Enhanced Rate Capability in B-Site High-Entropy Perovskite Oxide Ceramics: The Case of La(Co0.2Cr0.2Ni0.2Ga0.2Ge0.2)O3
Highlights
- The solid-state method was used to prepare two equimolar B-site perovskite high-entropy oxides.
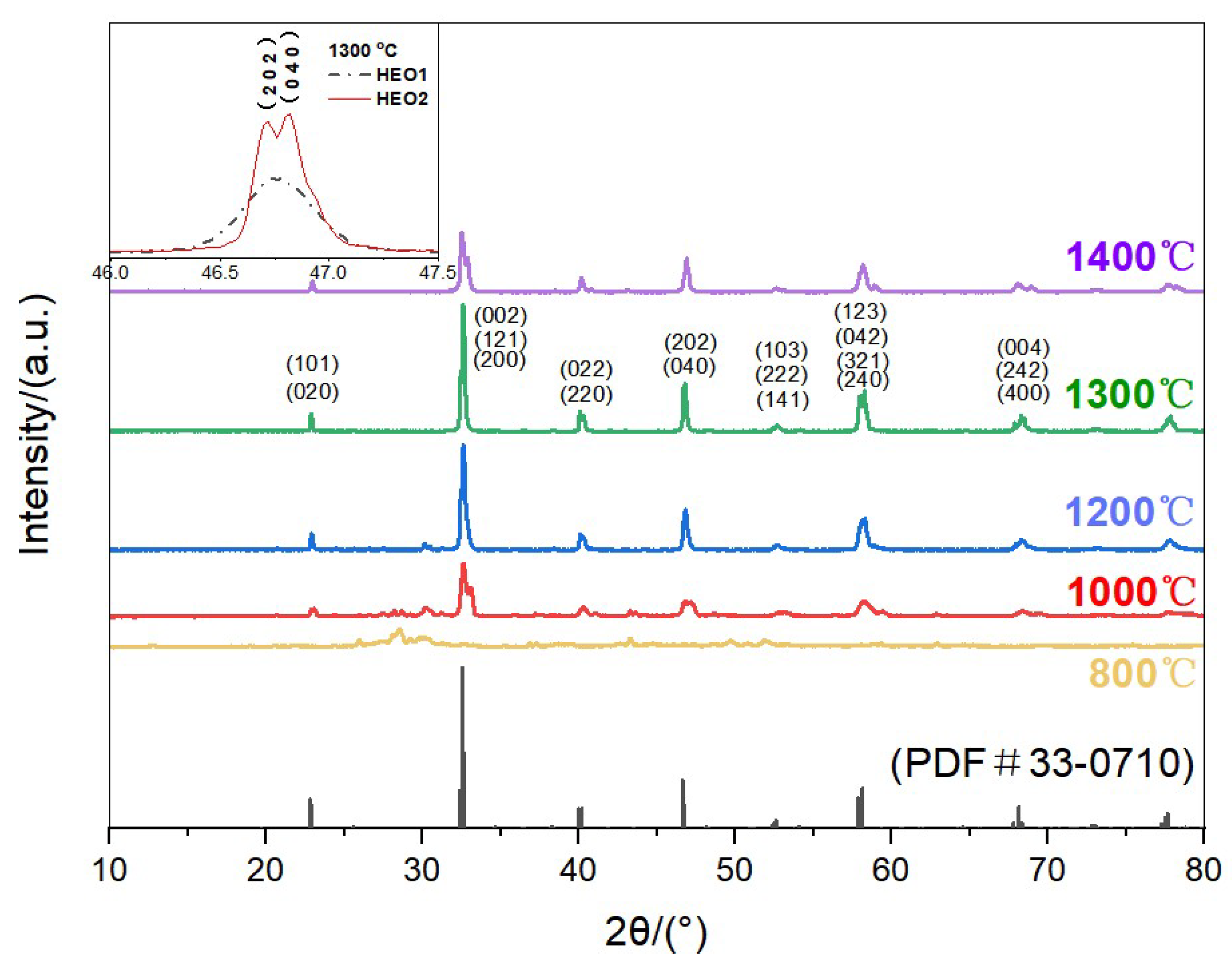
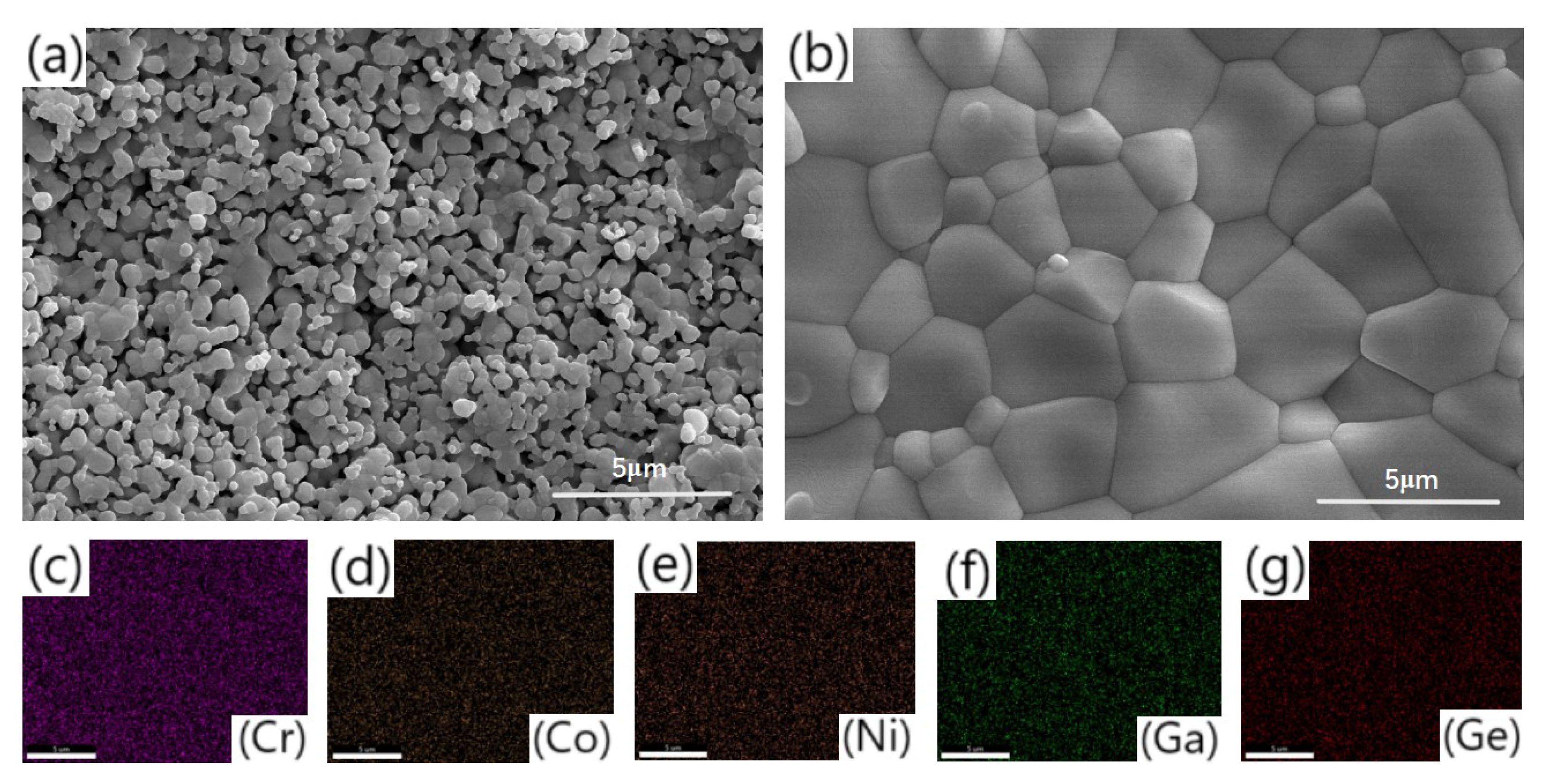
- Both samples sintered at 1300 °C show an orthorhombic structure with >97% relative density; one has larger grains.
