Assessing the Performance and Lifetime of Cellulose Nitrate Lacquer on Silver
Abstract
1. Introduction
- The optical properties of the lacquer, as the aesthetics of many silver objects are highly valued.
- The protective properties of the lacquer—as lacquers age, these decay and at a certain point the tarnishing rate of the silver below the lacquer will become unacceptable.
- The reversibility of the aged lacquer. The relative greenness of solvents has been considered [5,6] and is another important consideration. Fourteen parameters to consider for conservation have been defined by the GoGreen project [7]. This process was collaboratively developed with several hundred conservation professionals. Frigilene can initially be removed with acetone [8], but as it ages, more polar solvent mixes are required. At a certain point, steam cleaning is needed to fully remove the lacquer. Full removal is essential for cleaning and relacquering. Steam cleaning has some risks to certain types of silver objects [8]. Initial work on reversibility has been reported [9].
2. Materials and Methods
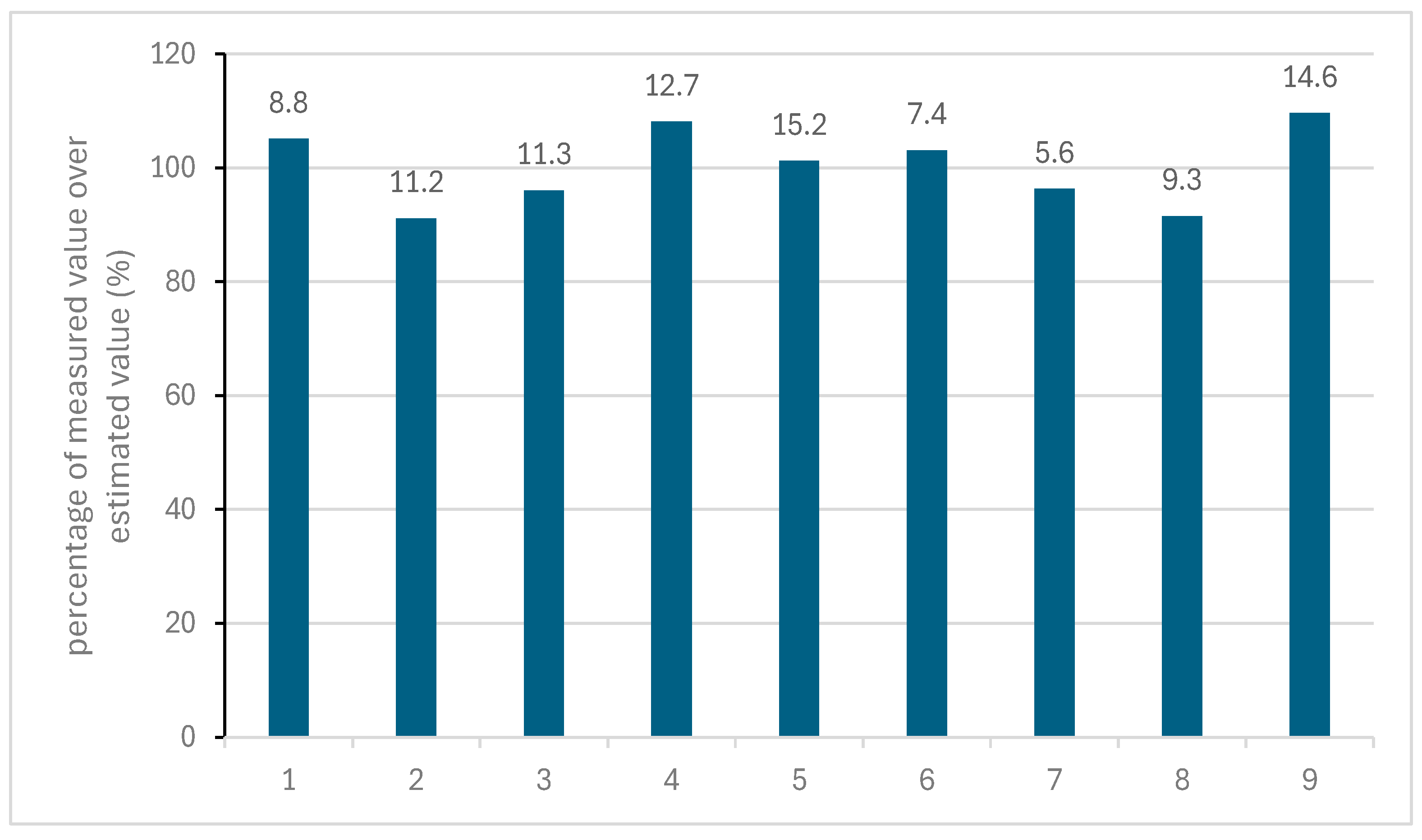
2.1. Thermal Aging and Modelling
2.2. Room Temperature Aging
2.3. Visual Perception
