Modified Cu-Sn Catalysts Enhance CO2RR Towards Syngas Generation
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
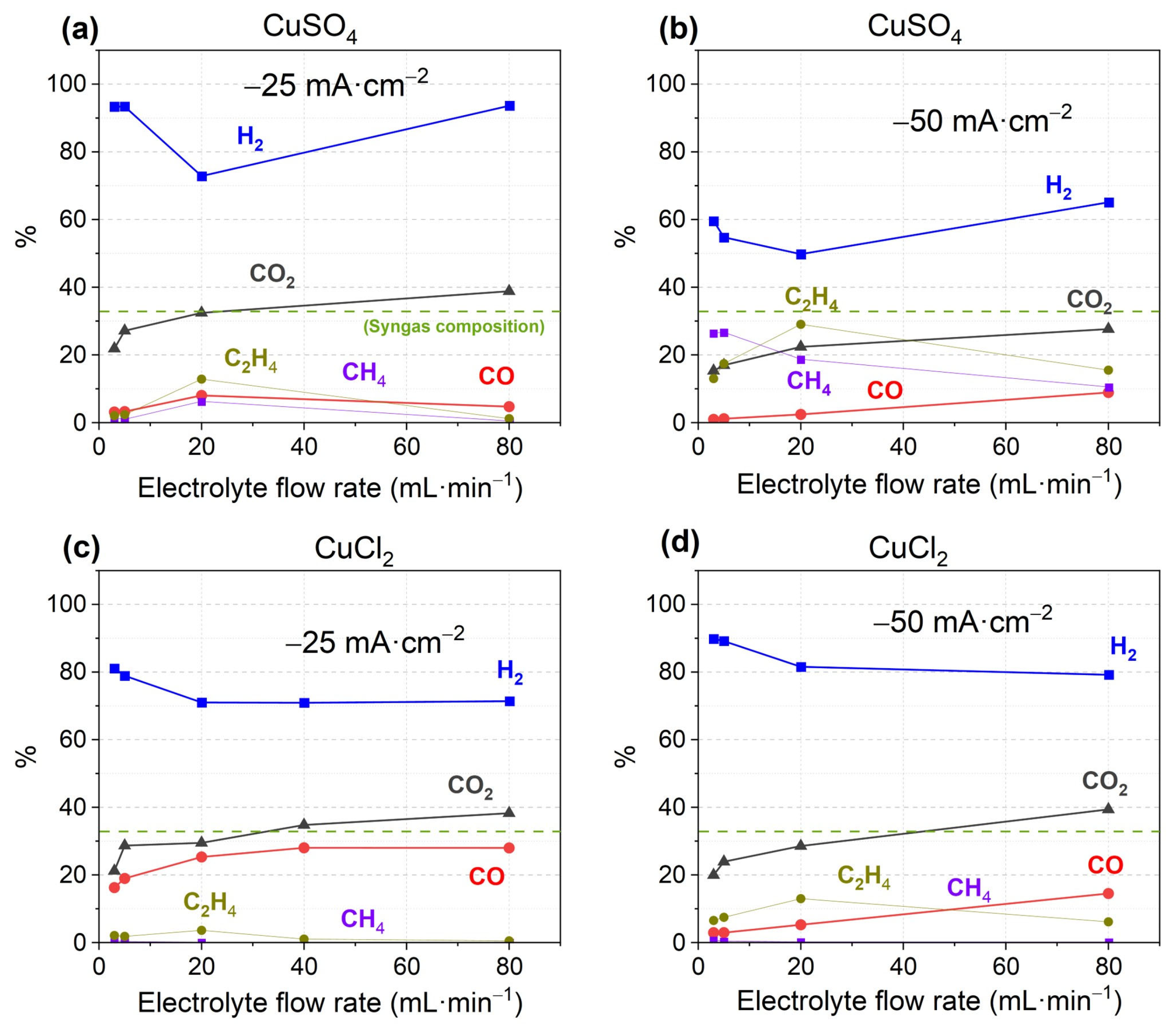
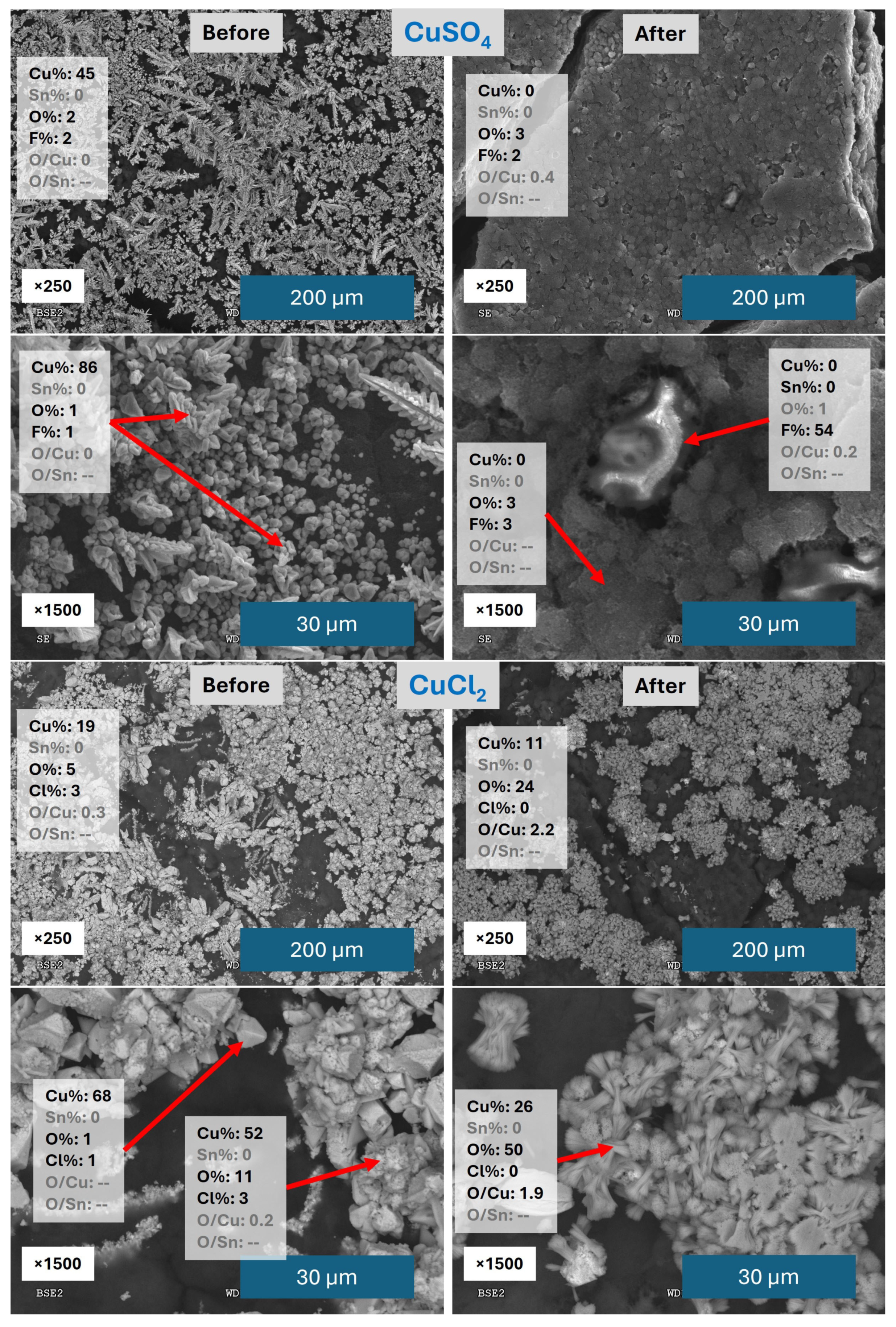
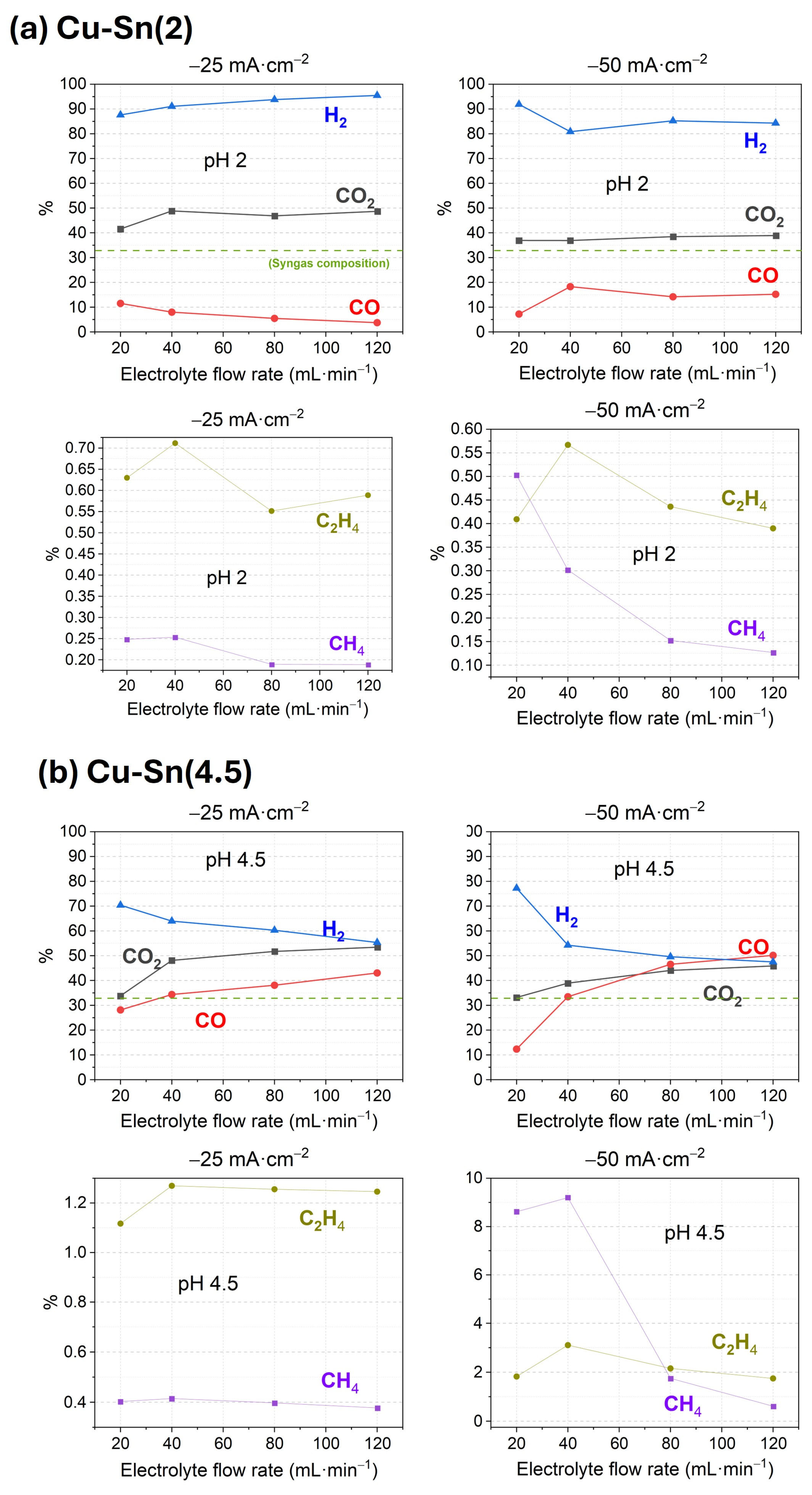
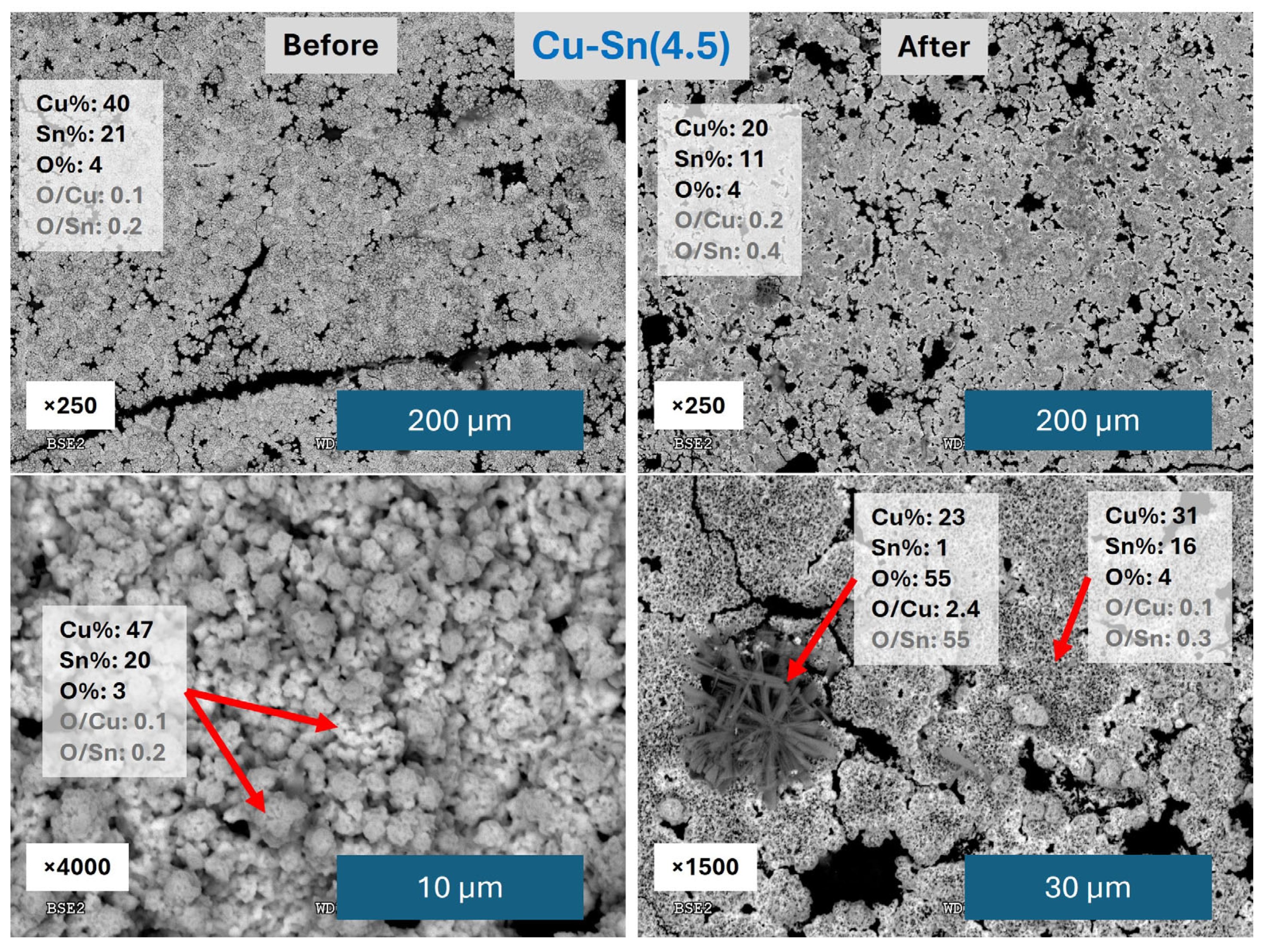
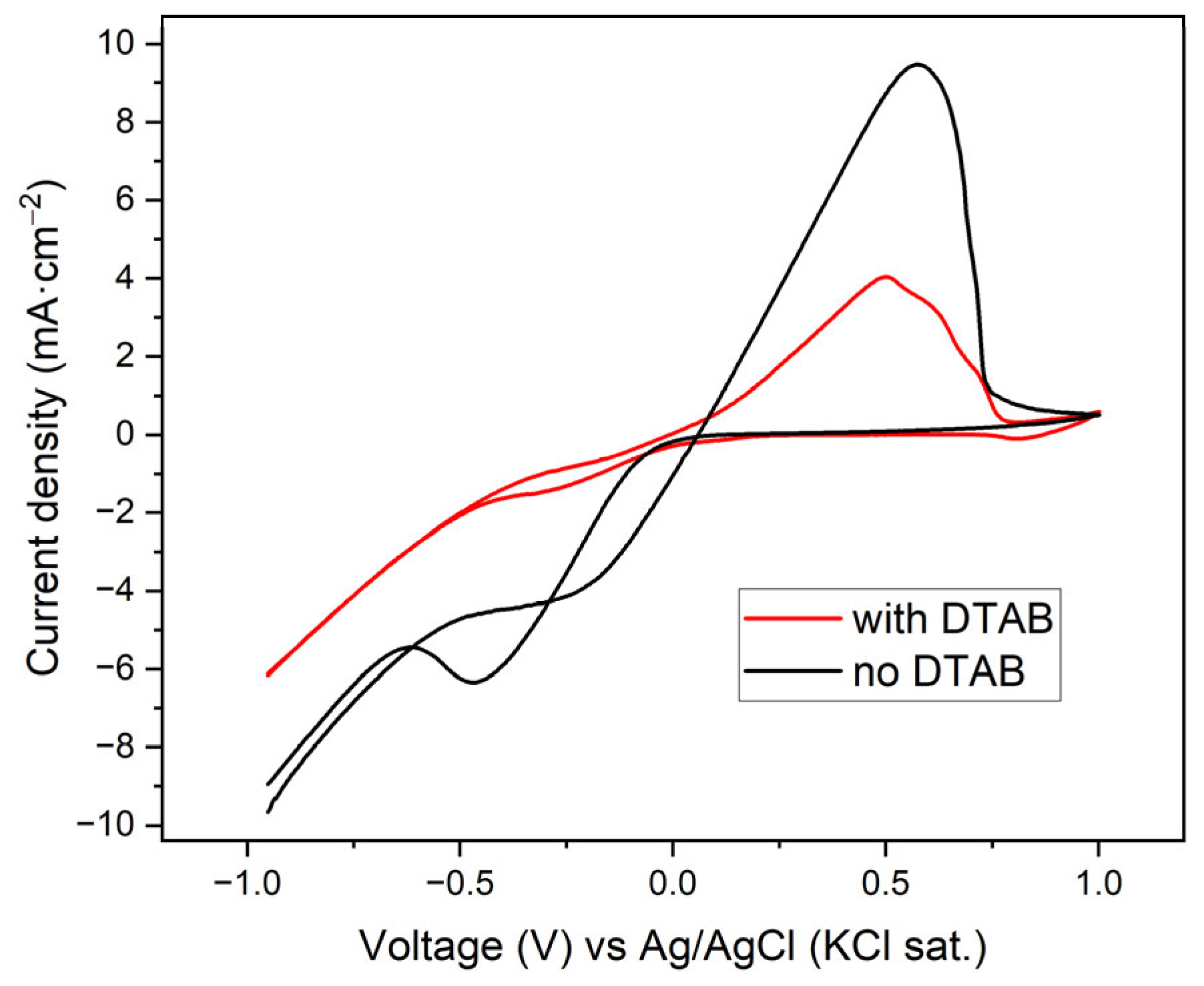
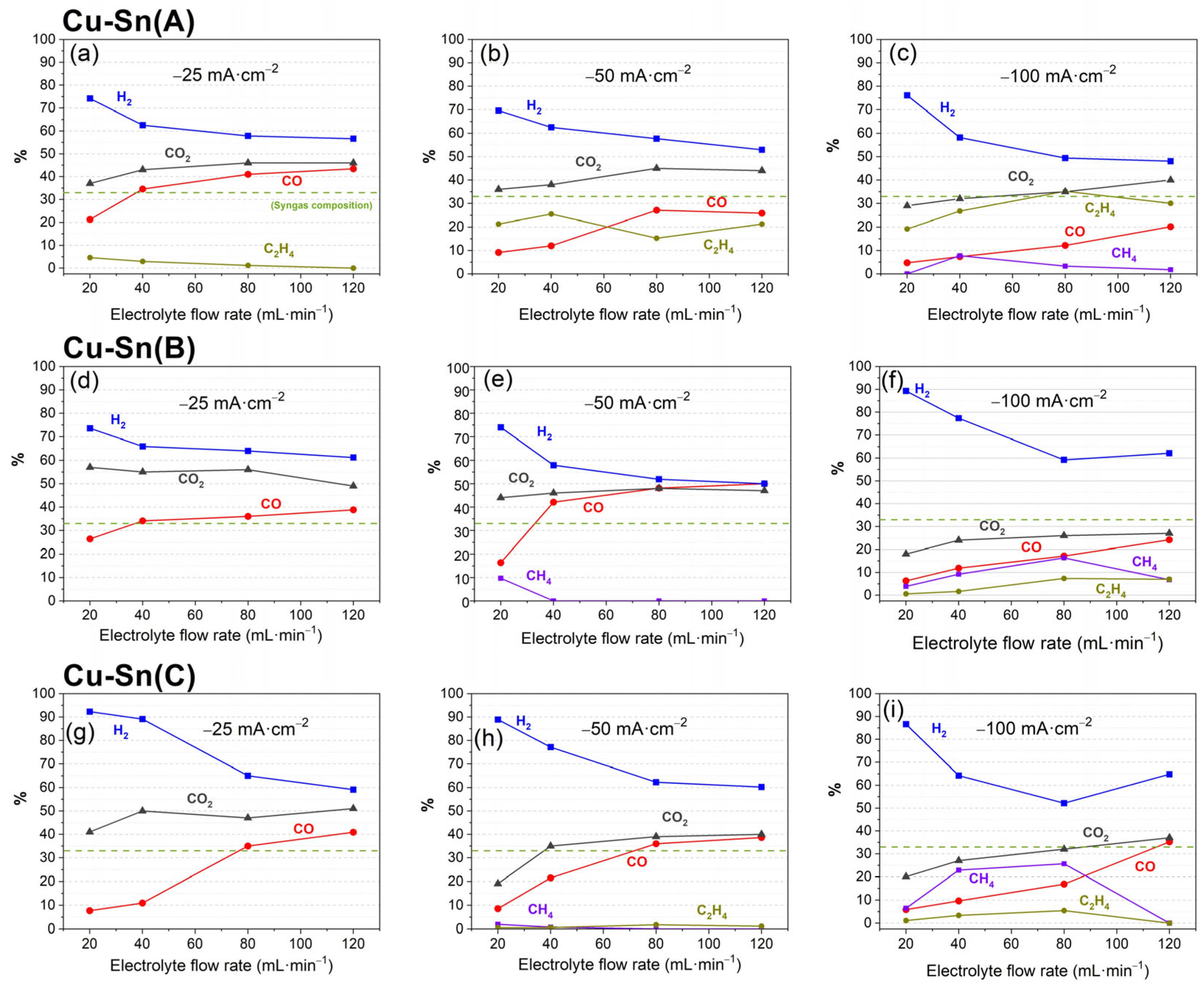
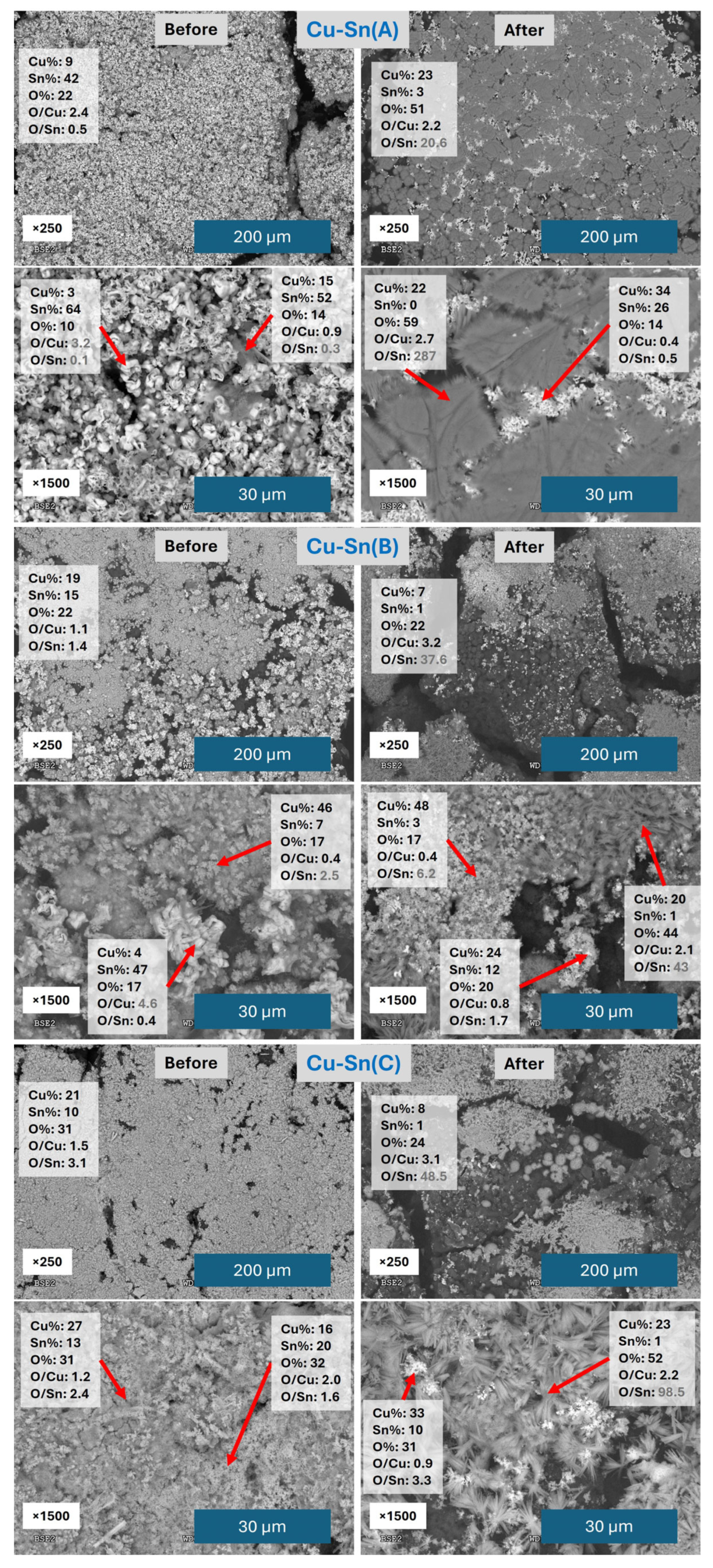
3.1. Performance of Catalysts Synthesized Under Constant Current Electrodeposition
3.2. Performance of Catalysts Synthesized Under Constant Voltage Electrodeposition
| Cathode Catalyst | Catholyte | FECO (%) | FECH4 (%) | FEC2H4 (%) | CO2 Outlet (%) | Current (mA·cm−2) | Cell Voltage (V) | Reference |
|---|---|---|---|---|---|---|---|---|
| Cu-Sn(B) | CO2 (g) sat. in 0.5 M KHCO3 and 0.01 M EDTA | 50 | 0 | 0 | 47 | −50 | −2.2 | This work |
| Cu-Sn(A) | CO2 (g) sat. in 0.5 M KHCO3 and 0.01 M EDTA | 12 | 3 | 35 | 35 | −100 | −2.7 | This work |
| Cu-Sn(C) | CO2 (g) sat. in 0.5 M KHCO3 and 0.01 M EDTA | 17 | 26 | 5 | 32 | −100 | −2.7 | This work |
| Cu*-Sn electrodeposited | CO2 (g) sat. in 0.5 M KHCO3 and 0.01 M EDTA | 62 | 0 | 1 | 49 | −25 | −2.0 | [54] |
| Ag composite | 3 M KHCO3 | 82 | nr ** | nr | nr | −100 | −3.4 | [64] |
| Porous Ag | 3 M KHCO3 | 60 | nr | nr | nr | −100 | −3.7 | [58] |
| Ag nanoparticles | 2 M KHCO3 | 46 | nr | nr | 41 | −200 | −3.8 | [68] |
| Electrodeposited Ag | 2 M KHCO3 with 0.02 M DTAB | 85 | nr | nr | 50 | −100 | −3.5 | [67] |
| Ag foam | 3 M KHCO3 | 15 | nr | nr | ~45 | −500 | −2.2 | [69] |
| Cu foam | 3 M KHCO3 with 3 mM CTAB | ~9 | 27 | nr | nr | −400 | −7.2 | [66] |
| Cu-Sn bronce | CO2 (g) with 0.1 M KHCO3 | 85 | nr | ~ 0 | nr | −6 | −0.8 V vs. RHE | [42] |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CO2RR | CO2 Reduction Reaction |
| FE | Faradaic Efficiency |
| SEM/EDX | Scanning Electron Microscopy/Energy-Dispersive X-ray Spectroscopy |
| CCS | Carbon Capture and Storage |
| CCU | Carbon Capture and Utilization |
| FT | Fischer-Tropsch |
| MEA | Membrane Electrode Assembly |
| HER | Hydrogen Evolution Reaction |
| OER | Oxygen Evolution Reaction |
| CV | Cyclic Voltammetry |
| CP | Chronopotentiometry |
| GDL | Gas Diffusion Layer |
| EDTA | Ethylenediaminetetraacetic Acid |
| DTAB | Dodecyltrimethylammonium Bromide |
| GC | Gas Chromatography |
| FID/TCD | Flame Ionization Detector/Thermal Conductivity Detector |
| CA | Chronoamperometry |
| XPS | X-ray Photoelectron Spectroscopy |
| RHE | Reversible Hydrogen Electrode |
