Cold-Plasma Method in Counteracting Prosthetic Stomatitis: Analysis of the Influence of Cold Plasma on Prosthetic Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Acrylic Resin
2.1.2. Acetal Resin
2.1.3. Metal Alloy
2.2. Methods
2.2.1. Cold Atmospheric Plasma Treatment
2.2.2. FT-IR
2.2.3. SEM-EDS
2.2.4. Surface Roughness
2.2.5. Contact Angle
2.2.6. Color Measurements
2.2.7. The 12 h Adhesion of Single-Species Candida albicans and Candida glabrata to Dental Materials
3. Results and Discussion
3.1. Influence of CAP on Physico-Chemical Properties of Prosthetic Materials
3.1.1. Changes in the Chemical Composition of the CAP-Treated Surfaces of the Prosthetic Materials
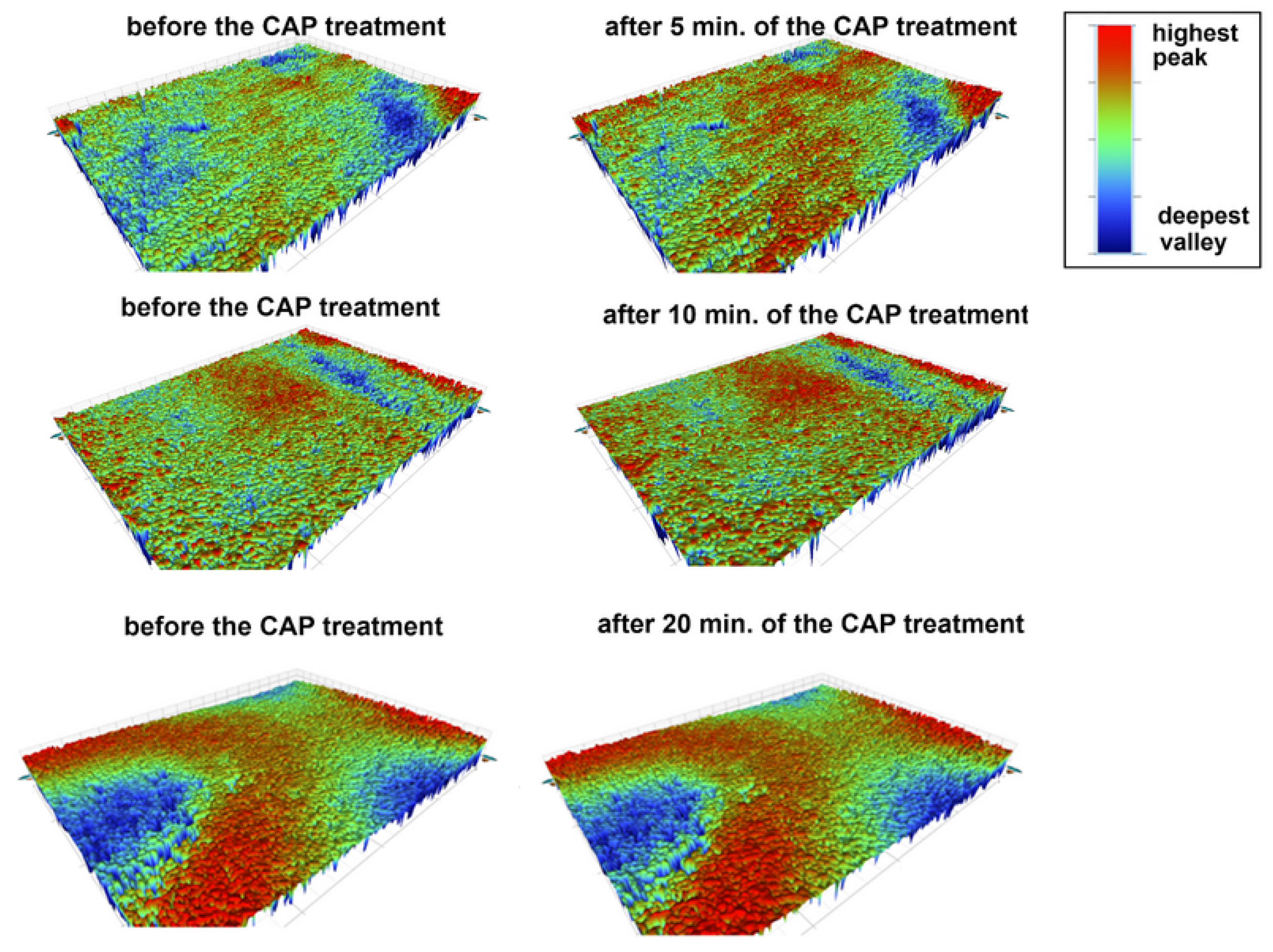
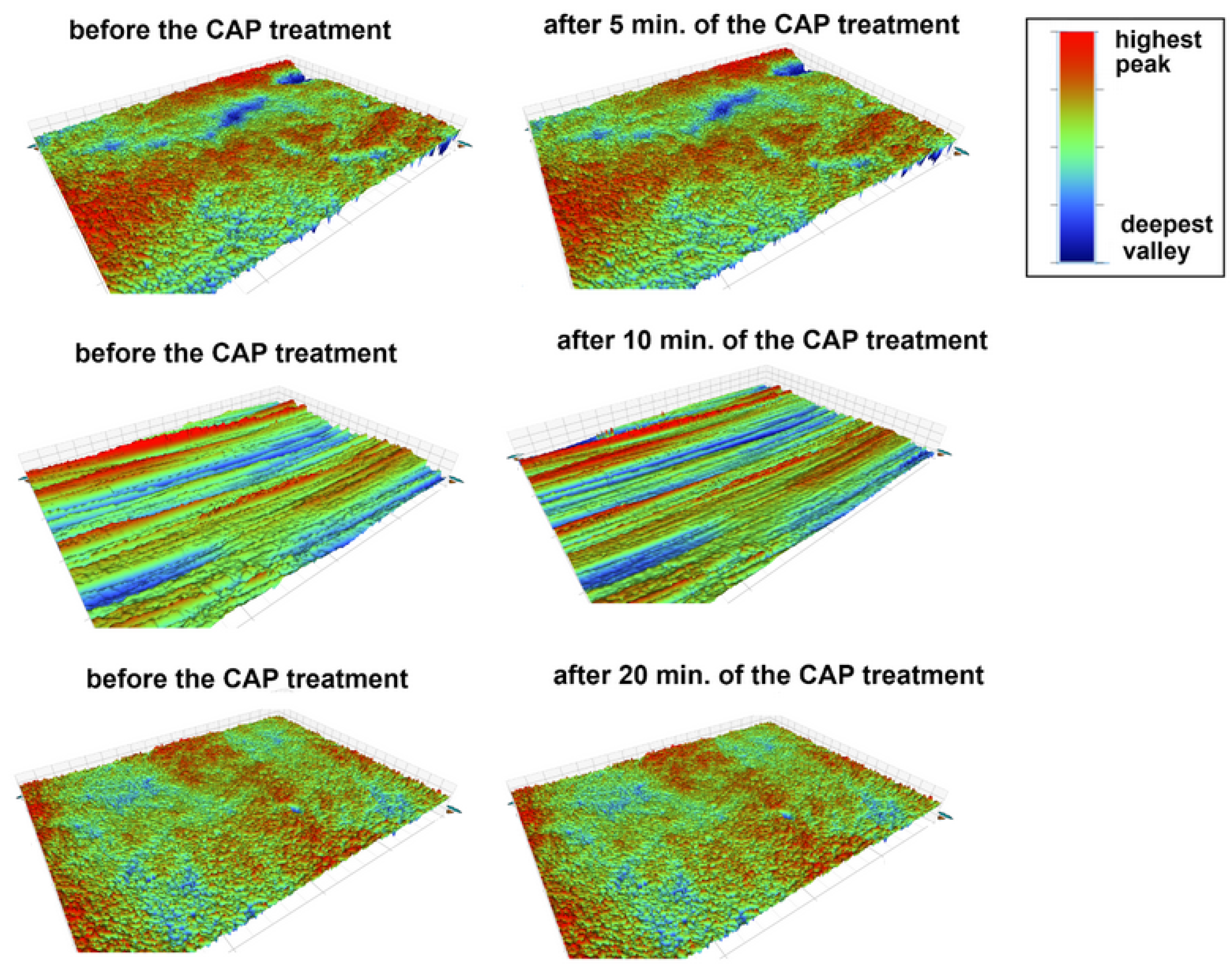
3.1.2. Changes in the Morphology of the CAP-Treated Surfaces of the Prosthetic Materials
3.1.3. Changes in the Wetting Properties of the CAP-Treated Surfaces of the Prosthetic Materials
3.1.4. Changes in the Color of the CAP-Treated Surfaces of the Prosthetic Materials
3.2. Influence of CAP on the 12 h Fungal Adhesion to the Prosthetic Materials
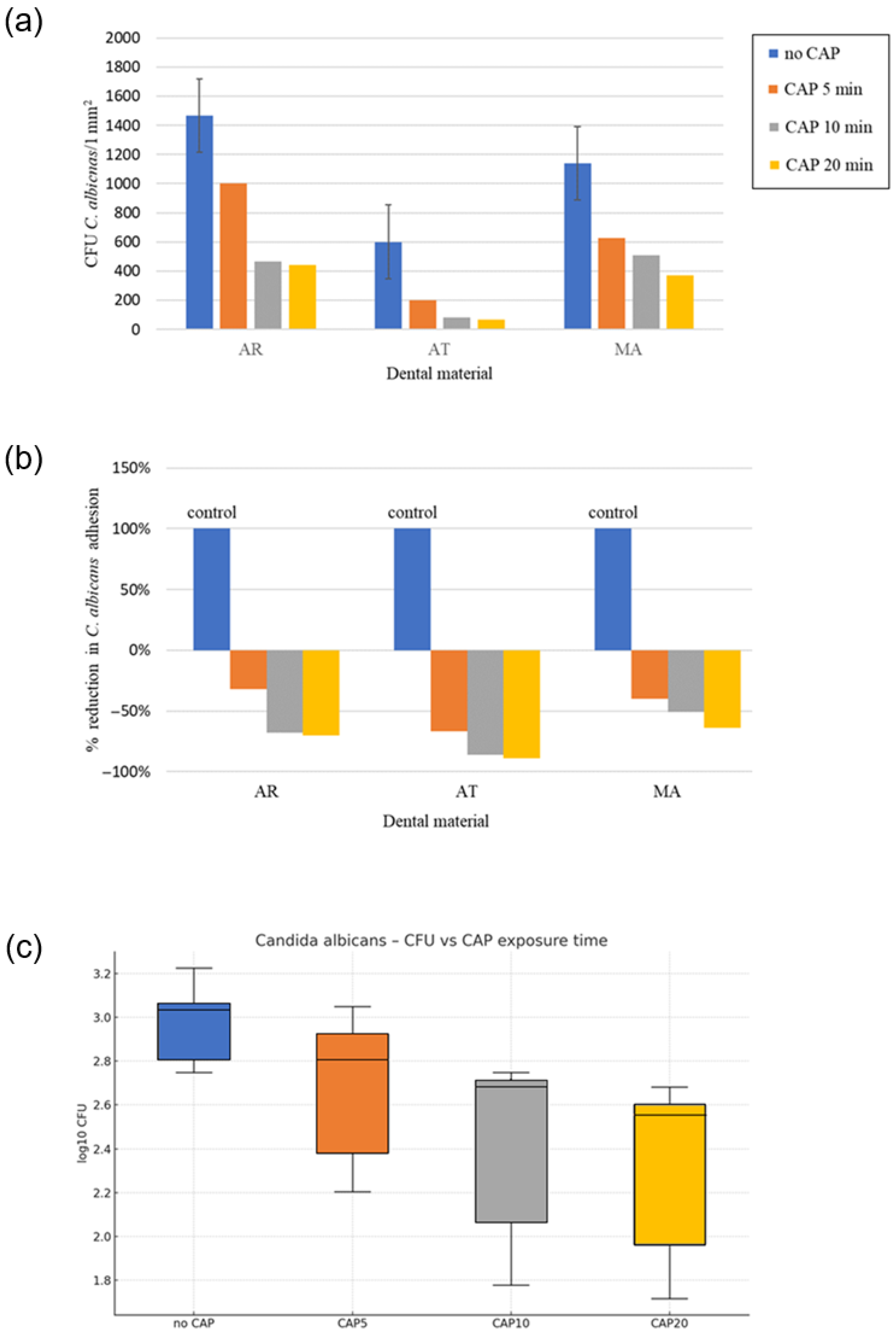
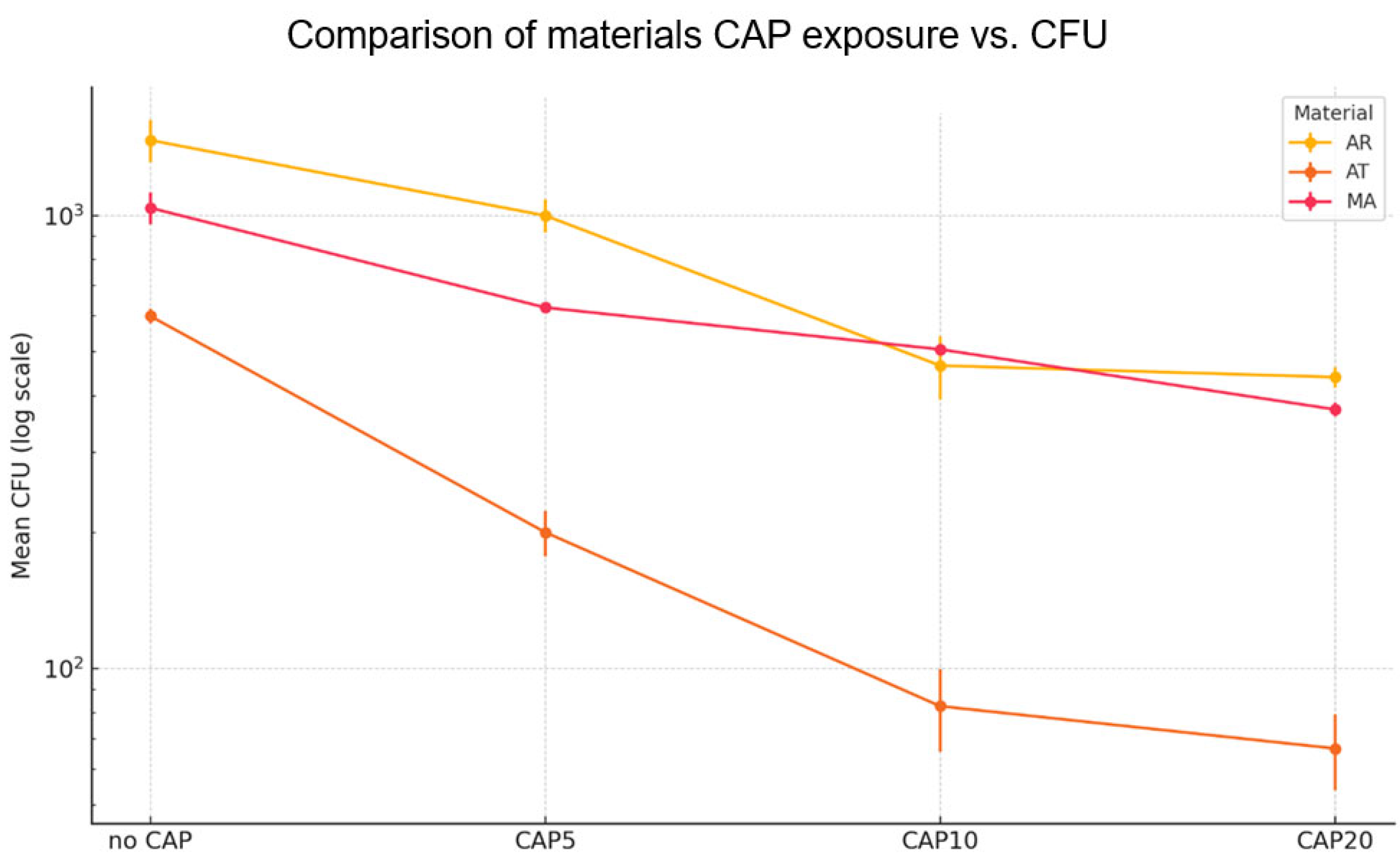
3.2.1. Effect of CAP on 12 h Adhesion of Reference C. albicans to the Prosthetic Materials
3.2.2. Effect of CAP on 12 h Adhesion of Reference C. glabrata to the Prosthetic Materials
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, P.; Krämer-Fernandez, P.; Klink, A.; Xu, Y.; Spintzyk, S. Repairability of a 3D Printed Denture Base Polymer: Effects of Surface Treatment and Artificial Aging on the Shear Bond Strength. J. Mech. Behav. Biomed. Mater. 2021, 114, 104227. [Google Scholar] [CrossRef]
- Nejatian, T.; Pezeshki, S.; Yaqin Syed, A.U. 5—Acrylic Denture Base Materials. In Advanced Dental Biomaterials; Khurshid, Z., Najeeb, S., Zafar, M.S., Sefat, F., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 79–104. ISBN 978-0-08-102476-8. [Google Scholar]
- Reynolds, I.R. A Review of Direct Orthodontic Bonding. Br. J. Orthod. 1975, 2, 171–178. [Google Scholar] [CrossRef]
- Tardelli, J.D.C.; dos Reis, A.C. Biomechanical, Esthetic, and Hygienic Considerations of Materials for Overdenture Bars: A Systematic Review. Dent. Rev. 2024, 4, 100082. [Google Scholar] [CrossRef]
- Thomas, S.; Nandini, V. Acetal Resin—A Quantum Leap in Aesthetic Restorative Dentistry: A Review. Int. J. Clin. Dent. Sci. 2011, 2, 56–59. [Google Scholar]
- Campo, E.A. 1—Polymeric Materials and Properties. In Selection of Polymeric Materials; Campo, E.A., Ed.; Plastics Design Library; William Andrew Publishing: Norwich, UK, 2008; pp. 1–39. ISBN 978-0-8155-1551-7. [Google Scholar]