2.4. Protective Effect
2.5. Nitrogen Oxides Concentration
3. Results and Discussion
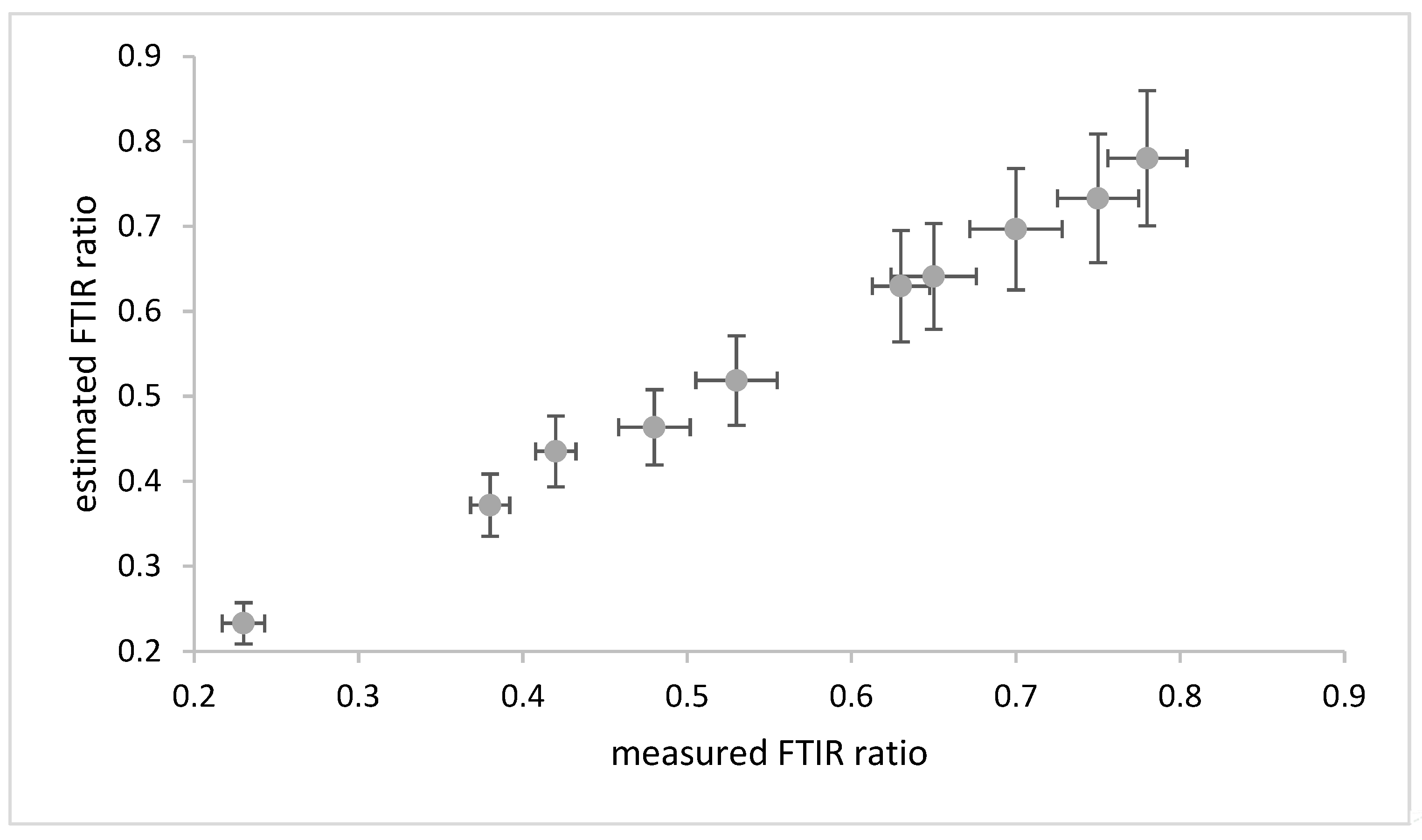
3.1. Thermal Aging and Modelling
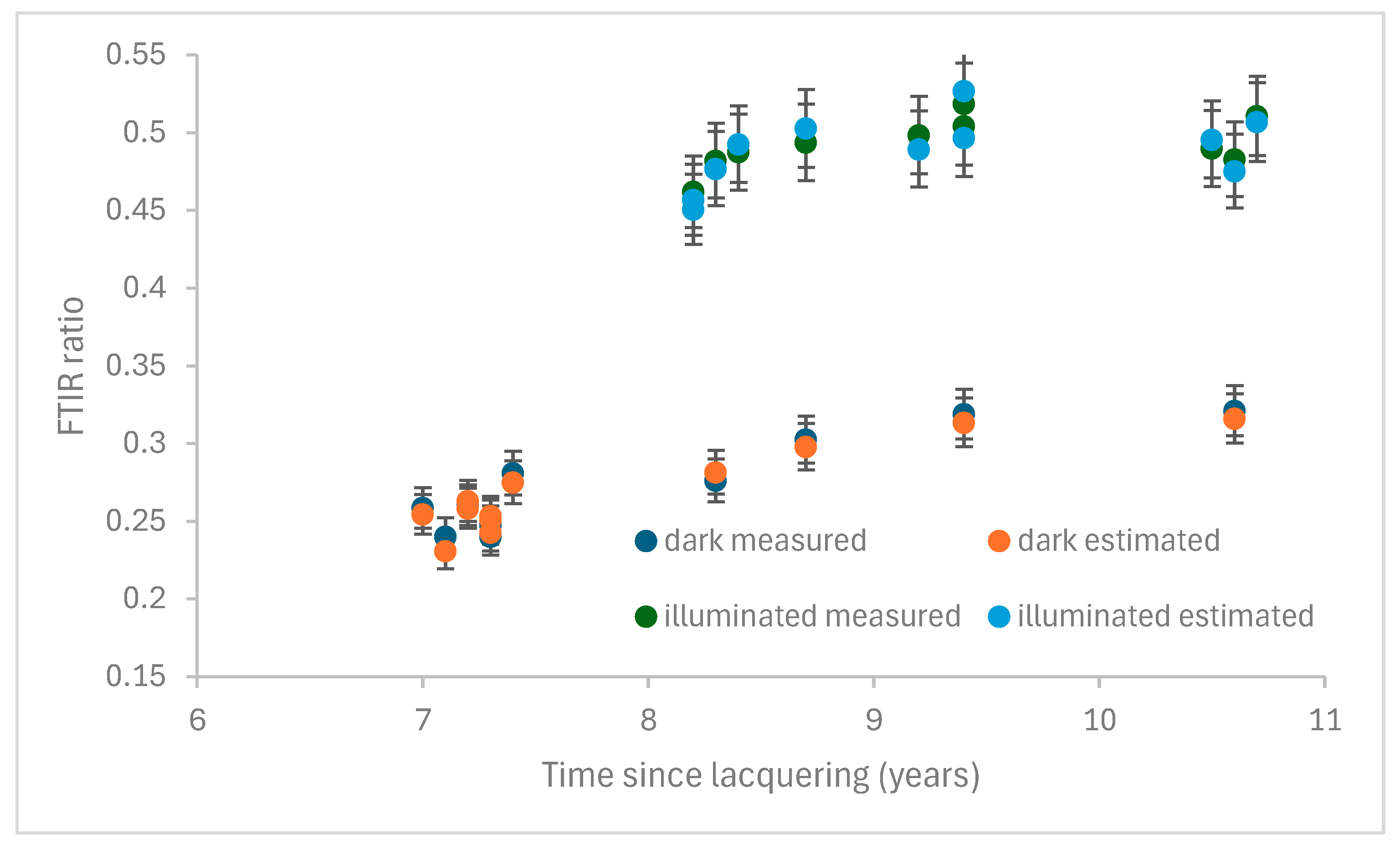
3.2. Room Temperature Aging
3.3. Visual Perception
3.4. Nitrogen Oxides Concentration
3.5. Protective Effect
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FTIR | Fourier Transform Infra-Red |
References
- Selwitz, C. Cellulose Nitrate in Conservation; Research in Conservation Series; Getty Conservation Institute: Marina del Rey, CA, USA, 1988; ISBN 978-0-89236-098-7. [Google Scholar]
- De Witte, E. The Protection of Silverware with Varnishes. Bull. (Inst. R. Du Patrim. Artist.) 1973, 14, 140–151. [Google Scholar]
- Eggers, G.; Kuiter, R.; Korenburg, C.; Ziegler, J.; Bette, S.; Stelzner, J. Metal Conservation, Cellulose Nitrate and the Oddy Test. In Proceedings of the Metal 2019 Proceedings of the Interim Meeting of the ICOM-CC Metals Working Group, Neuchâtel, Switzerland, 2–6 September 2019; Pulido & Nunes: Neuchâtel, Switzerland, 2019. [Google Scholar]