| MPL | Microporous Layer |
| PTFE | Polytetrafluoroethylene |
References
- Pearson, P.N.; Palmer, M.R. Atmospheric Carbon Dioxide Concentrations over the Past 60 Million Years. Nature 2000, 406, 695–699. [Google Scholar] [CrossRef]
- Zhang, Y. Carbon Dioxide Utilization: A Carbon-Neutral Energy Cycle. Nat. Rev. Chem. 2017, 1, 0057. [Google Scholar] [CrossRef]
- De Luna, P.; Hahn, C.; Higgins, D.; Jaffer, S.A.; Jaramillo, T.F.; Sargent, E.H. What Would It Take for Renewably Powered Electrosynthesis to Displace Petrochemical Processes? Science 2019, 364, eaav3506. [Google Scholar] [CrossRef]
- Jouny, M.; Luc, W.; Jiao, F. General Techno-Economic Analysis of CO2 Electrolysis Systems. Ind. Eng. Chem. Res. 2018, 57, 2165–2177. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Y.; Gaspard, P.; Kruse, N. The Oscillating Fischer-Tropsch Reaction. Science 2023, 382, 99–103. [Google Scholar] [CrossRef]
- de Klerk, A. Fischer–Tropsch Process. In Kirk-Othmer Encyclopedia of Chemical Technology; American Cancer Society: Atlanta, GA, USA, 2013; pp. 1–20. [Google Scholar]
- Lei, Y.; Wang, Z.; Bao, A.; Tang, X.; Huang, X.; Yi, H.; Zhao, S.; Sun, T.; Wang, J.; Gao, F. Recent Advances on Electrocatalytic CO2 Reduction to Resources: Target Products, Reaction Pathways and Typical Catalysts. Chem. Eng. J. 2023, 453, 139663. [Google Scholar] [CrossRef]
- Wei, P.; Li, H.; Lin, L.; Gao, D.; Zhang, X.; Gong, H.; Qing, G.; Cai, R.; Wang, G.; Bao, X. CO2 Electrolysis at Industrial Current Densities Using Anion Exchange Membrane Based Electrolyzers. Sci. China Chem. 2020, 63, 1711–1715. [Google Scholar] [CrossRef]
- Dinh, C.-T.; García De Arquer, F.P.; Sinton, D.; Sargent, E.H. High Rate, Selective, and Stable Electroreduction of CO2 to CO in Basic and Neutral Media. ACS Energy Lett. 2018, 3, 2835–2840. [Google Scholar] [CrossRef]
- Verma, S.; Lu, X.; Ma, S.; Masel, R.I.; Kenis, P.J.A. The Effect of Electrolyte Composition on the Electroreduction of CO2 to CO on Ag Based Gas Diffusion Electrodes. Phys. Chem. Chem. Phys. 2016, 18, 7075–7084. [Google Scholar] [CrossRef]
- Weekes, D.M.; Salvatore, D.A.; Reyes, A.; Huang, A.; Berlinguette, C.P. Electrolytic CO2 Reduction in a Flow Cell. Acc. Chem. Res. 2018, 51, 910–918. [Google Scholar] [CrossRef]
- Li, Y.C.; Lee, G.; Yuan, T.; Wang, Y.; Nam, D.-H.; Wang, Z.; García De Arquer, F.P.; Lum, Y.; Dinh, C.-T.; Voznyy, O.; et al. CO2 Electroreduction from Carbonate Electrolyte. ACS Energy Lett. 2019, 4, 1427–1431. [Google Scholar] [CrossRef]
