Use of Abandoned Copper Tailings as a Precursor to the Synthesis of Fly-Ash-Based Alkali Activated Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling of Raw Materials
2.2. Raw Materials and FA-AAMs Characterization
2.3. Preparation of FA-AAMs
2.4. Physical and Mechanical Properties of FA-AAMs
2.5. Leaching Test Conducted on FA-AAMs
2.6. Statistical Methods
3. Results and Discussion
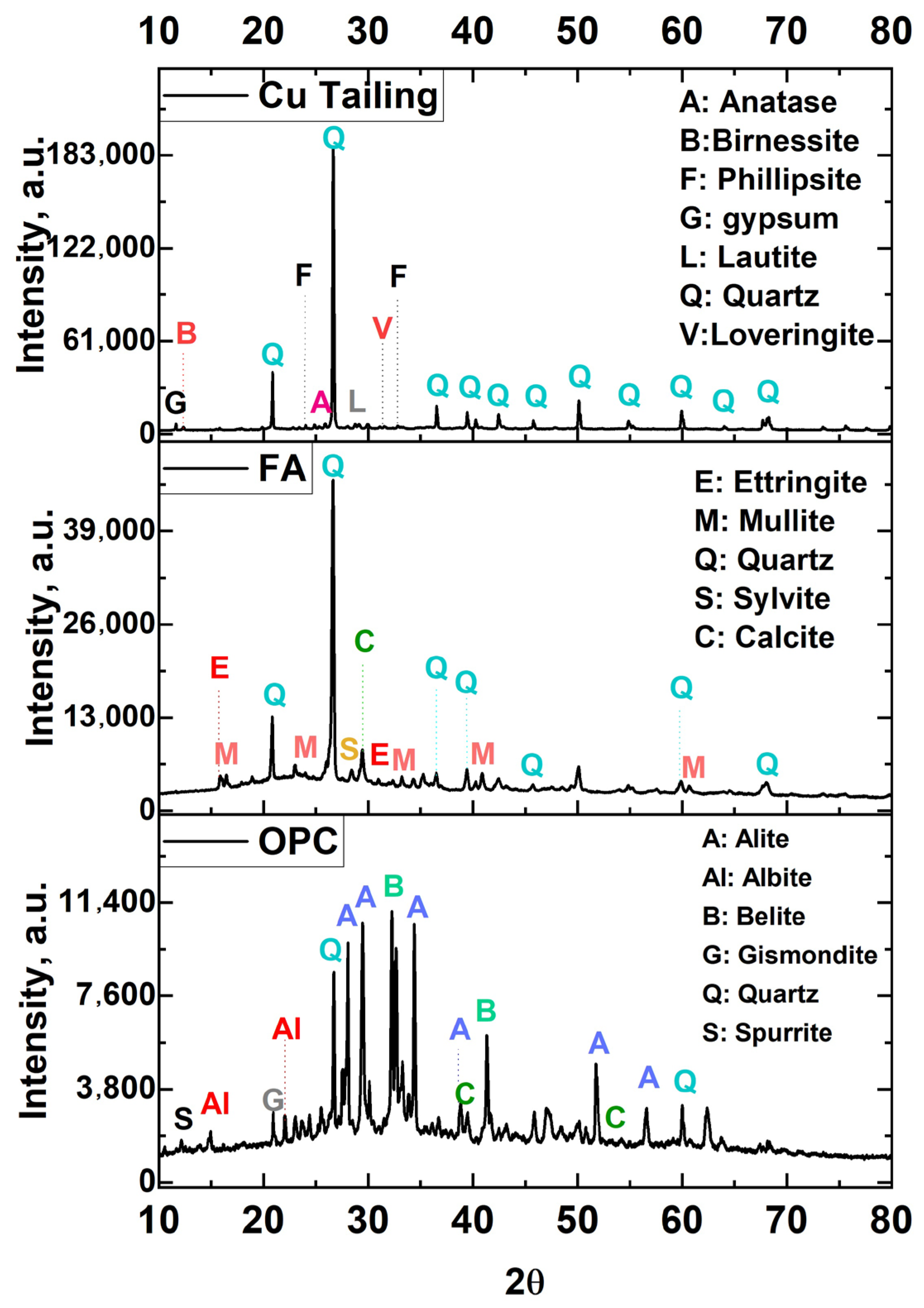
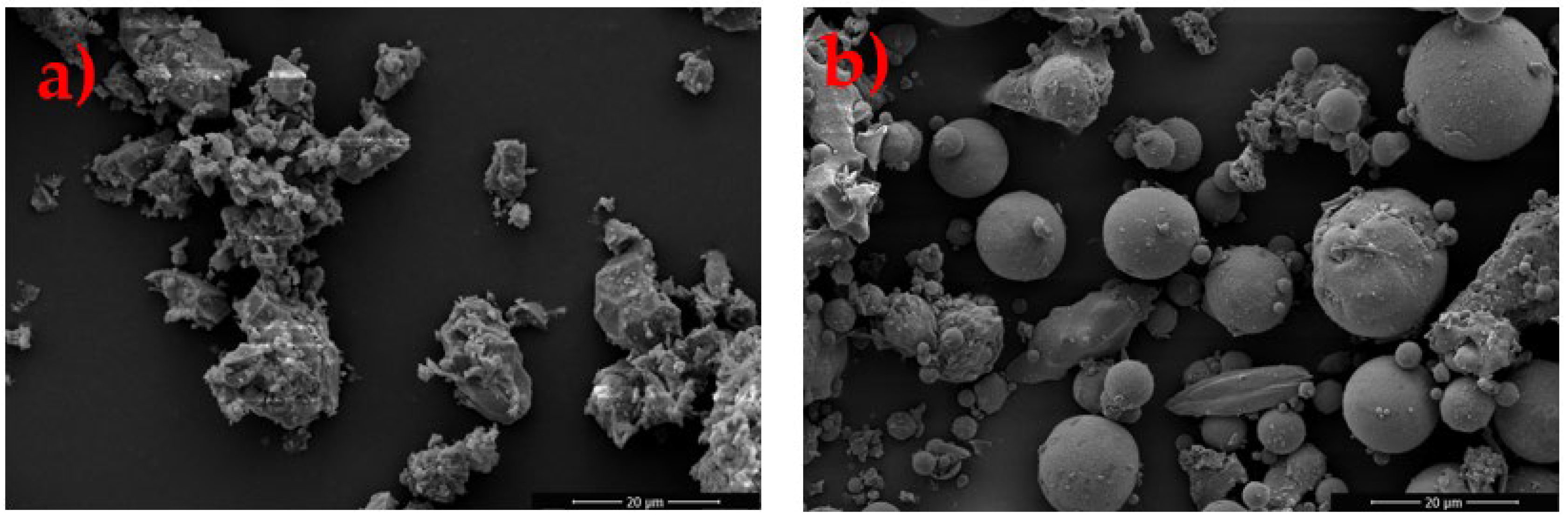
3.1. Chemical, Physical and Mineralogical Composition of Raw Materials
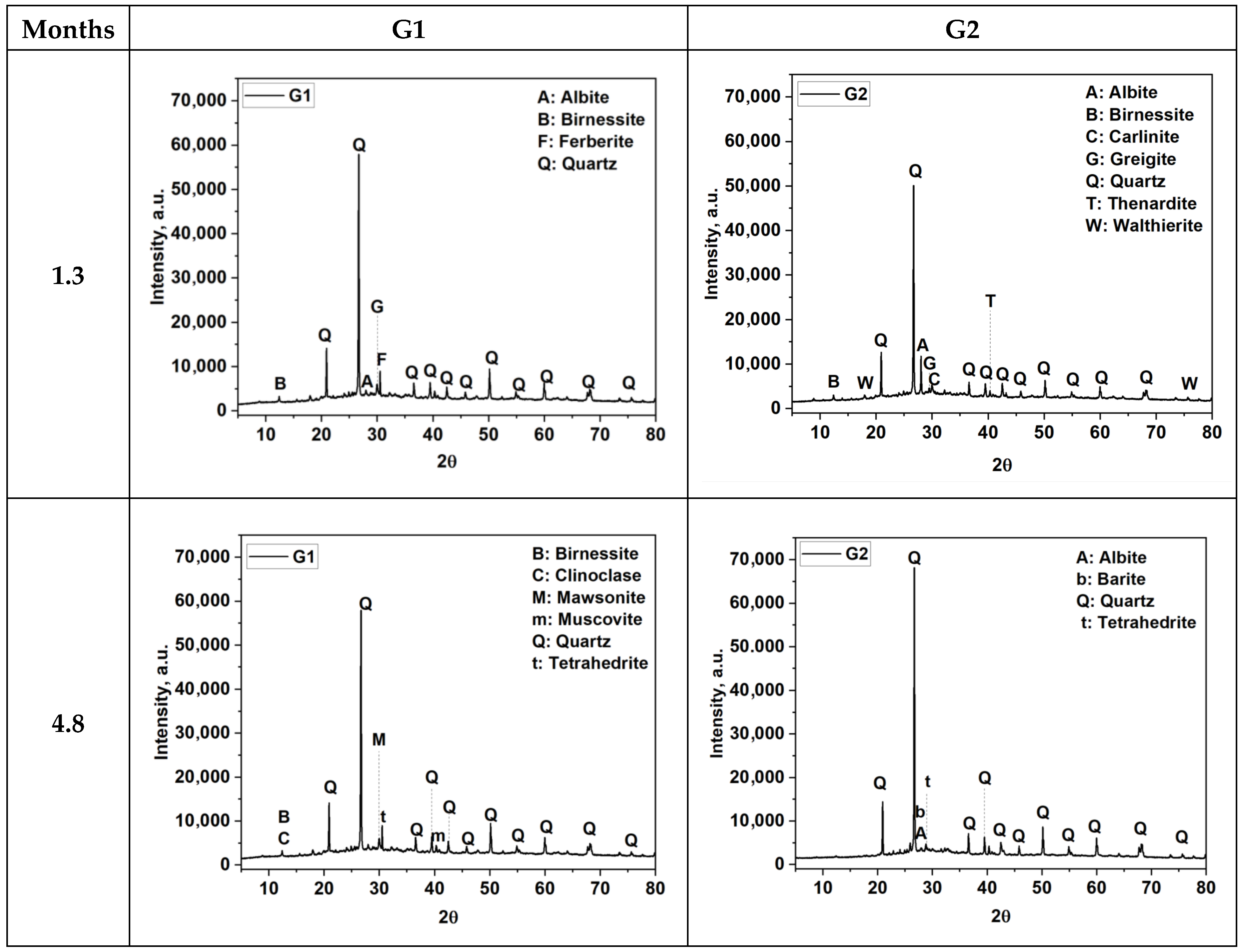
3.2. Mineralogical Composition of FA-AAMs
