Dry Reforming of Methane over Ni/WC Catalysts: Effect of Ni Content and CH4:CO2 Ratio
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of the Support
2.2. Synthesis of Catalysts
2.3. Characterization of Support and Catalysts
2.4. Catalytic Experiments
3. Results and Discussion
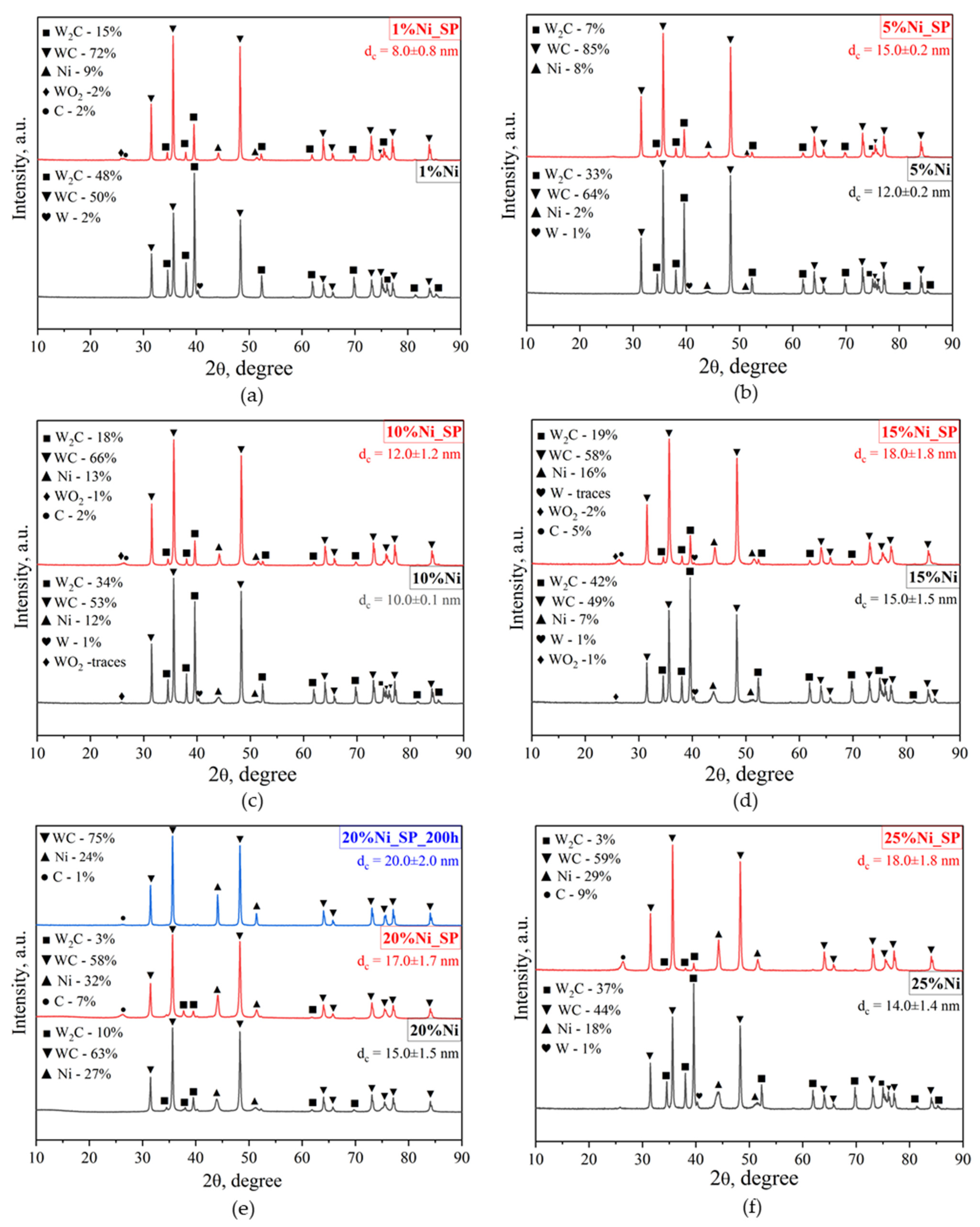
3.1. XRD
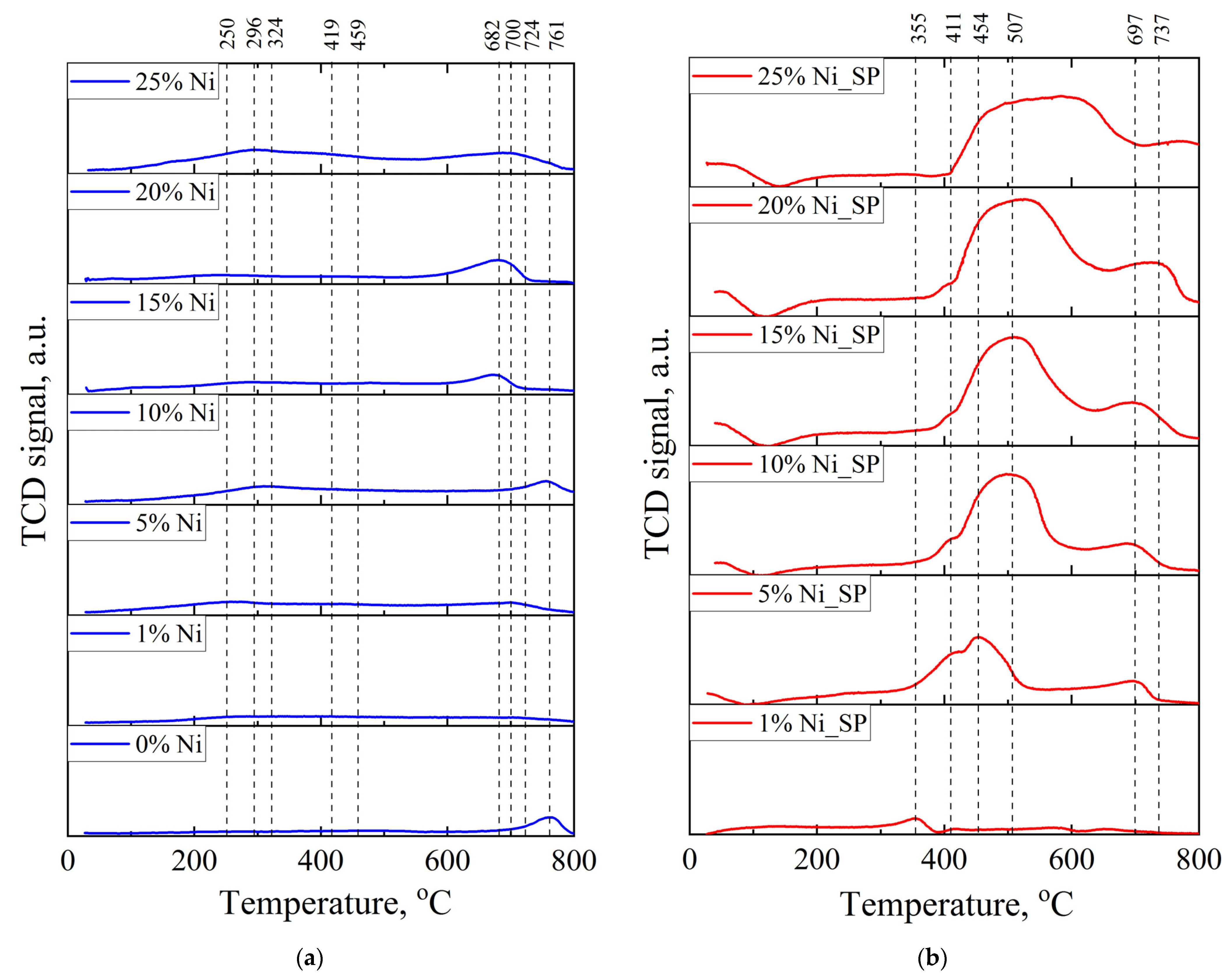
3.2. H2–TPR
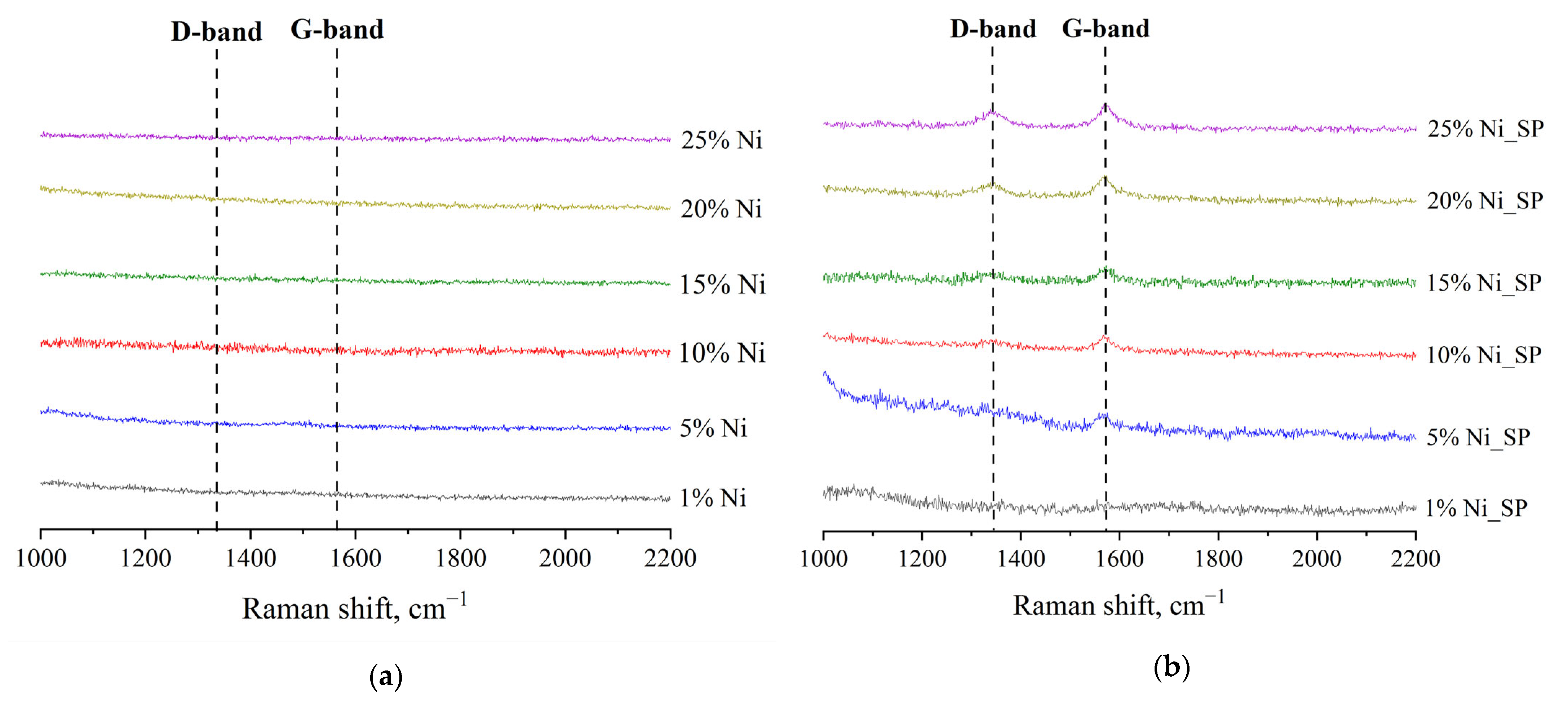
3.3. Raman Spectroscopy
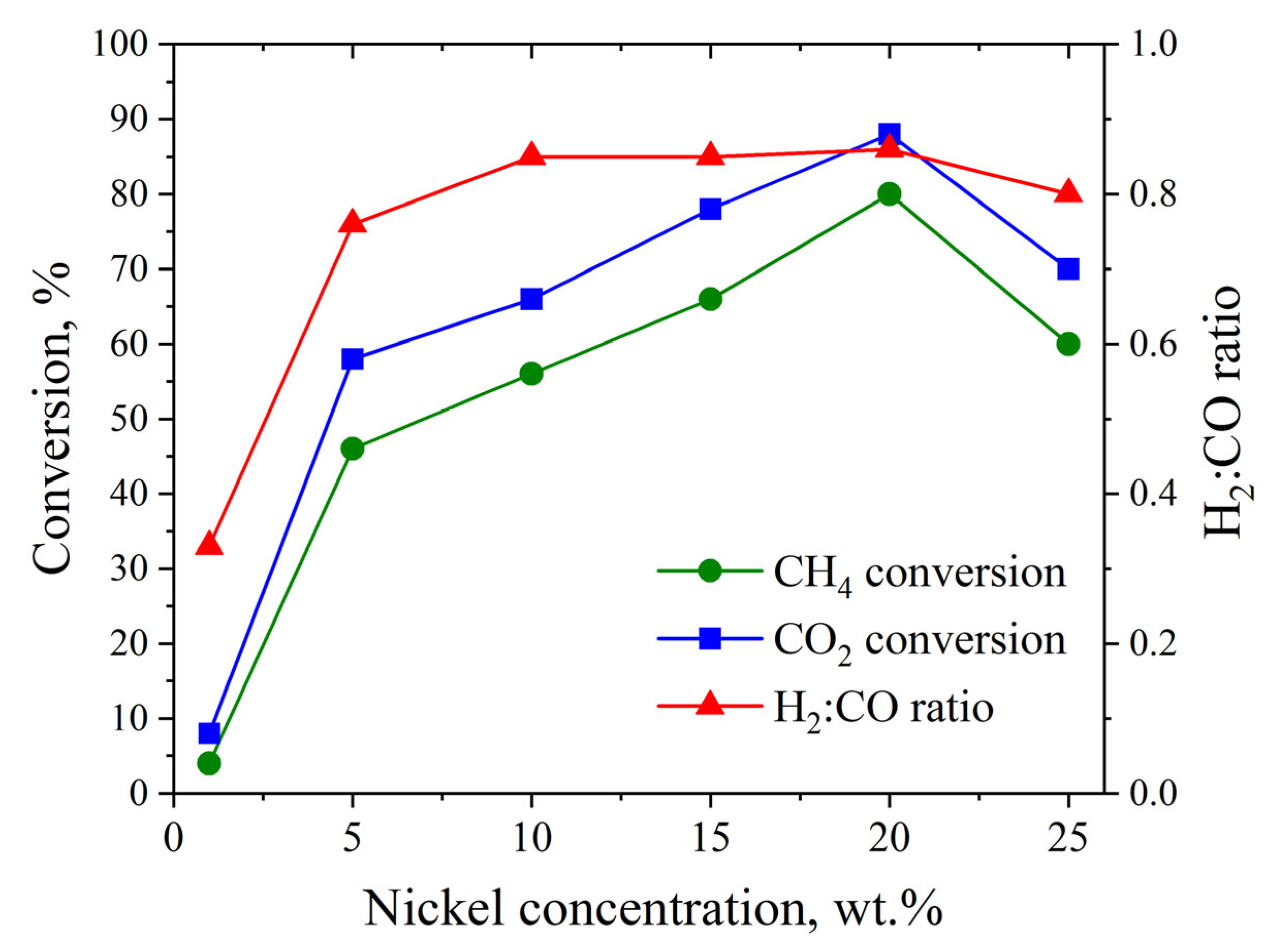
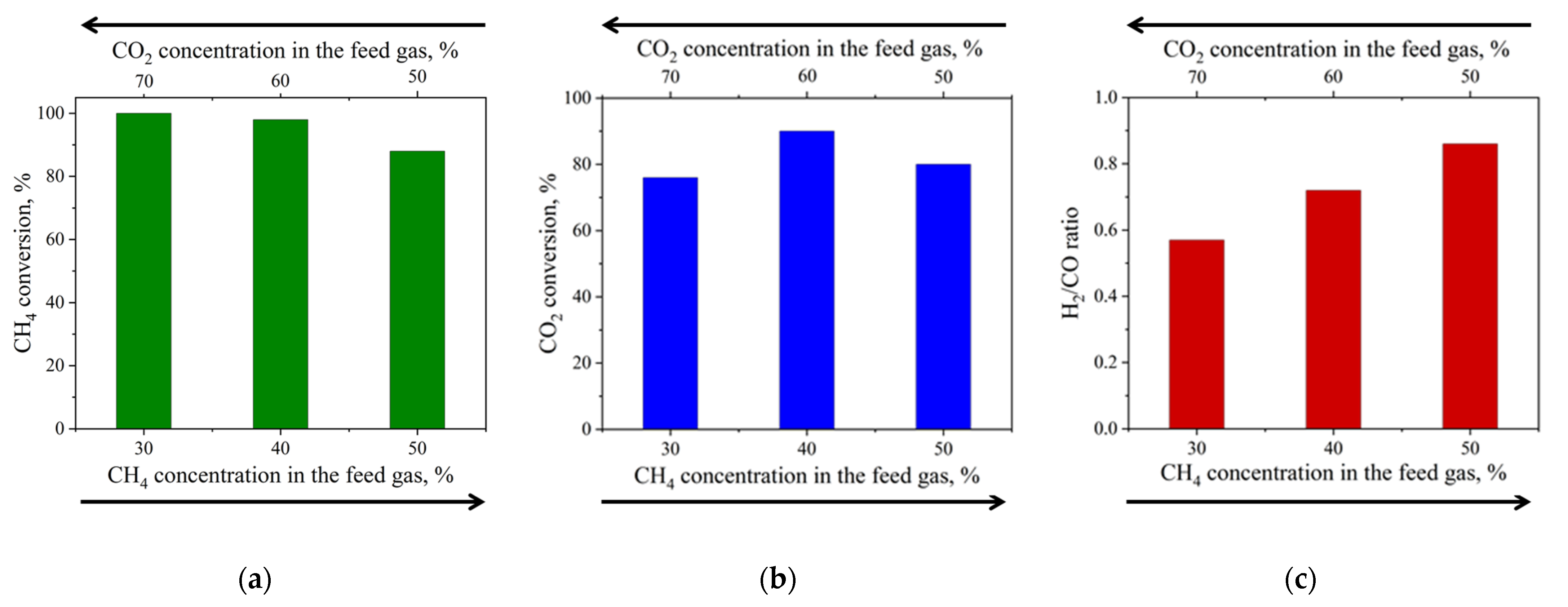
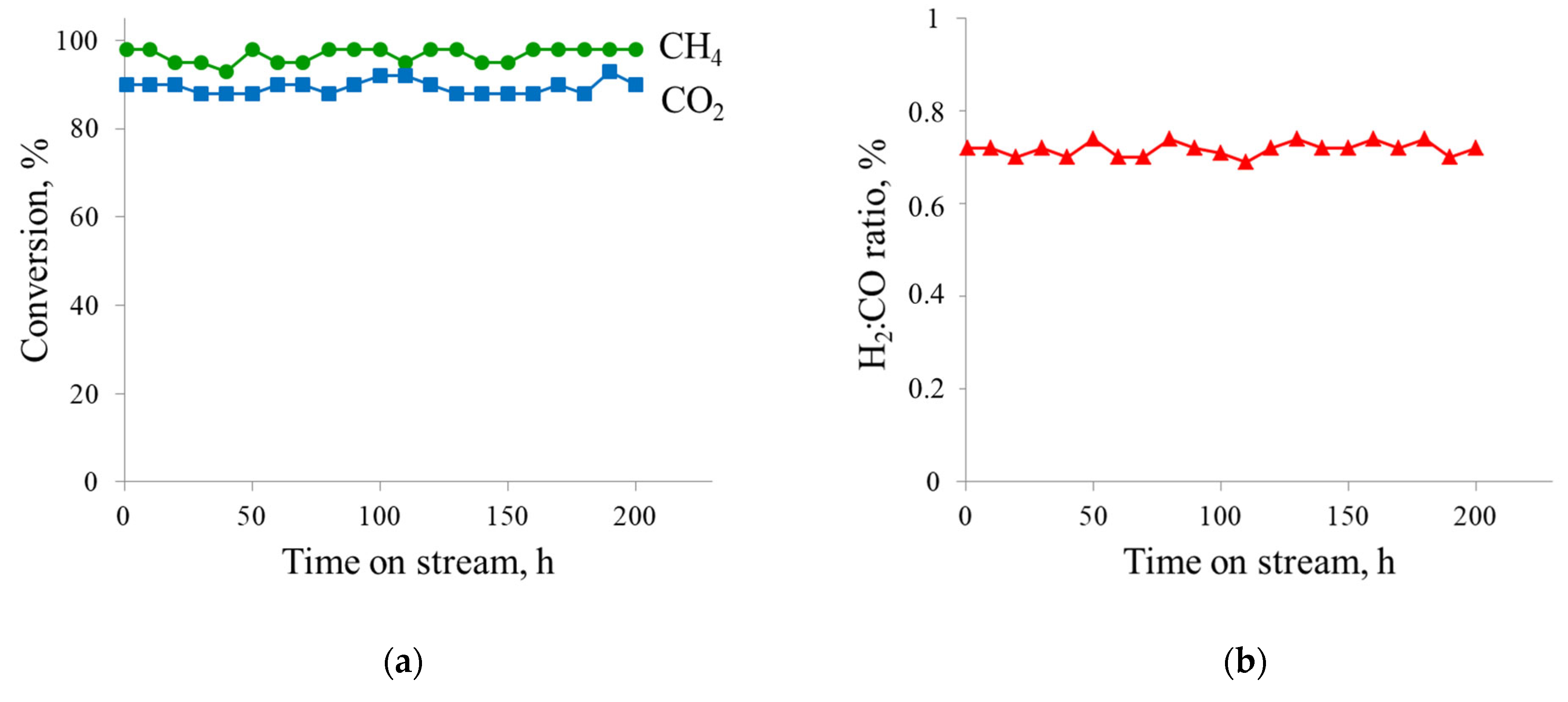
3.4. Catalytic Studies
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Norhasyima, R.S.; Mahlia, T.M.I. Advances in CO2 Utilization Technology: A Patent Landscape Review. J. CO2 Util. 2018, 26, 323–335. [Google Scholar] [CrossRef]
- Maestri, M.; Vlachos, D.G.; Beretta, A.; Groppi, G.; Tronconi, E. A C1 Microkinetic Model for Methane Conversion to Syngas on Rh/Al2O3. AIChE J. 2009, 55, 993–1008. [Google Scholar] [CrossRef]
- Naidja, A.; Krishna, C.R.; Butcher, T.; Mahajan, D. Cool Flame Partial Oxidation and Its Role in Combustion and Reforming of Fuels for Fuel Cell Systems. Prog. Energy Combust. Sci. 2003, 29, 155–191. [Google Scholar] [CrossRef]
- Fan, M.; Abdullah, A.Z.; Bhatia, S. Catalytic Technology for Carbon Dioxide Reforming of Methane to Synthesis Gas. ChemCatChem 2009, 1, 192–208. [Google Scholar] [CrossRef]
- Nedolivko, V.V.; Zasypalov, G.O.; Vutolkina, A.V.; Gushchin, P.A.; Vinokurov, V.A.; Kulikov, L.A.; Egazar’yants, S.V.; Karakhanov, E.A.; Maksimov, A.L.; Glotov, A.P. Carbon Dioxide Reforming of Methane. Russ. J. Appl. Chem. 2020, 93, 765–787. [Google Scholar] [CrossRef]
- Er-Rbib, H.; Bouallou, C.; Werkoff, F. Dry Reforming of Methane–Review of Feasibility Studies. Chem. Eng. 2012, 29, 163–168. [Google Scholar]
