Organic Field-Effect Transistors Based on Chemical-Plated Pt/Ag Electrodes
Highlights
- A simple silver mirror reaction was developed to fabricate high-quality silver electrodes for organic electronics.
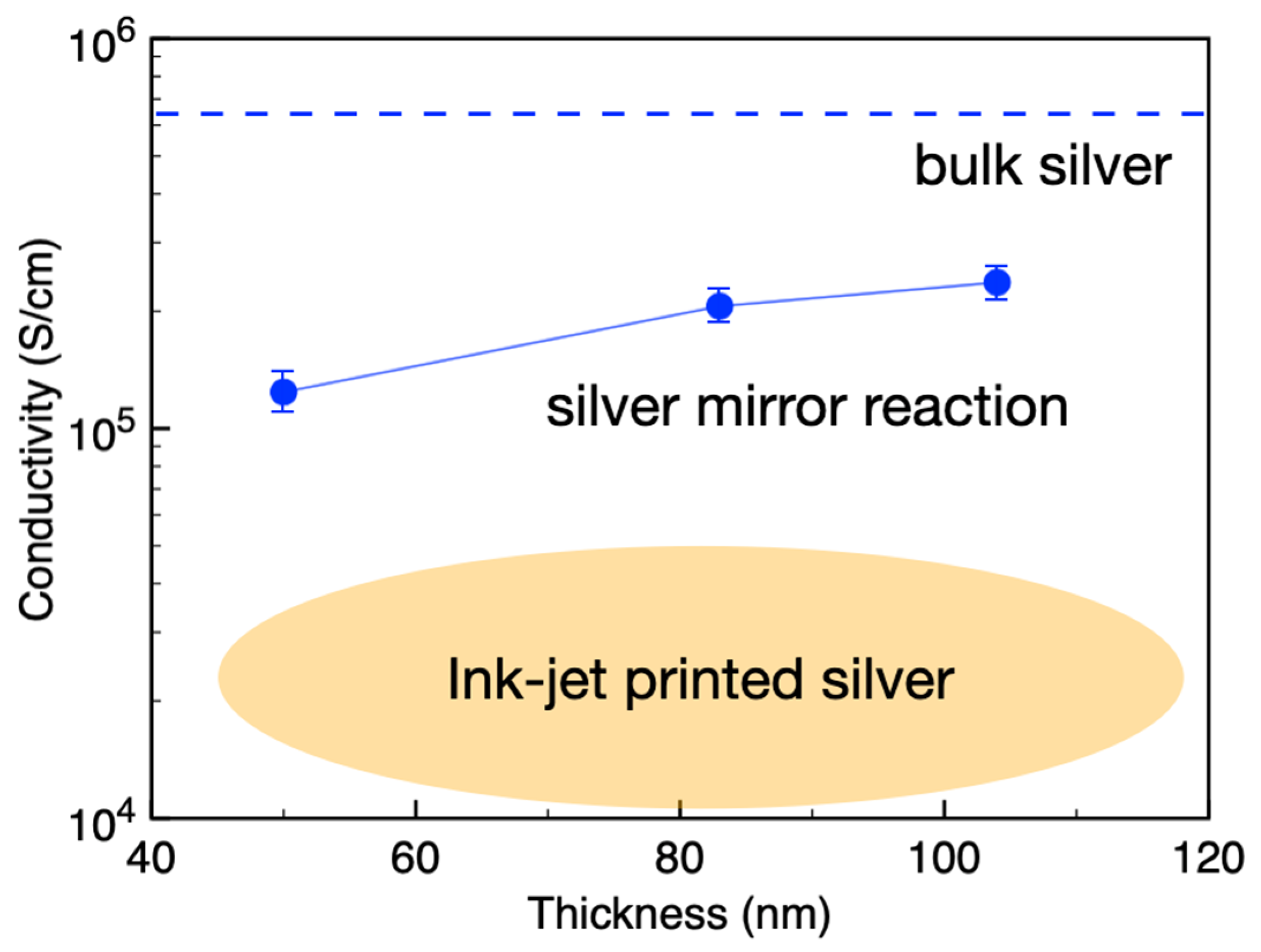
- The resulting solution-processed silver films exhibited electrical conductivity ten times higher than conventional inkjet-printed electrodes.