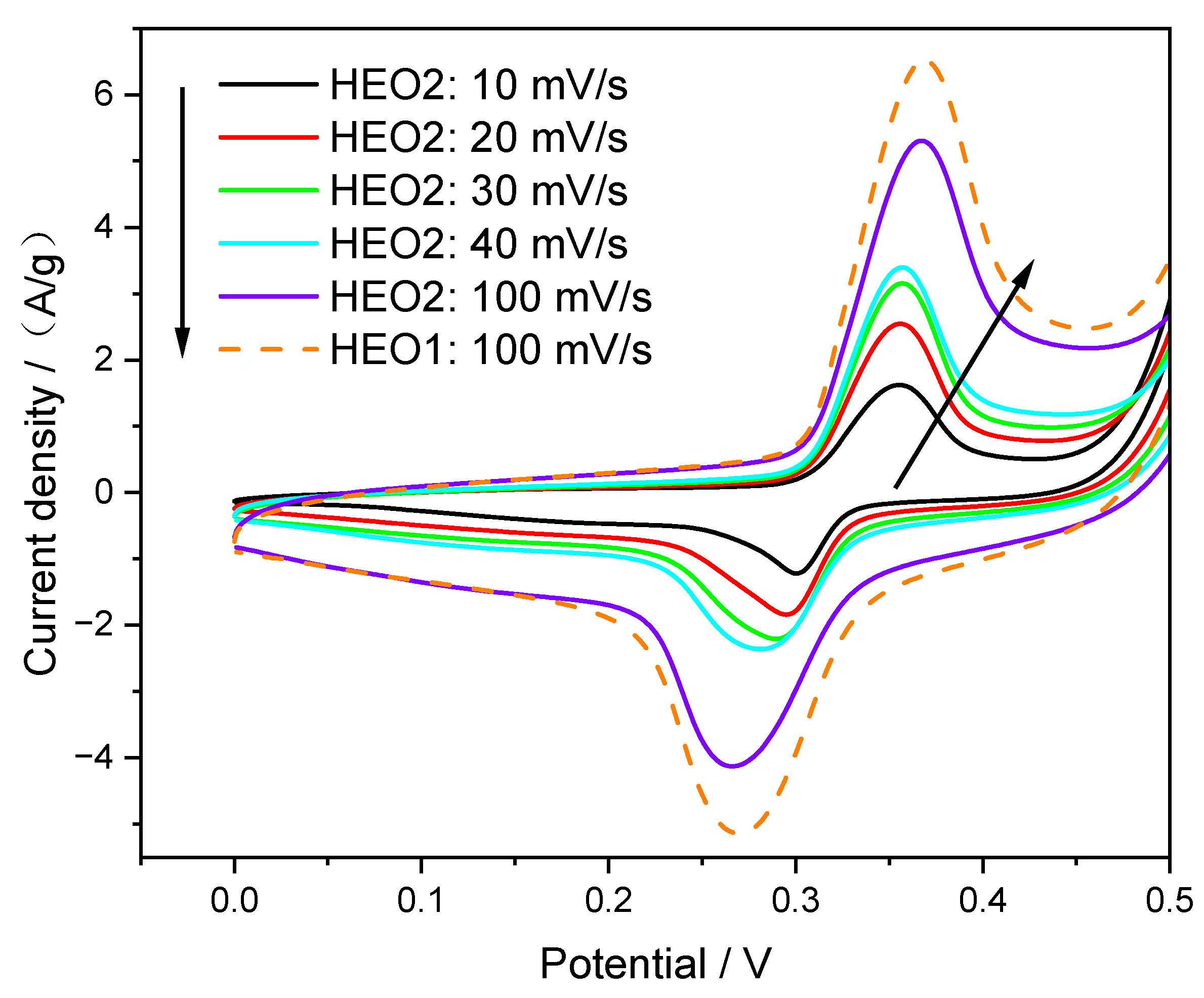
- The La(Co0.2Cr0.2Ni0.2Ga0.2Ge0.2)O3 electrode exhibits higher oxidation activity in CV.
