Highlights
- Innovative approach: Researchers are expanding eco-friendly chemistry with the development of such new methods as visible-light photography for mild organic reactions.
- Efficient synthesis of quinazoline derivatives: A green synthesis one-pot, three-component reaction method of quinazoline derivatives is described with curcumin dye-sensitized TiO2 nanoparticles and visible light.
- Optimal conditions achieved: This process allows the production of up to 97% of the product in 40 minutes of light and catalyst optimal conditions, presenting high efficiency.
- Sustainable and reusable: The curcumin dye-sensitized photocatalyst was capable of performing for multiple cycles and is potentially reusable.
- Green chemistry milestone: This study is advancing the sustainable and environmentally friendly manufacturing sector due to natural dye sensitization and visible-light photography.
Abstract
This study explores a sustainable method for synthesizing quinazoline derivatives through visible light-driven photocatalysis using curcumin-sensitized titanium dioxide (TiO2) nanoparticles. A one-pot, three-component reaction involving aldehydes, urea/thiourea, and dimedone was utilized to efficiently produce quinazoline compounds. The photocatalytic performance of curcumin-sensitized TiO2 (Cur-TiO2) was compared to pure TiO2 (P-TiO2), with Cur-TiO2 showing significantly enhanced activity. Under optimized conditions—light intensity of 100 mW/cm2, catalyst concentration of 1 mg/mL, and a reaction time of 40 min—a 97% product yield was achieved. The Cur-TiO2 catalyst demonstrated excellent reusability, maintaining high efficiency over four consecutive cycles with minimal performance loss. This work underscores the potential of natural dye sensitization to extend light absorption of TiO2 into the visible spectrum, providing an eco-friendly and cost-effective approach to sustainable organic synthesis.
1. Introduction
Quinazoline derivatives, characterized by their unique structure combining a quinazolinone core and an octahydrocyclopenta pyrrole ring system, are highly regarded for their diverse pharmacological and biological activities [1,2,3]. These compounds play pivotal roles in drug discovery, medicinal chemistry, materials science, and the development of synthetic methodologies [4,5,6,7,8]. Traditionally, their synthesis involves multi-step processes, including condensation reactions between amino acids or their derivatives and aldehydes or ketones, followed by cyclization, oxidation, or reduction to form the quinazoline scaffold [9,10,11]. While effective, these conventional methods often require harsh conditions and generate considerable waste. Recent advancements in sustainable chemistry have introduced greener and more efficient synthetic routes for quinazoline derivatives.
Visible light has emerged as a renewable energy resource for catalyzing organic synthesis, providing a sustainable alternative to traditional methods. Visible-light photocatalysis utilizes light-responsive photocatalysts, such as titanium dioxide (TiO2) nanoparticles, to drive chemical transformations under mild conditions, reducing the need for toxic reagents and minimizing environmental impact [12,13]. TiO2, known for its cost-effectiveness, non-toxicity, and exceptional photochemical properties, has become a prominent photocatalyst [14,15]. However, its utility is limited by its UV-specific absorption and rapid electron-hole recombination. To overcome these challenges, researchers have explored dye sensitization to enhance the functionality of TiO2 for use in the visible light spectrum [16,17,18].
The concept of dye-sensitized semiconductors gained prominence with the development of dye-sensitized solar cells (DSSCs) by Grätzel and O’Regan in 1991, demonstrating how dye molecules can broaden the absorption range of semiconductors like TiO2 [16]. This breakthrough has spurred innovations in dye design and semiconductor modifications, enabling applications in photocatalysis, pollutant degradation, and organic synthesis [17,19]. Dye molecules, when anchored onto TiO2, absorb visible light, become excited, and inject high-energy electrons into the semiconductor’s conduction band. These electrons facilitate redox reactions, including pollutant degradation, water splitting, and organic transformations, while the dye regenerates via interaction with a hole scavenger or redox mediator [20,21].
Natural dyes, such as curcumin, anthocyanins, and chlorophyll, have emerged as eco-friendly alternatives to synthetic dyes [22]. Curcumin, with its broad absorption spectrum of 420–525 nm, is a particularly promising sensitizer due to its alignment with green chemistry principles [23,24,25]. Studies by Goulart et al. [26] and Chawraba et al. [27] have demonstrated the ability of natural dyes to enhance TiO2 photocatalysis, providing sustainable solutions for environmental remediation and synthetic applications. Combining renewable dyes with the photocatalytic properties of TiO2 offers a viable pathway for eco-friendly chemical production under visible light [28].
This study focuses on synthesizing quinazoline derivatives using a simple, green, one-pot, three-component reaction involving dimedone, urea/thiourea, and aldehydes under visible light. The reaction employs curcumin dye-sensitized TiO2 nanoparticles, chosen for their efficient light absorption and energy transfer capabilities. The curcumin dye is prepared through a straightforward extraction process and used to sensitize TiO2 nanoparticles. The resulting Cur dye-TiO2 photocatalyst is characterized to establish the relationship between dye loading and photocatalytic performance.
The photocatalytic performance of Cur dye-TiO2 was evaluated by comparing it with pristine TiO2 nanoparticles under various reaction conditions. The study proposes a credible pathway for the photocatalytic process based on the identification of intermediate and reaction products. Additionally, the reusability of Cur dye-TiO2 was assessed over multiple cycles, demonstrating minimal performance loss and confirming its potential as a recyclable and sustainable photocatalyst for quinazoline synthesis. This research advances a greener approach to sustainable chemistry through visible-light photocatalysis, aligning with global efforts to develop environmentally friendly methodologies.
2. Method
2.1. Materials
The P25 TiO2 nanoparticles (composition of 80% anatase and 20% rutile) were provided by Evonik. Dimedone, urea/thiourea, and multiple aldehydes procured from Sigma Aldrich were utilized in the synthesis reactions.
2.2. Fabrication of Curcumin Dye (Cur Dye)
Curcumin dye can be prepared through a simple extraction process using turmeric powder, which is readily available in most grocery stores. Dissolve the desired amount of turmeric powder in a glass container using absolute ethanol with continuous stirring by a glass rod. Cover the container and let the mixture sit overnight to allow curcumin to dissolve in the solvent. Filter the mixture using filter paper and a funnel to separate the liquid (containing cur dye) from the solid residue. This step is important to remove any insoluble impurities. Collect the filtered liquid that contains curcumin dye in a separate container. Keep in mind that curcumin dye is sensitive to light and can degrade over time. Therefore, we store the dye in a dark, airtight container to preserve its color and properties.
2.3. Fabrication of Curcumin Dye-Sensitized TiO2 (Cur Dye-TiO2)
The following techniques have been carried out with the aim to sensitize the TiO2 nanoparticles with the Cur dye in accordance with Figure 1. An initial step involved combining the filtered curcumin dye solution with the TiO2 nanoparticles. The resultant mixture was then stirred until the nanoparticles were uniformly dispersed over the mixture. Next, Treatment with ultrasound waves has been used to further improve nanoparticle dispersion and facilitate the bonding of these particles with the molecules of the Cur dye. The TiO2 nanoparticles that had been loaded with absorbed Cur dye were extracted from the solution by centrifugation after the mixture was allowed to stand for a period of 24 h.
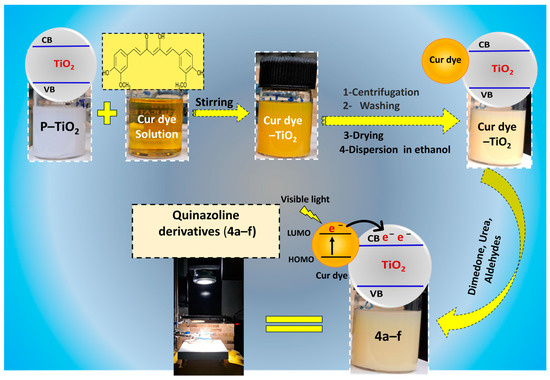
Figure 1.