- Li, T.; Lees, E.W.; Goldman, M.; Salvatore, D.A.; Weekes, D.M.; Berlinguette, C.P. Electrolytic Conversion of Bicarbonate into CO in a Flow Cell. Joule 2019, 3, 1487–1497. [Google Scholar] [CrossRef]
- Xie, K.; Miao, R.K.; Ozden, A.; Liu, S.; Chen, Z.; Dinh, C.-T.; Huang, J.E.; Xu, Q.; Gabardo, C.M.; Lee, G.; et al. Bipolar Membrane Electrolyzers Enable High Single-Pass CO2 Electroreduction to Multicarbon Products. Nat. Commun. 2022, 13, 3609. [Google Scholar] [CrossRef]
- Vermaas, D.A.; Smith, W.A. Synergistic Electrochemical CO2 Reduction and Water Oxidation with a Bipolar Membrane. ACS Energy Lett. 2016, 1, 1143–1148. [Google Scholar] [CrossRef]
- Welch, A.J.; Dunn, E.; DuChene, J.S.; Atwater, H.A. Bicarbonate or Carbonate Processes for Coupling Carbon Dioxide Capture and Electrochemical Conversion. ACS Energy Lett. 2020, 5, 940–945. [Google Scholar] [CrossRef]
- Sullivan, I.; Goryachev, A.; Digdaya, I.A.; Li, X.; Atwater, H.A.; Vermaas, D.A.; Xiang, C. Coupling Electrochemical CO2 Conversion with CO2 Capture. Nat. Catal. 2021, 4, 952–958. [Google Scholar] [CrossRef]
- Mou, K.; Chen, Z.; Yao, S.; Liu, L. Enhanced Electrochemical Reduction of Carbon Dioxide to Formate with In-Situ Grown Indium-Based Catalysts in an Aqueous Electrolyte. Electrochim. Acta 2018, 289, 65–71. [Google Scholar] [CrossRef]
- Marques Mota, F.; Nguyen, D.L.T.; Lee, J.-E.; Piao, H.; Choy, J.-H.; Hwang, Y.J.; Kim, D.H. Toward an Effective Control of the H2 to CO Ratio of Syngas through CO2 Electroreduction over Immobilized Gold Nanoparticles on Layered Titanate Nanosheets. ACS Catal. 2018, 8, 4364–4374. [Google Scholar] [CrossRef]
- Guerrette, J.P.; Percival, S.J.; Zhang, B. Voltammetric Behavior of Gold Nanotrench Electrodes. Langmuir 2011, 27, 12218–12225. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.T.; Zhang, B. Nanoelectrodes: Recent Advances and New Directions. Annu. Rev. Anal. Chem. 2012, 5, 253–272. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Xu, R. New Materials in Hydrothermal Synthesis. Acc. Chem. Res. 2001, 34, 239–247. [Google Scholar] [CrossRef]
- Chu, M.; Chen, C.; Wu, Y.; Yan, X.; Jia, S.; Feng, R.; Wu, H.; He, M.; Han, B. Enhanced CO2 Electroreduction to Ethylene via Strong Metal-Support Interaction. Green Energy Environ. 2022, 7, 792–798. [Google Scholar] [CrossRef]
- Mohanty, U.S. Electrodeposition: A Versatile and Inexpensive Tool for the Synthesis of Nanoparticles, Nanorods, Nanowires, and Nanoclusters of Metals. J. Appl. Electrochem. 2011, 41, 257–270. [Google Scholar] [CrossRef]
