3.1. Powder Characterization
The ASP60 and CuSn20 alloys (all compositions given in weight percent) were characterized using a combination of analytical techniques, including X-ray fluorescence spectroscopy (XRF), X-ray diffraction (XRD), and differential scanning calorimetry (DSC). The elemental compositions determined by XRF are summarized in
Table 1 for ASP60 and
Table 2 for CuSn20.
In the case of ASP60 high-speed steel, the experimentally determined chemical composition showed a good overall agreement with standard nominal values. However, a noticeable deviation was observed in the concentrations of vanadium (V) and tungsten (W), where the measured contents were 8.250 wt.% and 7.270 wt.%, respectively. These values exceed the typical nominal specification of approximately 6.50 wt.% for both elements. Such enrichment may influence the formation and distribution of secondary carbides, potentially affecting the alloy’s hardness, wear resistance, and response to heat treatment. Relatively high content of Co within the ASP60 might become a problem, as Co acts as a graphitization catalyzer resulting in the chipping and microcracking of the diamond grits, thus increasing the importance of further protective barriers.
The CuSn20 alloy composition remained within the expected compositional range for tin bronzes, with no significant deviations from standard specifications. The collected data serve as the baseline for understanding the subsequent structural and thermal analyses of these alloys.
In contrast, the CuSn20 alloy, which was employed as a porosity-reducing additive in the composite formulation, exhibited only minimal contamination (
Table 2). Trace amounts of silicon (Si) and phosphorus (P) were detected, with a combined concentration not exceeding 0.1 wt.%. Such low impurity levels are unlikely to significantly influence the alloy’s metallurgical behavior or its role in enhancing densification during the compaction process.
X-ray diffraction (XRD) phase analysis (
Figure 1) of the powder confirmed the presence of both α-Fe (ferrite, JCPDS card no. 04-002-1253) and retained γ-Fe (austenite, JCPDS card no. 01-074-5520) in the ASP60 alloy. Quantitative evaluation using the Rietveld refinement method revealed that α-Fe constituted approximately 25 wt.%, while retained γ-Fe accounted for 46 wt.% of the crystalline phases. In addition, vanadium carbide (VC) was identified (JCPDS card no. 04-001-2752), comprising 27 wt.% of the sample, along with a minor amount of cobalt carbide (Co
2C) (JCPDS card no. 03-065-8206).
The CuSn20 powder alloy exhibited a two-phase microstructure consisting of the δ-phase (Cu41Sn11) (JCPDS card no. 03-065-7047) and the ε-phase (Cu3Sn) (JCPDS card no. 04-001-2885). The presence of these intermetallic phases suggests that the original powder may have been produced under high cooling rates, typically achieved by gas atomization methods. These phases are typical in tin bronzes subjected to non-equilibrium solidification, and their coexistence may influence both thermal and mechanical behavior during composite fabrication.
Given that the ASP60 high-speed steel powder was produced via water atomization, it is reasonable to assume that the powder particles possessed oxidized surface layers upon fabrication. During high-temperature compaction, these surface oxides may undergo a thermally activated reduction reaction with carbon inherently present in the steel matrix. Such a reaction could lead to a reduction in oxygen content within the material, potentially enhancing its densification behavior.
To investigate this possibility, differential scanning calorimetry (DSC) analysis was performed on both ASP60 and CuSn20 alloys. For ASP60, a repeated heating cycle was conducted to determine whether any thermally induced reactions were irreversible and associated with oxide reduction.
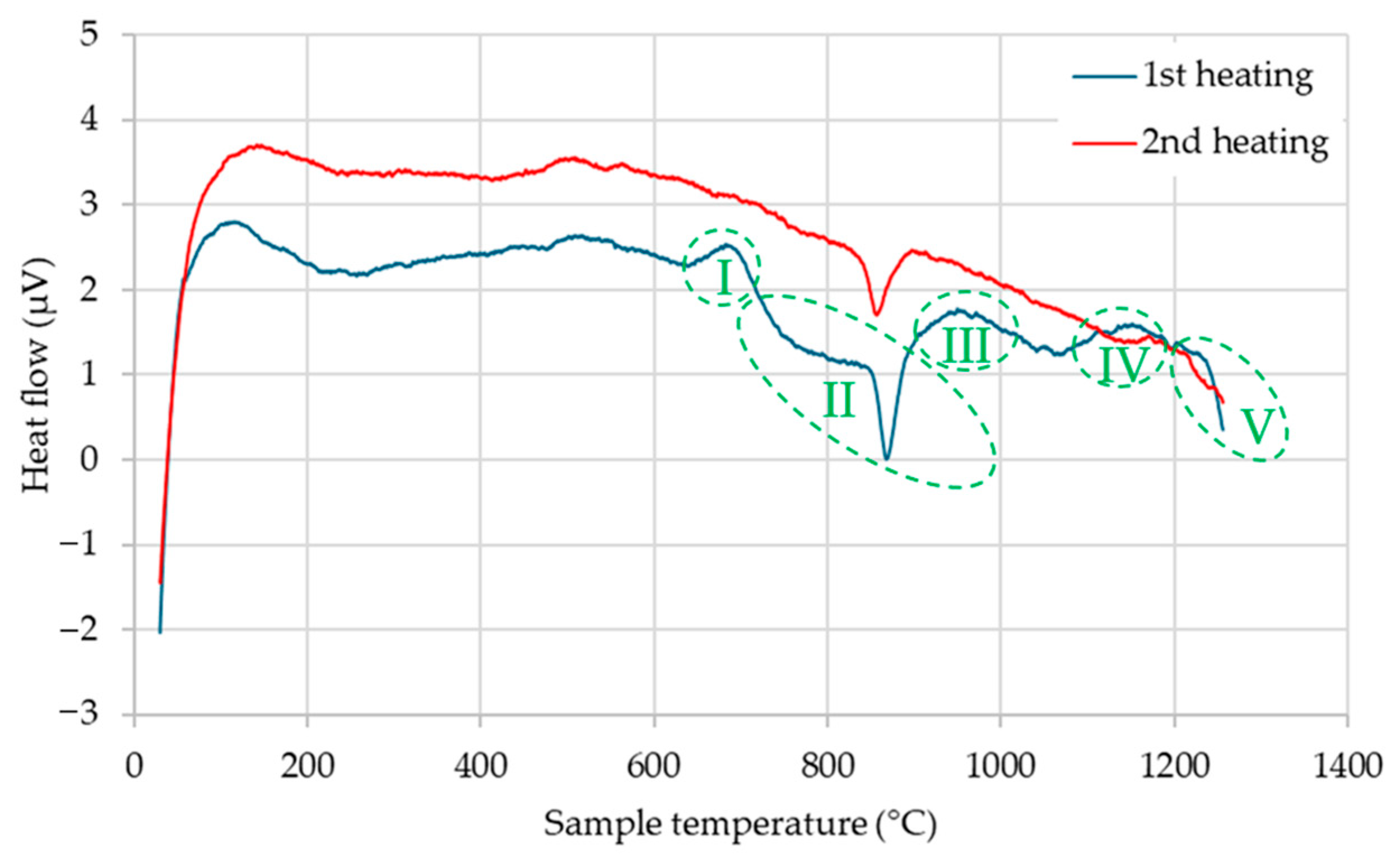
As shown in
Figure 2, the DSC thermogram obtained during the first heating of ASP60 reveals five distinct regions, labeled I–V. Regions I, III, and IV exhibit exothermic peaks initiating at approximately 650 °C, 900 °C, and 1100 °C, respectively. These peaks are indicative of chemical reactions, most likely corresponding to the reduction in various surface oxides by carbon present within the steel matrix. This interpretation is supported by the absence of these exothermic features in the second heating cycle (
Figure 2), confirming the irreversible nature of these events and strengthening the hypothesis that oxide reduction occurs predominantly during the initial thermal exposure. Similar observations reported in [
33] further support the effectiveness of a two-step heating approach in reducing oxide content in the powders, which is beneficial for minimizing diamond degradation via suppressed carbothermal reduction.
Below this temperature range, two additional endothermic regions, designated as II and V, are identified. Region II, starting at approximately 700 °C and extending up to 860 °C, corresponds to the onset of the martensite-to-austenite transformation, reaching its maximum transformation rate around 860 °C. In Region V, at temperatures exceeding 1200 °C, the dissolution of existing carbides begins. These carbides, identified by subsequent XRD analysis as V8C7 and Fe3W3C, are thermally destabilized at this stage, leading to their progressive dissolution into the austenitic matrix.
The observed reduction process suggests a concurrent depletion of carbon, which may cause a slight alteration in the steel’s chemical composition. However, the removal of surface oxides could be beneficial, as it enhances the compactability and sinterability of the alloy by reducing interparticle barriers and promoting stronger metallurgical bonding during sintering.
The contents of carbon, nitrogen, and oxygen in the ASP60 powders were determined by elemental analysis to evaluate their changes after the DSC analysis. As shown in
Table 3, the contents of both carbon and oxygen decreased, with carbon decreasing from 2.21 wt.% to 2.09 wt.% and oxygen decreasing from 0.285 wt.% to 0.120 wt.%. These results confirm the previously mentioned assumption that oxides within the material were reduced by the carbon present in the steel.