3.3. Mechanical Properties of FA-AAMs
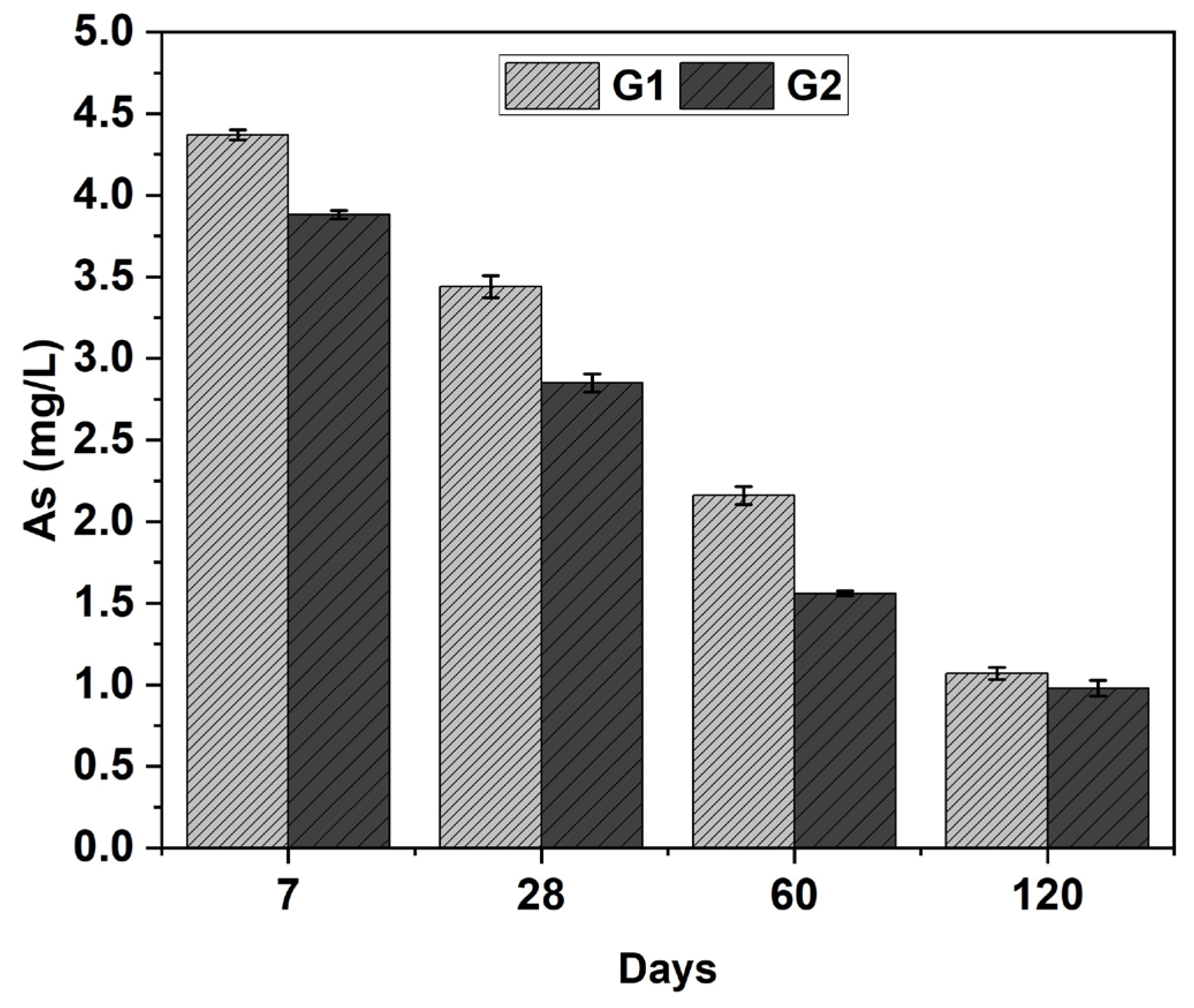
3.4. Leaching
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAMs | Alkali-activated materials |
| C–(A–)S–H | Calcium (alumino) silicate hydrate |
| C-(N)-A-S-H | Calcium (alkali) aluminosilicate hydrate |
| CaO | Calcium oxide |
| C–S–H | Calcium silicate hydrate |
| EDS | Energy Dispersive Spectroscopy |
| FA | Fly ash |
| FA-AAMs | Fly-ash-based alkali-activated materials |
| FESEM | Field Emission Scanning Electron Microscopy |
| HAAC | Hybrid alkali-activated cement |
| LOI | Loss on Ignition |
| MgO | Magnesium oxide |
