Unveiling Brass-Doped CoSb3-Based Thermoelectric Materials Using Solid-State Reaction
Abstract
1. Introduction
2. Experimental
3. Results and Discussion
3.1. Analysis of Phase Composition and Microstructure
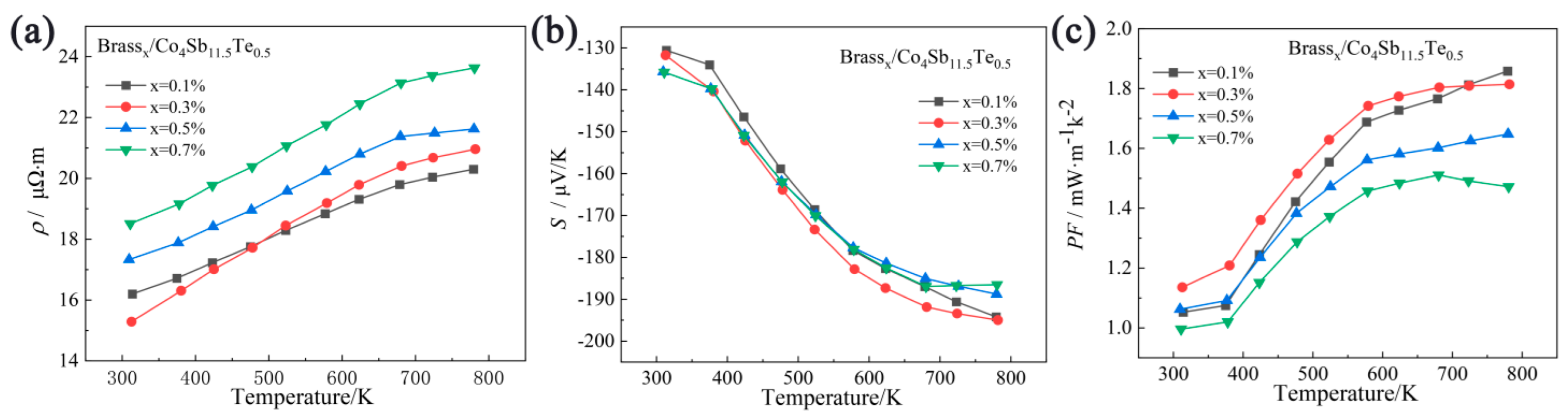
3.2. Analysis of Electrical Transport Properties
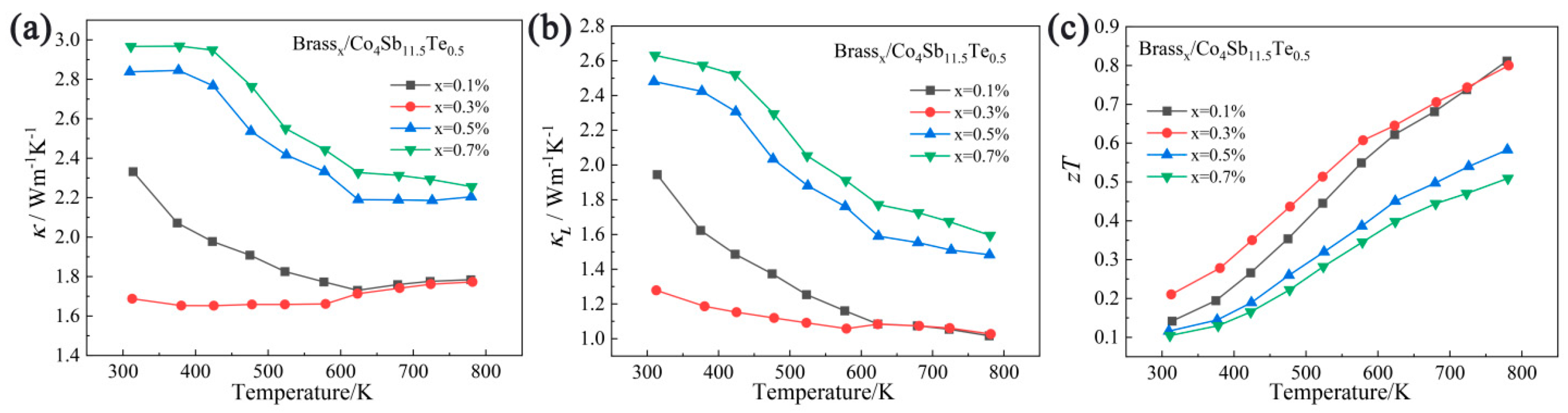
3.3. Analysis of Thermal Transport Properties
3.4. Analysis of Thermoelectric Figure of Merit (ZT Value)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef] [PubMed]
- González-Barrios, M.; Tabuyo-Martínez, M.; Ávila-Brande, D.; Prado-Gonjal, J. Perspective on Crystal Structures, Synthetic Methods, and New Directions in Thermoelectric Materials. Small Struct. 2024, 5, 2400136. [Google Scholar] [CrossRef]
- He, J.; Tritt, T.M. Advances in thermoelectric materials research: Looking back and moving forward. Science 2017, 357, eaak9997. [Google Scholar] [CrossRef]
- Wolf, M.; Hinterding, R.; Feldhoff, A. High power factor vs. high ZT—A review of thermoelectric materials for high-temperature application. Entropy 2019, 21, 1058. [Google Scholar] [CrossRef]
- Yin, Y.; Tudu, B.; Tiwari, A. Recent advances in oxide thermoelectric materials and modules. Vacuum 2017, 146, 356–374. [Google Scholar] [CrossRef]
- Finn, P.A.; Asker, C.; Wan, K.; Bilotti, E.; Fenwick, O.; Nielsen, C.B. Thermoelectric materials: Current status and future challenges. Front. Electron. Mater. 2021, 1, 677845. [Google Scholar] [CrossRef]
