Preparation of a Novel Carbon Nano Coating on Carbon Fiber Surface Based on Plasma Electrolysis Effect
Abstract
1. Introduction
2. Experiment
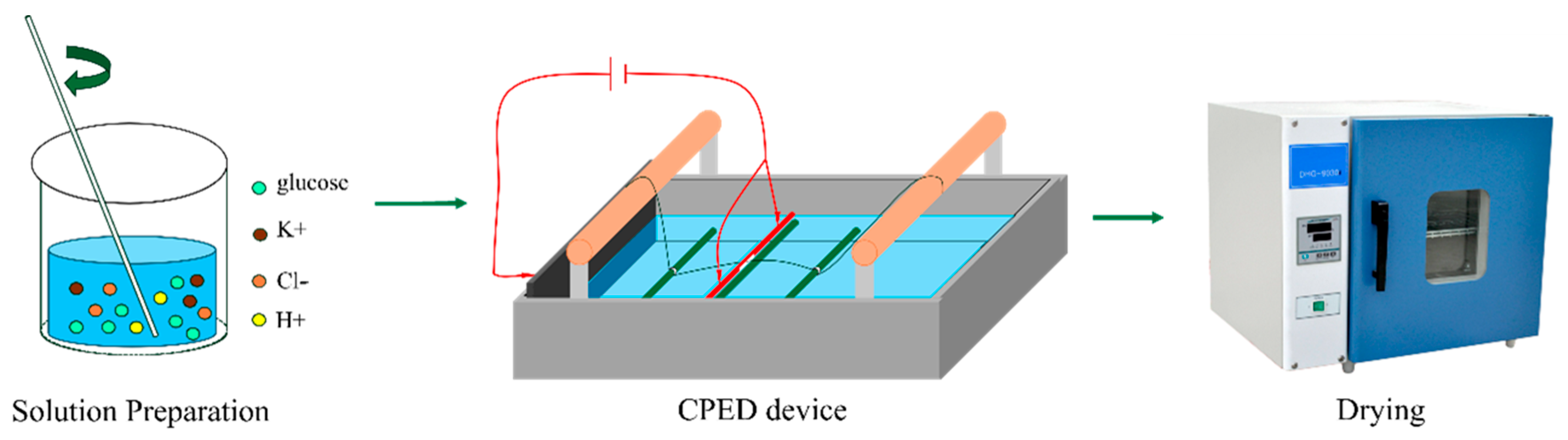
2.1. Materials Preparation
2.2. Experiment Procedure
2.3. Characterization Techniques
2.4. Performance Testing
3. Results and Discussion
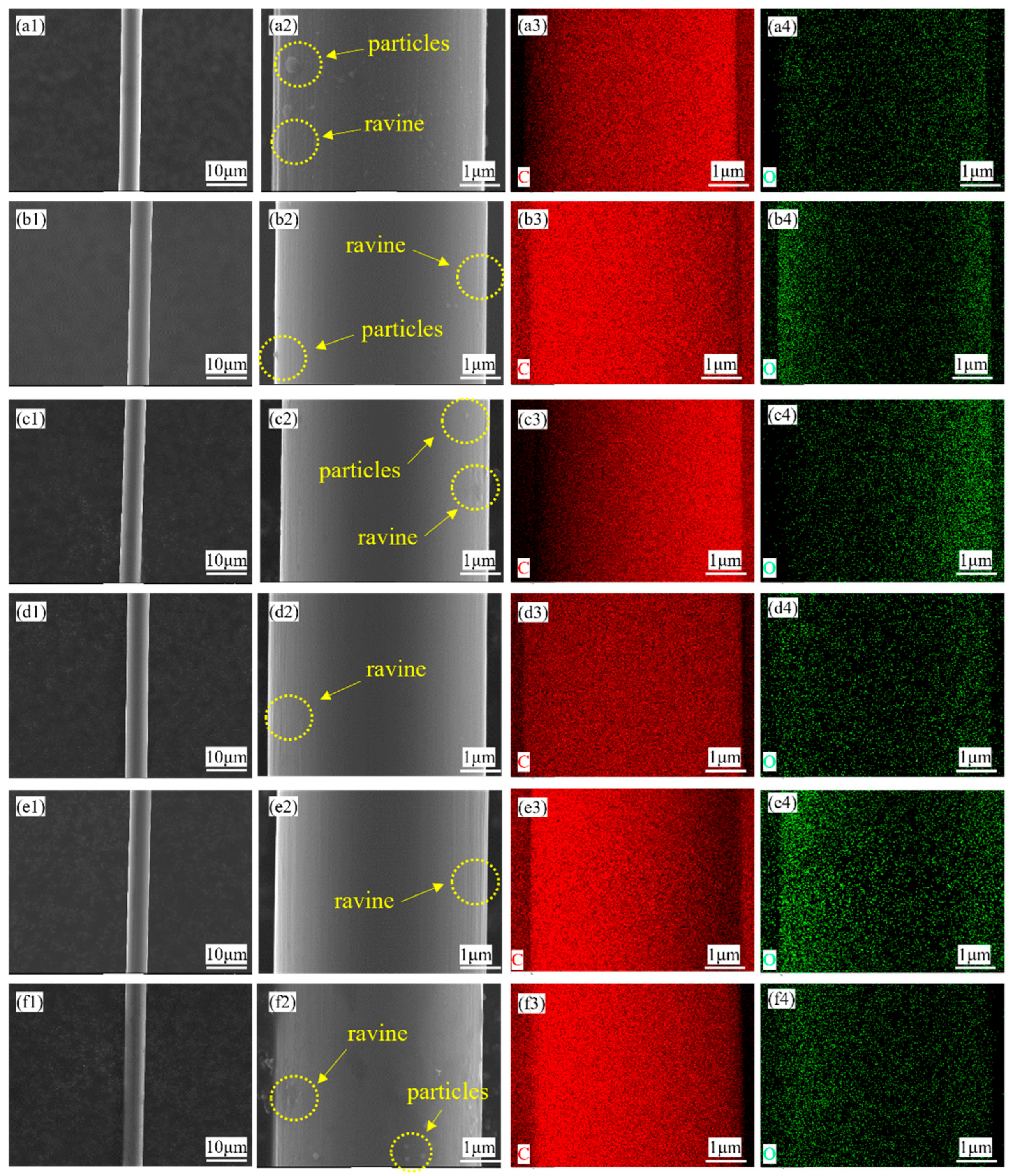
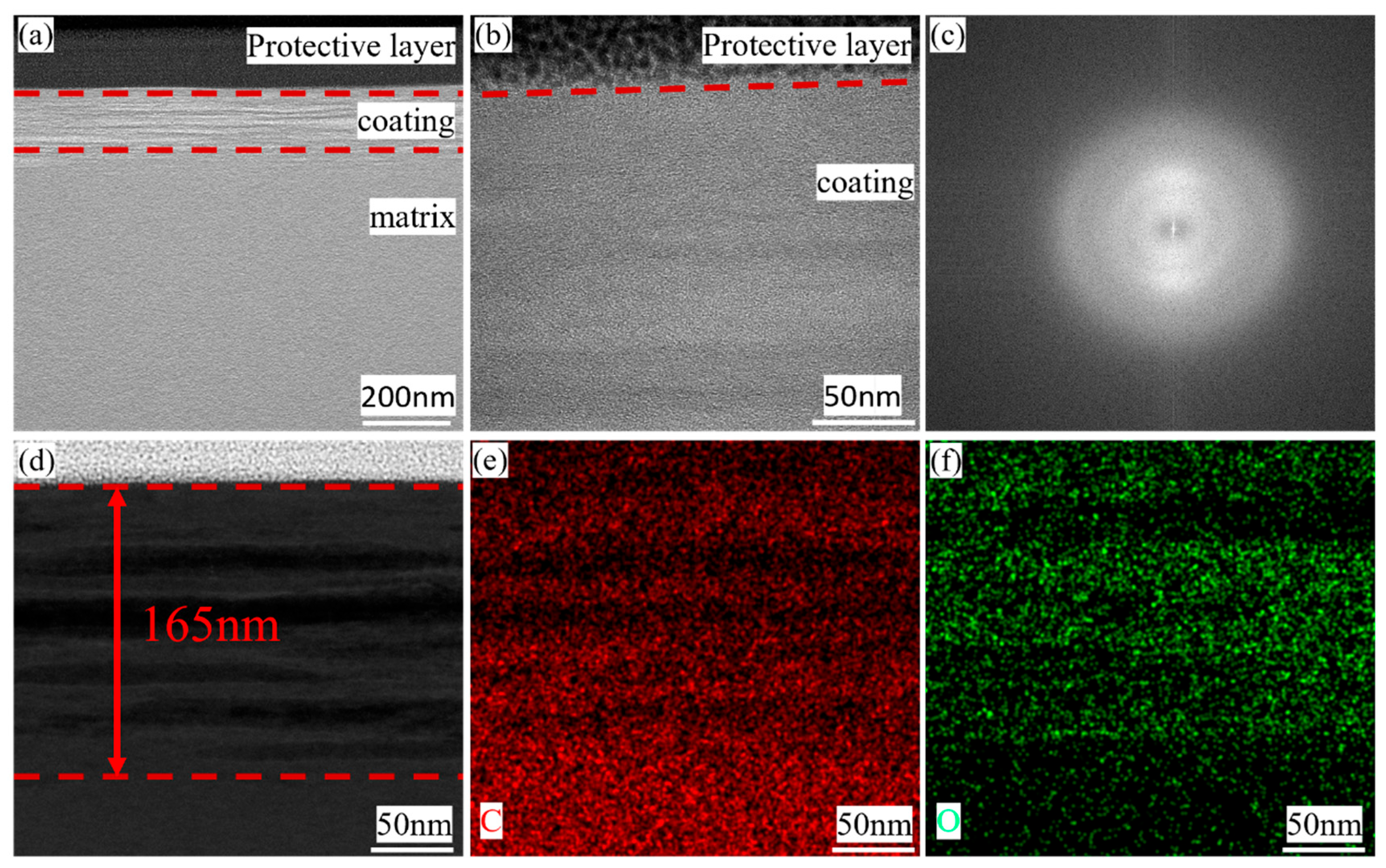
3.1. Micro Morphology of Carbon Coating
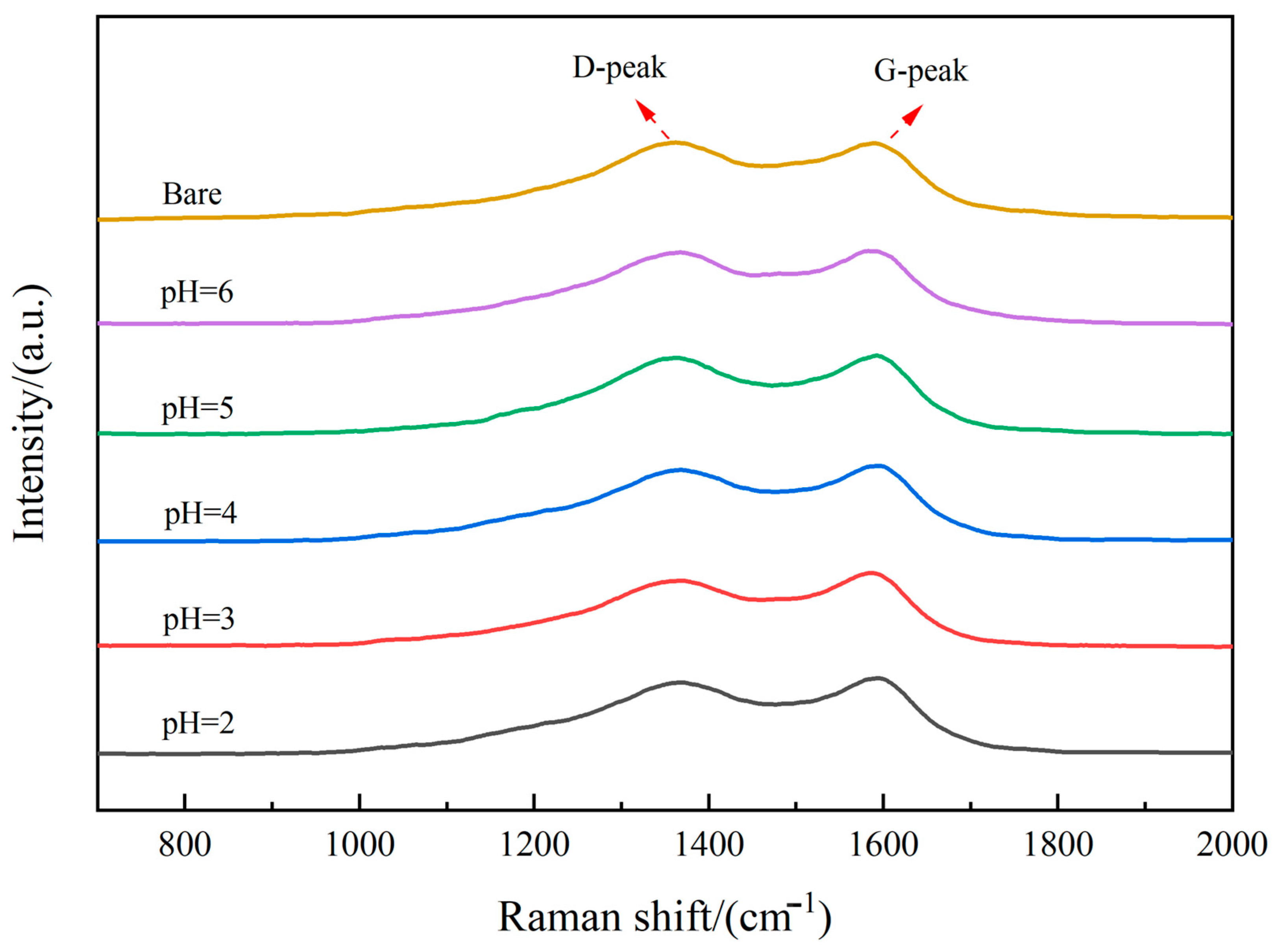
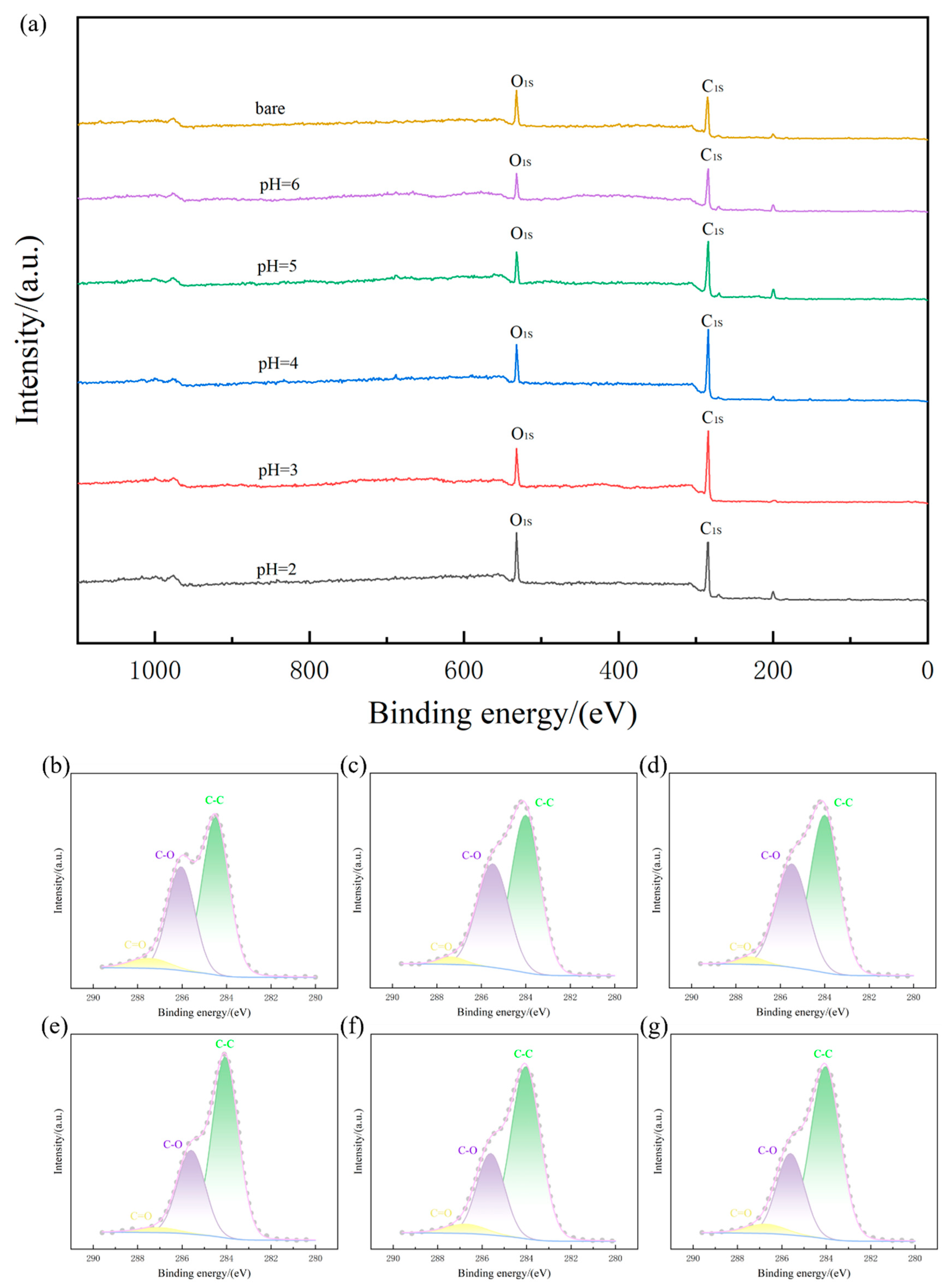
3.2. Phase Analysis
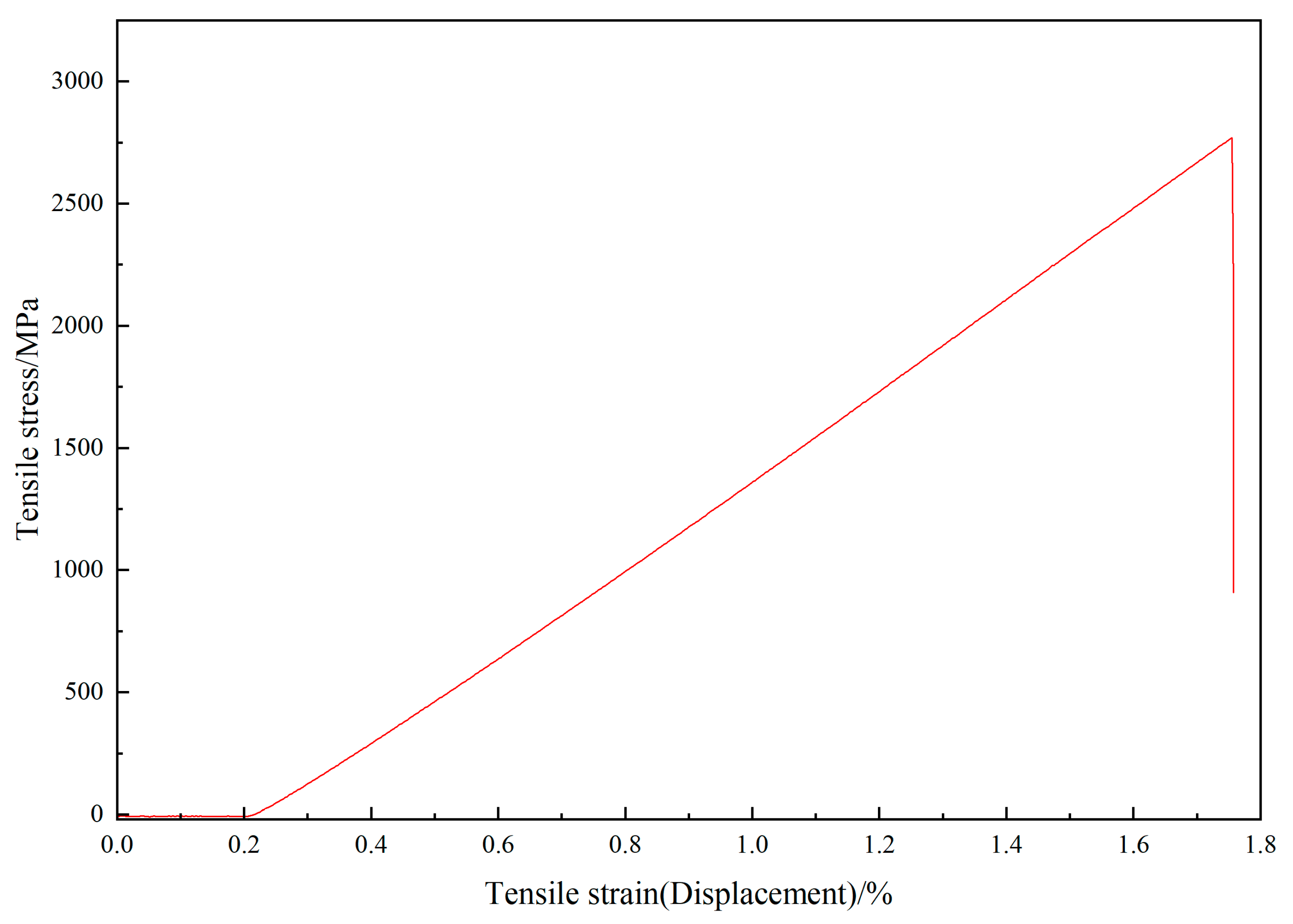
3.3. Performance of the Coating Testing
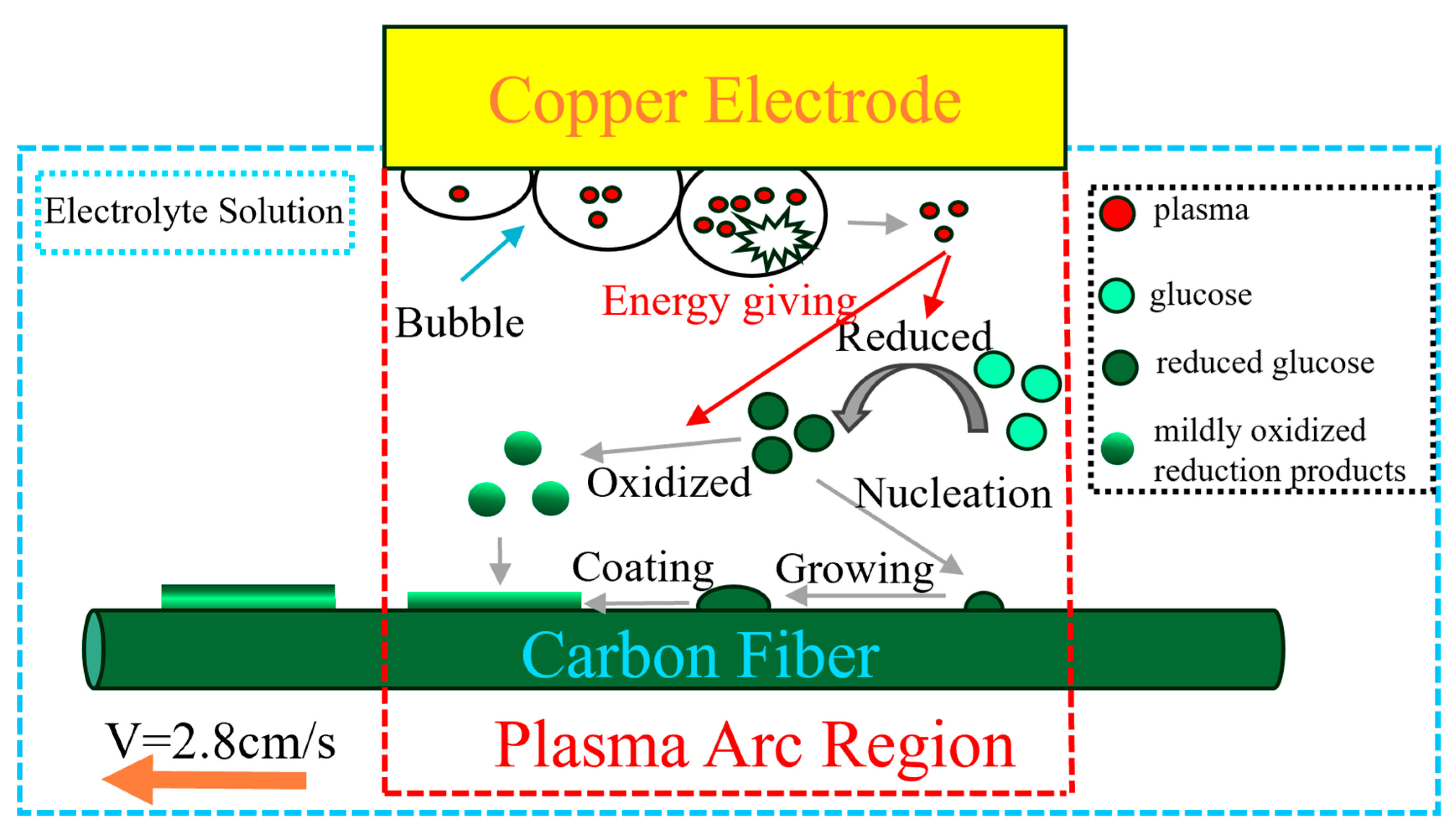
3.4. Analysis of Carbon Coating Deposition Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Corral, E.L.; Walker, L.S. Improved ablation resistance of C–C composites using zirconium diboride and boron carbide. J. Eur. Ceram. Soc. 2010, 30, 2357–2364. [Google Scholar] [CrossRef]
- Fitzer, E. The future of carbon-carbon composites. Carbon 1987, 25, 163–190. [Google Scholar] [CrossRef]
- Han, J.C.; He, X.D.; Du, S.Y. Oxidation and ablation of 3D carbon-carbon composite at up to 3000 °C. Carbon 1995, 33, 473–478. [Google Scholar] [CrossRef]
- Jacobson, N.S.; Curry, D.M. Oxidation microstructure studies of reinforced carbon/carbon. Carbon 2006, 44, 1142–1150. [Google Scholar] [CrossRef]
- Li, C.; Li, G.; Ouyang, H.; Lu, J. ZrB2 particles reinforced glass coating for oxidation protection of carbon/carbon composites. J. Adv. Ceram. 2019, 8, 102–111. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, Y.; Hu, H.; Fei, T.; Li, H. Oxidation resistance and mechanical properties of HfC nanowire-toughened ultra-high temperature ceramic coating for SiC-coated C/C composites. Appl. Surf. Sci. 2016, 360, 970–978. [Google Scholar] [CrossRef]
