Ecofriendly PEF- and PBF-Based Blends with Epoxidized Natural Rubber: Unraveling the Structure–Property Relationship
Abstract
1. Introduction
2. Materials and Methods
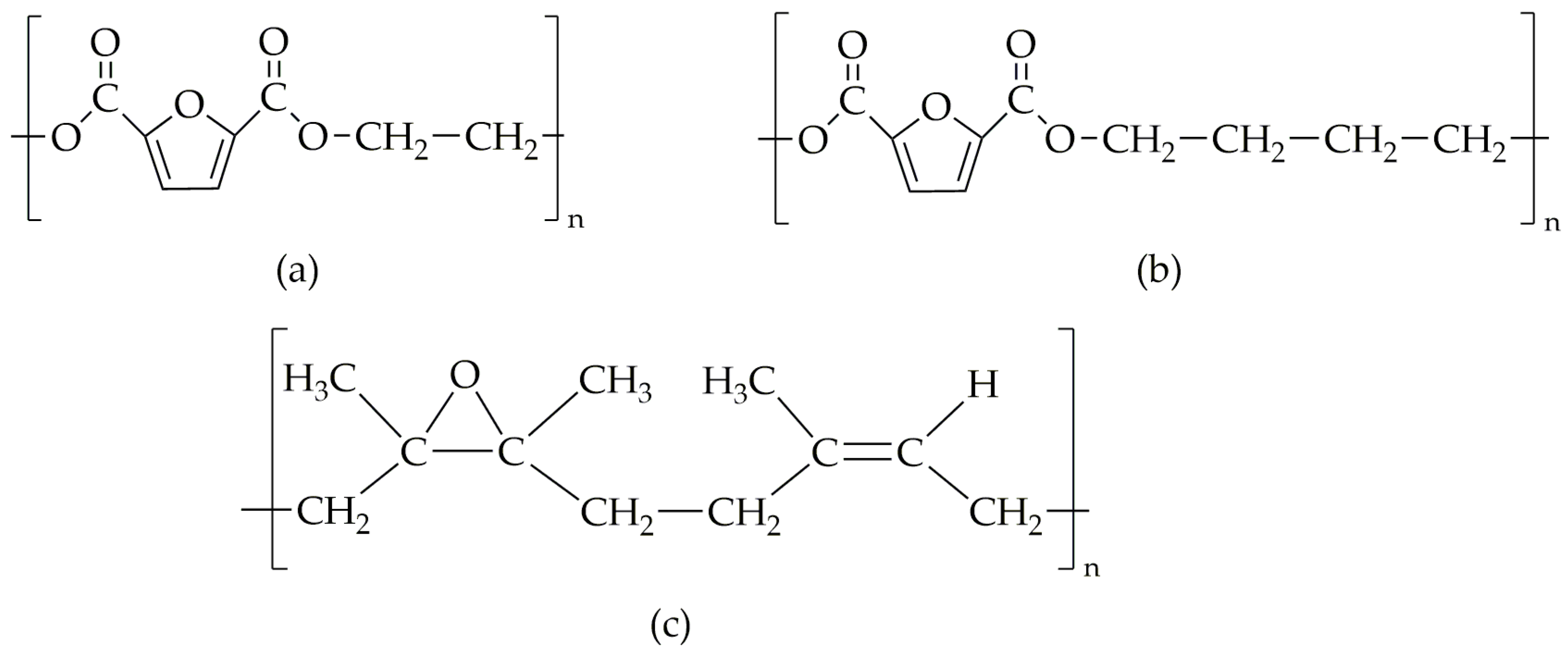
2.1. Materials
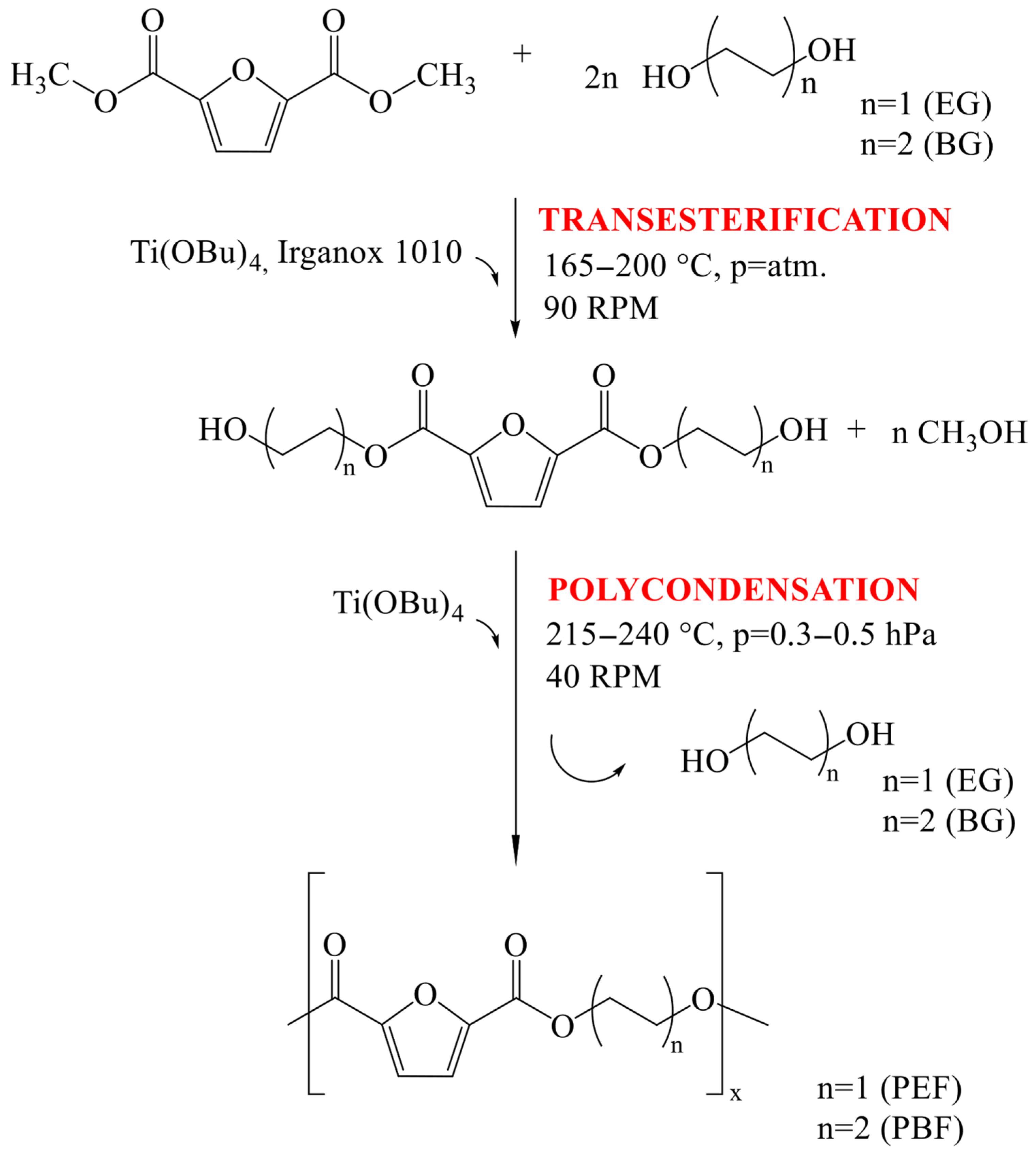
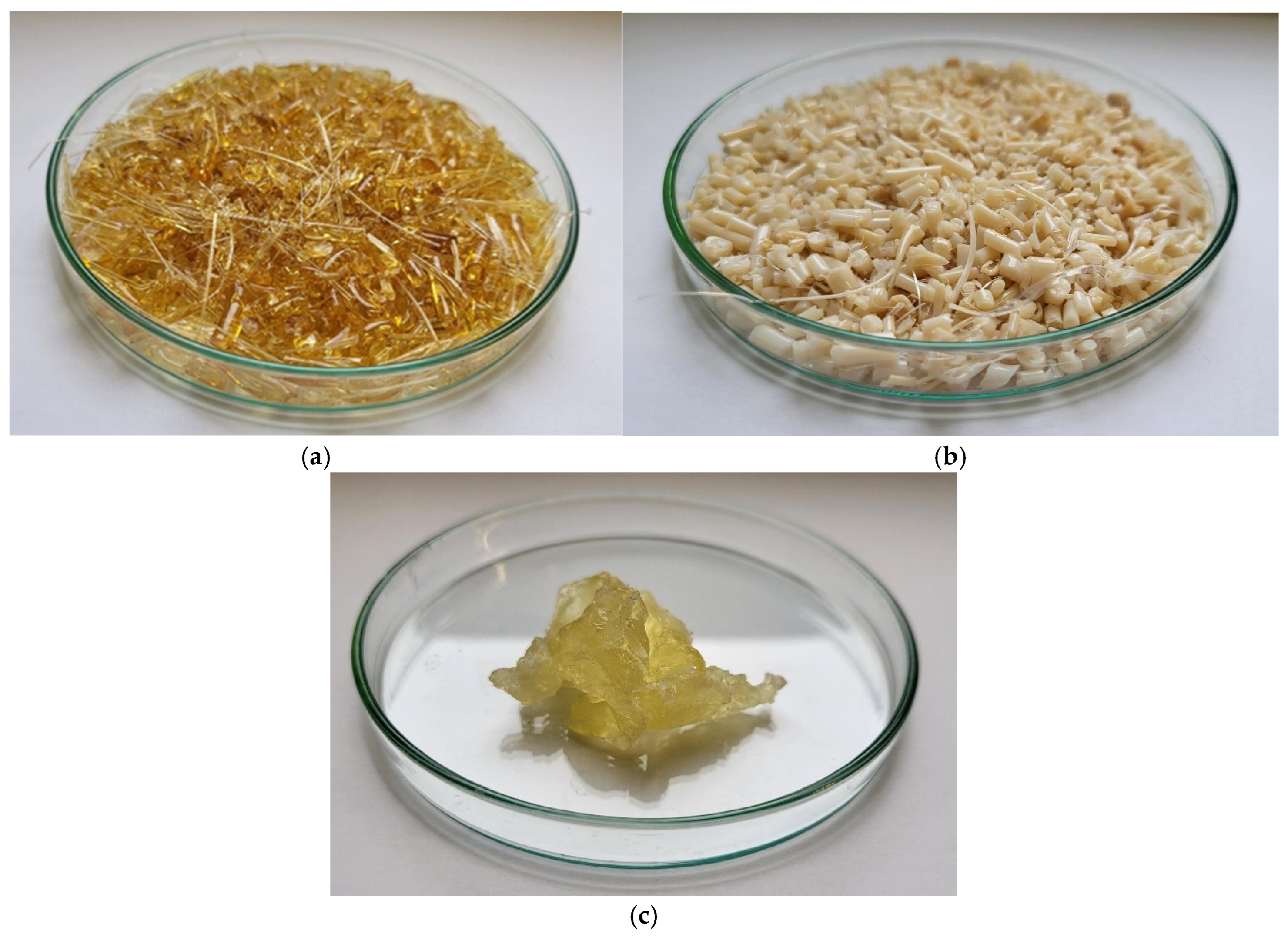
2.2. Preparation of Polyester/epNR Blends
2.3. Sample Preparation
2.4. Characterization Methods
3. Results and Discussion
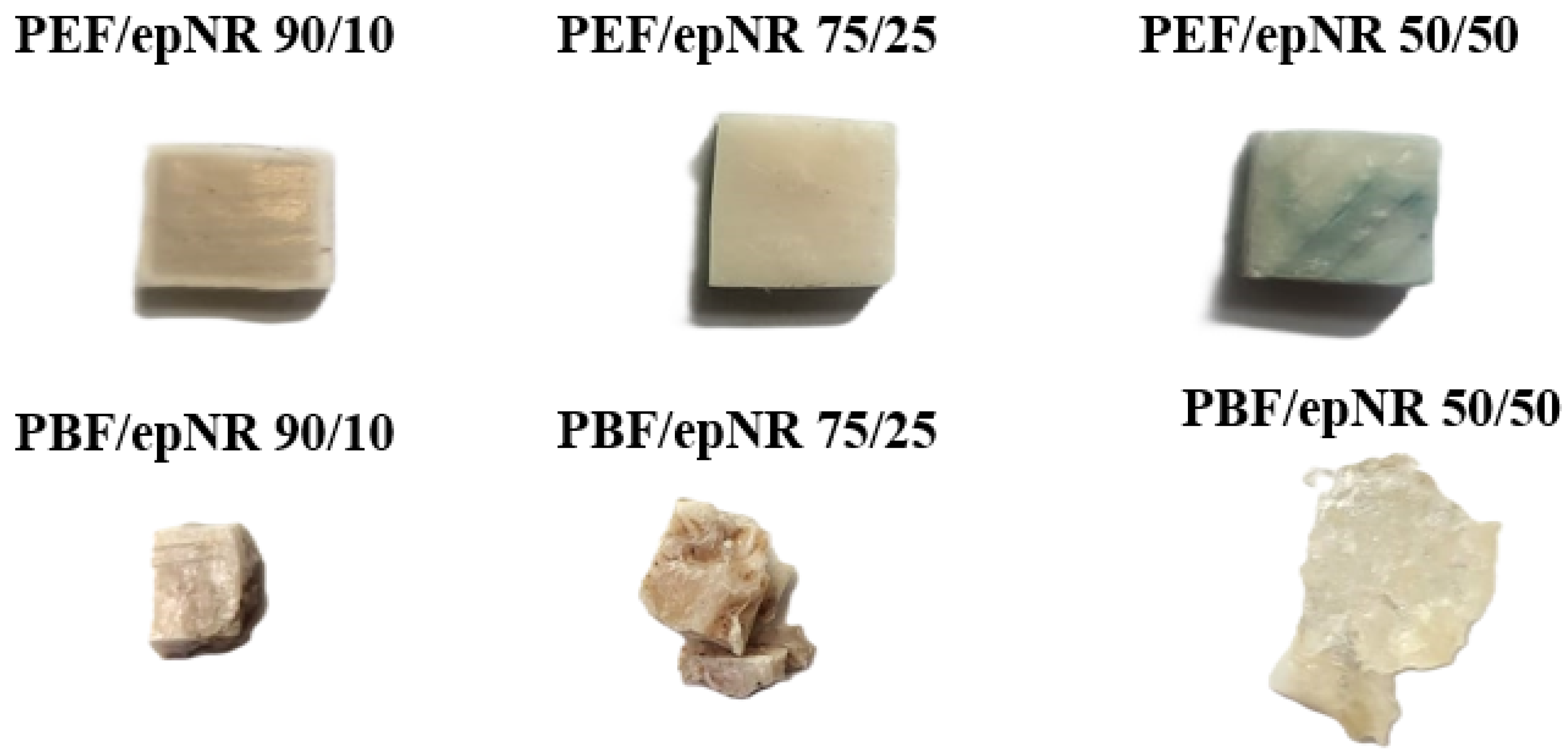
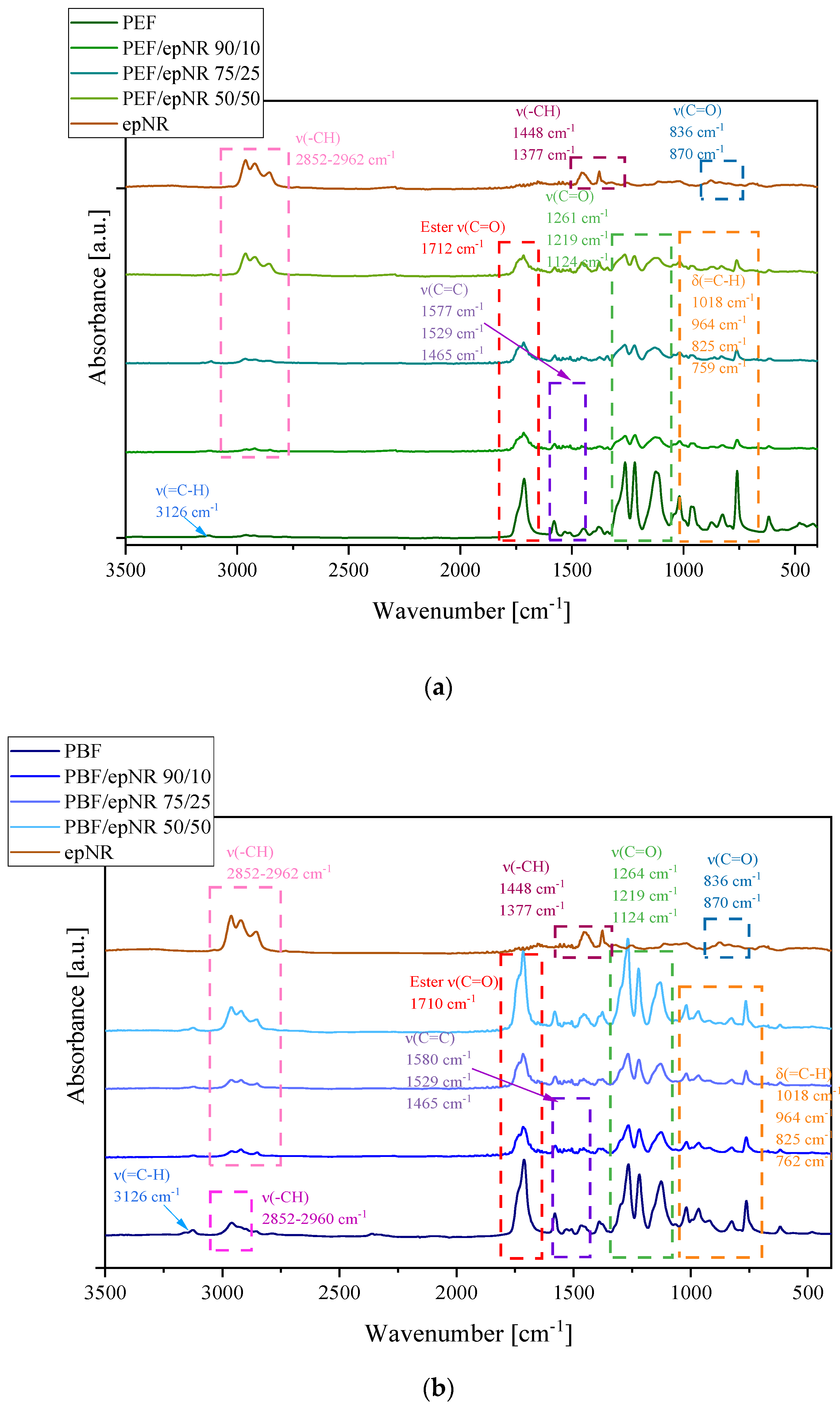
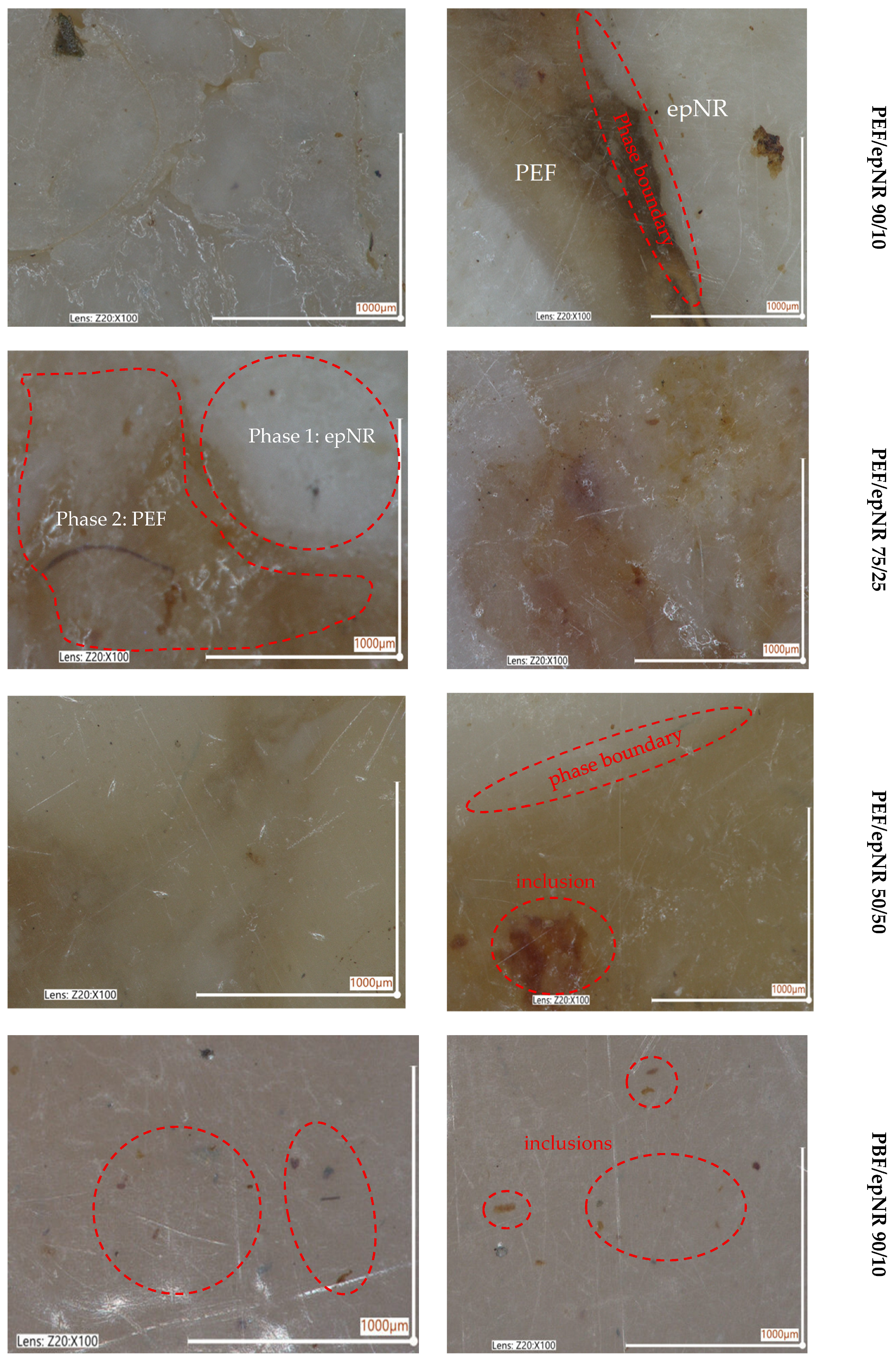
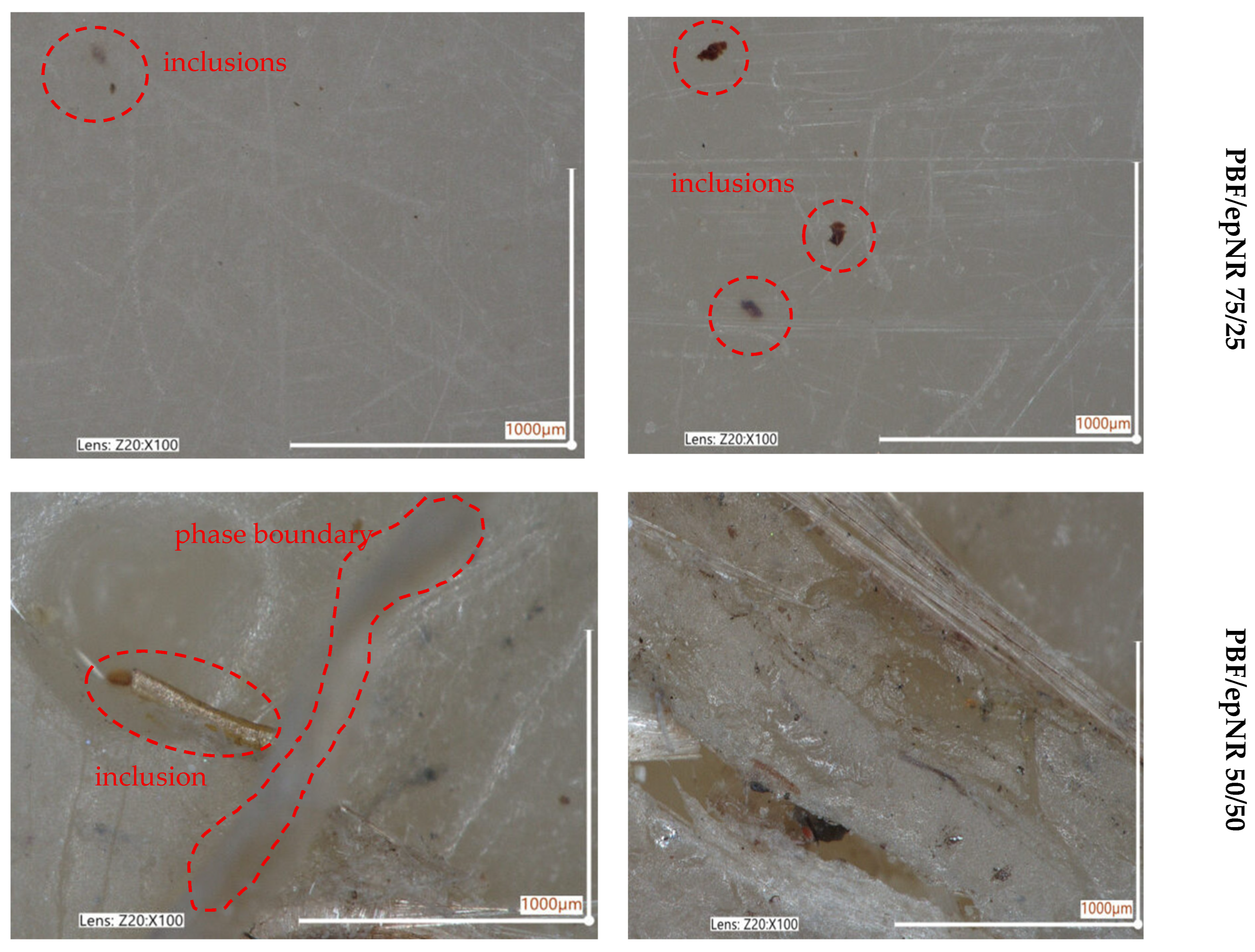
3.1. Solubility Assessment and Morphology
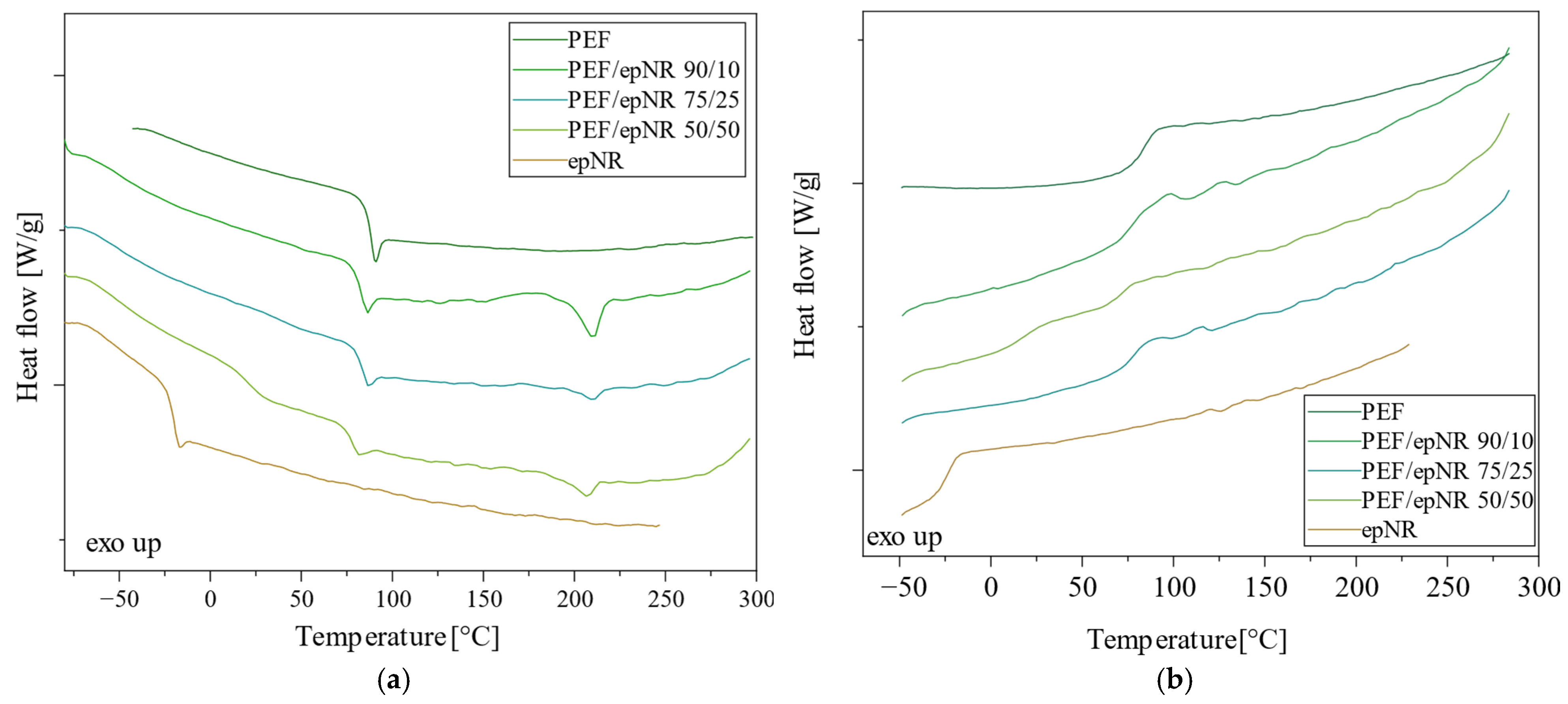
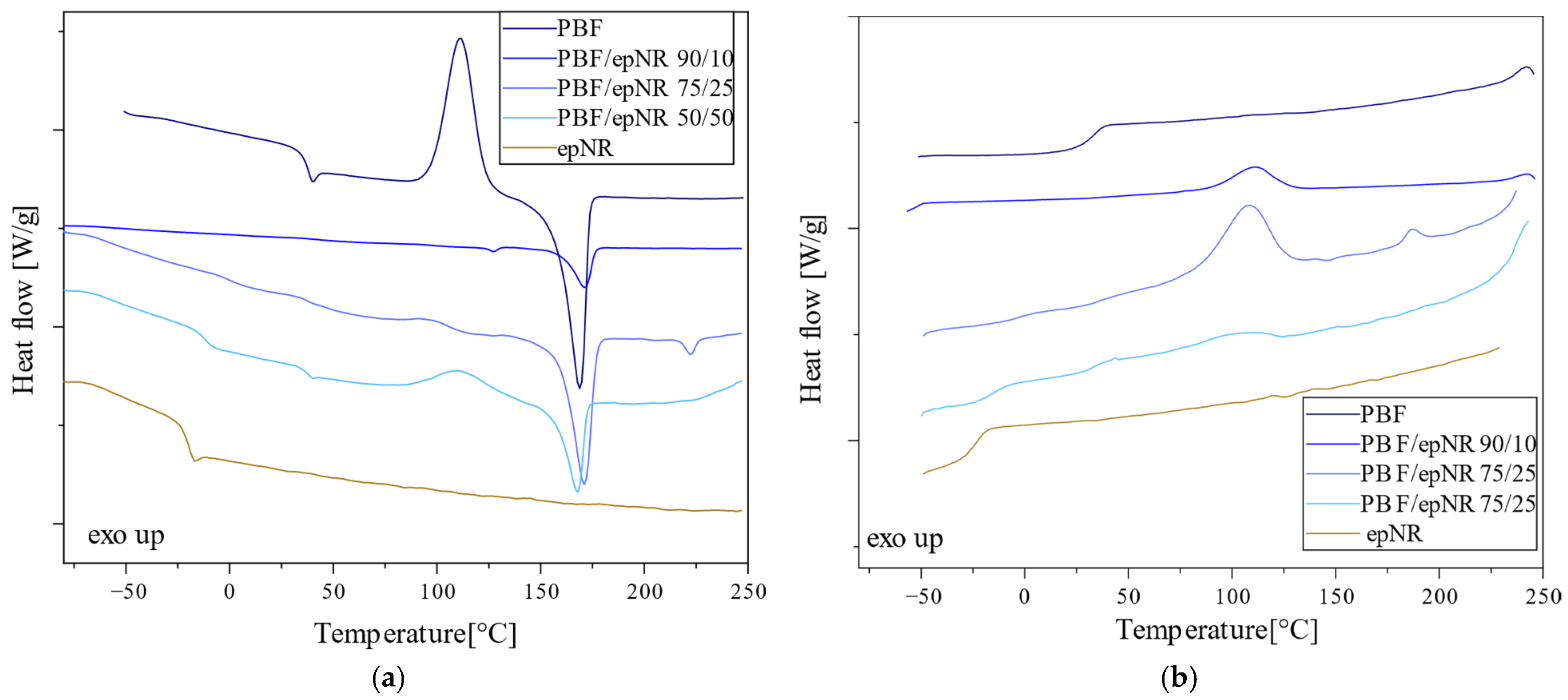
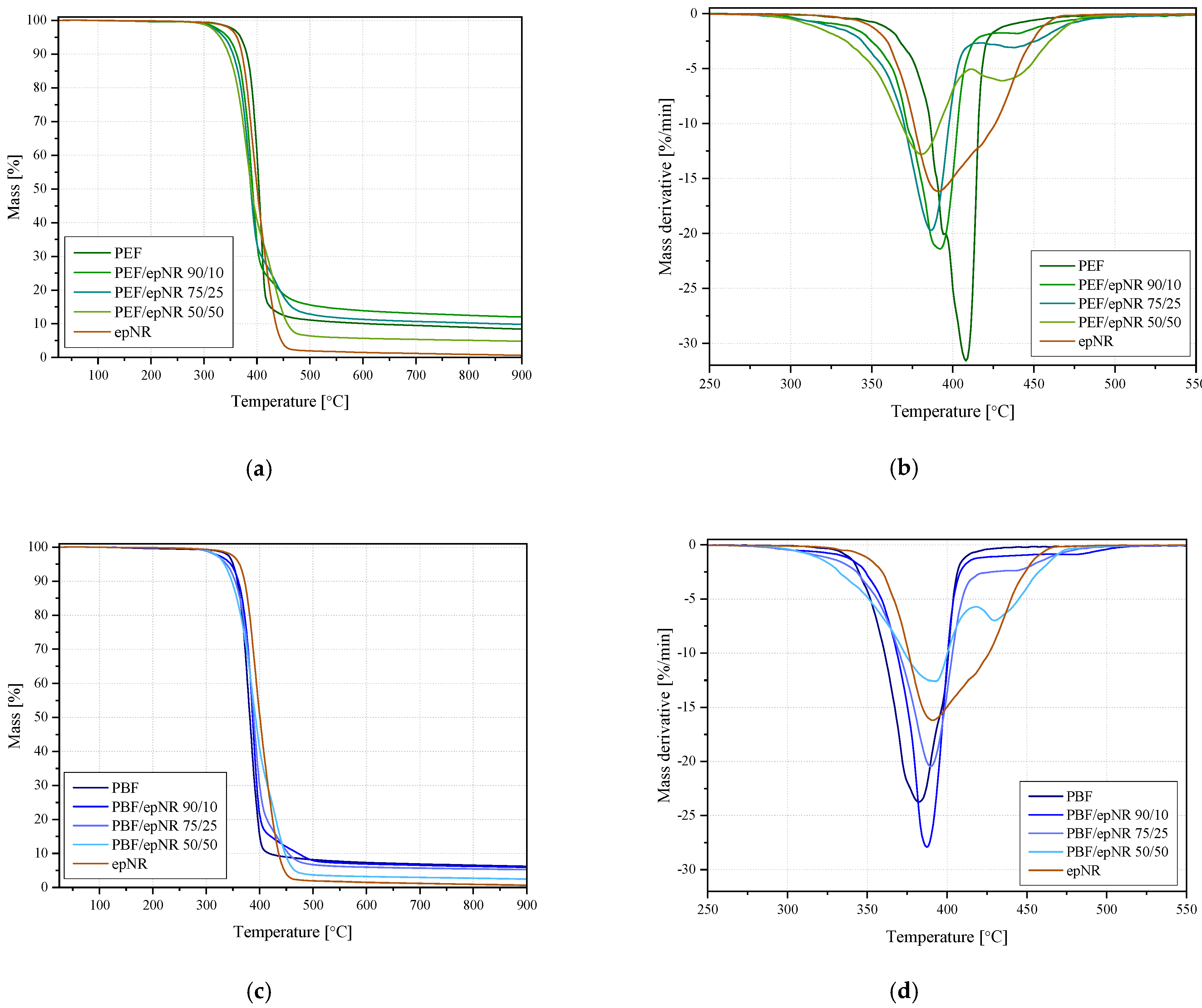
3.2. Thermal Properties
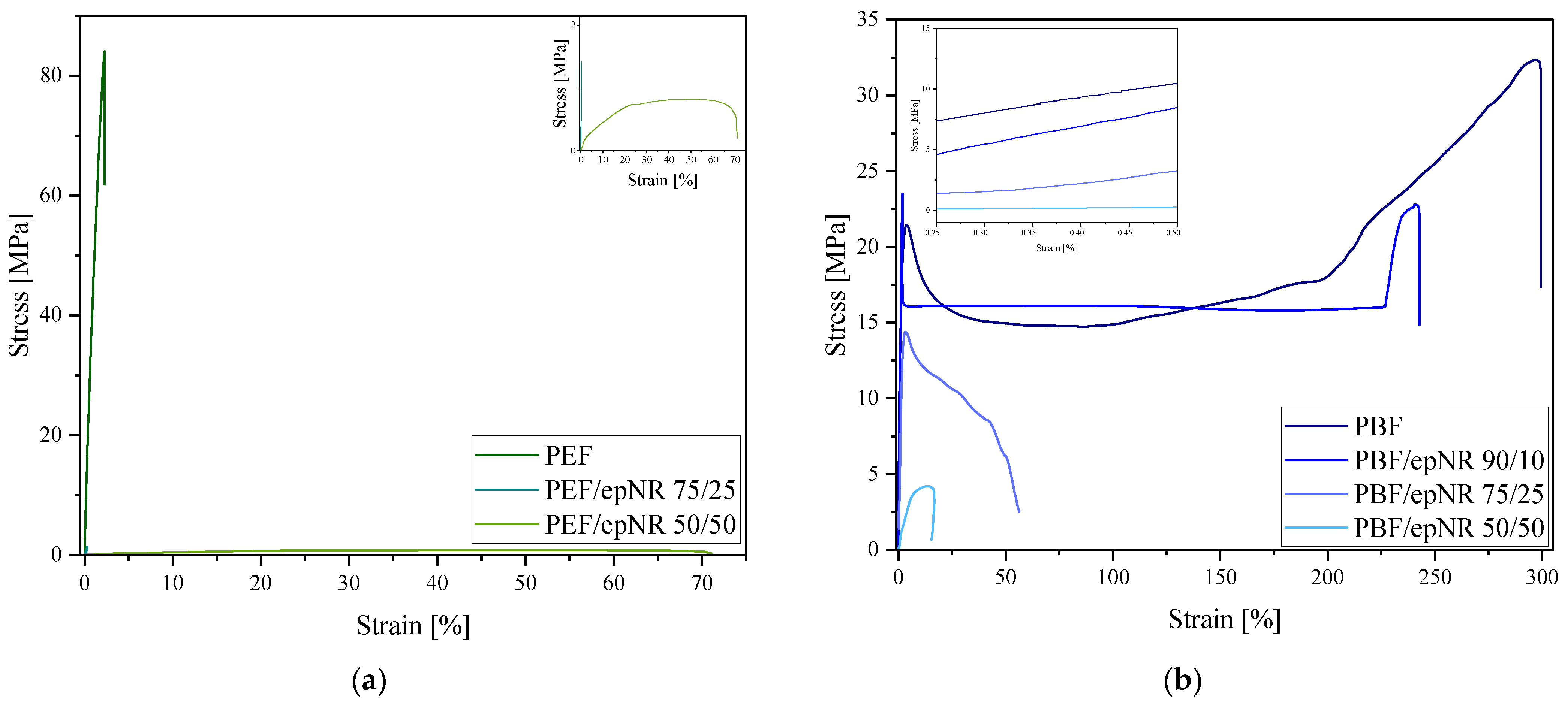
3.3. Physicochemical, Processing, and Mechanical Properties
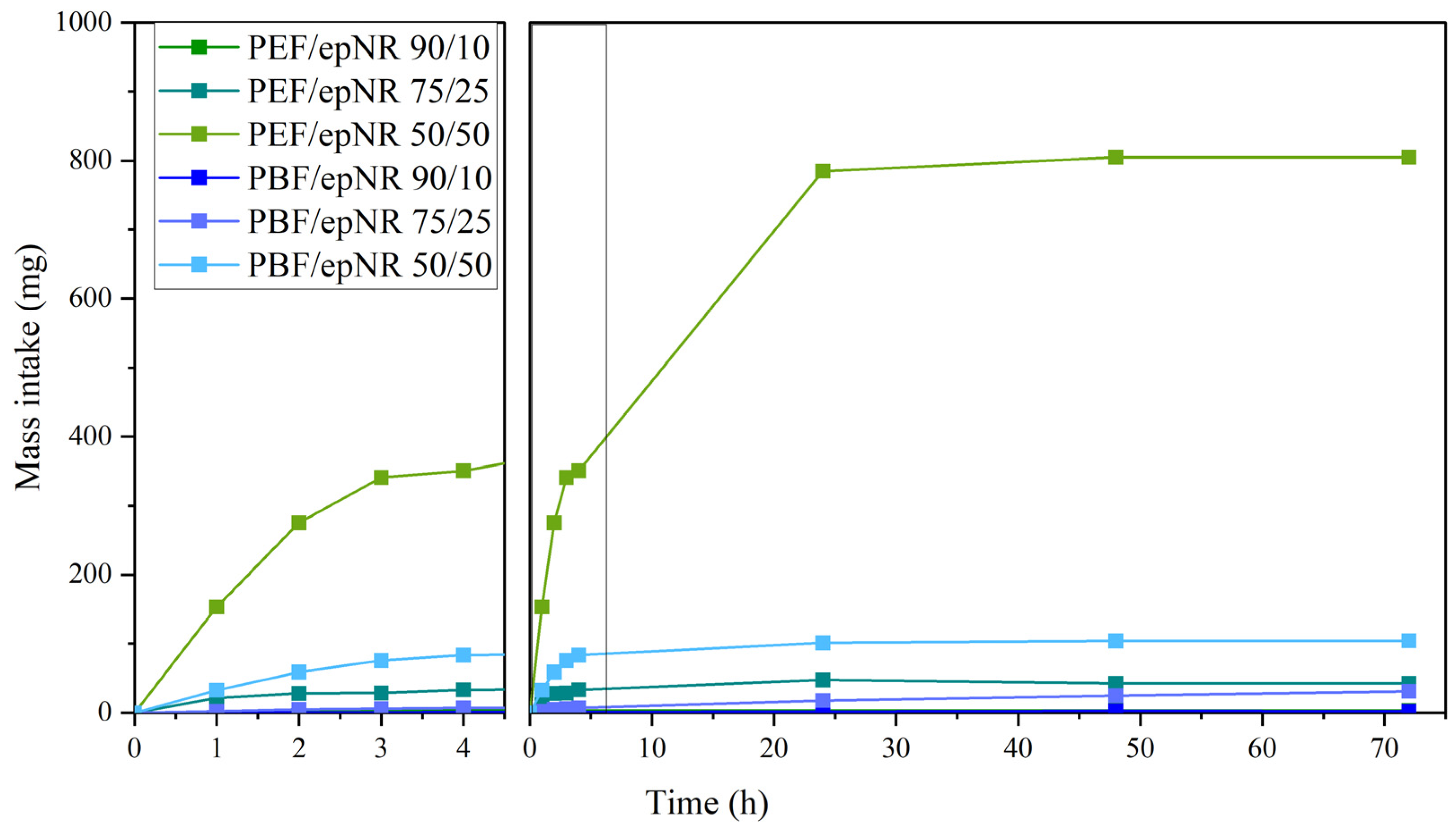
3.4. Mass Intake and Apparent Cross-Link Density
