Energy-Selective X-Ray Detection Using Chemically Tunable High-Z Nanocomposites
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of Tungstate Nanoparticles
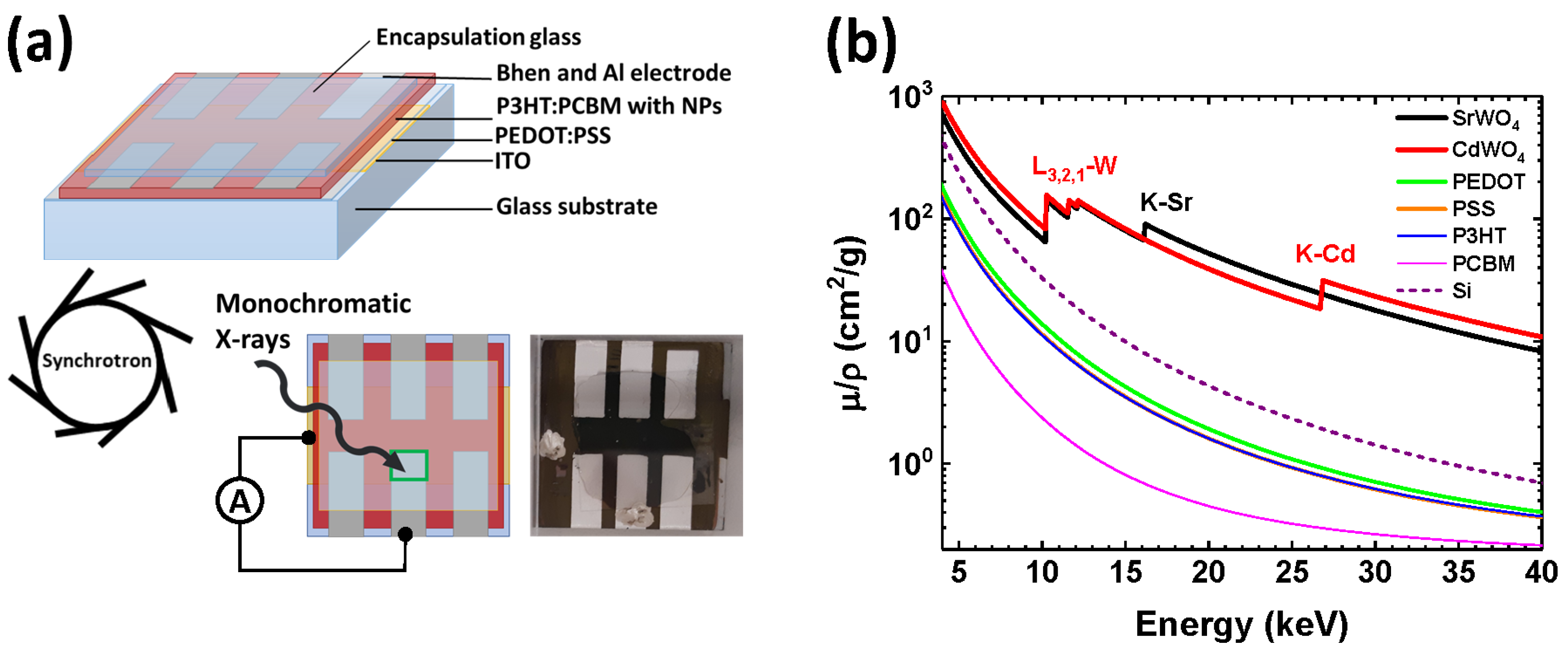
2.2. Fabrication of Hybrid Organic–Inorganic X-Ray Detectors
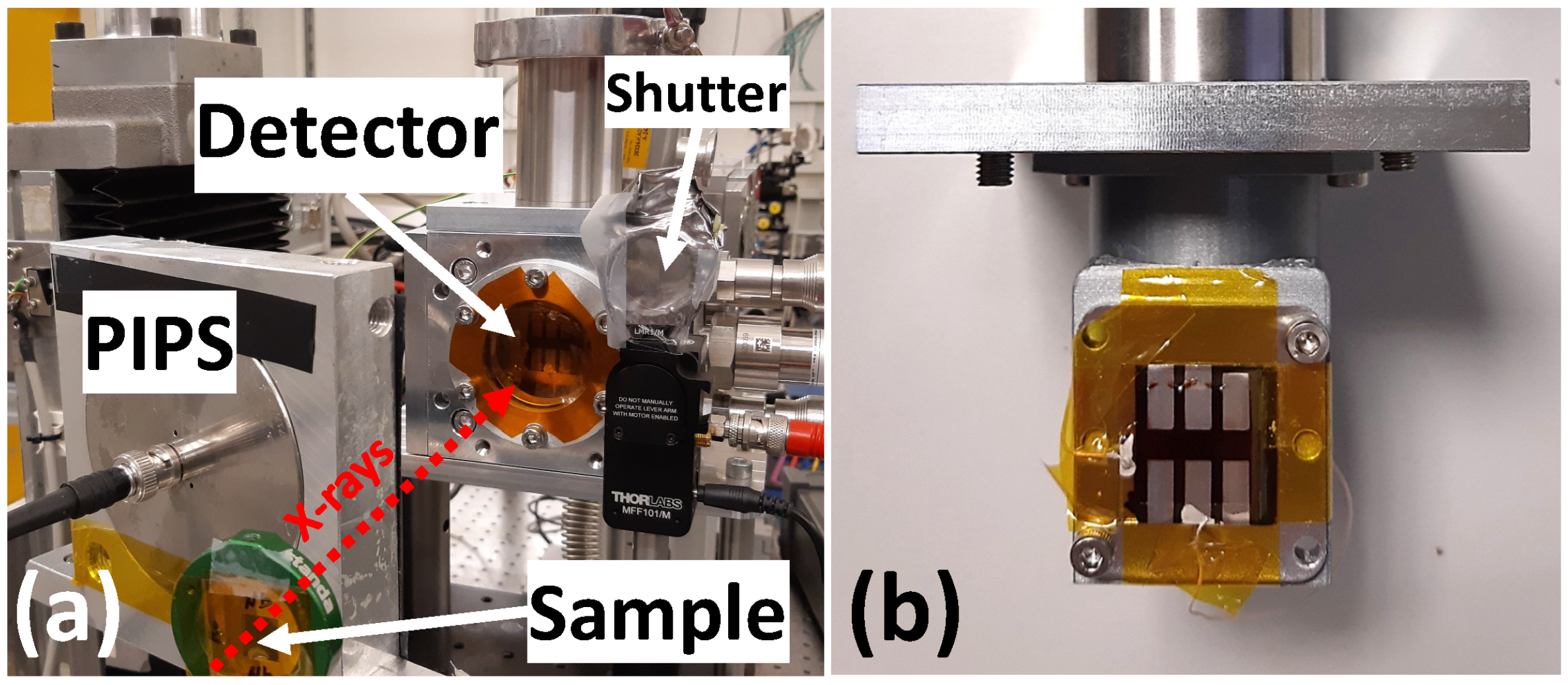
2.3. Testing of X-Ray Detectors for Different Applications
3. Results and Discussion
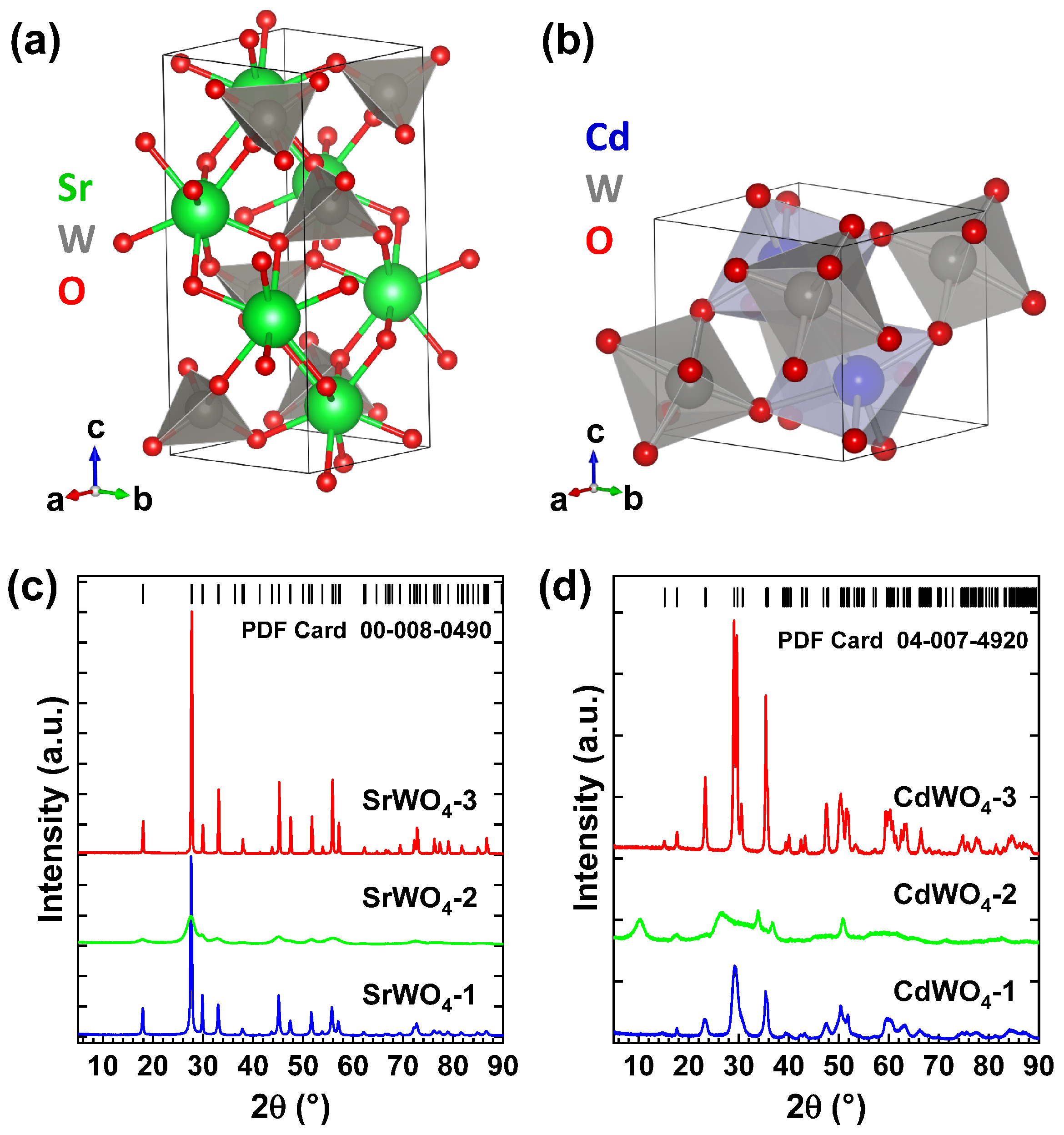
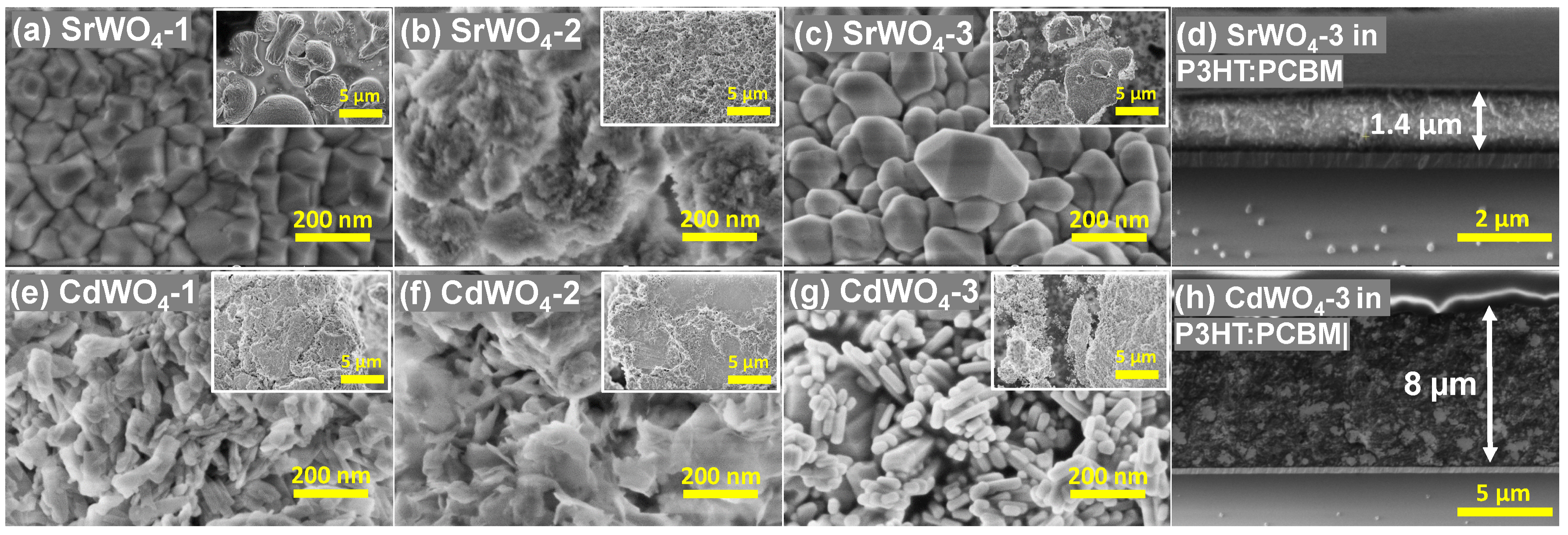
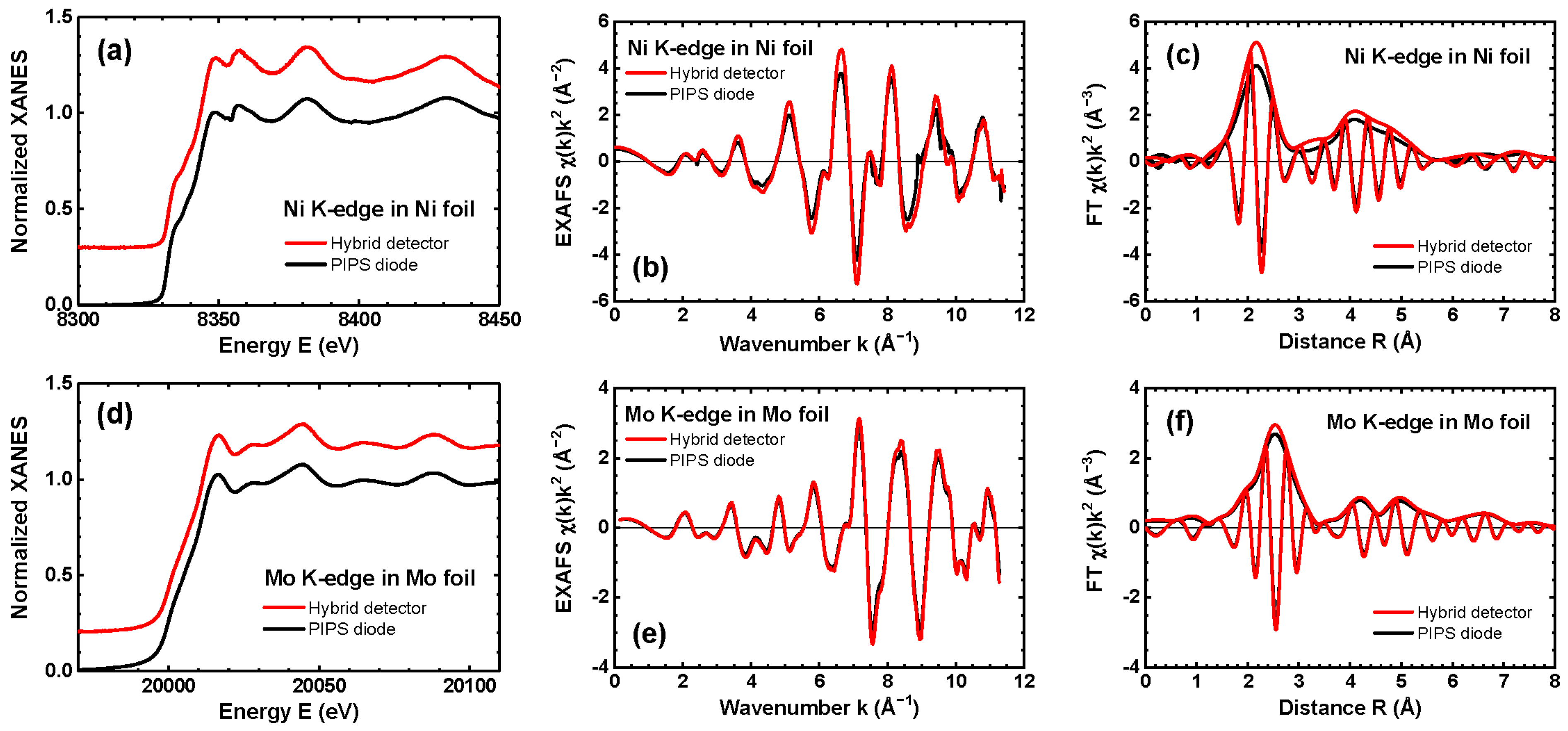
3.1. Characterization of Tungstate Nanoparticles
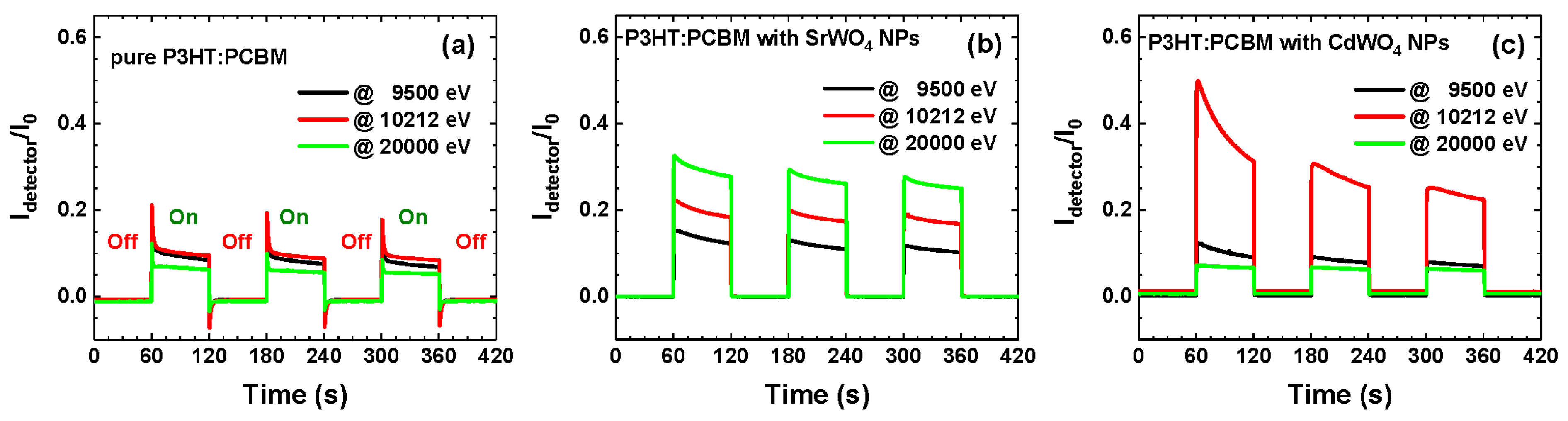
3.2. Hybrid Organic–Inorganic X-Ray Detector Response
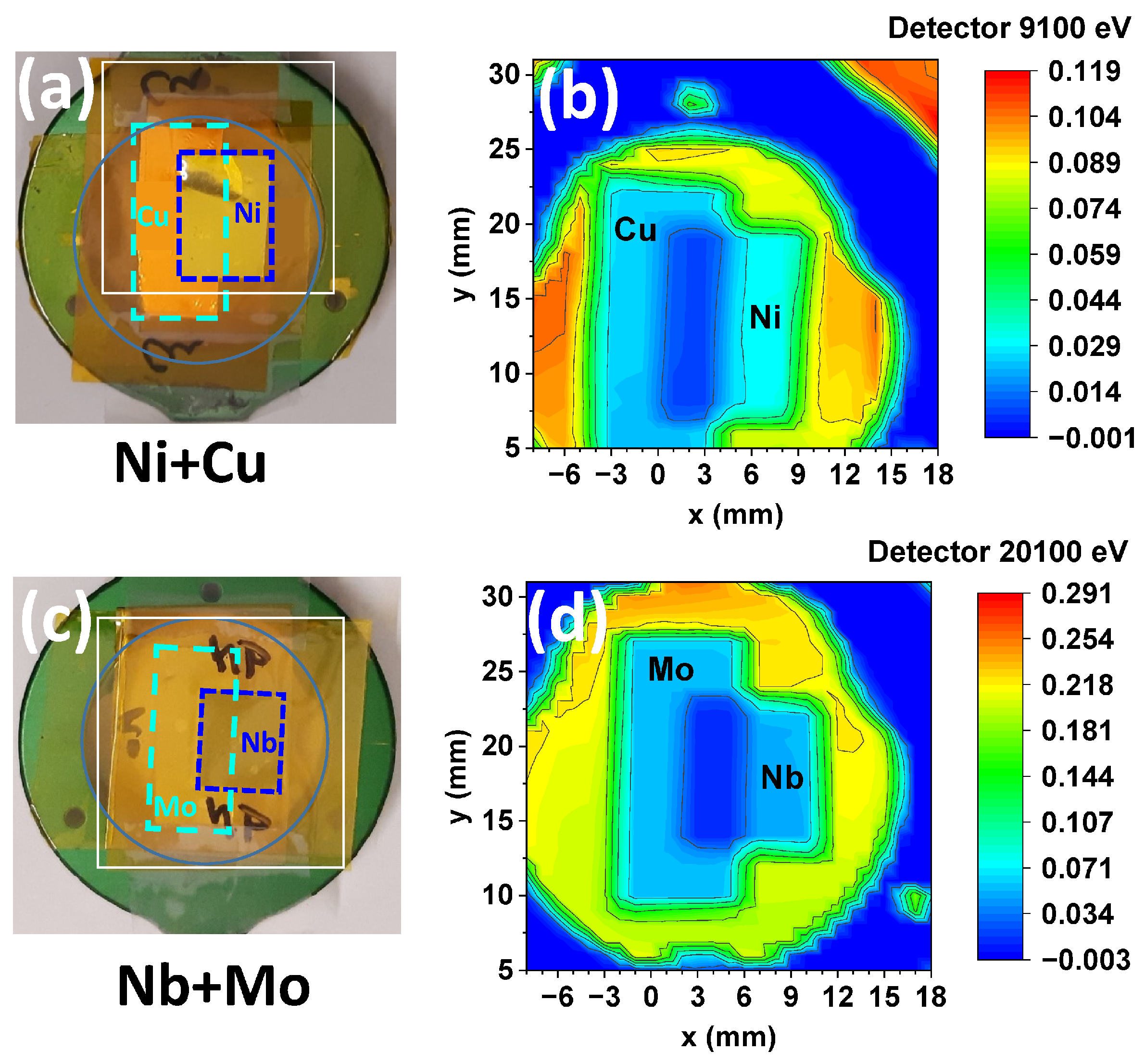
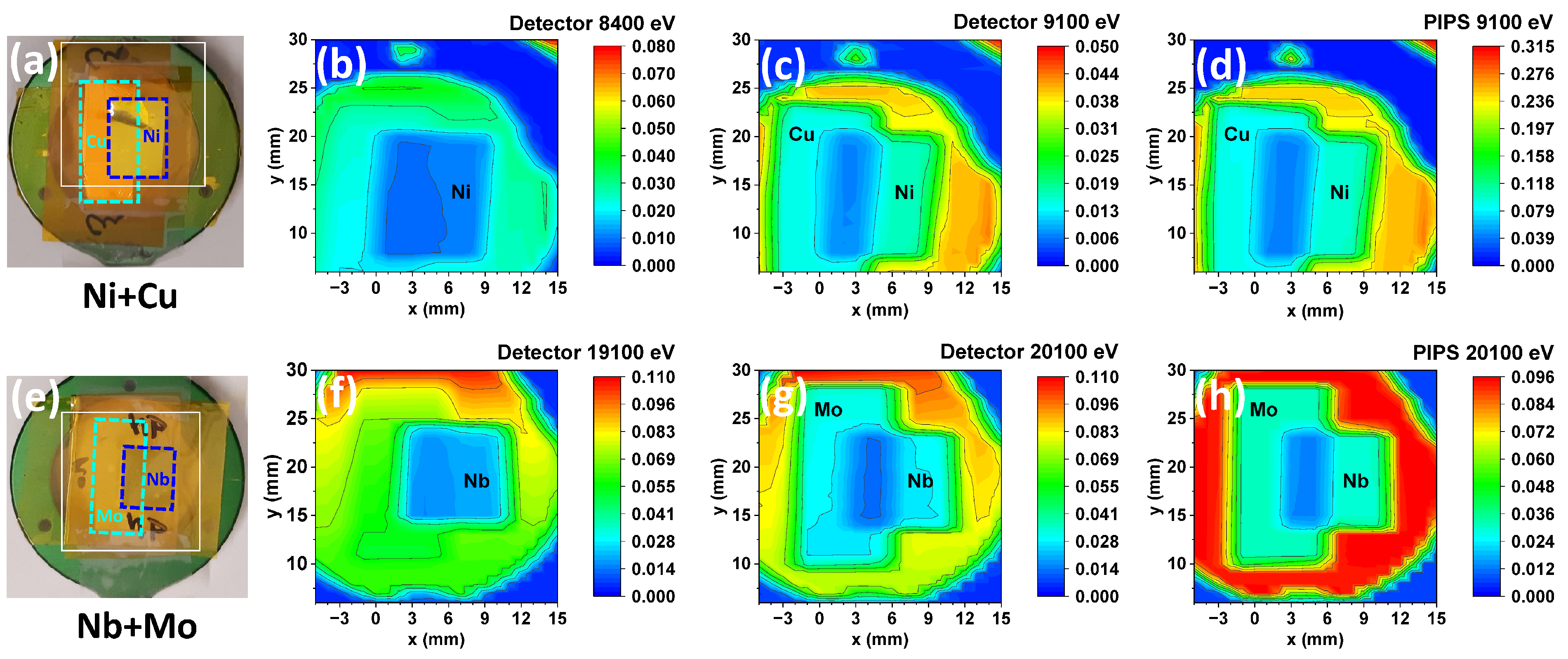
3.3. The Use of X-Ray Detectors for Spectroscopic and Imaging Applications
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EXAFS | extended X-ray absorption fine structure |
| ITO | indium tin oxide |
| NP | nanoparticle |
| P3HT:PCBM | Poly(3-hexylthiophene-2,5-diyl):Phenyl-C61-butyric acid methyl ester |
| PEDOT:PSS | Poly(3,4-ethylenedioxythiophene)-poly(styrenesulfonate) |
| PIPS | passivated implanted planar silicon |
| RT | room temperature |
| SEM | scanning electron microscopy |
| WL | white line |
| XANES | X-ray absorption near edge structure |
| XRD | X-ray diffraction |
References
- Wallentin, J.; Osterhoff, M.; Wilke, R.N.; Persson, K.M.; Wernersson, L.E.; Sprung, M.; Salditt, T. Hard X-ray detection using a single 100 nm diameter nanowire. Nano Lett. 2014, 14, 7071–7076. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Moch, J.G.; Johnson, S.S.; Chen, C.C. A review on X-ray detection using nanomaterials. Curr. Nanosci. 2017, 13, 364–372. [Google Scholar] [CrossRef]
