Effect of the Different Growth Shapes on the Electrochemical Behavior of Ti Thin Films for Medical Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Thin Film Deposition
2.2. Structural and Morphological Characterization
2.3. Electrochemical Characterization
3. Results and Discussion
3.1. Morphological Characterization
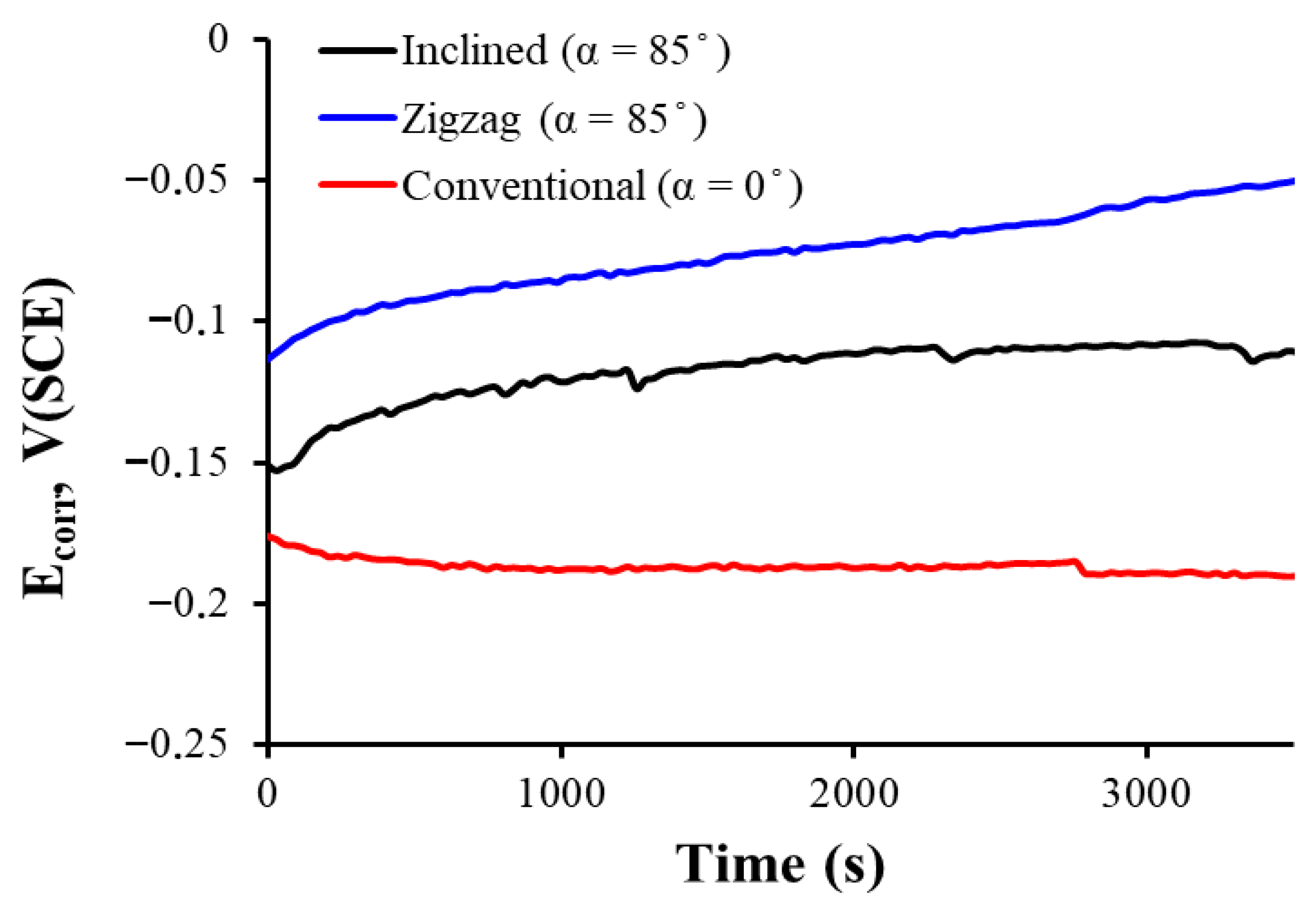
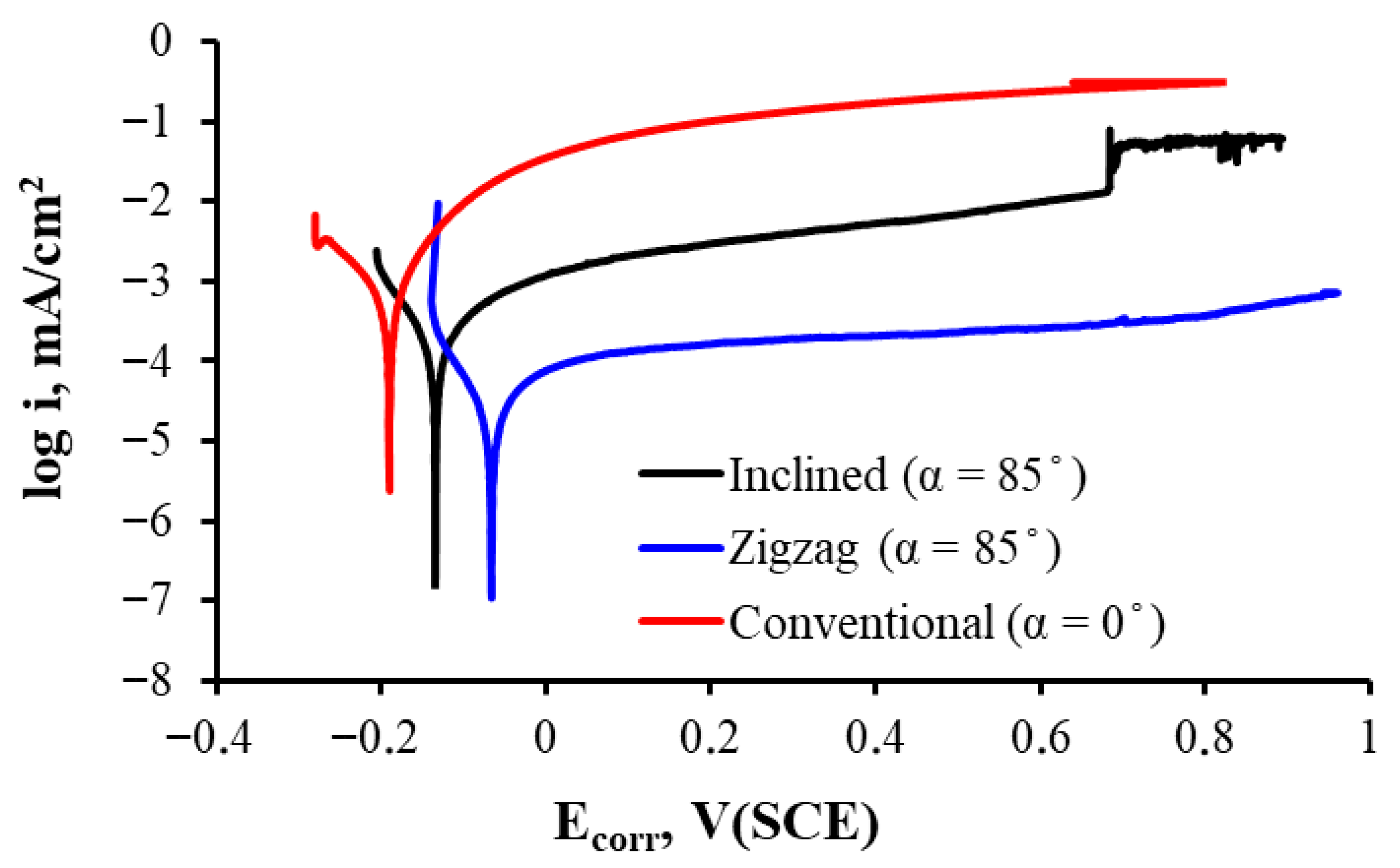
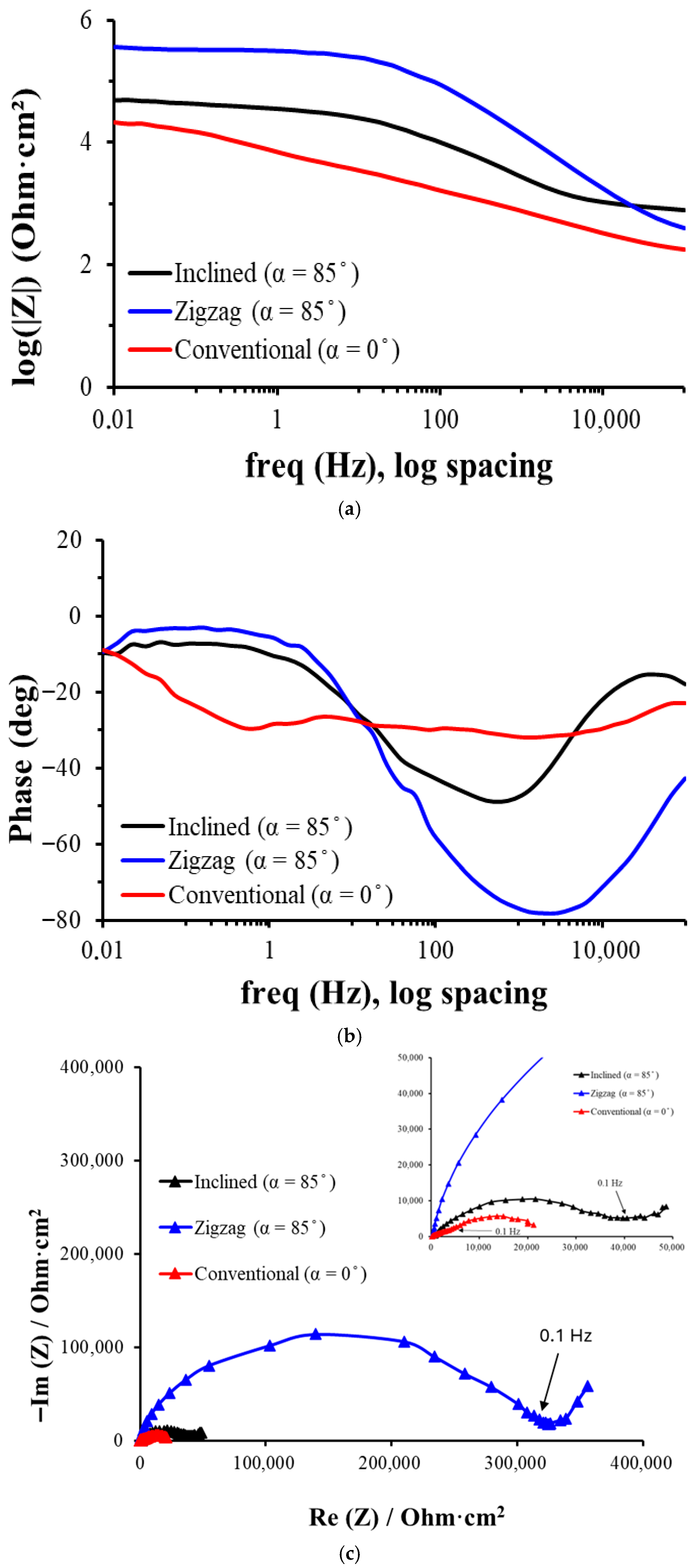
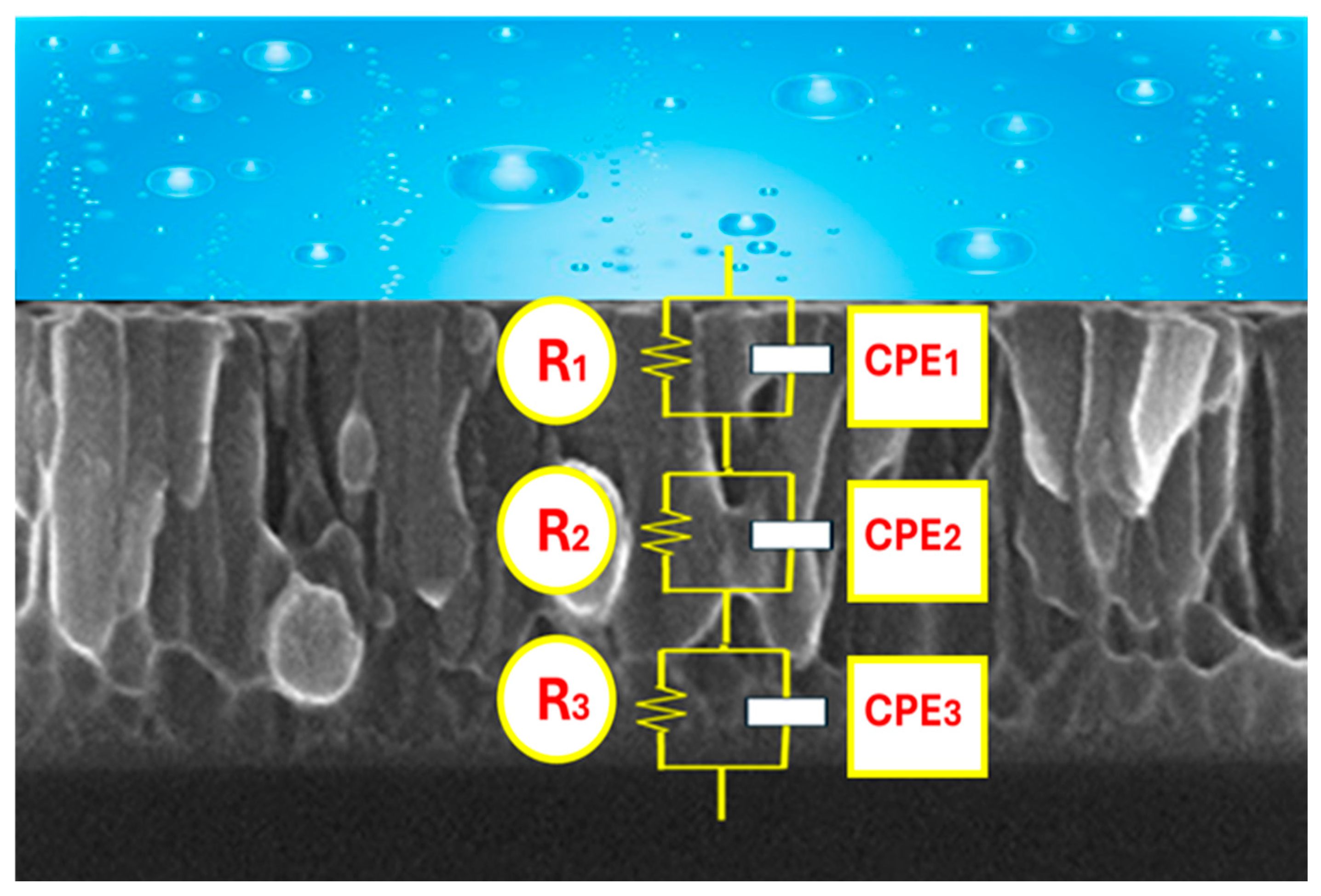
3.2. Corrosion Behavior
4. Conclusions
- For conventional geometry (α = 0°), a lower roughness and less developed morphology were observed, with thinner passive layers and lower Rp and Ecorr. Thus, in vivo, there is acceptable biocompatibility, but it is potentially inferior to the other geometries due to a lower ability to form stable and protective passive layers. The higher release of metal ions could induce mild inflammatory reactions or less bone integration in implants. It has a better mechanical behavior as it is more homogeneous and has less porous geometry.
- For the inclined geometry (α = 85°), intermediate roughness and diffusion characteristics have been observed, as well as more developed layers than in the conventional one, with improved corrosion resistance (higher Rp and R2). This implies an improvement in the expected cell integration and osteoblastic adhesion due to the more favorable surface texture, a better passivation that contributes to reducing the release of corrosion products, and a potential compromise between mechanical integrity and biological functionality.