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanders, J.H.; Cunniffe, J.; Carrejo, E.; Burke, C.; Reynolds, A.M.; Chandra Dey, S.; Nazrul Islam, M.; Wagner, O.; Argyropoulos, D. Biobased Polyethylene Furanoate: Production Processes, Sustainability, and Techno-Economics. Adv. Sustain. Syst. 2024, 8, 2400074. [Google Scholar] [CrossRef]
- Sousa, A.F.; Patrício, R.; Terzopoulou, Z.; Bikiaris, D.N.; Stern, T.; Wenger, J.; Loos, K.; Lotti, N.; Siracusa, V.; Szymczyk, A.; et al. Recommendations for Replacing PET on Packaging, Fiber, and Film Materials with Biobased Counterparts. Green Chem. 2021, 23, 8795–8820. [Google Scholar] [CrossRef]
- Gandini, A.; Armando, J.D.; Silvestre, C.P.N.; Souza, A.F.; Gomes, M. The Furan Counterpart of Poly(Ethylene Terephthalate): An Alternative Material Based on Renewable Resources. J. Polym. Sci. Part. A Polym. Chem. 2009, 47, 295–298. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Tsetsou, I.; Ioannidis, R.O.; Nikolaidis, G.N.; Exarhopoulos, S.; Kasmi, N.; Bikiaris, D.N.; Achilias, D.S.; Papageorgiou, G.Z. A New Era in Engineering Plastics: Compatibility and Perspectives of Sustainable Alipharomatic Poly(Ethylene Terephthalate)/Poly(Ethylene 2,5-Furandicarboxylate) Blends. Polymers 2021, 13, 1070. [Google Scholar] [CrossRef]