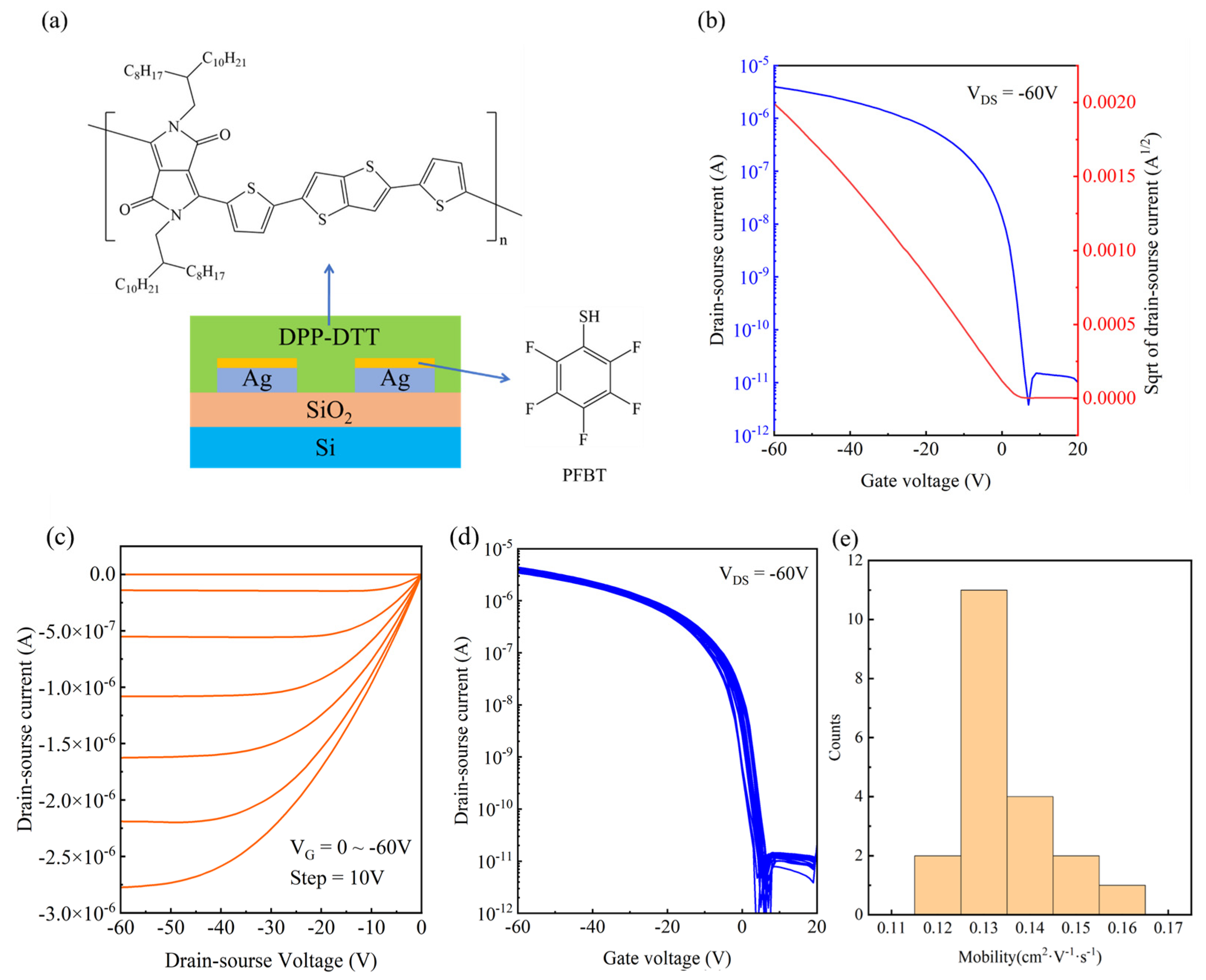
- When integrated into organic field-effect transistors (OFETs), the electrodes enabled devices with high mobility (0.13 cm²/V·s) and excellent uniformity (8.7% variation).
- Chemical platinum plating enabled precise work function tuning (+0.74 eV), highlighting the potential of this approach for surface engineering in organic and flexible electronics.