| N-A-S-H | Sodium aluminosilicate hydrate |
| OPC | Ordinary Portland cement |
| PDS | Particle size distribution |
| SCM | Supplementary cementitious materials |
| SH | Sodium hydroxide |
| SS | Sodium silicate |
| SSA | Specific surface area |
| TCLP | Toxicity Characteristic Leaching Procedure |
| XRF | X-ray diffraction |
References
- Xu, D.M.; Zhan, C.L.; Liu, H.X.; Lin, H.Z. A critical review on environmental implications, recycling strategies, and ecological remediation for mine tailings. Environ. Sci. Pollut. Res. 2019, 26, 35657–35669. [Google Scholar] [CrossRef] [PubMed]
- Sernageomin. Public Data on Tailings Deposits. Available online: https://www.sernageomin.cl/datos-publicos-deposito-de-relaves/ (accessed on 16 December 2024).
- Reyes, A.; Cuevas, J.; Fuentes, B.; Fernández, E.; Arce, W.; Guerrero, M.; Letelier, M.V. Distribution of potentially toxic elements in soils surrounding abandoned mining waste located in Taltal, Northern Chile. J. Geochem. Explor. 2021, 220, 106653. [Google Scholar] [CrossRef]
- Final Report—Update of the National SPPC Catastro. Available online: https://pras.mma.gob.cl/wp-content/uploads/2023/03/INFORME-FINAL-ACTUALIZACION-CATASTRO-NACIONAL-SPPC.pdf (accessed on 15 December 2024).