- Arora, S.; Prasad, R. An Overview on Dry Reforming of Methane: Strategies to Reduce Carbonaceous Deactivation of Catalysts. RSC Adv. 2016, 6, 108668–108688. [Google Scholar] [CrossRef]
- Pakhare, D.; Spivey, J. A Review of Dry (CO2) Reforming of Methane over Noble Metal Catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef]
- Shafiee, P.; Alavi, S.M.; Rezaei, M. Mechanochemical Synthesis Method for the Preparation of Mesoporous Ni–Al2O3 Catalysts for Hydrogen Purification via CO2 Methanation. J. Energy Inst. 2021, 96, 1–10. [Google Scholar] [CrossRef]
- Budiman, A.W.; Song, S.-H.; Chang, T.-S.; Shin, C.-H.; Choi, M.-J. Dry Reforming of Methane over Cobalt Catalysts: A Literature Review of Catalyst Development. Catal. Surv. Asia 2012, 16, 183–197. [Google Scholar] [CrossRef]
- Sharifianjazi, F.; Esmaeilkhanian, A.; Bazli, L.; Eskandarinezhad, S.; Khaksar, S.; Shafiee, P.; Yusuf, M.; Abdullah, B.; Salahshour, P.; Sadeghi, F. A Review on Recent Advances in Dry Reforming of Methane over Ni-and Co-Based Nanocatalysts. Int. J. Hydrogen Energy 2022, 47, 42213–42233. [Google Scholar] [CrossRef]
- Gonzalez-delaCruz, V.M.; Pereñiguez, R.; Ternero, F.; Holgado, J.P.; Caballero, A. In Situ XAS Study of Synergic Effects on Ni–Co/ZrO2 Methane Reforming Catalysts. J. Phys. Chem. C 2012, 116, 2919–2926. [Google Scholar] [CrossRef]
- Salaev, M.A.; Liotta, L.F.; Vodyankina, O.V. Lanthanoid-Containing Ni-Based Catalysts for Dry Reforming of Methane: A Review. Int. J. Hydrogen Energy 2022, 47, 4489–4535. [Google Scholar] [CrossRef]
- Li, M.; Sun, Z.; Hu, Y.H. Catalysts for CO2 Reforming of CH4: A Review. J. Mater. Chem. A 2021, 9, 12495–12520. [Google Scholar] [CrossRef]
- Owgi, A.H.K.; Jalil, A.A.; Hussain, I.; Hassan, N.S.; Hambali, H.U.; Siang, T.J.; Vo, D.V.N. Catalytic Systems for Enhanced Carbon Dioxide Reforming of Methane: A Review. Environ. Chem. Lett. 2021, 19, 2157–2183. [Google Scholar] [CrossRef]
