Development of Multifunctional Slag and Bauxite Residue-Based Geopolymers with Heavyweight Aggregate Enhancement
Highlights
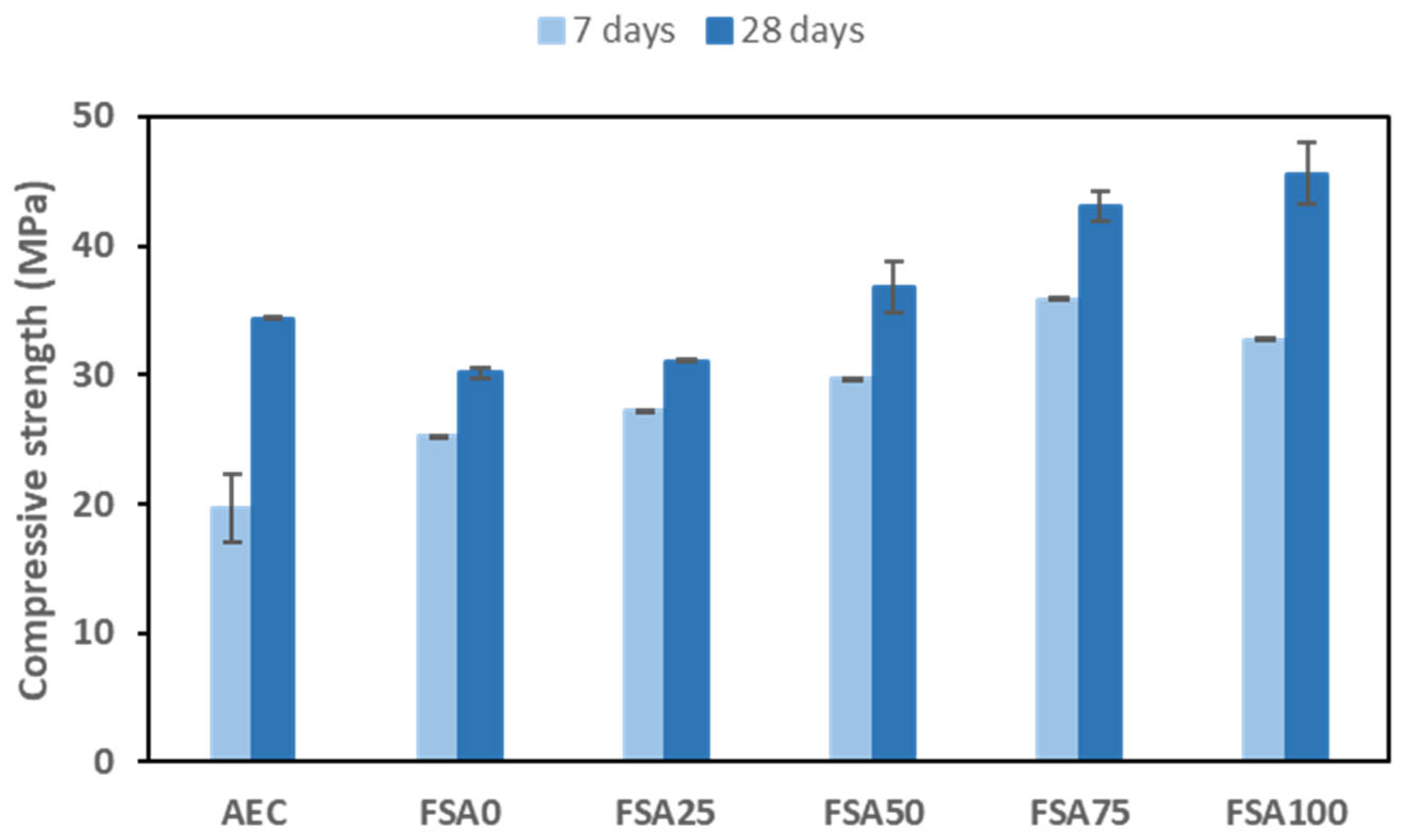
- Fe-rich spinel aggregates (FSAs) improved compressive strength up to 45.5 MPa at 28 days.
- The 100% FSA reduced electrical resistivity to 42 Ω·m for enhanced conductivity.
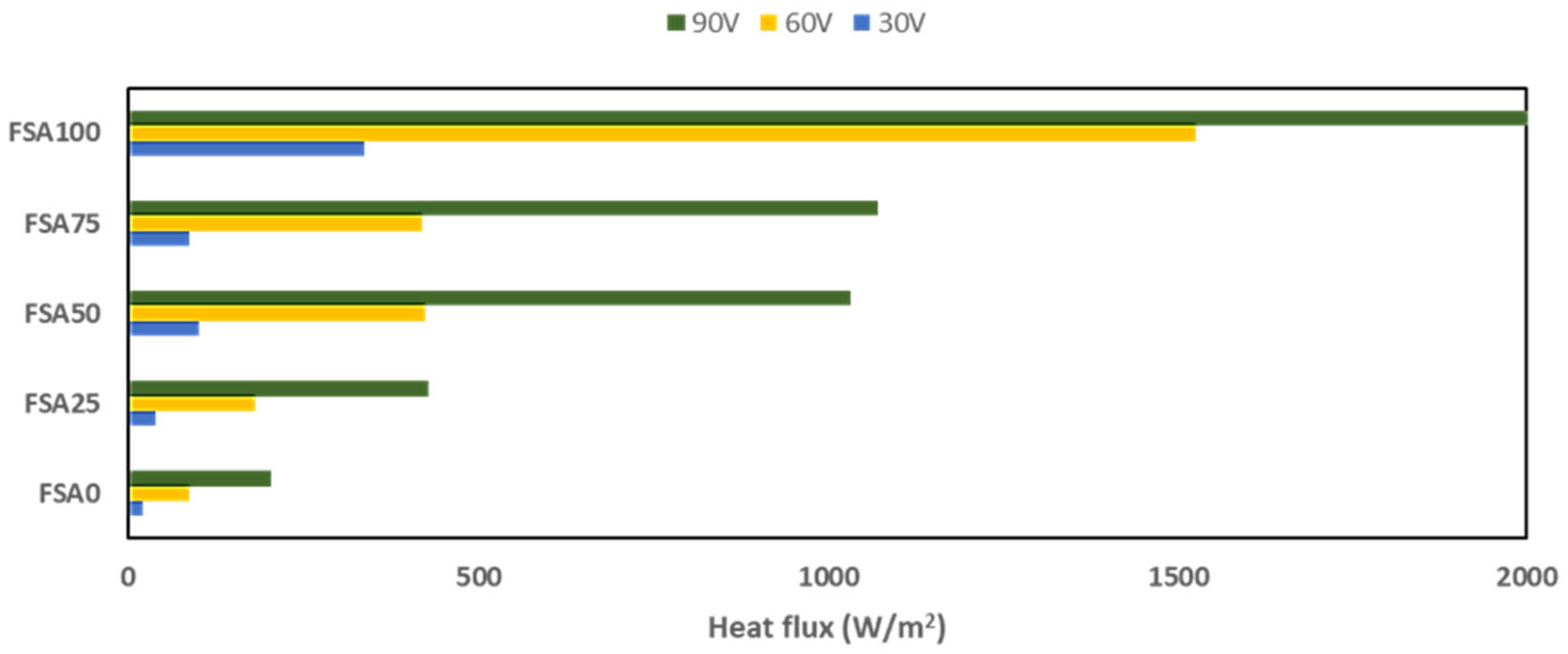
- Photothermal gain reached +9.8 °C in 300 s under 1 kW/m² of solar flux.