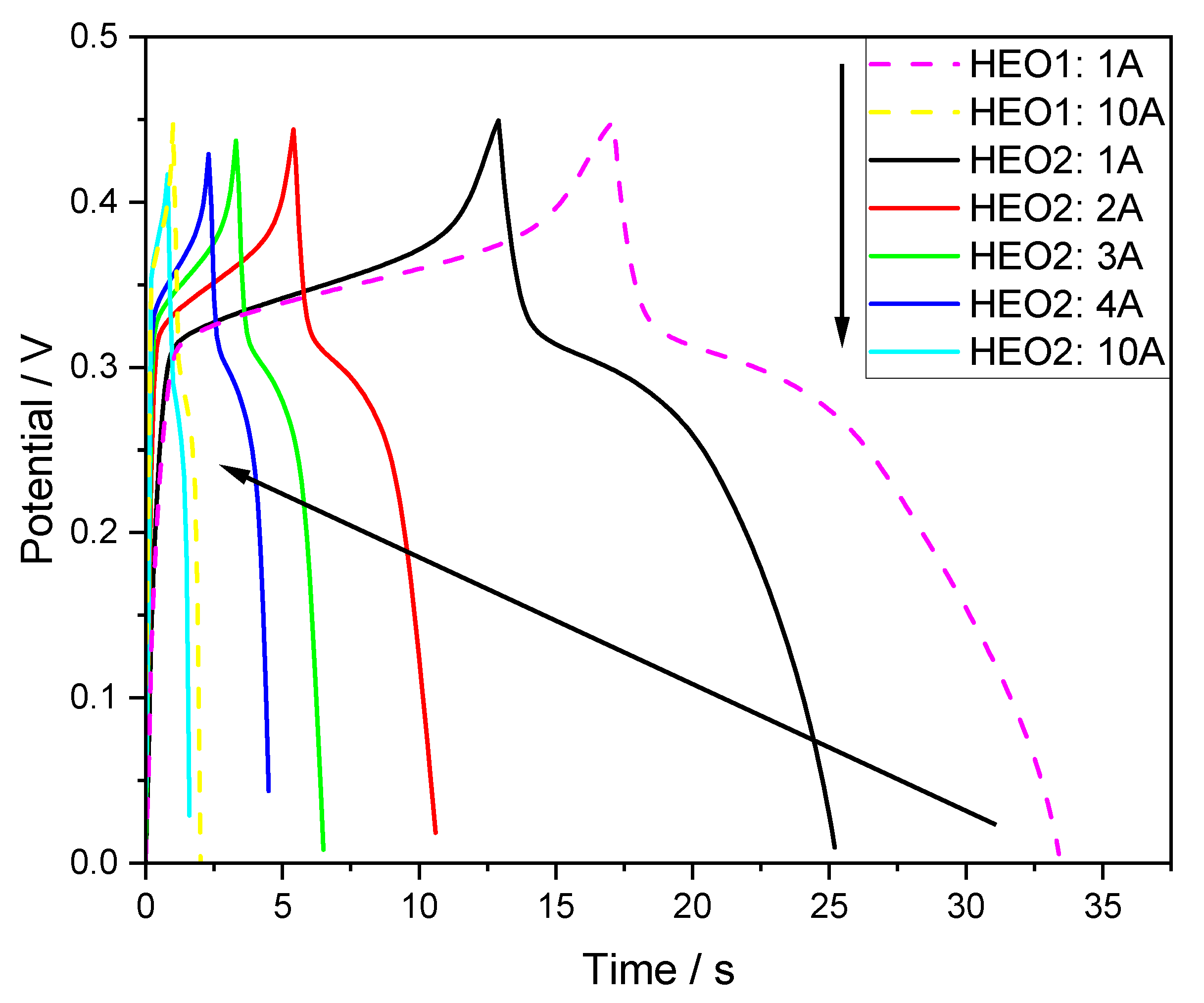
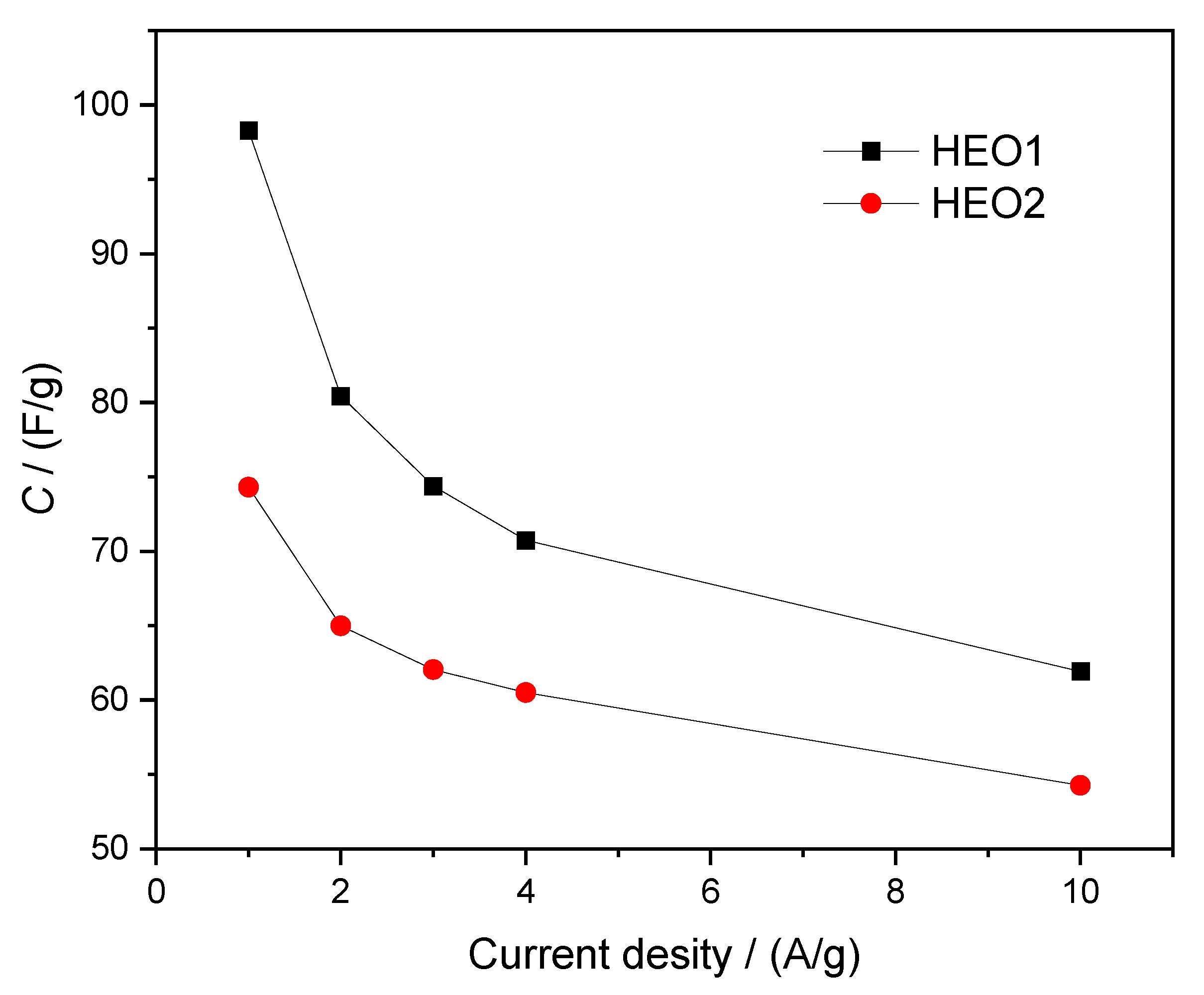
- It achieves better rate performance in GCD (10% higher retention).
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Phase and Morphology Characterization of La(Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)O3 and La(Co0.2Cr0.2Ni0.2Ga0.2Ge0.2)O3
3.2. Electrical Property Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsai, M.H.; Yeh, J.W. High-entropy alloys: A critical review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Huo, W.; Zhou, H.; Fang, F.; Xie, Z.; Jiang, J. Microstructure and mechanical properties of CoCrFeNiZrx eutectic high-entropy alloys. Mater. Des. 2017, 134, 226–233. [Google Scholar] [CrossRef]
- Rost, C.M.; Sachet, E.; Borman, T.; Moballegh, A.; Dickey, E.C.; Hou, D.; Jones, J.L.; Curtarolo, S.; Maria, J.P. Entropy-stabilized oxides. Nat. Commun. 2015, 6, 8485. [Google Scholar] [CrossRef]
- Zhu, M.; Du, C.; Zhou, R.; Li, D.; Wang, S.; Tian, C.; Chen, C. Synthesis and characterization of Ce1-x(Gd1/5Sm1/5Er1/5Y1/5Bi1/5)x O2-δ solid electrolyte for SOFCs. J. Rare Earths 2025, 43, 774–783. [Google Scholar] [CrossRef]
- Chen, H.; Lin, W.W.; Zhang, Z.H.; Jie, K.C.; Mullins, D.R.; Sang, X.H.; Yang, S.Z.; Jafta, C.J.; Bridges, C.A.; Hu, X.B.; et al. Mechanochemical synthesis of high entropy oxide materials under ambient conditions: Dispersion of catalysts via entropy maximization. ACS Mater. Lett. 2019, 1, 83–88. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, S.D.; Duan, C.Q.; Mao, J.; Dong, Y.; Dong, K.Z.; Wang, Z.Y.; Luo, S.H.; Liu, Y.G.; Qi, X.W. Spinel-structured high entropy oxide (FeCoNiCrMn)3O4 as anode towards superior lithium storage performance. J. Alloys Compd. 2020, 844, 156158. [Google Scholar] [CrossRef]
- Djenadic, R.; Sarkar, A.; Clemens, O.; Loho, C.; Botros, M.; Chakravadhanula, V.S.K.; Kubel, C.; Bhattacharya, S.S.; Gandhi, A.S.; Hahn, H. Multicomponent equiatomic rare earth oxides. Mater. Res. Lett. 2017, 5, 102–109. [Google Scholar] [CrossRef]
