A New Classification Method for High-Volume Fly Ash: Performance Based on Coal Source and Particle Size
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- Wb: The weights of the samples before burning.
- Wa: The weights of the samples after burning.
2.2. Fly Ash Modification System
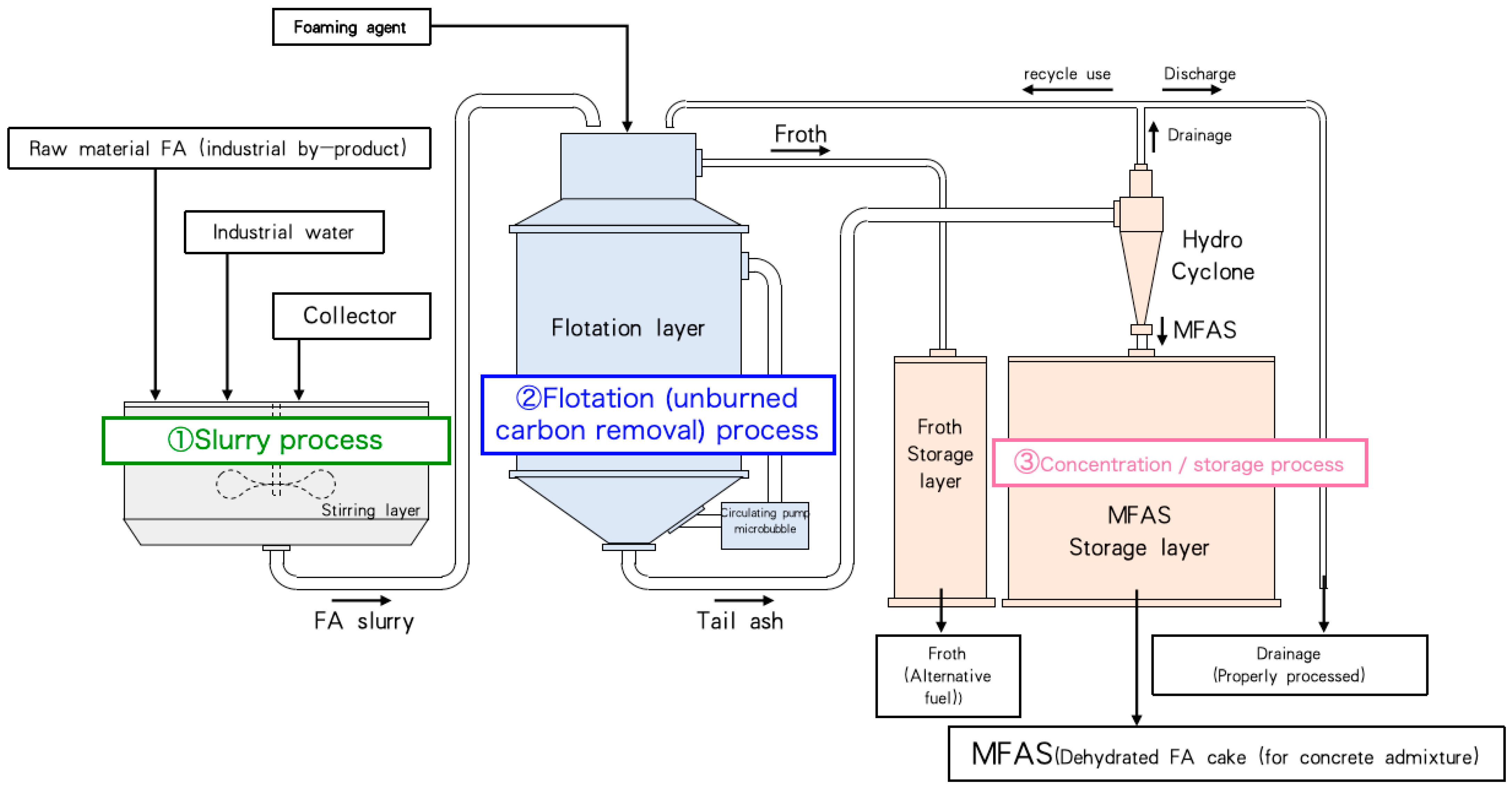
2.2.1. Structure of Fly Ash Modification System
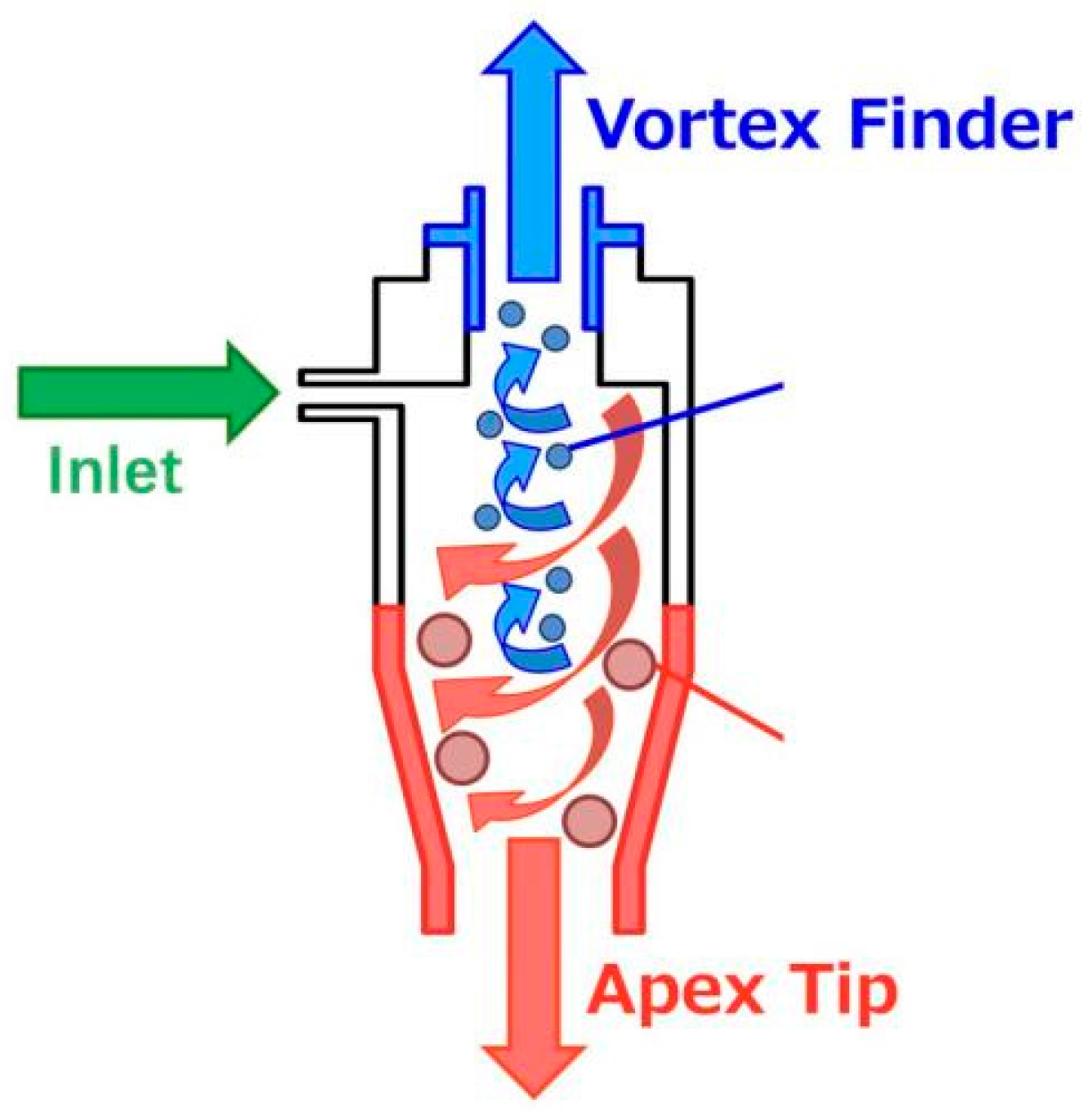
2.2.2. Classification, Concentration, and Dehydration Experiments
2.3. Experimental Methods
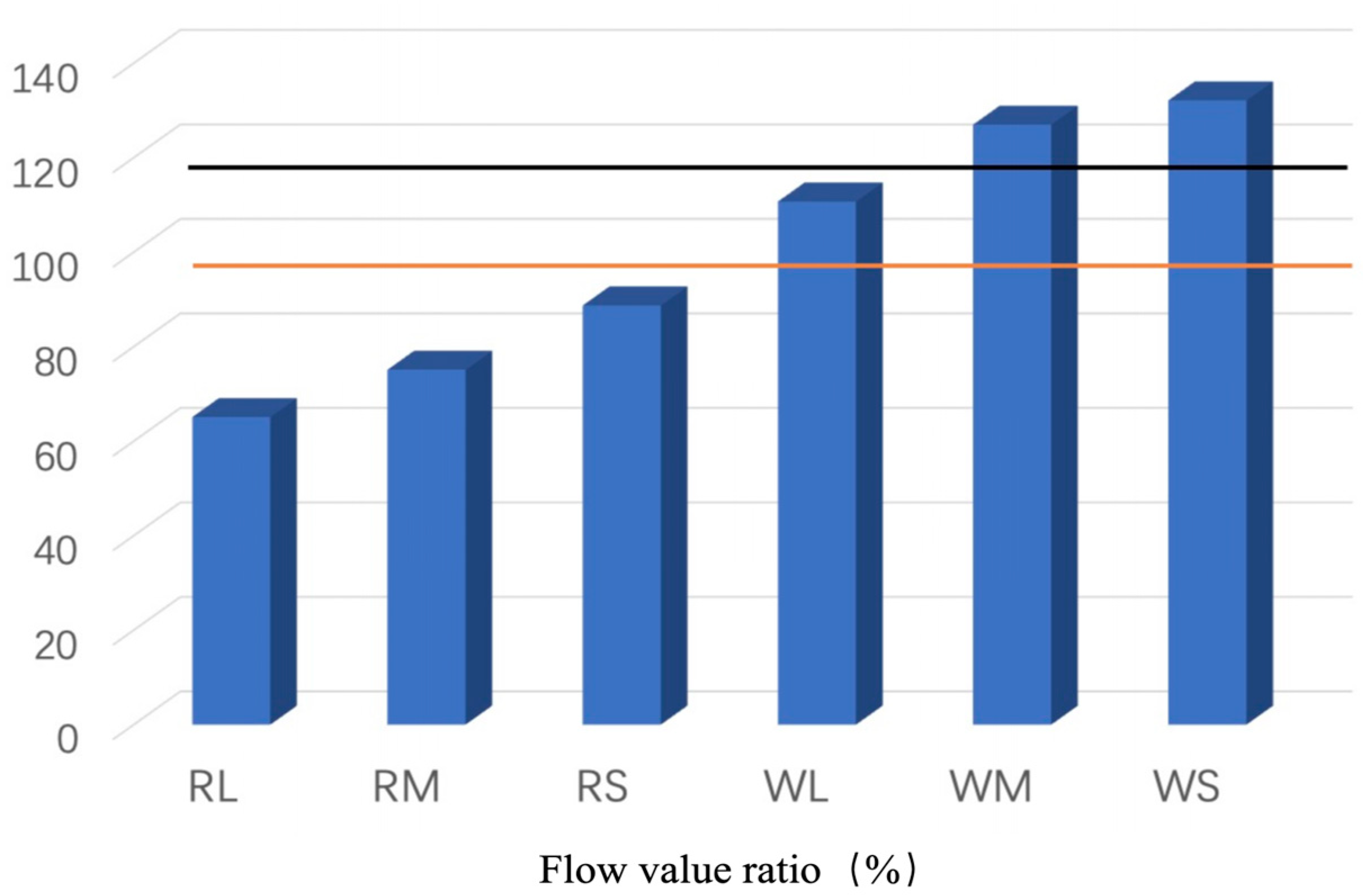
- F: Flow value ratio.
- l2: Flow value of the base mortar.