- Acid exposure increased mass and strength, unlike conventional concrete.
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Aluminosilicate Sources
2.1.2. Alkali Activator
2.1.3. Aggregates
2.1.4. Graphite
2.2. Methods
2.2.1. Mix Design Proportions
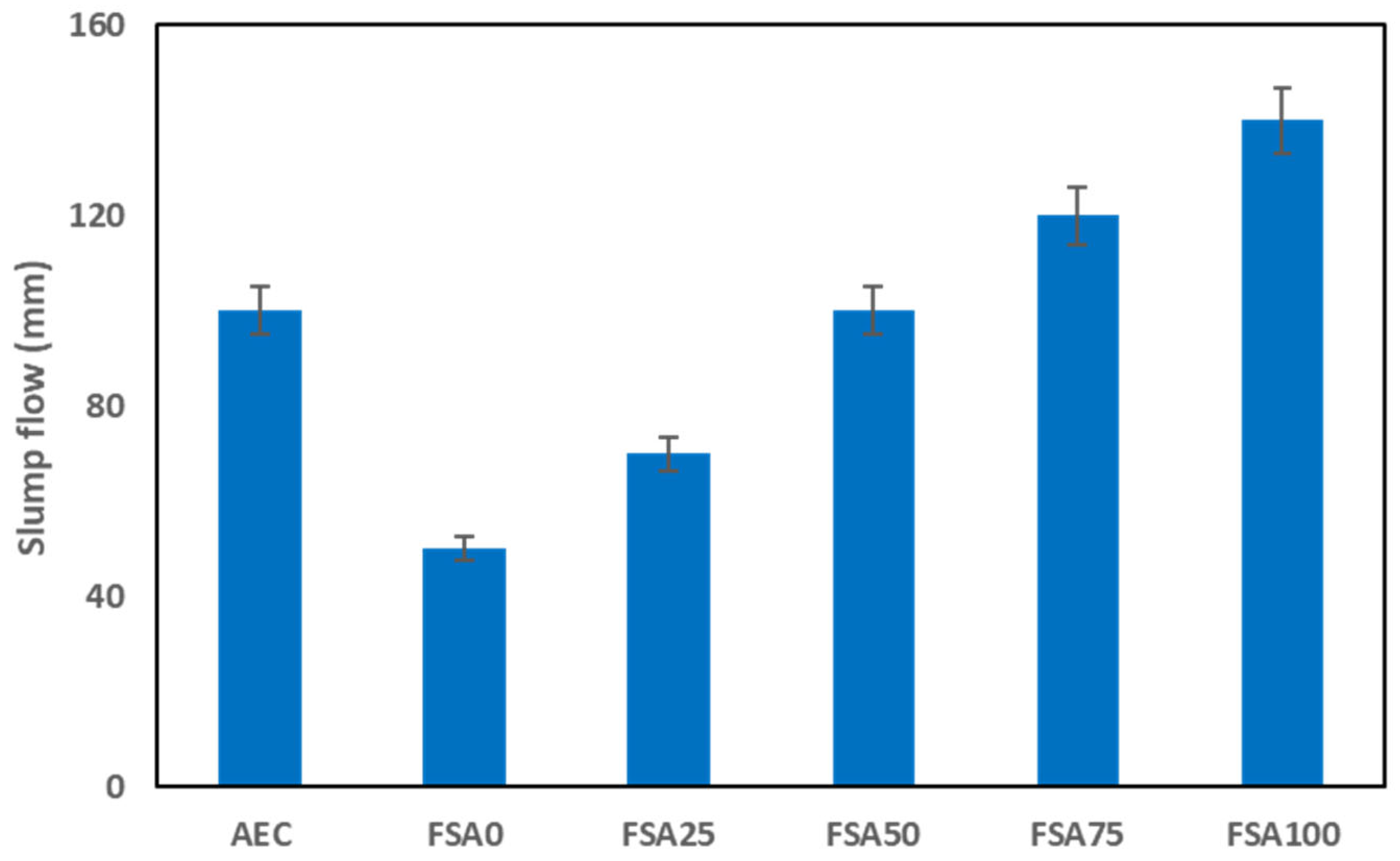
2.2.2. Slump Flow and Bulk Density
2.2.3. Compressive Strength Test
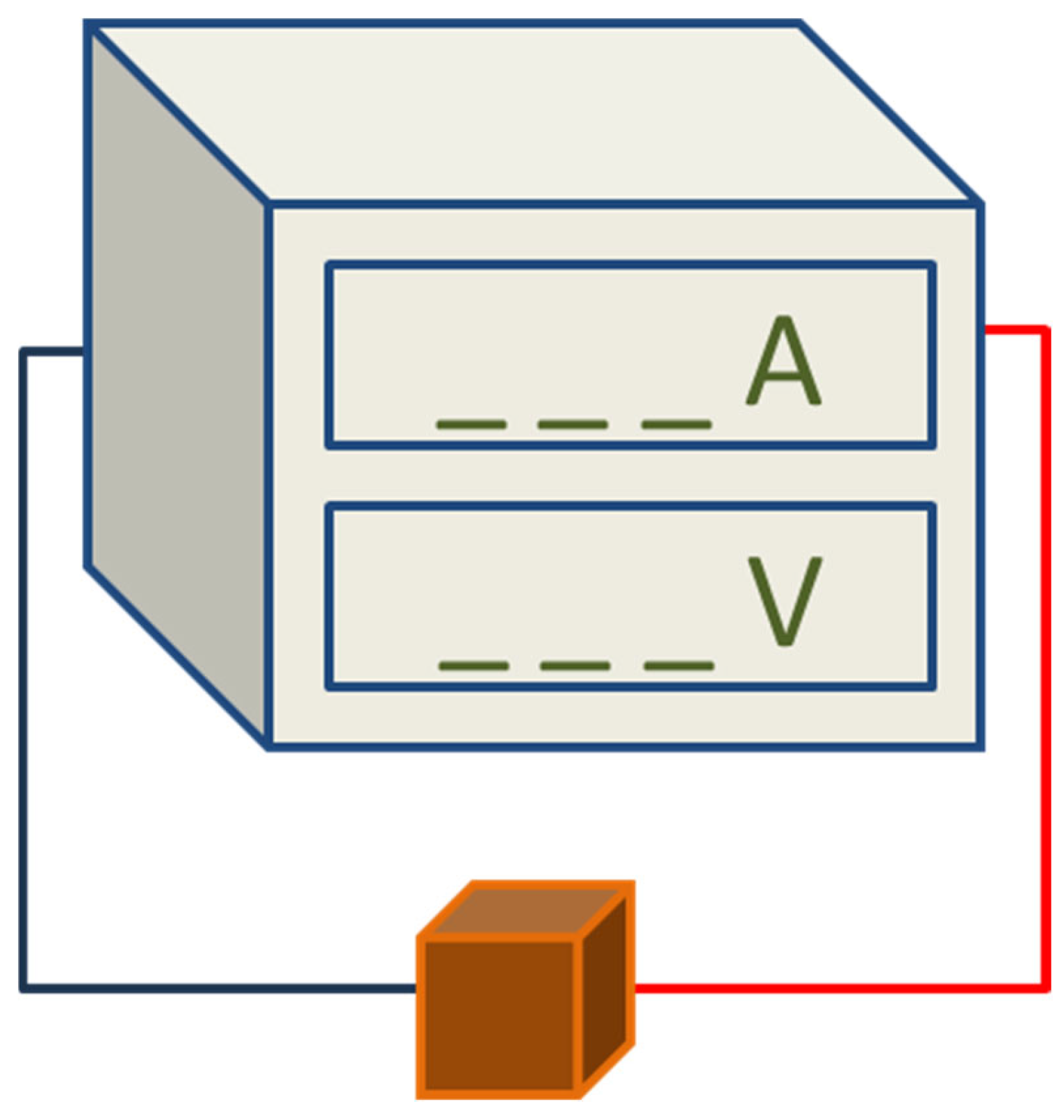
2.2.4. Electrical Property Tests
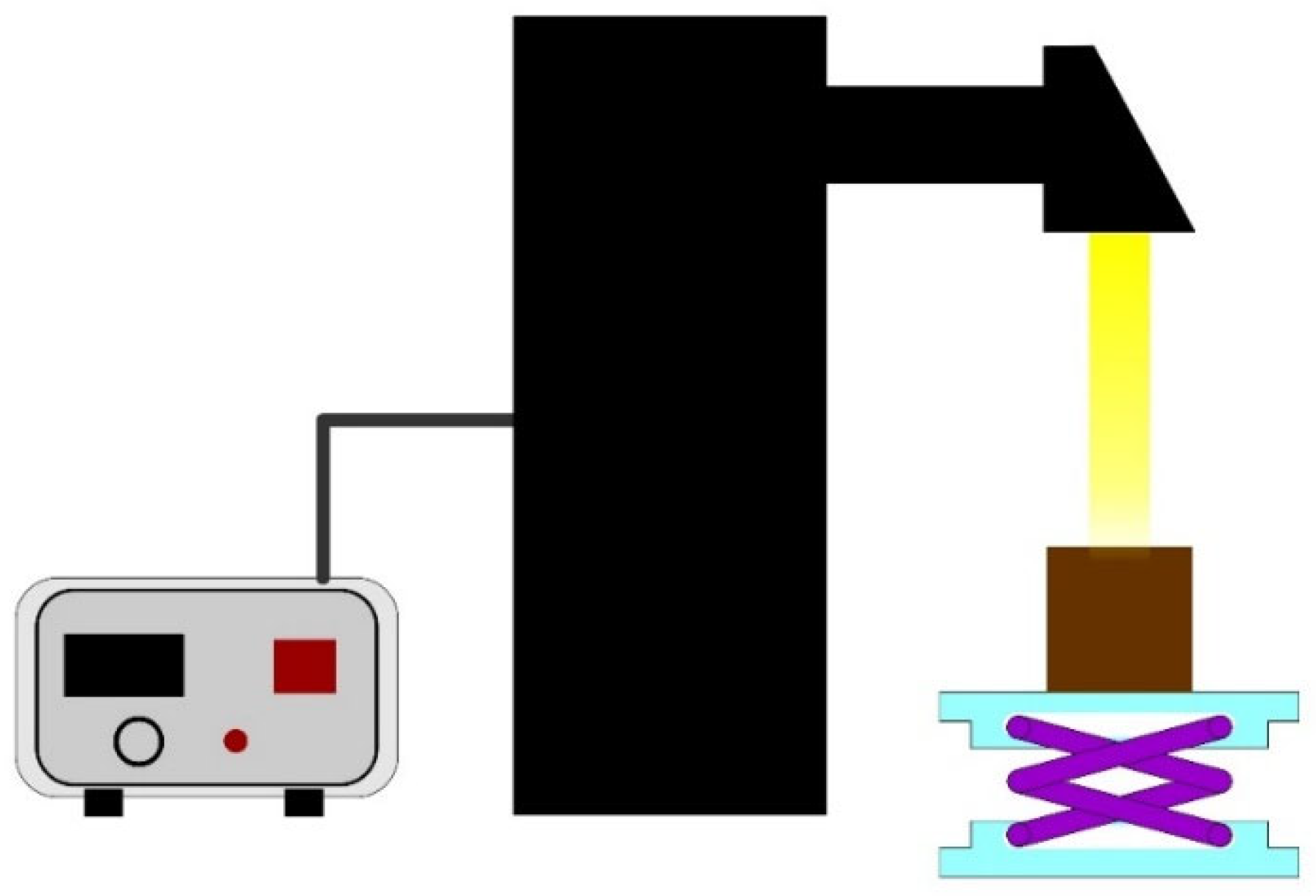
2.2.5. Photothermal Performance
2.2.6. Chemical Resistance
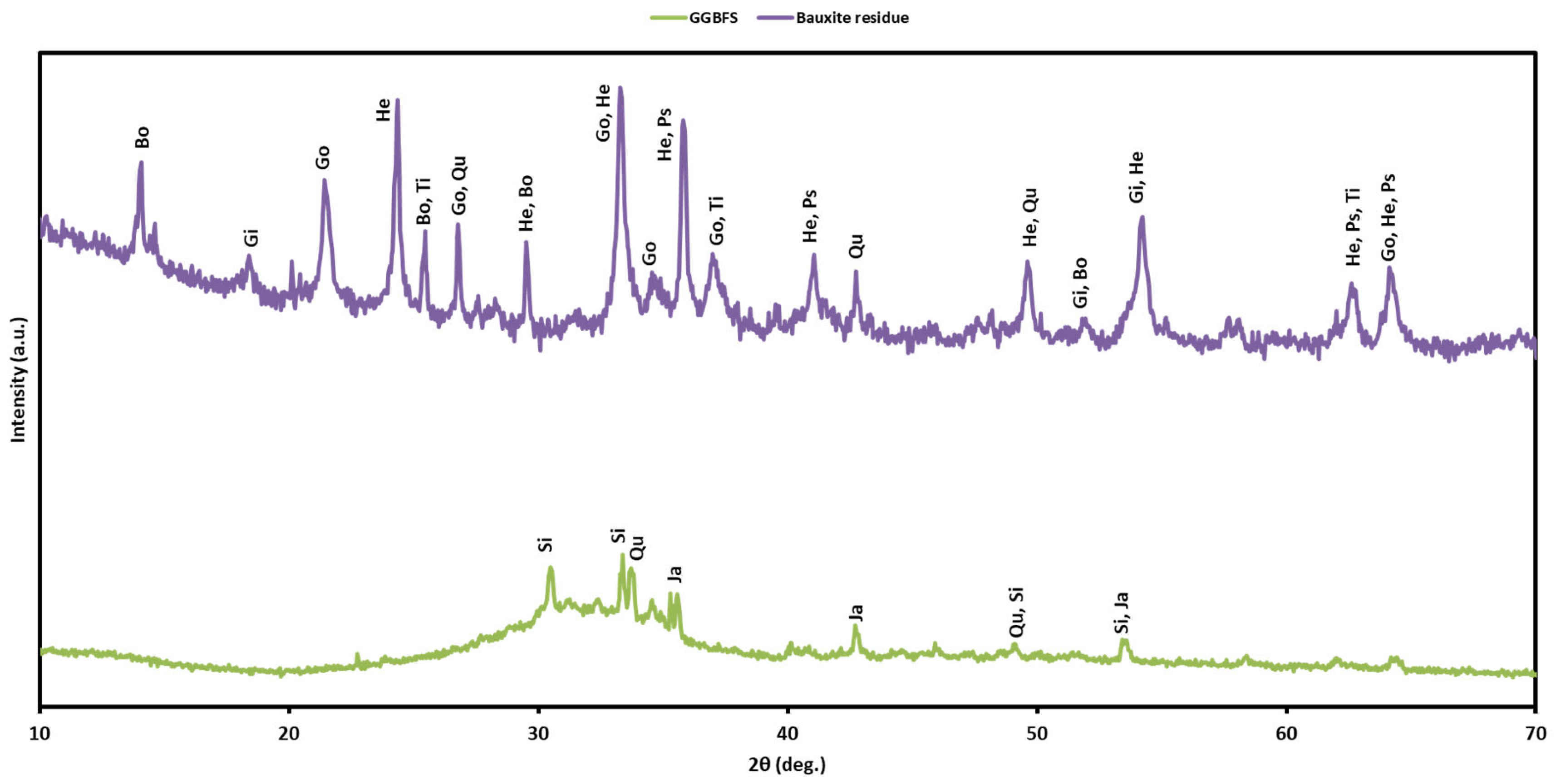
2.2.7. XRD Characterization
2.2.8. SEM Characterization
3. Results and Discussion
3.1. Slump Flow and Bulk Density of Fresh Mortars
3.2. Compressive Strength
3.3. Electrical Performance
3.4. Photothermal Conversion Performance
3.5. Changes in the Geopolymer Mass and Strength Under Acid Attack
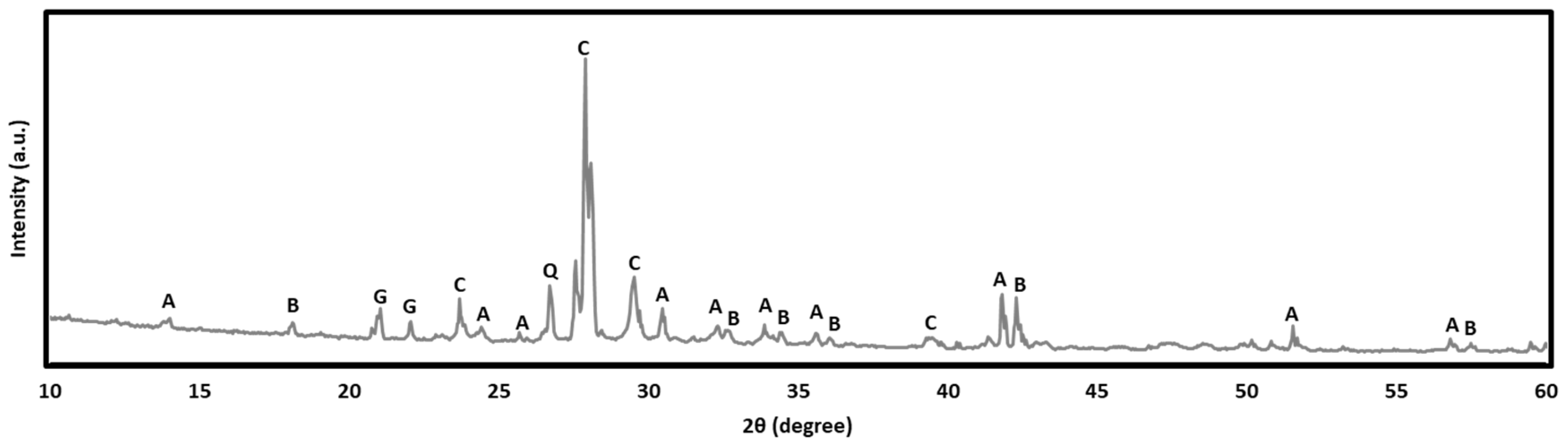
3.6. XRD Analysis
3.7. Microstructural Changes in the Geopolymer Samples Under Acid Attack Through SEM Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FSA | Fe-rich Spinel Aggregate |
| GGBFS | Ground Granulated Blast Furnace Slag |
| AEC | Air-entrained Concrete |
| OPC | Ordinary Portland Cement |
| XRD | X-ray Diffraction |
| SEM | Scanning Electron Microscopy |
| XRF | X-ray Fluorescence |
| CSA | Canadian Standards Association |
| CSH | Calcium–Silicate–Hydrate |
| HCl | Hydrochloric Acid |
References
- Miller, A.B.; Sears, M.E.; Morgan, L.L.; Davis, D.L.; Hardell, L.; Oremus, M.; Soskolne, C.L. Risks to Health and Well-Being from Radio-Frequency Radiation Emitted by Cell Phones and Other Wireless Devices. Front. Public Health 2019, 7, 223. [Google Scholar] [CrossRef] [PubMed]