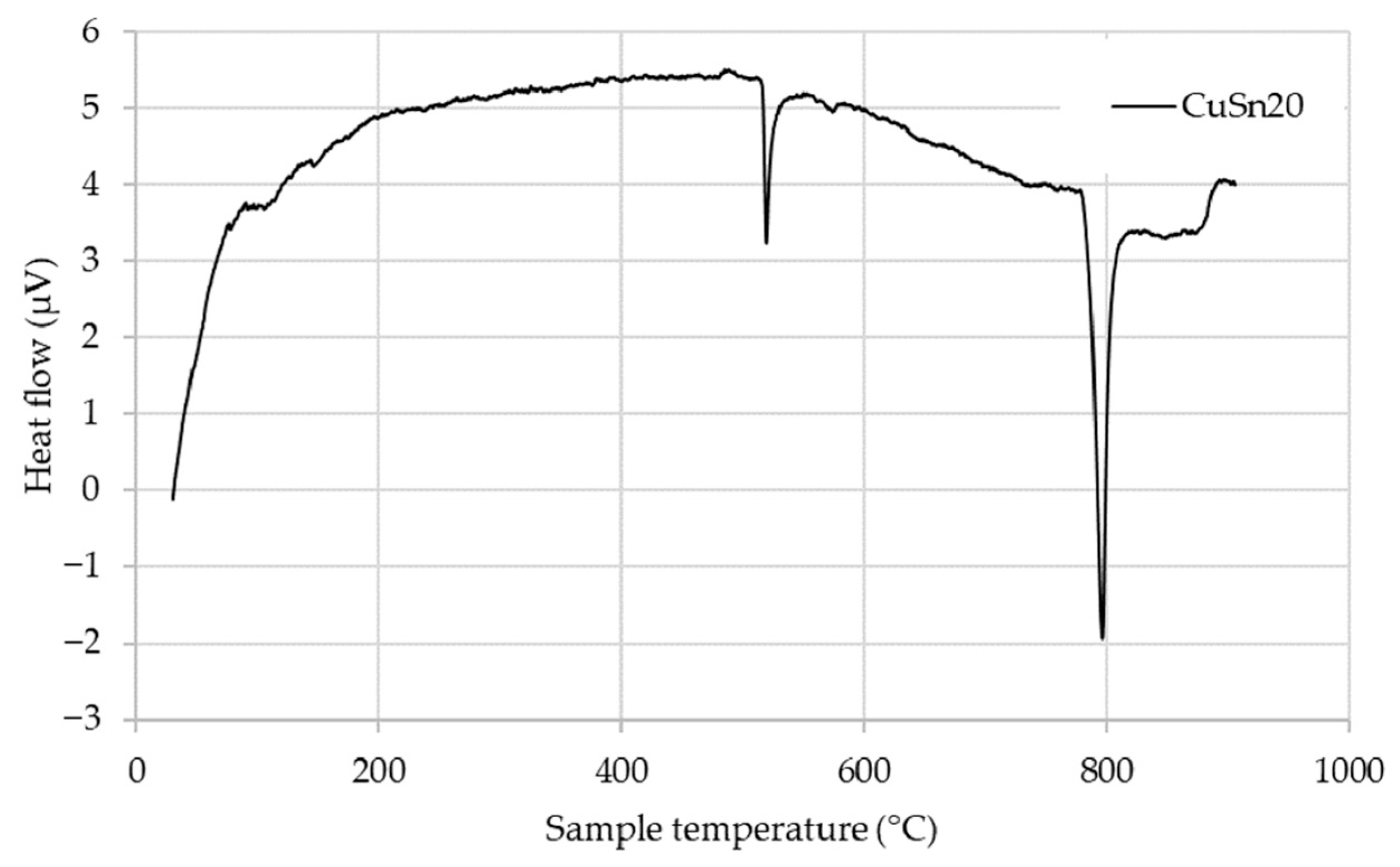
The CuSn20 alloy was also subjected to DSC (
Figure 3), which revealed two prominent endothermic peaks associated with thermal phase transformations and melting behavior. The thermal response is consistent with the metastable nature of the CuSn20 alloy, which likely originates from its specific powder production method. Upon heating, the alloy begins to transform toward a more thermodynamically stable phase equilibrium.
Initially, within the temperature range up to approximately 500 °C, the metastable structure gradually evolves into a mixture of α + δ and subsequently α + ε phases. A sharp endothermic peak is observed near this temperature, marking a critical transformation. The first major transformation, beginning slightly above 525 °C, corresponds to the α + δ → α + γ phase transition.
With continued heating, the system undergoes an additional reaction, transitioning from α + γ → α + β, followed by the onset of partial melting, characterized by the reaction α + β → α + L. This partial melting process is responsible for the second significant endothermic peak, occurring at approximately 778 °C, which aligns closely with the reported peritectic temperature for CuSn20 alloys.
Upon further heating above 930 °C, complete melting of the alloy occurs, in agreement with previously published data [
34,
35]. These observations are consistent with the findings in [
36], reporting two distinct endothermic peaks during DSC analysis of Cu–Sn-based alloys, confirming the reproducibility of this thermal behavior.
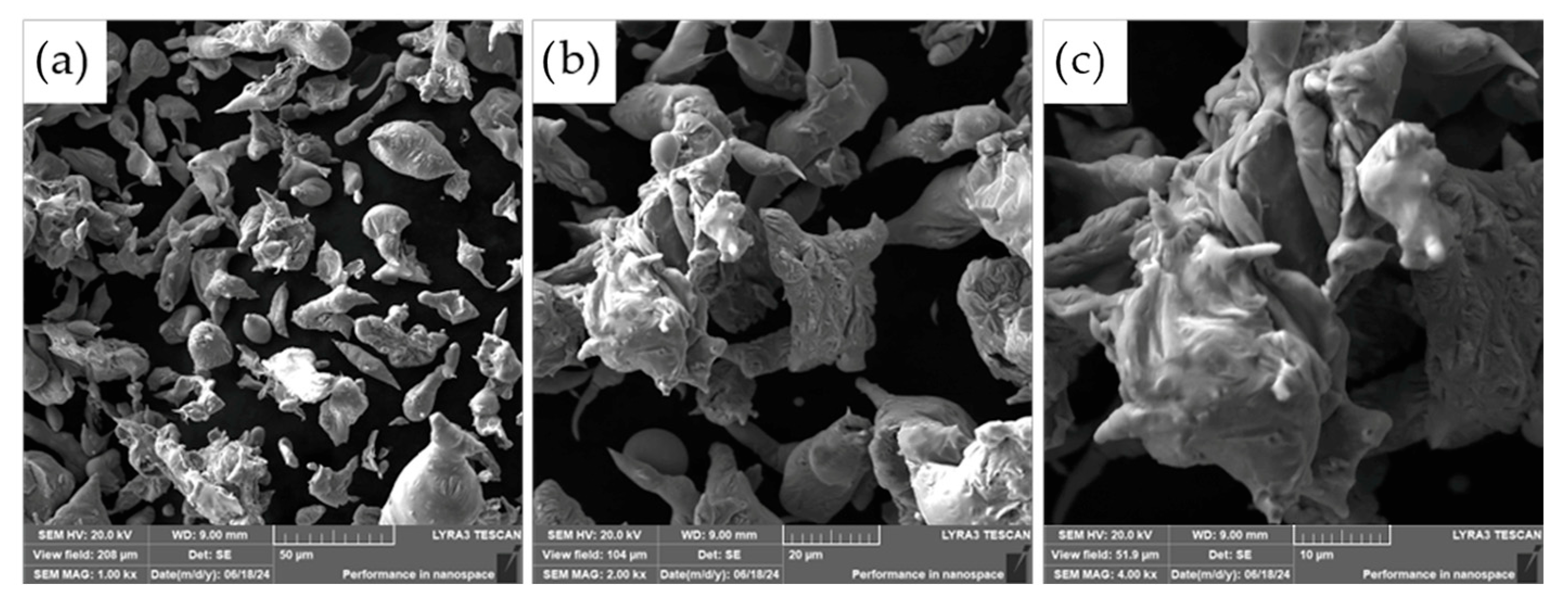
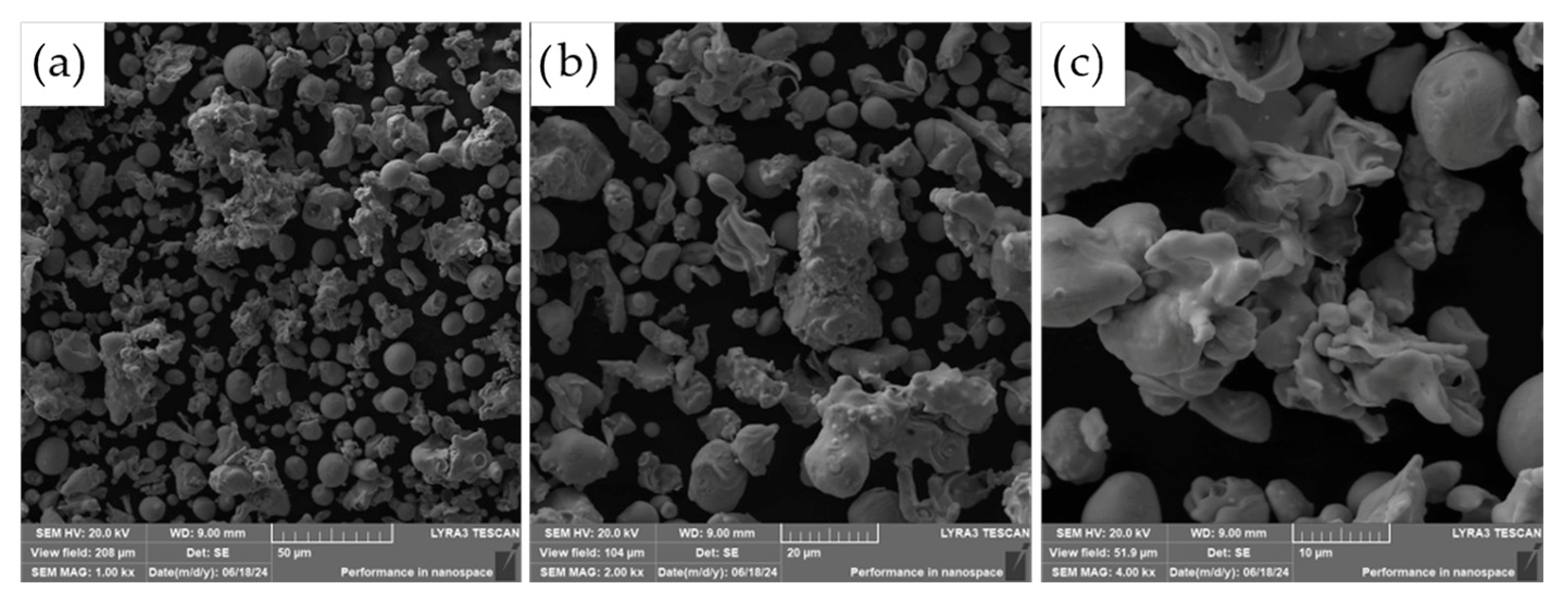
The morphology of the powder particles was further examined using scanning electron microscopy (SEM), as presented in
Figure 4. The observed microstructure of the ASP60 powders is consistent with the characteristics typically associated with the water atomization process. The particles exhibit predominantly irregular and rounded shapes, with a portion of larger particles displaying significant flattening. This deformation is likely a result of interactions between molten droplets and high-velocity water jets, as well as collisions with the walls of the atomization chamber during solidification.
