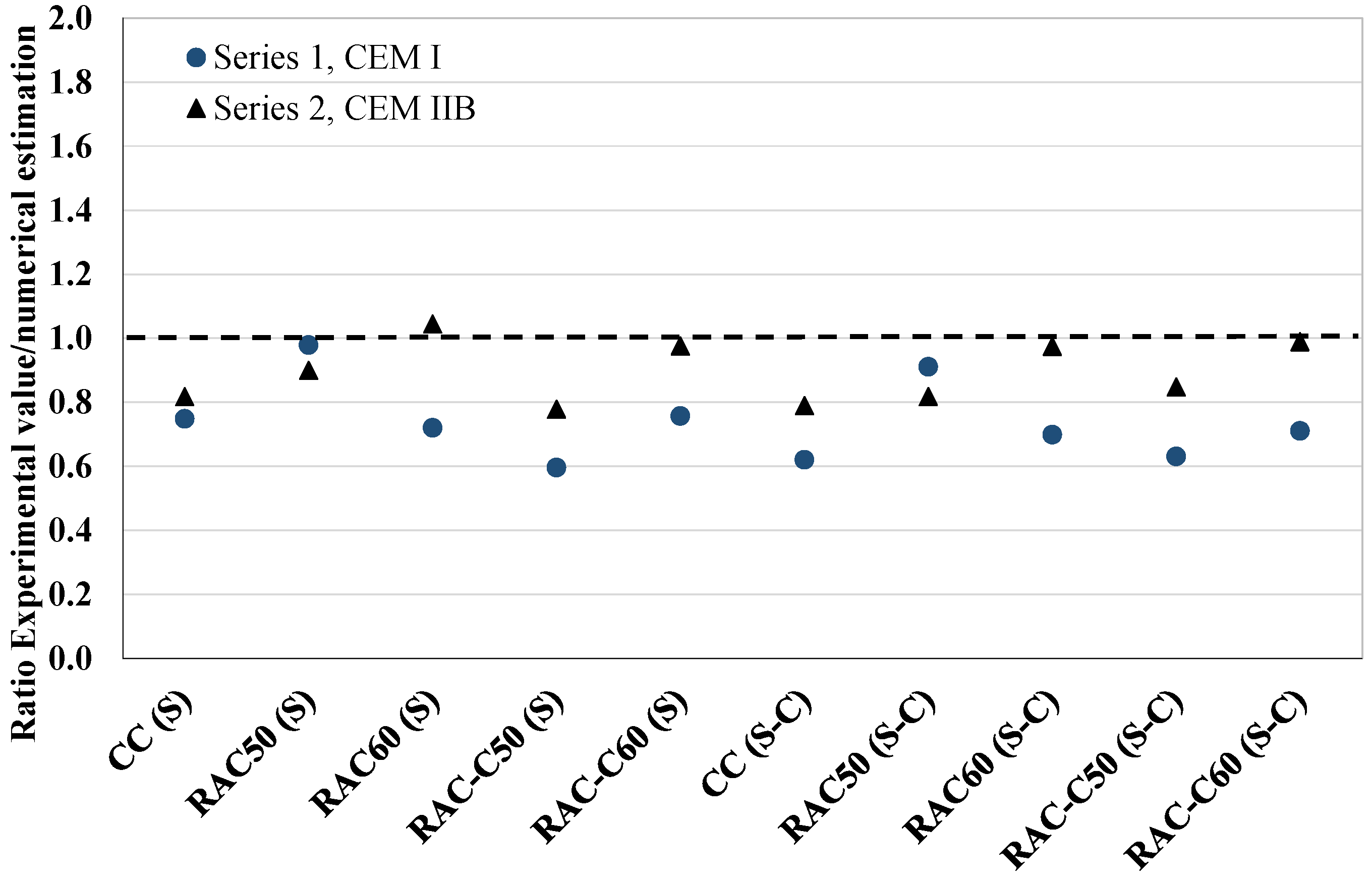
4. Test Procedure
5. Results
5.1. Physical Properties
5.2. Compressive Strength
5.3. Drying Shrinkage
5.4. Durability Properties
5.4.1. Capillary Water Absorption Coefficient—Sorptivity
5.4.2. Carbonation Resistance
6. Conclusions
- Through the accelerated carbonation process, the water absorption of RCA was reduced by 12.5%, decreasing from 6.5% to 5.6%.
- The CO2 curing process, conducted for 24 h at 20% CO2, 57% RH, and 20 °C, improved the surface of concrete specimens, guaranteeing a reduction in sorptivity capacity and a 2 mm carbonation depth.
- Comparable behaviour in the fresh state was achieved in SCC incorporating NA and high percentages (50% and 60%) of RCA and cRCA.