- For zigzag geometry (α = 85°, more complex pattern), higher roughness, surface porosity, and passive multilayer film with better electrochemical behavior have been observed. This implies excellent biocompatibility potential, thanks to lower metal ion release, higher effective surface area generating better interaction with proteins and cells, and possible stimulation of osteogenesis and enhanced cell adhesion. However, mechanical strength and long-term stability should be carefully evaluated, as a highly rough surface may be more prone to wear or delamination.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lüdecke, C.; Bossert, J.; Roth, M.; Jandt, K.D. Physical vapor deposited titanium thin films for biomedical applications: Reproducibility of nanoscale surface roughness and microbial adhesion properties. Appl. Surf. Sci. 2013, 80, 578–589. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, R.; Yuan, Z.; Shu, X.; Liu, E.; Han, Z. A comparative study of the corrosion performance of titanium (Ti), titanium nitride (TiN), titanium dioxide (TiO2) and nitrogen-doped titanium oxides (N–TiO2), as coatings for biomedical applications. Ceram. Int. 2015, 41, 11844–11851. [Google Scholar] [CrossRef]
- Bertapelle, M.; Borges, J.; Mirza-Rosca, J.; Vaz, F. Corrosion Behavior of a Titanium Nanostructured Surface Fabricated by Glancing Angle Sputter Deposition. Microsc. Microanal. 2024, 30, ozae044-595. [Google Scholar] [CrossRef]
- Arshi, N.; Lu, J.; Lee, C.G.; Yoon, J.H.; Koo, B.H.; Ahmed, F. Thickness effect on properties of titanium film deposited by d.c. magnetron sputtering and electron beam evaporation techniques. Bull. Mater. Sci. 2013, 36, 807–812. [Google Scholar] [CrossRef]
- Chawla, V.; Jayaganthan, R.; Chawla, A.K.; Chandra, R. Microstructural characterizations of magnetron sputtered Ti films on glass substrate. J. Mater Process Technol. 2009, 209, 3444–3451. [Google Scholar] [CrossRef]
- Liu, G.; Yang, Y.; Luo, X.; Huang, B.; Kou, Z.; Li, P. Improving the mechanical properties of titanium films by texture strengthening. Mater. Charact. 2017, 127, 365–370. [Google Scholar] [CrossRef]