The resulting morphology is representative of powders produced by water atomization and shares several similarities with powders fabricated via alternative atomization methods, such as gas atomization or centrifugal atomization. However, due to the aqueous environment employed in water atomization, some degree of surface oxidation is anticipated.
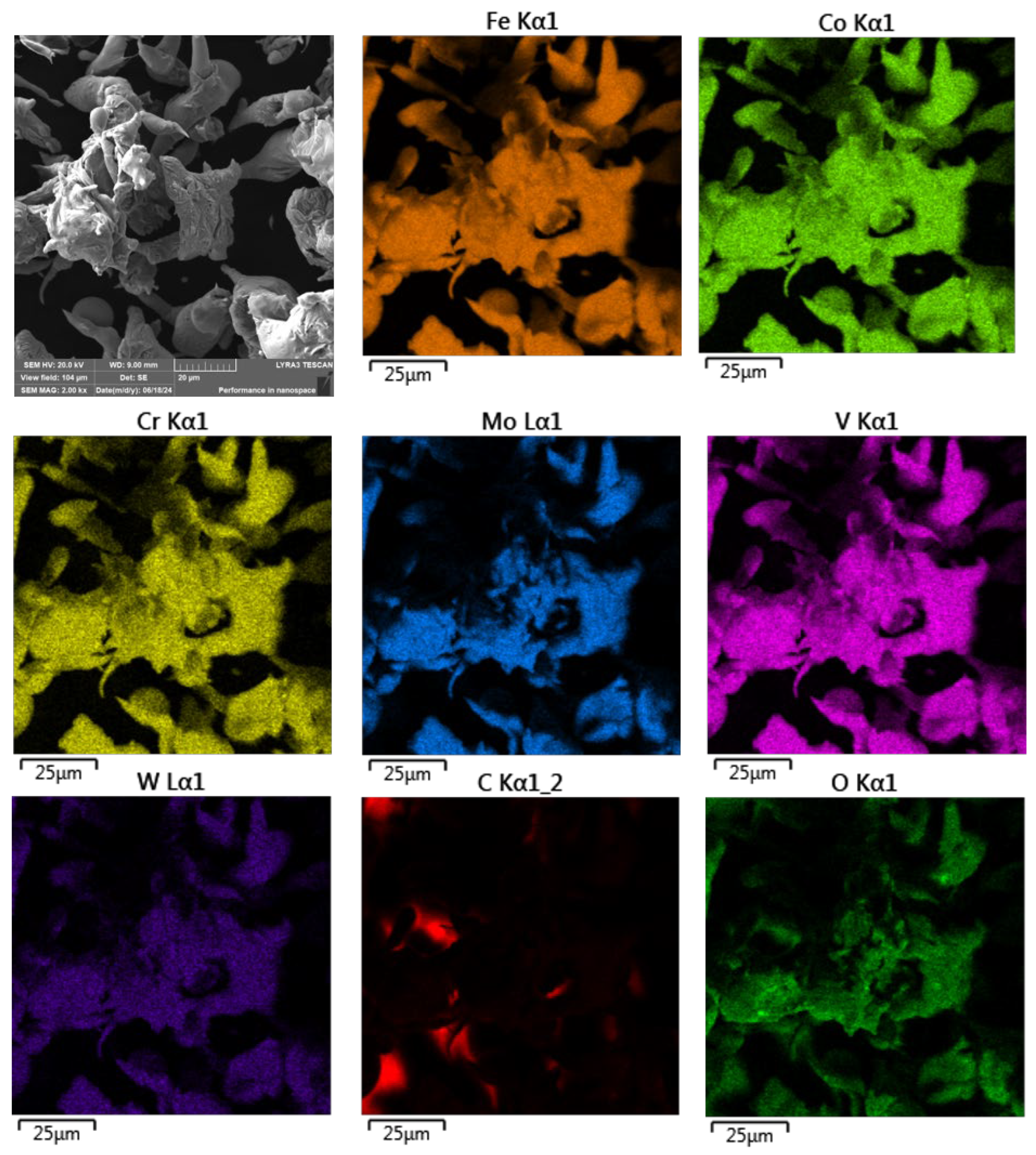
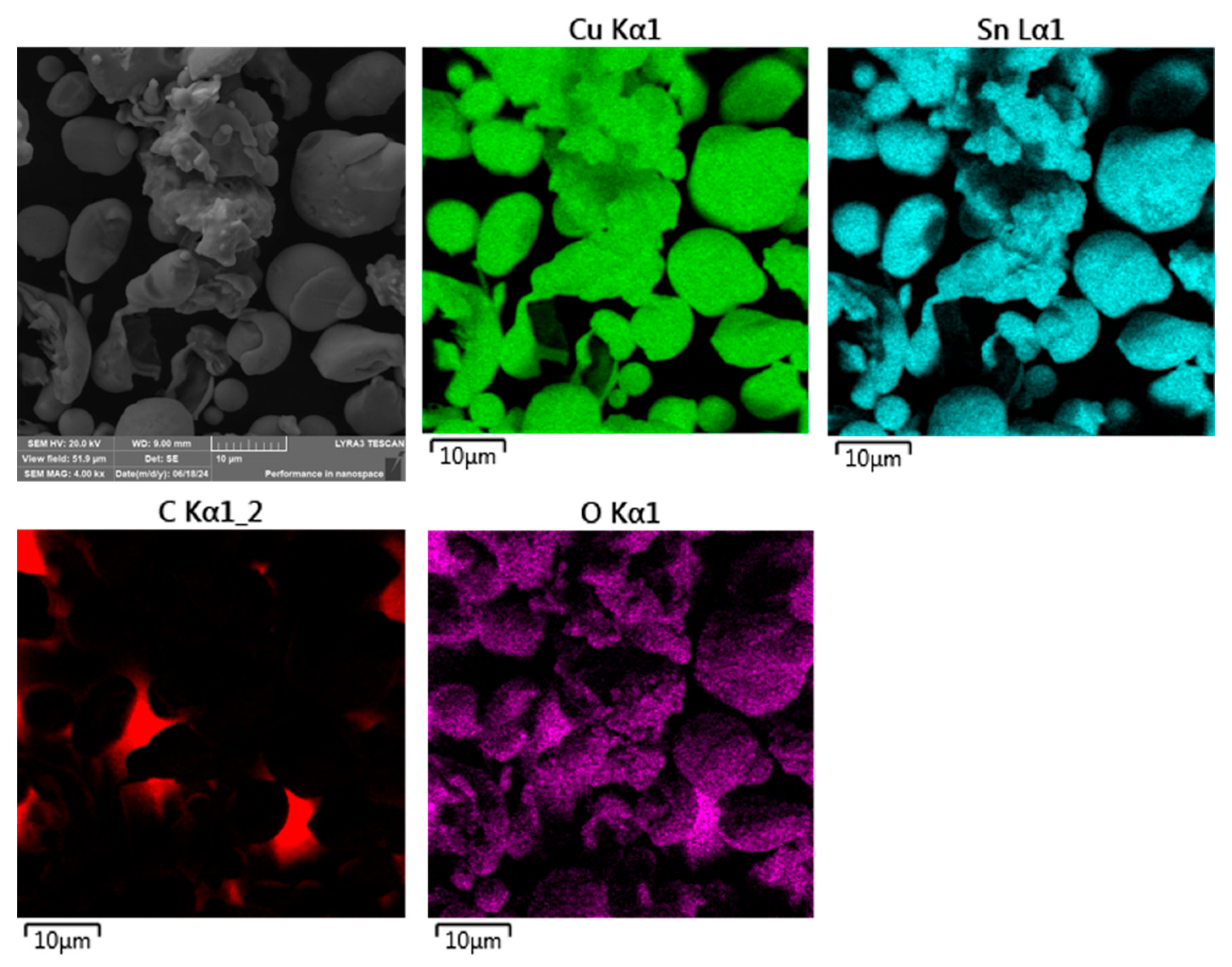
To investigate this, SEM analysis coupled with energy-dispersive X-ray spectroscopy (EDS) elemental mapping was employed to assess the distribution of oxidized elements on the particle surfaces. The resulting maps, shown in
Figure 5, provide qualitative evidence of oxide formation, particularly at the surface regions of the powder particles.
As illustrated in
Figure 5, the ASP60 powder particles exhibited overall good elemental homogeneity with respect to both their primary and alloying constituents. However, surface oxidation was clearly evident on several particles, in agreement with prior assumptions based on the water atomization process.
Although the oxidation appeared generally uniform across most particle surfaces, certain particles exhibited highly localized regions with significantly elevated oxygen concentrations relative to their surroundings. These localized oxidized zones provide further evidence of surface-level oxidation, likely resulting from the rapid solidification and exposure to the aqueous environment during atomization. The presence of such oxidation may influence the powder’s reactivity and sinterability in subsequent high-temperature processing steps.
A similar investigation was conducted on the CuSn20 alloy, which served as both a binder and a porosity-reducing agent in the compaction process. Due to its relatively low melting point and favorable flow characteristics, CuSn20 facilitates the rearrangement of ASP60 high-speed steel particles and aids in filling interstitial voids during hot pressing, thereby enhancing densification.
The morphology of the CuSn20 powder particles is presented in
Figure 6. Compared to the ASP60 powder, the CuSn20 particles are significantly smaller in size and exhibit a predominantly spherical to near-spherical shape. This morphology is indicative of a gas atomization production route, which is commonly used to produce powders with high flowability, narrow particle size distributions, and minimal surface oxidation. The observed characteristics are consistent with the intended role of CuSn20 in promoting uniform particle packing and improved sinterability due to the presence of a liquid phase within the composite system.