- Bogucki, Z.A.; Kownacka, M. Elastic Dental Prostheses—Alternative Solutions for Patients Using Acrylic Prostheses: Literature Review. Adv. Clin. Exp. Med. 2018, 27, 1441–1445. [Google Scholar] [CrossRef] [PubMed]
- Sobolewska, E.; Fraczak, B.; Czarnomysy-Furowicz, D.; Ey-Chmielewska, H.; Karakulska, J. Bacteria Adhesion to the Surface of Various Prosthetics Materials. Ann. Acad. Med. Stetin. 2007, 53, 68–71. [Google Scholar]
- Timbó, I.C.G.; Oliveira, M.S.C.S.; Regis, R.R. Effect of Sanitizing Solutions on Cobalt Chromium Alloys for Dental Prostheses: A Systematic Review of in Vitro Studies. J. Prosthet. Dent. 2024, 132, 704–713. [Google Scholar] [CrossRef]
- Al Jabbari, Y.S. Physico-Mechanical Properties and Prosthodontic Applications of Co-Cr Dental Alloys: A Review of the Literature. J. Adv. Prosthodont. 2014, 6, 138–145. [Google Scholar] [CrossRef]
- Bates, J.F.; Knapton, A.G. Metals and Alloys in Dentistry. Int. Met. Rev. 1977, 22, 39–60. [Google Scholar] [CrossRef]
- Wataha, J.C. Alloys for Prosthodontic Restorations. J. Prosthet. Dent. 2002, 87, 351–363. [Google Scholar] [CrossRef]
- Vaicelyte, A.; Janssen, C.; Le Borgne, M.; Grosgogeat, B. Cobalt–Chromium Dental Alloys: Metal Exposures, Toxicological Risks, CMR Classification, and EU Regulatory Framework. Crystals 2020, 10, 1151. [Google Scholar] [CrossRef]
- Skupien, J.A.; Valentini, F.; Boscato, N.; Pereira-Cenci, T. Prevention and Treatment of Candida Colonization on Denture Liners: A Systematic Review. J. Prosthet. Dent. 2013, 110, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Qian, K.; Pan, H.; Li, Y.; Wang, G.; Zhang, J.; Pan, J. Time-Related Surface Modification of Denture Base Acrylic Resin Treated by Atmospheric Pressure Cold Plasma. Dent. Mater. J. 2016, 35, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Laroussi, M. Plasma Medicine: A Brief Introduction. Plasma 2018, 1, 47–60. [Google Scholar] [CrossRef]
- Braný, D.; Dvorská, D.; Halašová, E.; Škovierová, H. Cold Atmospheric Plasma: A Powerful Tool for Modern Medicine. Int. J. Mol. Sci. 2020, 21, 2932. [Google Scholar] [CrossRef]
- Izadjoo, M.; Zack, S.; Kim, H.; Skiba, J. Medical Applications of Cold Atmospheric Plasma: State of the Science. J. Wound Care 2018, 27, S4–S10. [Google Scholar] [CrossRef]
- Stornelli, G.; Petrucci, G.; Caponio, V.C.A.; Sardella, E.; Balice, G.; Femminella, B.; Paolantonio, M.; Cela, I.; Acharya, T.R.; Jha, N.; et al. Translational application of cold atmospheric plasma in periodontology and implantology: Where are we? A systematic review of in vivo studies in human and animal models. J. Evid.-Based Dent. Pract. 2025, 25, 102096. [Google Scholar] [CrossRef]
- Sanesi, L.; Puca, V.; Caponio, V.C.A.; Pinti, M.; Balice, G.; Femminella, B.; Paolantonio, M.; Cela, I.; Kaushik, N.K.; Choi, E.H.; et al. Disinfection of Dental Root Canals by Cold Atmospheric Plasma: A Systematic Review and Meta-Analysis of Dental Biofilm. Front. Oral. Health 2024, 5, 1483078. [Google Scholar] [CrossRef]
