Effect of Copper Alloying on Hydrogen Embrittlement of Fe-28Mn-10Al-1C Austenitic Low-Density Steel
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrochemical Hydrogen Charging and TDS
2.3. The Slow Strain Rate Test (SSRT)
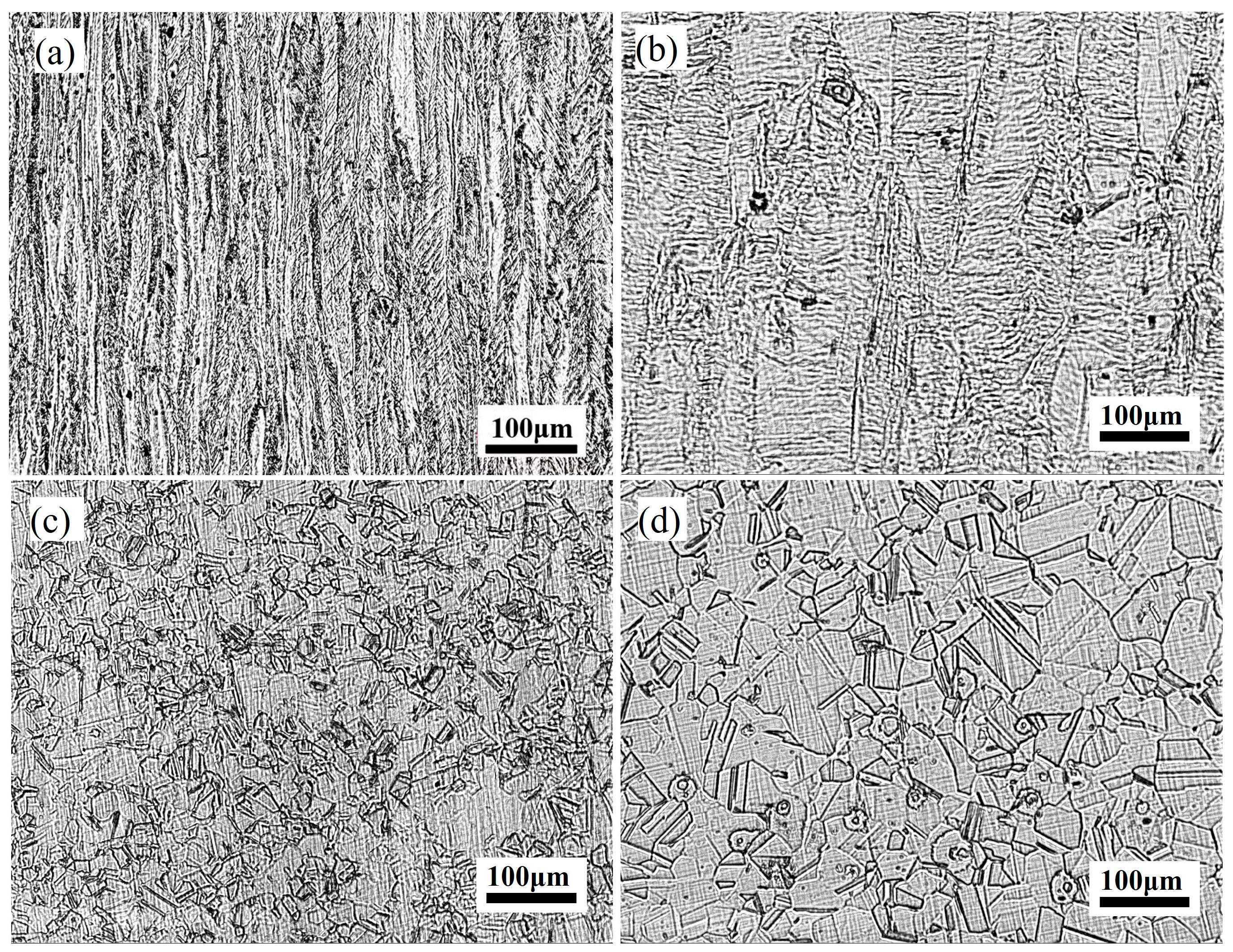
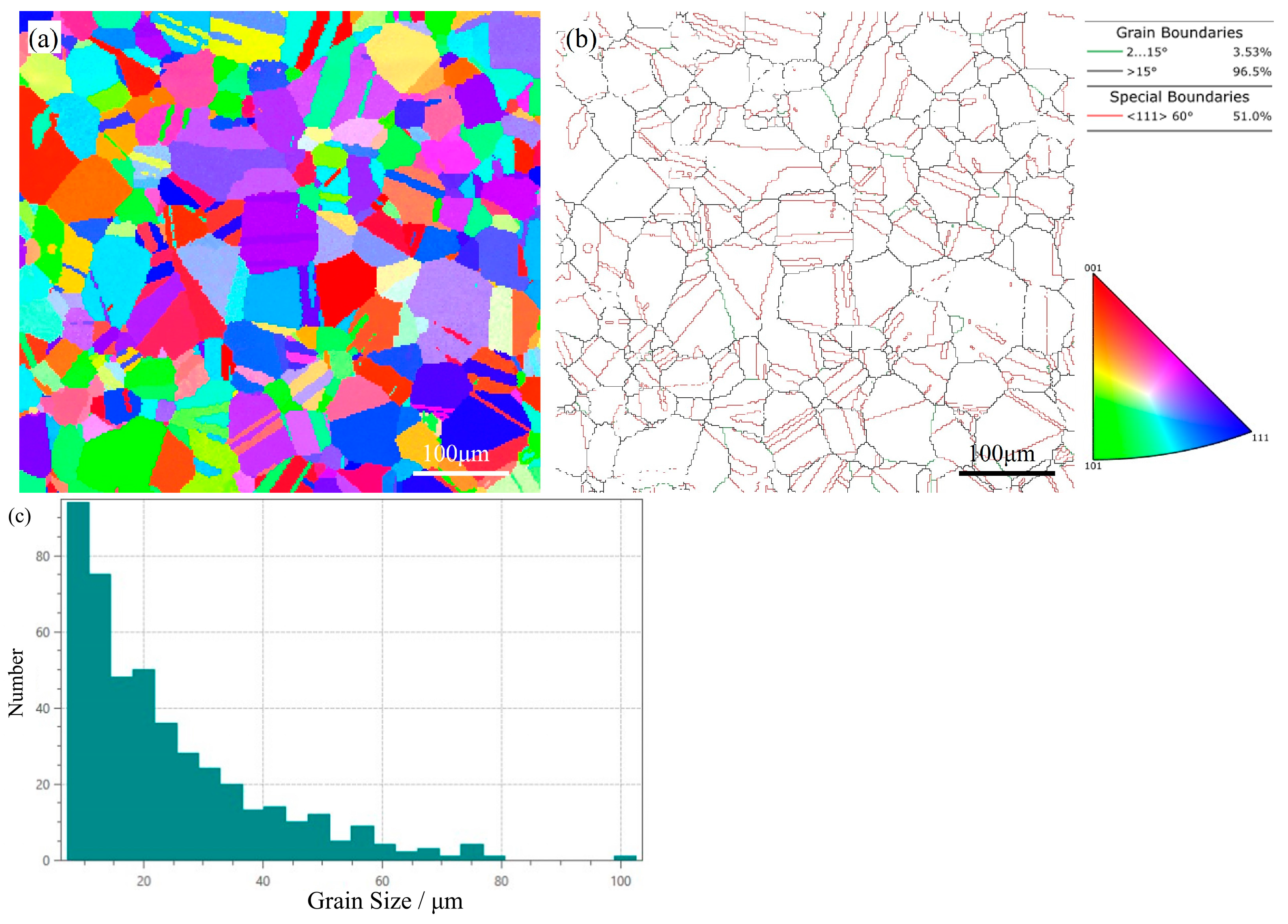
2.4. Microstructural Characterization
3. Results
3.1. Hydrogen Desorption Behavior
3.2. Hydrogen Embrittlement Susceptibility
4. Discussion
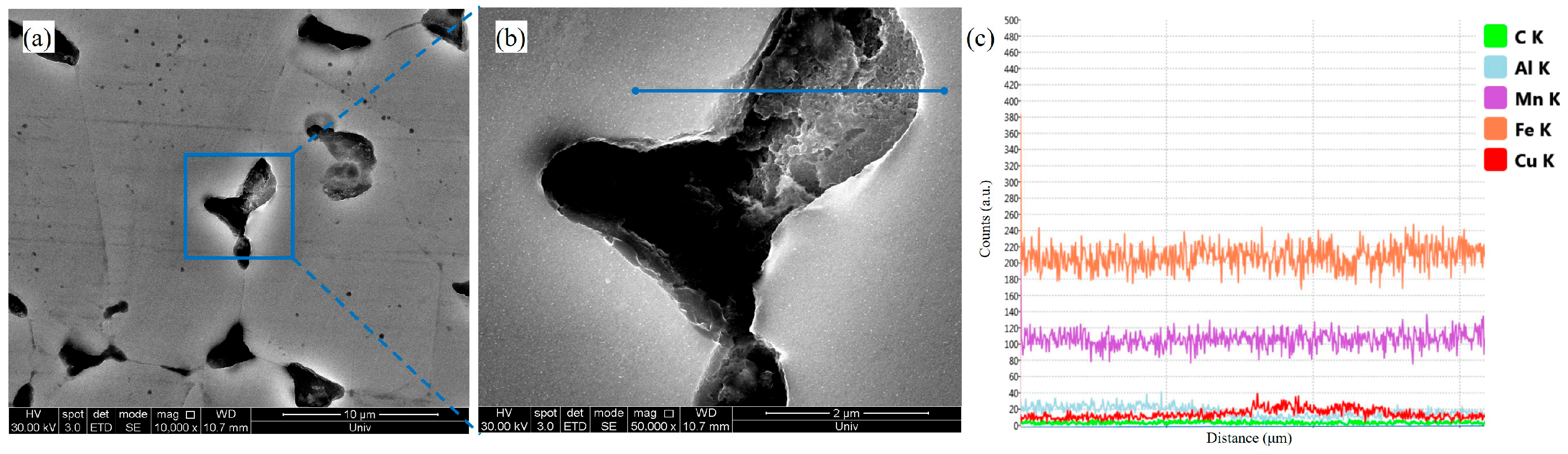
4.1. Influence of Annealing Treatment and Cu Addition on Hydrogen Content Variation in Steel
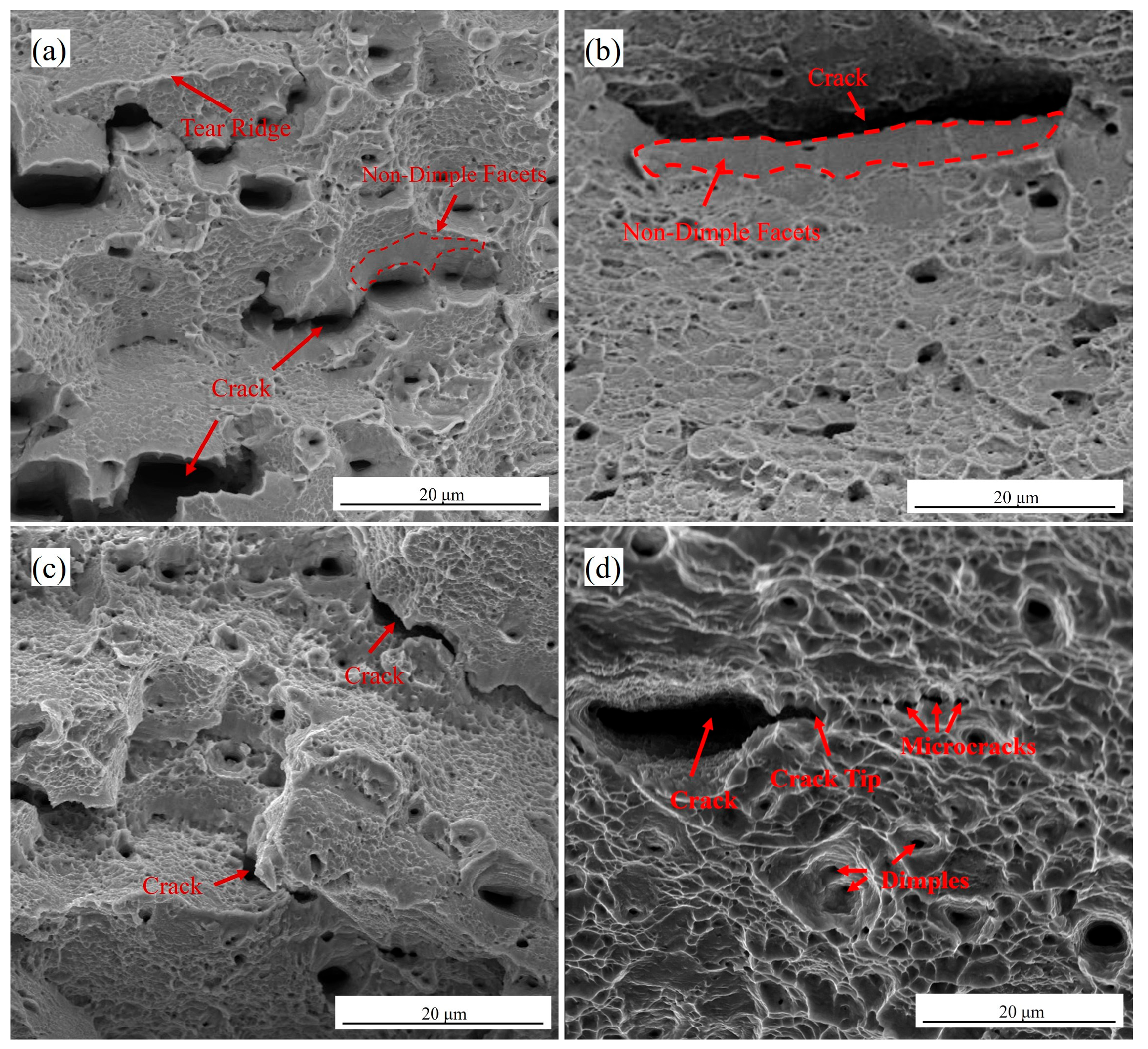
4.2. Hydrogen Embrittlement Mechanism Analysis
5. Conclusions
- Annealing treatment and Cu addition have little effect on the activation energy of reversible hydrogen traps; based on the activation energy, the types of reversible hydrogen traps are determined to be the same. Compared with 0Cu steels, the reversible hydrogen contents in LZ-3Cu and TH-3Cu decrease by 0.1 wt.ppm and 0.2 wt.ppm, respectively, indicating that Cu addition can inhibit the increase in reversible hydrogen content to a certain extent.
- After hydrogen charging, both the UTS and UE of the specimens decrease. The hydrogen embrittlement index (HEI) of LZ-0Cu decreases from 21.3% to 16.0% compared with that of LZ-3Cu, and the HEI of TH-0Cu decreases from 13.5% to 9.6% compared with that of TH-3Cu. TH-3Cu exhibits the lowest HEI. Cu precipitates homogenize the microstructure. This leads to a transition of hydrogen distribution from severe local concentration to a more uniform state. Meanwhile, as stable irreversible hydrogen traps, Cu precipitates can hinder hydrogen diffusion and segregation.