- Liu, J.; Li, P.; Bi, J.; Zhu, Q.; Han, B. Design and Preparation of Electrocatalysts by Electrodeposition for CO2 Reduction. Chem. A Eur. J. 2022, 28, e202200242. [Google Scholar] [CrossRef]
- Zhou, F.; Li, H.; Fournier, M.; MacFarlane, D.R. Electrocatalytic CO2 Reduction to Formate at Low Overpotentials on Electrodeposited Pd Films: Stabilized Performance by Suppression of CO Formation. ChemSusChem 2017, 10, 1509–1516. [Google Scholar] [CrossRef]
- Rabiee, H.; Zhang, X.; Ge, L.; Hu, S.; Li, M.; Smart, S.; Zhu, Z.; Yuan, Z. Tuning the Product Selectivity of the Cu Hollow Fiber Gas Diffusion Electrode for Efficient CO2 Reduction to Formate by Controlled Surface Sn Electrodeposition. ACS Appl. Mater. Interfaces 2020, 12, 21670–21681. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Guo, X.; Yang, J.; Wang, L. Electrodeposited Bi Dendrites/2D Black Phosphorus Nanosheets Composite Used for Boosting Formic Acid Production from CO2 Electroreduction. J. CO2 Util. 2020, 38, 32–38. [Google Scholar] [CrossRef]
- Rosen, J.; Hutchings, G.S.; Lu, Q.; Forest, R.V.; Moore, A.; Jiao, F. Electrodeposited Zn Dendrites with Enhanced CO Selectivity for Electrocatalytic CO2 Reduction. ACS Catal. 2015, 5, 4586–4591. [Google Scholar] [CrossRef]
- Dutta, A.; Morstein, C.E.; Rahaman, M.; Cedeño López, A.; Broekmann, P. Beyond Copper in CO2 Electrolysis: Effective Hydrocarbon Production on Silver-Nanofoam Catalysts. ACS Catal. 2018, 8, 8357–8368. [Google Scholar] [CrossRef]
- Saberi Safaei, T.; Mepham, A.; Zheng, X.; Pang, Y.; Dinh, C.-T.; Liu, M.; Sinton, D.; Kelley, S.O.; Sargent, E.H. High-Density Nanosharp Microstructures Enable Efficient CO2 Electroreduction. Nano Lett. 2016, 16, 7224–7228. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Pan, X.; Wang, J.; Bao, T.; Liu, X.; Tang, Z.; Xiu, H.; Li, J. Size Effect of Graphene Oxide on Graphene-Aerogel-Supported Au Catalysts for Electrochemical CO2 Reduction. Materials 2023, 16, 7042. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.; Morita, T.; Shimano, K.; Nagami, Y.; Yamasaki, S. Selective Ethylene Formation by Pulse-Mode Electrochemical Reduction of Carbon Dioxide Using Copper and Copper-Oxide Electrodes. J. Solid State Electrochem. 2007, 11, 554–557. [Google Scholar] [CrossRef]
- Yano, H.; Tanaka, T.; Nakayama, M.; Ogura, K. Selective Electrochemical Reduction of CO2 to Ethylene at a Three-Phase Interface on Copper(I) Halide-Confined Cu-Mesh Electrodes in Acidic Solutions of Potassium Halides. J. Electroanal. Chem. 2004, 565, 287–293. [Google Scholar] [CrossRef]
