Synthesis of Morpholinoamido- and Ester-Disubstituted ε-Caprolactones and Their Ring-Opening (Co)Polymerization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization Methods
2.3. Synthesis of Monomers
2.4. General Procedure of 4c Polymerization
2.5. Copolymerization Procedure
2.6. Micelle Preparation
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmidt, B.V.K.J. Hydrophilic Polymers. Polymers 2019, 11, 693. [Google Scholar] [CrossRef]
- Yang, T.-H. Recent Applications of Polyacrylamide as Biomaterials. Recent Pat. Mater. Sci. 2008, 1, 29–40. [Google Scholar] [CrossRef]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(Ethylene Glycol) in Drug Delivery: Pros and Cons as Well as Potential Alternatives. Angew. Chem. Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef]
- Gaberc-Porekar, V.; Zore, I.; Podobnik, B.; Menart, V. Obstacles and Pitfalls in the PEGylation of Therapeutic Proteins. Curr. Opin. Drug Discov. Dev. 2008, 11, 242–250. [Google Scholar]
- Albertsson, A.-C.; Varma, I.K. Aliphatic Polyesters: Synthesis, Properties and Applications. In Degradable Aliphatic Polyesters; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2002; Volume 157, pp. 1–40. [Google Scholar]
- Kurowiak, J.; Klekiel, T.; Będziński, R. Biodegradable Polymers in Biomedical Applications: A Review—Developments, Perspectives and Future Challenges. Int. J. Mol. Sci. 2023, 24, 16952. [Google Scholar] [CrossRef]
- Jerome, C.; Lecomte, P. Recent Advances in the Synthesis of Aliphatic Polyesters by Ring-Opening Polymerization. Adv. Drug Deliv. Rev. 2008, 60, 1056–1076. [Google Scholar] [CrossRef]
- Williams, C.K. Synthesis of Functionalized Biodegradable Polyesters. Chem. Soc. Rev. 2007, 36, 1573. [Google Scholar] [CrossRef] [PubMed]