- Nguyen, D.L.T.; Tran, A.V.; Vo, D.-V.N.; Nguyen, H.T.; Rajamohan, N.; Trinh, T.H.; Nguyen, T.L.; Le, Q.V.; Nguyen, T.M. Methane Dry Reforming: A Catalyst Challenge Awaits. J. Ind. Eng. Chem. 2024, 140, 169–189. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Amama, P.B. Recent Progress in the Design of Dry Reforming Catalysts Supported on Low-Dimensional Materials. J. CO2 Util. 2024, 81, 102734. [Google Scholar] [CrossRef]
- Guil-López, R.; Nieto, E.; Botas, J.A.; Fierro, J.L.G. On the Genesis of Molybdenum Carbide Phases during Reduction-Carburization Reactions. J. Solid State Chem. 2012, 190, 285–295. [Google Scholar] [CrossRef]
- Levy, R.B.; Boudart, M. Platinum-like Behavior of Tungsten Carbide in Surface Catalysis. Science 1973, 181, 547–549. [Google Scholar] [CrossRef] [PubMed]
- York, A.E.; Claridge, J.; Brungs, A.; Tsang, S.; Green, M.H. Molybdenum and Tungsten Carbides as Catalysts for the Conversion of Methane to Synthesis Gas Using Stoichiometric Feedstocks. Chem. Commun. 1997, 1, 39–40. [Google Scholar] [CrossRef]
- Yan, Q.; Lu, Y.; To, F.; Li, Y.; Yu, F. Synthesis of Tungsten Carbide Nanoparticles in Biochar Matrix as a Catalyst for Dry Reforming of Methane to Syngas. Catal. Sci. Technol. 2015, 5, 3270–3280. [Google Scholar] [CrossRef]
- Gavrilova, N.N.; Sapunov, V.N.; Skudin, V.V. Intensification of Dry Reforming of Methane on Membrane Catalyst. Chem. Eng. J. 2019, 374, 983–991. [Google Scholar] [CrossRef]
- Grigoryan, R.R.; Aloyan, S.G.; Harutyunyan, V.R.; Arsentev, S.D.; Tavadyan, L.A. Dry Reforming of Methane over Nanosized Tungsten Carbide Powders Obtained by Mechanochemical and Plasma-Mechanochemical Methods. Pet. Chem. 2019, 59, 1256–1263. [Google Scholar] [CrossRef]