- -
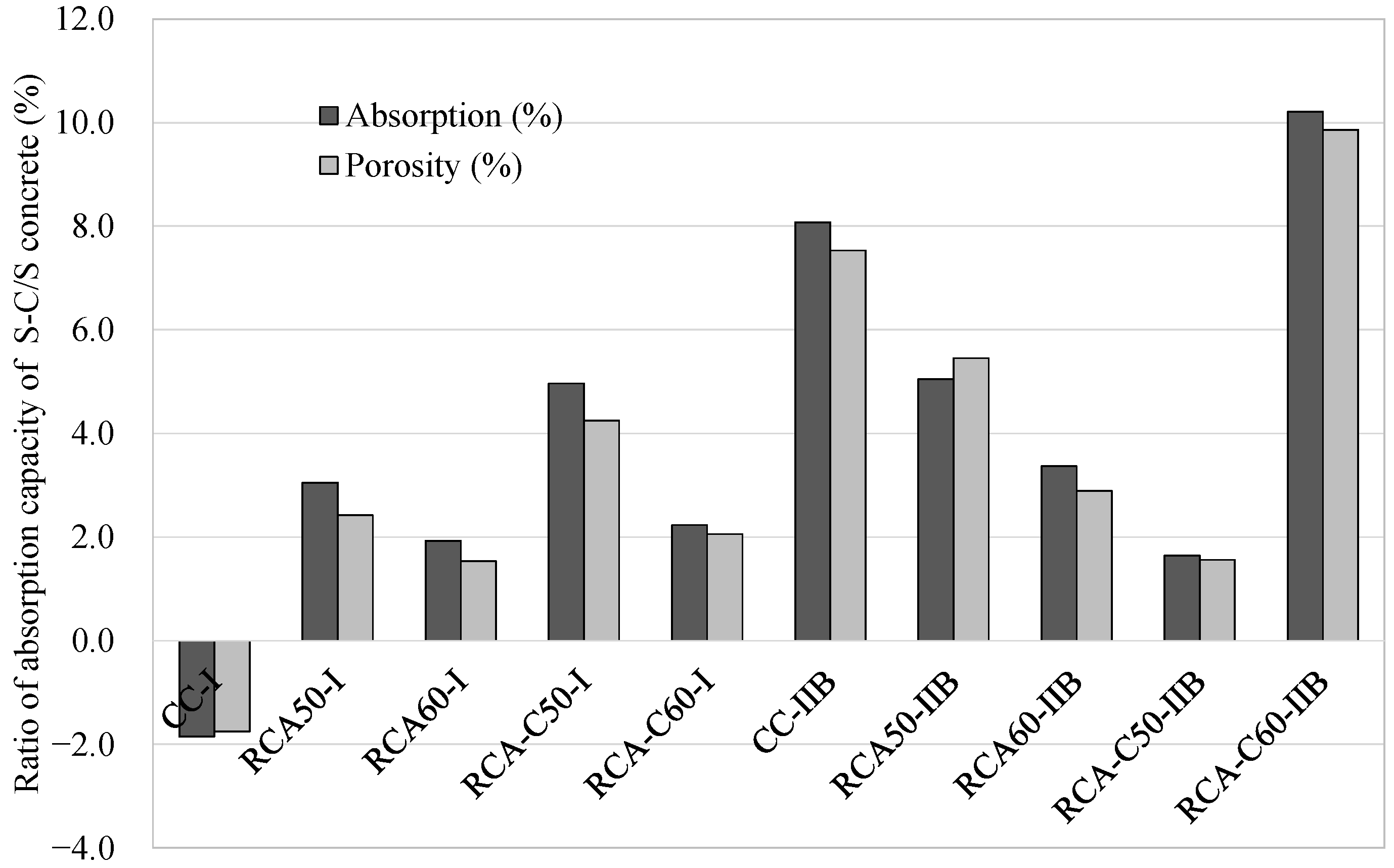
- Regarding physical properties—although CC had lower absorption than RCA and cRCA concretes, CO2 curing slightly improved all RACs, reducing absorption to below 4.5% with CEM I and 4.9% with CEM II.
- -
- Regarding compressive strength—all concretes in Series 1 and 2, including CC and RAC, met the 25 MPa strength requirement after 8 h of steam curing and reached around 60 MPa at 28 days with both the S and S-C processes. RAC-C50-I and RAC-C50-IIB showed the highest strength (97% and 98% of CC strength, respectively). cRCA concretes performing slightly better than those with RCA and S-C process led to a slight strength increase at 7 and 28 days compared to the S-process.
- -
- Regarding drying shrinkage—concretes with cRCA showed lower drying shrinkage than those with uncarbonated RCA, and CO2 curing further reduced shrinkage and weight loss. In Series 1, RAC-C50-I (with CEM I and cRCA) had the lowest shrinkage at 262 με, while in Series 2, despite higher shrinkage with CEM II, RAC50-II and RAC-C50 reached the lowest value of 375 με.
