Study on Loading of Na2WO4 and Silanization Treatment on Surface of Plasma Electrolytic Oxidation Coatings with Different Structures
Abstract
1. Introduction
2. Experiment and Method
2.1. Materials and Reagents
2.2. Preparation of PEO Coatings with Different Microstructures
2.3. Preparation of Na2WO4 Solution and Loading of Na2WO4
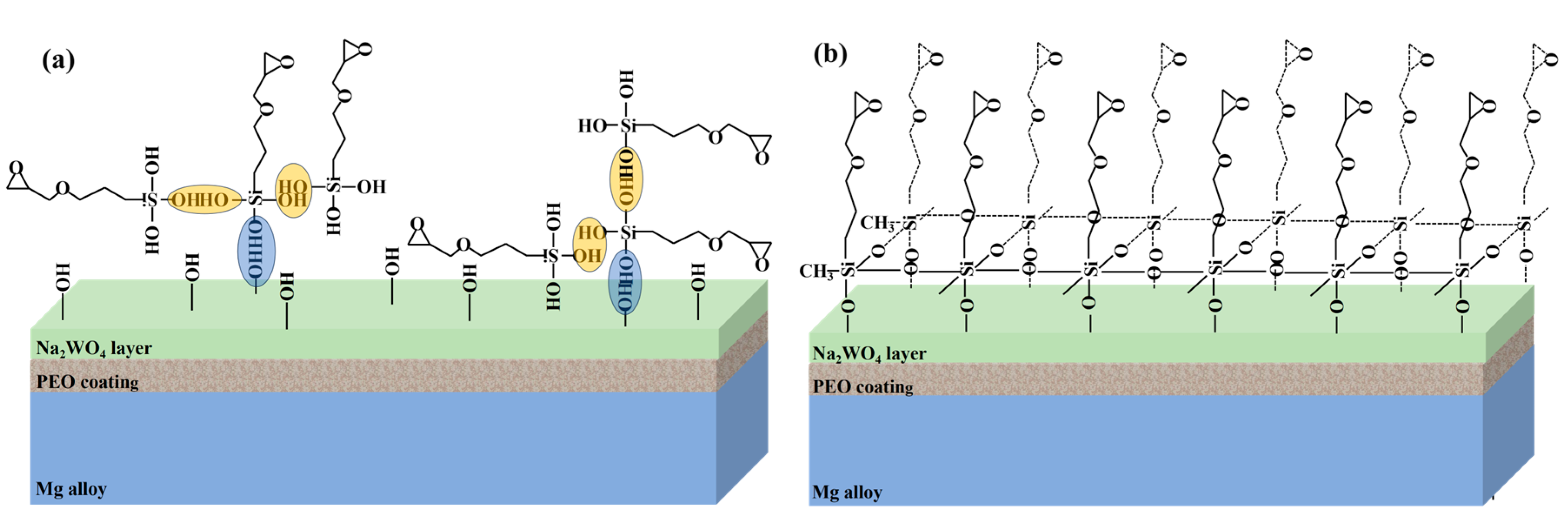
2.4. Preparation of Silane Solution and nP-W-SG Composite Coatings
2.5. Characterization