- Onaizi, A.M.; Amran, M.; Tang, W.; Betoush, N.; Alhassan, M.; Rashid, R.S.M.; Yasin, M.F.; Bayagoob, K.H.; Onaizi, S.A. Radiation-shielding concrete: A review of materials, performance, and the impact of radiation on concrete properties. J. Build. Eng. 2024, 97, 110800. [Google Scholar] [CrossRef]
- Ban, C.C.; Khalaf, M.A.; Ramli, M.; Ahmed, N.M.; Ahmad, M.S.; Ali, A.M.A.; Dawood, E.T.; Ameri, F. Modern heavyweight concrete shielding: Principles, industrial applications and future challenges; review. J. Build. Eng. 2021, 39, 102290. [Google Scholar] [CrossRef]
- Khalaf, M.A.; Ban, C.C.; Ramli, M. The constituents, properties and application of heavyweight concrete: A review. Constr. Build. Mater. 2019, 215, 73–89. [Google Scholar] [CrossRef]
- El-Sayed, T.A. Performance of heavy weight concrete incorporating recycled rice straw ash as radiation shielding material. Prog. Nucl. Energy 2021, 135, 103693. [Google Scholar] [CrossRef]
- Damion, T.; Chaunsali, P. Evaluating acid resistance of Portland cement, calcium aluminate cement, and calcium sulfoaluminate based cement using acid neutralisation. Cem. Concr. Res. 2022, 162, 107000. [Google Scholar] [CrossRef]
- Belaïd, F. How does concrete and cement industry transformation contribute to mitigating climate change challenges? Resour. Conserv. Recycl. Adv. 2022, 15, 200084. [Google Scholar] [CrossRef]
- Li, L.; Xie, J.; Zhang, B.; Feng, Y.; Yang, J. A state-of-the-art review on the setting behaviours of ground granulated blast furnace slag- and metakaolin-based alkali-activated materials. Constr. Build. Mater. 2023, 368, 130389. [Google Scholar] [CrossRef]