- Davis, C.C.; Dias Panariello, F.; Panariello, B. Exploring the Efficacy of Low-Temperature Plasmas on Oral Biofilms: A Scoping Review. Med. Sci. 2025, 13, 79. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, M.-A.; Han, G.-J.; Cho, B.-H. Plasma in Dentistry: A Review of Basic Concepts and Applications in Dentistry. Acta Odontol. Scand. 2014, 72, 1–12. [Google Scholar] [CrossRef]
- Kolb, J.F.; Mohamed, A.-A.H.; Price, R.O.; Swanson, R.J.; Bowman, A.; Chiavarini, R.L.; Stacey, M.; Schoenbach, K.H. Cold Atmospheric Pressure Air Plasma Jet for Medical Applications. Appl. Phys. Lett. 2008, 92, 241501. [Google Scholar] [CrossRef]
- Kang, S.-H.; Lee, H.-J.; Hong, S.-H.; Kim, K.-H.; Kwon, T.-Y. Influence of Surface Characteristics on the Adhesion of Candida Albicans to Various Denture Lining Materials. Acta Odontol. Scand. 2013, 71, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Wang, G.; Pan, J.; Ye, G.; Sun, K.; Zhang, J.; Wang, J. Cold Plasma-Induced Surface Modification of Heat-Polymerized Acrylic Resin and Prevention of Early Adherence of Candida Albicans. Dent. Mater. J. 2015, 34, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Qanber, L.M.; Hamad, T.I. Effect of Plasma Treatment on the Bond of Soft Denture Liner to Conventional and High Impact Acrylic Denture Materials. J. Baghdad Coll. Dent. 2021, 33, 9–17. [Google Scholar] [CrossRef]
- Wang, G.M.; Sun, P.P.; Pan, H.; Ye, G.P.; Sun, K.; Zhang, J.; Pan, J.; Fang, J. Inactivation of Candida Albicans Biofilms on Polymethyl Methacrylate and Enhancement of the Drug Susceptibility by Cold Ar/O2 Plasma Jet. Plasma Chem. Plasma Process 2016, 36, 383–396. [Google Scholar] [CrossRef]
- Delben, J.A.; Zago, C.E.; Tyhovych, N.; Duarte, S.; Vergani, C.E. Effect of Atmospheric-Pressure Cold Plasma on Pathogenic Oral Biofilms and In Vitro Reconstituted Oral Epithelium. PLoS ONE 2016, 11, e0155427. [Google Scholar] [CrossRef]
- Matthes, R.; Jablonowski, L.; Koban, I.; Quade, A.; Hübner, N.-O.; Schlueter, R.; Weltmann, K.-D.; von Woedtke, T.; Kramer, A.; Kocher, T. In Vitro Treatment of Candida Albicans Biofilms on Denture Base Material with Volume Dielectric Barrier Discharge Plasma (VDBD) Compared with Common Chemical Antiseptics. Clin. Oral. Investig. 2015, 19, 2319–2326. [Google Scholar] [CrossRef]
- Kamionka, J.; Matthes, R.; Holtfreter, B.; Pink, C.; Schlüter, R.; von Woedtke, T.; Kocher, T.; Jablonowski, L. Efficiency of Cold Atmospheric Plasma, Cleaning Powders and Their Combination for Biofilm Removal on Two Different Titanium Implant Surfaces. Clin. Oral. Investig. 2022, 26, 3179–3187. [Google Scholar] [CrossRef]