The CuSn20 powder alloy was further examined for elemental distribution using scanning electron microscopy (SEM) coupled with energy-dispersive X-ray spectroscopy (EDS), as shown in
Figure 7. The analysis confirmed a generally homogeneous distribution of the primary elements—copper (Cu) and tin (Sn)—throughout the powder particles. However, localized regions exhibiting elevated oxygen concentrations were also identified on the particle surfaces.
Such surface oxidation is characteristic of fine powders produced by gas atomization, where the high surface-area-to-volume ratio of the particles, combined with residual oxygen in the atomization environment, promotes the formation of surface oxides. These oxidized zones, although limited in extent, may influence the sintering behavior and interfacial bonding characteristics during composite consolidation.
The rheological properties of the ASP60 and CuSn20 powders were evaluated through measurements of their apparent (
Table 4) and tap densities (
Table 5), as well as particle size distribution. Apparent density measurements revealed relatively low bulk packing densities for both powders, with values of 1.270 ± 0.052 g/cm
3 for ASP60 and 3.183 ± 0.021 g/cm
3 for CuSn20. These low values reflect the initial loose packing state of the powders when poured without external compaction.
Subsequent tap density measurements, which involve mechanical vibration or tapping to promote particle settling and reduce interparticle voids, demonstrated a significant increase in packing efficiency. The tap density values increased to 1.949 ± 0.110 g/cm3 for ASP60 and 4.225 ± 0.510 g/cm3 for CuSn20. This increase reflects the improved particle rearrangement and packing stability under external agitation. Notably, the higher densities observed for CuSn20 in both apparent and tap conditions are consistent with its smaller particle size and spherical morphology, which favor more efficient packing compared to the larger, irregular particles of ASP60.
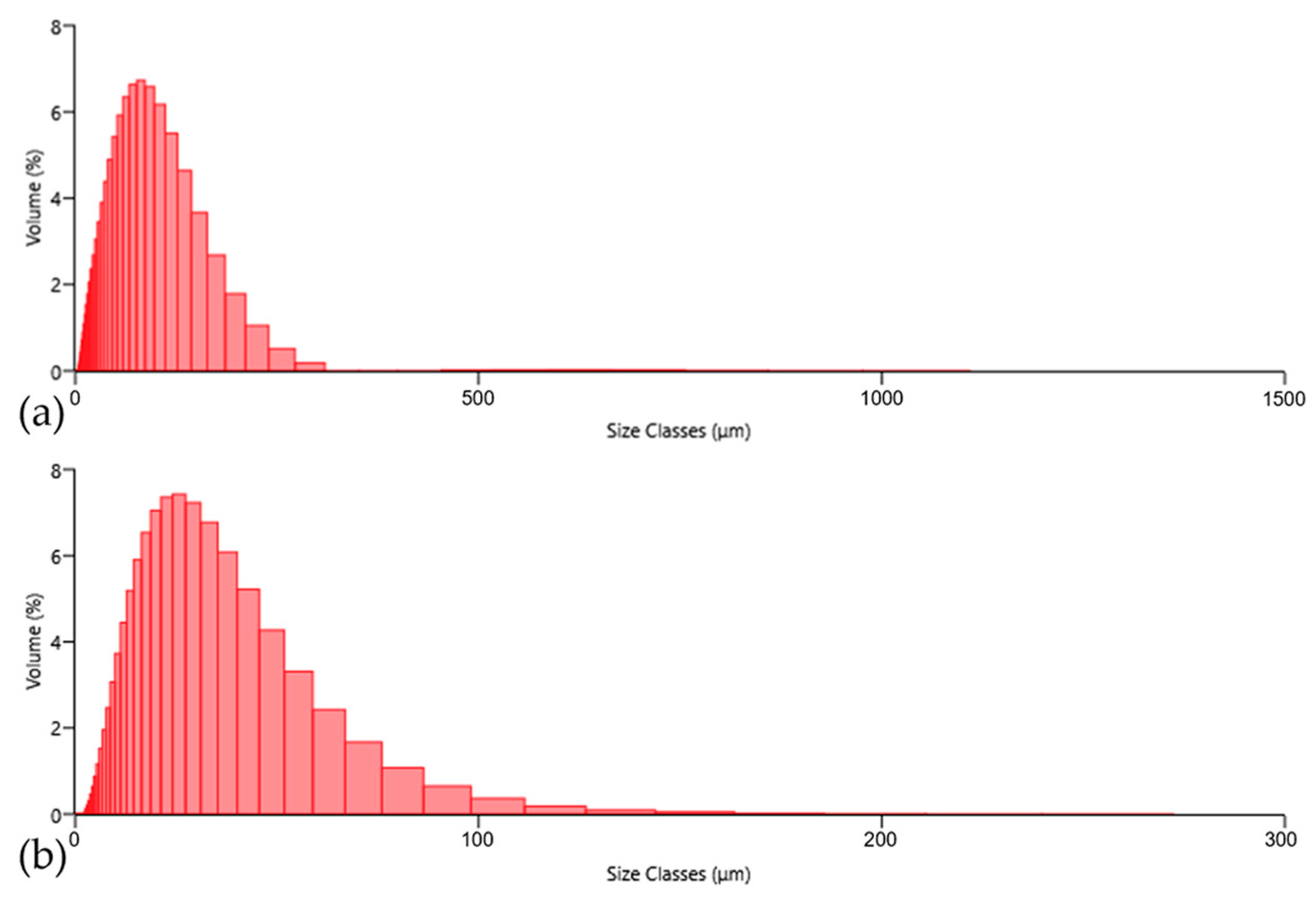
The particle size distributions of the ASP60 and CuSn20 powders were determined using laser diffraction analysis (Malvern Mastersizer 3000+), showing the histograms of the powder particle distributions in
Figure 8. The ASP60 powder exhibited particle sizes ranging from 2.8 to 309.5 μm, with a median diameter (D
50) of 62.0 μm, indicating a relatively broad particle size distribution. In contrast, the CuSn20 powder consisted of significantly finer particles, with sizes ranging from 1.4 to 211.1 μm and a D
50 value of 22.8 μm.
Based on these distributions, the specific surface areas of the powders were calculated, yielding 160.6 m2/kg for ASP60 and 352.7 m2/kg for CuSn20. The markedly higher surface area of the CuSn20 powder reflects its finer particle size and narrower distribution, which can influence its sintering behavior due to a more homogeneous distribution prior to its melting during the hot-pressing compaction.
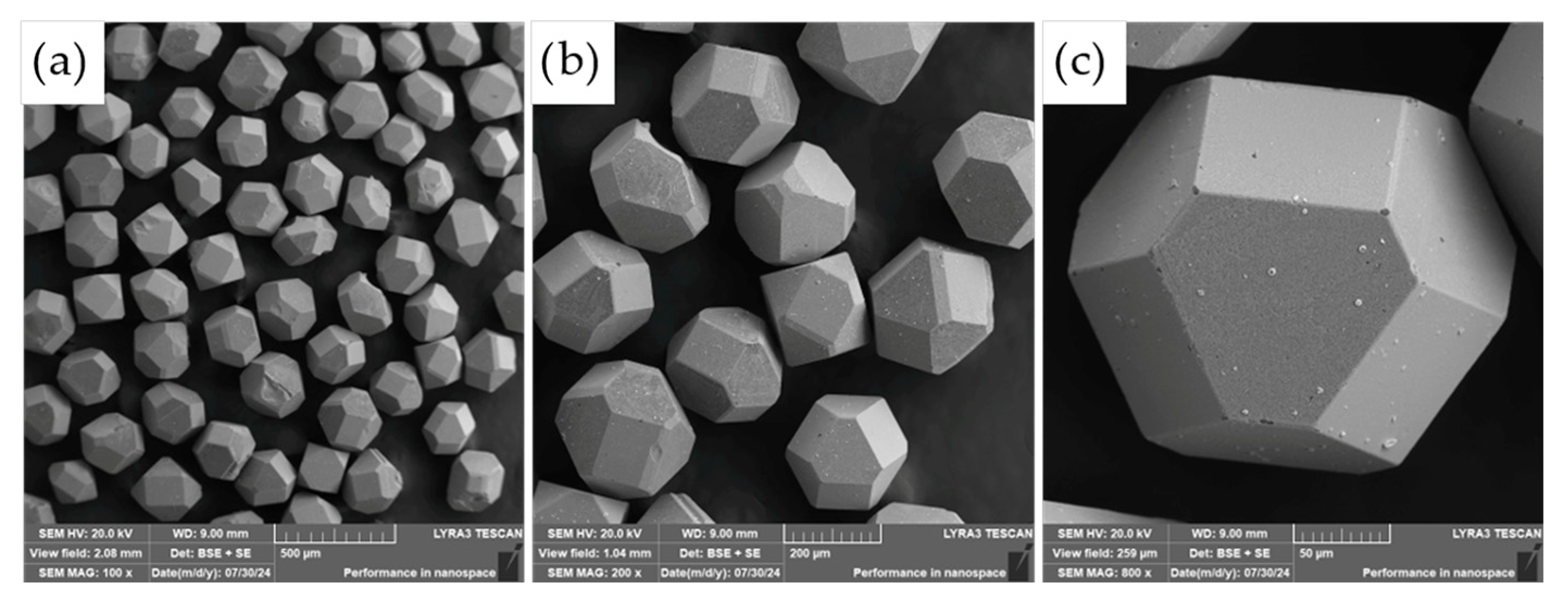
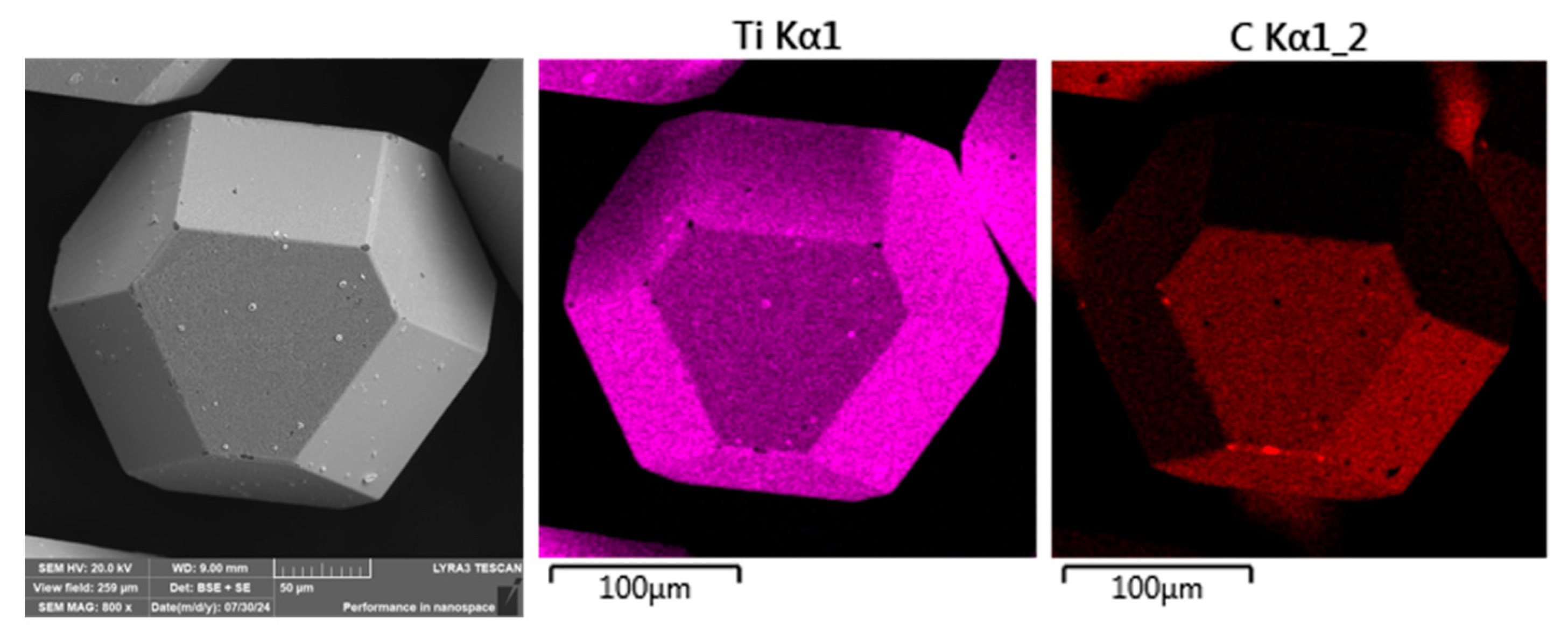
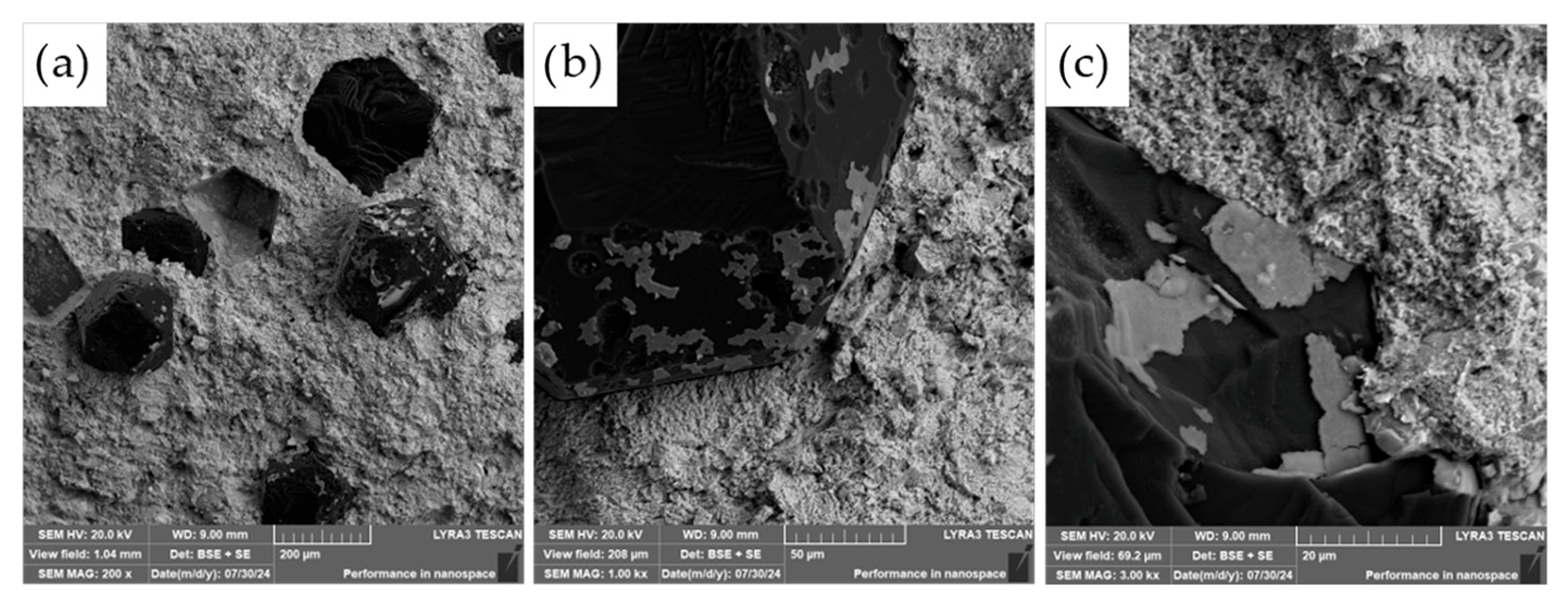