- Berardan, D.; Franger, S.; Meena, A.K.; Dragoe, N. Room temperature lithium superionic conductivity in high entropy oxides. J. Mater. Chem. A 2016, 4, 9536–9541. [Google Scholar] [CrossRef]
- Dabrowa, J.; Stygard, M.; Mikuta, A.; Knapik, A.; Mroczka, K.; Tejchman, W.; Danielewski, M.; Martin, M. Synthesis and microstructure of the (Co, Cr, Fe, Mn, Ni)3O4 high entropy oxide characterized by spinel structure. Mater. Lett. 2018, 216, 32–36. [Google Scholar] [CrossRef]
- Stygard, M.; Dabrowa, J.; Moździerz, M.; Zajusz, M.; Skubida, W.W.; Mroczka, K.; Berent, K.; Świerczek, K.; Danielewski, M. Formation and properties of high entropy oxides in Co–Cr–Fe–Mg–Mn–Ni–O system: (Cr, Fe, Mg, Mn, Ni)3O4 and (Co, Cr, Fe, Mg, Mn)3O4 high entropy spinels. J. Eur. Ceram. Soc. 2020, 40, 1644–1650. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, X.; Das, S.; Wang, Z.M.; Qi, X.; Du, Q. High remanent polarization and temperature-insensitive ferroelectric remanent polarization in BiFeO3-based lead-free perovskite. J. Mater. Chem. C 2019, 7, 10551–10560. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, X.Y.; Qi, X.W.; Zhu, H.G.; Li, Y.; Gu, Y.H. Enhanced ferroelectric, magnetic and magnetoelectric properties of multiferroic BiFeO3–BaTiO3–LaFeO3 ceramics. Ceram. Int. 2018, 44, 21269–21276. [Google Scholar] [CrossRef]
- Dong, G.X.; Ma, S.W.; Du, J.; Cui, J.D. Dielectric properties and energy storage density in ZnO-doped Ba0.3Sr0.7TiO3 ceramics. Ceram. Int. 2009, 35, 2069–2075. [Google Scholar] [CrossRef]
- Kreuer, K.D. Proton-conducting oxides. Annu. Rev. Mater. Res. 2003, 33, 333–359. [Google Scholar] [CrossRef]
- Wrighton, M.S.; Morse, D.L.; Ellis, A.B.; Ginley, D.S.; Abrahamson, H.B. Photoassisted electrolysis of water by ultraviolet irradiation of an antimony doped stannic oxide electrode. J. Am. Chem. Soc. 1976, 98, 44–48. [Google Scholar] [CrossRef]