2.6. Performance Test
3. Results
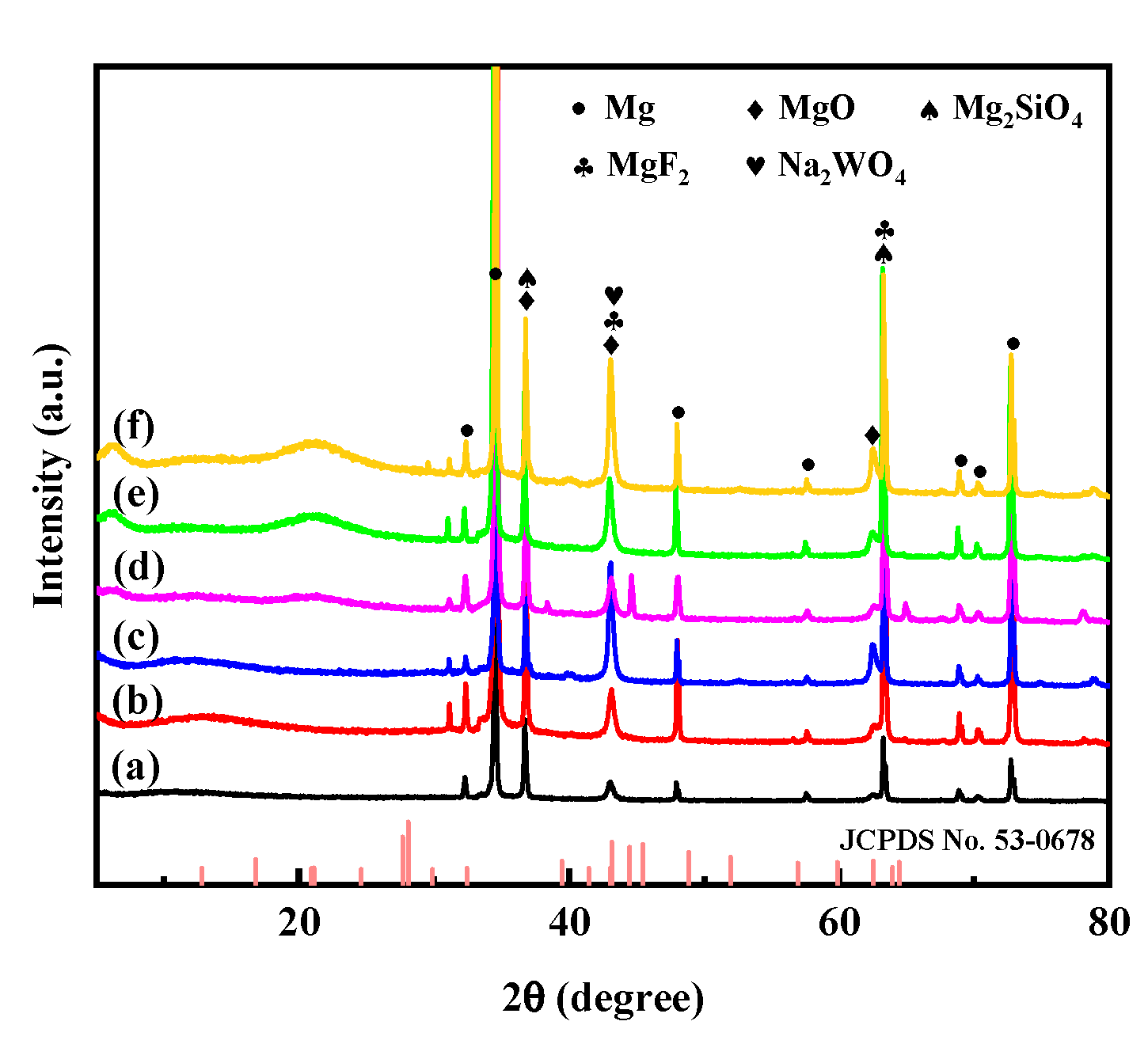
3.1. Phase Composition
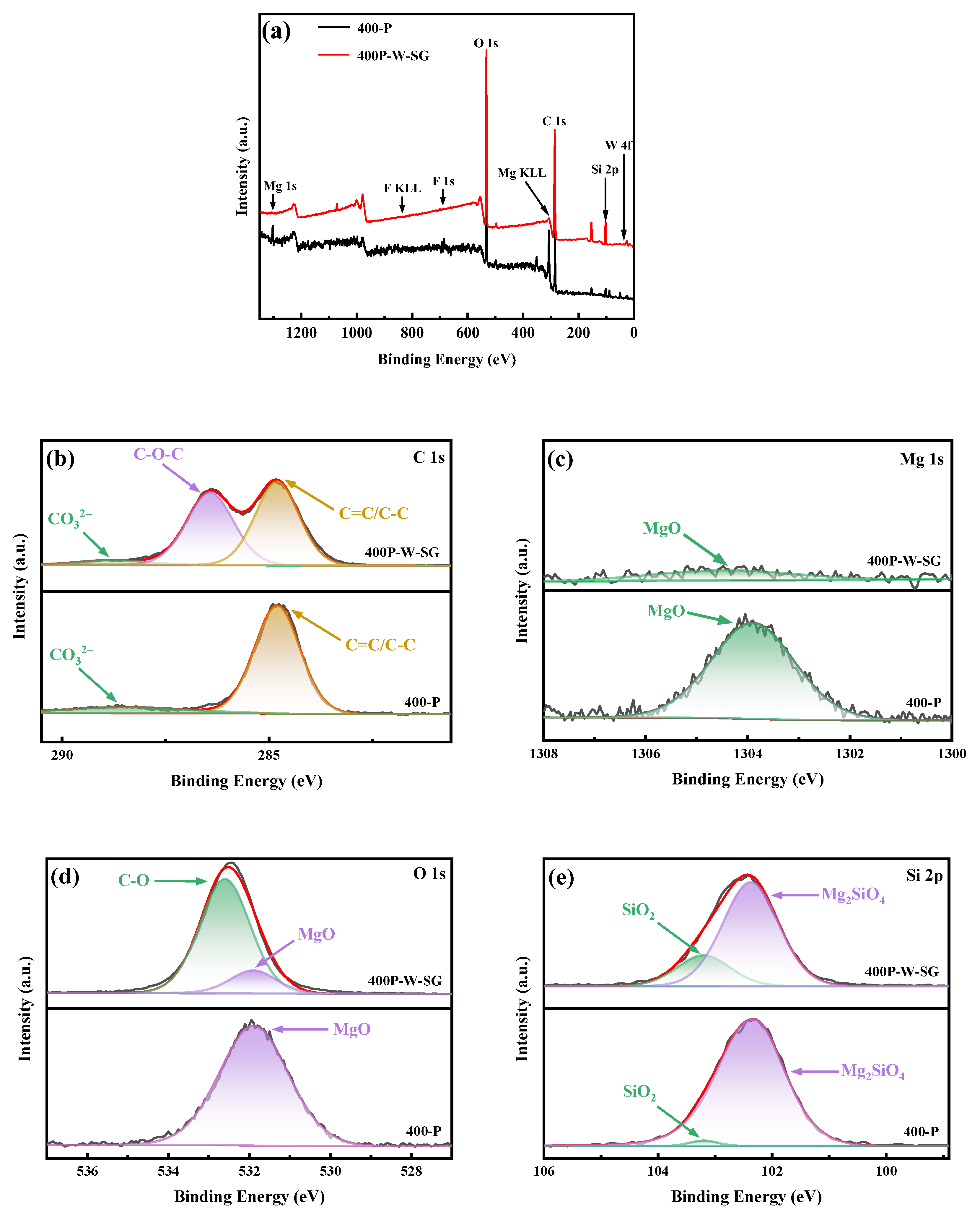
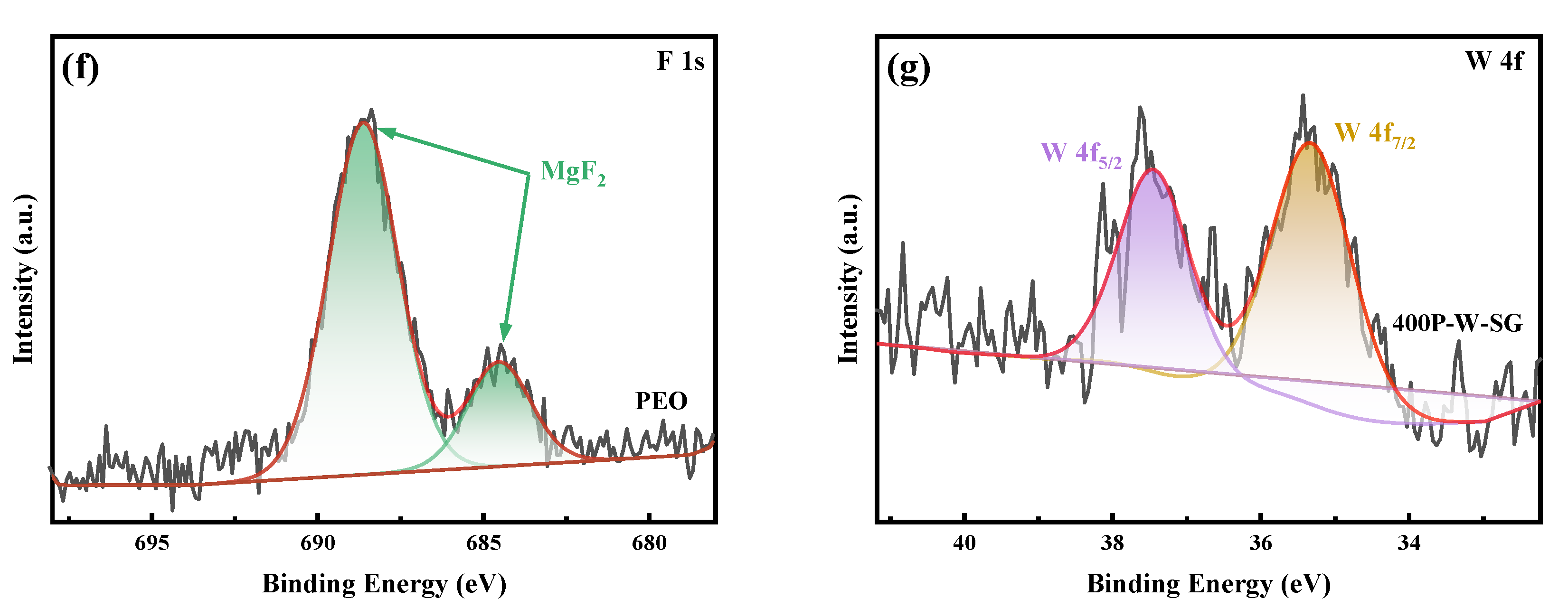
3.2. XPS Analysis
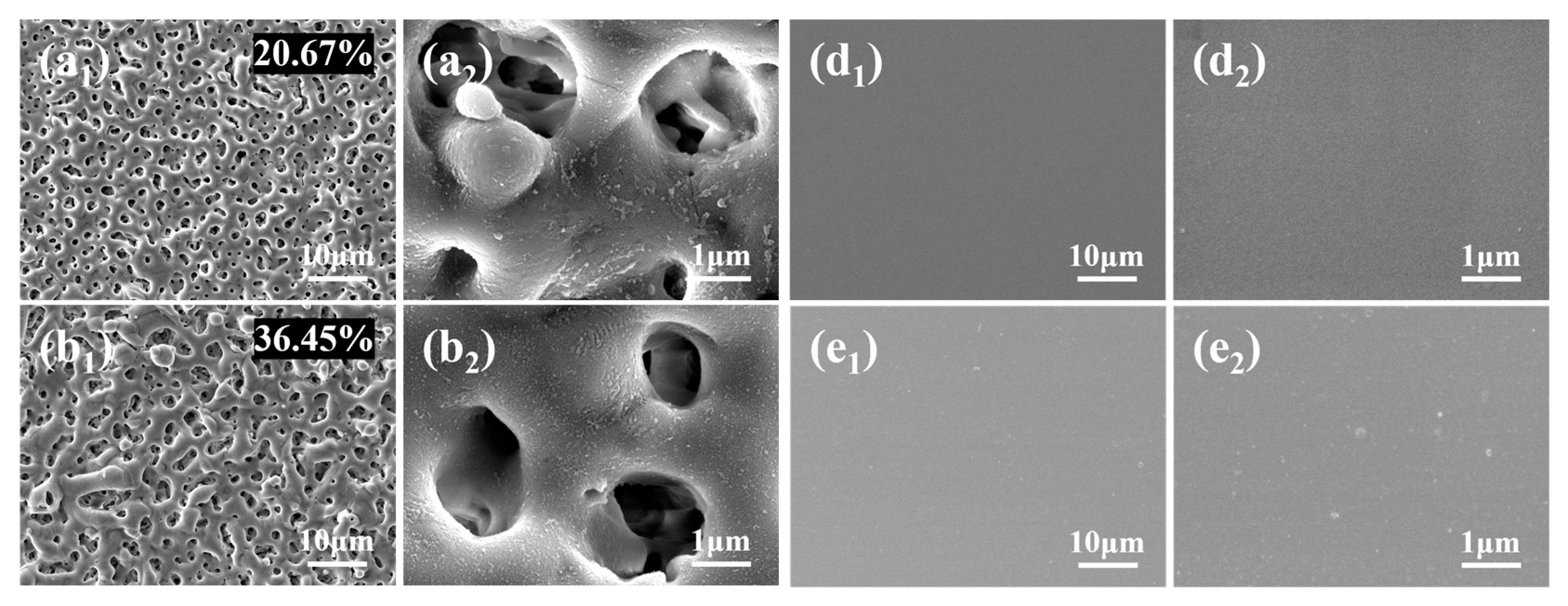
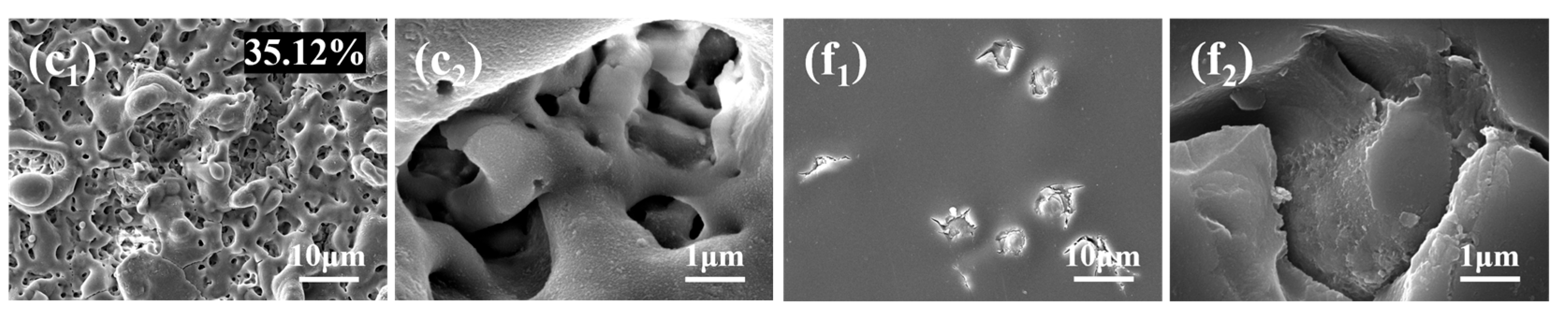
3.3. Surface Morphology and Elemental Composition and Distribution
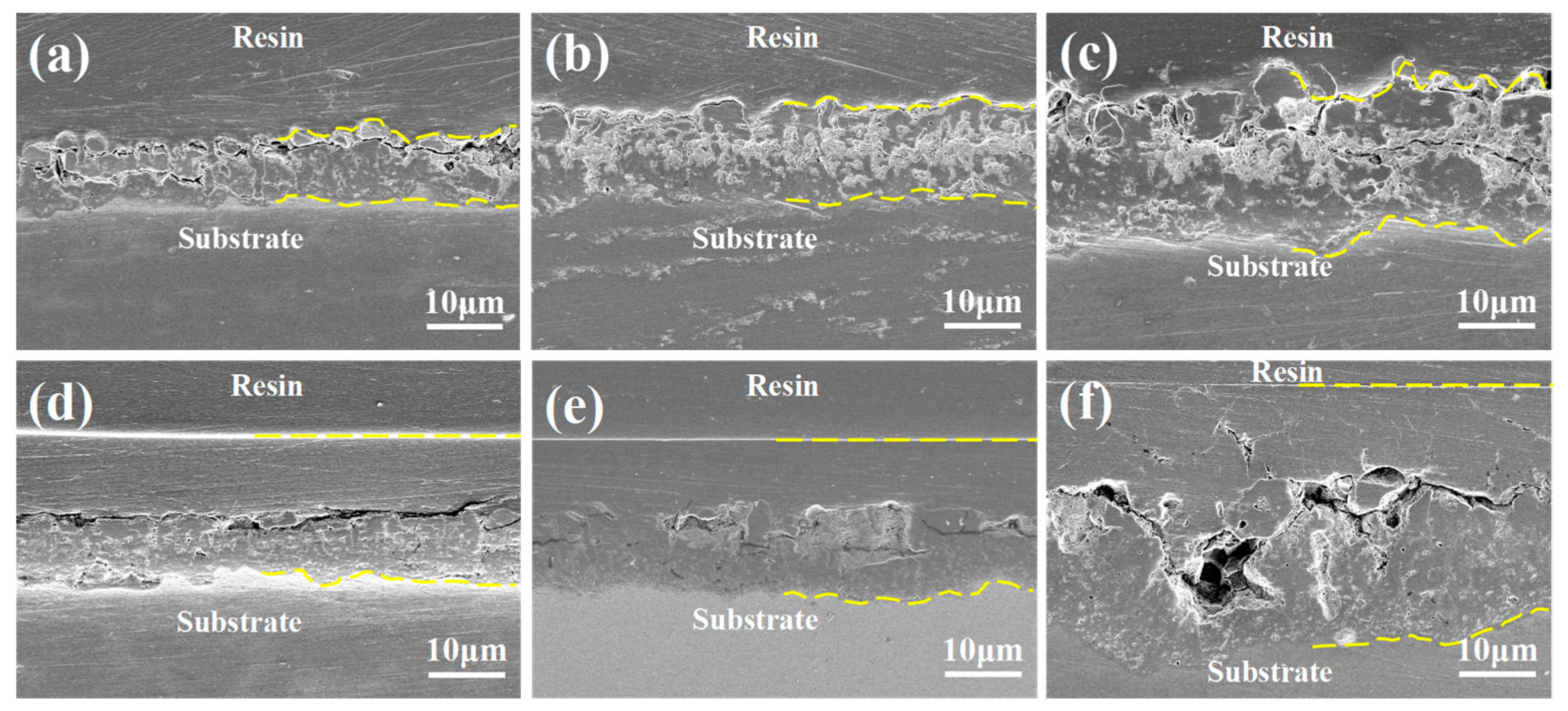
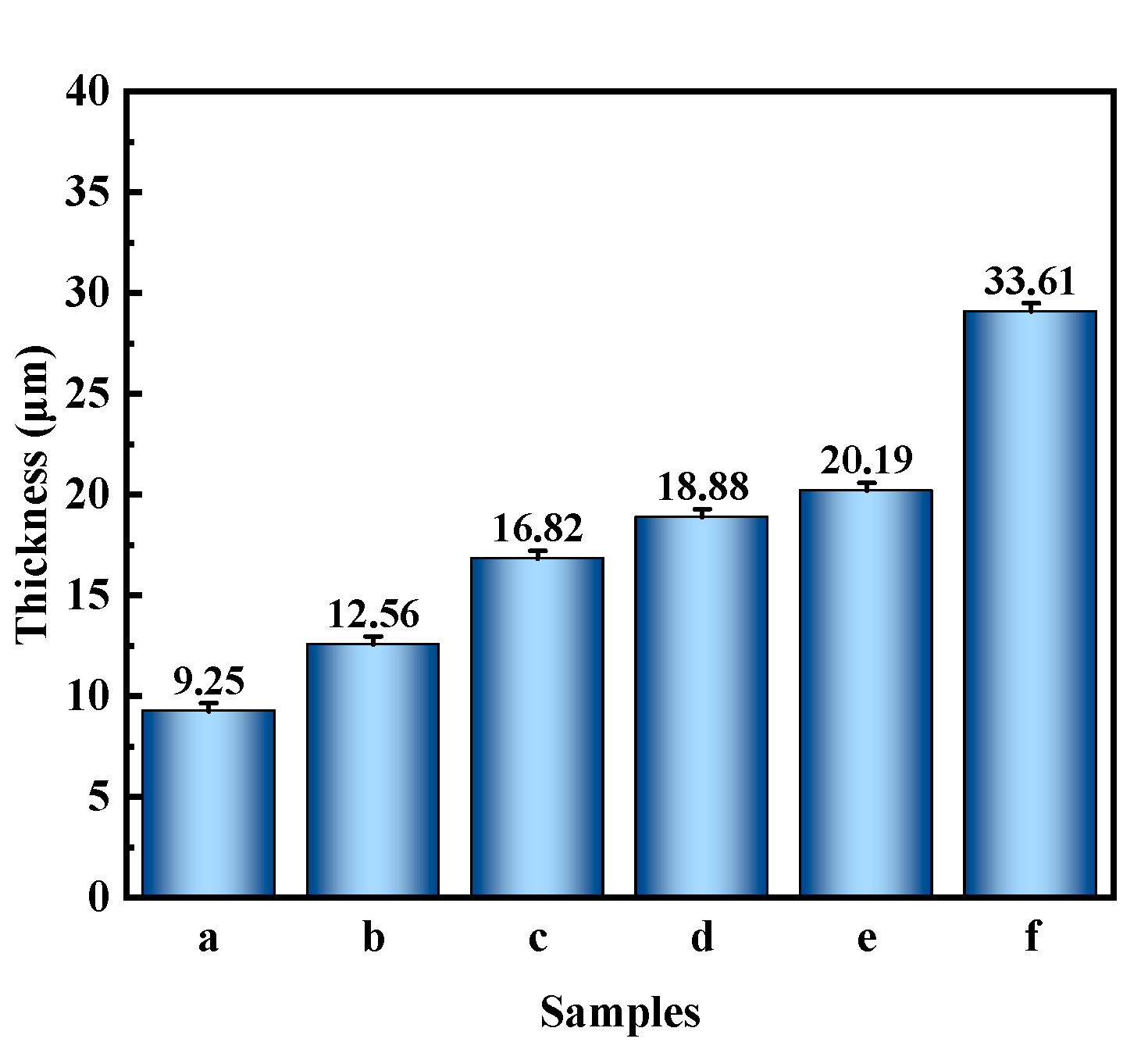
3.4. Cross-Sectional Morphology and Element Composition and Distribution