- Thickett, D.; Hallett, K. Managing Silver Tarnish. In Proceedings of the Transcending Boundaries: Integrated Approaches to Conservation, Beijing, China, 17–21 May 2021; Bridgland, J., Ed.; International Council of Museums: Beijing, China, 2021. [Google Scholar]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R.; Abou-Shehada, S.; Dunn, P.J. CHEM21 Selection Guide of Classical- and Less Classical-Solvents. Green Chem. 2015, 18, 288–296. [Google Scholar] [CrossRef]
- Fife, G. (Ed.) Greener Solvents in Conservation; Archetype: London, UK; Sustainability in Conservation: Basel, Switzerland, 2021. [Google Scholar]
- Home—GoGreen. Available online: https://gogreenconservation.eu/ (accessed on 27 August 2025).
- Ankersmit, B.; van Langh, R.; Ligterink, F.; de Groot, S.; Roelofs, W. Removal of Lacquers on Silver Using Steam; ICN Instituut Collectie Nederland: The Hague, The Netherlands, 2002; pp. 1–9. [Google Scholar]
- Thickett, D.; Lankaster, P.; Odlyha, M. Assessing and Predicting Sustainability for Maintaining Silver Collections. In Working Towards a Sustainable Past, Proceedings of the ICOM-CC 20th Triennial Conference Preprints, Valencia, Spain, 18–22 September 2023; Bridgland, J., Ed.; International Council of Museums: Paris, France, 2023. ISBN 978-2-491997-79-3. [Google Scholar]