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Characterization
3. Results
3.1. Optimization of the Silver Mirror Reaction Surface Morphology
3.2. Conductivity of the Silver Electrode
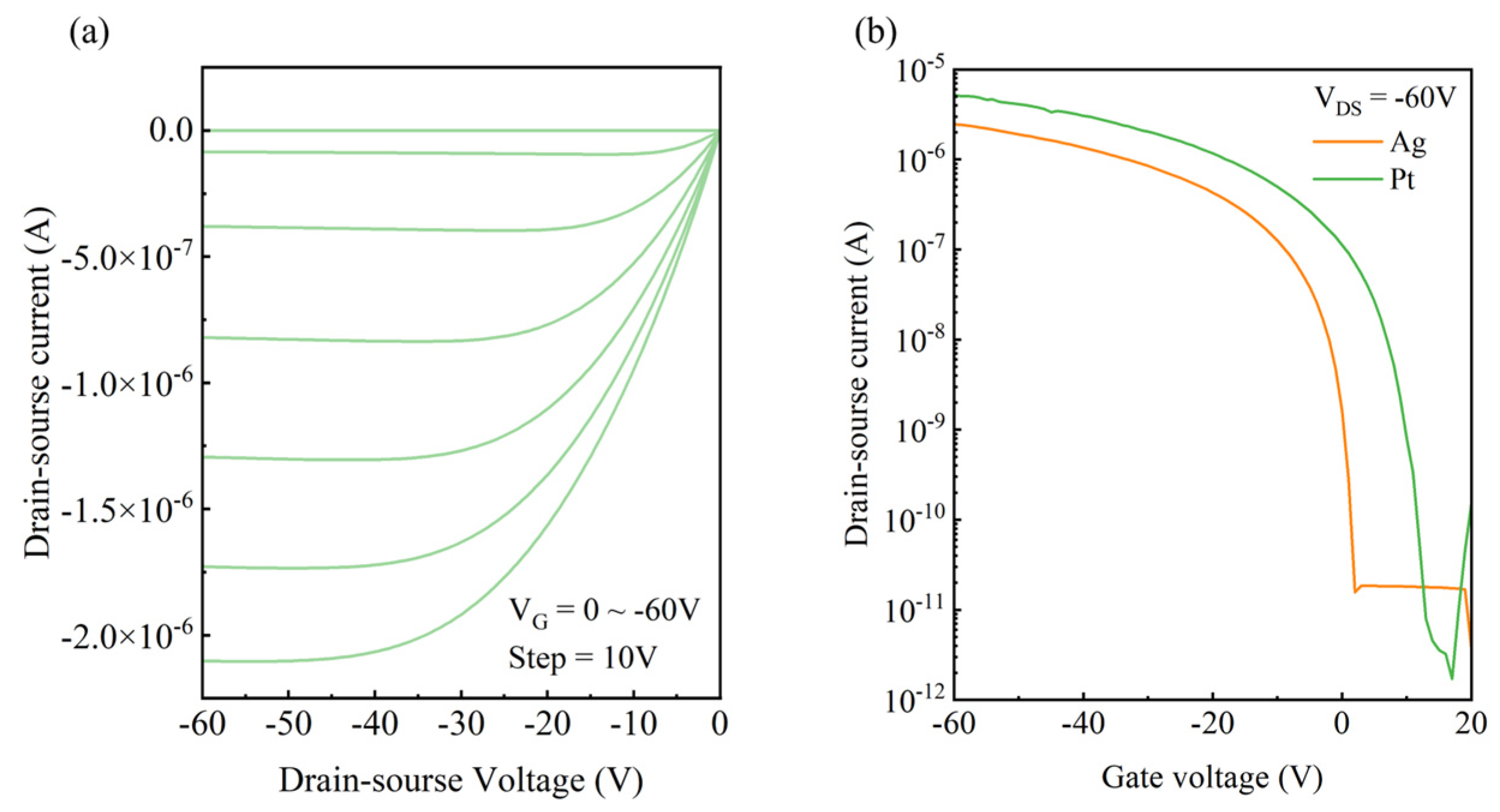
3.3. OFET Performance Characterization
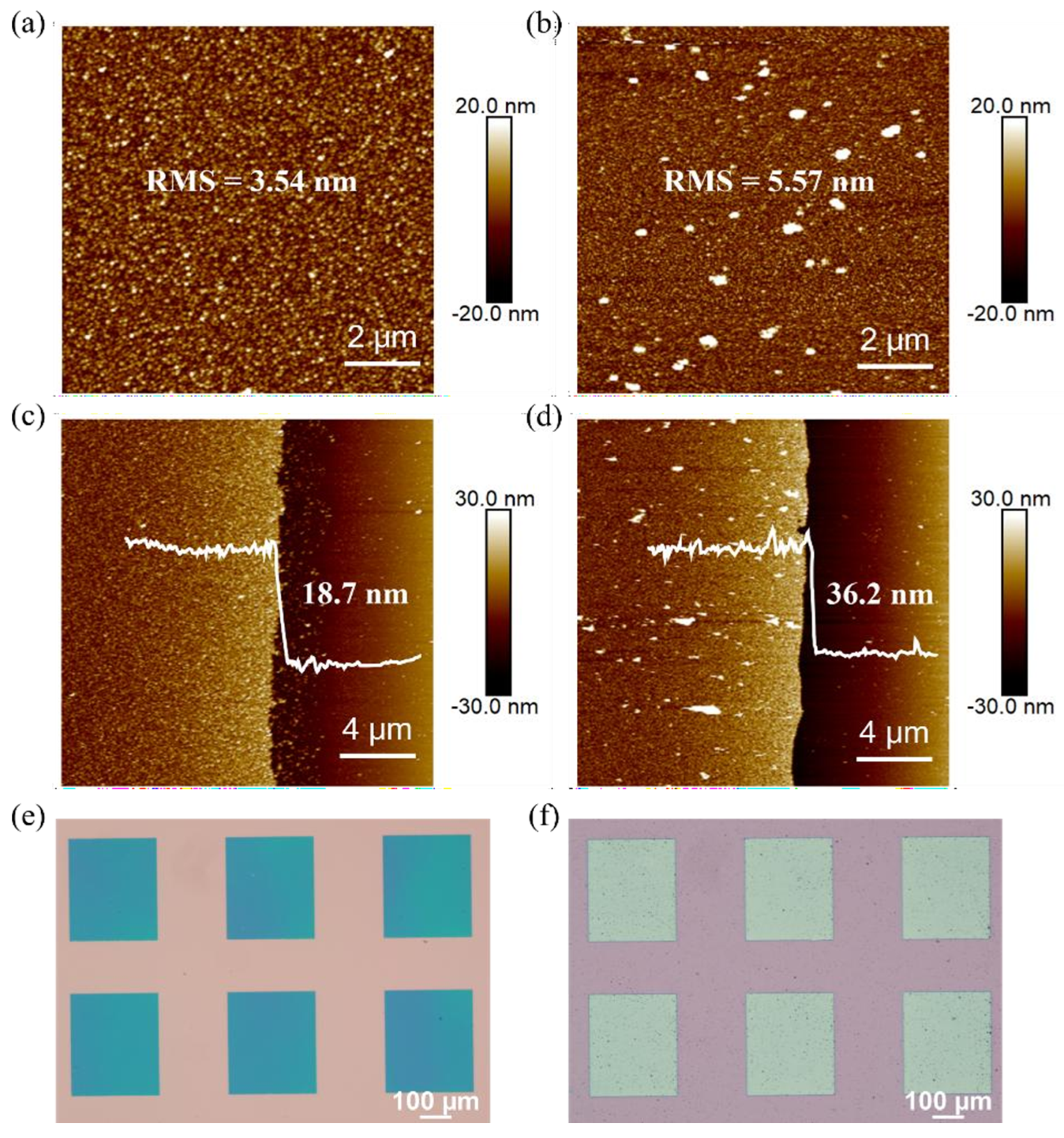
3.4. Chemical-Plated Platinum Electrode
3.5. Variation in Electrode Work Function
3.6. Chemical-Plated Platinum Electrode OFET Device
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pandey, M.; Syafutra, H.; Kumari, N.; Pandey, S.S.; Abe, R.; Benten, H.; Nakamura, M. Extreme Orientational Uniformity in Large-Area Floating Films of Semiconducting Polymers for Their Application in Flexible Electronics. ACS Appl. Mater. Interfaces 2021, 13, 38534–38543. [Google Scholar] [CrossRef]
- Tang, W.; Fu, Y.; Huang, Y.; Li, Y.; Song, Y.; Xi, X.; Yu, Y.; Su, Y.; Yan, F.; Guo, X. Solution Processed Low Power Organic Field-Effect Transistor Bio-Chemical Sensor of High Transconductance Efficiency. npj Flex. Electron. 2022, 6, 18. [Google Scholar] [CrossRef]
- Zhang, Y.-W.; Gao, K.-H.; Li, Z.-Q. Oxygen Sensitivity of Ga2O3 Nanosheet Field-Effect Transistor. Appl. Surf. Sci. 2025, 688, 162391. [Google Scholar] [CrossRef]
- Dacha, P.; Hambsch, M.; Pohl, D.; Haase, K.; Löffler, M.; Lan, T.; Feng, X.; Rellinghaus, B.; Mannsfeld, S.C.B. Tailoring the Morphology of a Diketopyrrolopyrrole-based Polymer as Films or Wires for High-Performance OFETs using Solution Shearing. Small Methods 2024, 8, 2300842. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, Z.; Xue, D.; Chu, M.; Zhang, Y.; Ji, L.; Wang, Q.; Jiang, X.; Sun, Y.; Miao, Q.; et al. Organic Heteroepitaxy Growth of High-Performance Responsive Thin Films with Solution Shearing Crystals as Templates. ACS Mater. Lett. 2022, 4, 1314–1321. [Google Scholar] [CrossRef]