- Thirimanne, H.; Jayawardena, K.D.G.I.; Parnell, A.J.; Bandara, R.M.I.; Karalasingam, A.; Pani, S.; Huerdler, J.E.; Lidzey, D.G.; Tedde, S.F.; Nisbet, A.; et al. High sensitivity organic-inorganic hybrid X-ray detectors with direct transduction and broadband response. Nat. Commun. 2018, 9, 2926. [Google Scholar] [CrossRef]
- Zapf, M.; Ritzer, M.; Liborius, L.; Johannes, A.; Hafermann, M.; Schonherr, S.; Segura-Ruiz, J.; Martinez-Criado, G.; Prost, W.; Ronning, C. Hot electrons in a nanowire hard X-ray detector. Nat. Commun. 2020, 11, 4729. [Google Scholar] [CrossRef]
- Chayanun, L.; Hrachowina, L.; Bjorling, A.; Borgstrom, M.T.; Wallentin, J. Direct three-dimensional imaging of an X-ray nanofocus using a single 60 nm diameter nanowire device. Nano Lett. 2020, 20, 8326–8331. [Google Scholar] [CrossRef]
- Butanovs, E.; Zolotarjovs, A.; Kuzmin, A.; Polyakov, B. Nanoscale X-ray detectors based on individual CdS, SnO2 and ZnO nanowires. Nucl. Instrum. Methods. Phys. Res. A 2021, 1014, 165736. [Google Scholar] [CrossRef]
- Min, S.; Kang, H.; Seo, B.; Cheong, J.; Roh, C.; Hong, S. A review of nanomaterial based scintillators. Energies 2021, 14, 7701. [Google Scholar] [CrossRef]
- Büchele, P.; Richter, M.; Tedde, S.F.; Matt, G.J.; Ankah, G.N.; Fischer, R.; Biele, M.; Metzger, W.; Lilliu, S.; Bikondoa, O.; et al. X-ray imaging with scintillator-sensitized hybrid organic photodetectors. Nat. Photon. 2015, 9, 843–848. [Google Scholar] [CrossRef]