A diagram illustrating the stepwise preparation of Cur dye−TiO2, used for the photocatalytic synthesis of quinazoline derivatives in the presence of visible light irradiation.
Following this, the nanoparticles underwent a series of washes with deionized water to eliminate any residual Cur dye or other impurities. Additionally, rinsing with ethanol was performed to eradicate any remaining color molecules or contaminants. Finally, the Cur dye-TiO2 sample was thoroughly washed and subsequently dried in an oven at 80 °C for 24 h. To ensure comparability between samples, P-TiO2 underwent the same preparation steps as the Cur dye-TiO2, except for curcumin dye addition.
2.4. Photocatalytic Synthesis of Quinazoline Derivatives
The photocatalytic synthesis of quinazoline (4a–f) was initiated by the utilization of visible light following the synthesis of nanoparticles made from Cur dye and TiO2. The detailed procedure is illustrated in Scheme 1. Dimedone (1) of 1 mmol, urea/thiourea (2) 1 mmol, aldehydes (3) 1 mmol, and Cur dye-TiO2 photocatalyst (10 mg dispersed in 10 mL ethanol, 1 mg/mL) were all included in the mixture. They mixed the stuff together. There was an improvement in the interaction between the reactant molecules and the TiO2 nanoparticles by the utilization of ultrasonic treatment. After that, the entire mixture was subjected to a photocatalytic reaction for a predetermined amount of time while being exposed to visible light through the utilization of a solar simulator which was equipped with a UV-cut-off filter. After the reaction, the mixture undertook filtration to remove any solid particles or debris that may have been present. The solution that was obtained was then extracted using dichloromethane. After that, the organic layer had been separated out, allowed to dry, and examined to confirm the structure of the quinazoline derivatives that were synthesized.
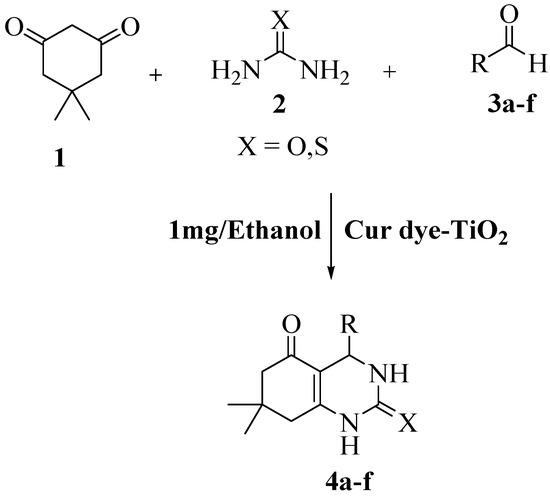
Scheme 1.
The illustration represents the input and outcome of the photocatalytic production of quinazoline derivatives using Cur dye TiO2 under visible light are shown.
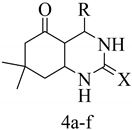
Spectroscopic Data of Synthesized Compounds
4-(4-chlorophenyl)-7,7-dimethyl-1,2,3,4,5,6,7,8-octahydroquinazoline-2,5-dione (4a)
White solid; FT-IR (cm−1, ATR); 3436 and 3330 (NH), 1578 (C=O, ring), 1466 (C=O, urea), 1369 (C=C); 1H NMR (DMSO-d6, 400 MHz): 6.96–7.19 (m, 4H, Ar-H), 5.92 (1H, s, CH), 2.51 (s, 2H, CH2), 2.33 (s, 2H, CH2), 1.03 (s, 6H, 2 × CH3); 13C NMR (DMSO-d6, 100 MHz): δ 187.65, 155.75, 135.08, 129.84, 129.71, 128.80, 128.21, 127.98, 126.53, 126.43, 123.07, 119.07, 114.75, 109.14, 47.11, 31.74, 28.25.
4-(4-fluorophenyl)-7,7-dimethyl-1,2,3,4,5,6,7,8-octahydroquinazoline-2,5-dione (4b)
White solid; FT-IR (cm−1, ATR); 3436 and 2955 (NH), 1580 (C=O, ring), 1504 (C=O, urea), 1367 (C=C); 1H NMR (DMSO-d6, 400 MHz): 6.75–7.20 (m, 4H, Ar-H), 5.91 (1H, s, CH), 2.50 (s, 2H, CH2), 2.28–2.38 (s, 2H, CH2), 1.04 (s, 6H, 2 × CH3); 13C NMR (DMSO-d6, 100 MHz): δ 187.68, 167.23, 159.41, 140.82, 137.38, 128.61, 128.53, 114.99, 110.17, 101.53, 59.36, 57.91, 47.06, 31.74, 28.24.
4-(4-hydroxy-3-methoxy)-7,7-dimethyl-1,2,3,4,5,6,7,8-octahydroquinazoline-2,5-dione (4c)
White solid; FT-IR (cm−1, ATR); 3188 and 2947 (NH), 1581 (C=O, ring), 1475 (C=O, urea), 1370 (C=C); 1H NMR (DMSO-d6, 400 MHz): 10.32 (s, 1H, OH), 6.88–6.90 (d, 1H, J = 7.88 Hz, Ar-H), 6.78–6.80 (d, 1H, J = 7.92 Hz, Ar-H), 6.53–6.55 (d, 1H, J = 7.56 Hz, Ar-H), 5.04 (s, 1H, CH), 3.30–3.79 (s, 3H, OCH3), 2.50 (s, 2H, CH2), 2.09–2.21 (s, 2H, CH2), 0.89–1.05 (s, 6H, 2 × CH3); 13C NMR (DMSO-d6, 100 MHz): δ 196.19, 164.97, 147.19, 139.69, 126.73, 124.17, 120.19, 111.08, 110.13, 56.00, 50.96, 32.06, 32.03, 29.58, 26.78.
4-(4-chlorophenyl)-7,7-dimethyl-5-oxo-1,2,3,4,5,6,7,8-octahydroquinazoline-2-thione (4d)
White solid; FT-IR (cm−1, ATR); 3431, 2949 (NH), 1651 (C=O, ring), 1467 (C=O, urea), 1358 (C=C); 1H NMR (DMSO-d6, 400 MHz): 7.17–7.30 (m, 4H, Ar-H), 4.51 (s, 1H, CH), 2.24–2.55 (s, 2H, CH2), 2.06–2.12 (s, 2H, CH2), 0.90–1.04 (s, 6H, 2×CH3); 13C NMR (DMSO-d6, 100 MHz): δ 196.51, 163.54, 143.72, 131.19, 130.37, 128.28, 127.97, 114.46, 50.49, 32.30, 31.42, 29.06, 27.00.
4-(4-fluorophenyl)-7,7-dimethyl-5-oxo-1,2,3,4,5,6,7,8-octahydroquinazoline-2-thione (4e)
White solid; FT-IR (cm−1, ATR); 3381, 2955 (NH), 1582 (C=O, ring), 1504 (C=O, urea), 1367 (C=C); 1H NMR (DMSO-d6, 400 MHz): 6.94–7.04 (m, 4H, Ar-H), 5.92 (s, 1H, CH), 2.32–2.50 (s, 2H, CH2), 2.06–2.08 (s, 2H, CH2), 0.85–1.04 (s, 6H, 2 × CH3); 13C NMR (DMSO-d6, 100 MHz): δ 187.67, 184.50,167.23, 159.39, 137.46, 128.61, 114.98, 101.53, 57.97, 50.92, 47.09, 31.74, 28.25.