- Geng, B.; Zhang, F.; Huang, C.; He, L.; Li, C.; Duan, S.; Ren, X.; Hu, W. Lattice Strain-Induced High-Performance Low-Operating-Voltage Organic Field-Effect Transistors by Solution-Sheared Organic Single Crystal. J. Mater. Chem. C 2024, 12, 5012–5018. [Google Scholar] [CrossRef]
- Lin, C.-C.; Afraj, S.N.; Velusamy, A.; Yu, P.-C.; Cho, C.-H.; Chen, J.; Li, Y.-H.; Lee, G.-H.; Tung, S.-H.; Liu, C.-L.; et al. A Solution Processable Dithioalkyl Dithienothiophene (DSDTT) Based Small Molecule and Its Blends for High Performance Organic Field Effect Transistors. ACS Nano 2021, 15, 727–738. [Google Scholar] [CrossRef]
- He, P.; Cao, J.; Ding, H.; Liu, C.; Neilson, J.; Li, Z.; Kinloch, I.A.; Derby, B. Screen-Printing of a Highly Conductive Graphene Ink for Flexible Printed Electronics. ACS Appl. Mater. Interfaces 2019, 11, 32225–32234. [Google Scholar] [CrossRef]
- Teng, Y.; Wang, X.; Zhang, Z.; Mei, S.; Nan, X.; Zhao, Y.; Zhang, X.; Xue, C.; Gao, L.; Li, J. Fully Printed Minimum Port Flexible Interdigital Electrode Sensor Arrays. Nanoscale 2024, 16, 7427–7436. [Google Scholar] [CrossRef]
- Tao, R.; Ning, H.; Chen, J.; Zou, J.; Fang, Z.; Yang, C.; Zhou, Y.; Zhang, J.; Yao, R.; Peng, J. Inkjet Printed Electrodes in Thin Film Transistors. IEEE J. Electron Devices Soc. 2018, 6, 774–790. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, X.; Su, W.; Cui, Z.; Lai, W.