- Shi, X.; Chen, L.; Uher, C. Recent advances in high-performance bulk thermoelectric materials. Int. Mater. Rev. 2016, 61, 379–415. [Google Scholar] [CrossRef]
- Mukherjee M, Srivastava A, Singh A K. Recent advances in designing thermoelectric materials. J. Mater. Chem. C 2022, 10, 12524–12555. [Google Scholar] [CrossRef]
- Qiu, P.; Shi, X.; Chen, L. Cu-based thermoelectric materials. Energy Storage Mater. 2016, 3, 85–97. [Google Scholar] [CrossRef]
- Zheng, Y.; Slade, T.J.; Hu, L.; Tan, X.Y.; Luo, Y.; Luo, Z.-Z.; Xu, J.; Yan, Q.; Kanatzidis, M.G. Defect engineering in thermoelectric materials: What have we learned? Chem. Soc. Rev. 2021, 50, 9022–9054. [Google Scholar] [CrossRef]
- Li, D.; Gong, Y.; Chen, Y.; Lin, J.; Khan, Q.; Zhang, Y.; Li, Y.; Zhang, H.; Xie, H. Recent progress of two-dimensional thermoelectric materials. Nano-Micro Lett. 2020, 12, 36. [Google Scholar] [CrossRef]
- Rogl, G.; Rogl, P. Skutterudites, a most promising group of thermoelectric materials. Curr. Opin. Green Sustain. Chem. 2017, 4, 50–57. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, M.; Liu, Z. Research progress on preparation methods of skutterudites. Inorganics 2022, 10, 106. [Google Scholar] [CrossRef]