- Zhang, J.-P.; Fu, Q.-G.; Qu, J.-L. Effect of temperature gradient on the erosion behavior of SiC coating for carbon/carbon composites in a combustion environment. Ceram. Int. 2016, 42, 18411–18417. [Google Scholar] [CrossRef]
- Chi, H.; Tian, X.; Mu, G.; Liu, S.; Liu, L.; Zhu, S.; Ma, Z.; Liu, Y. Ablation behavior of atmospheric plasma sprayed (Hf0.2Zr0.2Nb0.2Ta0.2Sc0.2)B2-SiC hybrid coating for carbon/carbon composites. J. Eur. Ceram. Soc. 2025, 45, 117446. [Google Scholar] [CrossRef]
- Huang, J.; Guo, L.; Li, K.; Yan, N.; Zhou, L.; Li, Y. Microstructures and oxidation behaviors of Al-modified and Al2O3-modified SiC coatings on carbon/carbon composites via pack cementation. Ceram. Int. 2021, 47, 8105–8112. [Google Scholar] [CrossRef]
- Qiang, X.; Li, H.; Liu, Y.; Zhang, N.; Li, X.; Tian, S.; Cong, Y. Oxidation and erosion resistance of multi-layer SiC nanowires reinforced SiC coating prepared by CVD on C/C composites in static and aerodynamic oxidation environments. Ceram. Int. 2018, 44, 16227–16236. [Google Scholar] [CrossRef]
- Qiang, X.; Dong, M.; Chen, X.; Yang, Z.; Zhai, H.; Wu, C.; Tian, S. CVD-grown SiC nanowires-reinforced SiC coating on C/C composites: Focusing on antioxidation, thermal shock and high-temperature gas erosion resistance. Surf. Coat. Technol. 2025, 495, 131584. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Ma, Z.; Zhu, S.; Liu, L.; Xie, M.; Zhang, Z.; Wang, R. Oxidation ablation resistance of ZrB2-HfB2-SiC-TaSi2 coating prepared on C/C composite surface. Surf. Coat. Technol. 2023, 466, 129615. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Ouyang, H.; Shen, T.; Chen, Z.; Bao, L.; Li, Y.; Wang, Q. An inorganic salt strategy to fabricate C/C-ZrC-SiC composites with enhanced mechanical and ablation resistance by PIP method. Ceram. Int. 2025, 51, 31522–31532. [Google Scholar] [CrossRef]
- Makarov, I.S.; Golova, L.K.; Bondarenko, G.N.; Skvortsov, I.Y.; Berkovich, A.K.; Bermeshev, M.V.; Mironova, M.V. Carbon—Silicon-Carbide Fibers Prepared from Solid Solutions of Cellulose in N-Methylmorpholine-N-Oxide with Added Tetraethoxysilane. Fibre Chem. 2017, 49, 231–236. [Google Scholar] [CrossRef]
- Jacobson, N.S.; Roth, D.J.; Rauser, R.W.; Cawley, J.D.; Curry, D.M. Oxidation through coating cracks of SiC-protected carbon/carbon. Surf. Coat. Technol. 2008, 203, 372–383. [Google Scholar] [CrossRef][Green Version]
- Li, L.; Liu, W.; Yang, F.; Jiao, W.; Hao, L.; Wang, R. Interfacial reinforcement of hybrid composite by electrophoretic deposition for vertically aligned carbon nanotubes on carbon fiber. Compos. Sci. Technol. 2020, 187, 107946. [Google Scholar] [CrossRef]
- Zou, M.; Zhao, W.; Wu, H.; Zhang, H.; Xu, W.; Yang, L.; Wu, S.; Wang, Y.; Chen, Y.; Xu, L.; et al. Single Carbon Fibers with a Macroscopic-Thickness, 3D Highly Porous Carbon Nanotube Coating. Adv. Mater. 2018, 30, 1704419. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Hu, P.; Dong, S.; Song, J.; Zhang, X. An efficient hydrothermal transformation approach for construction of controllable carbon coating on carbon fiber from renewable carbohydrate. Appl. Surf. Sci. 2019, 491, 478–487. [Google Scholar] [CrossRef]