- Chen, H. Lignocellulose Biorefinery Engineering. Principles and Applications, 1st ed.; Woodhead Publishing: Cambridge, UK, 2015. [Google Scholar]
- Paszkiewicz, S.; Irska, I.; Piesowicz, E. Environmentally Friendly Polymer Blends Based on Post-Consumer Glycol-Modified Poly(Ethylene Terephthalate) (PET-G) Foils and Poly(Ethylene 2,5-Furanoate) (PEF): Preparation and Characterization. Materials 2020, 13, 2673. [Google Scholar] [CrossRef]
- Han, W.; Liao, X. Degradation of Biobased Poly(Ethylene 2,5-furandicarboxylate) and Polyglycolide Acid Blends under Lipase Conditions. J. Appl. Polym. Sci. 2023, 140, e53698. [Google Scholar] [CrossRef]
- Poulopoulou, N.; Smyrnioti, D.; Nikolaidis, G.N.; Tsitsimaka, I.; Christodoulou, E.; Bikiaris, D.N.; Charitopoulou, M.A.; Achilias, D.S.; Kapnisti, M.; Papageorgiou, G.Z. Sustainable Plastics from Biomass: Blends of Polyesters Based on 2,5-Furandicarboxylic Acid. Polymers 2020, 12, 225. [Google Scholar] [CrossRef] [PubMed]