- Tong, R. New Chemistry in Functional Aliphatic Polyesters. Ind. Eng. Chem. Res. 2017, 56, 4207–4219. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, Y.; Huang, H.; Liu, L.; Chen, Y. Well-Defined Poly(α-Amino-δ-Valerolactone) via Living Ring-Opening Polymerization. Macromolecules 2018, 51, 2526–2532. [Google Scholar] [CrossRef]
- Xu, J.; Liang, W.; Zhang, J.; Dong, Z.; Lei, C. Synthesis of Side-Chain Functional Poly(ε-Caprolactone) via the Versatile and Robust Organo-Promoted Esterification Reaction. Eur. Polym. J. 2022, 179, 111526. [Google Scholar] [CrossRef]
- Toplishek, M.; Žagar, E.; Pahovnik, D. Synthesis of Dicyano-Substituted ε-Caprolactone and Its (Co)Polymers. Eur. Polym. J. 2019, 119, 438–444. [Google Scholar] [CrossRef]
- Gao, P.-F.; Wang, H.-R.; Xiao, X.-X.; Ren, W.-M.; Lu, X.-B.; Zhou, H. Synthesis and Ring-Opening Polymerization of Bisubstituted ε-Caprolactones Bearing Aryl and Cyano Groups. Polymer 2025, 319, 128034. [Google Scholar] [CrossRef]
- Yi, M.; Lu, Q.; Zhao, Y.; Cheng, C.; Zhang, S. Synthesis and Self-Assembly of the pH-Responsive Anionic Copolymers for Enhanced Doxorubicin-Loading Capacity. Langmuir 2018, 34, 7877–7886. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, K.; Reghu, S.; Lin, Y.; Chan-Park, M.B.; Liu, X.-W. Synthesis of Antibacterial Glycosylated Polycaprolactones Bearing Imidazoliums with Reduced Hemolytic Activity. Biomacromolecules 2019, 20, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Piva, O. Synthesis of Seven-Membered Ring Ethers and Lactones. In Synthesis of Saturated Oxygenated Heterocycles II; Cossy, J., Ed.; Topics in Heterocyclic Chemistry; Springer: Berlin/Heidelberg, Germany, 2014; Volume 36, pp. 283–320. [Google Scholar]
- Tian, D.; Dubois, P.; Grandfils, C.; Jérôme, R. Ring-Opening Polymerization of 1,4,8-Trioxaspiro[4.6]-9-Undecanone: A New Route to Aliphatic Polyesters Bearing Functional Pendent Groups. Macromolecules 1997, 30, 406–409. [Google Scholar] [CrossRef]