- l1: Flow rate value of the samples
3. Results and Discussion
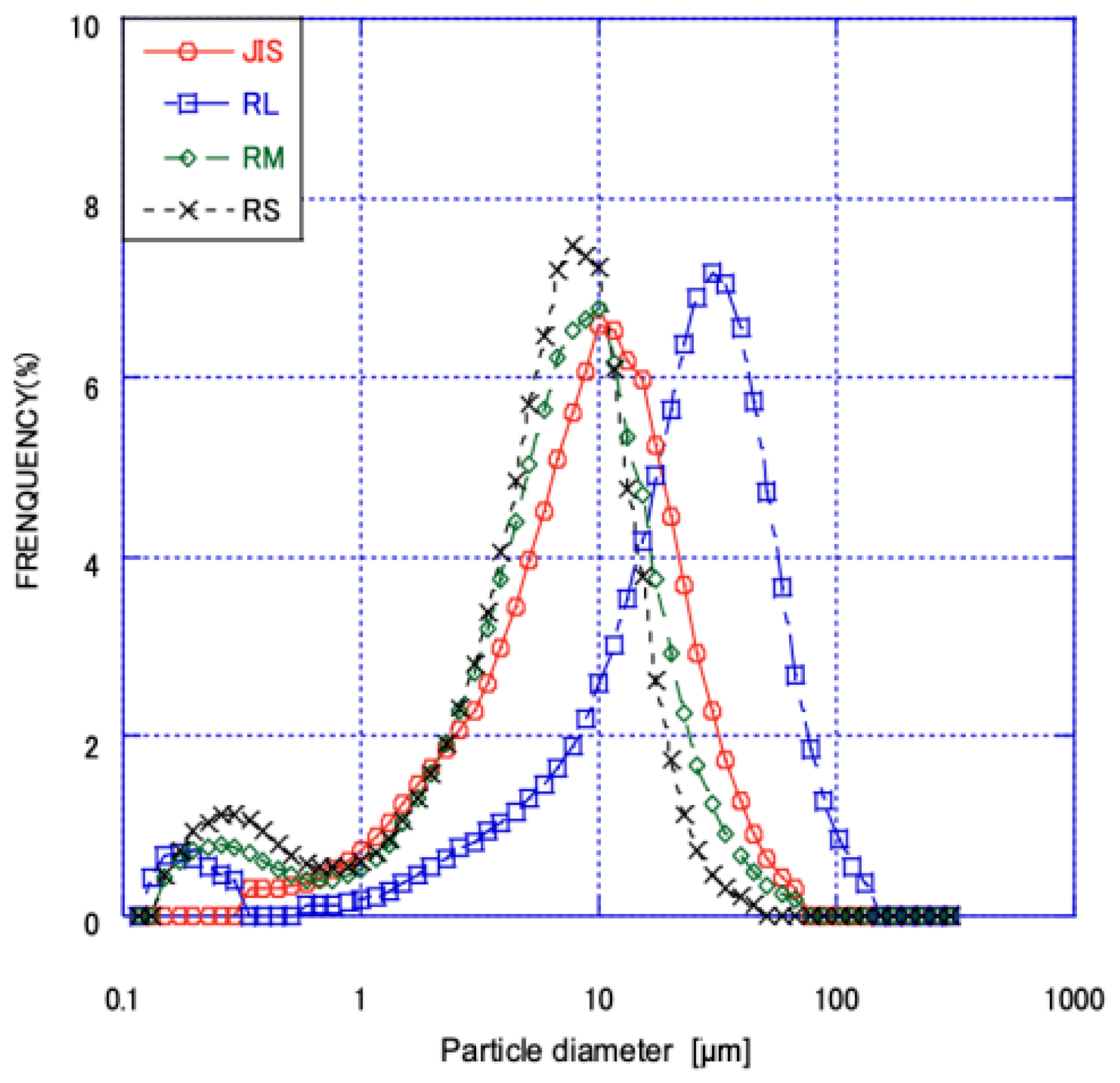
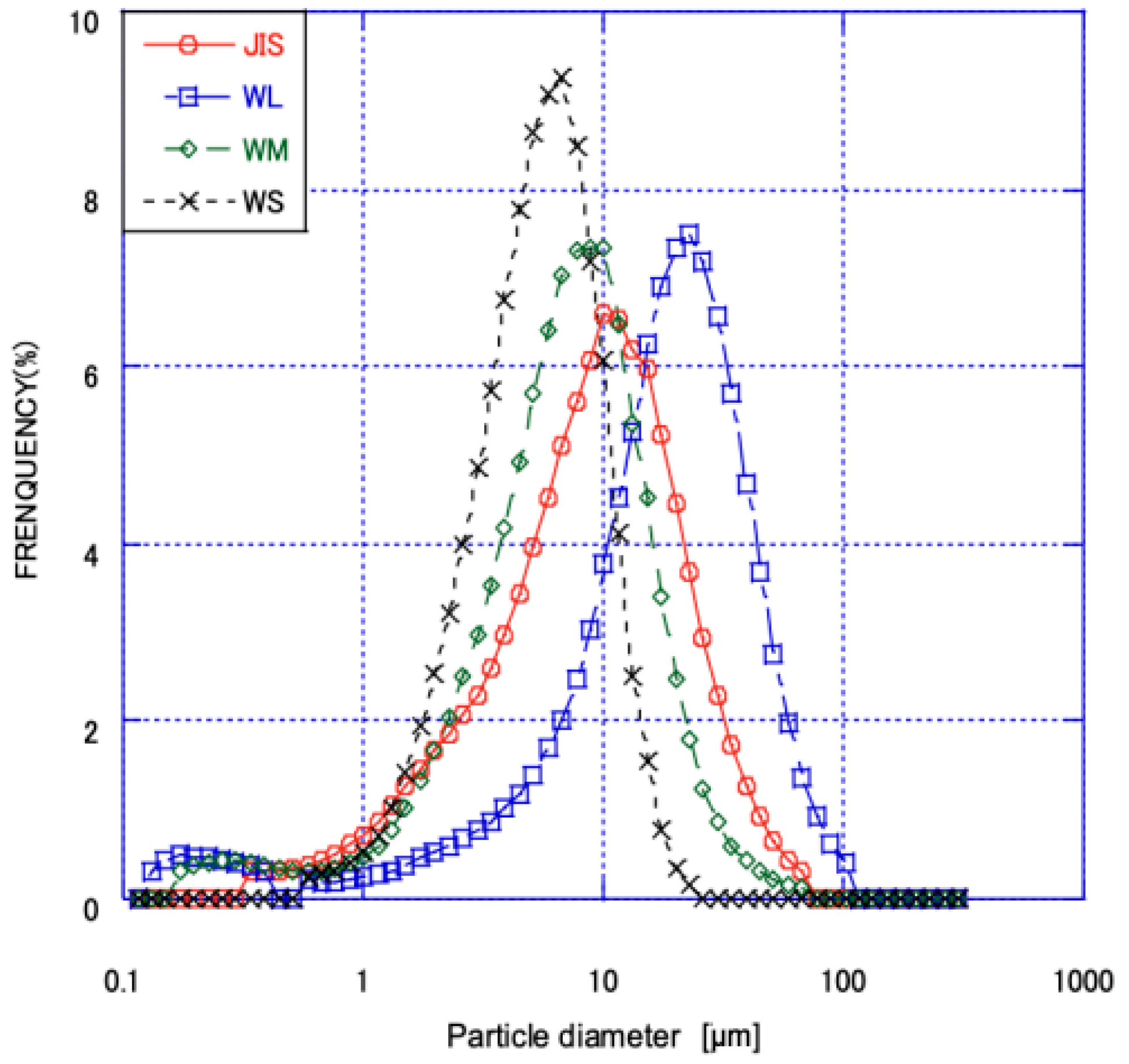
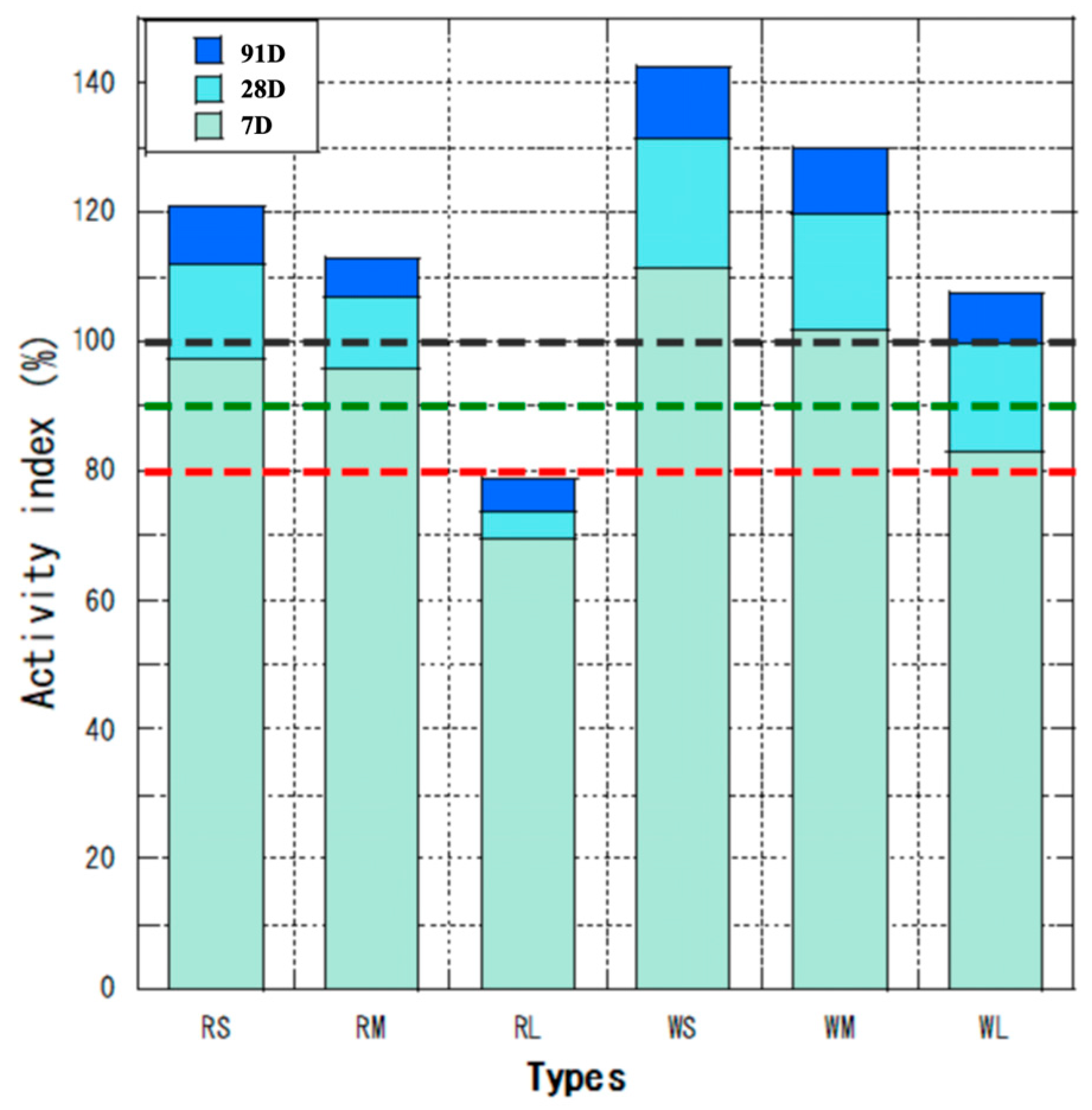
3.1. Analysis of the Physical Properties of Fly Ash