- Ajmal, S.; Yang, Y.; Tahir, M.A.; Li, K.; Bacha, A.-U.-R.; Nabi, I.; Liu, Y.; Wang, T.; Zhang, L. Boosting C2 Products in Electrochemical CO2 Reduction over Highly Dense Copper Nanoplates. Catal. Sci. Technol. 2020, 10, 4562–4570. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Dinh, C.-T.; Li, J.; Ozden, A.; Golam Kibria, M.; Seifitokaldani, A.; Tan, C.-S.; Gabardo, C.M.; Luo, M.; et al. Catalyst Synthesis under CO2 Electroreduction Favours Faceting and Promotes Renewable Fuels Electrosynthesis. Nat. Catal. 2019, 3, 98–106. [Google Scholar] [CrossRef]
- Wang, C.; Cao, M.; Jiang, X.; Wang, M.; Shen, Y. A Catalyst Based on Copper-Cadmium Bimetal for Electrochemical Reduction of CO2 to CO with High Faradaic Efficiency. Electrochim. Acta 2018, 271, 544–550. [Google Scholar] [CrossRef]
- Li, H.; Yue, X.; Qiu, Y.; Xiao, Z.; Yu, X.; Xue, C.; Xiang, J. Selective Electroreduction of CO2 to Formate over the Co-Electrodeposited Cu/Sn Bimetallic Catalyst. Mater. Today Energy 2021, 21, 100797. [Google Scholar] [CrossRef]
- Zeng, J.; Bejtka, K.; Ju, W.; Castellino, M.; Chiodoni, A.; Sacco, A.; Farkhondehfal, M.A.; Hernández, S.; Rentsch, D.; Battaglia, C.; et al. Advanced Cu-Sn Foam for Selectively Converting CO2 to CO in Aqueous Solution. Appl. Catal. B Environ. 2018, 236, 475–482. [Google Scholar] [CrossRef]
- Wang, J.; Ji, Y.; Shao, Q.; Yin, R.; Guo, J.; Li, Y.; Huang, X. Phase and Structure Modulating of Bimetallic CuSn Nanowires Boosts Electrocatalytic Conversion of CO2. Nano Energy 2019, 59, 138–145. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, X.; Liu, Z.; Wang, Q.; Xiao, X.; Pan, H.; Li, M.; Wang, J.; Shao, Y.; Peng, Z.; et al. A Highly Selective Tin-Copper Bimetallic Electrocatalyst for the Electrochemical Reduction of Aqueous CO2 to Formate. Appl. Catal. B Environ. 2019, 259, 118040. [Google Scholar] [CrossRef]
- Stojkovikj, S.; El-Nagar, G.A.; Firschke, F.; Pardo Pérez, L.C.; Choubrac, L.; Najdoski, M.; Mayer, M.T. Electrocatalyst Derived from Waste Cu–Sn Bronze for CO2 Conversion into CO. ACS Appl. Mater. Interfaces 2021, 13, 38161–38169. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y. Electrochemical CO2 Reduction on Metal Electrodes. In Modern Aspects of Electrochemistry; Vayenas, C.G., White, R.E., Gamboa-Aldeco, M.E., Eds.; Modern Aspects of Electrochemistry; Springer: New York, NY, USA, 2008; Volume 42, pp. 89–189. ISBN 978-0-387-49488-3. [Google Scholar]