- Winkler, M.; Raupp, Y.S.; Köhl, L.A.M.; Wagner, H.E.; Meier, M.A.R. Modified Poly(ε-Caprolactone)s: An Efficient and Renewable Access via Thia-Michael Addition and Baeyer–Villiger Oxidation. Macromolecules 2014, 47, 2842–2846. [Google Scholar] [CrossRef]
- Hao, J.; Servello, J.; Sista, P.; Biewer, M.C.; Stefan, M.C. Temperature-Sensitive Aliphatic Polyesters: Synthesis and Characterization of γ-Substituted Caprolactone Monomers and Polymers. J. Mater. Chem. 2011, 21, 10623. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Yan, J.; Lang, M. Synthesis and Solution Properties of pH Responsive Methoxy Poly(Ethylene Glycol)-b-Poly(γ-Amino-ε-Caprolactone). J. Mater. Chem. 2011, 21, 6677. [Google Scholar] [CrossRef]
- Detrembleur, C.; Mazza, M.; Lou, X.; Halleux, O.; Lecomte, P.; Mecerreyes, D.; Hedrick, J.L.; Jérôme, R. New Functional Aliphatic Polyesters by Chemical Modification of Copolymers of ε-Caprolactone with γ-(2-Bromo-2-Methylpropionate)-ε-Caprolactone, γ-Bromo-ε-Caprolactone, and a Mixture of β- and γ-Ene-ε-Caprolactone. Macromolecules 2000, 33, 7751–7760. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, Y.; Xu, H.; Zhou, C.; Lang, M. Synthesis of Well-Defined Carboxyl Poly(ε-Caprolactone) by Fine-Tuning the Protection Group. Polym. Chem. 2016, 7, 4630–4637. [Google Scholar] [CrossRef]
- Mahmud, A.; Xiong, X.-B.; Lavasanifar, A. Novel Self-Associating Poly(Ethylene Oxide)- Block -Poly(ε-Caprolactone) Block Copolymers with Functional Side Groups on the Polyester Block for Drug Delivery. Macromolecules 2006, 39, 9419–9428. [Google Scholar] [CrossRef]
- Surnar, B.; Jayakannan, M. Stimuli-Responsive Poly(Caprolactone) Vesicles for Dual Drug Delivery under the Gastrointestinal Tract. Biomacromolecules 2013, 14, 4377–4387. [Google Scholar] [CrossRef]