- Zhang, Q.; Pastor-Pérez, L.; Gu, S.; Ramirez Reina, T. Transition Metal Carbides (TMCS) Catalysts for Gas Phase CO2 Upgrading Reactions: A Comprehensive Overview. Catalysts 2020, 10, 955. [Google Scholar] [CrossRef]
- Czaplicka, N.; Rogala, A.; Wysocka, I. Metal (Mo, W, Ti) Carbide Catalysts: Synthesis and Application as Alternative Catalysts for Dry Reforming of Hydrocarbons—A Review. Int. J. Mol. Sci. 2021, 22, 12337. [Google Scholar] [CrossRef]
- Vasilevich, A.V.; Baklanova, O.N.; Lavrenov, A.V. Molybdenum Carbides: Synthesis and Application in Catalysis. Solid Fuel Chem. 2020, 54, 354–361. [Google Scholar] [CrossRef]
- Abdullah, M.; Sajjadi, B. Sustainable Conversion of Natural Gas to Hydrogen Using Transition Metal Carbides. Int. J. Hydrogen Energy 2024, 90, 61–103. [Google Scholar] [CrossRef]
- LaMont, D.C.; Thomson, W.J. Dry Reforming Kinetics over a Bulk Molybdenum Carbide Catalyst. Chem. Eng. Sci. 2005, 60, 3553–3559. [Google Scholar] [CrossRef]
- Bolatova, Z.; German, D.; Pakrieva, E.; Pak, A.; Larionov, K.; Carabineiro, S.A.C.; Bogdanchikova, N.; Kolobova, E.; Pestryakov, A. Ni, Co and Ni-Co-Modified Tungsten Carbides Obtained by an Electric Arc Method as Dry Reforming Catalysts. Catalysts 2022, 12, 1631. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Zhang, X.; Qiu, J.; Yu, L.; Shi, C. Ni Modified WCx Catalysts for Methane Dry Reforming. In Advances in CO2 Capture, Sequestration, and Conversion; ACS Publications: Washington, DC, USA, 2015; pp. 171–189. [Google Scholar]
- Iyer, M.V.; Norcio, L.P.; Punnoose, A.; Kugler, E.L.; Seehra, M.S.; Dadyburjor, D.B. Catalysis for Synthesis Gas Formation from Reforming of Methane. Top. Catal. 2004, 29, 197–200. [Google Scholar] [CrossRef]
- Shao, H.; Kugler, E.L.; Dadyburjor, D.B.; Rykov, S.A.; Chen, J.G. Correlating NEXAFS Characterization of Co–W and Ni–W Bimetallic Carbide Catalysts with Reactivity for Dry Reforming of Methane. Appl. Catal. A Gen. 2009, 356, 18–22. [Google Scholar] [CrossRef]