- Due to Cu alloying, the fracture morphology tends to transform from mixed brittle-ductile fracture to ductile fracture, and the fracture mechanism also changes from the coupled HEDE and HELP mechanism to the HELP mechanism. In conclusion, copper alloying can significantly improve the hydrogen embrittlement resistance of austenitic low-density steels.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ren, P.; Chen, X.P.; Wang, C.Y.; Zhou, Y.X.; Cao, W.Q.; Liu, Q. Evolution of Microstructure, Texture and Mechanical Properties of Fe–30Mn–11Al–1.2C Low-Density Steel during Cold Rolling. Mater. Charact. 2021, 174, 111013. [Google Scholar] [CrossRef]
- Kazakova, A.A.; Churyumov, A.Y. Microstructure and Mechanical Properties of Fe-30Mn-10Al-3.3Si-1C Light-Weight Steel. Materials 2025, 18, 1258. [Google Scholar] [CrossRef]
- Saxena, A.; Findley, K.O. A Model to Account for the Effects of Load Ratio and Hydrogen Pressure on the Fatigue Crack Growth Behavior of Pressure Vessel Steels. Materials 2024, 17, 4308. [Google Scholar] [CrossRef]
- Ahad, M.T.; Bhuiyan, M.M.H.; Sakib, A.N.; Becerril Corral, A.; Siddique, Z. An Overview of Challenges for the Future of Hydrogen. Materials 2023, 16, 6680. [Google Scholar] [CrossRef]
- Lee, Y.J.; Ha, J.; Choi, S.J.; Kim, H.I.; Ryu, S.; Kim, Y.; Youn, Y.-S. Decreasing Hydrogen Content within Zirconium Using Au and Pd Nanoparticles as Sacrificial Agents under Pressurized Water at High Temperature. Materials 2023, 16, 6164. [Google Scholar] [CrossRef]
- Nguyen, T.-D.; Ansari, N.; Lee, K.H.; Lee, D.-H.; Han, J.H.; Lee, S.Y. Effect of Strain Rate on Hydrogen Embrittlement of Ti6Al4V Alloy. Materials 2024, 17, 1100. [Google Scholar] [CrossRef]