- Shahin, M.; Lavasanifar, A. Novel Self-Associating Poly(Ethylene Oxide)-b-Poly(ɛ-Caprolactone) Based Drug Conjugates and Nano-Containers for Paclitaxel Delivery. Int. J. Pharm. 2010, 389, 213–222. [Google Scholar] [CrossRef]
- Ota, T.; Montagna, V.; Higuchi, Y.; Kato, T.; Tanaka, M.; Sardon, H.; Fukushima, K. Organocatalyzed Ring-Opening Reactions of γ-Carbonyl-Substituted ε-Caprolactones. RSC Adv. 2023, 13, 27764–27771. [Google Scholar] [CrossRef] [PubMed]
- Trollsås, M.; Lee, V.Y.; Mecerreyes, D.; Löwenhielm, P.; Möller, M.; Miller, R.D.; Hedrick, J.L. Hydrophilic Aliphatic Polyesters: Design, Synthesis, and Ring-Opening Polymerization of Functional Cyclic Esters. Macromolecules 2000, 33, 4619–4627. [Google Scholar] [CrossRef]
- Wang, H.; Calubaquib, E.L.; Bhadran, A.; Ma, Z.; Miller, J.T.; Zhang, A.; Biewer, M.C.; Stefan, M.C. Self-Assembly Behavior of Thermoresponsive Difunctionalized γ-Amide Polycaprolactone Amphiphilic Diblock Copolymers. Polym. Chem. 2023, 14, 514–522. [Google Scholar] [CrossRef]
- Swanson, J.P.; Cruz, M.A.; Monteleone, L.R.; Martinez, M.R.; Costanzo, P.J.; Joy, A. The Effect of Pendant Group Structure on the Thermoresponsive Properties of N -Substituted Polyesters. Polym. Chem. 2017, 8, 7195–7206. [Google Scholar] [CrossRef]
- Cruz, M.A.; Morris, D.L.; Swanson, J.P.; Kundu, M.; Mankoci, S.G.; Leeper, T.C.; Joy, A. Efficient Protein Encapsulation within Thermoresponsive Coacervate-Forming Biodegradable Polyesters. ACS Macro Lett. 2018, 7, 477–481. [Google Scholar] [CrossRef]
- Wen, L.; Zhang, S.; Xiao, Y.; He, J.; Zhu, S.; Zhang, J.; Wu, Z.; Lang, M. Organocatalytic Ring-Opening Polymerization Toward Poly(γ-Amide-ε-Caprolactone)s with Tunable Lower Critical Solution Temperatures. Macromolecules 2020, 53, 5096–5104. [Google Scholar] [CrossRef]