- Barbosa, R.D.; Baldanza, M.A.S.; de Resende, N.S.; Passos, F.B.; da Silva, V.L.D.S.T. Nickel–Promoted Molybdenum or Tungsten Carbides as Catalysts in Dry Reforming of Methane: Effects of Variation in CH4/CO2 Molar Ratio. Catal. Lett. 2021, 151, 1578–1591. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Zhang, G.; Liu, J.; Wang, Y.; Zhao, Y.; Li, G. Co–Ni/WC-AC catalysts for dry reforming of methane: The role of Ni species. Int. J. Hydrogen Energy 2023, 48, 15065–15076. [Google Scholar] [CrossRef]
- Li, S.; Zhang, G.; Wang, J.; Liu, J.; Lv, Y. Highly stable activity of cobalt based catalysts with tungsten on Tungsten Carbide-Activated Carbon for CO2 Reforming of CH4 to Produce Syngas. Int. J. Hydrogen Energy 2021, 46, 28613–28625. [Google Scholar] [CrossRef]
- Li, S.; Wang, J.; Zhang, G.; Liu, J.; Lv, Y.; Zhang, Y. Highly active and stable cobalt catalysts with a tungsten carbide-activated Carbide-Activated Carbon Support for Dry Reforming of Methane: Role of Tungsten Carbide. Fuel 2022, 311, 122512. [Google Scholar] [CrossRef]
- Yao, Z.; Jiang, J.; Zhao, Y.; Luan, F.; Zhu, J.; Shi, Y.; Gao, H.; Wang, H. Insights into the deactivation mechanism of metal carbide catalysts for dry reforming of methane via comparison of nickel-modified molybdenum and tungsten carbides. RSC Adv. 2016, 6, 19944–19951. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, Z.; Zhang, G.; Liu, J.; Wang, Y.; Zhao, Y.; Li, G.; Lv, Y. Highly Active and Stable Cobalt Catalysts with a Tungsten Carbide-Activated Carbon Support for Dry Reforming of Methane: Effect of the Different Promoters. Catal. Sci. Technol. 2022, 12, 4871–4883. [Google Scholar] [CrossRef]
- Shao, H. Bimetallic Carbides as Catalysts for Dry Reforming and Steam Reforming. Ph.D. Thesis, West Virginia University, Morgantown, WV, USA, 2006. [Google Scholar]