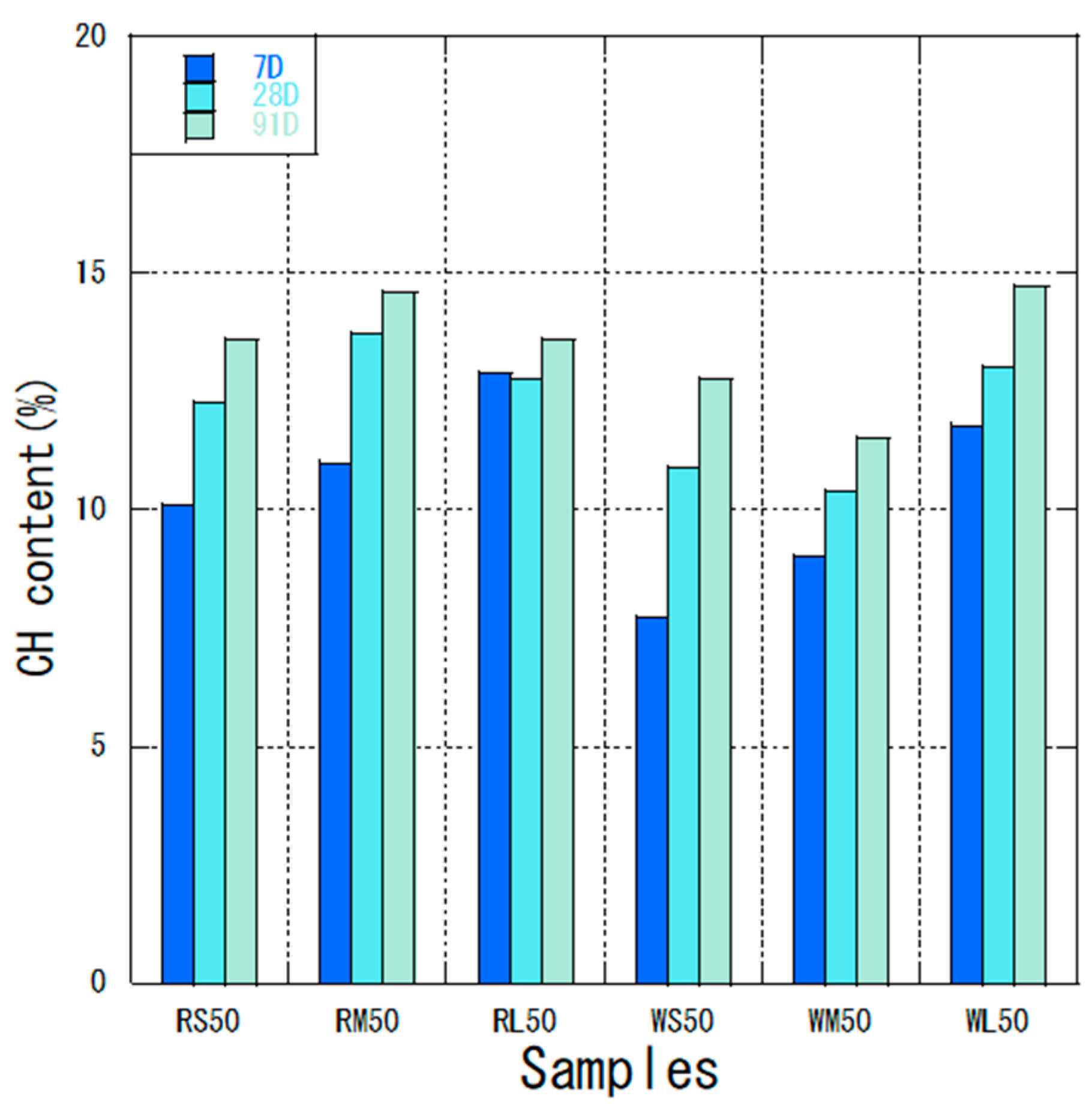
3.2. Thermal Analysis
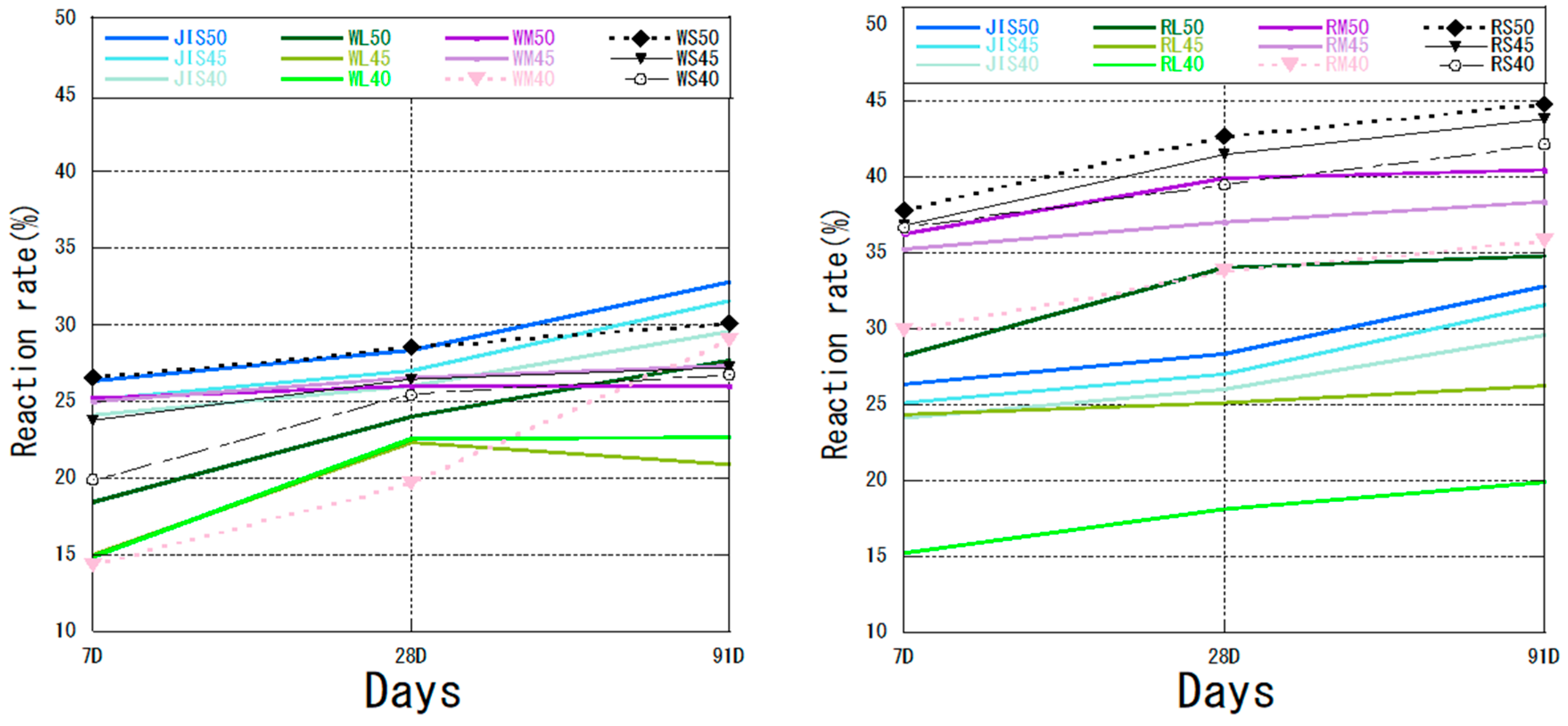
3.3. Reaction Rate
3.4. X-Ray Diffraction Analysis
4. Conclusions
- (1)
- Physical Properties: After processing, W ash generally exhibited higher activity and flow values compared to R ash. In particular, the classified fine fraction of W ash (WS) surpassed the specifications of JIS Class I fly ash, demonstrating that the flotation-classification process significantly enhances fly ash properties.