- Quan, C.; Deng, S.; Jiang, Y.; Jiang, C.; Shuai, M. Characteristics and high temperature oxidation behavior of Ni-Cr-Y2O3 nanocomposite coating prepared by cathode plasma electrolytic deposition. J. Alloys Compd. 2019, 793, 170–178. [Google Scholar] [CrossRef]
- Quan, C.; He, Y. Microstructure and characterization of a novel cobalt coating prepared by cathode plasma electrolytic deposition. Appl. Surf. Sci. 2015, 353, 1320–1325. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, D.; Liu, D.; Yang, Y.B.; Lin, M.T.; Gao, Y.; Luo, W.F.; Bai, Y. Preparation and thermal cycling performance of Ce-doped YSZ thermal barrier coatings by a novel cathode plasma electrolytic deposition. Ceram. Int. 2024, 50 Pt A, 35825–35830. [Google Scholar] [CrossRef]
- Xu, K.Y.; Lin, M.T.; Liu, Y.T.; Zhao, D.; Liu, M.; Wang, H.D.; Wang, Y.; Bai, Y. Enhanced thermal performance of CeYSZ coating on Ni-based high temperature alloy with a unique Y-doped Al2O3 barrier layer via cathodic plasma electrolytic deposition. Ceram. Int. 2024, 50 Pt C, 56123–56130. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, Y.; Liu, D.; Luo, W.F.; Wang, B.C.; Yang, Y.B.; Bai, Y.; Liu, M.; Wang, H.D. Effect of voltage and duty cycle on microstructure and corrosion resistance of ZrO2 coating by cathode plasma electrolytic deposition. Ceram. Int. 2024, 50, 7193–7197. [Google Scholar] [CrossRef]
- Bu, A.; Zhang, Y.; Xiang, Y.; Yang, Y.; Chen, W.; Cheng, H.; Wang, L. Formation of laminated nano-coatings for enhanced anti-oxidation and electromagnetic wave absorbing properties of carbon fiber. J. Mater. Res. Technol. 2020, 9, 9153–9161. [Google Scholar] [CrossRef]