- Kim, S.-G.; Kim, J.-Y.; Hwang, B. Effect of Tempering Temperature on Hydrogen Embrittlement of SCM440 Tempered Martensitic Steel. Materials 2023, 16, 5709. [Google Scholar] [CrossRef]
- Du, H.; An, N.; Wang, X.; Li, Y.; Liu, Z.; Jin, A.; Yang, R.; Pan, Y.; Li, X. Enhancing the SCC Resistance of the Anchor Steel with Microalloying in a Simulated Mine Environment. Materials 2023, 16, 5965. [Google Scholar] [CrossRef]
- Oh, D.-K.; Kim, S.-G.; Shin, S.-H.; Hwang, B. Hydrogen Embrittlement Behavior of API X70 Linepipe Steel under Ex Situ and In Situ Hydrogen Charging. Materials 2024, 17, 4887. [Google Scholar] [CrossRef]
- Zhou, C.; Zhou, H.; Zhang, L. The Impact of Impurity Gases on the Hydrogen Embrittlement Behavior of Pipeline Steel in High-Pressure H2 Environments. Materials 2024, 17, 2157. [Google Scholar] [CrossRef]
- Lee, M.; Kim, H.; Son, S.-W.; Ahn, J. The Influence of Cr2N Addition and Ni/Mn Ratio Variation on Mechanical and Corrosion Properties of HIP-Sintered 316L Stainless Steel. Materials 2025, 18, 2722. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, J.; Zhao, B.; Zhang, E. The Study on Fatigue Crack Growth Rate of 4130X Material under Different Hydrogen Corrosion Conditions. Materials 2024, 17, 257. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Chen, D.; Chiang, M.-H.; Cheng, G.-J.; Lin, H.-C.; Yen, H.-W. Response of Hydrogen Desorption and Hydrogen Embrittlement to Precipitation of Nanometer-Sized Copper in Tempered Martensitic Low-Carbon Steel. JOM 2019, 71, 1349–1356. [Google Scholar] [CrossRef]