- Chow, P.C.Y.; Someya, T. Organic photodetectors for next-generation wearable electronics. Adv. Mater. 2020, 32, 1902045. [Google Scholar] [CrossRef] [PubMed]
- Fraboni, B.; Ciavatti, A.; Merlo, F.; Pasquini, L.; Cavallini, A.; Quaranta, A.; Bonfiglio, A.; Fraleoni-Morgera, A. Organic semiconducting single crystals as next generation of low-cost, room-temperature electrical X-ray detectors. Adv. Mater. 2012, 24, 2289–2293. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Miyata, H.; Katsumata, M.; Nakano, S.; Matsuda, K.; Tamura, M. Organic semiconductors as real-time radiation detectors. Nucl. Instrum. Methods A 2014, 763, 304–307. [Google Scholar] [CrossRef]
- Basirico, L.; Ciavatti, A.; Cramer, T.; Cosseddu, P.; Bonfiglio, A.; Fraboni, B. Direct X-ray photoconversion in flexible organic thin film devices operated below 1V. Nat. Commun. 2016, 7, 13063. [Google Scholar] [CrossRef] [PubMed]
- Ciavatti, A.; Basirico, L.; Fratelli, I.; Lai, S.; Cosseddu, P.; Bonfiglio, A.; Anthony, J.E.; Fraboni, B. Boosting direct X-ray detection in organic thin films by small molecules tailoring. Adv. Funct. Mater. 2019, 29, 1806119. [Google Scholar] [CrossRef]
- Mills, C.A.; Al-Otaibi, H.; Intaniwet, A.; Shkunov, M.; Pani, S.; Keddie, J.L.; Sellin, P.J. Enhanced x-ray detection sensitivity in semiconducting polymer diodes containing metallic nanoparticles. J. Phys. D 2013, 46, 275102. [Google Scholar] [CrossRef]