- Poulopoulou, N.; Kantoutsis, G.; Bikiaris, D.N.; Achilias, D.S.; Kapnisti, M.; Papageorgiou, G.Z. Biobased Engineering Thermoplastics: Poly(Butylene 2,5-Furandicarboxylate) Blends. Polymers 2019, 11, 937. [Google Scholar] [CrossRef]
- Poulopoulou, N.; Nikolaidis, G.N.; Efstathiadou, V.L.; Kapnisti, M.; Papageorgiou, G.Z. Blending as a Process for Controlling the Properties of Poly(Ethylene 2,5-Furandicarboxylate) (PEF): Fully Biobased PEF/PBF Blends. Polymer 2023, 266, 125615. [Google Scholar] [CrossRef]
- Long, Y.; Zhang, R.; Huang, J.; Wang, J.; Zhang, J.; Rayand, N.; Hu, G.H.; Yang, J.; Zhu, J. Retroreflection in Binary Bio-Based PLA/PBF Blends. Polymer 2017, 125, 138–143. [Google Scholar] [CrossRef]
- Kanthiya, T.; Rachtanapun, P.; Boonrasri, S.; Kittikorn, T.; Chaiyaso, T.; Worajittiphon, P.; Tanadchangsaeng, N.; Thanakkasaranee, S.; Leksawasdi, N.; Phimolsiripol, Y.; et al. Reinforcement of Epoxidized Natural Rubber with High Antimicrobial Resistance Using Water Hyacinth Fibers and Chlorhexidine Gluconate. Polymers 2024, 16, 3089. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, K.; Xu, C.; Chen, Y. Supertoughened Biobased Poly(Lactic Acid)–Epoxidized Natural Rubber Thermoplastic Vulcanizates: Fabrication, Co-Continuous Phase Structure, Interfacial in Situ Compatibilization, and Toughening Mechanism. J. Phys. Chem. B 2015, 119, 12138–12146. [Google Scholar] [CrossRef]
- Kodsangma, A.; Homsaard, N.; Nadon, S.; Rachtanapun, P.; Leksawasdi, N.; Phimolsiripol, Y.; Insomphun, C.; Seesuriyachan, P.; Chaiyaso, T.; Jantrawut, P.; et al. Effect of Sodium Benzoate and Chlorhexidine Gluconate on a Bio-Thermoplastic Elastomer Made from Thermoplastic Starch-Chitosan Blended with Epoxidized Natural Rubber. Carbohydr. Polym. 2020, 242, 116421. [Google Scholar] [CrossRef]
- Kanthiya, T.; Kiattipornpithak, K.; Thajai, N.; Phimolsiripol, Y.; Rachtanapun, P.; Thanakkasaranee, S.; Leksawasdi, N.; Tanadchangsaeng, N.; Sawangrat, C.; Wattanachai, P.; et al. Modified Poly(Lactic Acid) Epoxy Resin Using Chitosan for Reactive Blending with Epoxidized Natural Rubber: Analysis of Annealing Time. Polymers 2022, 14, 1085. [Google Scholar] [CrossRef]
- Bitinis, N.; Verdejo, R.; Cassagnau, P.; Lopez-Manchado, M.A. Structure and Properties of Polylactide/Natural Rubber Blends. Mater. Chem. Phys. 2011, 129, 823–831. [Google Scholar] [CrossRef]