- Nguyen, T.-D.; Singh, C.; Lee, D.-H.; Kim, Y.S.; Lee, T.; Lee, S.Y. Deciphering Hydrogen Embrittlement Mechanisms in Ti6Al4V Alloy: Role of Solute Hydrogen and Hydride Phase. Materials 2024, 17, 1178. [Google Scholar] [CrossRef]
- Yang, M.; Liu, X.; Xing, L.; Chen, Z. Corrosion and Mechanical Behavior of the As-Cast and Solid-Solution-Treated AM50 Magnesium Alloy in Different Media. Materials 2023, 16, 2406. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, L. Effect of Hydrogen on High Cycle Fatigue Properties of L360 Pipeline Steel Notched Specimens. Materials 2024, 17, 5612. [Google Scholar] [CrossRef]
- Kong, X.; Jiang, H.; Lv, Y.; Xie, W.; Lu, S.; Xu, D. Research Progress on the Hydrogen Embrittlement Resistance Performance of High-Entropy Alloys. Materials 2025, 18, 2862. [Google Scholar] [CrossRef]
- Hempel, C.; Mandel, M.; Quitzke, C.; Wendler, M.; Kreschel, T.; Volkova, O.; Krüger, L. Hydrogen Diffusion in Deformed Austenitic TRIP Steel—A Study of Mathematical Prediction and Experimental Validation. Materials 2024, 17, 6114. [Google Scholar] [CrossRef]
- Villalobos, J.C.; Bedolla-Jacuinde, A.; Torres-Islas, Á.; Velasco-Plascencia, M.; Villanueva, H.; Rojas, H.; Del-Pozo, A. Evaluation of Hydrogen Embrittlement’s Effects on the Impact Toughness of Martensitic Ultra-High-Strength Steels as a Function of the Cathodic Charging Time. Materials 2025, 18, 764. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, M.; Li, J.; Shi, J.; Hui, W.; Dong, H. Hydrogen Embrittlement Susceptibility of 1500 MPa Grade 40CrNi3MoV Steels. Acta Metall. Sin. 2008, 44, 403–408. [Google Scholar]