- Hori, Y.; Wakebe, H.; Tsukamoto, T.; Koga, O. Electrocatalytic Process of CO Selectivity in Electrochemical Reduction of CO2 at Metal Electrodes in Aqueous Media. Electrochim. Acta 1994, 39, 1833–1839. [Google Scholar] [CrossRef]
- Mistry, H.; Varela, A.S.; Bonifacio, C.S.; Zegkinoglou, I.; Sinev, I.; Choi, Y.-W.; Kisslinger, K.; Stach, E.A.; Yang, J.C.; Strasser, P.; et al. Highly Selective Plasma-Activated Copper Catalysts for Carbon Dioxide Reduction to Ethylene. Nat. Commun. 2016, 7, 12123. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xue, S.; Barber, J.; Zhou, Y.; Meng, J.; Ke, X. An Overview of Cu-Based Heterogeneous Electrocatalysts for CO2 Reduction. J. Mater. Chem. A 2020, 8, 4700–4734. [Google Scholar] [CrossRef]
- Nitopi, S.; Bertheussen, E.; Scott, S.B.; Liu, X.; Engstfeld, A.K.; Horch, S.; Seger, B.; Stephens, I.E.L.; Chan, K.; Hahn, C.; et al. Progress and Perspectives of Electrochemical CO2 Reduction on Copper in Aqueous Electrolyte. Chem. Rev. 2019, 119, 7610–7672. [Google Scholar] [CrossRef]
- García De Arquer, F.P.; Dinh, C.-T.; Ozden, A.; Wicks, J.; McCallum, C.; Kirmani, A.R.; Nam, D.-H.; Gabardo, C.; Seifitokaldani, A.; Wang, X.; et al. CO2 Electrolysis to Multicarbon Products at Activities Greater than 1 A cm−2. Science 2020, 367, 661–666. [Google Scholar] [CrossRef]
- Azuma, M.; Hashimoto, K.; Hiramoto, M.; Watanabe, M.; Sakata, T. Electrochemical Reduction of Carbon Dioxide on Various Metal Electrodes in Low-Temperature Aqueous KHCO3 Media. J. Electrochem. Soc. 1990, 137, 1772–1778. [Google Scholar] [CrossRef]
- Masel, R.I.; Liu, Z.; Yang, H.; Kaczur, J.J.; Carrillo, D.; Ren, S.; Salvatore, D.; Berlinguette, C.P. An Industrial Perspective on Catalysts for Low-Temperature CO2 Electrolysis. Nat. Nanotechnol. 2021, 16, 118–128. [Google Scholar] [CrossRef]
- Ding, M.; Chen, Z.; Liu, C.; Wang, Y.; Li, C.; Li, X.; Zheng, T.; Jiang, Q.; Xia, C. Electrochemical CO2 Reduction: Progress and Opportunity with Alloying Copper. Mater. Rep. Energy 2023, 3, 100175. [Google Scholar] [CrossRef]
- Shang, L.; Lv, X.; Zhong, L.; Li, S.; Zheng, G. Efficient CO2 Electroreduction to Ethanol by Cu3 Sn Catalyst. Small Methods 2022, 6, 2101334. [Google Scholar] [CrossRef]
- Wuttig, A.; Surendranath, Y. Impurity Ion Complexation Enhances Carbon Dioxide Reduction Catalysis. ACS Catal. 2015, 5, 4479–4484. [Google Scholar] [CrossRef]
- Herranz, D.; Bernedo Biriucov, S.; Arranz, A.; Avilés Moreno, J.R.; Ocón, P. Syngas Production Improvement from CO2RR Using Cu-Sn Electrodeposited Catalysts. Materials 2024, 18, 105. [Google Scholar] [CrossRef]