- Lopes, C.; Gabor, C.; Cristea, D.; Costa, R.; Domingues, R.; Rodrigues, M.; Borges, J.; Alves, E.; Barradas, N.; Munteanu, D.; et al. Evolution of the mechanical properties of Ti-based intermetallic thin films doped with different metals to be used as biomedical devices. Appl. Surf. Sci. 2020, 505, 144617. [Google Scholar] [CrossRef]
- Wayman, C.M. Shape memory and related phenomena. Prog. Mater. Sci. 1992, 36, 203–224. [Google Scholar] [CrossRef]
- Boyer, R.R. An overview on the use of titanium in the aerospace industry. Mater. Sci. Eng. A 1996, 213, 103–114. [Google Scholar] [CrossRef]
- Benard, W.L.; Kahn, H.; Heuer, A.H.; Huff, M.A. Thin-film shape-memory alloy actuated micropumps. J. Microelectromechanical Syst. 1998, 7, 245–251. [Google Scholar] [CrossRef]
- Wei, Z.G.; Sandström, R.; Miyazaki, S. Shape-memory materials and hybrid composites for smart systems—Part I Shape-memory materials. J. Mater. Sci. 1998, 33, 3743–3762. [Google Scholar] [CrossRef]
- Hyun, S.; Brown, W.L.; Vinci, R.P. Stress relaxation in nanoscale aluminum films. In Reliability, Testing, and Characterization of MEMS/MOEMS III; Society of Photo Optical: Bellingham, WA, USA, 2003; Volume 5343, pp. 154–162. [Google Scholar] [CrossRef]
- Jeyachandran, Y.L.; Karunagaran, B.; Narayandass, S.; Mangalaraj, D.; Jenkins, T.; Martin, P. Properties of titanium thin films deposited by dc magnetron sputtering. Mater. Sci. Eng. A 2006, 431, 277–284. [Google Scholar] [CrossRef]
- Resnik, D.; Kovač, J.; Vrtačnik, D.; Aljančič, U.; Možek, M.; Zalar, A.; Amon, S. Investigation of interface properties of Ti/Ni/Ag thin films on Si substrate. Vacuum 2007, 82, 162–165. [Google Scholar] [CrossRef]