- Vasilica Arsenie, L.; Ladmiral, V.; Lacroix-Desmazes, P.; Catrouillet, S. Morpholine and Thiomorpholine Derived Polymers: Multifunctional Platforms for Biological Applications. Eur. Polym. J. 2023, 200, 112490. [Google Scholar] [CrossRef]
- Tanaka, H.; Hanasaki, M.; Isojima, T.; Takeuchi, H.; Shiroya, T.; Kawaguchi, H. Enhancement of Sensitivity of SPR Protein Microarray Using a Novel 3D Protein Immobilization. Colloids Surf. B Biointerfaces 2009, 70, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Singh, R.K. Morpholine as Ubiquitous Pharmacophore in Medicinal Chemistry: Deep Insight into the Structure-Activity Relationship (SAR). Bioorg. Chem. 2020, 96, 103578. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, K.; Thangagiri, B.; Mishra, A.K.; Ahn, B.-H.; Gal, Y.-S.; Lim, K.T. AB2-Type Miktoarm Poly(L-Lactide)-b-Poly(N-Acryloylmorpholine) Amphiphilic Star Block Copolymers as Nanocarriers for Drug Delivery. React. Funct. Polym. 2018, 132, 112–119. [Google Scholar] [CrossRef]
- Xu, F.; Li, H.; Luo, Y.-L.; Tang, W. Redox-Responsive Self-Assembly Micelles from Poly(N-Acryloylmorpholine-Block-2-Acryloyloxyethyl Ferrocenecarboxylate) Amphiphilic Block Copolymers as Drug Release Carriers. ACS Appl. Mater. Interfaces 2017, 9, 5181–5192. [Google Scholar] [CrossRef]
- Hasegawa, U.; Tateishi, N.; Uyama, H.; Van Der Vlies, A.J. Hydrolysis-Sensitive Dithiolethione Prodrug Micelles. Macromol. Biosci. 2015, 15, 1512–1522. [Google Scholar] [CrossRef]
- Efe, H.; Bicen, M.; Kahraman, M.V.; Kayaman-Apohan, N. Synthesis of 4-Acryloylmorpholine-Based Hydrogels and Investigation of Their Drug Release Behaviors. J. Braz. Chem. Soc. 2013, 24, 814–820. [Google Scholar] [CrossRef]