- Neves, A.; Ramos, A.M.; Callapez, M.E.; Friedel, R.; Réfrégiers, M.; Thoury, M.; Melo, M.J. Novel Markers to Early Detect Degradation on Cellulose Nitrate-Based Heritage at the Submicrometer Level Using Synchrotron UV–VIS Multispectral Luminescence. Sci. Rep. 2021, 11, 20208. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.; Angelin, E.M.; Roldão, É.; Melo, M.J. New Insights into the Degradation Mechanism of Cellulose Nitrate in Cinematographic Films by Raman Microscopy. J. Raman Spectrosc. 2019, 50, 202–212. [Google Scholar] [CrossRef]
- Quye, A.; Littlejohn, D.; Pethrick, R.A.; Stewart, R.A. Accelerated Ageing to Study the Degradation of Cellulose Nitrate Museum Artefacts. Polym. Degrad. Stab. 2011, 96, 1934–1939. [Google Scholar] [CrossRef]
- Feller, R. Accelerated Aging: Photochemical and Thermal Aspects; Getty Conservation Institute: Marinea del Ray, CA, USA, 1992. [Google Scholar]
- Thermal Transitions of Homopolymers: Glass Transition & Melting Point. Available online: https://www.sigmaaldrich.com/GB/en/technical-documents/technical-article/materials-science-and-engineering/polymer-synthesis/thermal-transitions-of-homopolymers?msockid=288c1e3735de614c36930aed34d2604e (accessed on 27 August 2025).
- Duncan, J. Personal Communication, 2025.
- Odlyha, M. Personal Communication, 2025.