- Qin, D.; Shi, W.; Lu, Y.; Cai, W.; Liu, Z.; Sui, J. Roles of interface engineering in performance optimization of skutterudite-based thermoelectric materials. Carbon Neutraliz. 2022, 1, 233–246. [Google Scholar] [CrossRef]
- Rogl, G.; Rogl, P.F. Filled Sb-based skutterudites from 1996–2022. Crystals 2022, 12, 1843. [Google Scholar] [CrossRef]
- Liang, T.; Su, X.; Yan, Y.; Zheng, G.; Zhang, Q.; Chi, H.; Tang, X.; Uher, C. Ultra-fast synthesis and thermoelectric properties of Te doped skutterudites. J. Mater. Chem. A 2014, 2, 17914–17918. [Google Scholar] [CrossRef]
- Matsubara, M.; Asahi, R. Optimization of filler elements in CoSb3-based skutterudites for high-performance n-type thermoelectric materials. J. Electron. Mater. 2016, 45, 1669–1678. [Google Scholar] [CrossRef]
- Rull-Bravo, M.; Moure, A.; Fernández, J.F.; Martín-González, M. Skutterudites as thermoelectric materials: Revisited. RSC Adv. 2015, 5, 41653–41667. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, T.; Wang, Y.; Ruan, X.; Jin, C.; Xia, A. High-performance n-type CoSb3-based thermoelectric material with vortex and strip-shaped grain structures. J. Eur. Ceram. Soc. 2023, 43, 1985–1991. [Google Scholar] [CrossRef]
- Liu, W.; Hu, J.; Zhang, S.; Deng, M.; Han, C.-G.; Liu, Y. New trends, strategies and opportunities in thermoelectric materials: A perspective. Mater. Today Phys. 2017, 1, 50–60. [Google Scholar] [CrossRef]