The commercially supplied diamond particles (Hyperion, 60/80 mesh), pre-coated with a titanium carbide (TiC) protective layer, were analyzed in detail using scanning electron microscopy (SEM) combined with energy-dispersive X-ray spectroscopy (EDS). As illustrated in
Figure 9, the diamonds exhibit the characteristic angular morphology typical of abrasive and cutting tool applications. The TiC coating serves a critical function as a thermal and chemical barrier, designed to suppress graphitization and chemical degradation of the diamond core during high-temperature compaction processes.
While the majority of the particles displayed a continuous and uniform TiC layer, minor flaws in the coating were observed on a subset of particles, particularly along sharp edges and corners (see
Figure 9c). These localized defects may act as vulnerable sites for chemical interaction, mechanical damage, or thermal degradation during sintering, potentially compromising the long-term stability and performance of the composite. Such observations highlight the importance of coating integrity in high-temperature applications where strong matrix–diamond interactions occur.
Detailed SEM–EDS elemental distribution mapping (
Figure 10) confirmed the presence of defects in the TiC protective coatings, as previously described. These flaws were observed not only along the sharp edges and corners of the diamond particles but also across some flat surface areas. The observed damage is most likely attributable to the batch-wise handling of the TiC-coated diamonds during storage, transport, or processing. Given the extreme hardness of diamond, individual particles may act as abrasive agents or impact sources, mechanically compromising the integrity of the relatively softer TiC coating.
Such mechanical interactions can result in localized chipping, cracking, or delamination of the TiC layer, particularly at geometrically exposed regions where the coating is more susceptible to stress concentrations. These defects may serve as entry points for diffusion or chemical reactions during high-temperature compaction, potentially undermining the protective function of the TiC barrier and reducing the overall thermal stability of the diamond reinforcements within the composite.
3.2. Optimization of Hot Pressing
To evaluate the influence of compaction temperature on residual porosity, uniaxial hot pressing was conducted at three different temperatures: 950 °C, 1000 °C, and 1050 °C. Powder batches of 30 g were loaded into a graphite mold and hot-pressed under a constant pressure of 35 MPa for a dwell time of 3 min at the specified temperatures. This experimental setup was designed to simulate typical conditions for the consolidation of powder-based composites.
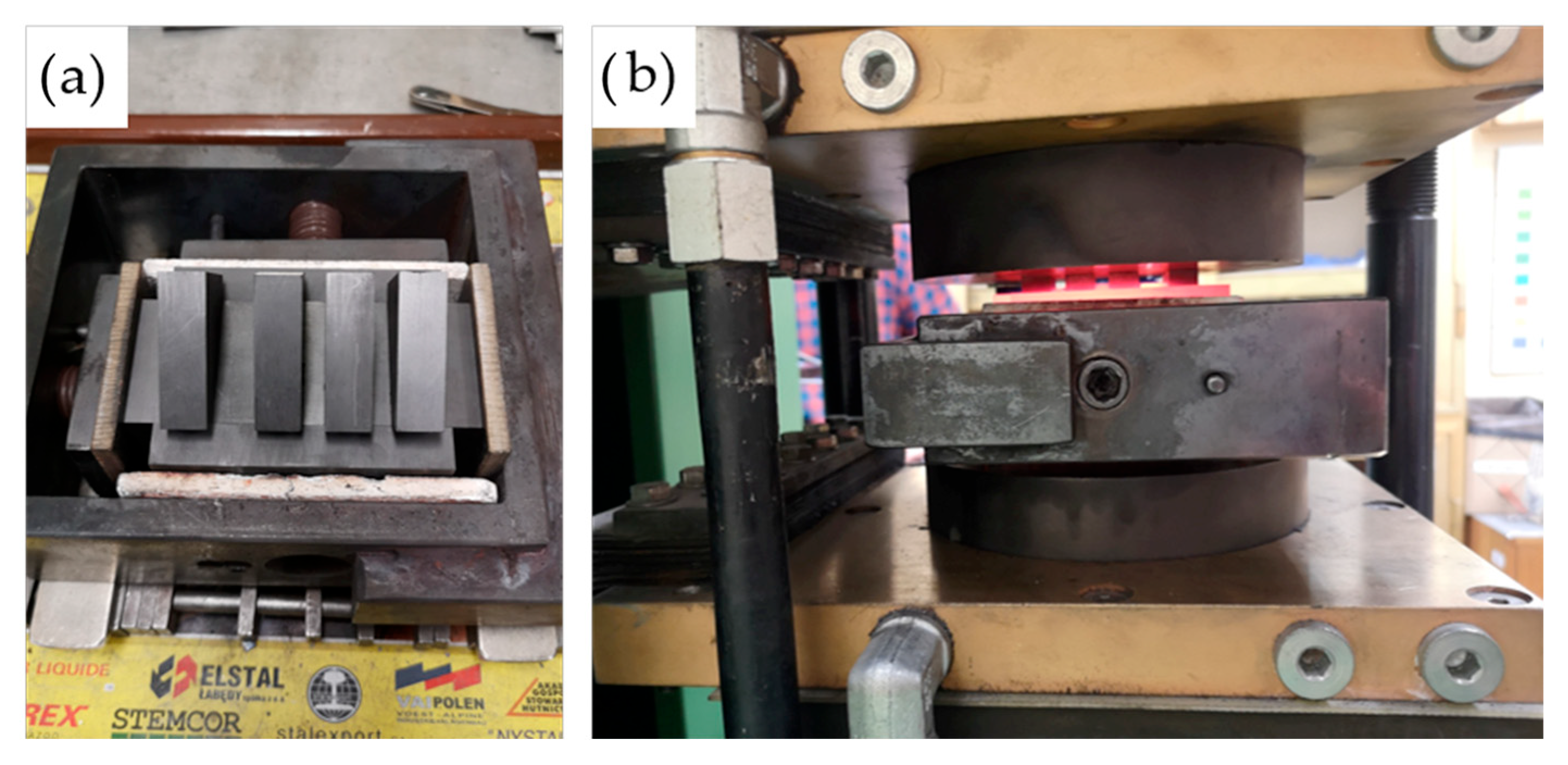
The complete mold assembly, hot-pressing apparatus, and compaction process are depicted in
Figure 11, illustrating the alignment of the tooling and the thermal–mechanical environment in which densification was achieved. These conditions were selected to assess the effect of increasing compaction temperature on pore elimination, densification behavior, and potential phase evolution during processing.