- Roduit, B.; Hartmann, M.; Folly, P.; Sarbach, A.; Baltensperger, R. Prediction of Thermal Stability of Materials by Modified Kinetic and Model Selection Approaches Based on Limited Amount of Experimental Points. Thermochim. Acta 2014, 579, 31–39. [Google Scholar] [CrossRef]
- Huelsmeyer, M.; Kuzman, D.; Bončina, M.; Martinez, J.; Steinbrugger, C.; Weusten, J.; Calero-Rubio, C.; Roche, W.; Niederhaus, B.; VanHaelst, Y.; et al. A Universal Tool for Stability Predictions of Biotherapeutics, Vaccines and in Vitro Diagnostic Products. Sci. Rep. 2023, 13, 10077. [Google Scholar] [CrossRef] [PubMed]
- Roduit, B.; Rickenbach, D.; Folly, P.; Sarbach, A.; Leubin, M.; Stucki, F.; Baltensperger, R. Application of the Master Kinetics Approach for the Prediction of the Thermal Behavior of the Propellants Belonging to the Same Class of Materials: Abstracts of the 2024 50th Annual NATAS Conference. Polymers 2024, 16, 2844. [Google Scholar] [CrossRef]
- Nunes, S.; Ramacciotti, F.; Neves, A.; Angelin, E.M.; Ramos, A.M.; Roldão, É.; Wallaszkovits, N.; Armijo, A.A.; Melo, M.J. A Diagnostic Tool for Assessing the Conservation Condition of Cellulose Nitrate and Acetate in Heritage Collections: Quantifying the Degree of Substitution by Infrared Spectroscopy. Herit. Sci. 2020, 8, 33. [Google Scholar] [CrossRef]
- Pope, D.; Gibbens, H.R.; Moss, R.L. The Tarnishing of Ag at Naturally-Occurring H2S and SO2 Levels. Corros. Sci. 1968, 8, 883–887. [Google Scholar] [CrossRef]
- Bradley, S. Preventive Conservation Research and Practice at the British Museum. Available online: https://cool.culturalheritage.org/jaic/articles/jaic44-03-002.html (accessed on 27 August 2025).
- Fei, P.; Liao, L.; Cheng, B.; Song, J. Quantitative Analysis of Cellulose Acetate with a High Degree of Substitution by FTIR and Its Application. Anal. Methods 2017, 9, 6194–6201. [Google Scholar] [CrossRef]
- Derrick, M.; Stulik, D.; Landry, J. Infrared Spectroscopy in Conservation Science; Getty Conservation Institute: Los Angeles, CA, USA, 1999; ISBN 978-0-89236-469-5. [Google Scholar]
- Bullock, S.; Saunders, D. Measurement of Cumulative Exposure Using Blue Wool Standards. In Proceedings of the ICOM Committee for Conservation 12th Triennial Meeting, Lyon, France, 29 August–3 September 1999. [Google Scholar]
- Pretzel, B. Analysis of Comparative Color Changes Occurring in a Set of 19th Century Photographs by Lady Hawarden. In Proceedings of the Imperfect Image: Photographs their Past, Present and Future, Windermere, UK, 6–10 April 1992; The Centre for Photographic Conservation: London, UK, 1992; pp. 165–181. [Google Scholar]
- Costa, V.; Dubus, M. Impact of the Environmental Conditions on the Conservation of Metal Artifacts: An Evaluation Using Electrochemical Techniques. In Proceedings of the Museum Microclimates, Copenhagen, Denmark, 19–23 November 2007; Padfield, T., Borchersen, K., Eds.; National Museum of Denmark: Copenhagen, Denmark, 2007. [Google Scholar]
- Palmes, E.D.; Gunnison, A.F.; DiMattio, J.; Tomczyk, C. Personal Sampler for Nitrogen Dioxide. Am. Ind. Hyg. Assoc. J. 1976, 37, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Gibson, L.T.; Cooksey, B.G.; Littlejohn, D.; Tennent, N.H. A Diffusion Tube Sampler for the Determination of Acetic Acid and Formic Acid Vapours in Museum Cabinets. Anal. Chim. Acta 1997, 341, 11–19. [Google Scholar] [CrossRef]
- ISO 12569:2017; Thermal Performance of Buildings and Materials—Determination of Specific Airflow Rate in Buildings—Tracer Gas Dilution Method. International Organization for Standardization: London, UK, 2024.