- Wysocka, I.; Czaplicka, N.; Pawelczyk, E.; Karczewski, J.; Sobczak, J.; Bielan, Z.; Maciejewski, M.; Kościelska, B.; Rogala, A. Novel Sugar-Based Nickel-Tungsten Carbide Catalysts for Dry Reforming of Hydrocarbons. J. Ind. Eng. Chem. 2023, 124, 431–446. [Google Scholar] [CrossRef]
- Zhang, S.; Shi, C.; Chen, B.; Zhang, Y.; Qiu, J. An Active and Coke-Resistant Dry Reforming Catalyst Comprising Nickel–Tungsten Alloy Nanoparticles. Catal. Commun. 2015, 69, 123–128. [Google Scholar] [CrossRef]
- Liang, N.; Xing, J. Study on Catalytic Roles of Tungsten Carbide Nanospheres for CH4/CO2 Reforming Over Ni Based Catalysts. Top. Chem. Mater. Eng. 2018, 1, 270–272. [Google Scholar]
- Pak, A.Y.; Kokorina, A.I. Effect of Energy on the Phase Composition of the Product of Arc Discharge Synthesis in the Tungsten–Carbon System Obtained in a Self-Shielding Autonomous Gas Environment. Inorg. Mater. Appl. Res. 2021, 12, 544–550. [Google Scholar] [CrossRef]
- Okamoto, H.; Kawamura, G.; Ishikawa, A.; Kudo, T. Characterization of Oxygen in WC Catalysts and Its Role in Electrocatalytic Activity for Methanol Oxidation. J. Electrochem. Soc. 1987, 134, 1645. [Google Scholar] [CrossRef]
- Xie, Z.; Li, M.; Zhou, Y.; Feng, Y.; Song, X.; Li, L.; Ding, W.; Wei, Z. Temperature-Induced Interface Hybridization of WC Boosts NiCu Activity for Alkaline Hydrogen Oxidation Reaction. Small Methods 2024, 8, 2400007. [Google Scholar] [CrossRef]
- Zhou, K.; Du, X.; Zhou, L.; Yang, H.; Lei, X.; Zeng, Y.; Li, D.; Hu, C. The Deoxygenation of Jatropha Oil to High Quality Fuel via the Synergistic Catalytic Effect of Ni, W2C and WC Species. Catalysts 2021, 11, 469. [Google Scholar] [CrossRef]
- Shishanov, M.V.; Luchkin, M.S.; Ivanova, A.N.; Morozov, A.A. Raman Spectroscopy as Method for the Analysis of Carbon Materials. Coke Chem. 2024, 10, 40–48. [Google Scholar]