Following compaction, the prism-shaped specimens were ground using a diamond grinding disc to ensure uniform surface finish and dimensional accuracy. Residual porosity was then determined using two complementary methods: dimensional analysis and the Archimedes method, with the results summarized in
Table 6. For the Archimedes measurements, the open porosity was sealed by immersing the samples in molten paraffin wax prior to submersion in water, ensuring a more accurate assessment of closed porosity.
Based on the porosity results obtained from specimens made of pure ASP60 powder, additional mixtures containing ASP60 and CuSn20 were prepared and hot-pressed to evaluate the effect of the alloying additive on densification. As shown in
Table 6, the incorporation of CuSn20 led to a notable reduction in residual porosity. This improvement is primarily attributed to the partial melting of the CuSn20 alloy during high-temperature compaction, which facilitates the rearrangement of ASP60 particles and promotes densification by filling interparticle voids. The molten phase acts as a transient liquid binder, enhancing packing efficiency and bonding within the composite structure.
The mechanical performance of the samples was evaluated using three-point bending tests, with the results summarized in
Table 7. The highest bending strength—374.51 ± 36.73 MPa—was achieved by the ASP60 + CuSn20 composite hot-pressed at 1050 °C, which also exhibited the lowest residual porosity among all tested compositions. The porosity of this sample was measured at 3.70% by the Archimedes method and 3.69% by dimensional analysis, indicating excellent agreement between the two techniques and highlighting the effectiveness of the densification process at this temperature.
In general, compaction at 1000 °C or higher was found to significantly enhance mechanical properties, regardless of the presence of CuSn20. However, the addition of CuSn20 further amplified these improvements by reducing residual porosity and promoting more efficient particle rearrangement and bonding during hot pressing.
It is important to note that substantial variation in apparent surface porosity was observed among the tested samples. This heterogeneity is attributed to the uniaxial compaction process, where frictional resistance—both between powder particles and between particles and the mold walls—impedes uniform pressure transmission. This results in non-uniform densification, particularly at the sample surfaces.
To account for this effect in mechanical testing, sample orientation was determined via optical microscopy. The more porous surface was deliberately positioned on the tensile side of the bending test setup, representing the most critical area for crack initiation. Consequently, the bending strength values reported in
Table 7 should be regarded as conservative lower-bound estimates, strongly influenced by residual porosity.
It is anticipated that substantially higher mechanical performance could be achieved through the use of advanced consolidation techniques, such as hot isostatic pressing (HIP), which can produce near-full-density materials with minimal porosity and enhanced structural integrity.
Based on the obtained results, Vickers hardness (HV30) and Rockwell hardness (HRC) measurements were conducted on both the tensile and compressive sides of the specimens sintered at 1000 °C and 1050 °C. These specific temperatures were selected due to the pronounced differences observed in surface porosity, which warranted further investigation into the localized mechanical response. The hardness data are summarized in
Table 8 (Vickers) and
Table 9 (Rockwell). Compared to other work [
37], the hereby reported samples were showing at least three times higher Vickers hardnesses compared to their low-alloy steel that has been hot-pressed at significantly lower temperatures within a range of 820–950 °C.