- Intaniwet, A.; Mills, C.A.; Shkunov, M.; Sellin, P.J.; Keddie, J.L. Heavy metallic oxide nanoparticles for enhanced sensitivity in semiconducting polymer x-ray detectors. Nanotechnology 2012, 23, 235502. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Li, Y.; Chen, H.; Yu, L.; Zhang, J.A. A high-sensitivity flexible direct X-ray detector based on Bi2O3/PDMS nanocomposite thin film. Nanomaterials 2021, 11, 1832. [Google Scholar] [CrossRef]
- Ankah, G.; Buchele, P.; Poulsen, K.; Rauch, T.; Tedde, S.; Gimmler, C.; Schmidt, O.; Kraus, T. PbS quantum dot based hybrid-organic photodetectors for X-ray sensing. Org. Electron. 2016, 33, 201–206. [Google Scholar] [CrossRef]
- Starkenburg, D.J.; Johns, P.M.; Baciak, J.E.; Nino, J.C.; Xue, J. Thin film organic photodetectors for indirect X-ray detection demonstrating low dose rate sensitivity at low voltage operation. J. Appl. Phys. 2017, 122, 225502. [Google Scholar] [CrossRef]
- Li, L.; Ye, S.; Qu, J.; Zhou, F.; Song, J.; Shen, G. Recent advances in perovskite photodetectors for image sensing. Small 2021, 17, 2005606. [Google Scholar] [CrossRef]
- Liu, J. Flexible, printable soft-X-ray detectors based on all-inorganic perovskite quantum dots. Adv. Mater. 2019, 31, 1901644. [Google Scholar] [CrossRef]
- Mescher, H.; Schackmar, F.; Eggers, H.; Abzieher, T.; Zuber, M.; Hamann, E.; Baumbach, T.; Richards, B.S.; Hernandez-Sosa, G.; Paetzold, U.W.; et al. Flexible inkjet-printed triple cation perovskite X-ray detectors. ACS Appl. Mater. Interfaces 2020, 12, 15774. [Google Scholar] [CrossRef]
- Basirico, L.; Ciavatti, A.; Fraboni, B. Solution-grown organic and perovskite X-ray detectors: A new paradigm for the direct detection of ionizing radiation. Adv. Mater. Technol. 2021, 6, 2000475. [Google Scholar] [CrossRef]