-Y. In-Depth Investigation of Inkjet-Printed Silver Electrodes over Large-Area: Ink Recipe, Flow, and Solidification. Adv. Mater. Interfaces 2022, 9, 2102548. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.; Wei, Z.; Sun, Y.; He, Y.; Di, C.-a.; Xu, W.; Hu, W.; Liu, Y.; Zhu, D. Inkjet-Printed Organic Electrodes for Bottom-Contact Organic Field-Effect Transistors. Adv. Funct. Mater. 2011, 21, 786–791. [Google Scholar] [CrossRef]
- Zhang, J.; Geng, B.; Duan, S.; Huang, C.; Xi, Y.; Mu, Q.; Chen, H.; Ren, X.; Hu, W. High-Resolution Organic Field-Effect Transistors Manufactured by Electrohydrodynamic Inkjet Printing of Doped Electrodes. J. Mater. Chem. C 2020, 8, 15219–15223. [Google Scholar] [CrossRef]
- Waldrip, M.; Yu, Y.; Dremann, D.; Losi, T.; Willner, B.; Caironi, M.; McCulloch, I.; Jurchescu, O.D. Designing Contact Independent High-Performance Low-Cost Flexible Electronics. Adv. Mater. 2024, 36, 2410442. [Google Scholar] [CrossRef]
- Liu, C.; Quan, H.; Shi, Y.; Chen, H.; Dong, H.; Wang, J.; Tao, Y.; Chen, C.; Wang, J. Highly Flexible Honeycomb-Structured Flexible Transparent Conductive Electrodes by Inkjet Printing for Light-Emitting Devices. J. Mater. Chem. C 2025, 13, 11198–11206. [Google Scholar] [CrossRef]
- Rojano Chávez, S.M.; Ayala, L.M.; Karthik, T.V.K.; Maldonado, A.; Gómez-Pozos, H. Zinc Oxide Thin Films Deposited by Sol–Gel Spin-Coating Technique for Propane and Carbon Monoxide Sensing Applications. J. Mater. Sci. Mater. Electron. 2024, 35, 797. [Google Scholar] [CrossRef]
- Chung, J.W.; Park, W.-T.; Park, J.-I.; Yun, Y.; Gu, X.; Lee, J.; Noh, Y.-Y. Thiophene-Thiazole-Based Semiconducting Copolymers for High-Performance Polymer Field-Effect Transistors. ACS Appl. Mater. Interfaces 2017, 9, 38728–38736. [Google Scholar] [CrossRef]