4-(4-hydroxy-3-methoxy)-7,7-dimethyl-5-oxo-1,2,3,4,5,6,7,8-octahydroquinazoline-2-thione (4f)
White solid; FT-IR (cm−1, ATR); 3374 and 3163 (NH), 1589 (C=O, ring), 1465 (C=O, urea), 1374 (C=C); 1H NMR (DMSO-d6, 400 MHz): 10.26 (s, 1H, OH), 7.24–7.26 (d, 1H, J = 7.96 Hz, Ar-H), 6.90–7.01 (d, 1H, J = 44.12 Hz, Ar-H), 6.52–6.54 (d, 1H, J = 7.56 Hz, Ar-H), 5.04 (s, 1H, CH), 3.78–3.85 (s, 3H, OCH3), 2.50 (s, 2H, CH2), 2.05–2.21 (s, 2H, CH2), 0.89–1.05 (s, 6H, 2 × CH3); 13C NMR (DMSO-d6, 100 MHz): δ 192.61, 184.48, 164.96, 151.22, 147.18, 139.68, 126.75, 124.17, 118.21, 110.13, 56.01, 50.96, 32.03, 29.58, 26.78.
The detailed NMR analysis of all synthesized compounds can be found in attached Supplementary Materials (NMR data 4a–f).
2.5. Characterization
A comprehensive set of analytical techniques was employed to examine the characteristics of P-TiO2 and Cur dye-TiO2. The study used Fourier transform infrared (FT-IR) with the Thermo Science (iD5 ATR diamond) Nicolet device (Waltham, MA, USA). For structural analysis, Raman spectroscopy was performed using the SENTERRA II Compact Raman Microscope by (Bruker, Billerica, MA, USA), where the sample powders on a glass slide were probed with a 532 nm laser at 2 mW power, capturing spectra at a resolution of 1 cm−1. A UV-visible spectrophotometry test was conducted with a Jasco V-570 spectrophotometer (Jasco Corporation, Tokyo, Japan). Thermal behavior was assessed through Thermogravimetric Analysis (TGA) and Differential Thermal Analysis (DTG) using the Netzsch Proteus 70 system (Netzsch Gerätebau GmbH, Selb, Germany) with Proteus software version 7.0. The samples were heated from 25 to 1000 °C at a rate of 10 °C/min. The Brunauer-Emmett-Telle (BET) surface area, pore radius, and pore volume of the catalyst were analyzed through N2-physisorption at 77 K using Quantachrome ASiQwin software (version 5.2) (Boynton Beach, FL, USA). International X-ray diffractometer with Cu Kα radiation (λ = 1.5406 Å) was utilized for X-ray diffraction (XRD) analysis, covering a scanning range of 2θ = 10–70° (Rigaku International, Tokyo, Japan) to ascertain the crystal structure and phase purity of the samples. The morphology of the samples was visualized using Scanning Electron Microscopy (SEM) with the FEI Quanta 250 (Hillsboro, OR, USA). X-ray Photoelectron Spectroscopy (XPS) measurement was performed by a Thermo K-Alpha spectrometer (Waltham, MA, USA). It utilized 1486.6 eV working energy and a 400 μm spot size. Charge adjustment was done during analysis. Furthermore, all binding energy estimations were calibrated to the C 1s energy (284.5 eV) as an internal standard. The Bruker-Plus (400 MHz) Nuclear Magnetic Resonance (NMR) spectrometer (Bruker Corporation, Billerica, MA, USA) was utilized to conduct an analysis of the chemical structures of the quinazoline derivatives that were synthesized. The spectrometer was used to record both 1H and 13C NMR spectra, with tetramethylsilane serving as the internal standard. These diverse methodologies provided detailed insights into the materials’ attributes and behavior.
3. Results and Discussion
3.1. Characterization of Curcumin Dye-Sensitized TiO2 Photocatalysts
This research advances our knowledge of the complex processes underlying the interaction between curcumin dye and TiO2 nanoparticles, highlighting its effectiveness in photocatalytically generating quinazoline derivatives. Insights obtained from FTIR are pivotal in understanding the dynamics of reactions triggered using visible light on the surface of Cur dye-TiO2, emphasizing the crucial role of employing natural dyes to promote environmentally friendly photocatalysis in the creation of organic compounds. The FTIR examination focused on the chemical structure and interactions among P-TiO2 nanoparticles, Cur dye, and the dye-enhanced TiO2, as shown in Figure 2a. The analysis of P-TiO2 spectra identified unique peaks, with a notable broad peak at 3343.56 cm−1 bringing attention to the existence of hydroxyl (-OH) groups and a band near 1639.20 cm−1 suggesting the bending vibrations of adsorbed water molecules. Another peak, which was located at around 652 cm−1, was indicative of the stretching vibrations of the Ti-O-Ti band.
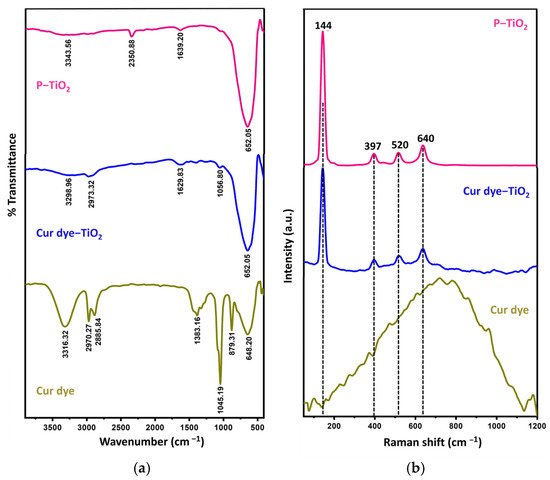
Figure 2.
(a) FTIR and (b) Raman analyses of the P-TiO2 nanoparticles, Cur dye, and Cur dye-TiO2, showcasing the intricate details of their chemical and molecular interactions.
The examination of Cur dye through FTIR showcased peaks reflecting its constituent functional groups. A significant broad peak at 3361.32 cm−1 was linked to phenolic hydroxyl group stretching vibrations, while additional peaks at 2970.27 cm−1 and 2885.84 cm−1 were associated with sp2 C-H bond stretching. The presence of a band at 1378 cm−1 signified the C-O bond elongation in phenol groups, and a distinct band at 1045.19 cm−1 identified the C-O stretching in phenyl alkyl ethers. Peaks at 648.20 and 879.31 cm−1 were linked to with the bending vibrations of aromatic C–H bonds.
The detection of OH, CH, and C-O functional groups in the Cur dye-TiO2 spectra underscored the successful bonding of Cur dye molecules with the TiO2 nanoparticles, suggesting these groups play a substantial role in enhancing the effectiveness of photocatalytic reactions on the TiO2 surface.
Raman spectroscopy contains insightful information regarding the molecular composition and interactions within Cur dye-TiO2, contributing to material characterization. Raman spectra, presented in Figure 2b, corroborate findings from FTIR spectra on P-TiO2, Cur dye, and Cur dye-TiO2. In the Raman spectrum for the P-TiO2, normal vibration modes, notably the prominent mode at 144 cm−1, indicate a well-resolved anatase phase. Additional peaks at 640, 520, and 397 cm−1 correspond to other vibration modes [29,30]. The Raman spectrum of Cur dye, spanning the 50–900 cm−1 range, reveals bending vibrations of phenyl rings (500–600 cm−1), out-of-plane bending (below 400 cm−1), and lattice modes and structural vibrations (600–700 cm−1) [31].
The Raman peaks in Cur dye-TiO2 exhibit broadness and slight wavenumber shifts after adding Cur dye, indicating effective Cur dye adsorption onto TiO2 nanoparticles. These findings confirm successful TiO2 nanoparticle sensitization with Cur dye, laying a strong groundwork for effective photocatalysis in xanthene derivative production using visible light.