- Brahimi, S.V.; Sriraman, K.R.; Yue, S. Hydrogen Embrittlement Characteristics of Two Tempered Martensitic Steel Alloys for High-Strength Bolting. Proc. Inst. Mech. Eng. C-J. Mech. Eng. Sci. 2017, 231, 3214–3227. [Google Scholar] [CrossRef]
- Brahimi, S.V.; Yue, S.; Sriraman, K.R. Alloy and Composition Dependence of Hydrogen Embrittlement Susceptibility in High-Strength Steel Fasteners. Philos. Trans. R. Soc. A-Math. Phys. Eng. Sci. 2017, 375, 20160407. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, Y.; Hou, H. Core-Shell Structure Nanoprecipitates in Fe-xCu-3.0Mn-1.5Ni-1.5Al Alloys: A Phase Field Study. Prog. Nat. Sci. Mater. Int. 2022, 32, 358–368. [Google Scholar] [CrossRef]
- Liu, D.; Tong, Z.; Han, D.; Ding, H.; Cai, M.; Zhao, K.; Li, H.; Niu, S. Study on the Influence of κ-Carbide on the High Temperature Flow Behavior of the Medium-Mn Lightweight Steel: Modeling and Characterization. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2024, 908, 146784. [Google Scholar] [CrossRef]
- Kim, H.; Suh, D.-W.; Kim, N.J. Fe–Al–Mn–C Lightweight Structural Alloys: A Review on the Microstructures and Mechanical Properties. Sci. Technol. Adv. Mater. 2013, 14, 14205. [Google Scholar] [CrossRef]
- Dieudonne, T.; Marchetti, L.; Wery, M.; Miserque, F.; Tabarant, M.; Chene, J.; Allely, C.; Cugy, P.; Scott, C.P. Role of Copper and Aluminum on the Corrosion Behavior of Austenitic Fe-Mn-C TWIP Steels in Aqueous Solutions and the Related Hydrogen Absorption. Corros. Sci. 2014, 83, 234–244. [Google Scholar] [CrossRef]
- Dieudonne, T.; Marchetti, L.; Wery, M.; Chene, J.; Allely, C.; Cugy, P.; Scott, C.P. Role of Copper and Aluminum Additions on the Hydrogen Embrittlement Susceptibility of Austenitic Fe-Mn-C TWIP Steels. Corros. Sci. 2014, 82, 218–226. [Google Scholar] [CrossRef]