- Hertel, T.; Pontikes, Y. Geopolymers, inorganic polymers, alkali-activated materials and hybrid binders from bauxite residue (red mud)–Putting things in perspective. J. Clean. Prod. 2020, 258, 120610. [Google Scholar] [CrossRef]
- Harmaji, A.; Jafari, R.; Simard, G. Valorization of Residue from Aluminum Industries: A Review. Materials 2024, 17, 5152. [Google Scholar] [CrossRef]
- Awoyera, P.; Adesina, A. A critical review on application of alkali activated slag as a sustainable composite binder. Case Stud. Constr. Mater. 2019, 11, e00268. [Google Scholar] [CrossRef]
- Neupane, K. Evaluation of environmental sustainability of one-part geopolymer binder concrete. Clean. Mater. 2022, 6, 100138. [Google Scholar] [CrossRef]
- Zhang, W.; Yao, X.; Yang, T.; Liu, C.; Zhang, Z. Increasing mechanical strength and acid resistance of geopolymers by incorporating different siliceous materials. Constr. Build. Mater. 2018, 175, 411–421. [Google Scholar] [CrossRef]
- Rawat, R.; Pasla, D. Evaluating the corrosion resistance and LCA of lightweight geopolymer concrete integrating sintered fly ash aggregate as coarse aggregate. Constr. Build. Mater. 2024, 449, 138370. [Google Scholar] [CrossRef]
- Adjei, S.; Abdelaal, A.; Elkatatny, S.; Abdelfattah, A.M. Durability of lightweight oil-well geopolymer system in sulfate environment. J. Pet. Explor. Prod. Technol. 2023, 13, 439–448. [Google Scholar] [CrossRef]
- Esteves, D.S.; Melo, A.; Alves, S.; Durães, N.; Paiva, M.C.; Sequeiros, E.W. Magnetic Field-Assisted Orientation and Positioning of Magnetite for Flexible and Electrically Conductive Sensors. Micromachines 2025, 16, 68. [Google Scholar] [CrossRef]