XPS provides a detailed view of the chemical and electronic configuration of materials. In Figure 3, the XPS analysis for both P-TiO2 and Cur dye-TiO2 is shown, encompassing survey scans and detailed spectra for Ti 2p, O 1s, and C 1s levels. These scans in Figure 3a,b verify the elemental composition of titanium, oxygen, and carbon within both samples, matching anticipated outcomes for the Cur dye-TiO2 blend. Specifically, Figure 3b highlights an increased carbon presence in Cur dye-TiO2 compared to P-TiO2 in Figure 3a, suggesting successful dye integration on the TiO2 surface.
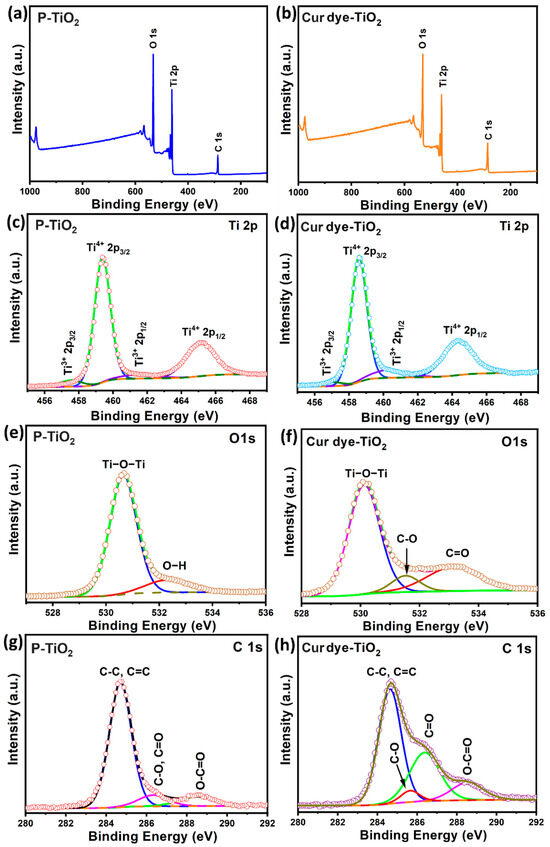
Figure 3.
(a–h) XPS spectra of the survey, Ti 2p, O 1s, and C 1s for P-TiO2, and Cur dye-TiO2 as shown.
The XPS spectrum for P-TiO2 against Ti 2p nanoparticles, displayed in Figure 3c, shows clear peaks at roughly 459.5 eV and 465.2 eV, indicative of the Ti 2p3/2 and Ti 2p1/2 orbitals, respectively. The 5.7 eV gap between these peaks is typical of Ti4+ in the TiO2 structure [32,33]. Peaks representing Ti3+ states are also observed, confirming the dual oxidation state presence in the TiO2 matrix, aligning with established literature. For Cur dye-TiO2, depicted in Figure 3d, the Ti 2p spectrum reveals pronounced Ti4+ peaks and less intense Ti3+ signals, with an overall shift towards lower binding energies when compared to the pure P-TiO2 spectrum. This shift underscores the effective dye sensitization of TiO2, laying the groundwork for improved photocatalytic performance using visible light for synthesizing quinazoline derivatives.
The O1s XPS spectrum of P-TiO2, presented in Figure 3e, exhibits a prominent peak at approximately 530.6 eV, corresponding to Ti-O bond energies, which confirms the oxide composition of the material. Additionally, a secondary peak at 532.5 eV, attributed to Ti-OH bonding, highlights the presence of hydroxyl groups, further supporting the coexistence of titanium and oxygen in distinct chemical environments [32]. Conversely, in the Cur dye-TiO2, the deconvoluted O1s spectrum (Figure 3f) reveals additional peaks at 530.2, 531.6, and 533.4 eV, indicating interactions between the TiO2 and dye molecules, showcasing Ti-O-Ti bonds and highlighting C-O and C=O bonding from the curcumin dye.
The spectrum related to C 1s of P-TiO2 (Figure 3g), suggests the existence of surface carbon, likely from environmental contaminants [33]. However, this does not detract from the overall analysis of the composition of nanoparticles. The Cur dye-TiO2 C 1s spectrum, illustrated in Figure 3h, exposes a variety of chemical environments for carbon and oxygen atoms within the dye, and additional peaks corresponding to C=O, C-O, C-C, and O-C=O bonds. The Cur dye-TiO2 XPS results show that the electronic structure has changed significantly because of the dye adsorption. This proves that the dye was able to sensitize TiO2 and that it has the potential to improve both photocatalytic and photoelectrochemical performance.
Figure 4 showcases the XRD patterns and SEM images of P-TiO2 and Cur dye-TiO2, providing key insights into their structural and morphological characteristics, further supported by FTIR, Raman, and XPS analyses. The XRD (Figure 4a) patterns reveal strong peaks corresponding to the anatase (A) and rutile (R) phases of TiO2, with anatase being the dominant phase, as evidenced by peaks such as A(101), A(200), and A(204). This mixed-phase composition, approximately 80% anatase and 20% rutile, is essential for effective charge separation, enhancing photocatalytic activity [32,34]. Importantly, the XRD pattern for Cur dye-TiO2 retains the crystalline structure of P-TiO2, with no additional peaks for curcumin, confirming its presence as an amorphous or molecularly dispersed layer on the TiO2 surface. This finding aligns with FTIR results, which identified characteristic vibrational shifts indicative of the molecular adsorption of curcumin, and XPS analysis, which revealed chemical interactions and charge transfer between curcumin and TiO2.
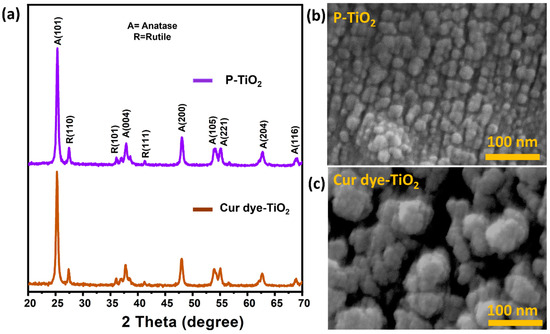
Figure 4.
(a) XRD and (b,c) SEM analysis of the P-TiO2 and Cur dye-TiO2, respectively.
The SEM images complement the XRD findings, illustrating distinct morphological differences between the samples. P-TiO2 (Figure 4b) exhibits uniform, densely packed nanoparticles with smooth surfaces, reflecting its pristine nature. In contrast, Cur dye-TiO2 (Figure 4c) displays aggregated particles with irregular and rough surfaces, a result of curcumin adsorption on the TiO2 surface, likely filling some pores. These structural and surface modifications, as highlighted by XRD, SEM, and complementary spectroscopic techniques, demonstrate the successful sensitization of TiO2 with curcumin, enabling enhanced photocatalytic activity under visible light.
Thermal stability and decomposition patterns of P-TiO2 nanoparticles and Cur dye-TiO2 were evaluated using TGA and DTG, as shown in Figure 5. The TGA profile for P-TiO2, shown in Figure 5a, demonstrates a sequential weight reduction up to about 500 °C, with initial losses between 40–200 °C attributed to the desorption of water and volatile compounds from the nanoparticle surface. A continuous weight decrease between 200–510 °C is likely due to the elimination of organic residues adsorbed during production or processing. The DTG curve highlights distinct peaks at 78 °C, 184 °C, and 350 °C, corresponding to water evaporation, volatile desorption, and organic decomposition, respectively. Overall, P-TiO2 shows minimal weight loss and retains a high residual mass of 98.17%, indicating excellent thermal stability.