- Lopes, C.; Alves, A.C.; Ferreira, A.; Alves, E.; Barradas, N.P.; Borsan, I.; Munteanu, D.; Vaz, F. The influence of the nanostructure design on the corrosion behaviour of TiN thin films prepared by glancing angle deposition. Mater. Chem. Phys. 2025, 329, 130100. [Google Scholar] [CrossRef]
- Zhijun, M.; Yanqing, Y.; Jiang, D.; Yan, C.; Yongqing, Z. The effect of magnetron sputter parameter on fiber coating character. Mater. Lett. 2004, 58, 2118–2121. [Google Scholar] [CrossRef]
- Musil, J.; Vlček, J.; Ježek, V.; Benda, M.; Kolega, M.; Boomsma, R. Production of Ti films with controlled texture. Surf. Coat. Technol. 1995, 76–77, 274–279. [Google Scholar] [CrossRef]
- Liu, G.; Yang, Y.; Huang, B.; Luo, X.; Ouyang, S.; Zhao, G.; Jin, N.; Li, P. Effects of substrate temperature on the structure, residual stress and nanohardness of Ti6Al4V films prepared by magnetron sputtering. Appl. Surf. Sci. 2016, 370, 53–58. [Google Scholar] [CrossRef]
- Chen, A.Y.; Bu, Y.; Tang, Y.; Wang, Y.; Liu, F.; Xie, X.; Gu, J. Deposition-rate dependence of orientation growth and crystallization of Ti thin films prepared by magnetron sputtering. Thin Solid Films 2015, 574, 71–77. [Google Scholar] [CrossRef]
- Chawla, V.; Jayaganthan, R.; Chawla, A.K.; Chandra, R. Morphological study of magnetron sputtered Ti thin films on silicon substrate. Mater. Chem. Phys. 2008, 111, 414–418. [Google Scholar] [CrossRef]
- Spârchez, C.; Lopes, C.; Gabor, C.; Munteanu, D.; Correa, M.; Vaz, F.; Ferreira, A. Characterization of GLAD-grown TiCu thin films for thermo-resistive sensing applications. Sens. Actuators A Phys. 2024, 376, 115661. [Google Scholar] [CrossRef]
- Pedrosa, P.; Ferreira, A.; Martin, N.; Pour Yazdi, M.A.; Billard, A.; Lanceros-Méndez, S.; Vaz, F. Nano-sculptured Janus-like TiAg thin films obliquely deposited by GLAD co-sputtering for temperature sensing. Nanotechnology 2018, 29, 355706. [Google Scholar] [CrossRef] [PubMed]