- (2)
- Reactivity: The use of high-volume fly ash (50% replacement) promoted prolonged pozzolanic reaction, especially with finer particles, as indicated by the continuous consumption of calcium hydroxide at later ages. Smaller particle sizes led to greater decreases in CH content, highlighting their enhanced reactivity.
- (3)
- Cement Hydration and Performance: Finer fly ash particles consistently resulted in higher consumption of C3S across all ages, indicating accelerated cement hydration and pozzolanic activity. The overall performance of R ash was superior to W ash, attributable to its inherent mineral composition. Classification significantly improved reactivity, with overflow (OF) fractions showing the highest reaction rates and pozzolanic consumption. While smaller particle sizes generally enhance pozzolanic reactivity, the plateauing of reaction rates in coarser fractions (e.g., RL) suggests that chemical and mineralogical factors also impose limitations. Thus, both particle size and phase composition must be considered to fully optimize fly ash performance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Osborne, D.; Tasker, C.; Simington, I.R.; Arnold, B.J.; Diez, M.A.; Schumacher, G.; Hamilton, C.J. Future directions toward more efficient and cleaner use of coal. In The Coal Handbook; Woodhead Publishing: Cambridge, UK, 2023. [Google Scholar]
- Watanabe, R. After the Fukushima disaster: Japan’s nuclear policy change from 2011 to 2012. Rev. Policy Res. 2016, 33, 623–645. [Google Scholar] [CrossRef]
- Aggregate or Estimated Results (Comprehensive Energy Statistics). Available online: https://www.enecho.meti.go.jp/statistics/total_energy/results.html (accessed on 5 June 2025).
- Blissett, R.S.; Rowson, N.A. A review of the multi-component utilisation of coal fly ash. Fuel 2012, 97, 1–23. [Google Scholar] [CrossRef]
- Dmitrienko, M.A.; Nyashina, G.S.; Strizhak, P.A. Major gas emissions from combustion of slurry fuels based on coal, coal waste, and coal derivatives. J. Clean. Prod. 2018, 177, 284–301. [Google Scholar] [CrossRef]
- Tsiridis, V.; Petala, M.; Samaras, P.; Sakellaropoulos, G. Evaluation of interactions between soil and coal fly ash leachates using column percolation tests. Waste Manag. 2015, 43, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Vilakazi, A.Q.; Ndlovu, S.; Chipise, L.; Shemi, A. The recycling of coal fly ash: A review on sustainable developments and economic considerations. Sustainability 2022, 14, 1958. [Google Scholar] [CrossRef]
- Mora, E.P.; Paya, J.; Monzo, J. Influence of different sized fractions of a fly ash on workability of mortars. Cem. Concr. Res. 1993, 23, 917–924. [Google Scholar] [CrossRef]
- Bigas, J.P.; Gallias, J.L. Effect of fine mineral additions on granular packing of cement mixtures. Mag. Concr. Res. 2002, 54, 155–164. [Google Scholar] [CrossRef]
- Moghaddam, F.; Sirivivatnanon, V.; Vessalas, K. The effect of fly ash fineness on heat of hydration, microstructure, flow and compressive strength of blended cement pastes. Case Stud. Constr. Mater. 2019, 10, e00218. [Google Scholar] [CrossRef]
- Ministry of the Environment. Available online: http://www.env.go.jp/earth/ccs/ccus-kaigi/post_50.html (accessed on 1 August 2025).
- Telesca, A.; Marroccoli, M.; Calabrese, D.; Valenti, G.L.; Montagnaro, F. Flue gas desulfurization gypsum and coal fly ash as basic components of prefabricated building materials. Waste Manag. 2013, 33, 628–633. [Google Scholar] [CrossRef]
- Batra, V.; Urbonaite, S.; Svensson, G. Characterization of unburned carbon in bagasse fly ash. Fuel 2008, 87, 2972–2976. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, F.; Tao, Y. Removal of unburned carbon from fly ash using enhanced gravity separation and the comparison with froth flotation. Fuel 2020, 259, 116282. [Google Scholar] [CrossRef]
- Ejtemaei, M.; Gharabaghi, M.; Irannajad, M. A review of zinc oxide mineral beneficiation using flotation method. Adv. Colloid Interface Sci. 2014, 206, 68–78. [Google Scholar] [CrossRef]
- Harris, T.; Wheelock, T.D. Process Conditions for the Separation of Carbon from Fly Ash by Froth Flotation. Int. J. Coal Prep. Util. 2008, 28, 133–152. [Google Scholar] [CrossRef]
- Lin, H.; Takasu, K.; Koyamada, H.; Suyama, H. Development of Flotation Device for Removing Unburnt Carbon in Fly Ash for Use in Hardened Cementitious Materials. Materials 2021, 14, 6517. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-H.; Kim, J.-K.; Park, Y.-D. Prediction of compressive strength of fly ash concrete by new apparent activation energy function. Cem. Concr. Res. 2003, 33, 965–971. [Google Scholar] [CrossRef]
- Wang, X.-Y. Effect of fly ash on properties evolution of cement based materials. Constr. Build. Mater. 2014, 69, 32–40. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, L.; Liu, J.; Liu, R.; Pang, B. Impact of particle size of fly ash on the early compressive strength of concrete: Experimental investigation and modelling. Constr. Build. Mater. 2022, 323, 126444. [Google Scholar] [CrossRef]