- Lottermoser, B.G. Mine Wastes: Characterization, Treatment and Environmental Impacts, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Khan, S.; Shah, M.T.; Brusseau, M.L.; Khan, A.; Shah, A. Human health risks associated with heavy metals in the environment. Sci. Total Environ. 2021, 797, 149129. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Van Deventer, J.S.J. Binder Chemistry—High-Calcium Alkali-Activated Materials. In Alkali Activated Materials; Springer: Dordrecht, The Netherlands, 2014; Volume 13, pp. 59–91. [Google Scholar] [CrossRef]
- Paiva, H.; Yliniemi, J.; Illikainen, M.; Rocha, F.; Ferreira, V.M. Mine Tailings Geopolymers as a Waste Management Solution for A More Sustainable Habitat. Sustainability 2019, 11, 995. [Google Scholar] [CrossRef]
- Simonsen, A.M.T.; Solismaa, S.; Hansen, H.K.; Jensen, P.E. Evaluation of mine tailings’ potential as supplementary cementitious materials based on chemical, mineralogical and physical characteristics. Waste Manag. 2020, 102, 710–721. [Google Scholar] [CrossRef]
- Kiventerä, J.; Perumal, P.; Yliniemi, J.; Illikainen, M. Mine tailings as a raw material in alkali activation: A review. Int. J. Miner. Metall. Mater. 2020, 27, 1009–1020. [Google Scholar] [CrossRef]
- Zhang, L.; Ahmari, S.; Zhang, J. Synthesis and characterization of fly ash modified mine tailings-based geopolymers. Constr. Build. Mater. 2011, 25, 3773–3781. [Google Scholar] [CrossRef]
- Ahmari, S.; Chen, R.; Zhang, L. Utilization of mine tailings as road base material. In GeoCongress 2012: State of the Art and Practice in Geotechnical Engineering; ASCE: Reston, VA, USA, 2012; pp. 3654–3661. [Google Scholar] [CrossRef]
- Huang, B.; Feng, Q.; An, D.; Zhang, J. Use of mine tailings as precast construction materials through alkali activation. Min. Metall. Explor. 2020, 37, 251–265. [Google Scholar] [CrossRef]
- Vargas, F.; López, M. Activation of Copper Tailings for Use as SCM: Mechanical Performance and Influencing Factors. J. Clean. Prod. 2018, 182, 427–436. [Google Scholar] [CrossRef]
- Tarvainen, T.; Reyes, A.; Sapon, S. Acceptable soil baseline levels in Taltal, Chile, and in Tampere, Finland. Appl. Geochem. 2020, 123, 104813. [Google Scholar] [CrossRef]
- Bah, A.; Jin, J.; Ramos, A.O.; Bao, Y.; Ma, M.; Li, F. Arsenic(V) immobilization in fly ash and mine tailing-based geopolymers: Performance and mechanism insight. Chemosphere 2022, 306, 135636. [Google Scholar] [CrossRef] [PubMed]
- Ahmari, S.; Zhang, L.; Zhang, J. Effects of activator type/concentration and curing temperature on alkali-activated binder based on copper mine tailings. J. Mater. Sci. 2012, 47, 5933–5945. [Google Scholar] [CrossRef]
- Onuaguluchi, O.; Eren, Ö. Cement mixtures containing copper tailings as an additive: Durability properties. Mater. Res. 2012, 15, 1029–1036. [Google Scholar] [CrossRef]
- Zeng, H.; Ramanathan, S.; Kim, H.J. Effect of copper mine tailings and copper slag on the hydration, microstructure, and mechanical property in Portland cement. Results Eng. 2025, 26, 104890. [Google Scholar] [CrossRef]
- Schneider, M.; Romer, M.; Tschudin, M.; Bolio, H. Sustainable cement production—Present and future. Cem. Concr. Res. 2011, 41, 642–650. [Google Scholar] [CrossRef]
- Lothenbach, B.; Scrivener, K.; Hooton, R.D. Supplementary cementitious materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- Benzaazoua, M.; Belem, T.; Bussière, B. Chemical factors that influence the performance of mine sulphidic paste backfill. Cem. Concr. Res. 2002, 32, 1133–1144. [Google Scholar] [CrossRef]
- Tariq, A.; Nehdi, M. Developing durable paste backfill from sulphidic tailings. In Proceedings of the Institution of Civil Engineers—Waste and Resource Management; Thomas Telford Ltd.: London, UK, 2007; Volume 160, pp. 155–166. [Google Scholar] [CrossRef]
- Argane, R.; Benzaazoua, M.; Hakkou, R.; Bouamrane, A. A comparative study on the practical use of low sulfide base-metal tailings as aggregates for rendering and masonry mortars. J. Clean. Prod. 2016, 112, 914–925. [Google Scholar] [CrossRef]
- Janković, K.; Stanković, S.; Bojović, D.; Stojanović, M.; Antić, L. The influence of nano-silica and barite aggregate on properties of ultra high performance concrete. Constr. Build. Mater. 2016, 126, 147–156. [Google Scholar] [CrossRef]
- Mohapatra, A.K.; Pradhan, B. Hybrid alkali activated cements (HAACs) system: A state-of-the-art review on fresh, mechanical, and durability behaviour. Constr. Build. Mater. 2022, 361, 129636. [Google Scholar] [CrossRef]
- Mehta, P.K. Reducing the Environmental Impact of Concrete—Google Académico. [En línea]. Available online: https://scholar.google.com/scholar_lookup?title=Reducing%20the%20environmental%20impact%20of%20concrete&author=P.K.%20Mehta&publication_year=2001&pages=61-66 (accessed on 16 September 2024).