- Tetuko, A.P.; Sebayang, A.M.S.; Fachredzy, A.; Setiadi, E.A.; Asri, N.S.; Sari, A.Y.; Purnomo, F.; Muslih, C.; Fajrin, M.A.; Sebayang, P. Encapsulation of paraffin-magnetite, paraffin, and polyethylene glycol in concretes as thermal energy storage. J. Energy Storage 2023, 68, 107684. [Google Scholar] [CrossRef]
- Alexandrovskaya, Y.M.; Pavley, Y.R.; Grigoriev, Y.V.; Grebenev, V.V.; Shatalova, T.B.; Obrezkova, M.V. Thermal behavior of magnetite nanoparticles with various coatings in the range 30–1000 °C. Thermochim. Acta 2022, 708, 179120. [Google Scholar] [CrossRef]
- Harmaji, A.; Kirana, M.C.; Jafari, R. Machine Learning to Predict Workability and Compressive Strength of Low- and High-Calcium Fly Ash–Based Geopolymers. Crystals 2024, 14, 830. [Google Scholar] [CrossRef]
- Kwek, S.Y.; Awang, H.; Cheah, C.B. Influence of Liquid-to-Solid and Alkaline Activator (Sodium Silicate to Sodium Hydroxide) Ratios on Fresh and Hardened Properties of Alkali-Activated Palm Oil Fuel Ash Geopolymer. Materials 2021, 14, 4253. [Google Scholar] [CrossRef]
- Harmaji, A.; Jafari, R.; Simard, G. Durable bauxite-residue geopolymers: Enhancing scaling resistance with fly ash and waste glass powder. Constr. Build. Mater. 2025, 491, 142661. [Google Scholar] [CrossRef]
- CSA A23; Concrete Materials and Methods of Concrete Construction/Test Methods and Standard Practices for Concrete. CSA Group: Toronto, ON, Canada, 2024. Available online: https://www.csagroup.org/store/product/2701210/?srsltid=AfmBOooGNMxnZylys79gZooMEt02t7qQ3FD9nV2hkaK6F2eCxiyB9PI9 (accessed on 26 August 2025).