- Jung, M.E.; McCombs, C.A.; Takeda, Y.; Pan, Y.-G. Use of Silyloxydienes in Synthesis. Total Syntheses of the Sesquiterpene (±)-Seychellene. J. Am. Chem. Soc. 1981, 103, 6677–6685. [Google Scholar] [CrossRef]
- Lu, A.; Wang, Z.; Zhou, Z.; Chen, J.; Wang, Q. Application of “Hydrogen Bonding Interaction” in New Drug Development: Design, Synthesis, Antiviral Activity, and SARs of Thiourea Derivatives. J. Agric. Food Chem. 2015, 63, 1378–1384. [Google Scholar] [CrossRef]
- Makiguchi, K.; Satoh, T.; Kakuchi, T. Diphenyl Phosphate as an Efficient Cationic Organocatalyst for Controlled/Living Ring-Opening Polymerization of δ-Valerolactone and ε-Caprolactone. Macromolecules 2011, 44, 1999–2005. [Google Scholar] [CrossRef]
- Anastasia, M.; Allevi, P.; Ciuffreda, P.; Fiecchi, A.; Scala, A. Synthesis of (2R,3S,22R,23R)- and (2R,3S,22S,23S)-2,3,22,23-Tetrahydroxy-B-Homo-8-Oxa-5α-Ergostan-7-Ones, Two New Brassinolide Analogs. J. Org. Chem. 1985, 50, 321–325. [Google Scholar] [CrossRef]
- Clyne, D.S.; Weiler, L. The Synthesis of 14-Membered Macrocyclic Ethers. Tetrahedron 1999, 55, 13659–13682. [Google Scholar] [CrossRef]
- Celiz, A.D.; Scherman, O.A. Controlled Ring-Opening Polymerization Initiated via Self-Complementary Hydrogen-Bonding Units. Macromolecules 2008, 41, 4115–4119. [Google Scholar] [CrossRef]
- Lou, X.; Detrembleur, C.; Lecomte, P.; Jérôme, R. Two-Step Backbiting Reaction in the Ring-Opening Polymerization of γ-Acryloyloxy-ɛ-Caprolactone Initiated with Aluminium Isopropoxide. Macromol. Rapid Commun. 2002, 23, 126–129. [Google Scholar] [CrossRef]
- Kularatne, R.N.; Washington, K.E.; Bulumulla, C.; Calubaquib, E.L.; Biewer, M.C.; Oupicky, D.; Stefan, M.C. Histone Deacetylase Inhibitor (HDACi) Conjugated Polycaprolactone for Combination Cancer Therapy. Biomacromolecules 2018, 19, 1082–1089. [Google Scholar] [CrossRef]