- Bu, A.; Zhang, Y.; Zhang, Y.; Shen, Y.; Chen, W.; Cheng, H.; Wang, L.; Wang, P.; Li, M.; Lu, L.; et al. Microstructure, properties and formation mechanism of SiO2/SiC nano-coating onto carbon fiber by non-electrode plasma electrolysis. J. Alloys Compd. 2019, 773, 346–351. [Google Scholar] [CrossRef]
- Li, J.; Hu, R.; Liu, H.; Zhou, M.; Gao, Z.; Luo, X. Plasma electrolytic deposition of α-Al2O3 on TiNb fibres and their mechanical properties. Ceram. Int. 2021, 47, 32915–32926. [Google Scholar] [CrossRef]
- Xiang, Y.; Zhang, Y.; Bu, A.; Deng, J.; Meng, Y.; Chen, W.; Cheng, H.; Wang, L.; Lu, L.; Li, M. Preparation, characterization and annealing behavior of nanostructured Al2O3 coating on quartz fiber by non-electrode plasma synthesis. Ceram. Int. 2019, 45, 15520–15525. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, C.; Chen, W.; Cheng, H.; Wang, L. A novel aqueous plasma electrolysis for carbon fiber. Chem. Eng. J. 2016, 304, 426–430. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, X.; Chen, W.; Cheng, H.; Wang, L. Synthesis of Y2O3-ZrO2-SiO2 composite coatings on carbon fiber reinforced resin matrix composite by an electro-plasma process. Appl. Surf. Sci. 2016, 371, 504–511. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, Y.; Shen, Y.; Chen, W.; Cheng, H.; Wang, L. Room-temperature aqueous plasma electrolyzing Al2O3 nano-coating on carbon fiber. Appl. Surf. Sci. 2017, 419, 357–364. [Google Scholar] [CrossRef]
- He, Z.; Cheng, G.; Jiang, Y.; Li, Y.; Zhu, J.; Meng, W.; Zhou, H.; Dai, L.; Wang, L. Novel 2D porous carbon nanosheet derived from biomass: Ultrahigh porosity and excellent performances toward V2+/V3+ redox reaction for vanadium redox flow battery. Int. J. Hydrogen Energy 2020, 45, 3959–3970. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Hu, Z.; Yu, J.C. Converting cellulose waste into a high-efficiency photocatalyst for Cr(VI) reduction via molecular oxygen activation. Appl. Catal. B Environ. 2021, 295, 120253. [Google Scholar] [CrossRef]