- Thickett, D. Specifying Air Exchange Rates for Showcases. In Chemical Interactions Between Cultural Artefacts and Indoor Environment; Uitgeverij Acco: Leuven, Belgium, 2020; pp. 25–48. [Google Scholar]
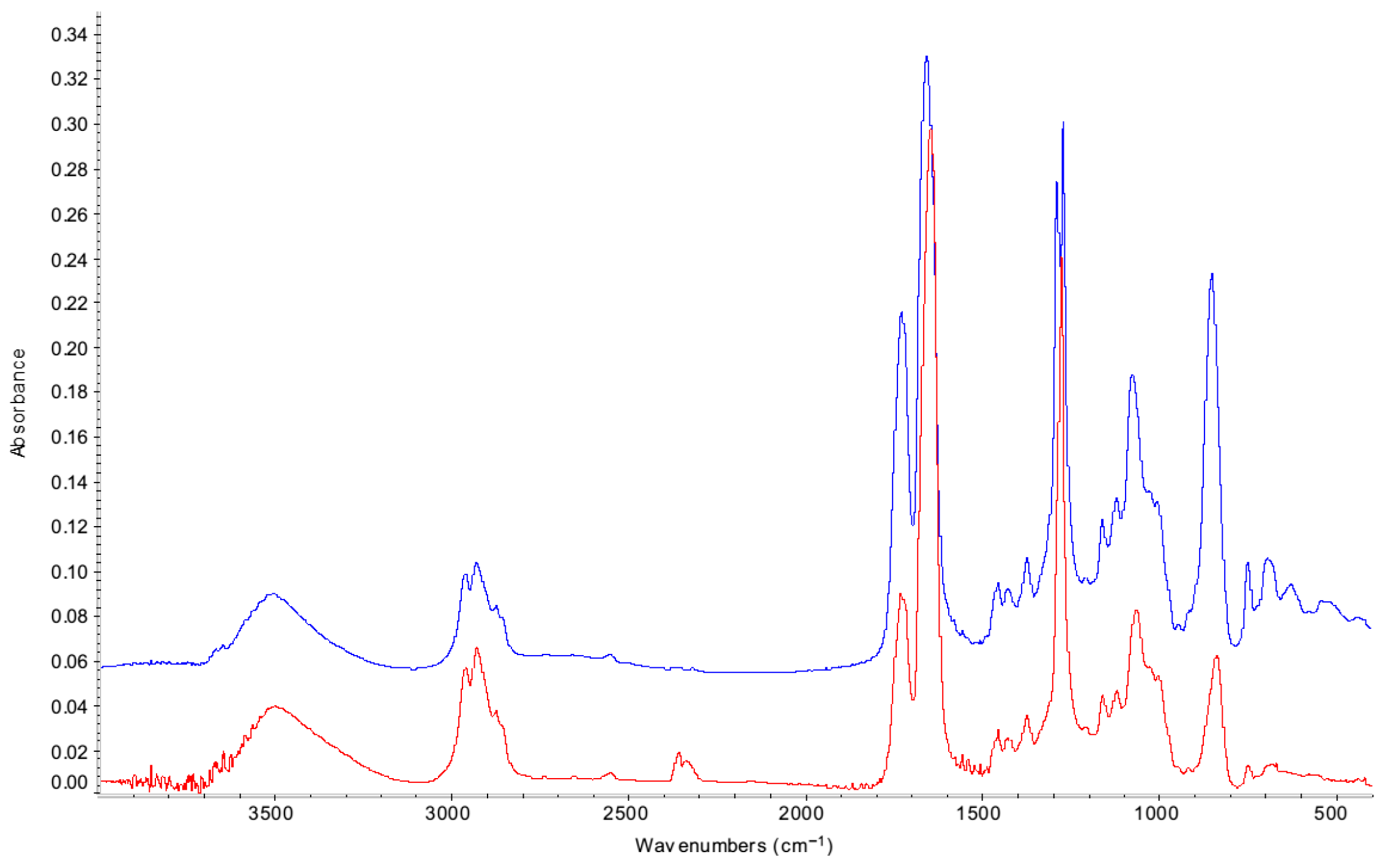
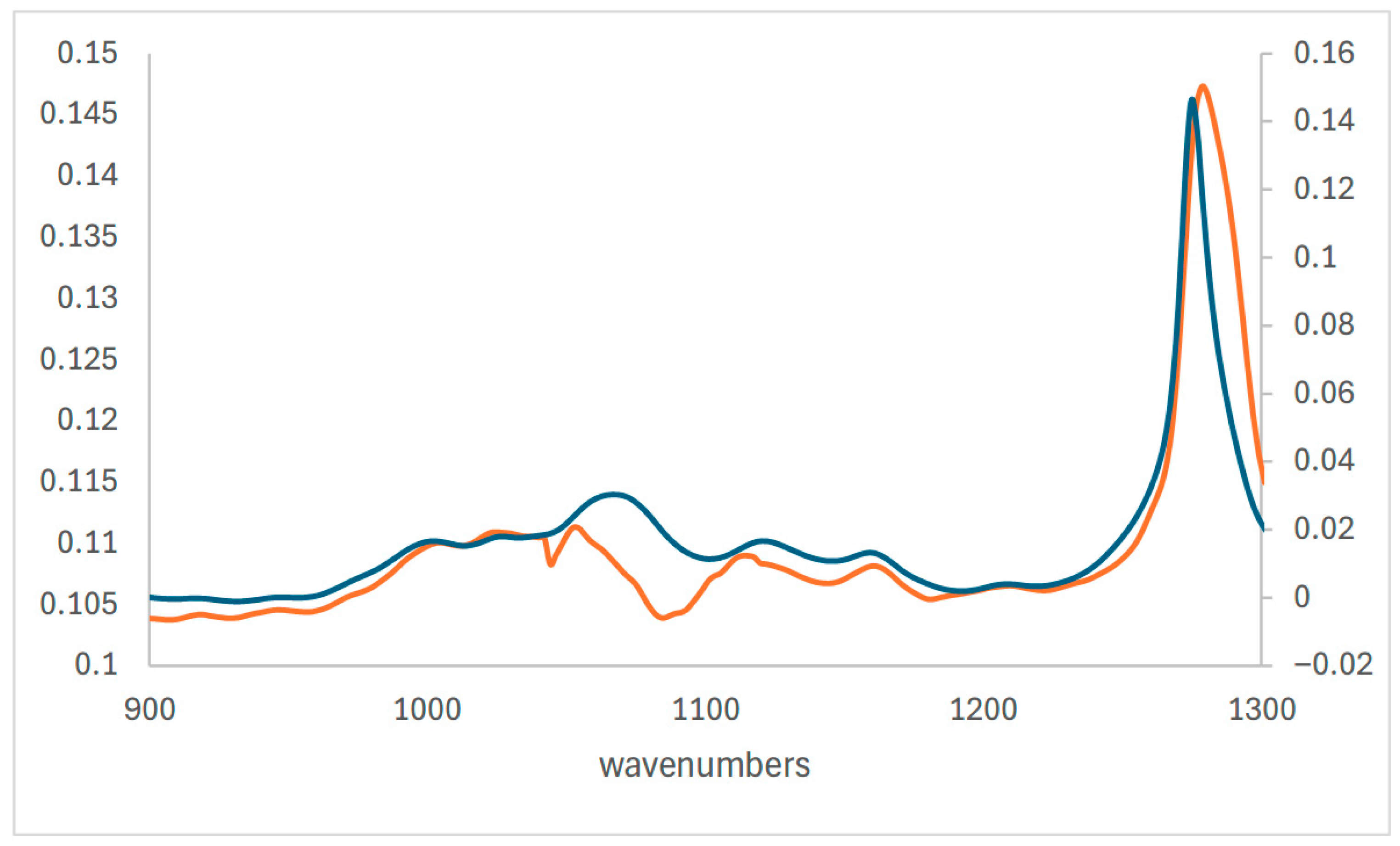


| 3462 | O-H |
| 2964 | C-H |
| 1728 | C=O initially from phthalate, then also degradation product |
| 1636 | NO2 |
| 1274 | NO2 |
| 1050 | COC |
| 829 | NO |
| Arbitrary Number | Length (m) | Width (m) | Height (m) | Lacquered Silver Area | Air Exchange Rate (day−1) | Materials, Top, Bottom, Sides (Back) |
|---|---|---|---|---|---|---|
| 1 | 1.0 | 1.0 | 2.4 | 0.43 | 0.02 | Metal, fabric, glass (fabric) |
| 2 | 1.0 | 1.0 | 2.4 | 1.54 | 0.03 | Metal, fabric, glass (fabric) |
| 3 | 1.5 | 1.0 | 1.5 | 0.20 | 0.21 | Wood, fabric, glass (wood) |
| 4 | 1.5 | 1.0 | 1.5 | 1.01 | 0.32 | Wood, fabric, glass (wood) |
| 5 | 1.5 | 1.0 | 1.5 | 0.50 | 0.40 | Wood, fabric, glass (wood) |
| 6 | 2.2 | 0.8 | 0.4 | 4.13 | 0.42 | Glass, fabric, glass |
| 7 | 1.5 | 0.6 | 0.6 | 2.12 | 0.60 | Glass, fabric, glass |
| 8 | 1.5 | 0.6 | 0.6 | 1.09 | 0.70 | Glass, fabric, glass |
| 9 | 0.8 | 0.8 | 2.0 | 0.10 | 0.80 | Metal, fabric, glass |
| Temperature (°C) | Time (days) | Ratio | Temperature (°C) | Time (days) | Ratio |