- Zhang, X.; Jones, G.O.; Hedrick, J.L.; Waymouth, R.M. Fast and Selective Ring-Opening Polymerizations by Alkoxides and Thioureas. Nat. Chem. 2016, 8, 1047–1053. [Google Scholar] [CrossRef]
- Akatsuka, M.; Aida, T.; Inoue, S. Alcohol/Methylaluminum Diphenolate Systems as Novel, Versatile Initiators for Synthesis of Narrow Molecular Weight Distribution Polyester and Polycarbonate. Macromolecules 1995, 28, 1320–1322. [Google Scholar] [CrossRef]
- Urakami, H.; Guan, Z. Living Ring-Opening Polymerization of a Carbohydrate-Derived Lactone for the Synthesis of Protein-Resistant Biomaterials. Biomacromolecules 2008, 9, 592–597. [Google Scholar] [CrossRef]
- Olaj, O.F.; Vana, P.; Zoder, M. Chain Length Dependent Propagation Rate Coefficient kp in Pulsed-Laser Polymerization: Variation with Temperature in the Bulk Polymerization of Styrene and Methyl Methacrylate. Macromolecules 2002, 35, 1208–1214. [Google Scholar] [CrossRef]
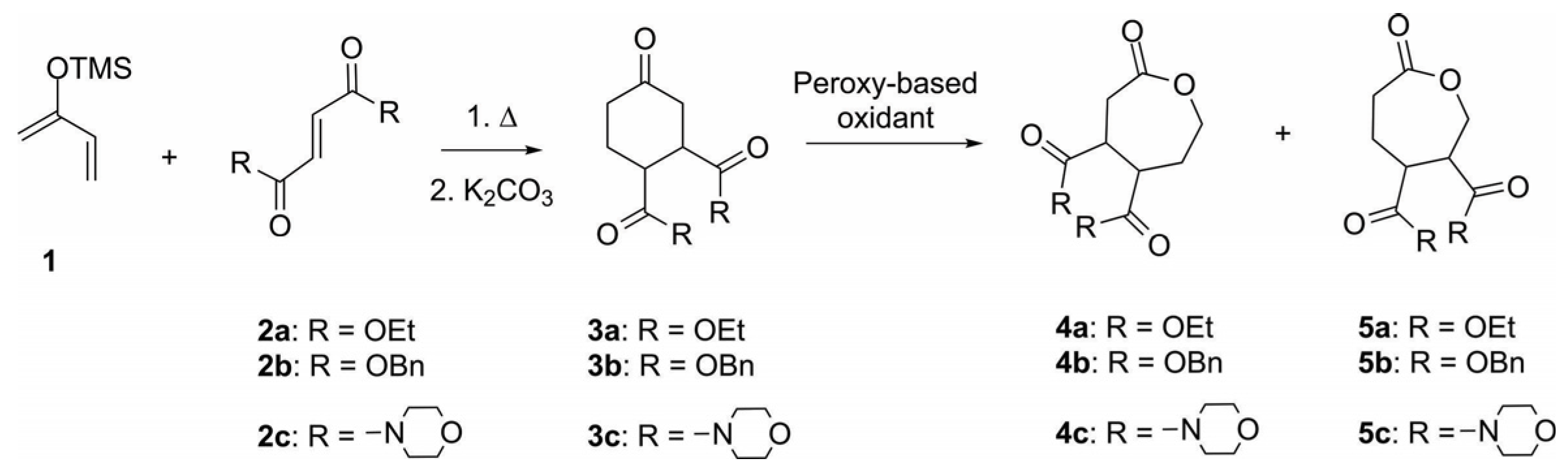
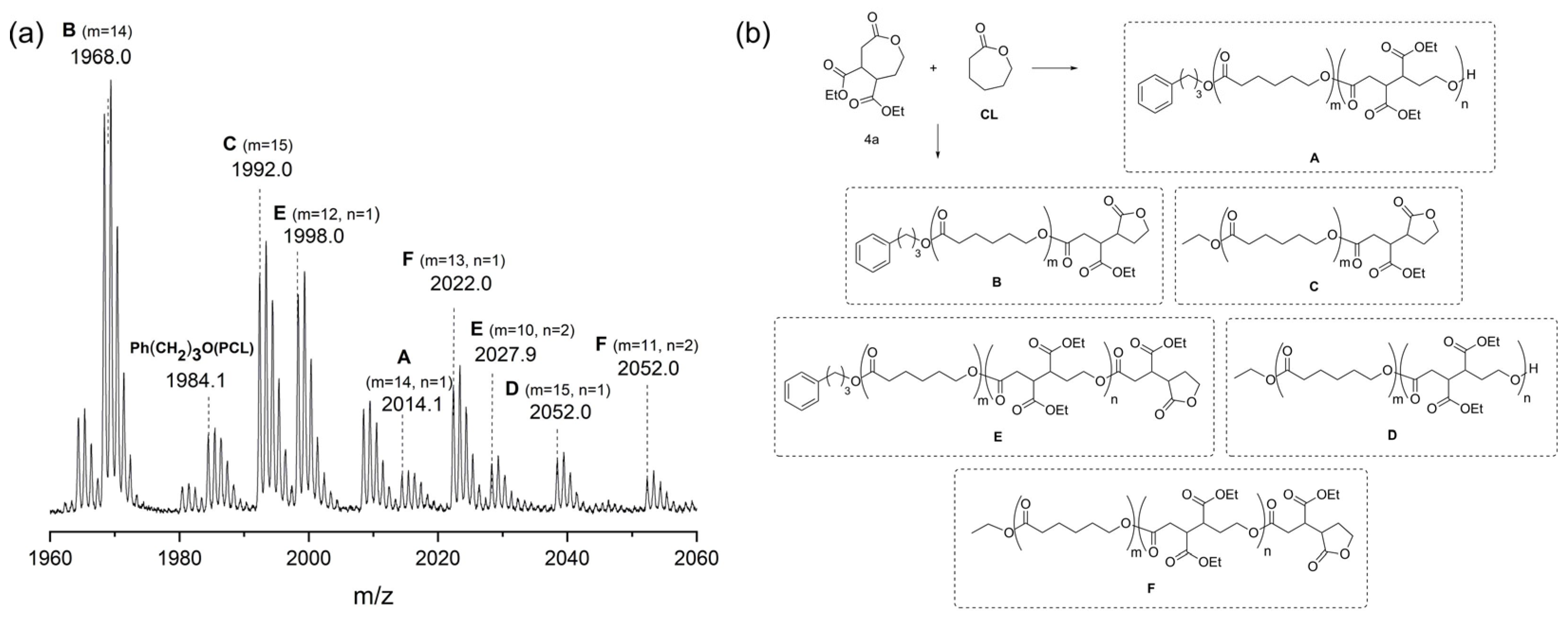
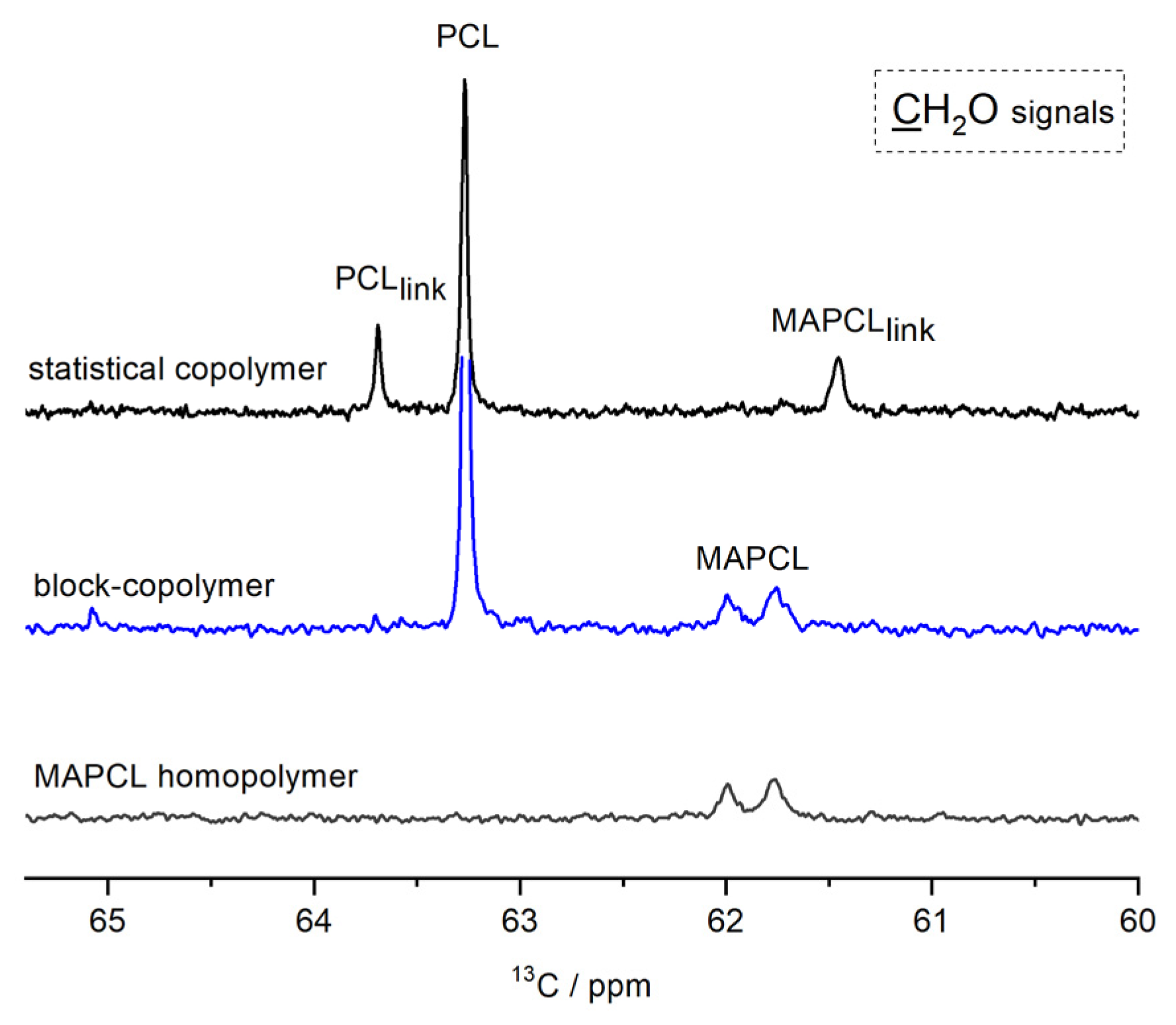
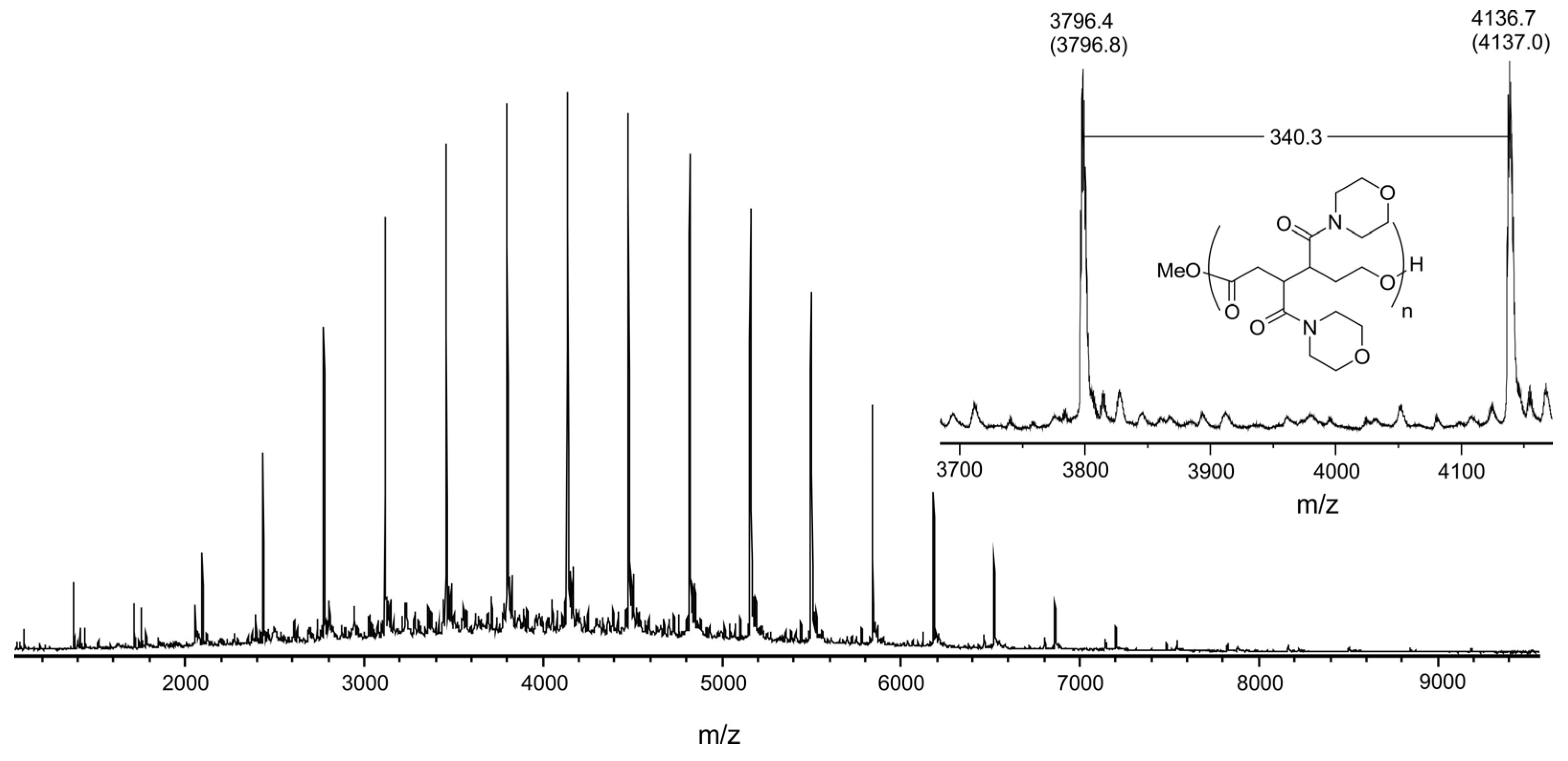
| Entry | R | Oxidant | Time, h | Reaction Mixture Composition, mol % 1 | ||
|---|---|---|---|---|---|---|
| 3 | 4 | 5 | ||||
| 1 | OEt | mCPBA | 48 | - | 83 | 17 |
| 2 | OBn | mCPBA | 48 | 38 | 53 | 9 |
| 3 | morpholine | mCPBA | 48 | - | 81 | 19 |
| 4 | OBn | TFPAA | 16 | - | 83 | 17 |
| 5 | OEt | TFPAA | 16 | - | 80 | 20 |
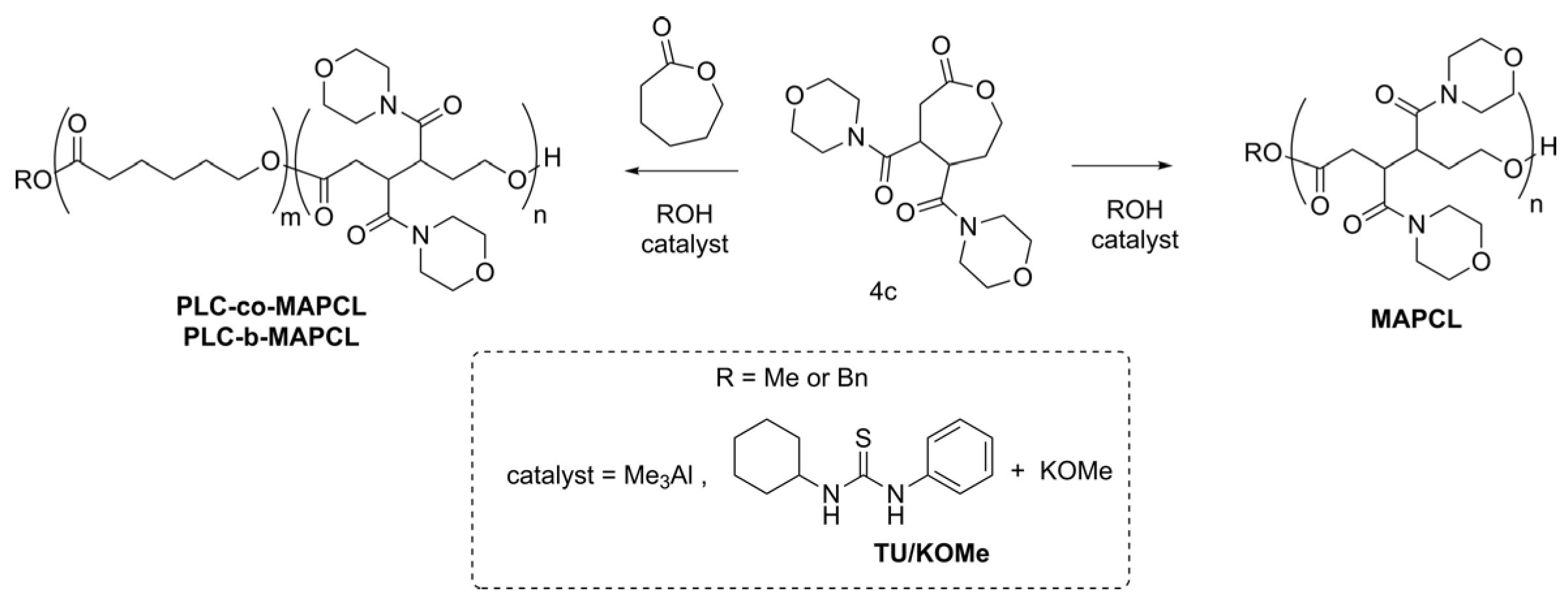
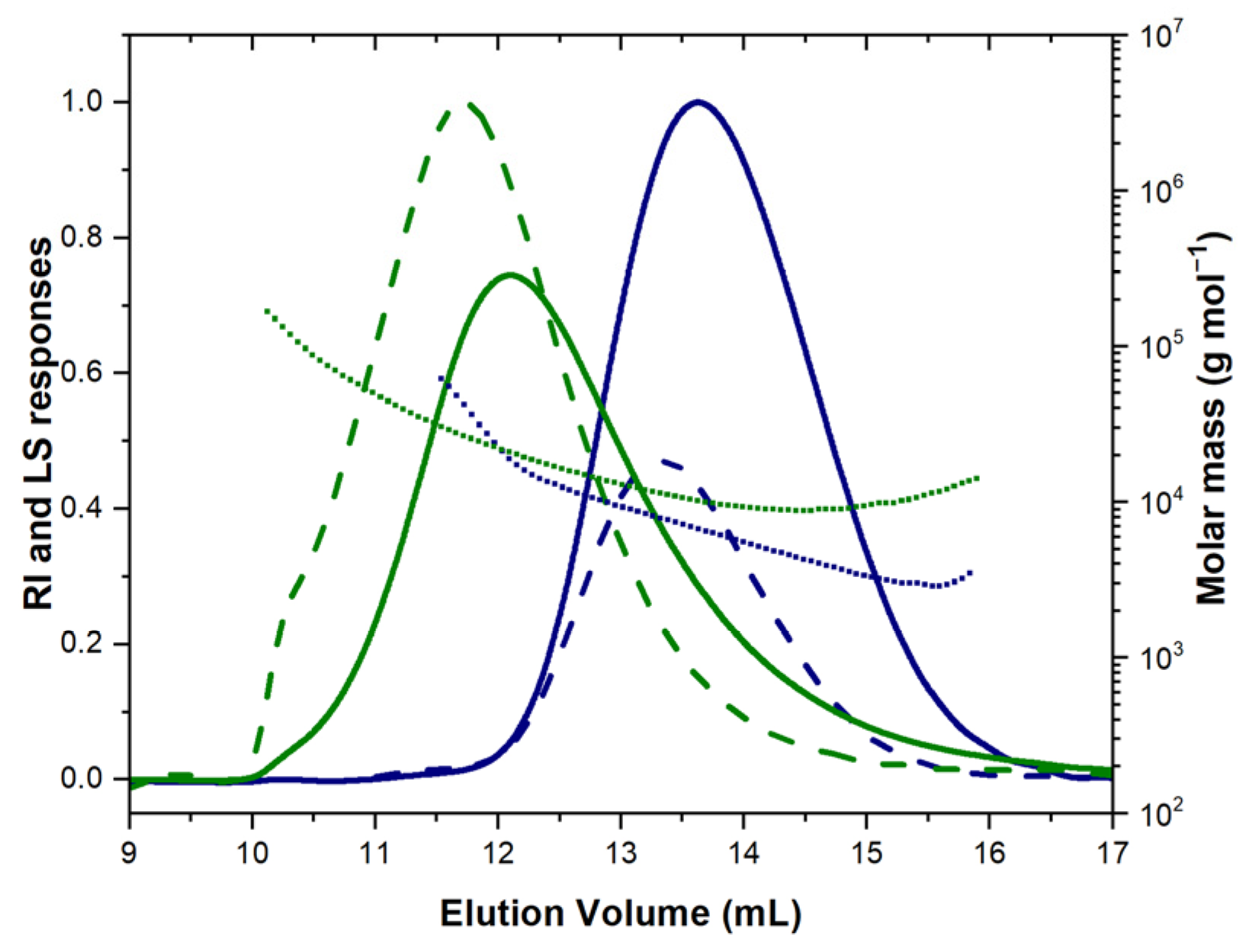
| Name | Monomer | Initiator | Catalyst (I:C:M Ratio) 1 | Solvent/ Temperature | Reaction Time | Conversion/Yield (%) | Mtheor (g mol−1) | 1H NMR Mn (g mol−1) | SEC/MALS-RI | Đ | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mn (g mol−1) | Mw (g mol−1) | ||||||||||