- Bitinis, N.; Sanz, A.; Nogales, A.; Verdejo, R.; Lopez-Manchado, M.A.; Ezquerra, T.A. Deformation Mechanisms in Polylactic Acid/Natural Rubber/Organoclay Bionanocomposites as Revealed by Synchrotron X-ray Scattering. Soft Matter 2012, 8, 8990–8997. [Google Scholar] [CrossRef]
- Bitinis, N.; Verdejo, R.; Maya, E.M.; Espuche, E.; Cassagnau, P.; Lopez-Manchado, M.A. Physicochemical Properties of Organoclay Filled Polylactic Acid/Natural Rubber Blend Bionanocomposites. Compos. Sci. Technol. 2012, 72, 305–313. [Google Scholar] [CrossRef]
- Juntuek, P.; Ruksakulpiwat, C.; Chumsamrong, P.; Ruksakulpiwat, Y. Effect of Glycidyl Methacrylate-grafted Natural Rubber on Physical Properties of Polylactic Acid and Natural Rubber Blends. J. Appl. Polym. Sci. 2012, 125, 745–754. [Google Scholar] [CrossRef]
- PN-EN 62811:2015; Performance Requirements for AC and/or DC-Supplied Electronic Controlgear for Discharge Lamps (Excluding Fluorescent Lamps)—Operating at Low-Frequency Square-Wave. Polish Committee for Standardization (PKN): Warsaw, Poland, 2015.