- Ji, L.; McDaniel, M.D.; Wang, S.J.; Posadas, A.B.; Li, X.H.; Huang, H.Y.; Lee, J.C.; Demkov, A.A.; Bard, A.J.; Ekerdt, J.G.; et al. A silicon-based photocathode for water reduction with an epitaxial SrTiO3 protection layer and a nanostructured catalyst. Nat. Nanotechnol. 2015, 10, 84–90. [Google Scholar] [CrossRef]
- Li, W.; Zhao, Z.; Zhao, J.; Wang, Y.; Wang, X. High entropy La(Cr0.2Mn0.2Fe0.2Co0.2Ni0.2)O3 with tailored eg occupancy and transition metal–oxygen bond properties for oxygen reduction reaction. J. Mater. Sci. Technol. 2024, 194, 236–246. [Google Scholar] [CrossRef]
- Sarkar, A.; Loho, C.; Velasco, L.; Thomas, T.; Bhattacharya, S.S.; Hahn, H.; Djenadic, R. Multicomponent equiatomic rare earth oxides with narrow band gap and associated praseodymium multivalency. Dalton Trans. 2017, 46, 12167–12176. [Google Scholar] [CrossRef]
- Jiang, S.C.; Hu, T.; Gild, J.; Zhou, N.X.; Nie, J.Y.; Qin, M.D.; Harrington, T.; Vecchio, K.; Luo, J. A new class of high-entropy perovskite oxides. Scr. Mater. 2018, 142, 116–120. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, F.; Miao, Y.; Liu, Y.; Yu, J.; Gao, F. Preparation and Electrical Properties of High Entropy La(Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)O3 Perovskite Ceramics Powder. J. Inorg. Mater. 2021, 36, 431–435. [Google Scholar] [CrossRef]
- Zhao, S.H.; Yang, Z.B.; Zhao, X.M.; Xu, W.W.; Xin, W.; Zhang, Q.Y. Green preparation and supercapacitive performance of NiCo2S4@ACF heterogeneous electrode materials. J. Inorg. Mater. 2019, 34, 130–136. [Google Scholar]
- Tao, K.Y.; Li, P.Y.; Kang, L.T.; Li, X.R.; Zhao, Q.F.; Dong, L.; Liang, W. Facile and low-cost combustion-synthesized amorphous mesoporous NiO/carbon as high mass-loading pseudocapacitor materials. J. Power Sources 2015, 293, 23–32. [Google Scholar] [CrossRef]
- Ma, X.J.; Kong, L.B.; Zhang, W.B.; Liu, M.C.; Luo, Y.C.; Kang, L. Design and synthesis of 3D Co3O4@MMoO4 (M=Ni, Co) nanocomposites as high-performance supercapacitor electrodes. Electrochim. Acta 2014, 130, 660–669. [Google Scholar] [CrossRef]