|---|---|---|---|---|---|
| 50 | 0 | 0.220 | 40 | 0 | 0.22 |
| 50 | 11.691 | 0.277 | 40 | 11.611 | 0.226 |
| 50 | 11.691 | 0.282 | 40 | 11.611 | 0.225 |
| 50 | 11.691 | 0.282 | 40 | 11.611 | 0.226 |
| 50 | 18.732 | 0.304 | 40 | 30.039 | 0.234 |
| 50 | 18.732 | 0.297 | 40 | 30.039 | 0.241 |
| 50 | 27.448 | 0.346 | 40 | 30.039 | 0.236 |
| 50 | 27.448 | 0.336 | 40 | 47.262 | 0.247 |
| 50 | 27.448 | 0.346 | 40 | 47.262 | 0.245 |
| 50 | 36.312 | 0.386 | 40 | 47.262 | 0.241 |
| 50 | 36.312 | 0.396 | 40 | 70.046 | 0.277 |
| 50 | 36.312 | 0.404 | 40 | 70.046 | 0.282 |
| 50 | 43.896 | 0.433 | 40 | 70.046 | 0.288 |
| 50 | 43.896 | 0.423 | 40 | 91.317 | 0.337 |
| 50 | 43.896 | 0.431 | 40 | 91.317 | 0.327 |
| 50 | 51.174 | 0.495 | 40 | 91.317 | 0.331 |
| 50 | 51.174 | 0.493 | 45 | 0 | 0.22 |
| 50 | 54.775 | 0.530 | 45 | 11.850 | 0.241 |
| 50 | 54.775 | 0.532 | 45 | 11.850 | 0.239 |
| 35 | 0 | 0.22 | 45 | 11.850 | 0.232 |
| 35 | 91.051 | 0.223 | 45 | 23.832 | 0.279 |
| 35 | 91.051 | 0.219 | 45 | 23.832 | 0.278 |
| 35 | 91.051 | 0.221 | 45 | 23.832 | 0.286 |
| 45 | 36.411 | 0.309 | |||
| 45 | 36.411 | 0.303 | |||
| 45 | 36.411 | 0.305 | |||
| 45 | 53.016 | 0.375 | |||
| 45 | 53.016 | 0.379 | |||
| 45 | 53.016 | 0.370 | |||
| 45 | 62.853 | 0.425 | |||