- -
- Regarding durability—RACs made with cRCA showed lower sorptivity than those with uncarbonated RCA, with further reductions from CO2 curing—RAC-C50 and RAC-C60 in Series 1 had the lowest values (0.05 mm/min0.5), while RAC50 in Series 2 reached 0.06 mm/min0.5.
- -
- Both RCA and cRCA improved carbonation resistance compared to CCs, and CO2 curing further enhanced this resistance. RAC-C50-I (Series 1) and RAC50-II (Series 2) achieved the lowest carbonation rates and an estimated 50-year lifespan, with the S-C process boosting their durability.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Belin, P.; Habert, G.; Thiery, M.; Roussel, N. Cement paste content and water absorption of recycled concrete coarse aggregates. Mater. Struct. Constr. 2014, 47, 1451–1465. [Google Scholar] [CrossRef]
- Griffiths, S.; Sovacool, B.K.; Furszyfer Del Rio, D.D.; Foley, A.M.; Bazilian, M.D.; Kim, J.; Uratani, J.M. Decarbonizing the cement and concrete industry: A systematic review of socio-technical systems, technological innovations, and policy options. Renew. Sustain. Energy Rev. 2023, 180, 113291. [Google Scholar] [CrossRef]
- Al-Kheetan, M.J.; Jweihan, Y.S.; Rabi, M.; Ghaffar, S.H. Durability Enhancement of Concrete with Recycled Concrete Aggregate: The Role of Nano-ZnO. Buildings 2024, 14, 353. [Google Scholar] [CrossRef]
- Etxeberria, M. Evaluation of eco-efficient concretes produced with fly ash and uncarbonated recycled aggregates. Materials 2021, 14, 7499. [Google Scholar] [CrossRef]
- Neville, A.M. Properties of Concrete, 5th ed.; Pearson Education Limited: London, UK, 2011. [Google Scholar]
- Liang, C.; Pan, B.; Ma, Z.; He, Z.; Duan, Z. Utilization of CO2 curing to enhance the properties of recycled aggregate and prepared concrete: A review. Cem. Concr. Compos. 2020, 105, 103446. [Google Scholar] [CrossRef]
- Lucy, Y.V.; Priyadharshini, E.; Michel, J.; Zengfeng, T.; Ellina, Z.; Hanein, T.; Chai, T.; Wei, L.; Zhidong, W.; Balci, E.; et al. Carbonated recycled concrete aggregates in construction: Potential and bottlenecks identified by RILEM TC 309-MCP. Mater. Struct. 2025, 6, 58. [Google Scholar] [CrossRef]
- Pu, Y.; Li, L.; Wang, Q.; Shi, X.; Luan, C.; Zhang, G.; Fu, L.; El-Fatah Abomohra, A. Accelerated carbonation technology for enhanced treatment of recycled concrete aggregates: A state-of-the-art review. Constr. Build. Mater. 2021, 282, 122671. [Google Scholar] [CrossRef]
- Tang, Y.; Xiao, J.; Zhang, H.; Wang, D.; Zhang, M.; Zhang, J. Effect of accelerated carbonation of fully recycled aggregates on fracture behaviour of concrete. Cem. Concr. Compos. 2024, 148, 105442. [Google Scholar] [CrossRef]
- Revilla-Cuesta, V.; Skaf, M.; Ortega-López, V.; Manso, J.M. Multi-parametric flowability classification of self-compacting concrete containing sustainable raw materials: An approach to real applications. J. Build. Eng. 2023, 63, 105524. [Google Scholar] [CrossRef]
- Kapoor, K.; Singh, S.P.; Singh, B.; Singh, P. Effect of recycled aggregates on fresh and hardened properties of self compacting concrete. Mater. Today Proc. 2020, 32, 600–607. [Google Scholar] [CrossRef]
- Etxeberria, M.; Vázquez, E.; Marí, A.; Barra, M. Influence of amount of recycled coarse aggregates and production process on properties of recycled aggregate concrete. Cem. Concr. Res. 2007, 37, 735–742. [Google Scholar] [CrossRef]
- Bravo, M.; De Brito, J.; Pontes, J.; Evangelista, L. Mechanical performance of concrete made with aggregates from construction and demolition waste recycling plants. J. Clean. Prod. 2015, 99, 59–74. [Google Scholar] [CrossRef]
- Li, L.; Poon, C.S.; Xiao, J.; Xuan, D. Effect of carbonated recycled coarse aggregate on the dynamic compressive behavior of recycled aggregate concrete. Constr. Build. Mater. 2017, 151, 52–62. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, C.; Li, Y.; Pan, X.; Poon, C.-S.; Xie, Z. Performance Enhancement of Recycled Concrete Aggregates through Carbonation. J. Mater. Civ. Eng. 2015, 27, 04015029. [Google Scholar] [CrossRef]
- Luo, S.; Ye, S.; Xiao, J.; Zheng, J.; Zhu, Y. Carbonated recycled coarse aggregate and uniaxial compressive stress-strain relation of recycled aggregate concrete. Constr. Build. Mater. 2018, 188, 956–965. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, H.; Tang, Y.; Deng, Q.; Wang, D.; Poon, C. sun Fully utilizing carbonated recycled aggregates in concrete: Strength, drying shrinkage and carbon emissions analysis. J. Clean. Prod. 2022, 377, 134520. [Google Scholar] [CrossRef]
- Xuan, D.; Zhan, B.; Poon, C.S. Durability of recycled aggregate concrete prepared with carbonated recycled concrete aggregates. Cem. Concr. Compos. 2017, 84, 214–221. [Google Scholar] [CrossRef]