- Yang, F.; Nie, Y.; Zhang, H.; Niu, W.; Shi, Q.; Ma, J.; Zheng, L.; Liang, W. Hydrogen Embrittlement of 27Cr−4Mo−2Ni Super Ferritic Stainless Steel. Materials 2024, 17, 1546. [Google Scholar] [CrossRef]
- Koyama, M.; Springer, H.; Merzlikin, S.V.; Tsuzaki, K.; Akiyama, E.; Raabe, D. Hydrogen Embrittlement Associated with Strain Localization in a Precipitation-Hardened Fe-Mn-Al-C Light Weight Austenitic Steel. Int. J. Hydrogen Energy 2014, 39, 4634–4646. [Google Scholar] [CrossRef]
- Ren, X.; Qi, Y.; Li, Y.; Zhou, J.; Gu, J. Co-Precipitation Mechanism of Cu-Rich Phase and κ-Carbide Precipitates in Fe-28Mn-10Al-1C-3Cu Austenitic Low-Density Steel. Mater. Lett. 2024, 366, 136522. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Huang, C.; Liu, P.-Y.; Yen, H.-W.; Niu, R.; Burr, P.; Moore, K.L.; Martinez-Paneda, E.; Atrens, A.; Cairney, J.M. Hydrogen Trapping and Embrittlement in Metals—A Review. Int. J. Hydrogen Energy 2025, 136, 789–821. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, X.; Yu, S.; Li, W. Effect of Microstructure on Hydrogen Trapping Behavior and Hydrogen Embrittlement Sensitivity of Medium Manganese Steel. Anti-Corros. Methods Mater. 2025, 72, 364–376. [Google Scholar] [CrossRef]
- Chen, B.; Qiu, F.; Xia, L.; Xu, L.; Jin, J.; Gou, G. In Situ Ultrasonic Characterization of Hydrogen Damage Evolution in X80 Pipeline Steel. Materials 2024, 17, 5891. [Google Scholar] [CrossRef]
- Nakamichi, H.; Yamada, K.; Sato, K. Sub-Nanometre Elemental Analysis of Cu Cluster in Fe-Cu-Ni Alloy Using Aberration Corrected STEM-EDS. J. Microsc. 2011, 242, 55–61. [Google Scholar] [CrossRef]