- Rao, X.; Zhong, Y.; Feng, H.; Wang, Y.; Tan, X.; Zhu, J.; Ang, R. Structure optimization and multi-frequency phonon scattering boosting thermoelectrics in self-doped CoSb3-based skutterudites. ACS Appl. Mater. Interfaces 2023, 15, 5301–5308. [Google Scholar] [CrossRef]
- Mao, J.; Wang, Y.; Kim, H.S.; Liu, Z.; Saparamadu, U.; Tian, F.; Dahal, K.; Sun, J.; Chen, S.; Liu, W.; et al. High thermoelectric power factor in Cu–Ni alloy originate from potential barrier scattering of twin boundaries. Nano Energy 2015, 17, 279–289. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, Z.; Zhu, Z.; Liu, W. Research status of high strength and anti-attrition brass. Nonferrous Met. Sci. Eng. 2012, 3, 23–29. [Google Scholar]
- Stavroulakis, P.; Toulfatzis, A.I.; Pantazopoulos, G.A.; Paipetis, A.S. Machinable leaded and eco-friendly brass alloys for high performance manufacturing processes: A critical review. Metals 2022, 12, 246. [Google Scholar] [CrossRef]
- Radhi, H.N.; Aljassani, A.M.H.; Mohammed, M.T. Effect of ECAP on microstructure, mechanical and tribological properties of aluminum and brass alloys: A review. Mater. Today Proc. 2020, 26, 2302–2307. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.; Ma, H.; Deng, S.; Wang, H.; Xiong, R.; Huang, S. Enhancing the thermoelectric performance of CoSb3-based skutterudite by decoupling the electrical and thermal properties by embedding Cu nanoparticles. Ceram. Int. 2021, 47, 28268–28273. [Google Scholar] [CrossRef]