- Jungbauer, G.; Moser, D.; Müller, S.; Pfister, W.; Sculean, A.; Eick, S. The Antimicrobial Effect of Cold Atmospheric Plasma against Dental Pathogens—A Systematic Review of In-Vitro Studies. Antibiotics 2021, 10, 211. [Google Scholar] [CrossRef]
- Darvell, B.W. Chapter 5—Acrylic. In Materials Science for Dentistry, 10th ed.; Darvell, B.W., Ed.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Cambridge, UK, 2018; pp. 121–142. ISBN 978-0-08-101035-8. [Google Scholar]
- Silva, N.D.S.; De Melo, B.S.T.; Oliva, A.; de Araújo, P.S.R. Sonication Protocols and Their Contributions to the Microbiological Diagnosis of Implant-Associated Infections: A Review of the Current Scenario. Front. Cell. Infect. Microbiol. 2024, 14, 1398461. [Google Scholar] [CrossRef]
- Al-Ali, A.; Kassab-Bashi, T. Fourier Transform Infra Red (FTIR) Spectroscopy of New Copolymers of Acrylic Resin Denture Base Materials. IJERSTE 2015, 4, 172–180. [Google Scholar]
- Torrisi, L.; Roszkowska, A.M.; Silipigni, L.; Cutroneo, M.; Torrisi, A. Effects of 365 Nm UV Lamp Irradiation of Polymethylmethacrylate (PMMA). Radiat. Eff. Defects Solids 2024, 179, 264–274. [Google Scholar] [CrossRef]
- Bakr, A.M.; Darwish, A.; Azab, A.A.; El Awady, M.E.; Hamed, A.A.; Elzwawy, A. Structural, Dielectric, and Antimicrobial Evaluation of PMMA/CeO2 for Optoelectronic Devices. Sci. Rep. 2024, 14, 2548. [Google Scholar] [CrossRef] [PubMed]
- Huszank, R.; Szilágyi, E.; Szoboszlai, Z.; Szikszai, Z. Investigation of Chemical Changes in PMMA Induced by 1.6 MeV He+ Irradiation by Ion Beam Analytical Methods (RBS-ERDA) and Infrared Spectroscopy (ATR-FTIR). Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2019, 450, 364–368. [Google Scholar] [CrossRef]
- Lim, K.; Hayat, M.D.; Jena, K.D.; Zhang, W.; Cao, P. On Amine Treated Polyoxymethylene (POM) Blends with Low Formaldehyde Emission for Metal Injection Moulding (MIM). J. Mater. Sci. 2022, 57, 15160–15170. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, S.; Cheng, H.; Peng, R.; Luo, Z. Thermally Triggered Vanishing Bulk Polyoxymethylene for Transient Electronics. Sci. Rep. 2019, 9, 18107. [Google Scholar] [CrossRef]
- Pang, Y.-J.; Xu, W.-S.; Yang, B.-T.; Ni, H.-Y.; Chen, J. Influence of Early Thermal-Oxidative Ageing on the Structure and Properties of Polyoxymethylene Copolymer. R. Soc. Open Sci. 2021, 8, 210034. [Google Scholar] [CrossRef]
- Kwiatkowski, M.; Terebun, P.; Mazurek, P.; Pawlat, J. Wettability of Polymeric Materials after Dielectric Barrier Discharge Atmospheric-Pressure Plasma Jet Treatment. Sens. Mater. 2018, 30, 1207–1212. [Google Scholar] [CrossRef]
- Kwiatkowski, M.; Pawłat, J.; Starek-Wójcicka, A.; Krajewska, M.; Terebun, P.; Zarzeczny, D.; Machoy, M.; Mazur-Lesz, A.; Matsuyama, N.; Murakami, T.; et al. Impact of DBD Plasma Jet Treatment on the Enamel Surface of Primary Teeth. Materials 2024, 17, 5173. [Google Scholar] [CrossRef]