- Chavhan, P.P.; Vyawahare, M.R. Correlation of Static and Dynamic modulus of Elasticity for Different SCC mixes. Recent Innov. Trends Comput. Commun. 2015, 3, 4914–4919. [Google Scholar]
- Thomas, M.D.A.; Matthews, J.D. Performance of pfa concrete in a marine environment—10-year results. Cem. Concr. Compos. 2004, 26, 5–20. [Google Scholar] [CrossRef]
- Thomas, M. Optimizing the Use of Fly Ash in Concrete; Portland Cement Association: Skokie, IL, USA, 2007. [Google Scholar]
- Gupta, V.; Siddique, S.; Chaudhary, S. Characterization of different types of fly ash collected from various sources in Central India. Mater. Today Proc. 2019, 18, 5076–5080. [Google Scholar] [CrossRef]
- Bayat, O.; Ucurum, M.; Poole, C. Effects of size distribution on flotation kinetics of Turkish sphalerite. Miner. Process. Extr. Met. 2004, 113, 53–59. [Google Scholar] [CrossRef]
- Luo, S.; Guo, M.-Z.; Ling, T.-C. Mechanical and microstructural performances of fly ash blended cement pastes with mixing CO2 during fresh stage. Constr. Build. Mater. 2022, 358, 129444. [Google Scholar] [CrossRef]
- Kang, S.; Ley, M.T.; Behravan, A. Predicting ion diffusion in fly ash cement paste through particle analysis. Constr. Build. Mater. 2021, 272, 121934. [Google Scholar] [CrossRef]
- Kiattikomol, K.; Jaturapitakkul, C.; Songpiriyakij, S.; Chutubtim, S. A study of ground coarse with dierent finenesses from various sources as pozzolanic materials. Cem. Concr. Compos. 2001, 23, 335–343. [Google Scholar] [CrossRef]
- Haha, M.B.; De Weerdt, K.; Lothenbach, B. Quantification of the degree of reaction of fly ash. Cem. Concr. Res. 2010, 40, 1620–1629. [Google Scholar] [CrossRef]
- Kim, T.; Olek, J. Effects of Sample Preparation and Interpretation of Thermogravimetric Curves on Calcium Hydroxide in Hydrated Pastes and Mortars. Transp. Res. Board 2012, 2290, 10–18. [Google Scholar] [CrossRef]
- Scrivener, K.; Snellings, R.; Lothenbach, B. A Practical Guide to Microstructural Analysis of Cementitious Materials; Taylor & Francis Group: Abingdon, UK, 2016. [Google Scholar]
- Ishizaki, M.; Asaga, K.; Daimon, M.; Takahashi, S. Measurement of reaction ratio of each compound in the hydration process of portland cement. In Proceedings of the Review of the 42nd General Meeting Technical Session, The Cement Association of Japan, New York, NY, USA, 11 November 1988; pp. 38–41. [Google Scholar]
- JIS R 5201:2015; Physical Testing Methods for Cement. Japanese Industrial Standards: Tokyo, Japan, 2015.
- JIS A 6201:2015; Fly Ash for Use in Concrete. Japanese Industrial Standards Committee: Tokyo, Japan, 2015.
- Asaga, K. Determination of Uncombined Quartz in Hydrothermal Reaction of Quartz and Portland. J. Ceram. Assoc. 1982, 90, 397–400. [Google Scholar] [CrossRef]
- Kobayakawa, M.; Ozu, H.; Habara, S. Effect of mixing and curing temperature on pozzolanic reaction rate of fly ash. J. Res. Taiheiyo Cem. Corp. 2000, 14–27. [Google Scholar]
- Ishikawa, Y. Survey on Quality Distribution of Fly Ash JIS Class II Products. In Technical Report of the Architectural Institute of Japan; Architectural Institute of Japan (AIJ): Tokyo, Japan, 2006; pp. 1–4. [Google Scholar]
- Xiangnan, J.; Koji, T.; Hiroki, S.; Hidehiro, K. The Effects of Curing Temperature on CH-Based Fly Ash Composites. Materials 2023, 16, 2645. [Google Scholar] [CrossRef]
- Yamazaki, H. Basic research on the effect of fine mineral powder on the strength of concrete. J. Jpn. Soc. Civ. Eng. 1962, 15–44. [Google Scholar]
- Yang, L.; Zhu, Z.; Li, D.; Yan, X.; Zhang, H. Effects of particle size on the flotation behavior of coal fly ash. Waste Manag. 2019, 85, 490–497. [Google Scholar] [CrossRef]
- Ferrarisa, C.F.; Oblab, K.H.; Hillb, R. The influence of mineral admixtures on the rheology of cement paste and concrete. Cem. Concr. Res. 2001, 31, 245–255. [Google Scholar] [CrossRef]
- Luxán, M.P.; Rojas, M.I.S.D.; Frías, M. Investigations on the fly ash-calcium hydroxide reactions. Cem. Concr. Res. 1989, 19, 69–80. [Google Scholar] [CrossRef]
- Ju-Yuan, H.; Scheetz, B.E.; Roy, D.M. Hydration of fly ash Portland cements. Cem. Concr. Res. 1984, 14, 505–512. [Google Scholar] [CrossRef]
- Tkaczewska, E.; Małolepszy, J. Hydration of coal–biomass fly ash cement. Constr. Build. Mater. 2009, 23, 2694–2700. [Google Scholar] [CrossRef]
- Wang, T.; Ishida, T.; Gu, R.; Luan, Y. Experimental investigation of pozzolanic reaction and curing temperature-dependence of low-calcium fly ash in cement system and Ca-Si-Al element distribution of fly ash-blended cement paste. Constr. Build. Mater. 2021, 267, 121012. [Google Scholar] [CrossRef]
- Kobayakawa, M. Pozzolanic Reaction Analysis Method for Fly Ash and Its Application to Existing Structures. Master’s Thesis, Tokyo Institute of Technology, Tokyo, Japan, 2009. [Google Scholar]
- Zeng, Q.; Li, K.; Fen-Chong, T.; Dangla, P. Determination of cement hydration and pozzolanic reaction extents for fly-ash cement pastes. Constr. Build. Mater. 2012, 27, 560–569. [Google Scholar] [CrossRef]