- Mingqing, S.; Xinying, M.; Xiaoying, W.; Zuofu, H.; Zhuoqiu, L. Experimental studies on the indoor electrical floor heating system with carbon black mortar slabs. Energy Build. 2008, 40, 1094–1100. [Google Scholar] [CrossRef]
- Li, C.; Ge, H.; Daquan, S.; Xuhong, Z. Novel conductive wearing course using a graphite, carbon fiber, and epoxy resin mixture for active de-icing of asphalt concrete pavement. Mater. Struct. 2021, 54, 48. [Google Scholar] [CrossRef]
- Frąc, M.; Szołdra, P.; Pichór, W. Smart Graphite-Cement Composites with Low Percolation Threshold. Materials 2022, 15, 2770. [Google Scholar] [CrossRef]
- Luo, T.; Wang, Q. Effects of Graphite on Electrically Conductive Cementitious Composite Properties: A Review. Materials 2021, 14, 4798. [Google Scholar] [CrossRef]
- Gao, J.; Huo, Y.; Liu, Y.; Yang, Y. Absorbing effect of air entraining agent on early frost expansion of mortar: Calculation model and application. Constr. Build. Mater. 2024, 415, 135100. [Google Scholar] [CrossRef]
- Harmaji, A.; Ekaputri, J.J. The Application of Microbes to the Fly Ash-Based Alkali-Activated Material Performance Containing Slag; Springer Nature: Singapore, 2023. [Google Scholar]
- Zhao, Z.; Remond, S.; Damidot, D.; Xu, W. Influence of fine recycled concrete aggregates on the properties of mortars. Constr. Build. Mater. 2015, 81, 179–186. [Google Scholar] [CrossRef]
- Anis, M.; Abdel-Raheem, M. A Review of Electrically Conductive Cement Concrete Pavement for Sustainable Snow-Removal and Deicing: Road Safety in Cold Regions. Transp. Res. Rec. 2024, 2678, 50–71. [Google Scholar] [CrossRef]
- Pan, D.; Deng, X.; Ge, Y.; He, Y.; Cui, X. Preparation of a low-cost electric compensation photothermal evaporator for all-weather-available and efficient evaporation. Sol. Energy 2023, 259, 151–164. [Google Scholar] [CrossRef]
- Aygörmez, Y.; Canpolat, O. Long-term sulfuric and hydrochloric acid resistance of silica fume and colemanite waste reinforced metakaolin-based geopolymers. Rev. Construcción 2021, 20, 291–307. [Google Scholar] [CrossRef]
- Aygörmez, Y.; Canpolat, O.; Al-Mashhadani, M.M. Assessment of geopolymer composites durability at one year age. J. Build. Eng. 2020, 32, 101453. [Google Scholar] [CrossRef]
- Zhang, P.; Zheng, Y.; Wang, K.; Zhang, J. A review on properties of fresh and hardened geopolymer mortar. Compos. Part B Eng. 2018, 152, 79–95. [Google Scholar] [CrossRef]
- Pundienė, I.; Pranckevičienė, J.; Kligys, M.; Girskas, G. Study of the Course of Cement Hydration in the Presence of Waste Metal Particles and Pozzolanic Additives. Materials 2022, 15, 2925. [Google Scholar] [CrossRef]
- Nabiun, R.; Nasajpour-Esfahani, N.; Jasim, D.J.; Mirvalad, S.; Garmestani, H.; Toghraie, D. Laboratory evaluation of mechanical properties and noise absorption in magnetite-enhanced concrete for pavement applications. Case Stud. Constr. Mater. 2024, 21, e03570. [Google Scholar] [CrossRef]
- Valizadeh, A.; Aslani, F.; Asif, Z.; Roso, M. Development of Heavyweight Self-Compacting Concrete and Ambient-Cured Heavyweight Geopolymer Concrete Using Magnetite Aggregates. Materials 2019, 12, 1035. [Google Scholar] [CrossRef]
- Ali, S.I.A.; Lublóy, É. Fire resistance properties of heavyweight magnetite concrete in comparison with normal basalt- and quartz-based concrete. J. Therm. Anal. Calorim. 2022, 147, 11679–11691. [Google Scholar] [CrossRef]
- Sas, Z.; Sha, W.; Soutsos, M.; Doherty, R.; Bondar, D.; Gijbels, K.; Schroeyers, W. Radiological characterisation of alkali-activated construction materials containing red mud, fly ash and ground granulated blast-furnace slag. Sci. Total Environ. 2019, 659, 1496–1504. [Google Scholar] [CrossRef]
- Liu, X.; Feng, P.; Chen, J.; Liu, Q.; Yu, X.; Cai, Y.; Zhu, H.; Qing, L.; Hong, J. A critical review on the interaction between calcium silicate hydrate (C-S-H) and different ions. Constr. Build. Mater. 2024, 413, 134931. [Google Scholar] [CrossRef]
- Abdullah, M.A.H.; Rashid, R.S.M.; Amran, M.; Hejazii, F.; Azreen, N.M.; Fediuk, R.; Voo, Y.L.; Vatin, N.I.; Idris, M.I. Recent Trends in Advanced Radiation Shielding Concrete for Construction of Facilities: Materials and Properties. Polymers 2022, 14, 2830. [Google Scholar] [CrossRef] [PubMed]
- Aslani, F.; Asif, Z. Properties of Ambient-Cured Normal and Heavyweight Geopolymer Concrete Exposed to High Temperatures. Materials 2019, 12, 740. [Google Scholar] [CrossRef] [PubMed]
- Castillo, H.; Collado, H.; Droguett, T.; Sánchez, S.; Vesely, M.; Garrido, P.; Palma, S. Factors Affecting the Compressive Strength of Geopolymers: A Review. Minerals 2021, 11, 1317. [Google Scholar] [CrossRef]
- Sikora, P.; Abd Elrahman, M.; Horszczaruk, E.; Brzozowski, P.; Stephan, D. Incorporation of magnetite powder as a cement additive for improving thermal resistance and gamma-ray shielding properties of cement-based composites. Constr. Build. Mater. 2019, 204, 113–121. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, X.; Guo, C.; Kang, D.; Zhang, W.; Lan, J.; Fang, Z. Behavior of hematite, magnetite, and reduced iron powder in geopolymers: Effects of mechanical properties and reaction mechanism. J. Clean. Prod. 2024, 444, 141178. [Google Scholar] [CrossRef]
- Nguyen, K.T.; Ahn, N.; Le, T.A.; Lee, K. Theoretical and experimental study on mechanical properties and flexural strength of fly ash-geopolymer concrete. Constr. Build. Mater. 2016, 106, 65–77. [Google Scholar] [CrossRef]
- Fiala, L.; Pommer, V.; Böhm, M.; Scheinherrová, L.; Černý, R. Self-heating alkali activated materials: Microstructure and its effect on electrical, thermal and mechanical properties. Constr. Build. Mater. 2022, 335, 127527. [Google Scholar] [CrossRef]
- Vlachakis, C.; Perry, M.; Biondi, L. Self-Sensing Alkali-Activated Materials: A Review. Minerals 2020, 10, 885. [Google Scholar] [CrossRef]
- Gomaa, M.M. Electrical properties of hematite and pure sand synthetic homogeneous mixture. Appl. Water Sci. 2022, 13, 43. [Google Scholar] [CrossRef]
- Ren, Z.; Zeng, H.; Zeng, X.; Chen, X.; Wang, X. Effect of Nanographite Conductive Concrete Mixed with Magnetite Sand Excited by Different Alkali Activators and Their Combinations on the Properties of Conductive Concrete. Buildings 2023, 13, 1630. [Google Scholar] [CrossRef]