The tensile side—corresponding to the surface opposite the upper movable piston during uniaxial compaction—consistently exhibited hardness values with higher relative standard deviations (RSDs) and wider 95% confidence intervals compared to the compressive side. This variability reflects the non-uniform porosity distribution within the specimens, which is significantly influenced by the position within the sample.
Such heterogeneity is a known limitation of uniaxial compaction, where friction between powder particles and the die wall results in uneven force transmission, leading to density gradients across the green body. These gradients persist through sintering and directly impact the mechanical properties at different regions of the compact. Moreover, the observed uneven pressure distribution would further affect the lifespan of diamonds due to the formation of tensile stresses on their surfaces in areas where a pore is present, leading to pressure drops and enhancing the localized graphitization of the diamond surface.
To overcome the limitations associated with uniaxial compaction—particularly the friction-induced anisotropy and resulting inhomogeneous densification—hot isostatic pressing (HIP) presents a highly effective alternative. Unlike uniaxial pressing, HIP applies uniform isostatic pressure in all directions, eliminating density gradients and promoting homogeneous microstructural development throughout the compact. As a result, HIP enables the production of near-full-density materials with minimal residual porosity and consistent mechanical properties across the entire volume. Given these advantages, it is anticipated that the application of HIP would lead to substantially enhanced mechanical performance, making it a superior consolidation technique for structural components requiring high reliability and integrity.
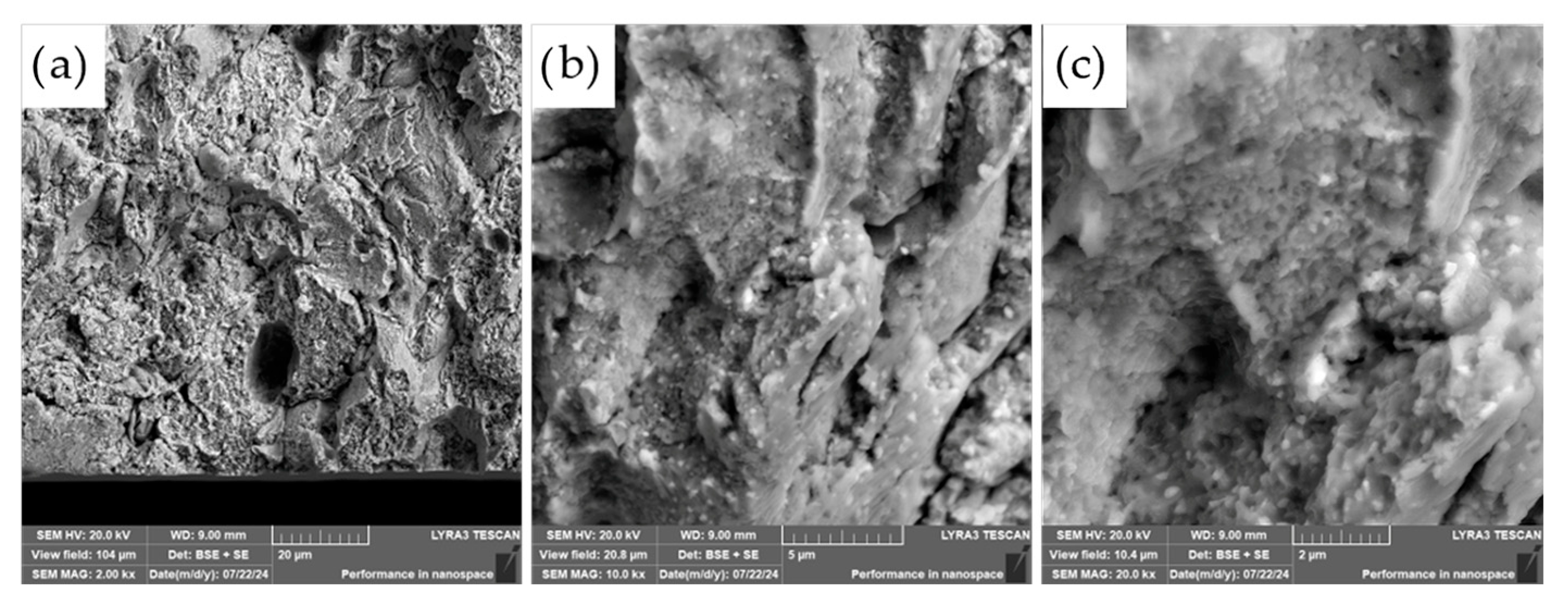
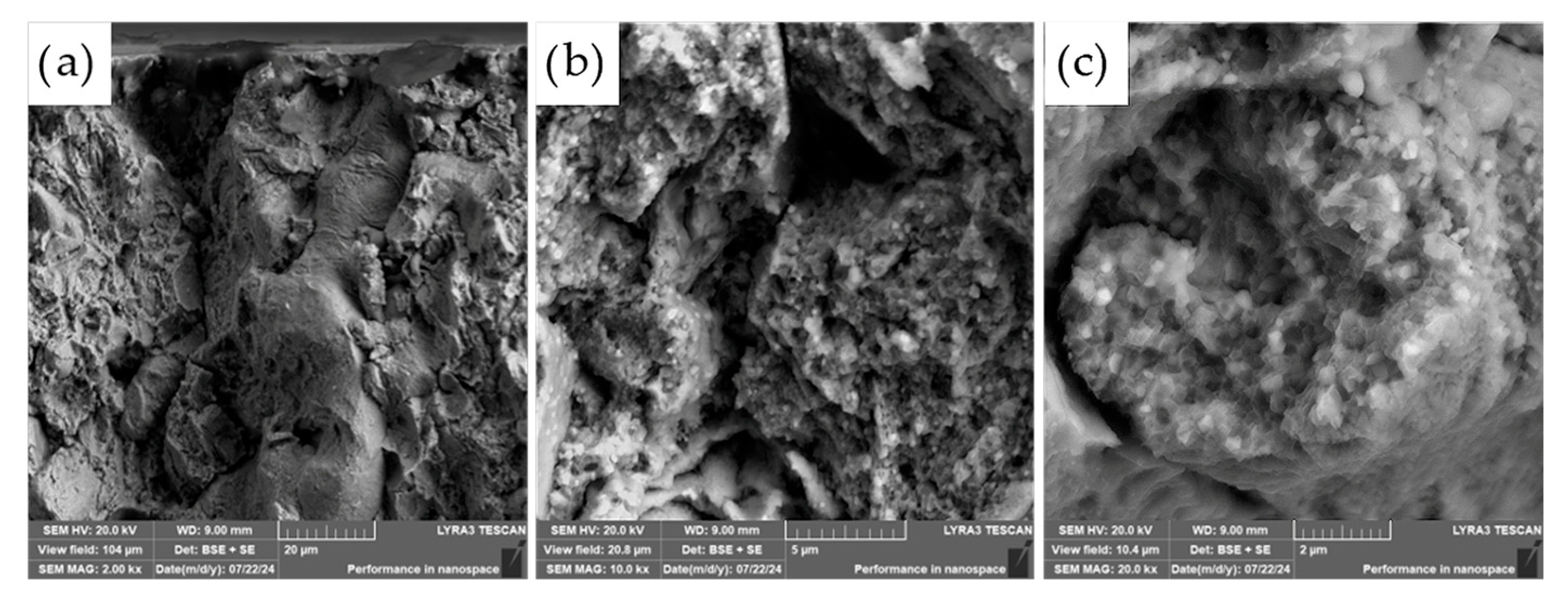
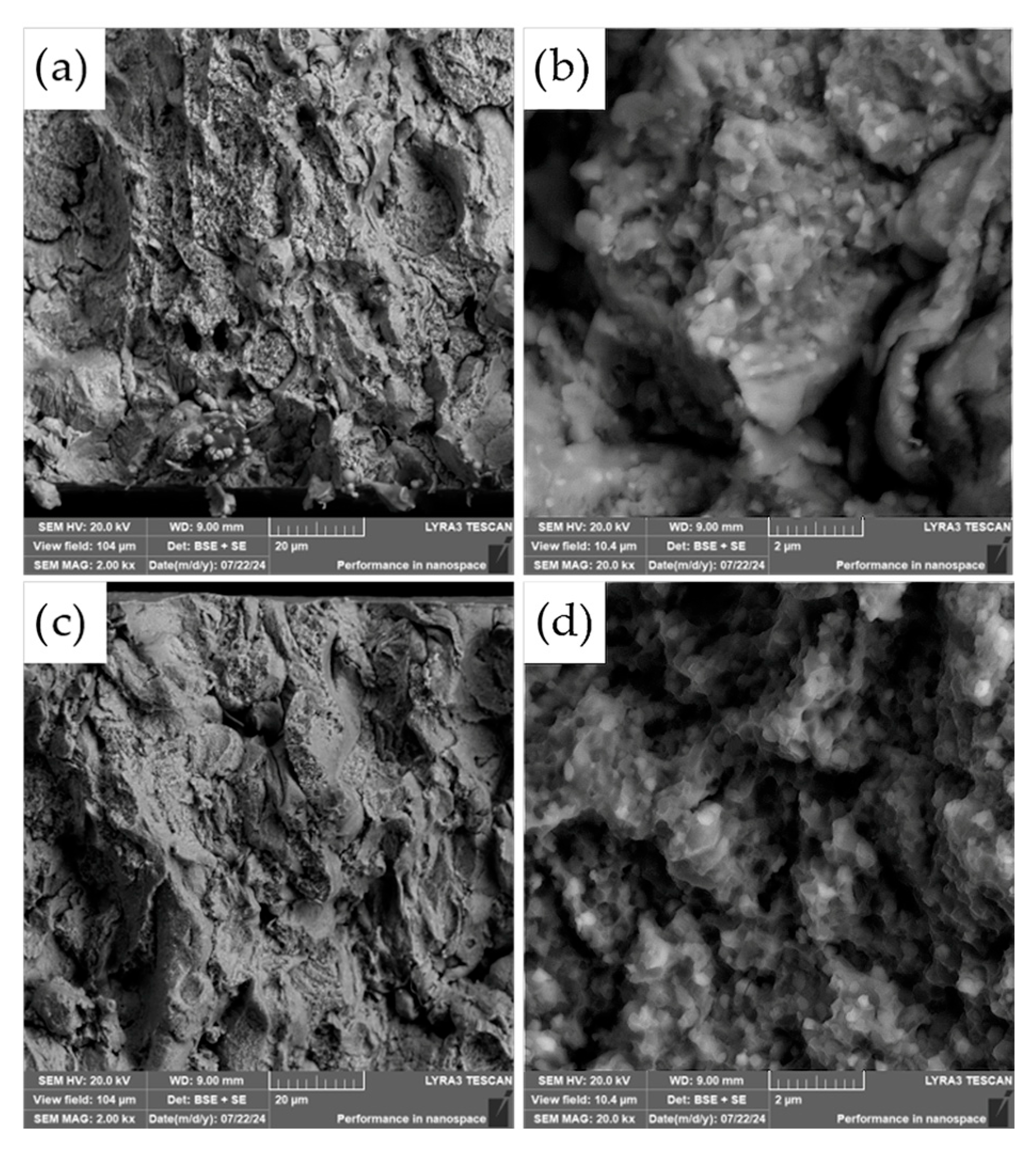
The fracture surfaces of the most promising specimens—ASP60 and ASP60 + CuSn20 sintered at 1000 °C and 1050 °C—were investigated using scanning electron microscopy (SEM) coupled with energy-dispersive X-ray spectroscopy (EDS). The analysis revealed that the overall fracture morphology remained largely consistent, regardless of the region examined (i.e., the more porous area, as shown in
Figure 12, versus the crack initiation site,
Figure 13) or the applied compaction temperature (see
Figure 14).
The observed fracture surfaces exhibited features characteristic of mixed-mode failure, combining transgranular fracture with quasi-cleavage facets. Additionally, numerous particle pull-outs were evident, leading to the formation of voids, indicative of weak interparticle bonding in localized regions. Notably, the presence of micro-dimples was also observed, which are typically associated with ductile fracture behavior. These dimples likely originate from the plastic deformation of retained austenite regions as well as decohesion around fine carbide particles.
The carbide particles, uniformly distributed across the fracture surface, displayed an average diameter of approximately 200 nm. Their homogeneous dispersion suggests effective particle bonding and consistent microstructural development throughout the sintered matrix. These findings underscore the complex fracture mechanisms at play and highlight the influence of retained austenite and second-phase particles on the overall fracture behavior of the specimens.
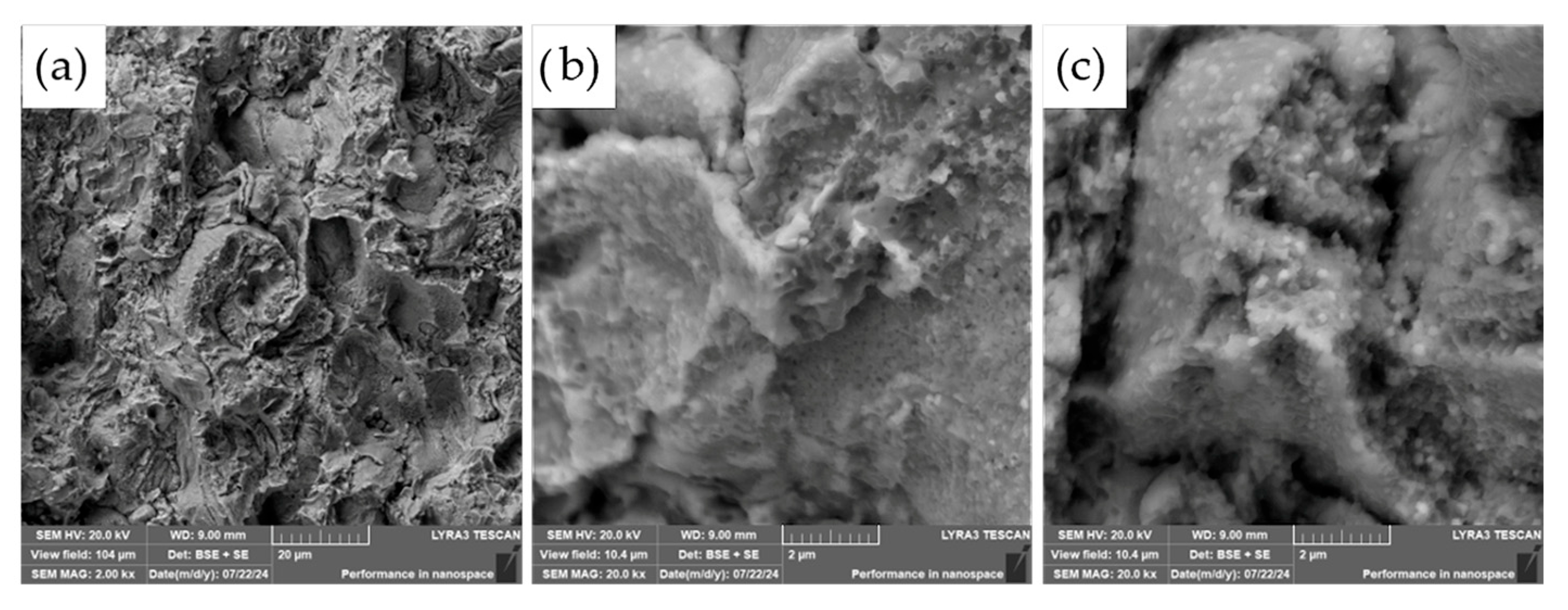
However, a notable distinction emerges when comparing the fracture surfaces of the ASP60 alloy with those of the ASP60 + CuSn20 composite. In the latter, distinct regions corresponding to the CuSn20 phase are evident, appearing as brighter areas in the SEM micrographs (
Figure 15). These regions indicate the presence of the CuSn20 alloy within the matrix, suggesting a heterogeneous microstructure in which the secondary phase is embedded within the ASP60 framework.