- Li, M.; Cao, Y.; Xie, K.; Tang, J. Molecular Engineering on Kinetics-Driven Self-Assembled Monolayers Working as Auxiliary Layers on Dielectrics in Organic Field-Effect Transistors. Adv. Electron. Mater. 2024, 10, 2300712. [Google Scholar] [CrossRef]
- Maiorano, E.; Gianvittorio, S.; Lanzi, M.; Tonelli, D.; Pick, H.; Lesch, A. Print-Light-Synthesis of Gold Thin Film Electrodes for Electrochemical Sensing. Adv. Mater. Technol. 2023, 8, 2202039. [Google Scholar] [CrossRef]
- Xiao, X.; Shen, X.; Tie, Y.; Zhao, Y.; Yang, R.; Li, Y.; Li, W.; Tang, L.; Li, R.; Wang, Y.-X.; et al. Stepwise Aggregation Control of PEDOT:PSS Enabled High-Conductivity, High-Resolution Printing of Polymer Electrodes for Transparent Organic Phototransistors. ACS Appl. Mater. Interfaces 2024, 16, 29217–29225. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Gu, J.; Fan, L.; Ma, J.; Lian, H.; Shi, W.; Wei, B. All-Printed Organic Photodetectors with Metal Electrodes Enabled by One-Step Solvent-Mediated Transfer Printing Technology. Nanoscale 2024, 16, 10682–10689. [Google Scholar] [CrossRef]
- Tang, W.; Feng, L.; Zhao, J.; Cui, Q.; Chen, S.; Guo, X. Inkjet Printed Fine Silver Electrodes for All-Solution-Processed Low-Voltage Organic Thin Film Transistors. J. Mater. Chem. C 2014, 2, 1995–2000. [Google Scholar] [CrossRef]
- Ji, D.; Jiang, L.; Guo, Y.; Dong, H.; Wang, J.; Chen, H.; Meng, Q.; Fu, X.; Tian, G.; Wu, D.; et al. “Regioselective Deposition” Method to Pattern Silver Electrodes Facilely and Efficiently with High Resolution: Towards All-Solution-Processed, High-Performance, Bottom-Contacted, Flexible, Polymer-Based Electronics. Adv. Funct. Mater. 2014, 24, 3783–3789. [Google Scholar] [CrossRef]