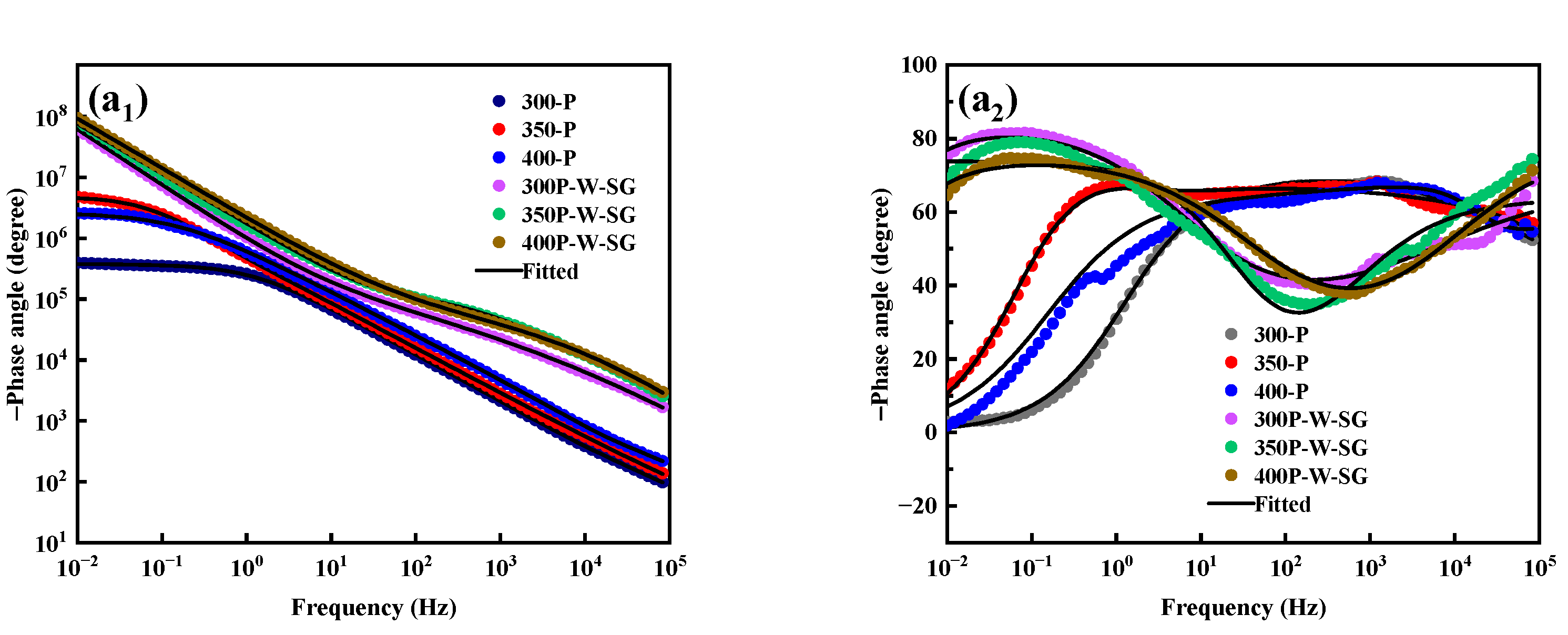
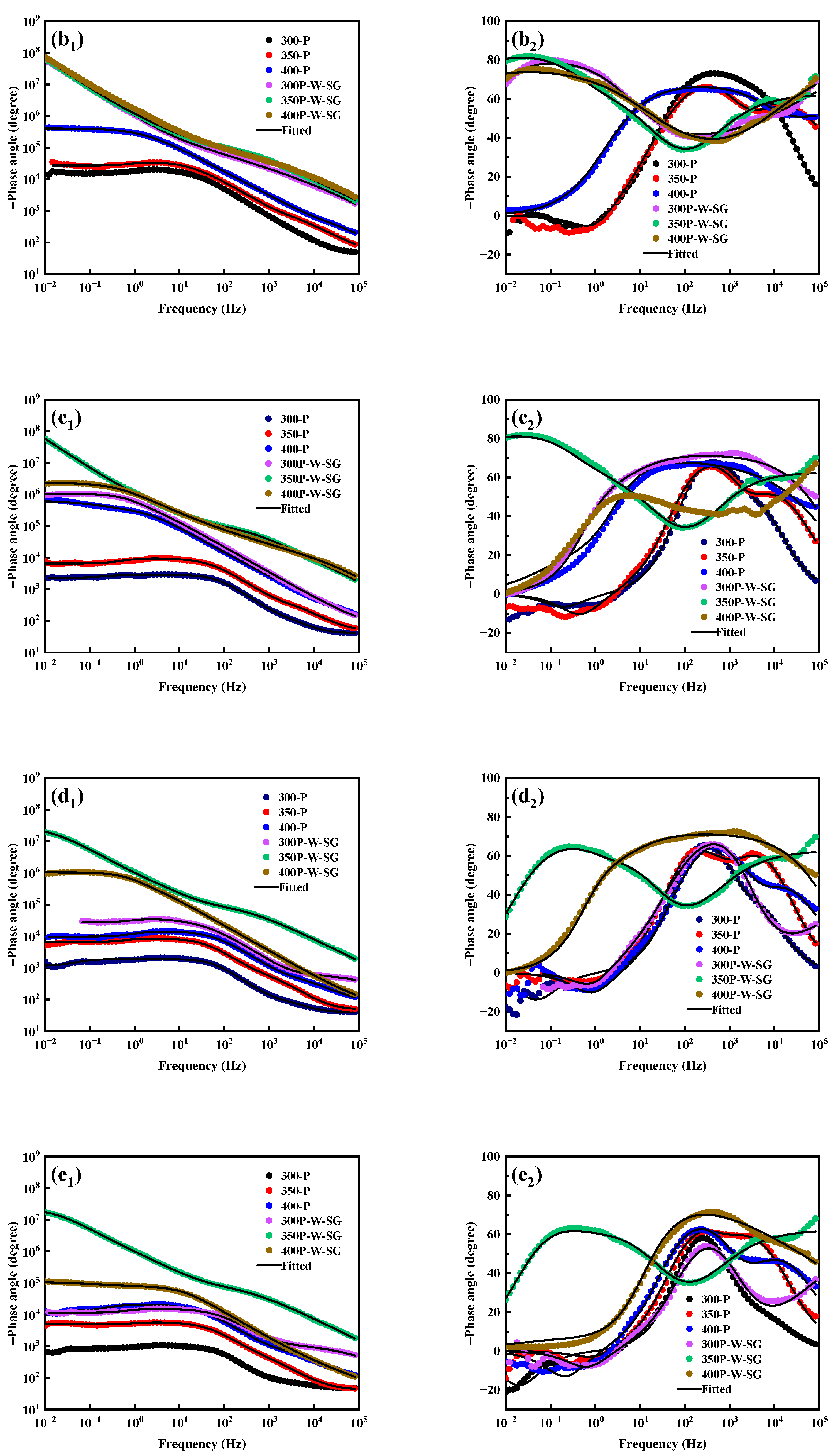
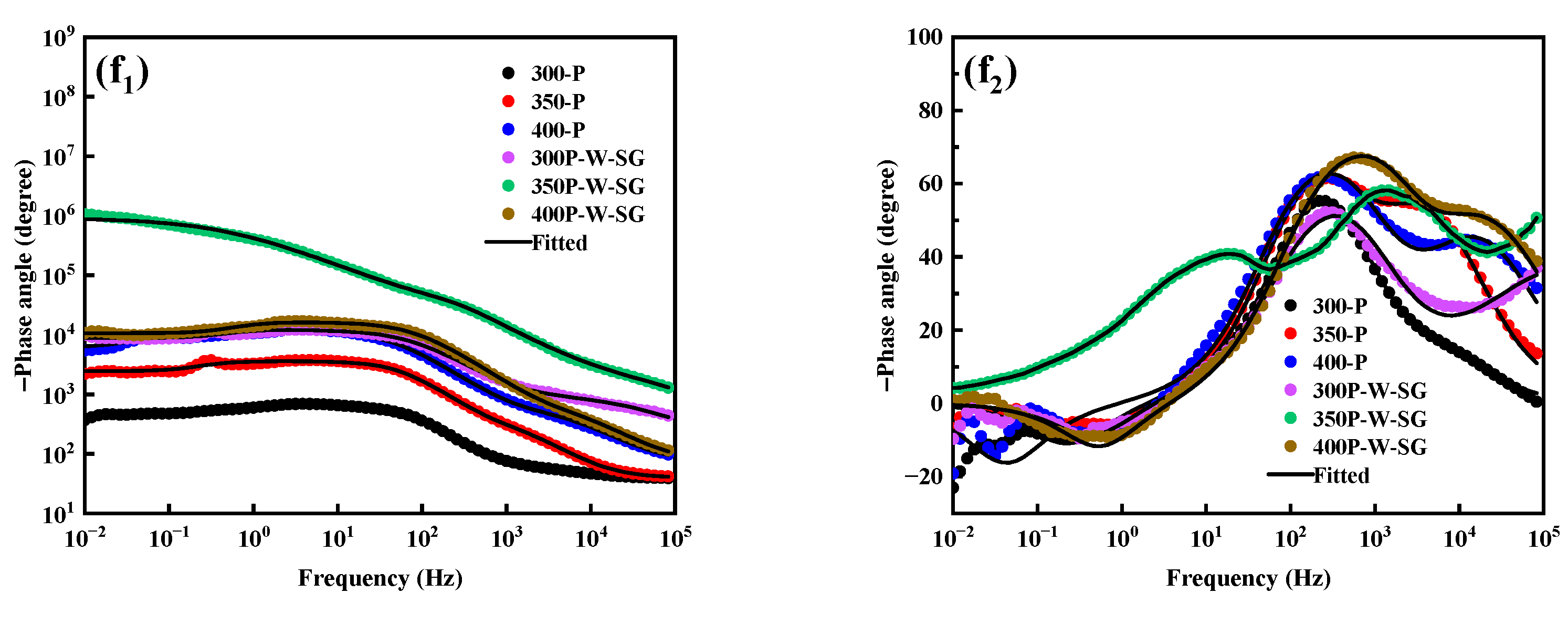
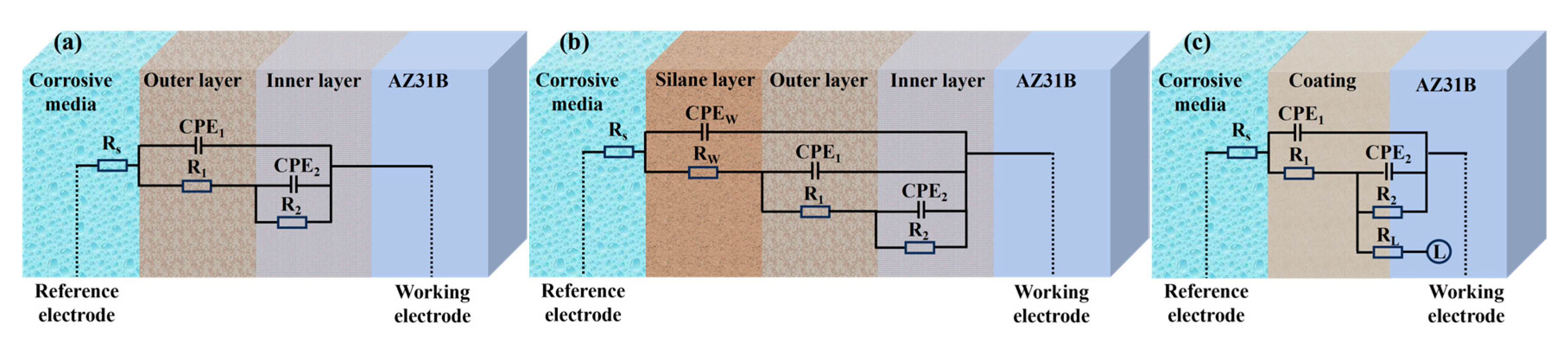
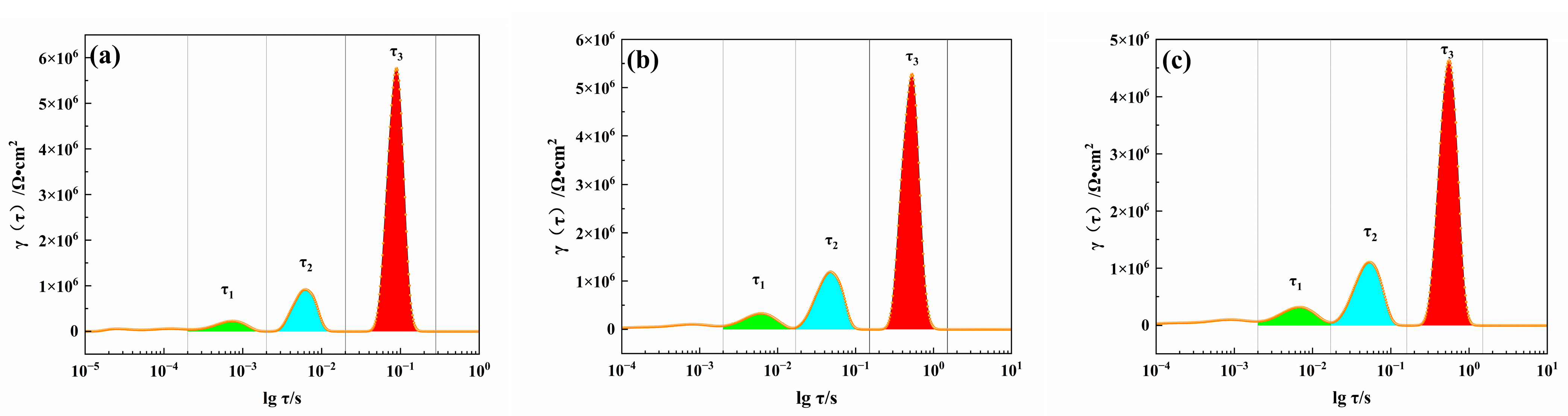
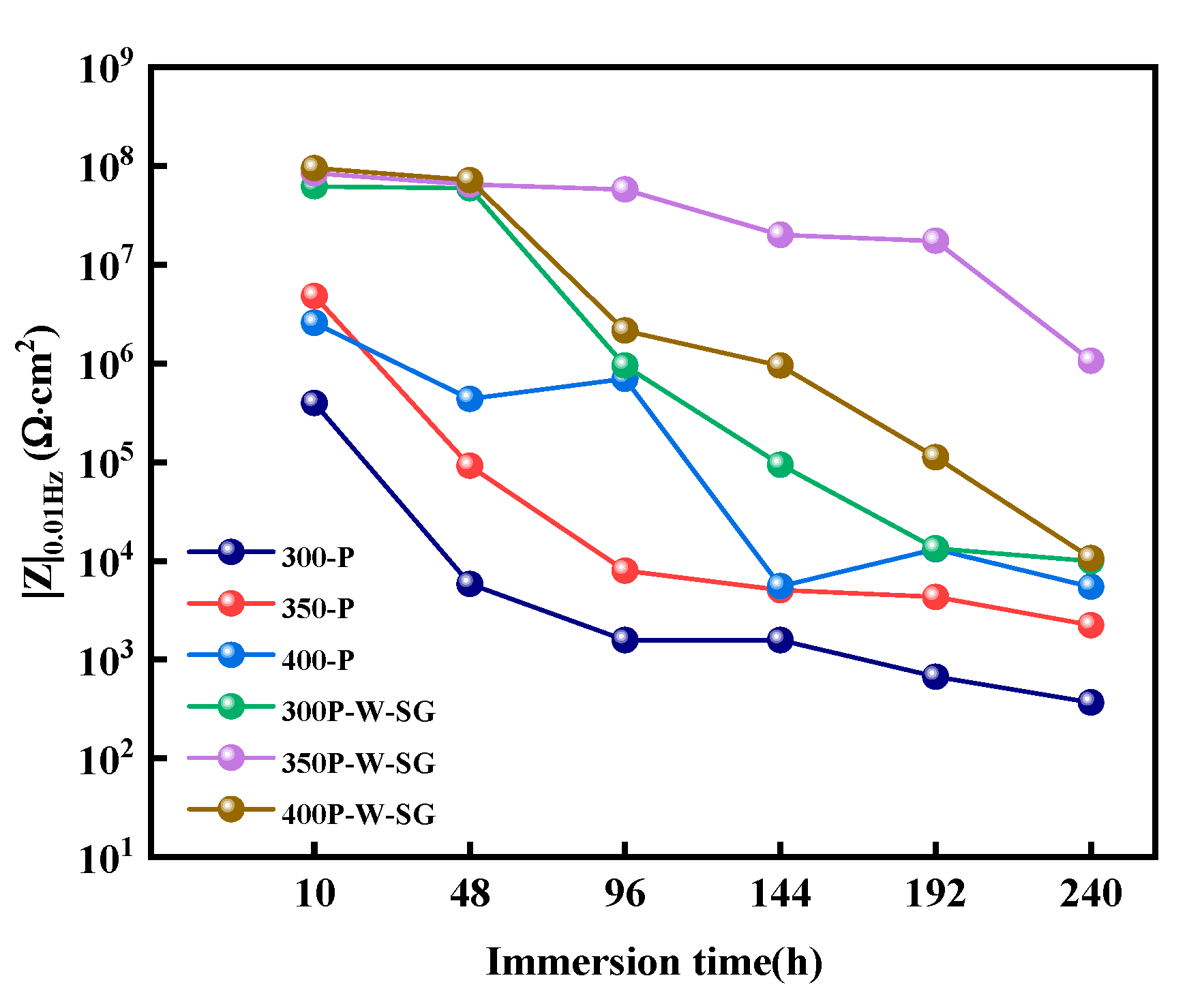
3.5. Corrosion Resistance
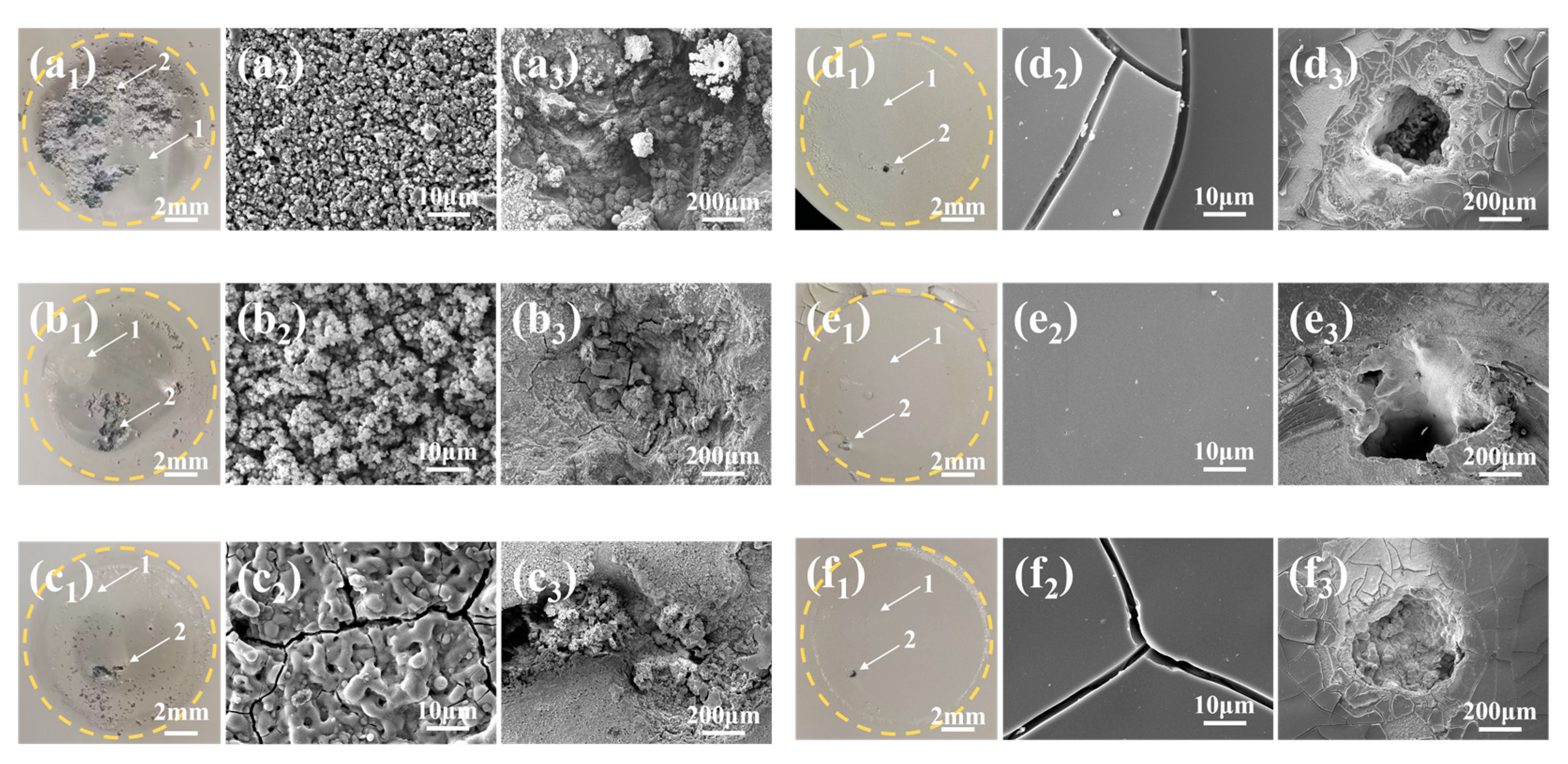
3.6. Corrosion Morphology and Elemental Composition and Distribution
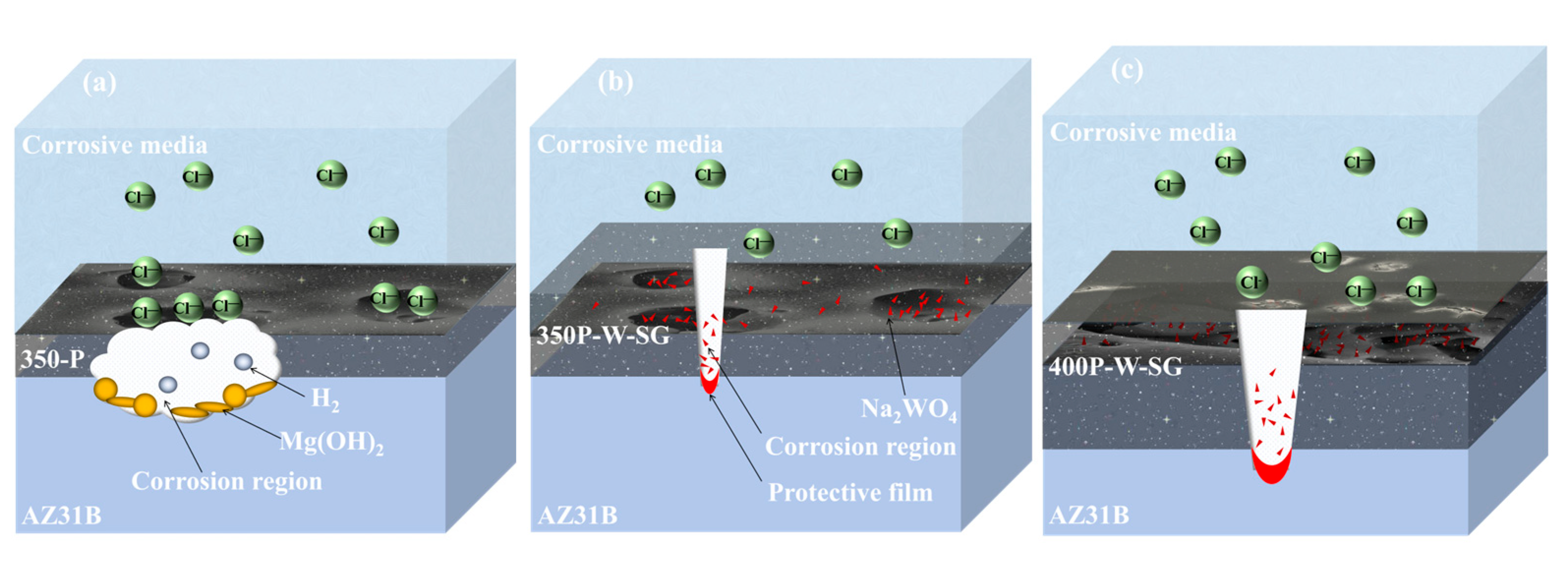
4. Discussion
5. Conclusions