- Correa, M.A.; Ferreira, A.; Tromer, R.M.; Machado, L.D.; Gamino, M.; Junior, S.A.N.F.; Bohn, F.; Vaz, F. Improving the room-temperature ferromagnetism in zno and low-doped zno:Ag films using glad sputtering. Materials 2021, 14, 5337. [Google Scholar] [CrossRef]
- Hawkeye, M.M.; Taschuk, M.T.; Brett, M.J. Post-Deposition Processing and Device Integration. In Glancing Angle Deposition of Thin Films; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 227–259. [Google Scholar] [CrossRef]
- Park, C.; Yoon, J.; Thomas, E.L. Enabling nanotechnology with self assembled block copolymer patterns. Polymer 2003, 44, 6725–6760. [Google Scholar] [CrossRef]
- El-Meligi, A.A. Corrosion Preventive Strategies as a Crucial Need for Decreasing Environmental Pollution and Saving Economics. AACE Clin. Case Rep. 2021, 7, 1. [Google Scholar] [CrossRef]
- Inturi, R.B.; Szklarska-Smialowska, Z. Localized Corrosion of Nanocrystalline 304 Type Stainless Steel Films. Corrosion 1992, 48, 398–403. [Google Scholar] [CrossRef]
- Kraack, M.; Boehni, H.; Muster, W.; Patscheider, J. Influence of molybdenum on the corrosion properties of stainless steel films. Surf. Coat. Technol. 1994, 68–69, 541–545. [Google Scholar] [CrossRef]
- Fujimoto, S.; Newman, R.; Smith, G.; Kaye, S.; Kheyrandish, H.; Colligon, J. Passivation thresholds in iron-chromium alloys prepared by ion-beam sputtering. Corros. Sci. 1993, 35, 51–55. [Google Scholar] [CrossRef]
- Ryan, M.P.; Laycock, N.J.; Newman, R.C.; Isaacs, H.S. The Pitting Behavior of Iron-Chromium Thin Film Alloys in Hydrochloric Acid. J. Electrochem. Soc. 1998, 145, 1566–1571. [Google Scholar] [CrossRef]
- Eliaz, N. Corrosion of metallic biomaterials: A review. Materials 2019, 12, 407. [Google Scholar] [CrossRef]