- Correia, D.M.; Nunes-Pereira, J.; Alikin, D.; Kholkin, A.L.; Carabineiro, S.A.C.; Rebouta, L.; Rodrigues, M.S.; Vaz, F.; Costa, C.M.; Lanceros-Méndez, S. Surface Wettability Modification of Poly(Vinylidene Fluoride) and Copolymer Films and Membranes by Plasma Treatment. Polymer 2019, 169, 138–147. [Google Scholar] [CrossRef]
- Vukušić, T.; Vesel, A.; Holc, M.; Ščetar, M.; Jambrak, A.R.; Mozetič, M. Modification of Physico-Chemical Properties of Acryl-Coated Polypropylene Foils for Food Packaging by Reactive Particles from Oxygen Plasma. Materials 2018, 11, 372. [Google Scholar] [CrossRef]
- Guschl, P.C.; Hicks, R.F.; MacDavid, S. Atmospheric Oxygen-Helium Plasma Surface Modification of Medical Plastics. In Proceedings of the 2008 IEEE 35th International Conference on Plasma Science, Karlsruhe, Germany, 15–19 June 2008; p. 1. [Google Scholar]
- Terpilowski, K.; Rymuszka, D.; Hołysz, L.; Ilnicki, M. Surface Properties of Metal Alloys Used in Aviation after Plasma Treatment. Surf. Interface Anal. 2017, 49, 647–653. [Google Scholar] [CrossRef]
- Wu, C.C.; Wu, C.I.; Sturm, J.C.; Kahn, A. Surface Modification of Indium Tin Oxide by Plasma Treatment: An Effective Method to Improve the Efficiency, Brightness, and Reliability of Organic Light Emitting Devices. Appl. Phys. Lett. 1997, 70, 1348–1350. [Google Scholar] [CrossRef]
- Onderwaater, W.G.; Taranovskyy, A.; van Baarle, G.C.; Frenken, J.W.M.; Groot, I.M.N. In Situ Optical Reflectance Difference Observations of CO Oxidation over Pd(100). J. Phys. Chem. C 2017, 121, 11407–11415. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Blawert, C.; Serdechnova, M.; Karlova, P.; Dovzhenko, G.; Florian Wieland, D.C.; Stojadinovic, S.; Vasilic, R.; Mojsilovic, K.; Zheludkevich, M.L. Formation of Plasma Electrolytic Oxidation Coatings on Pure Niobium in Different Electrolytes. Appl. Surf. Sci. 2022, 573, 151629. [Google Scholar] [CrossRef]
- Fernández-López, P.; Alves, S.A.; San-Jose, J.T.; Gutierrez-Berasategui, E.; Bayón, R. Plasma Electrolytic Oxidation (PEO) as a Promising Technology for the Development of High-Performance Coatings on Cast Al-Si Alloys: A Review. Coatings 2024, 14, 217. [Google Scholar] [CrossRef]
- Lau, Y.T.; Chin, O.H.; Lee, H.C.; Chiu, W.S.; Woo, H.J. Plasma Surface Treatment of Polystyrene in a Low Power Low Frequency Argon Glow Discharge. Appl. Surf. Sci. 2022, 578, 151963. [Google Scholar] [CrossRef]
- Gizer, S.G.; Bhethanabotla, V.R.; Ayyala, R.S.; Sahiner, N. Low-Pressure Plasma Treated Polycarbonate and Polymethyl Methacrylate (PMMA) Sheets with Different Surface Patterns to Change Their Surface Properties. Surf. Interfaces 2023, 37, 102646. [Google Scholar] [CrossRef]