- Zhang, T.; Cui, J.; Chen, M.; Yang, J.; Yan, Z.; Zhang, M. Durability of concrete containing carbonated recycled aggregates: A comprehensive review. Cem. Concr. Compos. 2025, 156, 105865. [Google Scholar] [CrossRef]
- Revilla-Cuesta, V.; Evangelista, L.; de Brito, J.; Skaf, M.; Manso, J.M. Shrinkage prediction of recycled aggregate structural concrete with alternative binders through partial correction coefficients. Cem. Concr. Compos. 2022, 129, 104506. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Q.; Zhou, Y. Influence of Curing Time on the Drying Shrinkage of Concretes with Different Binders and Water-to-Binder Ratios. Adv. Mater. Sci. Eng. 2017, 2017, 2695435. [Google Scholar] [CrossRef]
- Hooton, R.D.; Stanish, K.; Angel, J.P.; Prusinski, J. The effect of ground granulated blast furnace slag (Slag Cement) on the drying shrinkage of concrete—A critical review of the literature. Am. Concr. Inst. ACI Spec. Publ. 2009, 263, 79–94. [Google Scholar] [CrossRef]
- Vintimilla, C.; Etxeberria, M. Limiting the maximum fine and coarse recycled aggregates-Type A used in structural concrete. Constr. Build. Mater. 2023, 380, 131273. [Google Scholar] [CrossRef]
- Vintimilla, C.; Etxeberria, M. Durable Structural Recycled Concrete for Different Exposure Environments. Materials 2025, 18, 587. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.V.; Neves, R.; De Brito, J.; Dhir, R.K. Carbonation behaviour of recycled aggregate concrete. Cem. Concr. Compos. 2015, 62, 22–32. [Google Scholar] [CrossRef]
- Liang, C.; Lu, N.; Ma, H.; Ma, Z.; Duan, Z. Carbonation behavior of recycled concrete with CO2-curing recycled aggregate under various environments. J. CO2 Util. 2020, 39, 101185. [Google Scholar] [CrossRef]
- Russo, N.; Lollini, F. Effect of carbonated recycled coarse aggregates on the mechanical and durability properties of concrete. J. Build. Eng. 2022, 51, 104290. [Google Scholar] [CrossRef]
- Dundar, B.; Tugluca, M.S.; Ilcan, H.; Sahin, O.; Sahmaran, M. Valorization of low-quality recycled concrete aggregates in cement-based systems through carbonation: Assessment of engineering performance. J. Aust. Ceram. Soc. 2025. [Google Scholar] [CrossRef]
- Al Salaheen, M.; Alaloul, W.S.; Alzubi, K.M.; bahaa Aldin Malkawi, A.; Musarat, M.A. Advancing waste-based construction materials through carbon dioxide curing: A comprehensive review. Results Eng. 2023, 20, 101591. [Google Scholar] [CrossRef]
- Tam, V.W.Y.; Butera, A.; Le, K.N. An investigation of the shrinkage, concrete shrinkage reversibility and permeability of CO2-treated concrete. Constr. Build. Mater. 2023, 365, 130120. [Google Scholar] [CrossRef]
- Zhang, D.; Shao, Y. Effect of early carbonation curing on chloride penetration and weathering carbonation in concrete. Constr. Build. Mater. 2016, 123, 516–526. [Google Scholar] [CrossRef]
- Liu, Z.; Meng, W. Fundamental understanding of carbonation curing and durability of carbonation-cured cement-based composites: A review. J. CO2 Util. 2021, 44, 101428. [Google Scholar] [CrossRef]
- Sharma, D.; Goyal, S. Effect of accelerated carbonation curing on near surface properties of concrete. Eur. J. Environ. Civ. Eng. 2022, 26, 1300–1321. [Google Scholar] [CrossRef]
- Xian, X.; Zhang, D.; Lin, H.; Shao, Y. Ambient pressure carbonation curing of reinforced concrete for CO2 utilization and corrosion resistance. J. CO2 Util. 2022, 56, 101861. [Google Scholar] [CrossRef]
- Zhang, D.; Shao, Y. Surface scaling of CO2-cured concrete exposed to freeze-thaw cycles. J. CO2 Util. 2018, 27, 137–144. [Google Scholar] [CrossRef]
- Guo, B.; Chu, G.; Yu, R.; Wang, Y.; Yu, Q.; Niu, D. Effects of sufficient carbonation on the strength and microstructure of CO2-cured concrete. J. Build. Eng. 2023, 76, 107311. [Google Scholar] [CrossRef]
- Rostami, V.; Shao, Y.; Boyd, A.J. Carbonation Curing versus Steam Curing for Precast Concrete Production. J. Mater. Civ. Eng. 2012, 24, 1221–1229. [Google Scholar] [CrossRef]
- Zhang, D.; Shao, Y. Early age carbonation curing for precast reinforced concretes. Constr. Build. Mater. 2016, 113, 134–143. [Google Scholar] [CrossRef]
- Ahmad, S.; Assaggaf, R.A.; Maslehuddin, M.; Al-Amoudi, O.S.B.; Adekunle, S.K.; Ali, S.I. Effects of carbonation pressure and duration on strength evolution of concrete subjected to accelerated carbonation curing. Constr. Build. Mater. 2017, 136, 565–573. [Google Scholar] [CrossRef]
- EN 1097-6; Tests for Mechanical and Physical Properties of Aggregates. Part 6, Determination of Particle Density and Water Absorption. CEN-CENELEC Management Centre: Brussels, Belgium, 2022; p. 64.