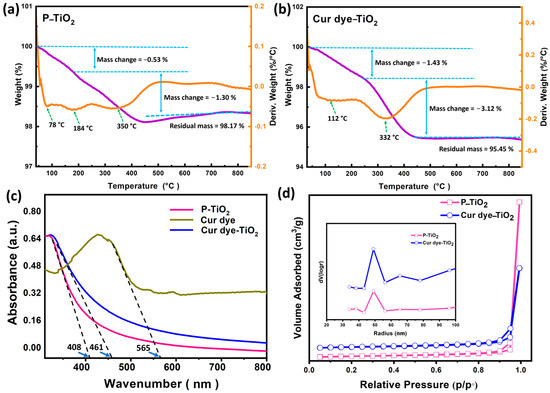
Figure 5.
(a,b) presented (TGA + DTA) curves for both P-TiO2 and Cur dye-TiO2. (c) UV-vis spectra display P-TiO2, Cur dye, and Cur dye-TiO2. (d) Nitrogen adsorption-desorption isotherms and pore size distribution curves (inset) for P-TiO2 and Cur dye-TiO2 materials, illustrating their surface area characteristics and mesoporous structure.
The TGA curve of Cur dye-TiO2, shown in Figure 5b, reveals two distinct weight loss stages. The first stage (40–220 °C) is attributed to the loss of adsorbed water and volatiles, like P-TiO2. The second stage (220–500 °C) corresponds to the decomposition of organic components from dye sensitization. The DTG profile supports these findings, showing endothermic peaks aligned with each weight loss phase, highlighting the influence of dye on the thermal properties of the composite. Specifically, Cur dye-TiO2 exhibits an initial weight loss (~1.43%) between 112–220 °C, followed by a more significant loss (~3.12%) between 220–500 °C, with a residual mass of 95.45%, indicating high thermal stability. In comparison, Pure TiO2 shows lower weight losses and a higher residual mass (98.17%), reflecting the absence of organic dye. While Cur dye-TiO2 undergoes greater weight loss due to dye incorporation, it retains excellent stability above 500 °C.
Figure 5c illustrates the UV-visible (UV-Vis) absorption spectra of P-TiO2, Cur dye, and Cur dye-TiO2, highlighting their light-absorption properties and bandgap calculations. P-TiO2 exhibits strong absorption in the UV region, consistent with its wide intrinsic bandgap of 3.04 eV and nanoscale particle effects, which indicate an enlarged surface area [20,34]. In contrast, the Cur dye spectrum shows broad absorption from 300 to 600 nm, with a distinct peak at 430 nm corresponding to a π–π* transition, confirming the presence of dye and its ability to absorb visible light effectively. For Cur dye-TiO2, the absorption edge shifts to 461 nm, significantly extending into the visible range compared to the 408 nm edge of pristine P-TiO2. This red shift indicates the successful sensitization of TiO2 by Cur dye, enhancing its capacity to harness visible light [23].
The bandgap energies (Eg) for Cur dye, P-TiO2, and Cur dye-TiO2 were calculated from their respective absorption edge wavelengths (λ edge) using the equation Eg (eV) = 1240/λedge (nm). The calculated bandgap energies are 2.19 eV, 3.04 eV, and 2.69 eV, respectively, consistent with the findings of Buddee et al. [23].These results underscore the role of Cur dye in narrowing the bandgap of TiO2, modifying its electronic structure, and extending its light absorption into the visible spectrum. Such enhancements significantly improve the photocatalytic potential of the composite, making it highly effective for visible-light-driven applications, such as organic synthesis.
The BET results, presented in Table 1, show comparable surface areas for P-TiO2 (50.41 m2/g) and Cur dye-TiO2 (48.23 m2/g), indicating that curcumin loading did not significantly affect the overall surface area. However, the reduction in pore volume for Cur dye-TiO2 suggests that curcumin molecules occupy and partially block the pores, a finding consistent with the morphological changes observed in the SEM images (Figure 4). The SEM analysis revealed aggregated and irregular particles in Cur dye-TiO2 compared to the smooth, uniform nanoparticles in P-TiO2, which aligns with the pore filling and structural modification inferred from the BET data. Furthermore, the N2 adsorption-desorption isotherms and pore size distribution curves (Figure 5d) confirm the mesoporous nature of both materials, with type IV isotherms and H3 hysteresis loops (p/p° > 0.9), supporting previous findings [35]. These results, together with SEM and BET analyses, highlight the structural and surface alterations induced by curcumin loading, which enhance the photocatalytic capabilities of the composite material.

Table 1.
The BET surface perimeters of the P-TiO2 and Cur dye-TiO2.
3.2. Photocatalytic Performance
The photocatalytic performance of Cur dye-TiO2 in synthesizing quinazoline derivatives is strongly correlated with its structural, optical, and surface property modifications as revealed through detailed characterizations. UV-Vis spectroscopy highlighted the extended absorption range of Cur dye-TiO2 into the visible spectrum, enhancing light harvesting under visible light illumination. XRD analysis confirmed the retention of the anatase phase, critical for efficient charge separation, while SEM images demonstrated morphological changes due to curcumin adsorption, which likely enhanced interactions between the catalyst and reactants. BET analysis further emphasized the impact of curcumin in altering the pore structure, optimizing the material’s surface properties for better catalytic activity. These enhancements collectively enabled the superior photocatalytic efficiency of Cur dye-TiO2, as demonstrated by its ability to achieve high yields under optimized conditions.
In this study, Cur dye-TiO2 was employed as a photocatalyst in a multicomponent reaction involving dimedone, urea/thiourea, and various substituted aldehydes to synthesize quinazoline derivatives (4a–f). The catalyst provided several advantages, including cost-effectiveness, environmental sustainability, and ease of recovery through simple filtration, thereby minimizing acidic waste and environmental impact. To test the adaptability of this approach, various aryl and heteroaryl aldehydes were successfully converted under optimized conditions, using 10 mg of Cur dye-TiO2 in 10 mL of ethanol (1 mg/mL) under visible light illumination. This method yielded high returns of quinazoline derivatives via a one-pot synthesis with straightforward product isolation.
To optimize the reaction conditions, model reactions were performed to evaluate the effects of catalyst concentration, reaction duration, and light intensity. These experiments confirmed that Cur dye-TiO2 is a highly effective photocatalyst under visible light, achieving excellent yields. The study demonstrates the potential of Cur dye-TiO2 as a sustainable and efficient catalyst for visible-light-driven organic synthesis, combining structural and optical innovations with practical advantages for green chemistry applications.
The photocatalytic performance of Cur dye-TiO2 in the synthesis of quinazoline derivatives was systematically evaluated by varying the photocatalyst concentration while maintaining constant light intensity. The results, shown in Figure 6a, indicate a clear relationship between photocatalyst concentration and reaction yield. In the absence of Cur dye-TiO2 (0 mg/mL), only 20% yield was achieved after 20 min, highlighting the limited photocatalytic activity of visible light alone. When the photocatalyst concentration increased to 1 mg/mL, the yield significantly rose to 97% after 40 min, demonstrating the critical role of Cur dye-TiO2 in enhancing the reaction efficiency. However, increasing the concentration beyond 1 mg/mL resulted in reduced yields (e.g., 95% at 2 mg/mL after 60 min, 93% at 3 mg/mL after 90 min, and 88% at 4 mg/mL after 120 min), indicating an optimal concentration of 1 mg/mL for maximum efficiency.