- ISO 1133-1:2011; Plastics—Determination of the Melt Mass-Flow Rate (MFR) and Melt Volume-Flow Rate (MVR) of Thermoplastics. Part 1: Standard Method; ISO: Geneva, Switzerland, 2011.
- ISO 527; Plastics—Determination of Tensile Properties—Part 1: General Principles. ISO: Geneva, Switzerland, 2019.
- ISO 48-4:2018; Rubber, Vulcanized or Thermoplastic—Determination of Hardness. Part 4: Indentation Hardness by Durometer Method (Shore Hardness); ISO: Geneva, Switzerland, 2018.
- Utracki, L.A. Polymer Blends and Alloys; Hanser: Munich, Germany, 1989. [Google Scholar]
- Holden, G. Thermoplastic Elastomers. In Rubber Technology; Morton, M., Ed.; Springer: Boston, MA, USA, 1987; Volume 53, ISBN 9788578110796. [Google Scholar]
- Fakirov, S. Handbook of Condensation Thermoplastic Elastomers; John Wiley & Sons: Hoboken, NJ, USA, 2006; pp. 1–619. [Google Scholar] [CrossRef]
- Van Krevelen, D.W. Properties of Polymers, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2009; ISBN 9780444828774. [Google Scholar]
- Hoy, K.L. New Values of the Solubility Parameters from Vapor Pressure Data. J. Paint Technol. 1970, 42, 76–118. [Google Scholar]
- Nolasco, M.M.; Rodrigues, L.C.; Araújo, C.F.; Coimbra, M.M.; Ribeiro-Claro, P.; Vaz, P.D.; Rudić, S.; Silvestre, A.J.D.; Bouyahya, C.; Majdoub, M.; et al. From PEF to PBF: What Difference Does the Longer Alkyl Chain Make a Computational Spectroscopy Study of Poly(Butylene 2,5-Furandicarboxylate). Front. Chem. 2022, 10, 1056286. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, S.; Shen, A.; Zhu, J.; Shen, Z.; Wang, J.; Liu, X. High Molecular Weight Poly(Butylene Terephthalate-co-butylene 2,5-furan Dicarboxylate) Copolyesters: From Synthesis to Thermomechanical and Barrier Properties. J. Appl. Polym. Sci. 2020, 137, 49365. [Google Scholar] [CrossRef]
- Sousa, A.F.; Coelho, J.F.J.; Silvestre, A.J.D. Renewable-Based Poly((Ether)Ester)s from 2,5-Furandicarboxylic Acid. Polymer 2016, 98, 129–135. [Google Scholar] [CrossRef]
- Irska, I.; Paszkiewicz, S.; Gorący, K.; Linares, A.; Ezquerra, T.A.; Jędrzejewski, R.; Rosłaniec, Z.; Piesowicz, E. Poly(Butylene Terephthalate)/Polylactic Acid Based Copolyesters and Blends: Miscibility-Structure-Property Relationship. Express Polym. Lett. 2020, 14, 26–47. [Google Scholar] [CrossRef]
- Khumpaitool, B.; Utara, S.; Jantachum, P. Thermal and Mechanical Properties of an Epoxidized Natural Rubber Composite Con-Taining a Li/Cr Co-Doped NiO-Based Filler. J. Met. Mater. Miner. 2018, 28, 87–94. [Google Scholar]
- Sripornsawat, B.; Thitithammawong, A.; Tulaphol, S.; Johns, J.; Nakaramontri, Y. Positive Synergistic Effects on Vulcanization, Mechanical and Electrical Properties of Using Deep Eutectic Solvent in Natural Rubber Vulcanizates. Polym. Test. 2021, 96, 107071. [Google Scholar] [CrossRef]
- Guo, B.; Cao, Y.; Jia, D.; Qiu, Q. Thermoplastic Elastomers Derived from Scrap Rubber Powder/LLDPE Blend with LLDPE-Graft-(Epoxidized Natural Rubber) Dual Compatibilizer. Macromol. Mater. Eng. 2004, 289, 360–367. [Google Scholar] [CrossRef]
- Parulekar, Y.; Mohanty, A.K. Biodegradable Toughened Polymers from Renewable Resources: Blends of Polyhydroxybutyrate with Epoxidized Natural Rubber and Maleated Polybutadiene. Green Chem. 2006, 8, 206–213. [Google Scholar] [CrossRef]
- Trovatti, E.; Cunha, A.G.; Carvalho, A.J.F.; Gandini, A. Furan-Modified Natural Rubber: A Substrate for Its Reversible Crosslinking and for Clicking It onto Nanocellulose. Int. J. Biol. Macromol. 2017, 95, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Fredi, G.; Dorigato, A.; Dussin, A.; Xanthopoulou, E.; Bikiaris, D.N.; Botta, L.; Fiore, V.; Pegoretti, A. Compatibilization of Polylactide/Poly(Ethylene 2,5-Furanoate) (PLA/PEF) Blends for Sustainable and Bioderived Packaging. Molecules 2022, 27, 6371. [Google Scholar] [CrossRef]
- Ahmed, S.; Cardinaels, R.; Abu-Jdayil, B.; Munam, A.; Iqbal, M.Z. Toughening Brittle Poly(Ethylene Furanoate) with Linear Low-Density Polyethylene via Interface Modulation Using Reactive Compatibilizers. ACS Omega 2025, 10, 5756–5769. [Google Scholar] [CrossRef]
- Zubkiewicz, A.; Paszkiewicz, S.; Szymczyk, A. The Effect of Annealing on Tensile Properties of Injection Molded Biopolyesters Based on 2,5-Furandicarboxylic Acid. Polym. Eng. Sci. 2021, 61, 1536–1545. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Wahbi, M.; Kasmi, N.; Papageorgiou, G.Z.; Bikiaris, D.N. Effect of Additives on the Thermal and Thermo-Oxidative Stability of Poly(Ethylene Furanoate) Biobased Polyester. Thermochim. Acta 2020, 686, 178549. [Google Scholar] [CrossRef]
- Bijarimi, M.; Ahmad, S.; Rasid, R. Mechanical, Thermal and Morphological Properties of Poly(Lactic Acid)/Epoxidized Natural Rubber Blends. J. Elastomers Plast. 2014, 46, 338–354. [Google Scholar] [CrossRef]
- Sousa, A.F.; Matos, M.; Freire, C.S.R.; Silvestre, A.J.D.; Coelho, J.F.J. New Copolyesters Derived from Terephthalic and 2,5-Furandicarboxylic Acids: A Step Forward in the Development of Biobased Polyesters. Polymer 2013, 54, 513–519. [Google Scholar] [CrossRef]
- Dong, L.; Wu, S.; Xu, T.; Dai, J.; Chen, W.; Lu, W. Unraveling the Yellowing Mechanism in Poly(Ethylene Furanoate) Induced by Trace Impurities of 2,5-Furandicarboxylic Acid. ACS Sustain. Chem. Eng. 2025, 13, 5677–5688. [Google Scholar] [CrossRef]
- Zhou, G.; Li, L.; Jiang, M.; Wang, G.; Wang, R.; Wu, G.; Zhou, G. Renewable Poly(Butene 2, 5-Furan Dicarboxylate) Nanocomposites Constructed by TiO2 Nanocubes: Synthesis, Crystallization, and Properties. Polym. Degrad. Stab. 2021, 189, 109591. [Google Scholar] [CrossRef]