- Huo, H.H.; Zhao, Y.Q.; Xu, C.L. 3D Ni3S2 nanosheet arrays supported on Ni foam for high-performance supercapacitor and non-enzymatic glucose detection. J. Mater. Chem. A 2014, 2, 15111–15117. [Google Scholar] [CrossRef]
- Zhang, L.X.; Zheng, W.H.; Jiu, H.F.; Ni, C.H.; Chang, J.X.; Qi, G.S. The synthesis of NiO and NiCo2O4 nanosheets by a new method and their excellent capacitive performance for asymmetric supercapacitor. Electrochim. Acta 2016, 215, 212–222. [Google Scholar] [CrossRef]
- Zhang, G.X.; Chen, Y.M.; He, Z.N.; Lin, C.; Chen, Y.G.; Guo, H.B. Surfactant dependence of nanostructured NiCo2S4 films on Ni foam for superior electrochemical performance. J. Inorg. Mater. 2018, 33, 289–294. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mok, B.-H.; Yao, T.; Fu, L.; Lu, C.-T.; Ouyang, H.; Pan, Z.; Tian, C. Enhanced Rate Capability in B-Site High-Entropy Perovskite Oxide Ceramics: The Case of La(Co0.2Cr0.2Ni0.2Ga0.2Ge0.2)O3. Materials 2025, 18, 3966. https://doi.org/10.3390/ma18173966
Mok B-H, Yao T, Fu L, Lu C-T, Ouyang H, Pan Z, Tian C. Enhanced Rate Capability in B-Site High-Entropy Perovskite Oxide Ceramics: The Case of La(Co0.2Cr0.2Ni0.2Ga0.2Ge0.2)O3. Materials. 2025; 18(17):3966. https://doi.org/10.3390/ma18173966
Chicago/Turabian StyleMok, Boon-How, Tengfa Yao, Longchao Fu, Cheng-Tsung Lu, Haoxian Ouyang, Zongying Pan, and Changan Tian. 2025. "Enhanced Rate Capability in B-Site High-Entropy Perovskite Oxide Ceramics: The Case of La(Co0.2Cr0.2Ni0.2Ga0.2Ge0.2)O3" Materials 18, no. 17: 3966. https://doi.org/10.3390/ma18173966
APA StyleMok, B.-H., Yao, T., Fu, L., Lu, C.-T., Ouyang, H., Pan, Z., & Tian, C. (2025). Enhanced Rate Capability in B-Site High-Entropy Perovskite Oxide Ceramics: The Case of La(Co0.2Cr0.2Ni0.2Ga0.2Ge0.2)O3. Materials, 18(17), 3966. https://doi.org/10.3390/ma18173966