- de la Rosa, J.E.; García-Cabezón, C.; García-Hernández, C.; Delgado-Pujol, E.J.; García-García, F.J.; Bocaccini, A.R.; Torres, Y. Enhancing corrosion resistance and bioactive behavior of porous metallic scaffolds through electrochemical coatings. Appl. Surf. Sci. Adv. 2025, 26, 100723. [Google Scholar] [CrossRef]
- Rodrigues, M.S.; Borges, J.; Proença, M.; Pedrosa, P.; Martin, N.; Romanyuk, K.; Kholkin, A.L.; Vaz, F. Nanoplasmonic response of porous Au-TiO2 thin films prepared by oblique angle deposition. Nanotechnology 2019, 30, 225701. [Google Scholar] [CrossRef]
- Pedrosa, P.; Fiedler, P.; Lopes, C.; Alves, E.; Barradas, N.P.; Haueisen, J.; Machado, A.V.; Fonseca, C.; Vaz, F. Ag:TiN-Coated Polyurethane for Dry Biopotential Electrodes: From Polymer Plasma Interface Activation to the First EEG Measurements. Plasma Process. Polym. 2016, 13, 341–354. [Google Scholar] [CrossRef]
- ASTM G102-89; Standard Reference Test Method for Making Potentiostatic and Potentiodynamic Anodic. ASTM Standards: West Conshohocken, PA, USA, 2004.
- ISO 16773-1-4:2016; Electrochemical Impedance Spectroscopy (EIS) on Coated and Uncoated Metallic Specimens. ISO: Geneva, Switzerland, 2016.
- Thornton, J.A. Influence of Apparatus Geometry and Deposition Conditions on The Structure and Topography of Thick Sputtered Coatings. J. Vac. Sci. Technol. 1974, 11, 666–670. [Google Scholar] [CrossRef]
- Petrov, I.; Barna, P.B.; Hultman, L.; Greene, J.E. Microstructural evolution during film growth. J. Vac. Sci. Technol. A 2003, 21, S117–S128. [Google Scholar] [CrossRef]
- Tucker, R.C. Surface cleaning and coating removal by lasers. In ASM Handbook Volume 5: Surface Engineering; ASM International: Almere, The Netherlands, 1994; pp. 431–464. [Google Scholar]
- Mahieu, S.; Ghekiere, P.; Depla, D.; de Gryse, R. Biaxial alignment in sputter deposited thin films. Thin Solid Films 2006, 515, 1229–1249. [Google Scholar] [CrossRef]