- Song, J.; Han, W.; Dong, S.; Fang, C.; Cheng, Y.; Liu, D.; Zhang, X. Constructing hydrothermal carbonization coatings on carbon fibers with controllable thickness for achieving tunable sorption of dyes and oils via a simple heat-treated route. J. Colloid. Interface Sci. 2020, 559, 263–272. [Google Scholar] [CrossRef]
- Jin, X.; Zhu, R.; Zhu, Y.; Li, M. Preparation and electrochemical corrosion of Cr–C coatings by cathode plasma electrolytic deposition for stainless steel bipolar plate in PEMFC. J. Mater. Res. Technol. 2025, 37, 1669–1681. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, X.; Zhang, Z.; Huang, K.; Peng, G.; Fang, T.; He, Y.; Wu, J. Formation and wear performance of diamond-like carbon films on 316L stainless steel prepared by cathodic plasma electrolytic deposition. Diam. Relat. Mater. 2019, 95, 135–140. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, C.; Zhang, J.; Lian, Y.; He, Y. C-Al2O3 coatings prepared by cathode plasma electrolytic deposition on TC4 substrate for better high temperature oxidation resistance. Surf. Coat. Technol. 2021, 405, 126585. [Google Scholar] [CrossRef]
- GB/T 23442-2009; Determination of Structure and Morphology for the Precursors of Polyacrylonitrile-Based Carbon Fibers. China Standard Press: Beijing, China, 2009.
- GB/T 3362-2017; Test Methods for Tensile Properties of Carbon Fiber Multifilament. China Standard Press: Beijing, China, 2009.