- PEO coatings prepared at different voltages exhibit distinct microstructures: The 300 V coating (300-P) is thinner, with smaller micropores and lower porosity; the 350 V coating (350-P) is thicker, with uniformly distributed surface micropores and the highest porosity (36.45%); and the 400 V coating (400-P) has an uneven surface, inconsistent micropore size, non-uniform distribution, and high roughness, but its greater thickness endows it with the best corrosion resistance.
- When Na2WO4 is loaded onto the micropores and surface of the PEO coating, followed by silanization treatment, the microstructure and corrosion resistance of the PEO coating are enhanced to varying extents. Specifically, the 350-P coating exhibits the highest porosity and the greatest Na2WO4 content. Consequently, the fabricated composite coating (350P-W-SG) features a dense and uniform structure. In contrast, the composite coating prepared at 400 V (400P-W-SG) has defects such as pits. This is attributed to the inherent surface imperfections of the 400-P coating. Therefore, the 350P-W-SG composite coating exhibits the best corrosion resistance due to its greater thickness, higher Na2WO4 content, and dense structure. After immersion in a 3.5 wt.% NaCl solution for 240 h, the 350P-W-SG composite coating still retains its integrity, and its low-frequency impedance modulus |Z|0.01Hz reaches as high as 1.06 × 106 Ω·cm2.