- Mahieu, S.; de Winter, G.; Depla, D.; de Gryse, R.; Denul, J. A model for the development of biaxial alignment in yttria stabilized zirconia layers, deposited by unbalanced magnetron sputtering. Surf. Coat. Technol. 2004, 187, 122–130. [Google Scholar] [CrossRef]
- Barranco, A.; Borras, A.; Gonzalez-Elipe, A.R.; Palmero, A. Perspectives on oblique angle deposition of thin films: From fundamentals to devices. Prog. Mater. Sci. 2016, 76, 59–153. [Google Scholar] [CrossRef]
- Alvarez, R.; García-Martín, J.M.; Macías-Montero, M.; Gonzalez-Garcia, L.; González, J.C.; Rico, V.; Perlich, J.; Cotrino, J.; González-Elipe, A.R.; Palmero, A. Growth regimes of porous gold thin films deposited by magnetron sputtering at oblique incidence: From compact to columnar microstructures. Nanotechnology 2013, 24, 045604. [Google Scholar] [CrossRef] [PubMed]
- Lifshitz, Y.; Edrei, R.; Hoffman, A.; Grossman, E.; Lempert, G.; Berthold, J.; Schultrich, B.; Jäger, H. Surface roughness evolution and growth mechanism of carbon films from hyperthermal species. Diam. Relat. Mater. 2007, 16, 1771–1776. [Google Scholar] [CrossRef]
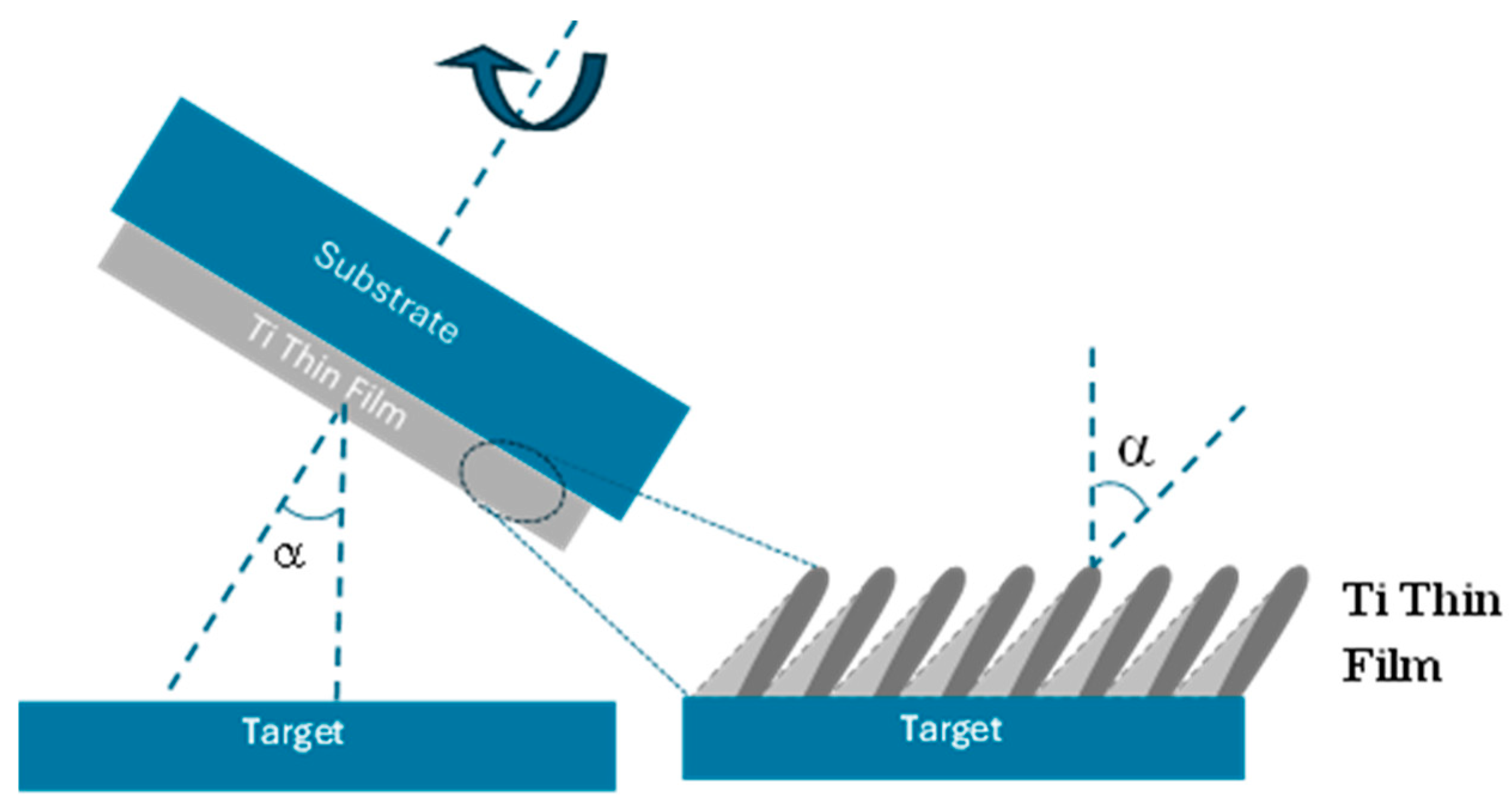

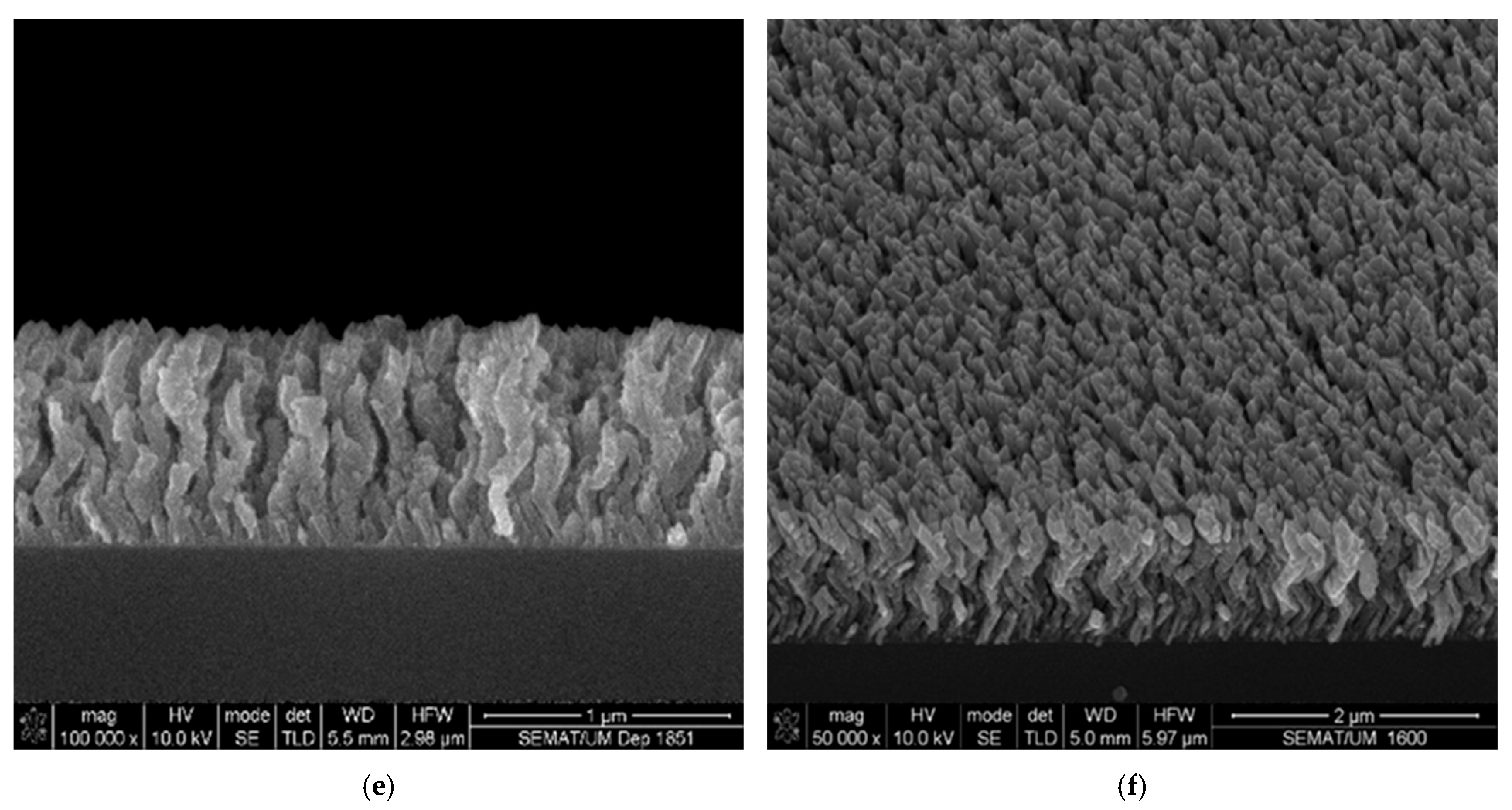
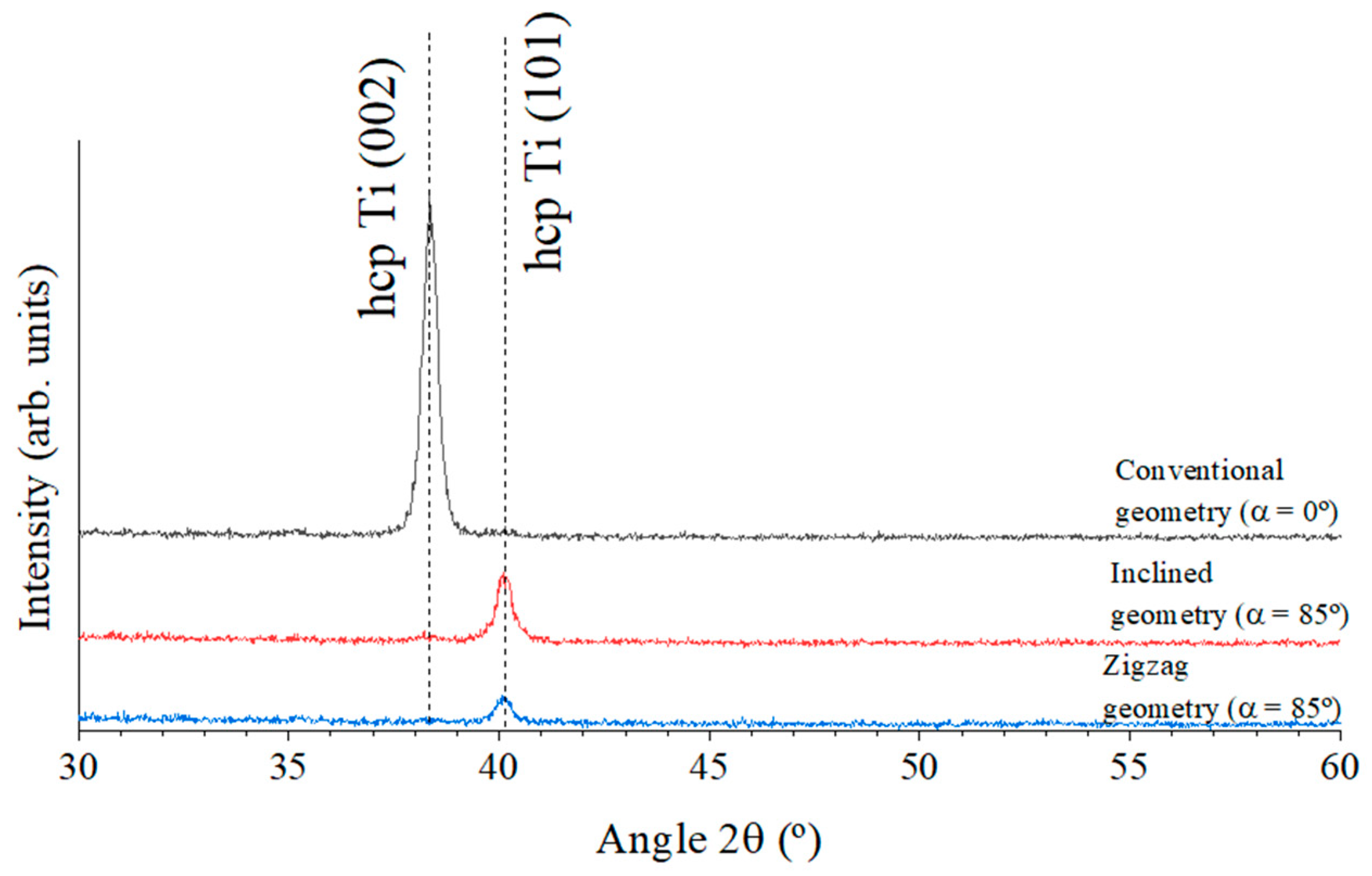
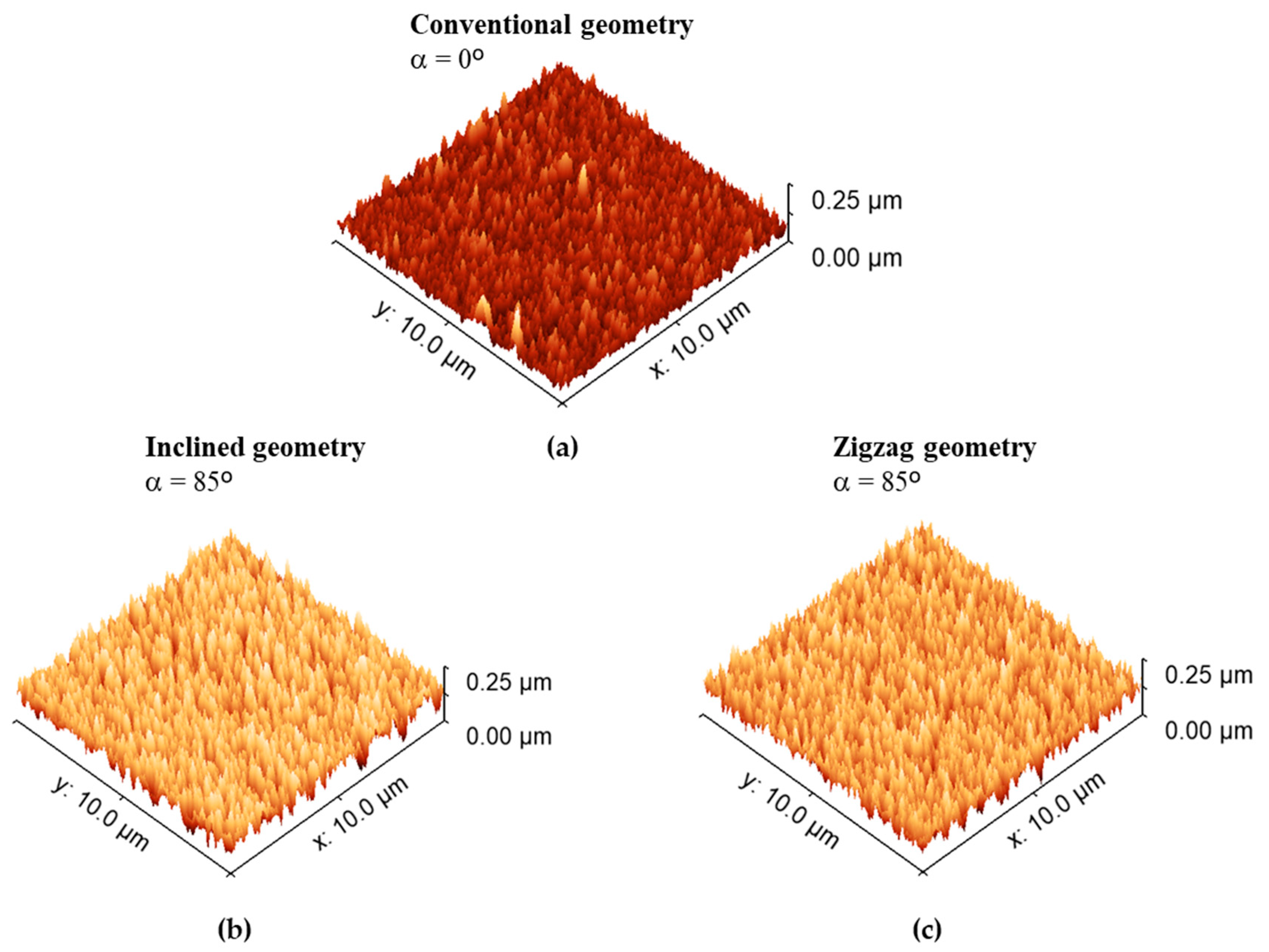
| Parameter | Conventional (α = 0°) | Inclined (α = 85°) | Zigzag (α = 85°) |
|---|---|---|---|
| Ecorr (mV vs. Ref.) | −191 ± 12 | −116 ± 8 | −49 ± 3 |
| Icorr (µA) | (7 ± 2.8) × 10−2 | (1.90 ± 0.8) × 10−2 | (5 ± 1.6) × 10−3 |
| βc (mV/dec) | 12.10 ± 2.4 | 6.40 ± 1.1 | 15.6 ± 2.7 |
| βa (mV/dec) | 11.10 ± 1.5 | 7.60 ± 0.6 | 17.1 ± 1.8 |
| Corrosion rate (mpy) | (9 ± 2.4) × 10−3 | (2.45 ± 0.21) × 10−3 | (6.44 ± 1.23) × 10−4 |
| Rp (Ohm) | (2.80 ± 0.21) × 104 | (6.4 ± 0.35) × 104 | (3.52 ± 0.66) × 105 |
| Parameters | Conventional (α = 0°) | Inclined (α = 85°) | Zigzag (α = 85°) |
|---|---|---|---|