- Ji, Y.; Qin, B.; Huang, R.; Zhang, J. Study on solid-state reaction synthesis and thermoelectric properties of Cu-filled skutterudite. Bull. Chin. Ceram. Soc. 2020, 39, 2343–2348. [Google Scholar]
- Qin, B.; Ji, Y.; Lei, Y.; Li, Y. Thermoelectric properties of (Cu, Te) co-doped CoSb3 synthesized by high-pressure and high-temperature. Mater. Sci. Semicond. Process. 2024, 180, 108556. [Google Scholar] [CrossRef]
- Luo, Z.Z.; Cai, S.; Hao, S.; Bailey, T.P.; Luo, Y.; Luo, W.; Yu, Y.; Uher, C.; Wolverton, C.; Dravid, V.P.; et al. Extraordinary role of Zn in enhancing thermoelectric performance of Ga-doped n-type PbTe. Energy Environ. Sci. 2022, 15, 368–375. [Google Scholar] [CrossRef]
- Narducci, D.; Giulio, F. Recent advances on thermoelectric silicon for low-temperature applications. Materials 2022, 15, 1214. [Google Scholar] [CrossRef]
- Nozariasbmarz, A.; Agarwal, A.; Coutant, Z.A.; Hall, M.J.; Liu, J.; Liu, R.; Malhotra, A.; Norouzzadeh, P.; Öztürk, M.C.; Ramesh, V.P.; et al. Thermoelectric silicides: A review. Jpn. J. Appl. Phys. 2017, 56, 05DA04. [Google Scholar] [CrossRef]
- Baghdadi, N.; Salah, N.; Alshahrie, A.; Koumoto, K. Microwave irradiation to produce high performance thermoelectric material based on Al doped ZnO nanostructures. Crystals 2020, 10, 610. [Google Scholar] [CrossRef]