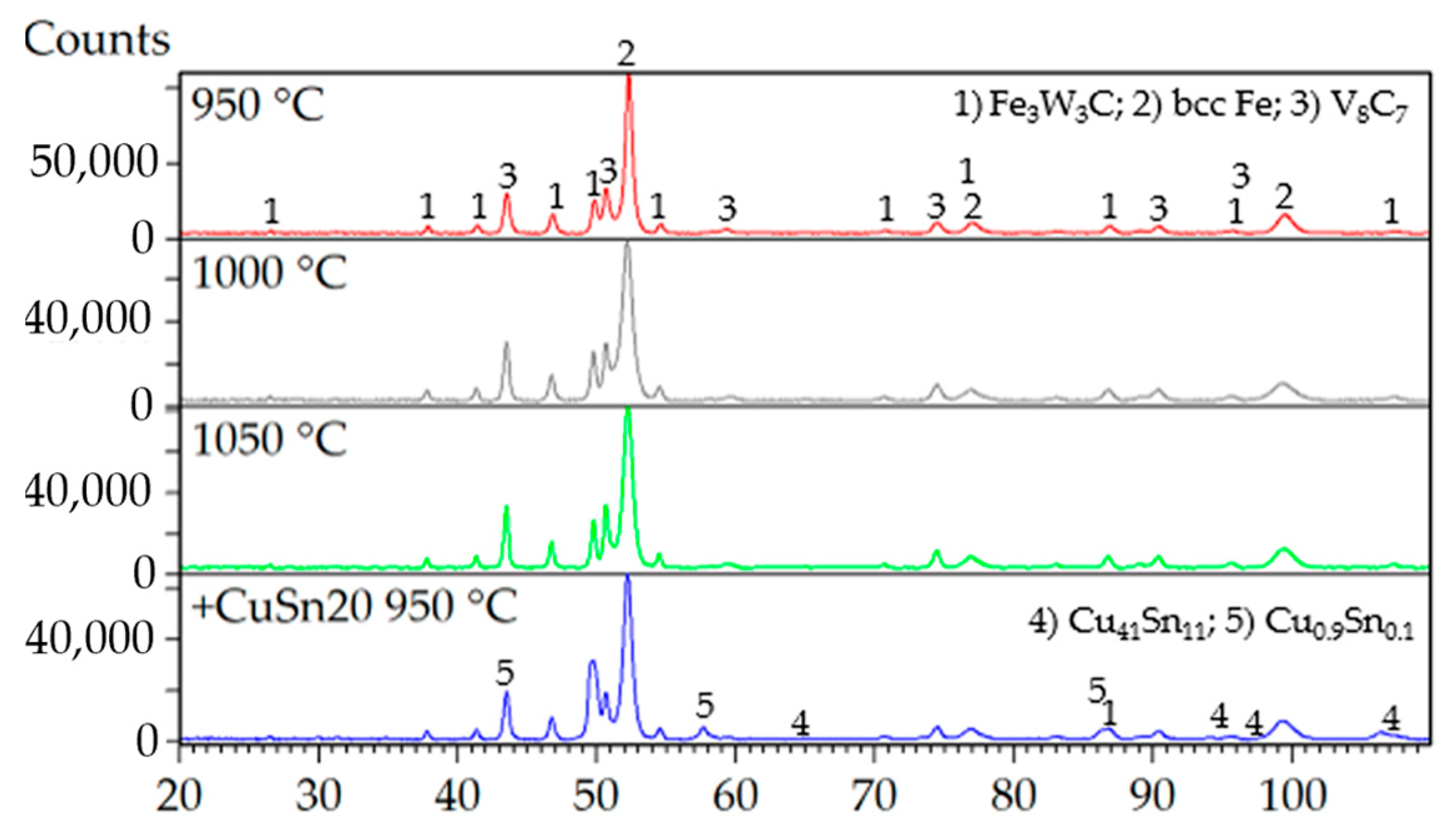
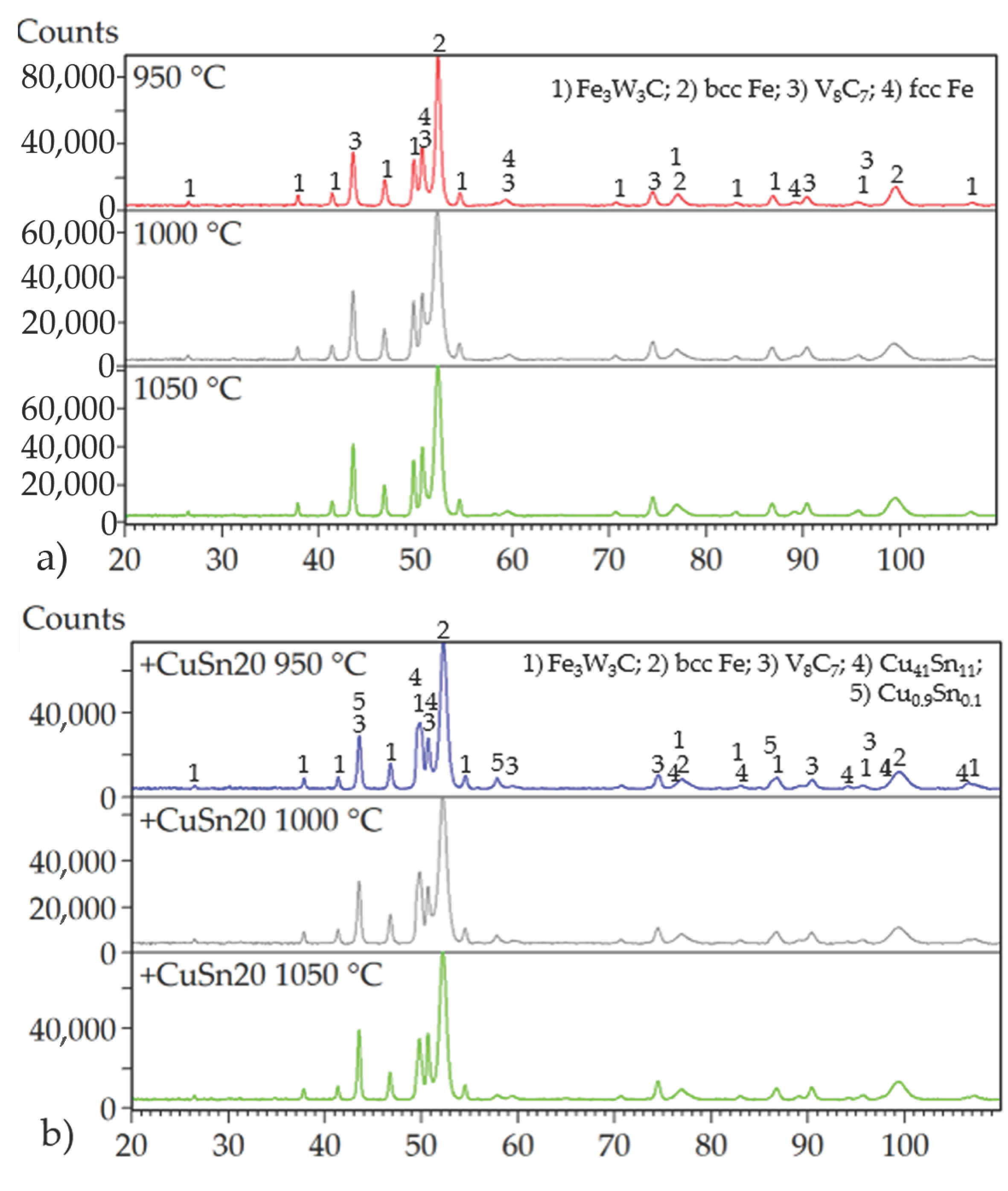
Evaluation of the mechanical properties clearly indicated that porosity is the dominant factor negatively affecting the overall performance of the investigated materials. Despite the variation in compaction temperatures, X-ray diffraction (XRD) analysis revealed that the phase composition of the specimens remained largely invariant (
Figure 16). The primary phases identified across all samples included a ternary carbide Fe
3W
3C (JCPDS card no. 04-006-1675), a binary vanadium carbide V
8C
7 (JCPDS card no. 01-089-2608), and body-centered cubic (BCC) Fe (JCPDS card no. 04-002-1253), corresponding to the presence of martensite. The presence of martensite could be explained by the ability of ASP60 high-speed steel to undergo a phase transformation of retained austenite during plastic deformation, which is introduced, e.g., due to grinding.
The addition of 9.8 wt.% CuSn20 alloy to the ASP60 matrix significantly reduced porosity and led to the formation of additional intermetallic phases. The majority of these phases corresponded to Cu0.9Sn0.1 (JCPDS card no. 04-018-6729), while a smaller fraction, amounting to several weight percent, was identified as Cu41Sn11 (JCPDS card no. 01-071-0094). The observed BCC Fe phase may be associated with low-carbon martensitic structures or other ferritic constituents, which are not easily distinguishable by conventional methods such as optical microscopy (OM) or scanning electron microscopy (SEM).
Nevertheless, upon deep etching, all the specimens revealed the presence of small fractions of retained austenite (
Figure 17) characterized by a face-centered cubic (FCC) crystal lattice. This observation suggests that some metastable austenitic regions were retained during cooling, potentially contributing to localized ductility within the otherwise hard matrix. Except for exposing the retained austenite, the phase composition was identical to that observed in the case of prepared specimens without deep etching.
Based on these findings, a compaction temperature of 1050 °C combined with the addition of 9.8 wt.% CuSn20 was identified as the most promising processing condition for the fabrication of a composite material reinforced with TiC-coated diamond particles. This combination offers an optimal balance between densification, phase stability, and microstructural uniformity.