| 45 | 62.853 | 0.418 | |||
| 45 | 72.532 | 0.517 | |||
| 45 | 72.532 | 0.511 | |||
| 45 | 72.532 | 0.503 |
| Site | Person | Acceptable FTIR Ratio | Unacceptable FTIR Ratio |
|---|---|---|---|
| Apsley House | Curator | 0.63 | 0.65 |
| Conservator | 0.68 | 0.70 | |
| Audley End House | Curator | 0.68 | 0.70 |
| Conservator | 0.65 | 0.66 | |
| Brodsworth Hall | Curator | 0.70 | 0.71 |
| Conservator | 0.68 | 0.70 | |
| Osbourne House | Curator | 0.66 | 0.68 |
| Conservator | 0.65 | 0.66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thickett, D.; Harvey, C. Assessing the Performance and Lifetime of Cellulose Nitrate Lacquer on Silver. Materials 2025, 18, 4155. https://doi.org/10.3390/ma18174155
Thickett D, Harvey C. Assessing the Performance and Lifetime of Cellulose Nitrate Lacquer on Silver. Materials. 2025; 18(17):4155. https://doi.org/10.3390/ma18174155
Chicago/Turabian StyleThickett, David, and Cathryn Harvey. 2025. "Assessing the Performance and Lifetime of Cellulose Nitrate Lacquer on Silver" Materials 18, no. 17: 4155. https://doi.org/10.3390/ma18174155
APA StyleThickett, D., & Harvey, C. (2025). Assessing the Performance and Lifetime of Cellulose Nitrate Lacquer on Silver. Materials, 18(17), 4155. https://doi.org/10.3390/ma18174155