- Fang, H.; Sheng, Z.; Wang, W.; Wei, C.; Li, S.; Geng, X.; Li, X.; Zhu, N.; Wen, G.; Dong, S.; et al. Formation and mechanism of carbon coating on carbon fibers through glucose-to-carbon conversion and its effect on the mechanical properties of Cf/ZrB2-SiC composites. J. Eur. Ceram. Soc. 2025, 45, 117569. [Google Scholar] [CrossRef]
- Nie, W.; Li, J.; Liu, Y.; Wang, M.; Liu, K.; Cui, H. Through carbon coating to significantly boost the electrochemical performance of nickel oxide. Adv. Powder Technol. 2025, 36, 104926. [Google Scholar] [CrossRef]
- Orlando, A.; Franceschini, F.; Muscas, C.; Pidkova, S.; Bartoli, M.; Rovere, M.; Tagliaferro, A. A Comprehensive Review on Raman Spectroscopy Applications. Chemosensors 2021, 9, 262. [Google Scholar] [CrossRef]
- Chen, L.; Fan, Z.; Mao, W.; Dai, C.; Chen, D.; Zhang, X. Analysis of Formation Mechanisms of Sugar-Derived Dense Carbons via Hydrogel Carbonization Method. Nanomaterials 2022, 12, 4090. [Google Scholar] [CrossRef] [PubMed]
| Name | Pureness/Model | Manufacturer |
|---|---|---|
| CF | HF40S | HSCARBONFIBRE |
| Glucose | CP | KANGMEI |
| KCl | 99.5% | Zhan Cheng (Tianjin) Technology (Tianjin, China) |
| HCl | 36.5% | Sinopharm Chemical Reagent Co., Ltd. |
| Deionized water | 99.9% | - |
| Name | Linear/(g/km) | Tensile Strength/(MPa) | Tensile Modulus/(GPa) |
|---|---|---|---|
| Value | 445 | 5600 | 295 |
| Sample Number | Bare | pH = 6 | pH = 5 | pH = 4 | pH = 3 | pH = 2 |
|---|---|---|---|---|---|---|
| Parameter | ||||||
| Concentration of KCl | 20 | 20 | 20 | 20 | 20 | 20 |
| Concentration of glucose | 0 | 17.5 | 17.5 | 17.5 | 17.5 | 17.5 |
| Voltage/(V) | 220 | 220 | 220 | 220 | 220 | 220 |
| pH | 7 | 6 | 5 | 4 | 3 | 2 |