- Sleczkowski, P.; Borkowski, M.; Zajaczkowska, H.; Ulanski, J.; Pisula, W.; Marszalek, T. Geometry Control of Source/Drain Electrodes in Organic Field-Effect Transistors by Electrohydrodynamic Inkjet Printing. Materials 2020, 13, 4974. [Google Scholar] [CrossRef]
- Podzorov, V.; Sysoev, S.E.; Loginova, E.; Pudalov, V.M.; Gershenson, M.E. Single-Crystal Organic Field Effect Transistors with the Hole Mobility ~8 cm2/VS. Appl. Phys. Lett. 2003, 83, 3504–3506. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, W.; Shi, X.; Ren, X.; Li, B.; Li, Y.; Jie, J.; Zhang, X.; Zhang, X. Molecule Upgrading Metal-Semiconductor Buried Contacts for High-Performance and High-Ideality Single-Crystal Organic Thin-Film Transistors. Natl. Sci. Rev. 2025, 12, nwaf207. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, X.; Rao, L.; Zhang, J.; Xiao, M.; Zhu, D.; Li, C.; Shi, X.; Liu, J.; Liu, J.; et al. Solution-Processed Top-Contact Electrodes Strategy for Organic Crystalline Field-Effect Transistor Arrays. Nano Res. 2022, 15, 858–863. [Google Scholar] [CrossRef]
- Zeng, J.; He, D.; Qiao, J.; Li, Y.; Sun, L.; Li, W.; Xie, J.; Gao, S.; Pan, L.; Wang, P.; et al. Ultralow Contact Resistance in Organic Transistors Via Orbital Hybridization. Nat. Commun. 2023, 14, 324. [Google Scholar] [CrossRef]
- Mallik, S.; Mandal, A.; Mandal, S.; Verma, S.P.; Das, A.; Guha, P.K.; Goswami, D.K. Transition of Activation Energy Induced by the Polarization of Au Nanoparticles in Organic Field-Effect Transistors at Low Temperatures. ACS Appl. Nano Mater. 2024, 7, 20186–20195. [Google Scholar] [CrossRef]