- Abdul-Maula, S.; Odler, I. Hydration reactions in fly-ash-Portland cements. In Proceedings of the Symposium on Effects of Fly Ash Incorporation in Cement and Concrete, Boston, MA, USA, 16–18 November 1981; pp. 102–111. [Google Scholar]
- Xu, A.; Sarkar, S.L.; Nilsson, L.-O. Effect of fly ash on the microstructure of cement mortar. Mater. Struct. 1993, 26, 414–424. [Google Scholar] [CrossRef]
- Cyr, M.; Lawrence, P.; Ringot, E. Mineral admixtures in mortars. Cem. Concr. Res. 2005, 35, 719–730. [Google Scholar] [CrossRef]
- Hanehara, S.; Tomosawa, F.; Kobayakawa, M.; Hwang, K. Effects of water/powder ratio, mixing ratio of fly ash, and Turing temperature on pozzolnaic reaction of fly ash in cement paste. Cem. Concr. Res. 2001, 31, 31–39. [Google Scholar] [CrossRef]
- Kakali, G.; Tsivilis, S.; Aggeli, E.; Bati, M. Hydration products of C3A, C3S and Portland cement in the presence of CaCO3. Cem. Concr. Res. 2000, 30, 1037–1077. [Google Scholar] [CrossRef]
- Pietersen, H.S. The Reactivity of Fly Ash in Cement; TU Delft: Delft, The Netherlands, 1993. [Google Scholar]
- Scrivener, K.L.; Füllmann, T.; Gallucci, E.; Walenta, G.; Bermejo, E. Quantitative study of Portland cement hydration by X-ray diffraction/Rietveld analysis and independent methods. Cem. Concr. Res. 2004, 34, 1541–1547. [Google Scholar] [CrossRef]
- Erdoğdu, K.; Türker, P. Effects of fly ash particle size on strength of Portland cement fly ash mortars. Cem. Concr. Res. 1998, 28, 1217–1222. [Google Scholar] [CrossRef]
- Chen, L.-C.; Papandreou, G.; Kokkinos, I.; Murphy, K.; Yuille, A.L. DeepLab: Semantic Image Segmentation with Deep Convolutional Nets, Atrous Convolution, and Fully Connected CRFs. IEEE Trans. Pattern Anal. Mach. Intell. 2018, 40, 834–848. [Google Scholar] [CrossRef]
- Tan, M.; Le, Q.V. EfficientNet: Rethinking Model Scaling for Convolutional Neural Networks. In Proceedings of the International Conference on Machine Learning, Long Beach, CA, USA, 9–15 June 2019. [Google Scholar]

| Symbol | Type | Physical Characteristics | |
|---|---|---|---|
| Cement | C | Portland cement | Density: 3.16 g/cm3 |
| Admixture | FA | Raw ash fly ash (W ash) | Density: 2.19 g/cm3 Specific surface area: 6810 g/cm3 Loss on ignition: 23.5% PH: 6.98% |
| Raw ash fly ash (R ash) | Density: 2.19 g/cm3 Specific surface area: 5500 g/cm3 Loss on ignition: 8.5% PH: 4.34% | ||
| Water | W | Tap water | - |
| Types | LOI (%) | |
|---|---|---|
| Raw fly ash | R ash | 8.5 |
| Modified fly ash | RS | 1.1 |
| RM | 1.2 | |
| RL | 2.8 | |
| Raw fly ash | W ash | 24.8 |
| Modified fly ash | WS | 1.5 |
| WM | 1.8 | |
| WL | 3.9 | |
| Type | W/C | W/B | Unit Weight (kg/m3) | ||
|---|---|---|---|---|---|
| W | C | FA | |||
| R/W40 | 0.80 | 0.40 | 180 | 225 | 225 |
| R/W45 | 0.90 | 0.45 | 180 | 200 | 200 |
| R/W50 | 1.00 | 0.50 | 180 | 180 | 180 |
| Types | LOI (%) | |
|---|---|---|
| Raw fly ash | R ash | 8.5 |
| Modified fly ash | RS | 1.1 |
| RM | 1.2 | |
| RL | 2.8 | |
| Raw fly ash | W ash | 24.8 |
| Modified fly ash | WS | 1.5 |
| WM | 1.8 | |
| WL | 3.9 | |
| JIS II | 1.3 | |
| Sample | 7D | 28D | 91D |
|---|---|---|---|
| JIS40 | 10.4 | 10.3 | 13.1 |
| RL40 | 9.6 | 13.4 | 13.9 |
| RM40 | 11.2 | 14.9 | 13.9 |
| RS40 | 9.0 | 10.9 | 12.7 |
| WL40 | 9.4 | 13.0 | 13.3 |
| WM40 | 8.2 | 11.2 | 11.6 |
| WS40 | 7.9 | 9.5 | 11.2 |
| JIS45 | 11.0 | 12.9 | 15.4 |
| RL45 | 11.1 | 15.4 | 16.5 |
| RM45 | 11.2 | 10.8 | 13.8 |
| RS45 | 9.5 | 10.7 | 13.5 |
| WL45 | 11.5 | 14.4 | 14.7 |
| WM45 | 9.1 | 11.1 | 11.7 |
| WS45 | 9.7 | 11.9 | 11.0 |
| JIS50 | 11.2 | 12.5 | 15.0 |
| RL50 | 12.9 | 12.8 | 13.6 |
| RM50 | 11.0 | 13.7 | 14.6 |
| RS50 | 10.1 | 12.3 | 13.6 |
| WL50 | 11.8 | 13.0 | 14.7 |
| WM50 | 9.0 | 10.4 | 11.5 |
| WS50 | 7.7 | 10.9 | 12.8 |
| Types | C3S (%) | ||
|---|---|---|---|
| 7D | 28D | 91D | |
| RS50 | 14.92 | 4.56 | 0.49 |
| RM50 | 15.42 | 5.01 | 0.75 |
| RL50 | 16.79 | 5.42 | 1 |
| WS50 | 10.88 | 3.45 | 0.47 |
| WM50 | 11.65 | 3.99 | 0.68 |
| WL50 | 14.69 | 5.02 | 1.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, X.; Zhang, C.; Yang, Y.; Zhang, J.; Tang, L.; Ji, D. A New Classification Method for High-Volume Fly Ash: Performance Based on Coal Source and Particle Size. Materials 2025, 18, 4145. https://doi.org/10.3390/ma18174145
Ji X, Zhang C, Yang Y, Zhang J, Tang L, Ji D. A New Classification Method for High-Volume Fly Ash: Performance Based on Coal Source and Particle Size. Materials. 2025; 18(17):4145. https://doi.org/10.3390/ma18174145
Chicago/Turabian StyleJi, Xiangnan, Chen Zhang, Yaru Yang, Jiahao Zhang, Lin Tang, and Dongxu Ji. 2025. "A New Classification Method for High-Volume Fly Ash: Performance Based on Coal Source and Particle Size" Materials 18, no. 17: 4145. https://doi.org/10.3390/ma18174145
APA StyleJi, X., Zhang, C., Yang, Y., Zhang, J., Tang, L., & Ji, D. (2025). A New Classification Method for High-Volume Fly Ash: Performance Based on Coal Source and Particle Size. Materials, 18(17), 4145. https://doi.org/10.3390/ma18174145