- Bian, Y.; Liu, K.; Ran, Y.; Li, Y.; Gao, Y.; Zhao, Z.; Shao, M.; Liu, Y.; Kuang, J.; Zhu, Z.; et al. Spatially nanoconfined N-type polymer semiconductors for stretchable ultrasensitive X-ray detection. Nat. Commun. 2022, 13, 7163. [Google Scholar] [CrossRef]
- Sleight, A.W. Accurate cell dimensions for ABO4 molybdates and tungstates. Acta Cryst. B 1972, 28, 2899–2902. [Google Scholar] [CrossRef]
- Nikl, M. Scintillation detectors for X-rays. Meas. Sci. Technol. 2006, 17, R37. [Google Scholar] [CrossRef]
- Bakradze, G.; Welter, E.; Kuzmin, A. Peculiarities of the local structure in new medium- and high-entropy, low-symmetry tungstates. J. Phys. Chem. Solids 2023, 172, 111052. [Google Scholar] [CrossRef]
- Kaczmarek, A.M.; Van Deun, R. Rare earth tungstate and molybdate compounds—From 0D to 3D architectures. ChemInform 2013, 42, 8835–8848. [Google Scholar] [CrossRef]
- Yan, B.; Wu, H.; Ma, P.; Niu, J.; Wang, J. Recent advances in rare earth co-doped luminescent tungsten oxygen complexes. Inorg. Chem. Front. 2021, 8, 4158–4176. [Google Scholar] [CrossRef]
- Pudza, I.; Pudzs, K.; Tokmakovs, A.; Strautnieks, N.R.; Kalinko, A.; Kuzmin, A. Nanocrystalline CaWO4 and ZnWO4 tungstates for hybrid organic-inorganic X-ray detectors. Materials 2023, 16, 667. [Google Scholar] [CrossRef]
- Tong, W.; Li, L.; Hu, W.; Yan, T.; Li, G. Systematic control of monoclinic CdWO4 nanophase for optimum photocatalytic activity. J. Phys. Chem. C 2010, 114, 1512–1519. [Google Scholar] [CrossRef]
- Su, Y.; Li, G.; Xue, Y.; Li, L. Tunable physical properties of CaWO4 nanocrystals via particle size control. J. Phys. Chem. C 2007, 111, 6684–6689. [Google Scholar] [CrossRef]
- Doebelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Caliebe, W.A.; Murzin, V.; Kalinko, A.; Gorlitz, M. High-flux XAFS-beamline P64 at PETRA III. AIP Conf. Proc. 2019, 2054, 060031. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, P.; López-Solano, J.; Radescu, S.; Mujica, A.; Muñoz, A.; Errandonea, D.; Pellicer-Porres, J.; Segura, A.; Fer-rer-Roca, C.; Manjón, F.J.; et al. Theoretical and experimental study of CaWO4 and SrWO4 under pressure. J. Phys. Chem. Solids 2006, 67, 2164–2171. [Google Scholar] [CrossRef]
- Daturi, M.; Busca, G.; Borel, M.M.; Leclaire, A.; Piaggio, P. Vibrational and XRD study of the system CdWO4-CdMoO4. J. Phys. Chem. B 1997, 101, 4358–4369. [Google Scholar] [CrossRef]
- Sun, L.; Guo, Q.; Wu, X.; Luo, S.; Pan, W.; Huang, K.; Lu, J.; Ren, L.; Cao, M.; Hu, C. Synthesis and photoluminescent properties of strontium tungstate nanostructures. J. Phys. Chem. C 2007, 111, 532–537. [Google Scholar] [CrossRef]
- Wang, Y.; Guan, X.; Li, L.; Lin, H.; Wang, X.; Li, G. Solvent-driven polymorphic control of CdWO4 nanocrystals for photocatalytic performances. New J. Chem. 2012, 36, 1852–1858. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.; Hao, H. Investigations on dehydration processes of trisodium citrate hydrates. Front. Chem. Sci. Eng. 2012, 6, 276–281. [Google Scholar] [CrossRef]
- Ameri, T.; Khoram, P.; Min, J.; Brabec, C.J. Organic ternary solar cells: A review. Adv. Mater. 2013, 25, 4245–4266. [Google Scholar] [CrossRef]
- Chantler, C.T.; Olsen, K.; Dragoset, R.A.; Chang, J.; Kishore, A.R.; Kotochigova, S.A.; Zucker, D.S. X-Ray Form Factor, Attenuation and Scattering Tables (Version 2.1); NIST: Gaithersburg, MD, USA, 2005. [CrossRef]