- Ye, W.; Guo, X.; Ma, T. A Review on Electrochemical Synthesized Copper-Based Catalysts for Electrochemical Reduction of CO2 to C2+ Products. Chem. Eng. J. 2021, 414, 128825. [Google Scholar] [CrossRef]
- Bae, S.; Kim, Y.; Kim, S.; Chung, C.M.; Cho, K. Enhanced Sulfate Ion Adsorption Selectivity in Capacitive Deionization with Ball-Milled Activated Carbon. Desalination 2022, 540, 116014. [Google Scholar] [CrossRef]
- Gao, D.; Sinev, I.; Scholten, F.; Arán-Ais, R.M.; Divins, N.J.; Kvashnina, K.; Timoshenko, J.; Roldan Cuenya, B. Selective CO2 Electroreduction to Ethylene and Multicarbon Alcohols via Electrolyte-Driven Nanostructuring. Angew. Chem. 2019, 131, 17203–17209. [Google Scholar] [CrossRef]
- Zhang, Z.; Lees, E.W.; Habibzadeh, F.; Salvatore, D.A.; Ren, S.; Simpson, G.L.; Wheeler, D.G.; Liu, A.; Berlinguette, C.P. Porous Metal Electrodes Enable Efficient Electrolysis of Carbon Capture Solutions. Energy Environ. Sci. 2022, 15, 705–713. [Google Scholar] [CrossRef]
- Tabassum, H.; Yang, X.; Zou, R.; Wu, G. Surface Engineering of Cu Catalysts for Electrochemical Reduction of CO2 to Value-Added Multi-Carbon Products. Chem Catal. 2022, 2, 1561–1593. [Google Scholar] [CrossRef]
- Qiu, M.; Wang, N.; Cui, Z.; Liu, J.; Hou, L.; Liu, J.; Hu, R.; Zhang, H.; Zhao, Y. Evolution of Copper Oxide Nanoneedle Mesh with Subtle Regulated Lyophobicity for High Efficiency Liquid Separation. J. Mater. Chem. A 2018, 6, 817–822. [Google Scholar] [CrossRef]
- Cudennec, Y.; Lecerf, A.; Gerault, Y. Synthesis of Cu(OH)2 and CuO by Soft Chemistry. Eur. J. Solid State Inorg. Chem. 1995, 32, 1013–1022. [Google Scholar]
- Zanellato, G.; Schiavi, P.G.; Zanoni, R.; Rubino, A.; Altimari, P.; Pagnanelli, F. Electrodeposited Copper Nanocatalysts for CO2 Electroreduction: Effect of Electrodeposition Conditions on Catalysts’ Morphology and Selectivity. Energies 2021, 14, 5012. [Google Scholar] [CrossRef]
- Li, T.; Shao, M. A Minireview on Electrochemical CO2 Conversion Based on Carbonate/Bicarbonate Media. EES. Catal. 2024, 2, 564–572. [Google Scholar] [CrossRef]
- Lees, E.W.; Goldman, M.; Fink, A.G.; Dvorak, D.J.; Salvatore, D.A.; Zhang, Z.; Loo, N.W.X.; Berlinguette, C.P. Electrodes Designed for Converting Bicarbonate into CO. ACS Energy Lett. 2020, 5, 2165–2173. [Google Scholar] [CrossRef]