| Y1 (Ohm·cm2) | 1.5 × 10−4 | 1.8 × 10−4 | 1.3 × 10−7 |
| n1 | 0.68 | 0.52 | 0.89 |
| R1 (Ohm·cm2) | 8.6 × 103 | 1.3 × 104 | 1.2 × 105 |
| Y2 (S·secn/cm2) | 7.0 × 10−5 | 9.2 × 10−10 | 6.3 × 10−8 |
| n2 | 0.42 | 1.00 | 0.96 |
| R2 (Ohm·cm2) | 2.8 × 103 | 3.7 × 102 | 3.1 × 105 |
| Y3 (S·secn/cm2) | 1.4 × 10−6 | 2.1 × 10−6 | 9.0 × 10−5 |
| n3 | 0.60 | 0.69 | 0.39 |
| R3 (Ohm·cm2) | 5.7 × 103 | 1.5 × 104 | 5.0 × 104 |
| χ2 | 3.7 × 10−4 | 1.3 × 10−3 | 8.5 × 10−4 |
| Parameter | Conventional (α = 0°) | Inclined (α = 85°) | Zigzag (α = 85°) |
|---|---|---|---|
| Thickness (μm) | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 |
| Roughness (nm) | 8 ± 0.6 | 32 ± 1.0 | 30 ± 1.0 |
| Crystallinity | 002 | 101 | 101 |
| Corrosion rate (mpy) | 0.19 ± 0.03 | 0.04 ± 0.01 | 0.006 ± 0.0005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertapelle, M.; Borges, J.; Mirza-Rosca, J.C.; Vaz, F. Effect of the Different Growth Shapes on the Electrochemical Behavior of Ti Thin Films for Medical Applications. Materials 2025, 18, 3959. https://doi.org/10.3390/ma18173959
Bertapelle M, Borges J, Mirza-Rosca JC, Vaz F. Effect of the Different Growth Shapes on the Electrochemical Behavior of Ti Thin Films for Medical Applications. Materials. 2025; 18(17):3959. https://doi.org/10.3390/ma18173959
Chicago/Turabian StyleBertapelle, Matteo, Joel Borges, Julia Claudia Mirza-Rosca, and Filipe Vaz. 2025. "Effect of the Different Growth Shapes on the Electrochemical Behavior of Ti Thin Films for Medical Applications" Materials 18, no. 17: 3959. https://doi.org/10.3390/ma18173959
APA StyleBertapelle, M., Borges, J., Mirza-Rosca, J. C., & Vaz, F. (2025). Effect of the Different Growth Shapes on the Electrochemical Behavior of Ti Thin Films for Medical Applications. Materials, 18(17), 3959. https://doi.org/10.3390/ma18173959