- Xu, Q.; Ming, C.; Xing, T.; Qiu, P.; Xiao, J.; Shi, X.; Chen, L. Thermoelectric properties of phosphorus-doped van der Waals crystal Ta4SiTe4. Mater. Today Phys. 2021, 19, 100417. [Google Scholar] [CrossRef]
- Mukherjee, S.; Parasuraman, R.; Umarji, A.M.; Rogl, G.; Rogl, P.; Chattopadhyay, K. Effect of Fe alloying on the thermoelectric performance of Cu2Te. J. Alloys Compd. 2020, 817, 152729. [Google Scholar] [CrossRef]
- Wei, J.; Yang, L.; Ma, Z.; Song, P.; Zhang, M.; Ma, J.; Yang, F.; Wang, X. Review of current high-ZT thermoelectric materials. J. Mater. Sci. 2020, 55, 12642–12704. [Google Scholar] [CrossRef]
- Kruszewski, M.J.; Zybała, R. Review of rapid fabrication methods of skutterudite materials. Mater. Today Proc. 2021, 44, 3475–3482. [Google Scholar] [CrossRef]
- Jasper, S.; Subash, R.; Muthuneelakandan, K.; Vijayakumar, D.; Ida, S.J. The Mechanical Properties of Brass Alloys: A Review. Eng. Proc. 2025, 93, 11. [Google Scholar]
- Yu, Y.; He, D.-S.; Zhang, S.; Cojocaru-Mirédin, O.; Schwarz, T.; Stoffers, A.; Wang, X.-Y.; Zheng, S.; Zhu, B.; Scheu, C.; et al. Simultaneous optimization of electrical and thermal transport properties of Bi0. 5Sb1. 5Te3 thermoelectric alloy by twin boundary engineering. Nano Energy 2017, 37, 203–213. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, C.; Zhang, S.; Zhu, M.; Wuttig, M.; Scheu, C.; Raabe, D.; Snyder, G.J.; Gault, B.; Cojocaru-Mirédin, O. Revealing nano-chemistry at lattice defects in thermoelectric materials using atom probe tomography. Mater. Today 2020, 32, 260–274. [Google Scholar] [CrossRef]