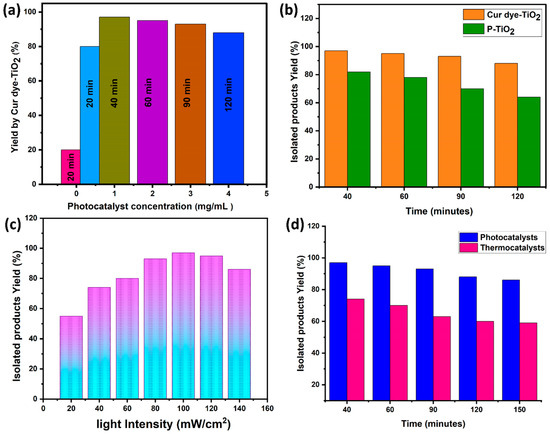
Figure 6.
Illustrates the experimental arrangement for synthesizing compounds (4a–f) using the Cur dye-TiO2 photocatalyst under visible light at optimized conditions. (a) explores how the Cur dye-TiO2 concentration affects the yield (%) of the target products, (b) monitors the reaction time with Cur dye-TiO2 and P-TiO2, (c) investigates the impact of light intensity on the yield (%) of target products, and (d) examines the influence of photocatalytic versus thermocatalytic synthesis on the yield (%).
Figure 6b compares the photocatalytic performance of Cur dye-TiO2 and unmodified P-TiO2 in synthesizing quinazoline derivatives. While both catalysts facilitated the reactions, Cur dye-TiO2 consistently outperformed P-TiO2 in terms of reaction rate and yield. After 40 min of visible light exposure, Cur dye-TiO2 achieved a 97% yield compared to 82% for P-TiO2, underscoring the significant enhancement in catalytic efficiency attributed to curcumin sensitization. This improvement is linked to the increased visible light absorption and surface property modifications induced by the dye, as established in earlier characterizations.
Figure 6c highlights the influence of light intensity on photocatalytic efficiency. A clear correlation was observed, with yields increasing from 55% at 20 mW/cm2 to 97% at 100 mW/cm2, reflecting the dependence of the reaction rate on photon absorption. However, at intensities beyond 100 mW/cm2, a slight decrease in efficiency was noted, possibly due to competing processes such as heat generation. These results demonstrate the importance of optimizing light intensity to maximize photon interactions and photocatalytic activity.
Figure 6d compares the photocatalytic and thermocatalytic approaches for quinazoline synthesis under identical temperature and light conditions. Photocatalysis consistently yielded higher yield (%) than thermocatalysis, confirming the superior efficiency of Cur dye-TiO2 under visible light. This advantage is attributed to the enhanced light absorption and charge transfer properties of Cur dye-TiO2, as evidenced in UV-Vis and XPS analyses. Thermocatalysis, reliant solely on thermal energy, demonstrated lower efficiency, emphasizing the unique benefits of photocatalytic processes.
The effect of aldehyde substituents on the photocatalytic synthesis of quinazoline derivatives was also investigated (Table 2). Substituents with electron-withdrawing or electron-donating groups influenced reaction times and yields. Aldehydes with electron-withdrawing groups generally exhibited faster reaction rates and higher yields (e.g., entries 1, 2, 4, 5), whereas those with electron-donating groups (entries 3, 6) required longer reaction times. These results underscore the adaptability of the method for structurally diverse substrates and its potential for efficient synthesis of quinazoline derivatives under visible-light-driven photocatalysis.

Table 2.
Cur dye-TiO2 photocatalyzed synthesis of quinazoline derivatives.
Additionally, the table highlights a notable trend where compounds incorporating urea (entries 1–3 in Table 2) achieved higher yields and faster reaction completions compared to those involving thiourea counterparts (entries 4–6 in Table 2). This observation suggests that the photocatalytic efficiency and the speed of quinazoline derivative formation are markedly influenced by the specific functional groups attached to the aldehydes, likely due to a combination of steric and electronic effects on the reaction dynamics.
These insights, consistent with findings from prior research involving various metal catalysts, underline the adaptability and efficacy of Cur dye-TiO2 in facilitating the synthesis of quinazoline derivatives. The study not only showcases the influence of aldehyde substituents on the photocatalytic activity but also points towards optimizing catalyst design for enhanced selectivity and efficiency in similar synthetic pathways. The results from this comprehensive evaluation provide valuable guidance for the improvement of catalyst efficacy and selectivity in the production of quinazoline derivatives and potentially other organic compounds.
3.2.1. Mechanistic Approach
We present a mechanism demonstrating how curcumin dye sensitization of TiO2 nanoparticles under visible light catalyzes the efficient synthesis of quinazoline derivatives through a one-pot, three-component condensation reaction, as depicted in Scheme 2. When curcumin dye adheres to the surface of TiO2 nanoparticles, it extends the absorption spectrum into the visible light region. Acting as a photosensitizer, curcumin absorbs visible light and transfers the energy to TiO2, exciting electrons from the valence band to the conduction band. Consequently, electron-hole pairs are generated within the TiO2 nanoparticles. The excited electrons in the conduction band exhibit high reducing potential, while the holes in the valence band possess significant oxidizing capabilities. The holes (h+) in the valence band can engage with ethanol, serving as a hole scavenger, thus participating in oxidation reactions to minimize charge carrier recombination [20,23]. Simultaneously, photoexcited electrons interact with molecular oxygen (O2) adsorbed on the TiO2 nanoparticle surface, generating superoxide radical anions (O2•−). These reactive species play a crucial role in activating the aldehyde (RCHO), facilitating its subsequent condensation reaction with urea. In this process, the carbonyl group of the aldehyde reacts with the nucleophilic amine group of urea, leading to the formation of an intermediate imine. Dimedone (a cyclic diketone, shown in red) reacts with the intermediate formed from aldehyde and urea. This step likely proceeds via nucleophilic attack, leading to a more complex intermediate structure. Dimedone is commonly involved in multi-component reactions to form fused rings. The condensation product undergoes cyclization, leading to the formation of the quinazoline core structure. This step involves the closure of a ring system, likely facilitated by dehydration (loss of water), forming the fused heterocycle characteristic of quinazoline derivatives. After cyclization, the final product is a quinazoline derivative (green nitrogenous structure). The “X” in the structure indicates variability in substituents (either oxygen or sulfur, based on the image), which can alter the electronic properties and biological activity of the final compound. This proposed mechanism illustrates a sustainable chemistry approach by harnessing visible light, a renewable energy source, to activate TiO2 nanoparticles sensitized with natural dye, curcumin. Furthermore, the one-pot synthesis reduces the number of synthetic steps and the requirement for additional reagents, thereby minimizing waste generation and environmental impact.
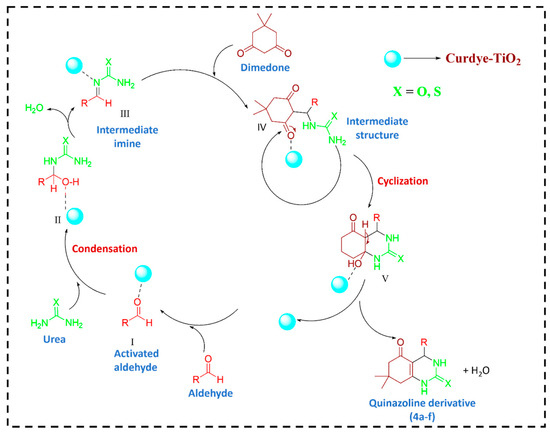
Scheme 2.
The suggested mechanism for quinazoline derivative (4a–f) synthesis utilizing Cur dye-TiO2 under visible light irradiation.