- Basyigit, Z.O. Enhancing the Coloration of Polypropylene Surfaces through Low-Frequency Plasma Modification with Disperse Dyes. Int. J. Adhes. Adhes. 2024, 135, 103837. [Google Scholar] [CrossRef]
- Duangkanya, K.; Kopwitthaya, A.; Chanhorm, S.; Infahsaeng, Y. Oxygen Plasma Treatment Time Induced Hydrophilicity of Polydimethylsiloxane (PDMS) Thin Films for Liquid Lenses Application. Mater. Today Proc. 2022, 65, 2442–2445. [Google Scholar] [CrossRef]
- Bhatt, P.; Kumar, V.; Subramaniyan, V.; Nagarajan, K.; Sekar, M.; Chinni, S.V.; Ramachawolran, G. Plasma Modification Techniques for Natural Polymer-Based Drug Delivery Systems. Pharmaceutics 2023, 15, 2066. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y.; Zou, T.; Fu, J.; Liu, Z. Increasing Strength and Fracture Toughness of Carbon Fibre-Reinforced Plastic Adhesively Bonded Joints by Combining Peel-Ply and Oxygen Plasma Treatments. Appl. Surf. Sci. 2023, 612, 155768. [Google Scholar] [CrossRef]
- Eslami, H.; Fakhrzadeh, V.; Nazari, E.; Katebi, K.; Alinejad, M. Cold Plasma Treatment for Candida Biofilm on Resin Base Dentures: A Systematic Review. J. Lasers Med. Sci. 2025, 16, e10. [Google Scholar] [PubMed]
- Zamperini, C.A.; Machado, A.L.; Vergani, C.E.; Pavarina, A.C.; Giampaolo, E.T.; da Cruz, N.C. Adherence in Vitro of Candida Albicans to Plasma Treated Acrylic Resin. Effect of Plasma Parameters, Surface Roughness and Salivary Pellicle. Arch. Oral. Biol. 2010, 55, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Trebulová, K.; Krčma, F.; Skoumalová, P.; Kozáková, Z.; Machala, Z. Effects of Different Cold Atmospheric-Pressure Plasma Sources on the Yeast Candida Glabrata. Plasma Process. Polym. 2023, 20, e2300048. [Google Scholar] [CrossRef]
- Chew, S.Y.; Ho, K.L.; Cheah, Y.K.; Sandai, D.; Brown, A.J.P.; Than, L.T.L. Physiologically Relevant Alternative Carbon Sources Modulate Biofilm Formation, Cell Wall Architecture, and the Stress and Antifungal Resistance of Candida Glabrata. Int. J. Mol. Sci. 2019, 20, 3172. [Google Scholar] [CrossRef]
- Gad, M.M.; Fouda, S.M. Current Perspectives and the Future of Candida Albicans-Associated Denture Stomatitis Treatment. Dent. Med. Probl. 2020, 57, 95–102. [Google Scholar] [CrossRef]
- ISO 25178-2:2021; Geometrical Product Specifications (GPS)—Surface Texture: Areal—Part 2: Terms, Definitions and Surface Texture Parameters. ISO: Geneva, Switzerland, 2021. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:25178:-2:ed-2:v1:en (accessed on 31 August 2025).








| AR-C [wt.%] | AR-5 [wt.%] | AR-10 [wt.%] | AR-20 [wt.%] | |
|---|---|---|---|---|
| C | 65.17 ± 0.78 | 64.93 ± 0.78 | 65.82 ± 0.82 | 66.56 ± 0.91 |