- Qin, B.; Ji, Y.; Bai, Z.; Huang, R.; Zhang, J. Thermoelectric properties of nanostructured CoSb3 fast prepared by solid-state reaction. Rare Met. Mater. Engineering. 2019, 48, 3118–3123. [Google Scholar]
- Zhang, Q. Synthesis and Performance Study of CoSb3-Based Skutterudite Thermoelectric Materials. Ph.D. Thesis, Yanshan University, Qinhuangdao, China, 2015. [Google Scholar]
- Fortulan, R.; Aminorroaya Yamini, S. Recent progress in multiphase thermoelectric materials. Materials 2021, 14, 6059. [Google Scholar] [CrossRef] [PubMed]
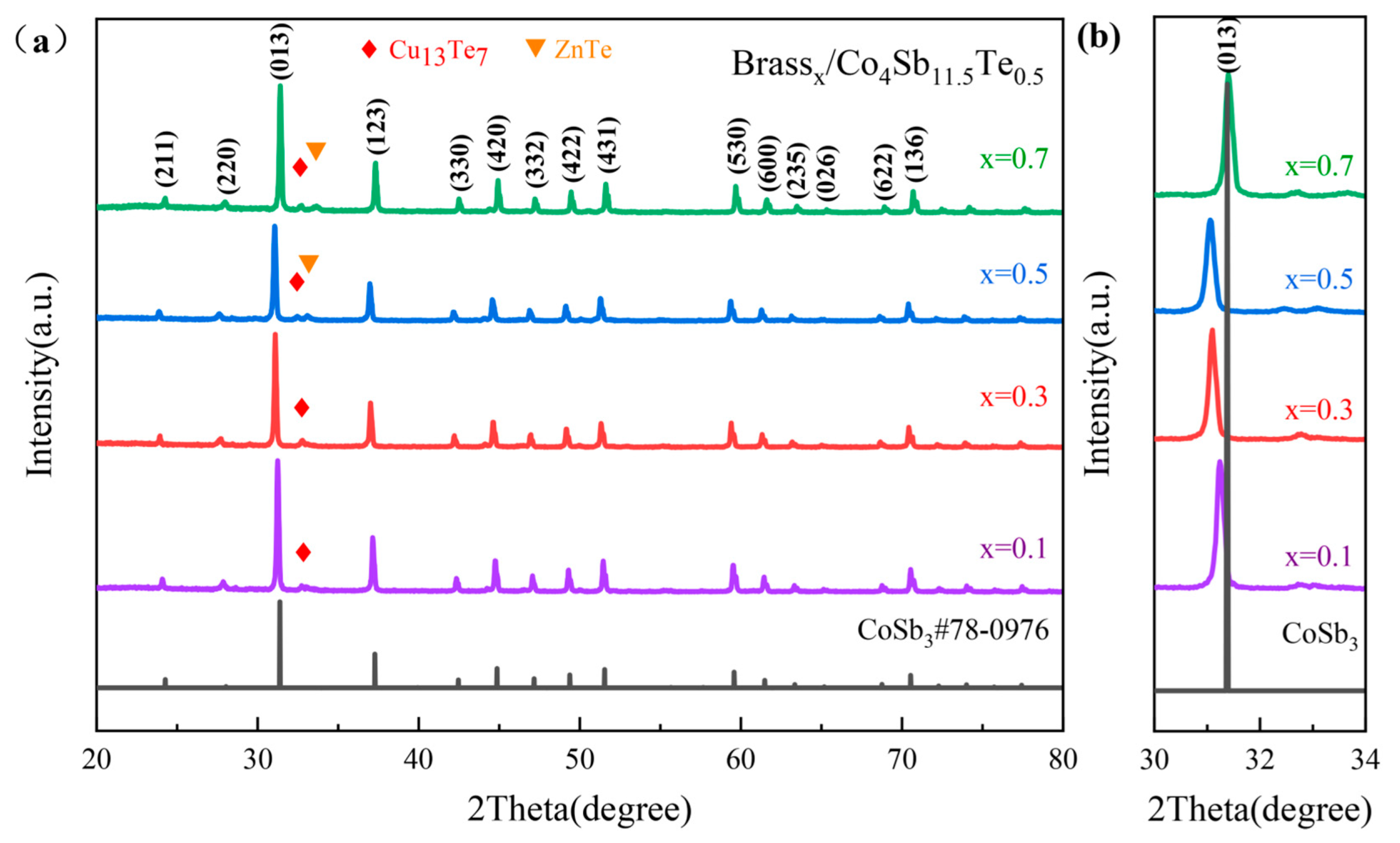
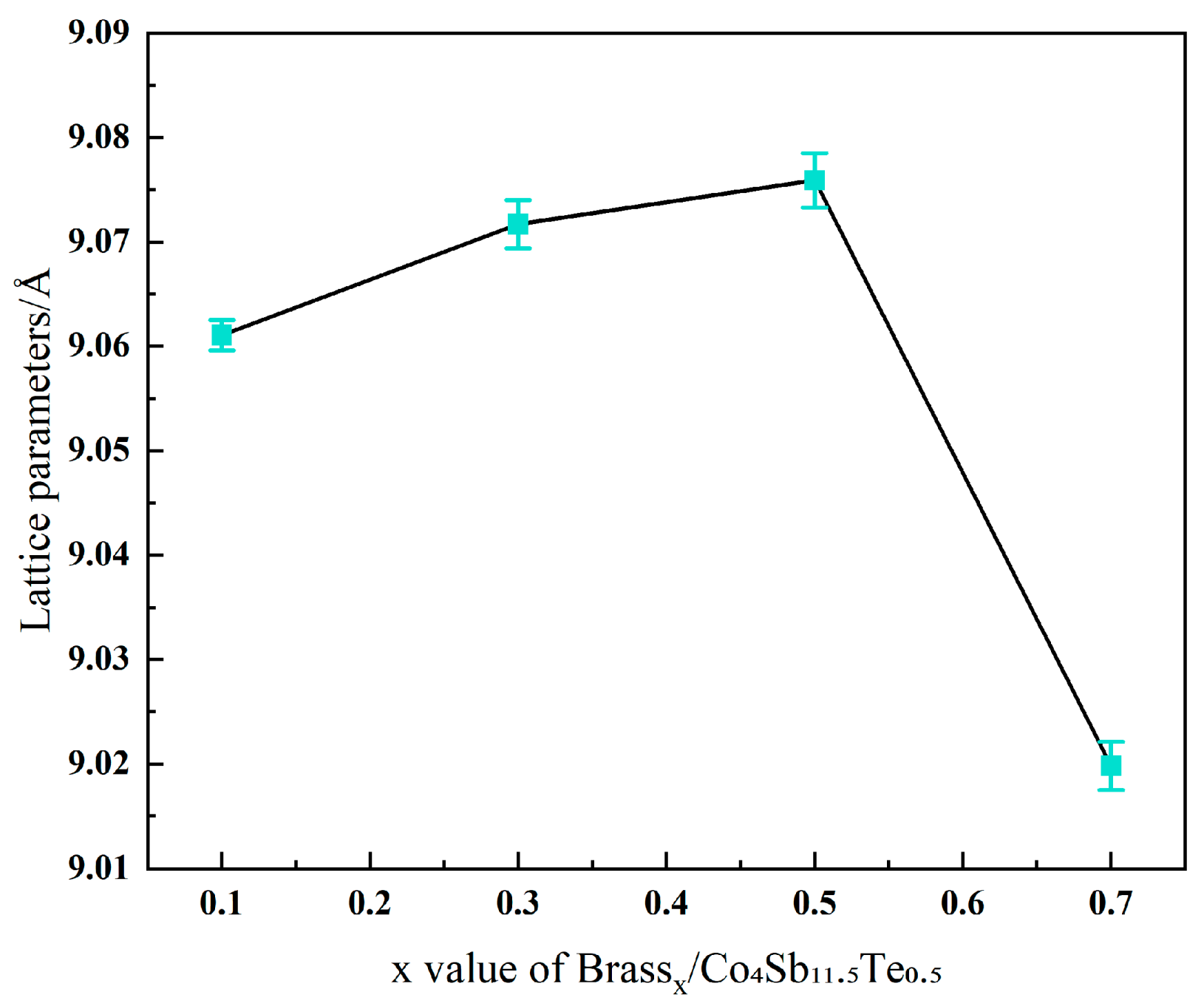
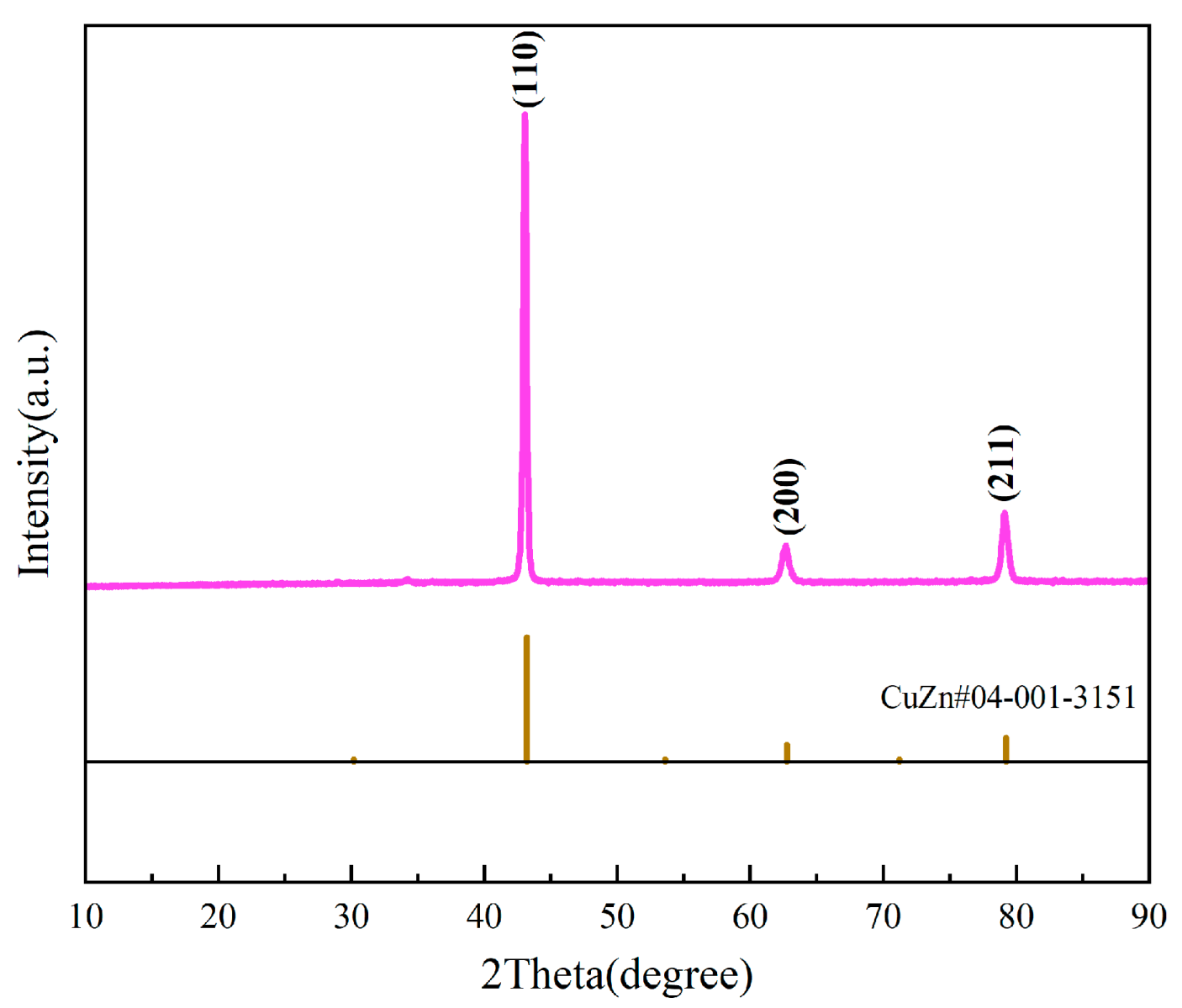
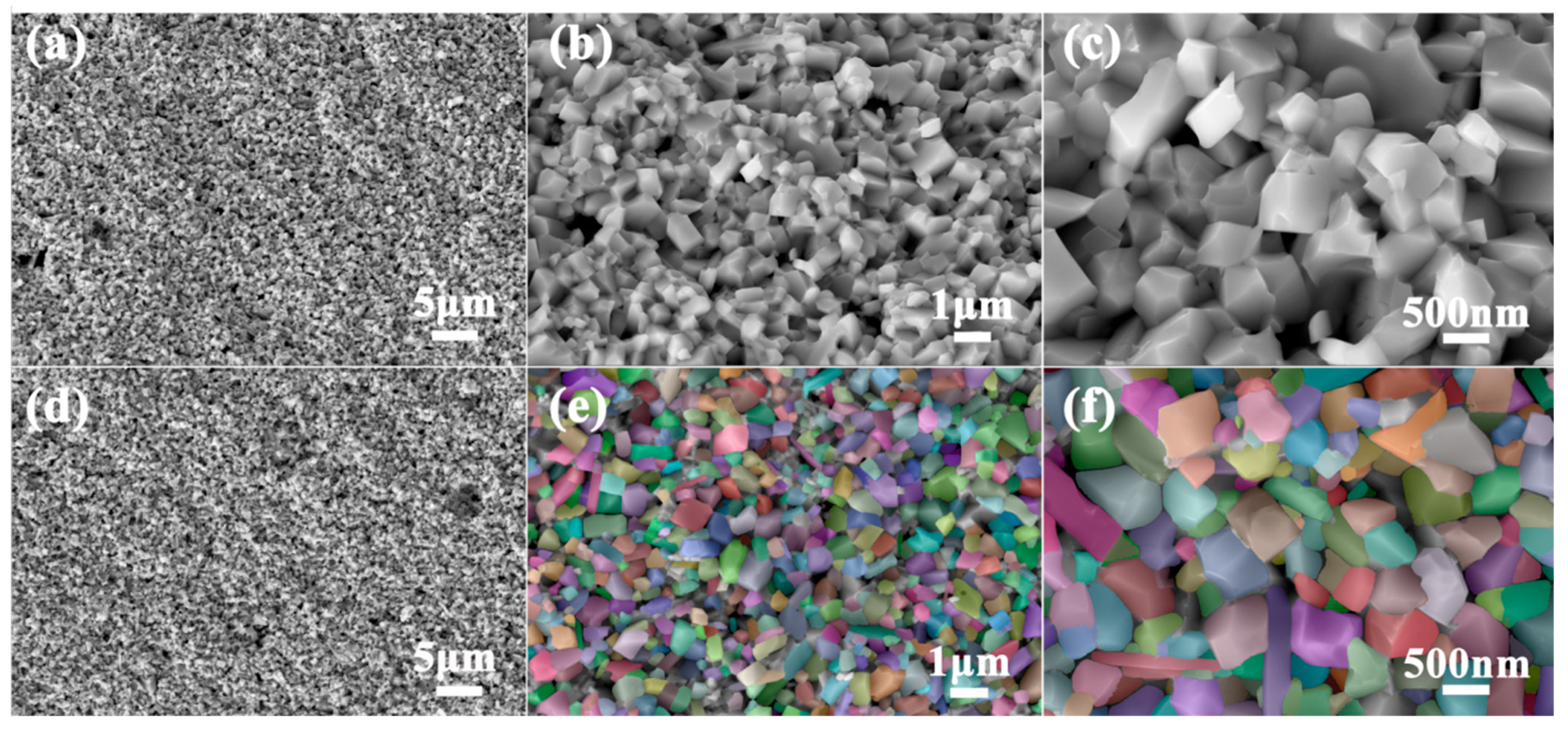


| Component | Test Results (Mass%) | Elemental Spectrum | Intensity |
|---|---|---|---|
| Zn | 50.5 | Zn-KA | 40.1096 |
| Cu | 48.8 | Cu-KA | 38.7163 |
| Si | 0.649 | Si-KA | 0.5156 |
| Al | 0.0222 | Al-KA | 0.0104 |
| P | 0.0205 | P-KA | 0.0163 |
| Fe | 0.0201 | Fe-KA | 0.0160 |
| Samples | Principal Chemical Elements | Atoms per Formula Unit | Corresponding at.% |
|---|---|---|---|
| Brass0.1/Co4Sb11.5Te0.5 | Cu | 0.01307 | 0.08166 |
| Zn | 0.01314 | 0.08214 | |