- UNE-EN 933-1; Tests for Geometrical Properties of Aggregates. Part 1: Determination of Particle Size Distribution. Sieving Method. European Standards: Madrid, Spain, 2012.
- UNE-EN 12620:2003+A1; Aggreegates for Concrete. European Standards: Madrid, Spain, 2009.
- Ministerio de la Presidencia and Relaciones con las Cortes y Memoria Democrática. Código Estructural. Boletín Oficial del Estado. 2021. No. 190, pp. 97664–99452. Available online: https://www.boe.es (accessed on 2 July 2025).
- Wang, D.; Noguchi, T.; Nozaki, T.; Higo, Y. Investigation of the carbonation performance of cement-based materials under high temperatures. Constr. Build. Mater. 2021, 272, 121634. [Google Scholar] [CrossRef]
- Xuan, D.; Zhan, B.; Poon, C.S. Development of a new generation of eco-friendly concrete blocks by accelerated mineral carbonation. J. Clean. Prod. 2016, 133, 1235–1241. [Google Scholar] [CrossRef]
- UNE-EN 206:2013+A2; Concrete. Specification, Performance, Production and Conformity. European Standards: Madrid, Spain, 2021.
- SCCEPG. The European Guidelines for Self-Compacting Concrete; European Project Group: BIBM The European Precast Concrete Organisation; CEMBUREAU The European Cement Association; ERMCO The European Ready-mix Concrete Organisation; EFCA The European Federation of Concrete Admixture Associations; EFNARC The European Federation of Specialist Construction Chemicals and Concrete Systems: Brussels, Belgium, 2005. [Google Scholar]
- UNE-EN 12350-8; Testing Fresh Concrete. Part 8: Self-Compacting Concrete Slump-Flow Test. European Standards: Madrid, Spain, 2020.
- Safiuddin, M.D.; Salam, M.A.; Jumaat, M.Z. Effects of recycled concrete aggregate on the fresh properties of self-consolidating concrete. Arch. Civ. Mech. Eng. 2011, 11, 1023–1041. [Google Scholar] [CrossRef]
- UNE-EN 12390-7; Testing Hardened Concrete. Part 7: Density of Hardened Concrete. European Standards: Madrid, Spain, 2020.
- UNE-EN 12390-3; Testing Hardened Concrete. Part 3, Compressive Strength of Test Specimens. European Standards: Madrid, Spain, 2020; p. 20.
- UNE-EN 12390-16; Testing Hardened Concrete. Part 16: Determination of the Shrinkage of Concrete. European Standards: Madrid, Spain, 2020.
- UNE-EN ISO 15148; Hygrothermal Performance of Building Materials and Products. Determination of Water Absorption Coefficient by Partial Immersion. European Standards: Madrid, Spain, 2003; pp. 1–16.
- UNE-EN 12390-12; Testing Hardened Concrete Part 12: Determination of the Carbonation Resistance of Concrete Accelerated Carbonation Method. European Standards: Madrid, Spain, 2020.
- Han, X.; Yan, J.; Huo, Y.; Chen, T. Effect of carbonation curing regime on 3D printed concrete: Compressive strength, CO2 uptake, and characterization. J. Build. Eng. 2024, 98, 111341. [Google Scholar] [CrossRef]
- UNE-EN 14630; Products and Systems for the Protection and Repair of Concrete Structures. Test Methods. Determination of Carbonation Depth in Hardened Concrete by the Phenolphthalein Method. European Standards: Madrid, Spain, 2007; pp. 1–16.