- Pawelczyk, E.; Wysocka, I.; Dymerski, T.; Gębicki, J. Catalytic Activity of Ni-MgAl2O4 Modified with Transition Metal (Ti, Mo, W) Carbides as Potential Catalysts for Resource Recovery via Dry Reforming of Waste Plastics. Catal. Today 2024, 427, 114414. [Google Scholar] [CrossRef]

| Catalyst | Phase Detected by XRD | wi/Σw (%) by XRD | Phase Observed by SAED | SSA (m2/g) | Pore Size (nm) | Pore Volume (cm3/g) | Average Size of Ni NPs b |
|---|---|---|---|---|---|---|---|
| 20%Ni/WC_DP a | Ni (cubic) WC (hexagonal) W2C (hexagonal) | 27 63 10 | Ni (220) W2C (201), (203), (300), (102) WC (100), (101), (111) | 38.4 | 9.9 | 0.05 | 15.4 |
| Catalyst | Reaction Conditions | Conversion, % | H2:CO | Ref. | |
|---|---|---|---|---|---|
| CH4 | CO2 | ||||
| 20%Ni a | P = 1 atm T = 800 °C CH4:CO2 = 0.43–1.00 WHSV = 12,000 mL·h−1·gcat−1 TOS = 200 h | 88–100 | 76–90 | 0.57–0.86 | This work |
| 20%Ni/WC_DP a | P = 1 atm T = 800 °C CH4:CO2 = 1 WHSV = 3600–12,000 mL·h−1·gcat−1 TOS = 80 h | 80–96 | 88–94 | 0.79–1.00 | [29] |
| Ni–WCx | P = 1 atm T = 800 °C CH4:CO2 = 1 WHSV = 36,000 mL·h−1·gcat−1 TOS = 25 h | 45 | 48 | 0.6 | [30] |
| Co6W6C | P = 5 atm T = 850 °C CH4:CO2 = 1 WHSV = 11,200 scc·h−1·gcat−1 TOS = 100 h | 82 | 78 | 1.00 | [31] |
| Ni–WC | P = 1 atm T = 800 °C CH4:CO2 = 0.67–1.5 Flow = 50 mL·min−1 TOS = 20 h | 60–98 | 75–85 | 0.70–0.80 | [33] |
| Co/WC–AC | P = 1 atm T = 800 °C CH4:CO2 = 1 Flow = 120 mL·min−1 TOS = 24 h | 86 | 86 | 0.90 | [34] |
| Ni-Co/WC–AC | P = 1 atm T = 800 °C CH4:CO2 = 1 Flow = 120 mL·min−1 TOS = 24 h | 92 | 91 | 0.98 | [34] |
| Co/WC–AC | P = 1 atm T = 800 °C CH4:CO2 = 1 GHSV = 2400 mL·h−1·gcat−1 Flow = 100 mL·min−1 TOS = 12 h | 95 | 95 | 0.92 | [35] |
| Ce–Co/WC–AC | P = 1 atm T = 800 °C CH4:CO2 = 1 Flow =120 mL·min−1 TOS = 10 h | 91 | 93 | 0.92 | [36] |