| Brass0.3/Co4Sb11.5Te0.5 | Cu | 0.03920 | 0.2450 |
| Zn | 0.03942 | 0.2464 | |
| Brass0.5/Co4Sb11.5Te0.5 | Cu | 0.06534 | 0.4084 |
| Zn | 0.06571 | 0.4107 | |
| Brass0.5/Co4Sb11.5Te0.5 | Cu | 0.09148 | 0.5717 |
| Zn | 0.09200 | 0.5750 |
| Total particles | 175 |
| Particle size information | |
| Average particle size | 371.133 nm |
| Particle size standard deviation | 181.213 nm |
| P10 | 182.926 nm |
| P50 | 505.964 nm |
| P90 | 829.002 nm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, D.; Ji, Y.; Qin, B.; Fan, J.; Lv, X.; Huang, R. Unveiling Brass-Doped CoSb3-Based Thermoelectric Materials Using Solid-State Reaction. Materials 2025, 18, 3928. https://doi.org/10.3390/ma18173928
Zhao D, Ji Y, Qin B, Fan J, Lv X, Huang R. Unveiling Brass-Doped CoSb3-Based Thermoelectric Materials Using Solid-State Reaction. Materials. 2025; 18(17):3928. https://doi.org/10.3390/ma18173928
Chicago/Turabian StyleZhao, Dan, Yonghua Ji, Bingke Qin, Jiaxin Fan, Xiaodong Lv, and Run Huang. 2025. "Unveiling Brass-Doped CoSb3-Based Thermoelectric Materials Using Solid-State Reaction" Materials 18, no. 17: 3928. https://doi.org/10.3390/ma18173928
APA StyleZhao, D., Ji, Y., Qin, B., Fan, J., Lv, X., & Huang, R. (2025). Unveiling Brass-Doped CoSb3-Based Thermoelectric Materials Using Solid-State Reaction. Materials, 18(17), 3928. https://doi.org/10.3390/ma18173928