- Samusawa, I.; Nakayama, S. Influence of Plastic Deformation and Cu Addition on Corrosion of Carbon Steel in Acidic Aqueous Solution. Corros. Sci. 2019, 159, 108122. [Google Scholar] [CrossRef]
- Shi, X.; Yan, W.; Wang, W.; Shan, Y.; Yang, K. Novel Cu-Bearing High-Strength Pipeline Steels with Excellent Resistance to Hydrogen-Induced Cracking. Mater. Des. 2016, 92, 300–305. [Google Scholar] [CrossRef]
- Benbelaid, S.; Belouchrani, M.A.; Assoul, Y.; Bezzazi, B. Modeling Damage of the Hydrogen Enhanced Localized Plasticity in Stress Corrosion Cracking. Int. J. Damage Mech. 2011, 20, 831–844. [Google Scholar] [CrossRef]
- Nagao, A.; Dadfarnia, M.; Somerday, B.P.; Sofronis, P.; Ritchie, R.O. Hydrogen-Enhanced-Plasticity Mediated Decohesion for Hydrogen-Induced Intergranular and “Quasi-Cleavage” Fracture of Lath Martensitic Steels. J. Mech. Phys. Solids 2018, 112, 403–430. [Google Scholar] [CrossRef]
- Stark, A.; Sonnweber-Ribic, P.; Elsaesser, C. Theoretical Study of Individual and Combined Effects of HELP- and HEDE-Based Damage Models on the Fatigue Behavior of Ferritic Steel by Hydrogen. Int. J. Hydrogen Energy 2025, 109, 27–39. [Google Scholar] [CrossRef]
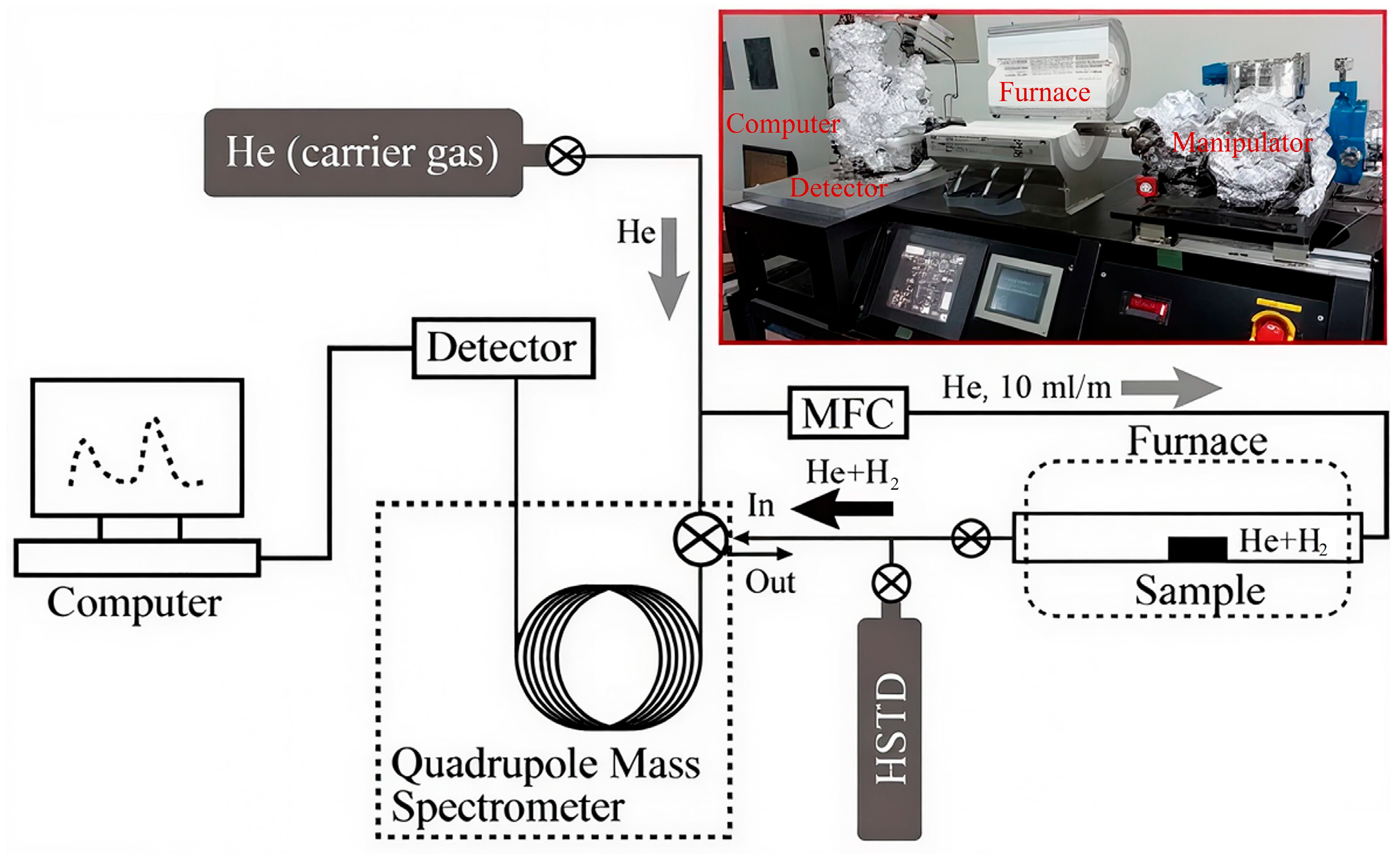
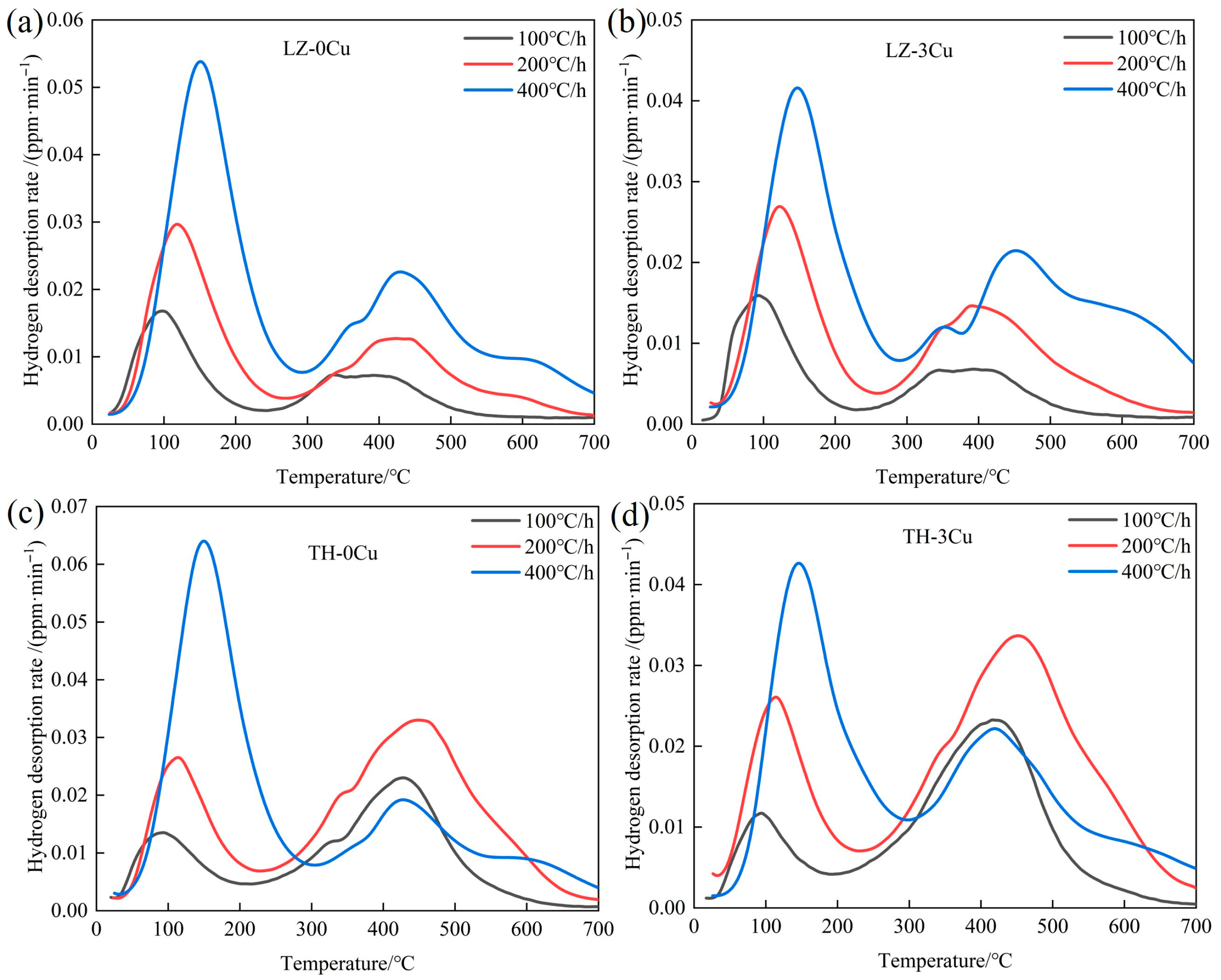
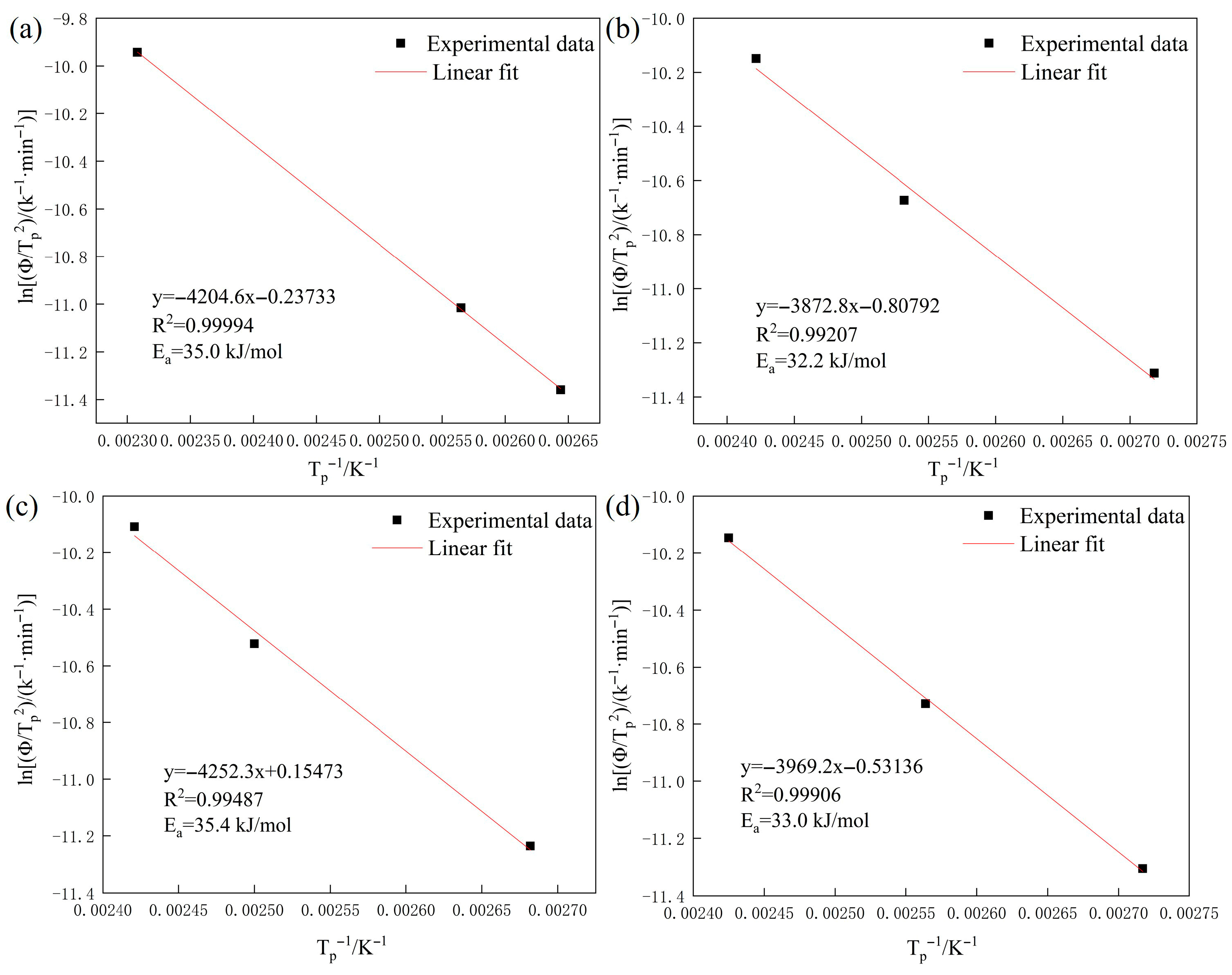
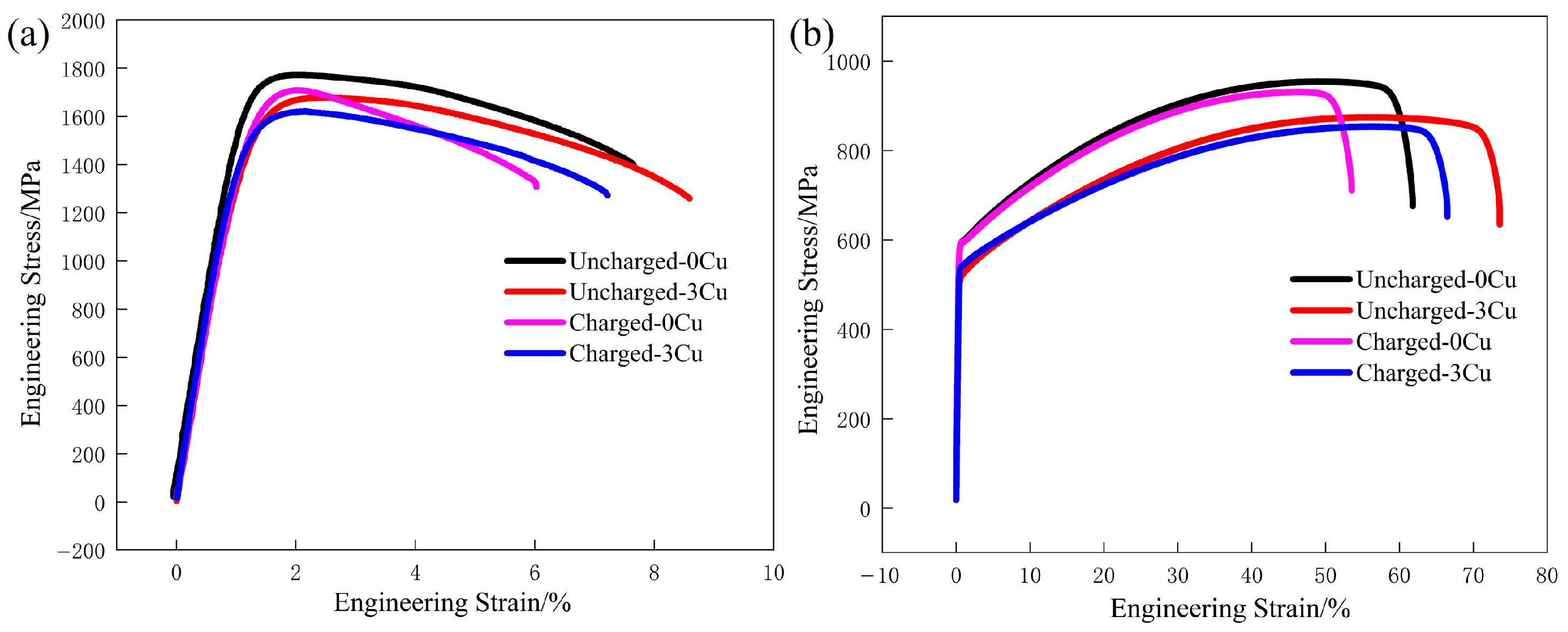
| Alloy | Fe | Mn | Al | C | Cu |
|---|---|---|---|---|---|
| 0Cu | Bal. | 28.15 | 10.02 | 0.98 | 0 |
| 3Cu | Bal. | 28.38 | 10.08 | 1.04 | 3.01 |
| Specimens | Reversible Hydrogen Content (wt.ppm) | Irreversible Hydrogen Content (wt.ppm) | Total Hydrogen Content (wt.ppm) |
|---|---|---|---|
| LZ-0Cu | 1.2 ± 0.1 | 1.1 ± 0.2 | 2.3 ± 0.3 |