- Omana, L.; Chandran, A.; John, R.E.; Wilson, R.; George, K.C.; Unnikrishnan, N.V.; Varghese, S.S.; George, G.; Simon, S.M.; Paul, I. Recent Advances in Polymer Nanocomposites for Electromagnetic Interference Shielding: A Review. ACS Omega 2022, 7, 25921–25947. [Google Scholar] [CrossRef]
- Qureshi, N.; Dhand, V.; Subhani, S.; Senthil Kumar, R.; Raghavan, N.; Kim, S.; Doh, J. Exploring Conductive Filler-Embedded Polymer Nanocomposite for Electrical Percolation via Electromagnetic Shielding-Based Additive Manufacturing. Adv. Mater. Technol. 2024, 9, 2400250. [Google Scholar] [CrossRef]
- Al-Ghamdi, A.A.; Al-Hartomy, O.A.; Al-Solamy, F.R.; Dishovsky, N.; Malinova, P.; Atanasova, G.; Atanasov, N. Conductive carbon black/magnetite hybrid fillers in microwave absorbing composites based on natural rubber. Compos. Part B Eng. 2016, 96, 231–241. [Google Scholar] [CrossRef]
- Kim, H.-S.; Jang, C.; Kim, H.G.; Woo, B.-H. Snow-melting performance of the thermally conductive concrete pavement–experimental evaluation in field application. Constr. Build. Mater. 2024, 411, 134508. [Google Scholar] [CrossRef]
- Wang, Z.; Bai, E.; Huang, H.; Wang, T.; Sun, H. Study on the electromagnetic property and microwave heating efficiency of concrete with magnetite aggregate. Constr. Build. Mater. 2022, 342, 128080. [Google Scholar] [CrossRef]
- Dehghanpour, H.; Yilmaz, K.; Afshari, F.; Ipek, M. Electrically conductive concrete: A laboratory-based investigation and numerical analysis approach. Constr. Build. Mater. 2020, 260, 119948. [Google Scholar] [CrossRef]
- Sassani, A.; Ceylan, H.; Kim, S.; Arabzadeh, A.; Taylor, P.C.; Gopalakrishnan, K. Development of Carbon Fiber-modified Electrically Conductive Concrete for Implementation in Des Moines International Airport. Case Stud. Constr. Mater. 2018, 8, 277–291. [Google Scholar] [CrossRef]
- An, M.; Huang, H.; Wang, Y.; Zhao, G. Effect of thermal cycling on the properties of high-performance concrete: Microstructure and mechanism. Constr. Build. Mater. 2020, 243, 118310. [Google Scholar] [CrossRef]
- Wu, T.; Huang, R.; Chi, M.; Weng, T. A study on electrical and thermal properties of conductive concrete. Comput. Concr. 2013, 12, 337–349. [Google Scholar] [CrossRef]
- Xu, B.; Han, J.; Kumar, A.; Li, P.; Yang, Y. Thermal storage using sand saturated by thermal-conductive fluid and comparison with the use of concrete. J. Energy Storage 2017, 13, 85–95. [Google Scholar] [CrossRef]
- Şahan, N.; Fois, M.; Paksoy, H. Improving thermal conductivity phase change materials—A study of paraffin nanomagnetite composites. Sol. Energy Mater. Sol. Cells 2015, 137, 61–67. [Google Scholar] [CrossRef]
- Gao, H.; Yin, T.; Ma, J.; Zhou, Y.; Li, K.; Bao, J. Research Progress of Photothermal Superhydrophobic Surfaces for Anti-Icing/Deicing. Molecules 2025, 30, 1865. [Google Scholar] [CrossRef]
- GivKashi, M.R.; Tohidloo, M. The effect of freeze-thaw cycles and sulfuric acid attack separately on the compressive strength and microstructure of 3D-printed air-entrained concrete. Constr. Build. Mater. 2024, 440, 137411. [Google Scholar] [CrossRef]
- Yang, H.; Che, Y.; Leng, F. Calcium leaching behavior of cementitious materials in hydrochloric acid solution. Sci. Rep. 2018, 8, 8806. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, Y.; Zhang, Z.; Huang, J.; Zhang, P.; Ma, Y.; Wang, H. Study of acidic degradation of alkali-activated materials using synthetic C-(N)-A-S-H and N-A-S-H gels. Compos. Part B Eng. 2022, 230, 109510. [Google Scholar] [CrossRef]
- Wattanasiriwech, D.; Yomthong, K.; Wattanasiriwech, S. Adsorption efficiency and photocatalytic activity of fly ash-based geopolymer foam mortar. Ceram. Int. 2021, 47, 27361–27371. [Google Scholar] [CrossRef]
- Pan, Z.; Wang, S.; Liu, Y.; Li, B.; Jia, Z.; Zhang, Y.; Wang, J. The hydration, pore structure and strength of cement-based material prepared with waste soaking solution from acetic acid treatment of regenerated aggregates. J. Clean. Prod. 2019, 235, 866–874. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Y.; Zhang, Z.; Zhang, P.; Ma, Y.; Wang, A.; Wang, H. Intrinsic sulfuric acid resistance of C-(N)-A-S-H and N-A-S-H gels produced by alkali-activation of synthetic calcium aluminosilicate precursors. Cem. Concr. Res. 2023, 165, 107068. [Google Scholar] [CrossRef]
- Park, J.A.; Pimenta, M.M.; Bezerra, A.C.d.S. Acid Activation in Low-Carbon Binders: A Systematic Literature Review. Buildings 2024, 14, 83. [Google Scholar] [CrossRef]
- Bingöl, Ş.; Bilim, C.; Duran Atiş, C.; Durak, U.; İlkentapar, S.; Karahan, O. An investigation of resistance of sodium meta silicate activated slag mortar to acidic and basic mediums. Rev. Construcción 2020, 19, 127–133. [Google Scholar] [CrossRef]
- Damion, T.; Soda, P.R.K.; Gupta, S. Enhanced acid resistance of geopolymer containing recycled masonry wastes as high-volume replacement of natural sand. J. Build. Eng. 2025, 105, 112396. [Google Scholar] [CrossRef]
- de Souza Oliveira, A.; Dweck, J.; Fairbairn, E. de M.R.; Gomes, O. da F.M.; Toledo Filho, R.D. Crystalline admixture effects on crystal formation phenomena during cement pastes’ hydration. J. Therm. Anal. Calorim. 2020, 139, 3361–3375. [Google Scholar] [CrossRef]
- Jin, B.; Wu, S.; Fan, S.; Hang, F.; Chen, H. Strength and Expansion of LHEC with Different Gypsum Contents Under Thermal Curing. Materials 2024, 17, 5766. [Google Scholar] [CrossRef]
- Shamsudin, M.A.; Mustapha, F.; Abdullah, M.N.; Mustapha, M. Sustainable Aluminosilicate Coatings from Palm Oil Waste for Enhanced Thermal and Microstructure Properties. Materials 2025, 18, 821. [Google Scholar] [CrossRef]
- Zuo, Y.; Ye, G. Pore Structure Characterization of Sodium Hydroxide Activated Slag Using Mercury Intrusion Porosimetry, Nitrogen Adsorption, and Image Analysis. Materials 2018, 11, 1035. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Ma, B.; Wang, J.; Cai, Q.; Jiang, J.; Qian, B.; Cheng, G.; Hu, Y.; Ma, F.; Sun, J.; et al. The influence of ZSM-5 waste on the properties of fly ash-based foamed geopolymer. J. Clean. Prod. 2022, 355, 131800. [Google Scholar] [CrossRef]
- Davidovits, J.; Davidovits, R. Ferro-Sialate Geopolymers. Geopolymer Institute Library. 2020. Available online: https://www.geopolymer.org/wp-content/uploads/Ferro-sialate.pdf (accessed on 10 July 2025).