| MAPCL1 | 4c | BnOH | Me3Al (1:1:15) | ClCH2CH2Cl/ 60 °C | 5.5 h | 84/50 | 4400 | 4500 | 5900 | 7100 | 1.20 |
| MAPCL2 | 4c | BnOH | Me3Al (1:4:58) | ClCH2CH2Cl/ 60 °C | 5 days | 85/79 | 16,800 | 14,800 | 14,900 | 20,000 | 1.34 |
| PCL-co-MAPCL | 4c (25 mol%) + CL (75 mol%) | BnOH | Me3Al (1:4:74) | ClCH2CH2Cl/ 60 °C | 16 h | 98 (CL) 98 (4c)/93 | 12,300 | 13,100 | 12,800 | 17,100 | 1.34 |
| PCL-b-MAPCL1 | 4c | PCL Mn = 4.3 kg mol−1 Đ = 1.03) | Me3Al (1:1.6:24) | ClCH2CH2Cl/ 60 °C | 14 h | 85/83 | 9800 | 12,400 | 9800 | 10,900 | 1.11 |
| MAPCL3 | 4c | - | TU/KOMe (1:21) | CH2Cl2/RT | 2 h | 70/62 | 5000 | - 2 | 4700 | 5400 | 1.15 |
| PCL-b-MAPCL2 | 1. CL | - | TU/KOMe (44:1 (CL)) | Toluene/RT | 3 h | 97 (CL) | 4900 | - 2 | 4500 | 5300 | 1.18 |
| 2. 4c | (12:1 (4c)) | Toluene-CH2Cl2/RT | 80 (4c)/78 | 8900 | - 2 | 7600 | 8200 | 1.07 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orehova, M.; Žagar, E.; Pahovnik, D. Synthesis of Morpholinoamido- and Ester-Disubstituted ε-Caprolactones and Their Ring-Opening (Co)Polymerization. Materials 2025, 18, 4067. https://doi.org/10.3390/ma18174067
Orehova M, Žagar E, Pahovnik D. Synthesis of Morpholinoamido- and Ester-Disubstituted ε-Caprolactones and Their Ring-Opening (Co)Polymerization. Materials. 2025; 18(17):4067. https://doi.org/10.3390/ma18174067
Chicago/Turabian StyleOrehova, Maria, Ema Žagar, and David Pahovnik. 2025. "Synthesis of Morpholinoamido- and Ester-Disubstituted ε-Caprolactones and Their Ring-Opening (Co)Polymerization" Materials 18, no. 17: 4067. https://doi.org/10.3390/ma18174067
APA StyleOrehova, M., Žagar, E., & Pahovnik, D. (2025). Synthesis of Morpholinoamido- and Ester-Disubstituted ε-Caprolactones and Their Ring-Opening (Co)Polymerization. Materials, 18(17), 4067. https://doi.org/10.3390/ma18174067