- Cantero, B.; Sáez del Bosque, I.F.; Matías, A.; Sánchez de Rojas, M.I.; Medina, C. Water transport mechanisms in concretes bearing mixed recycled aggregates. Cem. Concr. Compos. 2020, 107, 103486. [Google Scholar] [CrossRef]
- Zeyad, A.M.; Tayeh, B.A.; Adesina, A.; de Azevedo, A.R.G.; Amin, M.; Hadzima-Nyarko, M.; Saad Agwa, I. Review on effect of steam curing on behavior of concrete. Clean. Mater. 2022, 3, 100042. [Google Scholar] [CrossRef]
- Kosmatka, S.H.; Wilson, M.L. Design and Control of Concrete Mixtures—The Guide to Applications, Methods and Materials; Portland Cement Association: Washington, DC, USA, 2011. [Google Scholar]
- von Greve-Dierfeld, S.; Lothenbach, B.; Vollpracht, A.; Wu, B.; Huet, B.; Andrade, C.; Medina, C.; Thiel, C.; Gruyaert, E.; Vanoutrive, H.; et al. Understanding the carbonation of concrete with supplementary cementitious materials: A critical review by RILEM TC 281-CCC. Mater. Struct. 2020, 53, 136. [Google Scholar] [CrossRef]
- Lye, C.-Q.; Dhir, R.K.; Ghataora, G.S. Shrinkage of recycled aggregate concrete. Proc. Inst. Civ. Eng.-Struct. Build. 2016, 169, 867–891. [Google Scholar] [CrossRef]
- Kim, J. Influence of quality of recycled aggregates on the mechanical properties of recycled aggregate concretes: An overview. Constr. Build. Mater. 2022, 328, 127071. [Google Scholar] [CrossRef]
- McDonald, D.; Weiss, J. Report on Factors Affecing Shrinage and Creep of Hardened Concrete; American Concrete Institute: Farmington Hills, MI, USA, 2005; pp. 1–12. [Google Scholar]
- EN 1992-1-1; Eurocode 2 Design of Concrete Structures—Part 1-1: General Rules and Rules for Buildings, Bridges and Civil Engineering Structures. CEN-CENELEC Management Centre: Brussels, Belgium, 2023.
- Poon, C.S.; Kou, S.C. Properties of steam cured recycled aggregate concrete. In Proceedings of the International Conference on Sustainable Waste Management and Recycling: Construction Demolition Waste, London, UK, 14–15 September 2004; Limbachiya, M.C., Roberts, J.J., Eds.; Emerald Publishing Limited: Leeds, UK, 2004. [Google Scholar]
- Aragoncillo, A.M.; Cleary, D.; Thayasivam, U.; Lomboy, G. Water sorptivity prediction model for concrete with all coarse recycled concrete aggregates. Constr. Build. Mater. 2023, 394, 132128. [Google Scholar] [CrossRef]
- Alexander, M.G.; Ballim, Y.; Stanish, K. A framework for use of durability indexes in performance-based design and specifications for reinforced concrete structures. Mater. Struct. Constr. 2008, 41, 921–936. [Google Scholar] [CrossRef]
- Meng, Y.; Ling, T.C.; Mo, K.H.; Tian, W. Enhancement of high temperature performance of cement blocks via CO2 curing. Sci. Total Environ. 2019, 671, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Lei, B.; Zhang, C. On carbonation behavior of recycled aggregate concrete. Sci. China Technol. Sci. 2012, 55, 2609–2616. [Google Scholar] [CrossRef]
- Etxeberria, M.; Castillo, S. How the Carbonation Treatment of Different Types of Recycled Aggregates Affects the Properties of Concrete. Sustainability 2023, 15, 3169. [Google Scholar] [CrossRef]
- Parrot, J.L. A Review of Carbonation in Reinforced Concrete; Cement and Concrete Association: London, UK, 1987. [Google Scholar]