| O | 34.83 ± 0.77 | 35.07 ± 0.73 | 34.18 ± 0.85 | 33.44 ± 0.81 |
| AT-C [wt.%] | AT-5 [wt.%] | AT-10 [wt.%] | AT-20 [wt.%] | |
| C | 46.95 ± 0.46 | 47.47 ± 0.49 | 47.74 ± 0.86 | 46.59 ± 0.53 |
| O | 53.05 ± 0.53 | 52.53 ± 0.41 | 52.26 ± 0.78 | 53.41 ± 0.67 |
| MA-C [wt.%] | MA-5 [wt.%] | MA-10 [wt.%] | MA-20 [wt.%] | |
| Co | 49.69 ± 0.72 | 49.78 ± 0.61 | 50.14 ± 0.62 | 50.07 ± 0.63 |
| Cr | 24.47 ± 0.39 | 24.53 ± 0.42 | 24.27 ± 0.35 | 24.82 ± 0.48 |
| O | 9.72 ± 0.76 | 9.96 ± 0.45 | 9.77 ± 0.52 | 9.69 ± 0.64 |
| C | 6.55 ± 0.65 | 5.54 ± 0.45 | 5.55 ± 0.44 | 5.29 ± 0.31 |
| Mo | 4.06 ± 0.08 | 4.06 ± 0.01 | 4.46 ± 0.47 | 4.07 ± 0.04 |
| Si | 3.14 ± 0.09 | 3.22 ± 0.06 | 3.53 ± 0.07 | 3.18 ± 0.03 |
| Al | 1.73 ± 0.27 | 2.21 ± 0.34 | 1.54 ± 0.12 | 1.89 ± 0.13 |
| Fe | 0.64 ± 0.04 | 0.70 ± 0.01 | 0.74 ± 0.05 | 0.69 ± 0.06 |
| AR-5 | before CAP | after 5 min of CAP |
|---|---|---|
| Ra | 1.06 | 1.08 |
| Rq | 1.39 | 1.42 |
| Rt | 22.62 | 40.41 |
| Rv | −15.23 | −33.48 |
| AR-10 | before CAP | after 10 min of CAP |
| Ra | 0.71 | 0.73 |
| Rq | 0.92 | 0.96 |
| Rt | 26.27 | 43.03 |
| Rv | −20.84 | −37.85 |
| AR-20 | before CAP | after 20 min of CAP |
| Ra | 1.76 | 1.82 |
| Rq | 2.23 | 2.31 |
| Rt | 23.46 | 32.47 |
| Rv | −15.85 | −34.36 |
| AT-5 | before CAP | after 5 min of CAP |
| Ra | 5.69 | 5.63 |
| Rq | 7.18 | 7.12 |
| Rt | 57.31 | 56.36 |
| Rv | −30.13 | −29.34 |
| AT-10 | before CAP | after 10 min of CAP |
| Ra | 4.23 | 4.17 |
| Rq | 5.36 | 5.98 |
| Rt | 47.93 | 47.52 |
| Rv | −18.72 | −20.57 |
| AT-20 | before CAP | after 20 min of CAP |
| Ra | 5.32 | 5.27 |
| Rq | 7.43 | 7.49 |
| Rt | 77.99 | 76.17 |
| Rv | −32.75 | −31.32 |
| MA-5 | before CAP | after 5 min of CAP |
| Ra | 0.53 | 0.42 |
| Rq | 0.69 | 0.54 |
| Rt | 9.18 | 8.38 |
| Rv | −5.88 | −5.15 |
| MA-10 | before CAP | after 10 min of CAP |
| Ra | 1.75 | 1.82 |
| Rq | 2.18 | 2.28 |
| Rt | 19.87 | 20.03 |
| Rv | −7.51 | −8.74 |
| MA-20 | before CAP | after 20 min of CAP |
| Ra | 0.42 | 0.41 |
| Rq | 0.55 | 0.54 |
| Rt | 8.86 | 8.83 |
| Rv | −5.10 | −5.63 |
| Color Coordinates | AR-C | AR-5 | AR-10 | AR-20 |
|---|---|---|---|---|
| Brightness (L) | 36.86 ± 0.29 a | 38.19 ± 0.35 b | 39.26 ± 0.50 c | 37.85 ± 0.10 b |
| Chromatic component a | 8.25 ± 0.07 b | 8.38 ± 0.18 b | 6.68 ± 0.32 a | 8.48 ± 0.27 b |
| Chromatic component b | 0.59 ± 0.09 b | 0.93 ± 0.13 c | −2.16 ± 0.08 a | 0.56 ± 0.08 b |
| AT-C | AT-5 | AT-10 | AT-20 | |
| Brightness (L) | 59.66 ± 0.49 b | 59.00 ± 0.20 ab | 58.91 ± 0.28 ab | 58.66 ± 0.03 a |
| Chromatic component a | 0.97 ± 0.03 b | 0.96 ± 0.06 b | 0.86 ± 0.03 b | 0.64 ± 0.14 a |
| Chromatic component b | 5.69 ± 0.11 b | 6.04 ± 0.19 c | 4.86 ± 0.37 ab | 4.59 ± 0.61 a |
| MA-C | MA-5 | MA-10 | MA-20 | |
| Brightness (L) | 53.09 ± 0.80 a | 53.69 ± 1.08 a | 52.47 ± 1.02 a | 65.17 ± 0.89 b |