- Cai, W.; Gong, X.; Cao, Y. Polymer solar cells: Recent development and possible routes for improvement in the performance. Sol. Energy Mater. Sol. Cells 2010, 94, 114–127. [Google Scholar] [CrossRef]
- X-Ray Data Booklet, 3rd ed.; Lawrence Berkeley National Laboratory, University of California: Berkeley, CA, USA, 2009.
- Materlik, G.; Müller, J.E.; Wilkins, J.W. L-edge absorption spectra of the rare earths: Assessment of the single-particle picture. Phys. Rev. Lett. 1983, 50, 267–270. [Google Scholar] [CrossRef]
- Balerna, A.; Bernieri, E.; Burattini, E.; Kuzmin, A.; Lusis, A.; Purans, J.; Cikmach, P. XANES studies of MeO3−x (Me = W, Re, Ir) crystalline and amorphous oxides. Nucl. Instrum. Methods Phys. Res. Sect. A 1991, 308, 240–242. [Google Scholar] [CrossRef]
- Kalinko, A.; Kuzmin, A. Static and dynamic structure of ZnWO4 nanoparticles. J. Non-Cryst. Solids 2011, 357, 2595–2599. [Google Scholar] [CrossRef][Green Version]
- Jayawardena, K.D.G.I.; Thirimanne, H.M.; Tedde, S.F.; Huerdler, J.E.; Parnell, A.J.; Bandara, R.M.I.; Mills, C.A.; Silva, S.R.P. Millimeter-scale unipolar transport in high sensitivity organic-inorganic semiconductor X-ray detectors. ACS Nano 2019, 13, 6973–6981. [Google Scholar] [CrossRef] [PubMed]
- Haneef, H.F.; Zeidell, A.M.; Jurchescu, O.D. Charge carrier traps in organic semiconductors: A review on the underlying physics and impact on electronic devices. J. Mater. Chem. C 2020, 8, 759–787. [Google Scholar] [CrossRef]