- Van Den Heede, P.; De Belie, N. A service life based global warming potential for high-volume fly ash concrete exposed to carbonation. Constr. Build. Mater. 2014, 55, 183–193. [Google Scholar] [CrossRef]




| Cement | SiO2 | CaO | Fe2O3 | Al2O3 | MgO | SO3 | Na2O | K2O | LOI |
|---|---|---|---|---|---|---|---|---|---|
| I | 20.87 | 61.97 | 3.13 | 3.67 | 1.48 | 3.56 | 0.08 | 0.79 | 3.51 |
| II/B-M | 23.07 | 55.96 | 3.15 | 4.66 | 1.43 | 3.63 | 0.16 | 0.85 | 6.02 |
| Physical Property | FNA | CNA | RCA | CRCA |
|---|---|---|---|---|
| Dry Density (kg/dm3) | 2.69 | 2.66 | 2.32 | 2.37 |
| Absorption (%) | 1.49 | 1.49 | 6.45 | 5.65 |
| Property | Standard | Test Age (Days) | Specimen * | Specimen Size (mm) |
|---|---|---|---|---|
| Density and absorption | EN 12390-7 [50] | 28 | 2 | 100 × 100 × 100 |
| Compressive strength | EN 12390-3 [51] | 8 h, 1, 7 and 28 | 2, 4, 2, 4 | 100 × 100 × 100 |
| Drying Shrinkage | EN 12390-16 [52] | 0–56 | 2 | 75 × 75 × 254 |
| Sorptivity | EN ISO 15148 [53] | 28 | 2 | 100 × 100 × 100 |
| Carbonation resistance | EN 12390-12 [54] | 7, 28, 56 | 2 | 100 × 100 × 200 |
| Mix/Curing Process | Sorptivity (mm/min0.5) | Carbonation Depth (mm) | ||
|---|---|---|---|---|
| (S-1dC) | (S-2dC) | (S-C 1d) | (S-C 2d) | |
| CC-I | 0.0490 | 0.0493 | 2.0 | 3.6 |
| RAC-C50-I | 0.0538 | 0.0456 | 2.0 | 3.8 |
| CC-IIB | 0.0571 | 0.0405 | 2.7 | 3.2 |
| CRCA50-IIB | 0.0659 | 0.0565 | 3.0 | 4.2 |
| Mix/Curing Process | Dry Density (Kg/dm3) | Absorption (%) | Accesible Porosity (%) | Sorptivity (mm/min0.5) | ||||
|---|---|---|---|---|---|---|---|---|
| (S) | (S-C) | (S) | (S-C) | (S) | (S-C) | (S) | (S-C) | |
| Series 1 | ||||||||
| CC-I | 2.41 | 2.41 | 3.54 | 3.61 | 8.55 | 8.70 | 0.087 | 0.055 |
| RAC50-I | 2.34 | 2.35 | 3.99 | 3.87 | 9.33 | 9.10 | 0.094 | 0.066 |
| RAC60-I | 2.32 | 2.33 | 4.26 | 4.18 | 9.90 | 9.75 | 0.093 | 0.060 |
| RAC-C50-I | 2.33 | 2.34 | 4.67 | 4.44 | 10.87 | 10.41 | 0.088 | 0.053 |
| RAC-C60-I | 2.34 | 2.34 | 4.26 | 4.12 | 9.94 | 9.65 | 0.075 | 0.045 |
| Series 2 | ||||||||
| CC-IIB | 2.36 | 2.37 | 4.41 | 4.06 | 10.42 | 9.63 | 0.099 | 0.066 |
| RAC50-IIB | 2.30 | 2.30 | 5.21 | 4.95 | 12.01 | 11.35 | 0.112 | 0.062 |
| RAC60-IIB | 2.31 | 2.32 | 4.38 | 4.23 | 10.13 | 9.83 | 0.114 | 0.081 |
| RAC-C50-IIB | 2.33 | 2.33 | 4.51 | 4.44 | 10.50 | 10.34 | 0.107 | 0.081 |
| RAC-C60-IIB | 2.30 | 2.31 | 5.13 | 4.61 | 11.78 | 10.62 | 0.120 | 0.082 |
| Concrete Type | Compressive Strength (MPa) | |||||
|---|---|---|---|---|---|---|
| 8 h | 1 d | 7 d | 28 d | |||
| S | S | S | S-C | S | S-C | |
| CC-I | 29.8 (0.2) | 37.4 (3.9) | 56.7 (1) | 57.5 (3.2) | 61.3 (2.5) | 65.9 (3.8) |
| RAC50-I | 27.6 (0.7) | 34.2 (1.1) | 51.8 (1.7) | 52.7 (2.2) | 58.6 (2.2) | 59.9 (1.4) |
| RAC60-I | 28.4 (2.3) | 34.1 (1.4) | 49.9 (0.5) | 53.2 (0.1) | 57.8 (1.5) | 60.0 (1.5) |
| RAC-C50-I | 30.9 (0.2) | 37.4 (1.2) | 52.2 (0.5) | 55.4 (1.5) | 62.9 (0.6) | 64.1 (1.0) |
| RAC-C60-I | 28.0 (0.5) | 35.1 (0.5) | 50.3 (2.1) | 52.1 (0.5) | 59.7 (1.5) | 60.4 (1.8) |
| CC-IIB | 28.1 (2.2) | 35.5 (1.6) | 52.0 (0.3) | 53.5 (0.5) | 61.7 (1.9) | 62.5 (3.9) |
| RAC50-IIB | 27.8 (0.3) | 34.7 (0.3) | 48.3 (0.1) | 50.1 (0.3) | 58.8 (1.9) | 59.7 (2.4) |