- Further research is required to develop more corrosion-resistant and self-healing PEO-based composite coatings. This can be achieved by screening more effective single corrosion inhibitors or composite corrosion inhibitors, optimizing the preparation process parameters of the silane layer—such as selecting superior silane coupling agents, adjusting pH values, and controlling temperatures—or even replacing the sol–gel layer with highly corrosion-resistant and wear-resistant resin layers or layered double hydroxide layers.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kiani, F.; Lin, J.; Vahid, A.; Munir, K.; Wen, C.; Li, Y. Microstructures, mechanical properties, corrosion, and biocompatibility of extruded Mg-Zr-Sr-Ho alloys for biodegradable implant applications. J. Magnes. Alloys 2023, 11, 110–136. [Google Scholar] [CrossRef]
- Yang, Y.; Xiong, X.; Chen, J.; Peng, X.; Chen, D.; Pan, F. Research advances in magnesium and magnesium alloys worldwide in 2020. J. Magnes. Alloys 2021, 9, 705–747. [Google Scholar] [CrossRef]
- Wu, L.; Zhao, J.; Xie, Y.; Yang, Z. Progress of electroplating and electroless plating on magnesium alloy. Trans. Nonferrous Met. Soc. China 2010, 20, s630–s637. [Google Scholar] [CrossRef]
- Lee, Y.K.; Lee, K.; Jung, T. Study on microarc oxidation of AZ31B magnesium alloy in alkaline metal silicate solution. Electrochem. Commun. 2008, 10, 1716–1719. [Google Scholar] [CrossRef]
- Narayanan, T.S.; Park, I.S.; Lee, M.H. Strategies to improve the corrosion resistance of microarc oxidation (MAO) coated magnesium alloys for degradable implants: Prospects and challenges. Prog. Mater. Sci. 2014, 60, 1–71. [Google Scholar] [CrossRef]
- Treviño, M.; Mercado-Solis, R.D.; Colás, R.; Pérez, A.; Talamantes, J.; Velasco, A. Erosive wear of plasma electrolytic oxidation layers on aluminium alloy 6061. Wear 2013, 301, 434–441. [Google Scholar] [CrossRef]
- Zhu, J.; Jia, H.; Liao, K.; Li, X. Improvement on corrosion resistance of micro-arc oxidized AZ91D magnesium alloy by a pore-sealing coating. J. Alloys Compd. 2021, 889, 161460. [Google Scholar] [CrossRef]
- Yang, W.; Wang, A.; Jiang, B. Corrosion resistance of composite coating on magnesium alloy using combined microarc oxidation and inorganic sealing. Trans. Nonferrous Met. Soc. China 2012, 22, s760–s763. [Google Scholar] [CrossRef]
- Sopchenski, L.; Robert, J.; Touzin, M.; Tricoteaux, A.; Olivier, M.G. Improvement of wear and corrosion protection of PEO on AA2024 via sol-gel sealing. Surf. Coat. Technol. 2021, 417, 127195. [Google Scholar] [CrossRef]
- Pan, Y.; Lu, H.; Cheng, B.; Wang, C.; Dai, K.; Xu, G. Synergistic effect of polyurethane on pore-sealing and lubrication of microarc oxidation coating. Langmuir 2024, 40, 23268–23278. [Google Scholar] [CrossRef]
- Liu, J.; Yin, H.; Xu, Z.; Shao, Y.; Wang, Y. Improving the corrosion resistance of micro-arc oxidization film on AZ91D Mg alloy through silanization. Metals 2024, 14, 569. [Google Scholar] [CrossRef]
- Telmenbayar, L.; Ramu, A.G.; Erdenebat, T.O.; Choi, D. Anticorrosive lanthanum embedded PEO/GPTMS coating on magnesium alloy by plasma electrolytic oxidation with silanization. Mater. Today Commun. 2022, 33, 104662. [Google Scholar] [CrossRef]
- Huang, J.F.; Dun, Y.; Wan, Q.Y.; Wu, Z.R.; Zhao, X.H.; Tang, Y.M.; Zhang, X.F.; Zuo, Y. Improved Corrosion Resistance of MAO Coating on Mg-Li Alloy by RGO Modified Silanization. J. Alloys Compd. 2022, 929, 167283. [Google Scholar] [CrossRef]
- Wang, L.; Li, S.; Fu, J. Self-healing anti-corrosion coatings based on micron-nano containers with different structural morphologies. Prog. Org. Coat. 2023, 175, 107381. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, X.; Lamaka, S.V.; Ju, P.; Blawert, C.; Zhang, T.; Wang, F.; Zheludkevich, M.L. Active protection of Mg alloy by composite PEO coating loaded with corrosion inhibitors. Appl. Surf. Sci. 2019, 504, 144462. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Gnedenkov, S.V. Protective properties of inhibitor-containing composite coatings on a Mg alloy. Corros. Sci. 2016, 102, 348–354. [Google Scholar] [CrossRef]
- Gong, Y.; Geng, J.; Huang, J.; Chen, Z.; Wang, H. Self-healing performance and corrosion resistance of novel CeO2- sealed MAO film on aluminum alloy. Surf. Coat. Technol. 2021, 417, 127208. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, C.; Sui, P. Corrosion resistance and mechanisms of smart micro-arc oxidation/epoxy resin coatings on AZ31 Mg alloy: Strategic positioning of nanocontainers. J. Magnes. Alloys 2023, 11, 4562–4574. [Google Scholar] [CrossRef]
- Akbarzadeh, S.; Coelho, L.B.; Dangreau, L. Self-healing plasma electrolytic oxidation (PEO) coating developed by an assembly of corrosion inhibitive layer and sol-gel sealing on AA2024. Corros. Sci. 2023, 222, 111424. [Google Scholar] [CrossRef]
- Wang, Z.; An, L.; Chang, C. Study on Synergistically Improving Corrosion Resistance of Microarc Oxidation Coating on Magnesium Alloy by Loading of Sodium Tungstate and Silane Treatment. Materials 2025, 18, 361. [Google Scholar] [CrossRef]
- Shu, Y.F.; Fiang, B.; Wang, C.; Song, R.G. Effects of voltage on microstructure and properties of micro-arc oxidation ceramic coatings on AZ31B magnesium alloy under constant current-constant voltage operation mode. Anti-Corros. Methods Mater. 2023, 70, 513–521. [Google Scholar] [CrossRef]
- An, L.; Ma, Y.; Yan, X. Effects of electrical parameters and their interactions on plasma electrolytic oxidation coatings on aluminum substrates. Met. Soc. China 2020, 30, 883–895. [Google Scholar] [CrossRef]