- Nanayakkara, M.P.A.; Matjačić, L.; Wood, S.; Richheimer, F.; Castro, F.A.; Jenatsch, S.; Zufle, S.; Kilbride, R.; Parnell, A.J.; Masteghin, M.G.; et al. Ultra-low dark current organic-inorganic hybrid X-Ray detectors. Adv. Funct. Mater. 2020, 31, 2008482. [Google Scholar] [CrossRef]
- Xiang, L.; Huang, X.; Wang, Y.; Xin, Z.; Chai, G.; Xu, Y.; Wang, K.; Chen, J.; Liu, C.; Wang, X.; et al. X-ray sensitive hybrid organic photodetectors with embedded CsPbBr3 perovskite quantum dots. Org. Electron. 2021, 98, 106306. [Google Scholar] [CrossRef]
- Tan, Z.; Budnick, J.I.; Heald, S.M. Structural parameter determination in fluorescence EXAFS of concentrated samples. Rev. Sci. Instrum. 1989, 60, 1021–1025. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pudza, I.; Pudzs, K.; Tokmakovs, A.; Kalinko, A.; Kuzmin, A. Energy-Selective X-Ray Detection Using Chemically Tunable High-Z Nanocomposites. Materials 2025, 18, 4118. https://doi.org/10.3390/ma18174118
Pudza I, Pudzs K, Tokmakovs A, Kalinko A, Kuzmin A. Energy-Selective X-Ray Detection Using Chemically Tunable High-Z Nanocomposites. Materials. 2025; 18(17):4118. https://doi.org/10.3390/ma18174118
Chicago/Turabian StylePudza, Inga, Kaspars Pudzs, Andrejs Tokmakovs, Aleksandr Kalinko, and Alexei Kuzmin. 2025. "Energy-Selective X-Ray Detection Using Chemically Tunable High-Z Nanocomposites" Materials 18, no. 17: 4118. https://doi.org/10.3390/ma18174118
APA StylePudza, I., Pudzs, K., Tokmakovs, A., Kalinko, A., & Kuzmin, A. (2025). Energy-Selective X-Ray Detection Using Chemically Tunable High-Z Nanocomposites. Materials, 18(17), 4118. https://doi.org/10.3390/ma18174118