| RAC60-IIB | 30.5 (1.7) | 33.8 (2.8) | 51.6 (0.7) | 52.4 (0.2) | 59.1 (1.0) | 59.8 (0.6) |
| RAC-C50-IIB | 28.5 (1.2) | 34.3 (1.8) | 51.2 (0.8) | 54.2 (0.6) | 60.2 (0.8) | 61.6 (0.6) |
| RAC-C60-IIB | 26.2 (0.1) | 32.8 (0.7) | 50.2 (0.4) | 50.9 (0.8) | 58.7 (0.9) | 61.2 (1.3) |
| Mix/Curing Process | Shrinkage (με) 56d | Mass Loss (%) | |||
|---|---|---|---|---|---|
| (S) | (S-C) | Variation (%) | (S) | (S-C) | |
| Series 1 | |||||
| CC-I | −335 | −264 | 21 | 2.4% | 2.1% |
| RAC50-I | −451 | −413 | 8 | 3.1% | 2.8% |
| RAC60-I | −335 | −317 | 5 | 3.3% | 2.7% |
| RAC-C50-I | −262 | −274 | −5 | 2.8% | 2.5% |
| RAC-C60-I | −344 | −321 | 7 | 3.3% | 2.9% |
| Series 2 | |||||
| CC-IIB | −364 | −348 | 4 | 2.5% | 2.0% |
| RAC50-IIB | −413 | −372 | 10 | 3.0% | 2.3% |
| RAC60-IIB | −478 | −443 | 7 | 3.0% | 2.9% |
| RAC-C50-IIB | −352 | −378 | −7 | 3.1% | 2.9% |
| RAC-C60-IIB | −449 | −443 | 1 | 3.3% | 2.9% |
| Concrete Reference | Series 1 Cem I | Series 2 Cem IIB | ||||||
|---|---|---|---|---|---|---|---|---|
| Carbonation Depth (mm) at 70 Days | kacc (mm/dia0.5) | Carbonation Depth (mm) at 70 Days | kacc (mm/dia0.5) | |||||
| (S) | (S-C) | (S) | (S-C) | (S) | (S-C) | (S) | (S-C) | |
| CC | 14.61 | 13.48 | 1.64 | 1.34 | 13.79 | 13.02 | 1.57 | 1.31 |
| RAC50 | 13.41 | 12.33 | 1.54 | 1.20 | 13.08 | 11.57 | 1.44 | 1.14 |
| RAC60 | 12.84 | 12.81 | 1.48 | 1.25 | 12.95 | 12.52 | 1.46 | 1.27 |
| RAC-C50 | 11.73 | 10.97 | 1.28 | 1.07 | 13.84 | 13.03 | 1.56 | 1.37 |
| RAC-C60 | 12.80 | 11.20 | 1.42 | 1.12 | 14.25 | 13.23 | 1.58 | 1.28 |
| Concrete Reference | Serie 1 Cem I | Serie 2 Cem IIB | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonation Coefficient. | Carbonation Depth (mm) | Carb. Coeffic. | Carbonation Depth (mm) | |||||||||
| KnatTHEO (mm/Year0.5) | 50 Years | 100 Years | KnatTHEO (mm/Year0.5) | 50 Years | 100 Years | |||||||
| (S) | (S-C) | (S) | (S-C) | (S) | (S-C) | (S) | (S-C) | (S) | (S-C) | (S) | (S-C) | |
| CC | 3.8 | 3.1 | 26.4 | 21.7 | 37.3 | 30.7 | 3.6 | 3.0 | 25.3 | 21.1 | 35.8 | 29.8 |
| RAC50 | 3.5 | 2.7 | 24.9 | 19.4 | 35.2 | 27.4 | 3.3 | 2.6 | 23.3 | 18.4 | 32.9 | 26.0 |
| RAC60 | 3.4 | 2.9 | 23.9 | 20.2 | 33.8 | 28.5 | 3.4 | 2.9 | 23.7 | 20.6 | 33.5 | 29.0 |
| RAC-C50 | 2.9 | 2.5 | 20.1 | 17.3 | 29.3 | 24.5 | 3.5 | 3.1 | 25.2 | 22.2 | 35.6 | 31.4 |
| RAC-C60 | 3.2 | 2.6 | 23.0 | 18.4 | 32.5 | 26.1 | 3.6 | 2.9 | 25.6 | 20.6 | 36.1 | 29.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saavedra, R.; Etxeberria, M. Carbonation Treatments for Durable Low-Carbon Recycled Aggregate Concrete. Materials 2025, 18, 4168. https://doi.org/10.3390/ma18174168
Saavedra R, Etxeberria M. Carbonation Treatments for Durable Low-Carbon Recycled Aggregate Concrete. Materials. 2025; 18(17):4168. https://doi.org/10.3390/ma18174168
Chicago/Turabian StyleSaavedra, Ruth, and Miren Etxeberria. 2025. "Carbonation Treatments for Durable Low-Carbon Recycled Aggregate Concrete" Materials 18, no. 17: 4168. https://doi.org/10.3390/ma18174168
APA StyleSaavedra, R., & Etxeberria, M. (2025). Carbonation Treatments for Durable Low-Carbon Recycled Aggregate Concrete. Materials, 18(17), 4168. https://doi.org/10.3390/ma18174168