3.2.2. The Recyclability Studies of Cur Dye-TiO2 as Photocatalyst
To investigate the practicality and sustainability of Cur dye-TiO2 photocatalyst, we examined its reusability, a criterion vital for decreasing costs and environmental effect. Multiple rounds of photocatalytic synthesis of quinazolines under visible light were performed to explore Cur dye-TiO2 reusability. Four cycles of reuse were conducted while maintaining the time of irradiation consistent at 40 min and utilizing an optimized concentration of Cur dye-TiO2 (1 mg/mL).
Figure 7 presents the results for the recycling experiments involving the Cur dye-TiO2 as a photocatalyst. After each cycle, the used Cur dye-TiO2 photocatalyst was retrieved with a remarkable yield (97%) and underwent further drying before reuse. Across three successive recycling stages, the Cur dye-TiO2 photocatalyst consistently sustained its performance and yield.
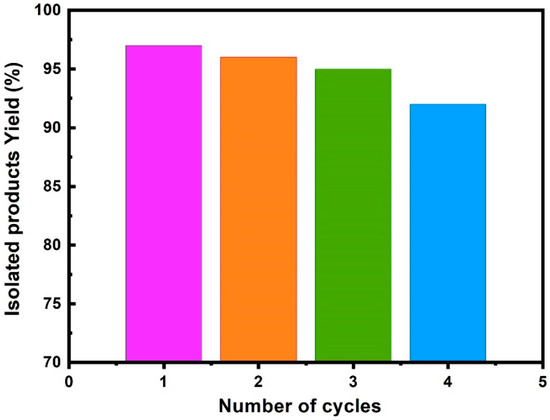
Figure 7.
Reusability tests of Cur dye-TiO2 as a photocatalyst at optimized conditions.
In the initial recycling round, the yield percentage peaked at 96%, remaining robust at 95% and 92% in the subsequent two cycles. These findings underscore the exceptional recycling of Cur dye-TiO2, highlighting its capacity to sustain photocatalytic performance over numerous iterations. This research underscores the promise of Cur dye-TiO2 as a dependable and effective environmentally friendly photocatalyst production using visible light, further enhanced by its reusability for multiple cycles.
4. Conclusions
This work achieves a notable advancement in sustainable chemistry by employing visible light with the photocatalyst dye-TiO2 for synthesizing quinazoline derivatives under mild and eco-friendly conditions. After meticulous optimization involving a time of reaction (40-m), an intensity of visible light (100 mW/cm−2), and a concentration of photocatalyst (1 mg/mL), we determined the ideal experimental parameters for the photocatalytic production process. Our comparative examination underscored the efficient photocatalysis performance of Cur dye-TiO2 over P-TiO2 nanoparticles, confirming the significance of sensitization for successful organic reactions. Additionally, our examination of Cur dye-TiO2 reusability showcased impressive stability and endurance. The photocatalyst retained consistent activity over four consecutive uses, with minimal decline noted. This capacity for reuse presents notable cost-effectiveness and environmental benefits, rendering it an appealing choice for real-world applications. Overall, this work showcases the effective fusion of a combination of natural dye sensitization and photocatalysis using visible light, connecting green chemistry with synthetic organic chemistry. It advances sustainable chemical synthesis and lays the framework for future developments.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ma17246235/s1
Author Contributions
Conceptualization, M.A.A.; methodology, M.A.A. and A.I.A.; software, A.I.A., T.F.Q. and M.A.A.; validation, M.A.A. and M.A.B.; formal analysis, M.A.A., T.F.Q. and A.I.A.; investigation, M.A.A., M.A.B. and A.I.A.; supervision, M.A.B. and M.A.A.; project administration, M.A.B. and A.I.A.; Funding acquisition, M.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research is funded by Prince Sattam bin Abdulaziz University through the project number (PSAU/2023/01/230642).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be provided upon request.
Acknowledgments
The authors extend their appreciation to Prince Sattam bin Abdulaziz University for funding this research work through the project number (PSAU/2023/01/230642).
Conflicts of Interest
The authors declare no conflict of interest.
References
- el-Sabbagh, O.I.; Shabaan, M.A.; Kadry, H.H.; Al-Din, E.S. New Octahydroquinazoline Derivatives: Synthesis and Hypotensive Activity. Eur. J. Med. Chem. 2010, 45, 5390–5396. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kaur, N.; Banerjee, P. Regioselective Brønsted Acid-Catalyzed Annulation of Cyclopropane Aldehydes with N′-Aryl Anthranil Hydrazides: Domino Construction of Tetrahydropyrrolo[1,2-a] Quinazolin-5(1H) Ones. J. Org. Chem. 2020, 85, 3393–3406. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, R.; Gheisvandi, Z.; Ghadermazi, M. Covalently Bonded Sulfonic Acid onto the Surface of Magnetic Nanosilica Obtained from Rice Husk: CoFe2O4@RH-Pr-SO3H as Novel Acid Catalyst for Synthesis of Octahydroquinazolinone and 3,4-Dihydropyrimidinone. J. Mol. Struct. 2022, 1265, 133421. [Google Scholar] [CrossRef]
- Alsibaee, A.M.; Al-Yousef, H.M.; Al-Salem, H.S. Quinazolinones, the Winning Horse in Drug Discovery. Molecules 2023, 28, 978. [Google Scholar] [CrossRef]
- Liu, K.; Li, D.; Zheng, W.; Shi, M.; Chen, Y.; Tang, M.; Yang, T.; Zhao, M.; Deng, D.; Zhang, C.; et al. Discovery, Optimization, and Evaluation of Quinazolinone Derivatives with Novel Linkers as Orally Efficacious Phosphoinositide-3-Kinase Delta Inhibitors for Treatment of Inflammatory Diseases. J. Med. Chem. 2021, 64, 8951–8970. [Google Scholar] [CrossRef]
- Bhat, M.; Belagali, S.L.; Mamatha, S.V.; Sagar, B.K.; Sekhar, E.V. Chapter 7—Importance of Quinazoline and Quinazolinone Derivatives in Medicinal Chemistry. In Studies in Natural Products Chemistry; Atta-ur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 71, pp. 185–219. [Google Scholar]
- Lei, M.; Ma, L.; Hu, L. An Efficient and Environmentally Friendly Procedure for Synthesis of Pyrimidinone Derivatives by Use of a Biginelli-Type Reaction. Monatsh. Chem. 2010, 141, 1005–1008. [Google Scholar] [CrossRef]
- Bazgiri, A.; Bananejad, B.; Pouramiri, B. Acidic Ionic Liquids Catalyzed One-Pot and Three-Component Synthesis of Octahydroquinazolin-2,5-Dione Derivatives Under Ambient Conditions. Available online: http://www.eurekaselect.com (accessed on 8 August 2017).