- Mehta, A.; Siddique, R. An overview of geopolymers derived from industrial by-products. Constr. Build. Mater. 2016, 127, 183–198. [Google Scholar] [CrossRef]
- Ma, C.-K.; Awang, A.Z.; Omar, W. Structural and material performance of geopolymer concrete: A review. Constr. Build. Mater. 2018, 186, 90–102. [Google Scholar] [CrossRef]
- Provis, J.L. Alkali Activated Materials: State-of-the-Art Report, RILEM TC 224-AAM; Springer: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Kiventerä, J.; Golek, L.; Yliniemi, J.; Ferreira, V.; Deja, J.; Illikainen, M. Utilization of sulphidic tailings from gold mine as a raw material in geopolymerization. Int. J. Miner. Process. 2016, 149, 104–110. [Google Scholar] [CrossRef]
- Bernal, S.A.; Mejía de Gutiérrez, R.; Rodríguez, E.D. Materiales de activación alcalina: Cementando un futuro sostenible. Rev. Fac. Ing. Univ. Valle. 2013, 15, 211–223. [Google Scholar] [CrossRef]
- Yavna, V.A.; Kasprzhitskii, A.S.; Lazorenko, G.I.; Kochur, A.G. Study of IR spectra of a polymineral natural association of phyllosilicate minerals. Opt. Spectrosc. 2015, 118, 529–536. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers: Ceramic-Like Inorganic Polymers. J. Ceram. Sci. Technol. 2017, 8, 335–350. [Google Scholar] [CrossRef]
- van Jaarsveld, J.G.S.; van Deventer, J.S.J. Effect of the Alkali Metal Activator on the Properties of Fly Ash-Based Geopolymers. Ind. Eng. Chem. Res. 1999, 38, 3932–3941. [Google Scholar] [CrossRef]
- Silva, P.D.; Sagoe-Crenstil, K.; Sirivivatnanon, V. Kinetics of geopolymerization: Role of Al2O3 and SiO2. Cem. Concr. Res. 2007, 37, 512–518. [Google Scholar] [CrossRef]
- Kalinkina, E.V.; Gurevich, B.I.; Kalinkin, A.M. Alkali-Activated Binder Based on Milled Antigorite. Minerals 2018, 8, 503. [Google Scholar] [CrossRef]
- Kinnunen, P.; Ismailov, A.; Solismaa, S.; Sreenivasan, H.; Räisänen, M.L.; Levänen, E.; Illikainen, M. Recycling mine tailings in chemically bonded ceramics—A review. J. Clean. Prod. 2018, 174, 634–649. [Google Scholar] [CrossRef]
- Ouffa, N.; Benzaazoua, M.; Belem, T.; Trauchessec, R.; Lecomte, A. Alkaline dissolution potential of aluminosilicate minerals for the geosynthesis of mine paste backfill. Mater. Today Commun. 2020, 24, 101221. [Google Scholar] [CrossRef]
- Tunsu, C.; Menard, Y.; Eriksen, D.Ø.; Ekberg, C.; Petranikova, M. Recovery of critical materials from mine tailings: A comparative study of the solvent extraction of rare earths using acidic, solvating and mixed extractant systems. J. Clean. Prod. 2019, 218, 425–437. [Google Scholar] [CrossRef]
- Tchakoute Kouamo, H.; Mbey, J.A.; Elimbi, A.; Kenne Diffo, B.B.; Njopwouo, D. Synthesis of volcanic ash-based geopolymer mortars by fusion method: Effects of adding metakaolin to fused volcanic ash. Ceram. Int. 2013, 39, 1613–1621. [Google Scholar] [CrossRef]
- Marjanović, N.; Komljenović, M.; Baščarević, Z.; Nikolić, V. Improving reactivity of fly ash and properties of ensuing geopolymers through mechanical activation. Constr. Build. Mater. 2014, 57, 151–162. [Google Scholar] [CrossRef]
- Naghsh, M.; Shams, K. Synthesis of a kaolin-based geopolymer using a novel fusion method and its application in effective water softening. Appl. Clay Sci. 2017, 146, 238–245. [Google Scholar] [CrossRef]
- Krishna, R.S.; Shaikh, F.; Mishra, J.; Lazorenko, G.; Kasprzhitskii, A. Mine tailings-based geopolymers: Properties, applications and industrial prospects. Ceram. Int. 2021, 47, 17826–17843. [Google Scholar] [CrossRef]