- Gutiérrez-Sánchez, O.; De Mot, B.; Bulut, M.; Pant, D.; Breugelmans, T. Engineering Aspects for the Design of a Bicarbonate Zero-Gap Flow Electrolyzer for the Conversion of CO2 to Formate. ACS Appl. Mater. Interfaces 2022, 14, 30760–30771. [Google Scholar] [CrossRef] [PubMed]
- Lees, E.W.; Liu, A.; Bui, J.C.; Ren, S.; Weber, A.Z.; Berlinguette, C.P. Electrolytic Methane Production from Reactive Carbon Solutions. ACS Energy Lett. 2022, 7, 1712–1718. [Google Scholar] [CrossRef]
- Larrea, C.; Avilés-Moreno, J.R.; Ocón, P. Strategies to Enhance CO2 Electrochemical Reduction from Reactive Carbon Solutions. Molecules 2023, 28, 1951. [Google Scholar] [CrossRef]
- Larrea, C.; Torres, D.; Avilés-Moreno, J.R.; Ocón, P. Multi-Parameter Study of CO2 Electrochemical Reduction from Concentrated Bicarbonate Feed. J. CO2 Util. 2022, 57, 101878. [Google Scholar] [CrossRef]
- Zhang, Z.; Lees, E.W.; Ren, S.; Mowbray, B.A.W.; Huang, A.; Berlinguette, C.P. Conversion of Reactive Carbon Solutions into CO at Low Voltage and High Carbon Efficiency. ACS Cent. Sci. 2022, 8, 749–755. [Google Scholar] [CrossRef]
| Catalyst | Solutions | Use of DTAB | N° of CAs 1200 s | Potential vs. Ag/AgCl (V) | Picture of Electrodeposited Carbon Cloth | Zoom with Optical Microscope (50×) |
|---|---|---|---|---|---|---|
| Cu-Sn(A) | CuSO4 50 mM SnCl2 50 mM | Only in CuSO4 | CuSO4 (2 CAs) SnCl2 (1 CA) | CuSO4 (−0.900 V) SnCl2 (−0.700 V) |  |  |
| Cu-Sn(B) | CuSO4 50 mM SnCl2 50 mM | Both | CuSO4 (2 CAs) SnCl2 (1 CA) | CuSO4 (−0.500 V) SnCl2 (−0.600 V) |  |  |
| Cu-Sn(C) | CuSO4 50 mM SnCl2 50 mM | Both | CuSO4 (2 CAs) SnCl2 (1 CA) | CuSO4 (−0.600 V) SnCl2 (−0.650 V) |  | 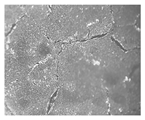 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herranz, D.; Maroto, A.; Rodriguez, M.; Avilés Moreno, J.R.; Ocón, P. Modified Cu-Sn Catalysts Enhance CO2RR Towards Syngas Generation. Materials 2025, 18, 4070. https://doi.org/10.3390/ma18174070
Herranz D, Maroto A, Rodriguez M, Avilés Moreno JR, Ocón P. Modified Cu-Sn Catalysts Enhance CO2RR Towards Syngas Generation. Materials. 2025; 18(17):4070. https://doi.org/10.3390/ma18174070
Chicago/Turabian StyleHerranz, Daniel, Antonio Maroto, Martina Rodriguez, Juan Ramón Avilés Moreno, and Pilar Ocón. 2025. "Modified Cu-Sn Catalysts Enhance CO2RR Towards Syngas Generation" Materials 18, no. 17: 4070. https://doi.org/10.3390/ma18174070
APA StyleHerranz, D., Maroto, A., Rodriguez, M., Avilés Moreno, J. R., & Ocón, P. (2025). Modified Cu-Sn Catalysts Enhance CO2RR Towards Syngas Generation. Materials, 18(17), 4070. https://doi.org/10.3390/ma18174070