- Otsuka, K.; Sugihara, T.; Inoue, T.; Jia, W.; Matsushita, S.; Saito, T.; Lee, M.; Taniguchi, T.; Watanabe, K.; Pitner, G.; et al. Coaxial Boron Nitride Nanotubes as Interfacial Dielectric Layers to Lower Interface Trap Density in Carbon Nanotube Transistors. Nano Res. 2023, 16, 12840–12848. [Google Scholar] [CrossRef]
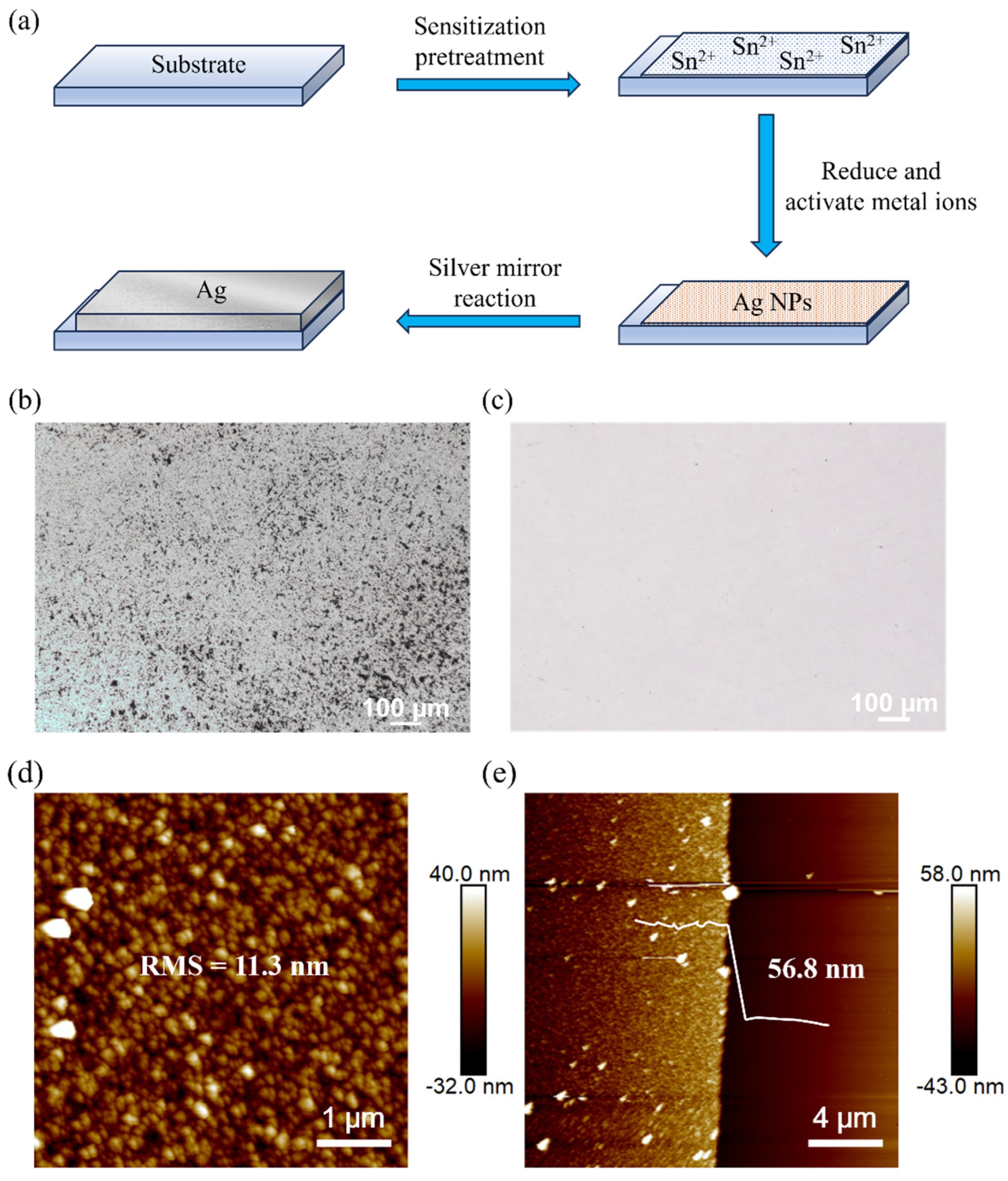

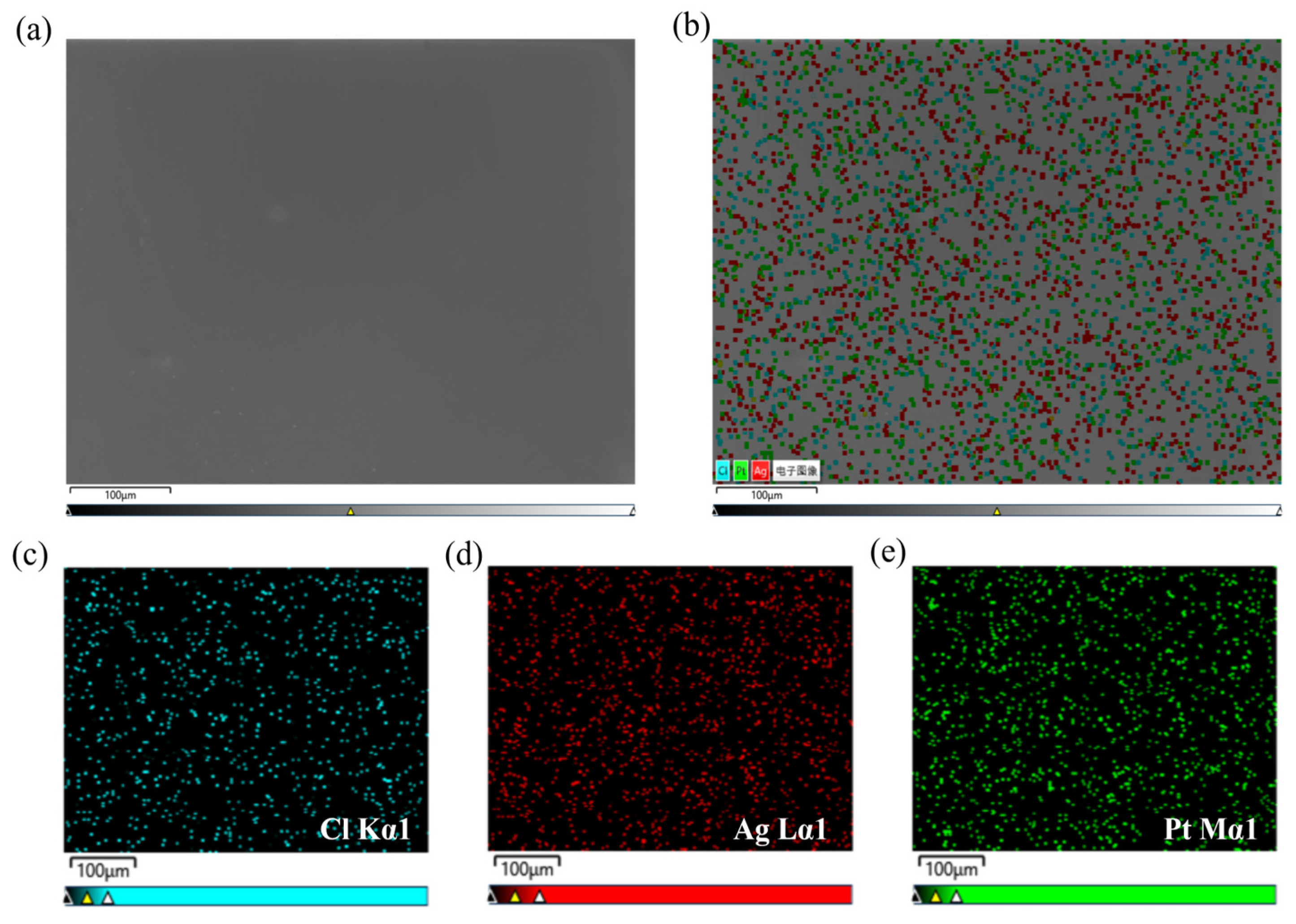

| Vsilver nitrate:Vammonia | Reaction Time/min | Roughness/nm | Thickness/nm |
|---|---|---|---|
| 5:1 | 5 | 11.3 ± 2.56 | 56.8 ± 4.7 |
| 5:2 | 5 | 6.58 ± 1.79 | 45.7 ± 3.9 |
| 5:3 | 5 | 6.01 ± 1.26 | 43.8 ± 2.2 |
| 5:4 | 5 | 4.73 ± 1.14 | 39.9 ± 2.3 |
| 5:5 | 5 | 4.42 ± 0.64 | 38.7 ± 1.9 |
| 5:6 | 5 | 5.07 ± 0.81 | 39.6 ± 2.1 |
| 5:1 | 3 | 9.22 ± 1.24 | 38.8 ± 3.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Ren, X. Organic Field-Effect Transistors Based on Chemical-Plated Pt/Ag Electrodes. Materials 2025, 18, 4130. https://doi.org/10.3390/ma18174130
Zhao C, Ren X. Organic Field-Effect Transistors Based on Chemical-Plated Pt/Ag Electrodes. Materials. 2025; 18(17):4130. https://doi.org/10.3390/ma18174130
Chicago/Turabian StyleZhao, Chenyang, and Xiaochen Ren. 2025. "Organic Field-Effect Transistors Based on Chemical-Plated Pt/Ag Electrodes" Materials 18, no. 17: 4130. https://doi.org/10.3390/ma18174130
APA StyleZhao, C., & Ren, X. (2025). Organic Field-Effect Transistors Based on Chemical-Plated Pt/Ag Electrodes. Materials, 18(17), 4130. https://doi.org/10.3390/ma18174130