- Barrie, E.; Cappuyns, V.; Vassilieva, E.; Adriaens, R.; Hollanders, S.; Garcés, D.; Paredes, C.; Pontikes, Y.; Elsen, J.; Machiels, L. Potential of inorganic polymers (geopolymers) made of halloysite and volcanic glass for the immobilisation of tailings from gold extraction in Ecuador. Appl. Clay Sci. 2015, 109, 95–106. [Google Scholar] [CrossRef]
- Borges, P.H.R.; Ramos, F.C.R.; Caetano, T.R.; Panzerra, T.H.; Santos, H. Reuse of iron ore tailings in the production of geopolymer mortars. REM Int. Eng. J. 2019, 72, 581–587. [Google Scholar] [CrossRef]
- Lazorenko, G.; Kasprzhitskii, A.; Yatsenko, E.A.; Wensheng, L.; Chaudhary, S. Towards coal mining waste valorization: Gangue as resource for the production of geopolymer and related alkali-activated materials. Green Technol. Sustain. 2025, 3, 100205. [Google Scholar] [CrossRef]
- Bazan, P.; Figiela, B.; Kozub, B.; Łach, M.; Mróz, K.; Melnychuk, M.; Korniejenko, K. Geopolymer foam with low thermal conductivity based on industrial waste. Materials 2024, 17, 6143. [Google Scholar] [CrossRef]
- Falah, M.; Obenaus-Emler, R.; Kinnunen, P.; Illikainen, M. Effects of Activator Properties and Curing Conditions on Alkali-Activation of Low-Alumina Mine Tailings. Waste Biomass Valor. 2020, 11, 5027–5039. [Google Scholar] [CrossRef]
- Ahmari, S.; Zhang, L. Durability and leaching behavior of mine tailings-based geopolymer bricks. Constr. Build. Mater. 2013, 44, 743–750. [Google Scholar] [CrossRef]
- Ahmari, S.; Zhang, L. Production of eco-friendly bricks from copper mine tailings through geopolymerization. Constr. Build. Mater. 2012, 29, 323–331. [Google Scholar] [CrossRef]
- Aseniero, J.P.J.; Opiso, E.M.; Banda, M.H.T.; Tabelin, C.B. Potential utilization of artisanal gold-mine tailings as geopolymeric source material: Preliminary investigation. SN Appl. Sci. 2018, 1, 35. [Google Scholar] [CrossRef]
- Manaviparast, H.R.; Miranda, T.; Pereira, E.; Cristelo, N. A comprehensive review on mine tailings as a raw material in the alkali activation process. Appl. Sci. 2024, 14, 5127. [Google Scholar] [CrossRef]
- ASTM C150; Standard Specification for Portland Cement. ASTM International: West Conshohocken, PA, USA, 2022; pp. 1–9. Available online: https://compass.astm.org/document/?contentCode=ASTM%7CC0150_C0150M-22%7Cen-US (accessed on 21 January 2024).
- INN—Instituto Nacional de Normalización. NCh-ISO 14004:2016. Environmental Management Systems—General Guidelines on Implementation. Santiago (Chile): INN; 2016. eCommerce INN. Available online: https://ecommerce.inn.cl/nch-iso14004201660650 (accessed on 24 March 2025).
- PD CEN TR 15310-1-2006; Characterization of Waste—Sampling of Waste Materials—Part 1: Guidance on Selection and Application of Criteria for Sampling Under Various Conditions. British Standards Institution (BSI): London, UK, 2006.
- Gao, Y.; Luo, J.; Zhu, X.; Zhang, J.; Fan, K.; Ma, M. A Review on the Effect of Organic Admixtures Containing Different Functional Groups on the Hydration Behaviors of Portland Cement. Revic 2025, 2024, 0014. [Google Scholar] [CrossRef]
- INN—Instituto Nacional de Normalización. NCh148:2021. Hydraulic Cements—Sampling and Testing Methods. Santiago (Chile): INN; 2021. eCommerce INN. Available online: https://ecommerce.inn.cl/nch148202163150 (accessed on 20 January 2025).