- Mishra, S.; Sahu, A. A Review of Magnetically Recyclable Nanocatalysts for the Synthesis of Quinazoline and Its Derivatives. Curr. Org. Chem. 2023, 27, 914–930. [Google Scholar] [CrossRef]
- Dawoud, N.T.A. An Efficient and Environmentally Friendly Procedure for Synthesis of Quinazolinone Derivatives by Use of a Biginelli-Type Reaction. Chem. Sci. Trans. 2013, 2, 129–134. [Google Scholar] [CrossRef]
- Mozafari, R.; Heidarizadeh, F. One Pot Synthesis of Octahydroquinazolinone Derivatives Using (Me (Im)12) H4CuPW11O39 as a Surfactant Type Catalyst. J. Clust. Sci. 2016, 27, 1629–1643. [Google Scholar] [CrossRef]
- Cheng, W.-M.; Shang, R. Transition Metal-Catalyzed Organic Reactions under Visible Light: Recent Developments and Future Perspectives. ACS. Catal. 2020, 10, 9170–9196. [Google Scholar] [CrossRef]
- Prier, C.K.; Rankic, D.A.; MacMillan, D.W.C. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 2013, 113, 5322–5363. [Google Scholar] [CrossRef] [PubMed]
- Humayun, M.; Raziq, F.; Khan, A.; Luo, W. Modification Strategies of TiO2 for Potential Applications in Photocatalysis: A Critical Review. Green Chem. Lett. Rev. 2018, 11, 86–102. [Google Scholar] [CrossRef]
- Zhou, Q.; Fang, Z.; Li, J.; Wang, M. Applications of TiO2 Nanotube Arrays in Environmental and Energy Fields: A Review. Micropor. Mesopor. Mater. 2015, 202, 22–35. [Google Scholar] [CrossRef]
- O’Regan, B.; Graetzel, M. A Low-Cost, High-Efficiency Solar Cell Based on Dye-Sensitized Colloidal TiO2 Films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Kamat, P.V. Meeting the Clean Energy Demand: Nanostructure Architectures for Solar Energy Conversion. J. Phys. Chem. C 2007, 111, 2834–2860. [Google Scholar] [CrossRef]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An Overview on Limitations of TiO2-Based Particles for Photocatalytic Degradation of Organic Pollutants and the Corresponding Countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, L.; Xie, E.; Duan, H.; Han, W.; Zhao, J. A Simple Method to Prepare Uniform-Size Nanoparticle TiO2 Electrodes for Dye-Sensitized Solar Cells. J. Power. Sources 2009, 189, 1256–1263. [Google Scholar] [CrossRef]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-Light Activation of TiO2 Photocatalysts: Advances in Theory and Experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Diaz-Angulo, J.; Gomez-Bonilla, I.; Jimenez-Tohapanta, C.; Mueses, M.; Pinzon, M.; Machuca-Martinez, F. Visible-Light Activation of TiO2 by Dye-Sensitization for Degradation of Pharmaceutical Compounds. Photochem. Photobiol. Sci. 2019, 18, 897–904. [Google Scholar] [CrossRef]
- Krishnan, S.; Shriwastav, A. Application of TiO2 Nanoparticles Sensitized with Natural Chlorophyll Pigments as Catalyst for Visible Light Photocatalytic Degradation of Methylene Blue. J. Environ. Chem. Eng. 2021, 9, 104699. [Google Scholar] [CrossRef]
- Buddee, S.; Wongnawa, S.; Sriprang, P.; Sriwong, C. Curcumin-Sensitized TiO2 for Enhanced Photodegradation of Dyes under Visible Light. J. Nanopart. Res. 2014, 16, 2336. [Google Scholar] [CrossRef]
- Erez, Y.; Simkovitch, R.; Shomer, S.; Gepshtein, R.; Huppert, D. Effect of Acid on the Ultraviolet–Visible Absorption and Emission Properties of Curcumin. J. Phys. Chem. A 2014, 118, 872–884. [Google Scholar] [CrossRef] [PubMed]
- Sinha, D.; De, D.; Ayaz, A. Performance and Stability Analysis of Curcumin Dye as a Photo Sensitizer Used in Nanostructured ZnO Based DSSC. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 193, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Goulart, S.; Jaramillo Nieves, L.J.; Dal Bó, A.G.; Bernardin, A.M. Sensitization of TiO2 Nanoparticles with Natural Dyes Extracts for Photocatalytic Activity under Visible Light. Dyes. Pigm. 2020, 182, 108654. [Google Scholar] [CrossRef]
- Chawraba, K.; Medlej, H.; Toufaily, J.; Lalevee, J.; Hamieh, T. TiO2 Sensitized by Natural Dye Extracted from Cinnamon Bark for Photodegradation of Methylene Blue in Water Under LED Irradiation. Chem. Afr. 2024, 7, 2087–2101. [Google Scholar] [CrossRef]
- Sanders, A.M.; Magnanelli, T.J.; Bragg, A.E.; Tovar, J.D. Photoinduced Electron Transfer within Supramolecular Donor–Acceptor Peptide Nanostructures under Aqueous Conditions. J. Am. Chem. Soc. 2016, 138, 3362–3370. [Google Scholar] [CrossRef]
- Hardcastle, F. Raman Spectroscopy of Titania (TiO2) Nanotubular Water-Splitting Catalysts. J. Arkansas Acad. Sci. 2011, 65, 43–48. [Google Scholar] [CrossRef]
- Chu, L.; Qin, Z.; Yang, J.; Li, X. Anatase TiO2 Nanoparticles with Exposed {001} Facets for Efficient Dye-Sensitized Solar Cells. Sci. Rep. 2015, 5, 12143. [Google Scholar] [CrossRef]
- Van Nong, H.; Hung, L.X.; Thang, P.N.; Chinh, V.D.; Vu, L.V.; Dung, P.T.; Van Trung, T.; Nga, P.T. Fabrication and Vibration Characterization of Curcumin Extracted from Turmeric (Curcuma Longa) Rhizomes of the Northern Vietnam. Springerplus 2016, 5, 1147. [Google Scholar] [CrossRef]
- Zhu, L.; Lu, Q.; Lv, L.; Wang, Y.; Hu, Y.; Deng, Z.; Lou, Z.; Hou, Y.; Teng, F. Ligand-Free Rutile and Anatase TiO2 Nanocrystals as Electron Extraction Layers for High Performance Inverted Polymer Solar Cells. RSC Adv. 2017, 7, 20084–20092. [Google Scholar] [CrossRef]
- Ben Saber, N.; Mezni, A.; Alrooqi, A.; Altalhi, T. Ternary Pt@TiO2/rGO Nanocomposite to Boost Photocatalytic Activity for Environmental and Energy Use. J. Inorg. Organomet. Polym. 2021, 31, 3802–3809. [Google Scholar] [CrossRef]
- Velmurugan, R.; Krishnakumar, B.; Kumar, R.; Swaminathan, M. Solar Active Nano-TiO2 for Mineralization of Reactive Red 120 and Trypan Blue. Arab. J. Chem. 2012, 5, 447–452. [Google Scholar] [CrossRef]
- Obuya, E.A.; Harrigan, W.; Andala, D.M.; Lippens, J.; Keane, T.C.; Jones, W.E. Photodeposited Pd Nanoparticle Catalysts Supported on Photoactivated TiO2 Nanofibers. J. Mol. Catal. A Chemical. 2011, 340, 89–98. [Google Scholar] [CrossRef]
- Arunkumar, R.; Babu, R.S.; Usha Rani, M. Investigation on Al2O3 Doped PVC–PBMA Blend Polymer Electrolytes. J. Mater. Sci. Mater. Electron. 2017, 28, 3309–3316. [Google Scholar] [CrossRef]
- Alharthi, A.I.; Alotaibi, M.A.; Alansi, A.M.; Qahtan, T.F.; Ali, I.; Al-Shalwi, M.N.; Bakht, M.A. Solar-Driven Thermocatalytic Synthesis of Octahydroquinazolinone Using Novel Polyvinylchloride (PVC)-Supported Aluminum Oxide (Al2O3) Catalysts. Materials 2023, 16, 2835. [Google Scholar] [CrossRef]
- Kuraitheerthakumaran, A.; Pazhamalai, S.; Manikandan, H.; Gopalakrishnan, M. Rapid and Efficient One-Pot Synthesis of Octahydroquinazolinone Derivatives Using Lanthanum Oxide under Solvent-Free Condition. J. Saudi Chem. Soc. 2014, 18, 920–924. [Google Scholar] [CrossRef]
- Karki, B.S.; Verma, S.; Agrwal, A.; Kasana, V. One Pot Three Component Organocatalyzed Synthesis of Octahydroquinazolinones. Int. J. Chem. Stud. 2017, 5, 280–290. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).