| Chromatic component a | 0.66 ± 0.12 b | 0.79 ± 0.21 b | 0.95 ± 0.09 b | 0.18 ± 0.01 a |
| Chromatic component b | −5.45 ± 1.42 ab | −4.81 ± 0.81 ab | −3.86 ± 0.07 b | −6.83 ± 0.06 a |
| Mean ± Sem CFU and %-Reduction vs. Control | ||||
|---|---|---|---|---|
| Material | Treatment | Mean CFU | Sem CFU | % Reduction |
| AR | no CAP | 1467 | 157.2 | 0 |
| AR | CAP5 | 1000 | 83.3 | 32 |
| AR | CAP10 | 467 | 74.2 | 68 |
| AR | CAP20 | 440 | 23.1 | 70 |
| AT | no CAP | 600 | 23.1 | 0 |
| AT | CAP5 | 200 | 23.1 | 67 |
| AT | CAP10 | 83 | 17 | 86 |
| AT | CAP20 | 67 | 12.7 | 89 |
| MA | no CAP | 1040 | 83.3 | 0 |
| MA | CAP5 | 627 | 13.3 | 40 |
| MA | CAP10 | 507 | 13.3 | 51 |
| MA | CAP20 | 373 | 13.3 | 64 |
| Mean ± Sem CFU and %-Reduction vs. Control–C. glabrata | ||||
|---|---|---|---|---|
| Material | Treatment | Mean CFU | Sem | % Reduction |
| AR | no CAP | 600 | — | 0 |
| AR | CAP5 | 440 | — | 27 |
| AR | CAP10 | 120 | — | 80 |
| AR | CAP20 | 40 | — | 93 |
| AT | no CAP | 360 | — | 0 |
| AT | CAP5 | 44 | — | 88 |
| AT | CAP10 | 12 | — | 97 |
| AT | CAP20 | 8 | — | 98 |
| MA | no CAP | 480 | — | 0 |
| MA | CAP5 | 280 | — | 42 |
| MA | CAP10 | 120 | — | 75 |
| MA | CAP20 | 36 | — | 93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazur-Lesz, A.; Pawłat, J.; Terebun, P.; Zarzeczny, D.; Grządka, E.; Starek-Wójcicka, A.; Kwiatkowski, M.; Malinowska, I.; Mnichowska-Polanowska, M.; Machoy, M. Cold-Plasma Method in Counteracting Prosthetic Stomatitis: Analysis of the Influence of Cold Plasma on Prosthetic Materials. Materials 2025, 18, 4162. https://doi.org/10.3390/ma18174162
Mazur-Lesz A, Pawłat J, Terebun P, Zarzeczny D, Grządka E, Starek-Wójcicka A, Kwiatkowski M, Malinowska I, Mnichowska-Polanowska M, Machoy M. Cold-Plasma Method in Counteracting Prosthetic Stomatitis: Analysis of the Influence of Cold Plasma on Prosthetic Materials. Materials. 2025; 18(17):4162. https://doi.org/10.3390/ma18174162
Chicago/Turabian StyleMazur-Lesz, Agnieszka, Joanna Pawłat, Piotr Terebun, Dawid Zarzeczny, Elżbieta Grządka, Agnieszka Starek-Wójcicka, Michał Kwiatkowski, Irena Malinowska, Magdalena Mnichowska-Polanowska, and Monika Machoy. 2025. "Cold-Plasma Method in Counteracting Prosthetic Stomatitis: Analysis of the Influence of Cold Plasma on Prosthetic Materials" Materials 18, no. 17: 4162. https://doi.org/10.3390/ma18174162
APA StyleMazur-Lesz, A., Pawłat, J., Terebun, P., Zarzeczny, D., Grządka, E., Starek-Wójcicka, A., Kwiatkowski, M., Malinowska, I., Mnichowska-Polanowska, M., & Machoy, M. (2025). Cold-Plasma Method in Counteracting Prosthetic Stomatitis: Analysis of the Influence of Cold Plasma on Prosthetic Materials. Materials, 18(17), 4162. https://doi.org/10.3390/ma18174162