- Patrisia, Y.; Law, D.W.; Gunasekara, C.; Wardhono, A. Long-term durability of iron-rich geopolymer concrete in sulphate, acidic and peat environments. J. Build. Eng. 2024, 97, 110744. [Google Scholar] [CrossRef]
- Tome, S.; Nana, A.; Tchakouté, H.K.; Temuujin, J.; Rüscher, C.H. Mineralogical evolution of raw materials transformed to geopolymer materials: A review. Ceram. Int. 2024, 50, 35855–35868. [Google Scholar] [CrossRef]
- Zhou, Y.; Liang, Y.; Wu, Z.; Wang, X.; Guan, R.; Li, C.; Qiao, F.; Wang, J.; Fu, Y.; Baek, J.-B. Amorphous/Crystalline Heterostructured Nanomaterials: An Emerging Platform for Electrochemical Energy Storage. Small 2025, 21, 2411941. [Google Scholar] [CrossRef]
- Pouyanne, A.; Boudache, S.; Hilloulin, B.; Loukili, A.; Roziere, E. Experimental Investigation on the Effects of Mineral Water Composition on the Leaching of Cement-Based Materials. Materials 2024, 17, 1548. [Google Scholar] [CrossRef]
- Ma, Q.; Yang, W.; Duan, Z.; Liu, H.; Hua, M.; Deng, Q. Influence of Alkali-Activators on Acid Rain Resistance of Geopolymer-Recycled Pervious Concrete with Optimal Pore Size. Materials 2022, 15, 8368. [Google Scholar] [CrossRef]
- Yang, W.; Zhu, P.; Liu, H.; Wang, X.; Ge, W.; Hua, M. Resistance to Sulfuric Acid Corrosion of Geopolymer Concrete Based on Different Binding Materials and Alkali Concentrations. Materials 2021, 14, 7109. [Google Scholar] [CrossRef]
- Santhanam, M.; Cohen, M.D.; Olekm, J. Sulfate attack research—whither now? Cem. Concr. Res. 2001, 31, 845–851. [Google Scholar] [CrossRef]
- Matalkah, F.; Salem, T.; Soroushian, P. Acid resistance and corrosion protection potential of concrete prepared with alkali aluminosilicate cement. J. Build. Eng. 2018, 20, 705–711. [Google Scholar] [CrossRef]
- Liu, J.; Peng, C.; Jiang, J.; Zhang, X.; He, D.; Zhou, K.; Chen, W. Recovery of iron and aluminum from iron-rich bauxite residue by an integrated phase reconstruction approach. Sci. Total Environ. 2023, 904, 166702. [Google Scholar] [CrossRef] [PubMed]
- Yoosuk, P.; Suksiripattanapong, C.; Hiroki, G.; Phoo-ngernkham, T.; Thumrongvut, J.; Sukontasukkul, P.; Chindaprasirt, P. Performance of polypropylene fiber-reinforced cellular lightweight fly ash geopolymer mortar under wet and dry cycles. Case Stud. Constr. Mater. 2024, 20, e03233. [Google Scholar] [CrossRef]
- Ninan, C.M.; Ramaswamy, K.; Sajeeb, R. Influence of acid replenishment and concentration on the degradation kinetics of cement mortar exposed to acetic acid. Mater. Today Proc. 2022, 65, 908–914. [Google Scholar] [CrossRef]










| Oxide (%) | Bauxite Residue | GGBFS |
|---|---|---|
| SiO2 | 11.1 | 33.8 |
| Al2O3 | 17.2 | 9.9 |
| Fe2O3 | 44.4 | 1.1 |
| MgO | 0.05 | 5.9 |
| CaO | 2.61 | 46.2 |
| Na2O | 6.44 | 0.6 |
| K2O | 0.06 | - |
| LOI | 11.9 | - |
| Sample | Portland Cement (kg/m3) | Fine Aggregate (kg/m3) | Coarse Aggregate (kg/m3) | Water (kg/m3) | Sika Air 60 (kg/m3) | Sika Viscocrete (kg/m3) |
|---|---|---|---|---|---|---|
| AEC | 420 | 950 | 804.3 | 157 | 0.15 | 3.12 |
| Sample | Bauxite Residue (kg/m3) | GGBFS (kg/m3) | Fine Aggregate (kg/m3) | Fine Magnetite (kg/m3) | NaOH (kg/m3) | Na2SiO3 (kg/m3) | Graphite (kg/m3) |
|---|---|---|---|---|---|---|---|
| FSA0 | 384 | 576 | 1920 | 0 | 112 | 224 | 9.6 |
| FSA25 | 384 | 576 | 1440 | 480 | 112 | 224 | 9.6 |
| FSA50 | 384 | 576 | 960 | 960 | 112 | 224 | 9.6 |
| FSA75 | 384 | 576 | 480 | 1440 | 112 | 224 | 9.6 |
| FSA100 | 384 | 576 | 0 | 1920 | 112 | 224 | 9.6 |
| Sample | Tmin (°C) | Tmax (°C) | ΔT (°C) |
|---|---|---|---|
| AEC | 23.5 | 28.1 | 4.6 |
| FSA0 | 23.5 | 29.6 | 6.1 |
| FSA25 | 23.5 | 30.2 | 6.7 |
| FSA50 | 23.7 | 31 | 7.3 |
| FSA75 | 23.7 | 31.3 | 7.6 |
| FSA100 | 24.1 | 33.9 | 9.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harmaji, A.; Jafari, R.; Simard, G. Development of Multifunctional Slag and Bauxite Residue-Based Geopolymers with Heavyweight Aggregate Enhancement. Materials 2025, 18, 4087. https://doi.org/10.3390/ma18174087
Harmaji A, Jafari R, Simard G. Development of Multifunctional Slag and Bauxite Residue-Based Geopolymers with Heavyweight Aggregate Enhancement. Materials. 2025; 18(17):4087. https://doi.org/10.3390/ma18174087
Chicago/Turabian StyleHarmaji, Andrie, Reza Jafari, and Guy Simard. 2025. "Development of Multifunctional Slag and Bauxite Residue-Based Geopolymers with Heavyweight Aggregate Enhancement" Materials 18, no. 17: 4087. https://doi.org/10.3390/ma18174087
APA StyleHarmaji, A., Jafari, R., & Simard, G. (2025). Development of Multifunctional Slag and Bauxite Residue-Based Geopolymers with Heavyweight Aggregate Enhancement. Materials, 18(17), 4087. https://doi.org/10.3390/ma18174087