| LZ-3Cu | 1.1 ± 0.1 | 1.0 ± 0.1 | 2.1 ± 0.2 |
| TH-0Cu | 1.3 ± 0.2 | 1.7 ± 0.2 | 3.0 ± 0.4 |
| TH-3Cu | 1.1 ± 0.1 | 2.5 ± 0.5 | 3.6 ± 0.6 |
| State | Specimens | UTS (MPa) | YS (MPa) | UE (%) | HEI (%) |
|---|---|---|---|---|---|
| LZ-0Cu | 1772 ± 45 | 1554 ± 39 | 7.65 ± 0.41 | - | |
| Original state | LZ-3Cu | 1677 ± 41 | 1450 ± 36 | 8.59 ± 0.52 | - |
| TH-0Cu | 954 ± 22 | 586 ± 25 | 61.90 ± 1.83 | - | |
| TH-3Cu | 874 ± 25 | 517 ± 23 | 73.50 ± 1.77 | - | |
| LZ-0Cu | 1708 ± 43 | 1430 ± 32 | 6.02 ± 0.63 | 21.31 | |
| After hydrogen charging | LZ-3Cu | 1621 ± 37 | 1336 ± 35 | 7.21 ± 0.55 | 16.07 |
| TH-0Cu | 930 ± 31 | 584 ± 21 | 53.51 ± 2.11 | 13.55 | |
| TH-3Cu | 853 ± 28 | 529 ± 27 | 66.48 ± 1.98 | 9.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, J.; Jiang, S.; Qi, Y.; Ren, X.; Li, Y. Effect of Copper Alloying on Hydrogen Embrittlement of Fe-28Mn-10Al-1C Austenitic Low-Density Steel. Materials 2025, 18, 4139. https://doi.org/10.3390/ma18174139
Gu J, Jiang S, Qi Y, Ren X, Li Y. Effect of Copper Alloying on Hydrogen Embrittlement of Fe-28Mn-10Al-1C Austenitic Low-Density Steel. Materials. 2025; 18(17):4139. https://doi.org/10.3390/ma18174139
Chicago/Turabian StyleGu, Jiahao, Sifan Jiang, Yanfei Qi, Xiqiang Ren, and Yungang Li. 2025. "Effect of Copper Alloying on Hydrogen Embrittlement of Fe-28Mn-10Al-1C Austenitic Low-Density Steel" Materials 18, no. 17: 4139. https://doi.org/10.3390/ma18174139
APA StyleGu, J., Jiang, S., Qi, Y., Ren, X., & Li, Y. (2025). Effect of Copper Alloying on Hydrogen Embrittlement of Fe-28Mn-10Al-1C Austenitic Low-Density Steel. Materials, 18(17), 4139. https://doi.org/10.3390/ma18174139