| Speed/(cm/s) | 2.8 | 2.8 | 2.8 | 2.8 | 2.8 | 2.8 |
| Name | Bare | pH = 6 | pH = 5 | pH = 4 | pH = 3 | pH = 2 |
|---|---|---|---|---|---|---|
| ID/IG | 1.008 | 0.979 | 0.976 | 0.947 | 0.901 | 0.937 |
| Name | Bare | pH = 6 | pH = 5 | pH = 4 | pH = 3 | pH = 2 |
|---|---|---|---|---|---|---|
| C | 76.5 | 81.2 | 81.5 | 81.79 | 82.28 | 78.18 |
| O | 23.5 | 18.8 | 18.5 | 18.21 | 17.72 | 21.82 |
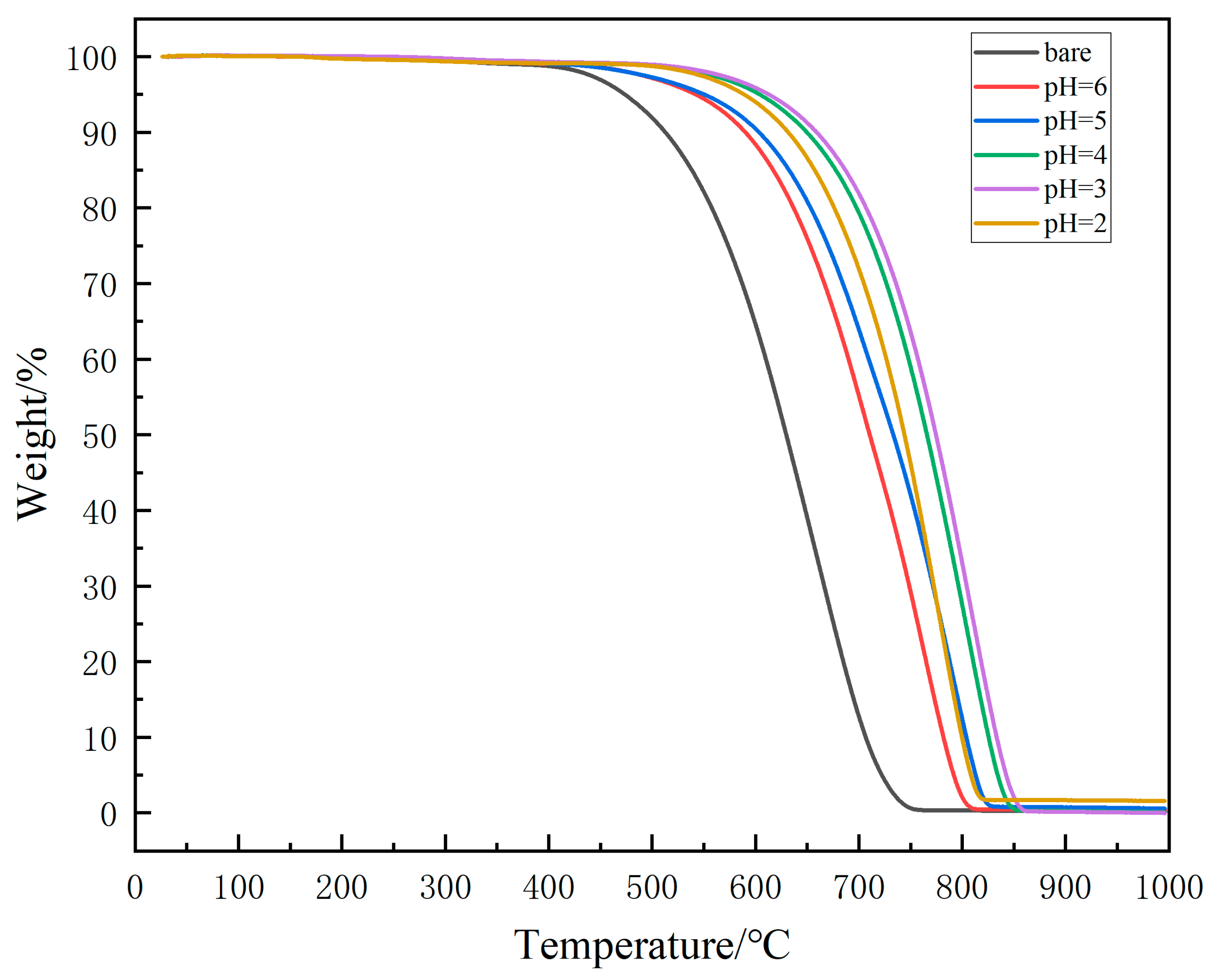
| Name | Bare | pH = 6 | pH = 5 | pH = 4 | pH = 3 | pH = 2 |
|---|---|---|---|---|---|---|
| Initial oxidation temperature/°C | 540 | 610 | 626 | 681 | 696 | 661 |
| Complete weightlessness temperature/°C | 749 | 807 | 825 | 853 | 859 | 820 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Zhou, Q.; Li, M.; Li, D.; Huang, C.; Wei, X.; Chen, W. Preparation of a Novel Carbon Nano Coating on Carbon Fiber Surface Based on Plasma Electrolysis Effect. Materials 2025, 18, 4093. https://doi.org/10.3390/ma18174093
He X, Zhou Q, Li M, Li D, Huang C, Wei X, Chen W. Preparation of a Novel Carbon Nano Coating on Carbon Fiber Surface Based on Plasma Electrolysis Effect. Materials. 2025; 18(17):4093. https://doi.org/10.3390/ma18174093
Chicago/Turabian StyleHe, Xin, Qian Zhou, Maoyuan Li, Dongqin Li, Chiyuhao Huang, Xiaolin Wei, and Weiwei Chen. 2025. "Preparation of a Novel Carbon Nano Coating on Carbon Fiber Surface Based on Plasma Electrolysis Effect" Materials 18, no. 17: 4093. https://doi.org/10.3390/ma18174093
APA StyleHe, X., Zhou, Q., Li, M., Li, D., Huang, C., Wei, X., & Chen, W. (2025). Preparation of a Novel Carbon Nano Coating on Carbon Fiber Surface Based on Plasma Electrolysis Effect. Materials, 18(17), 4093. https://doi.org/10.3390/ma18174093