| Ni–WC | P = 1 atm T = 800 °C CH4:CO2 = 1 WHSV = 6000 scc·h−1·gcat−1 Flow = 120 mL·min−1 TOS = 12 h | 82 | 90 | n.d. b | [37] |
| Y–Co/WC–AC | P = 1 atm T = 800 °C CH4:CO2 = 1 GHSV = 4800 mL·h−1·gcat−1 Flow =120 mL·min−1 TOS = 10 h | 92 | 94 | 0.85 | [38] |
| Co–WC | P = 3.4 atm T = 850 °C CH4:CO2 = 1 WHSV = 9000 scc·h−1·gcat−1 TOS = 100 h | 78 | 70 | 1.65 | [39] |
| Ni–WC | P = 3.4 atm T = 850 °C CH4:CO2 = 1 WHSV = 9000 scc·h−1·gcat−1 TOS = 100 h | 85 | 78 | 1.62 | [39] |
| Ni–WC | T = 800 °C CH4:CO2 = 1 GHSV = 5000 mL·h−1·gcat−1 TOS = 16 h | 75 | 50 | 85 | [40] |
| Ni17W3/SiO2 | P = 1 atm T = 800 °C CH4:CO2 = 1 WHSV = 96,000 mL·h−1·gcat−1 TOS = 30 h | 60 | 70 | n.d. b | [41] |
| Ni–WCx nanospheres | P = 1 atm T = 800 °C CH4:CO2 = 1 WHSV = 18,000 mL·h−1·gcat−1 TOS = 29 h | 68 | 80 | n.d. b | [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolatova, Z.; Kuznetsova, S.; Vedishcheva, O.; Carabineiro, S.A.C.; Kolobova, E.; Pestryakov, A. Dry Reforming of Methane over Ni/WC Catalysts: Effect of Ni Content and CH4:CO2 Ratio. Materials 2025, 18, 3990. https://doi.org/10.3390/ma18173990
Bolatova Z, Kuznetsova S, Vedishcheva O, Carabineiro SAC, Kolobova E, Pestryakov A. Dry Reforming of Methane over Ni/WC Catalysts: Effect of Ni Content and CH4:CO2 Ratio. Materials. 2025; 18(17):3990. https://doi.org/10.3390/ma18173990
Chicago/Turabian StyleBolatova, Zhanar, Svetlana Kuznetsova, Olga Vedishcheva, Sónia A. C. Carabineiro, Ekaterina Kolobova, and Alexey Pestryakov. 2025. "Dry Reforming of Methane over Ni/WC Catalysts: Effect of Ni Content and CH4:CO2 Ratio" Materials 18, no. 17: 3990. https://doi.org/10.3390/ma18173990
APA StyleBolatova, Z., Kuznetsova, S., Vedishcheva, O., Carabineiro, S. A. C., Kolobova, E., & Pestryakov, A. (2025). Dry Reforming of Methane over Ni/WC Catalysts: Effect of Ni Content and CH4:CO2 Ratio. Materials, 18(17), 3990. https://doi.org/10.3390/ma18173990