- Mingo, B.; Guo, Y.; Leiva-Garcia, R. Smart functionalization of ceramic-coated AZ31 magnesium alloy. ACS Appl. Mater. Interfaces 2020, 12, 30833–30846. [Google Scholar] [CrossRef] [PubMed]
- Gnedenkov, S.V.; Sinebryukhov, S.L.; Sergienko, V.I. Electrochemical impedance simulation of a metal oxide heterostructure/electrolyte interface: A review. Russ. J. Electrochem. 2006, 42, 197–211. [Google Scholar] [CrossRef]
- Liang, J.; Srinivasan, P.B.; Blawert, C.; Dietzel, W. Comparison of electrochemical corrosion behaviour of MgO and ZrO2 coatings on AM50 magnesium alloy formed by plasma electrolytic oxidation. Corros. Sci. 2009, 51, 2483–2492. [Google Scholar] [CrossRef]
- Lee, K.M.; Ko, Y.G.; Shin, D.H. Microstructural characteristics of oxide layers formed on Mg-9 wt% Al-1 wt% Zn alloy via two-step plasma electrolytic oxidation. J. Alloys Compd. 2014, 615, S418–S422. [Google Scholar] [CrossRef]
- Pezzato, L.; Rigon, M.; Martucci, A.; Brunelli, K.; Dabalà, M. Plasma Electrolytic Oxidation (PEO) as pre-treatment for sol-gel coating on aluminum and magnesium alloys. Surf. Coat. Technol. 2019, 366, 114–123. [Google Scholar] [CrossRef]
- Zanotto, F.; Grassi, V.; Frignani, A.; Zucchi, F. Protection of the AZ31 magnesium alloy with cerium modified silane coatings. Mater. Chem. Phys. 2011, 129, 1–8. [Google Scholar] [CrossRef]
- Liu, D.; Song, Y.; Shan, D.; Han, E. Self-healing coatings prepared by loading interphase inhibitors into MAO coating of AM60 Mg alloy. J. Electrochem. Soc. 2018, 165, C412–C421. [Google Scholar] [CrossRef]
- Wang, T.; Wang, W. Distribution of Relaxation Time of Polydimethylsiloxane Coatings During Self-healing Process. J. Chin. Soc. Corros. Prot. 2023, 43, 337–344. [Google Scholar] [CrossRef]
- Ma, J.; Pang, X.; Chen, Z.; Du, L.; Qiu, P. Super adhesive, self-healing elastomer based on synergistic dual dynamic interactions for corrosion-resistant coatings. Appl. Mater. Today 2025, 44, 102682. [Google Scholar] [CrossRef]
- Brusciotti, F.; Snihirova, D.V.; Xue, H.; Montemor, M.F.; Lamaka, S.V.; Ferreira, M.G.S. Hybrid epoxy-silane coatings for improved corrosion protection of Mg alloy. Corros. Sci. 2013, 67, 82–90. [Google Scholar] [CrossRef]
- Li, C.Y.; Fan, X.L.; Cui, L.Y.; Zeng, R.C. Corrosion resistance and electrical conductivity of a nano ATO-doped MAO/methyltrimethoxysilane composite coating on magnesium alloy AZ31. Corros. Sci. 2020, 168, 108570. [Google Scholar] [CrossRef]
- Osipenko, M.A.; Karczewski, J.; Dominów, M.; Prze´ sniak-Welenc, M.; Makarava, I.V.; Kurilo, I.; Kharytonau, D.S.; Ryl, J. Multisine impedimetric monitoring with an in-depth distribution of relaxation times analysis of WE43 and AZ31 magnesium alloys corrosion. Measurement 2023, 222, 113683. [Google Scholar] [CrossRef]
- An, L.; Chang, C.; Kang, D.; Wang, Z.; Meng, L.; Peng, J. Study on Corrosion Resistance of Micro-arc Oxidation Modified Magnesium Alloy in Three Kinds of Saturated Salt Solutions. Mater. Sci. Eng. R Rep. 2023, 37, 21070250. [Google Scholar] [CrossRef]
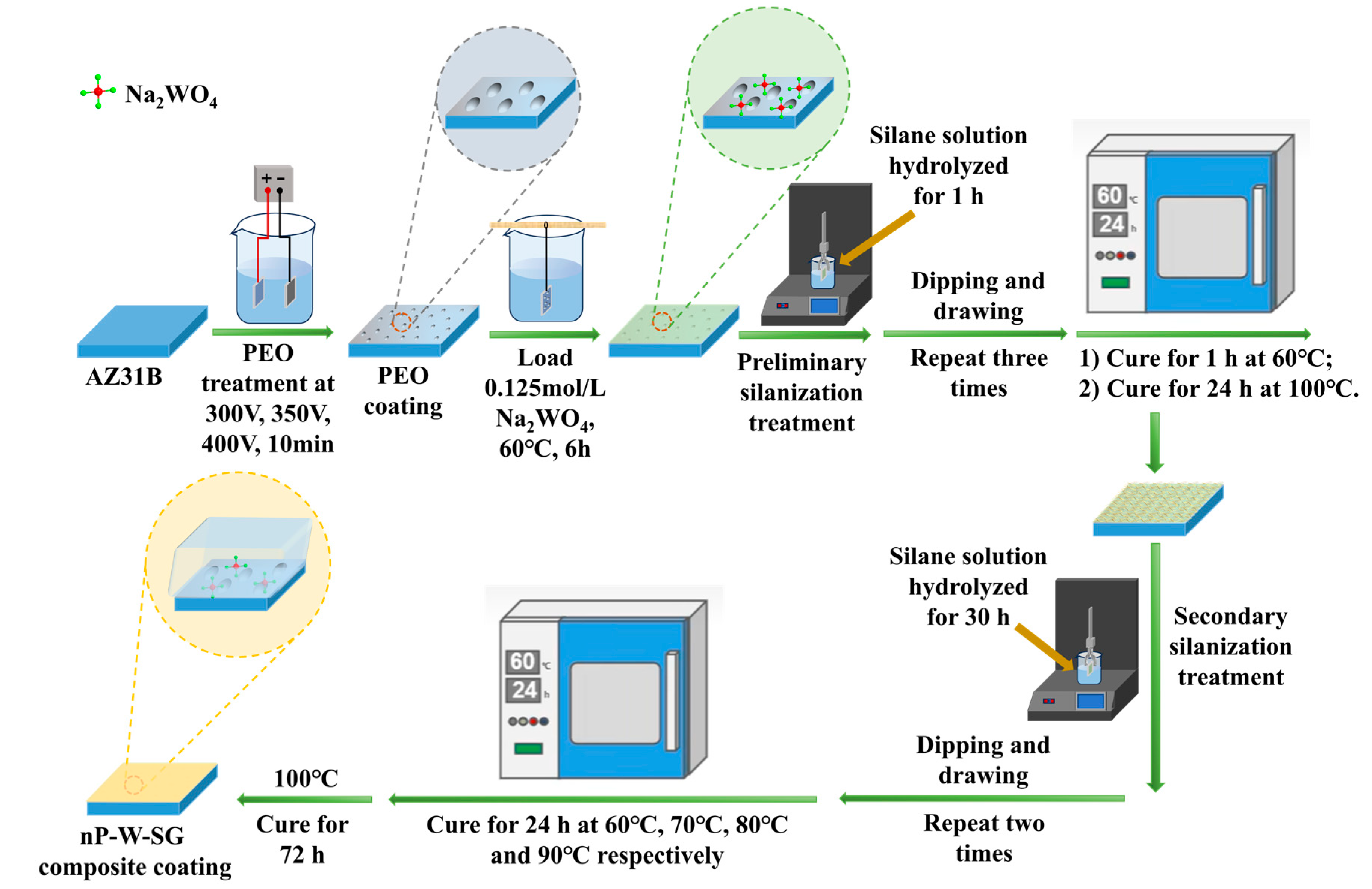
| Ab.Name | Voltage/V | Loading of Na2WO4 | Silanization Treatment |
|---|---|---|---|
| 300-P | 300 | - | - |
| 350-P | 350 | - | - |
| 400-P | 400 | - | - |
| 300P-W-SG | 300 | + | + |
| 350P-W-SG | 350 | + | + |
| 400P-W-SG | 400 | + | + |
| Sample | Mg (wt.%) | Al (wt.%) | F (wt.%) | O (wt.%) | Si (wt.%) | C (wt.%) | W (wt.%) |
|---|---|---|---|---|---|---|---|
| 300-P | 44.84 | 1.42 | 3.95 | 37.88 | 11.91 | - | - |
| 350-P | 44.10 | 1.42 | 4.32 | 37.94 | 12.22 | - | - |
| 400-P | 43.82 | 1.61 | 3.91 | 38.08 | 12.58 | - | - |
| 300P-W-SG | 0.04 | 0.10 | 0.03 | 37.21 | 21.29 | 39.61 | 1.74 |
| 350P-W-SG | 0.02 | 0.11 | 0.11 | 36.26 | 20.59 | 40.24 | 2.68 |
| 400P-W-SG | 0.57 | 0.11 | 0.09 | 37.19 | 21.16 | 38.74 | 2.13 |
| Sample | Mg (wt.%) | Al (wt.%) | Si (wt.%) | O (wt.%) | F (wt.%) | C (wt.%) | W (wt.%) |
|---|---|---|---|---|---|---|---|
| 300-P | 36.49 | 0.69 | 0.82 | 10.40 | 0.90 | 50.69 | - |
| 350-P | 33.58 | 0.49 | 1.23 | 14.63 | 1.85 | 48.22 | - |
| 400-P | 30.67 | 0.70 | 2.01 | 18.73 | 2.20 | 45.69 | - |
| 300P-W-SG | 34.32 | 0.62 | 2.96 | 15.71 | 1.62 | 43.95 | 0.82 |
| 350P-W-SG | 41.59 | 0.72 | 4.77 | 12.85 | 0.87 | 37.85 | 1.36 |
| 400P-W-SG | 27.27 | 0.48 | 6.81 | 23.80 | 2.72 | 37.60 | 1.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, D.; Wang, Z.; Qiao, J.; An, L.; Chang, C.; Meng, L.; Wang, Z.; Yang, Y. Study on Loading of Na2WO4 and Silanization Treatment on Surface of Plasma Electrolytic Oxidation Coatings with Different Structures. Materials 2025, 18, 4146. https://doi.org/10.3390/ma18174146
Lei D, Wang Z, Qiao J, An L, Chang C, Meng L, Wang Z, Yang Y. Study on Loading of Na2WO4 and Silanization Treatment on Surface of Plasma Electrolytic Oxidation Coatings with Different Structures. Materials. 2025; 18(17):4146. https://doi.org/10.3390/ma18174146
Chicago/Turabian StyleLei, Donghao, Ziyi Wang, Jinjun Qiao, Lingyun An, Chenggong Chang, Leichao Meng, Zhanying Wang, and Yanping Yang. 2025. "Study on Loading of Na2WO4 and Silanization Treatment on Surface of Plasma Electrolytic Oxidation Coatings with Different Structures" Materials 18, no. 17: 4146. https://doi.org/10.3390/ma18174146
APA StyleLei, D., Wang, Z., Qiao, J., An, L., Chang, C., Meng, L., Wang, Z., & Yang, Y. (2025). Study on Loading of Na2WO4 and Silanization Treatment on Surface of Plasma Electrolytic Oxidation Coatings with Different Structures. Materials, 18(17), 4146. https://doi.org/10.3390/ma18174146