- Chebbi, Y.; Kasmi, N.; Majdoub, M.; Papageorgiou, G.Z.; Achilias, D.S.; Bikiaris, D.N. Solid-State Polymerization of Poly(EthyleneFuranoate) Biobased Polyester, III: Extended Study OnEffect of Catalyst Type on Molecular Weight Increase. Polymers 2019, 11, 438. [Google Scholar] [CrossRef]
- DuPont. DuPontTM Hytrel® 6356 Thermoplastic Polyester Elastomer Product Information; DuPont: Wilmington, DE, USA, 2014. [Google Scholar]
- Campus Datasheets Online. CAMPUS® Datasheet Hytrel® 4556-TPC Celanese Product Texts; Celanese Corporation: Irving, TX, USA, 2022. [Google Scholar]
- Paszkiewicz, S.; Kramek, G.; Walkowiak, K.; Irska, I.; Piesowicz, E.; Rzonsowska, M.; Dudziec, B.; Barczewski, M. Thermal and Mechanical Properties of PLA/ENR Thermoplastic Vulcanizates Compatibilized with Tetrafunctional Double-Decker Silsesquioxanes. Polimery 2024, 68, 593–606. [Google Scholar] [CrossRef]


| Stage | Temperature [°C] | Pressure [bar] | Time [s] | |
|---|---|---|---|---|
| Stage I | 205 | 15 | 180 | Series PEF/ epNR |
| Stage II | 205 | 20 | 120 | |
| Stage III | 175 | 20 | 60 | |
| Stage I | 185 | 15 | 180 | Series PBF/ epNR |
| Stage II | 185 | 20 | 120 | |
| Stage III | 155 | 20 | 60 |
| Solubility Parameters | epNR [MPa−1] | PEF [MPa−1] | PBF [MPa−1] |
|---|---|---|---|
| δtot. | 19.54 | 27.33 | 25.49 |
| δp | 5.45 | 17.03 | 15.24 |
| δh | 8.88 | 15.67 | 13.54 |
| δd | 16.53 | 14.21 | 15.30 |
| ΔδepNR/PEF or PBF | 13.63 | 10.91 |
| Sample | Tg1 (°C) | ΔCp1 (J/g°C) | Tg1 (°C) | ΔCp1 (J/g°C) | Tcc (°C) | ΔHcc (J/g) | Tm (°C) | ΔHm (J/g) | Tc (°C) | ΔHc (J/g) | OOT (°C) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| epNR | −20.9 | 0.454 | - | - | - | - | - | - | - | - | 191.7 |
| PEF | - | - | 86.7 | 0.414 | - | - | - | - | - | - | 242.9 |
| PEF/epNR 90/10 | - | - | 82.1 | 0.251 | - | - | 210.2 | 5.14 | - | - | 238.0 |
| PEF/epNR 75/25 | - | - | 82.2 | 0.26 | - | - | 209.7 | 1.68 | - | - | 202.5 |
| PEF/epNR 50/50 | −23.1 | 0.160 | 77.5 | 0.261 | - | - | 207.6 | 2.23 | - | - | 198.6 |
| PBF | - | - | 36.2 | 0.312 | 111.4 | 39.74 | 169 | 33.75 | - | - | 264.8 |
| PBF/epNR 90/10 | - | - | 36 | 0.002 | - | - | 171.2 | 9.29 | 111.6 | 7.32 | 211.9 |
| PBF/epNR 75/25 | −3.2 | 0.096 | 35.5 | 0.053 | - | - | 171 | 34 | 108.3 | 28.91 | 214.4 |
| PBF/epNR 50/50 | −23.1 | 0.160 | 36.6 | 0.123 | 109.5 | 11.17 | 167 | 14.5 | 110.4 | 3.67 | 214.0 |
| Sample | T2% (°C) | T5% (°C) | T10% (°C) | Residue (%) | DTG1 (°C; %/min) | DTG2 (°C; %/min) |
|---|---|---|---|---|---|---|
| epNR | 344.8 | 362.4 | 371.9 | 0.63 | 391.1; −16.19 | - |
| PEF | 348.0 | 369.1 | 380.7 | 8.44 | 394.3; −20.08 | 408.2; −31.58 |
| PEF/epNR 90/10 | 319.9 | 345.7 | 361.6 | 12.05 | 392.1; −21.42 | 439.6; −1.81 |
| PEF/epNR 75/25 | 314.2 | 338.4 | 354.9 | 9.84 | 386.6; −19.73 | 437.8; −3.09 |
| PEF/epNR 50/50 | 311.5 | 330.7 | 346.8 | 4.81 | 380.7; −12.80 | 430.4; −6.12 |
| PBF | 337.9 | 350.3 | 358.4 | 6.2 | 382.4;−23.75 | - |
| PBF/epNR 90/10 | 316.9 | 345.9 | 360.2 | 5.94 | 387.4; −27.93 | 478.9; −0.90 |
| PBF/epNR 75/25 | 314.4 | 338.0 | 369.0 | 5.26 | 389.8; −20.46 | 442.2; −2.39 |
| PBF/epNR 50/50 | 316.5 | 334.0 | 347.9 | 2.46 | 392.6; −12.6 | 429.9; −7.00 |
| Sample | LVN (dL/g) | d (g/cm3) | MFR (g/10 min) | MVR (cm3/10 min) | E (GPa) | σb (MPa) | εb (%) | H (ShD) |
|---|---|---|---|---|---|---|---|---|
| epNR | 0.426 | - | - | - | 5.43 ± 0.03 | 84.07 ± 4.43 | 2.27 ± 0.14 | - |
| PEF | 0.402 | 1.403 | 20.4 a 18.7 b | 22.2 a 49.8 b | - | - | - | 74 ± 1 |
| PEF/epNR 90/10 | 0.439 | 1.380 | 4.2 d | 3.7 d | 0.14 ± 0.01 | 1.36 ± 0.07 | 0.12 ± 0.01 | 70 ± 1 |
| PEF/epNR 75/25 | 0.463 | 1.298 | - | - | 0.01 ± 0.01 | 0.82 ± 0.04 | 71.28 ± 5.47 | 67 ± 1 |
| PEF/epNR 50/50 | 0.606 | 1.171 | - | - | 2.35 ± 0.12 | 50.19 ± 0.21 | 291.55 ± 27.87 | 44 ± 1 |
| PBF | 0.637 | 1.343 | 6.0 a 14.9 b 38.7 c | 6.7 a 16.4 b 35.8 c | 1.62 ± 0.14 | 25.01 ± 0.92 | 274.28 ± 10.18 | 67 ± 1 |
| PBF/epNR 90/10 | 0.803 | 1.274 | 3.4 a 11.1 b | 3.4 a 11.7 b | 0.51 ± 0.01 | 13.36 ± 1.27 | 52.11 ± 3.34 | 64 ± 1 |
| PBF/epNR 75/25 | 0.581 | 1.231 | 0.8 a 3.2 b | 0.6 a 3.3 b | 0.06 ± 0.01 | 4.83 ± 0.34 | 14.98 ± 1.07 | 62 ± 1 |
| PBF/epNR 50/50 | 0.426 | 1.141 | 0.7 b | 0.4 b | 5.43 ± 0.03 | 84.07 ± 4.43 | 2.27 ± 0.14 | 64 ± 1 |
| Sample | Apparent Cross-Link Density |
|---|---|
| PEF/epNR 90/10 | 0.071 |
| PEF/epNR 75/25 | 0.208 |
| PEF/epNR 50/50 | 2.358 |
| PBF/epNR 90/10 | 0.070 |
| PBF/epNR 75/25 | 0.207 |
| PBF/epNR 50/50 | 0.599 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paszkiewicz, S.; Walkowiak, K.; Irska, I.; Śmigielski, J.; Piesowicz, E.; Hejna, A.; Dudziec, B.; Barczewski, M. Ecofriendly PEF- and PBF-Based Blends with Epoxidized Natural Rubber: Unraveling the Structure–Property Relationship. Materials 2025, 18, 4040. https://doi.org/10.3390/ma18174040
Paszkiewicz S, Walkowiak K, Irska I, Śmigielski J, Piesowicz E, Hejna A, Dudziec B, Barczewski M. Ecofriendly PEF- and PBF-Based Blends with Epoxidized Natural Rubber: Unraveling the Structure–Property Relationship. Materials. 2025; 18(17):4040. https://doi.org/10.3390/ma18174040
Chicago/Turabian StylePaszkiewicz, Sandra, Konrad Walkowiak, Izabela Irska, Jakub Śmigielski, Elżbieta Piesowicz, Aleksander Hejna, Beata Dudziec, and Mateusz Barczewski. 2025. "Ecofriendly PEF- and PBF-Based Blends with Epoxidized Natural Rubber: Unraveling the Structure–Property Relationship" Materials 18, no. 17: 4040. https://doi.org/10.3390/ma18174040
APA StylePaszkiewicz, S., Walkowiak, K., Irska, I., Śmigielski, J., Piesowicz, E., Hejna, A., Dudziec, B., & Barczewski, M. (2025). Ecofriendly PEF- and PBF-Based Blends with Epoxidized Natural Rubber: Unraveling the Structure–Property Relationship. Materials, 18(17), 4040. https://doi.org/10.3390/ma18174040