- Leiva, C.; Rodriguez-Galán, M.; Arenas, C.; Alonso-Fariñas, B.; Peceño, B. A mechanical, leaching and radiological assessment of fired bricks with a high content of fly ash. Ceram. Int. 2018, 44, 13313–13319. [Google Scholar] [CrossRef]
- Obenaus-Emler, R.; Illikainen, M.; Falah, M.; Kinnunen, P.; Heiskanen, K. Geopolymers from mining tailings for more sustainable raw material supply. MATEC Web Conf. 2019, 274, 05001. [Google Scholar] [CrossRef]
- Gado, R.A.; Hebda, M.; Łach, M.; Mikuła, J. Alkali Activation of Waste Clay Bricks: Influence of the Silica Modulus, SiO2/Na2O, H2O/Na2O Molar Ratio, and Liquid/Solid Ratio. Materials 2020, 13, 383. [Google Scholar] [CrossRef]
- Murugesan, S.; Ramaswamy, J.; Parshwanath, R.N. Effect of change in the silica modulus of sodium silicate solution on the microstructure of fly ash geopolymers. J. Build. Eng. 2021, 44, 102939. [Google Scholar] [CrossRef]
- Alaneme, G.U.; Olonade, K.A.; Esenogho, E.; Okereke, A.C.; Afolayan, O.D.; Oluwafemi, J.O.; Akinseye, A.S.; Ayeni, O.J. Proposed simplified methodological approach for designing geopolymer concrete mixtures. Sci. Rep. 2024, 14, 15191. [Google Scholar] [CrossRef] [PubMed]
- Thapa, S.; Debnath, S.; Kulkarni, S.M.; Solanki, H.; Nath, S. Mechanical properties of geopolymer concrete incorporating supplementary cementitious materials as binding agents. Discov. Civ. Eng. 2024, 1, 62. [Google Scholar] [CrossRef]
- ASTM C109/C109M-02; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50-mm] Cube Specimens). ASTM International: West Conshohocken, PA, USA, 2002.
- ASTM C187-23; Standard Test Method for Amount of Water Required for Normal Consistency of Hydraulic Cement Paste. ASTM International: West Conshohocken, PA, USA, 2023.
- ASTM C191-19; Standard Test Methods for Time of Setting of Hydraulic Cement by Vicat Needle. ASTM International: West Conshohocken, PA, USA, 2019.
- ASTM C20-00; Standard Test Methods for Apparent Porosity, Water Absorption, Apparent Specific Gravity, and Bulk Density of Burned Refractory Brick and Shapes by Boiling Water. ASTM International: West Conshohocken, PA, USA, 2000.
- Zelder, S.; Rosin, A.; Helling, D.; Schafföner, S. Mineral Composite Plaster Containing Hollow Glass Microspheres and CSA Cement for Building Insulation. Appl. Sci. 2022, 12, 1152. [Google Scholar] [CrossRef]
- Ministerio de Salud de Chile. Supreme Decree No. 148: Approves the Sanitary Regulation on the Management of Hazardous Waste; Ministerio de Salud: Santiago, Chile, 2003; Available online: https://www.ispch.cl/sites/default/files/normativa_anamed/establecimientos_autorizacion_y_fiscalizacion/Decreto%20Supremo%20148.pdf (accessed on 20 January 2025).
- United States Environmental Protection Agency (EPA). Method 1311: Toxicity Characteristic Leaching Procedure (TCLP), Test Methods for Evaluating Solid Waste, Physical/Chemical Methods. Revision 0; EPA: Washington, DC, USA, 1992. Available online: https://www.epa.gov/hw-sw846/sw-846-test-method-1311-toxicity-characteristic-leaching-procedure (accessed on 9 November 2024).
- ASTM C618-19; Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete. ASTM International: West Conshohocken, PA, USA, 2019.
- Provis, J.L.; Palomo, A.; Shi, C. Advances in understanding alkali-activated materials. Cem. Concr. Res. 2015, 78, 110–125. [Google Scholar] [CrossRef]
- Kiventerä, J.; Lancellotti, I.; Catauro, M.; Poggetto, F.D.; Leonelli, C.; Illikainen, M. Alkali activation as new option for gold mine tailings inertization. J. Clean. Prod. 2018, 187, 76–84. [Google Scholar] [CrossRef]
- Jamieson, H.E.; Walker, S.R.; Parsons, M.B. Mineralogical Characterization of Mine Waste. Appl. Geochem. 2015, 57, 85–105. [Google Scholar] [CrossRef]
- Clare, K.E.; Sherwood, P.T. The effect of organic matter on the setting of soil-cement mixtures. J. Appl. Chem. 1954, 4, 625–630. [Google Scholar] [CrossRef]
- Celik, I.B. The effects of particle size distribution and surface area upon cement strength development. Powder Technol. 2009, 188, 272–276. [Google Scholar] [CrossRef]
- Snellings, R. Assessing, Understanding and Unlocking Supplementary Cementitious Materials. RILEM Tech. Lett. 2016, 1, 50–55. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D. Geopolymerisation: A review and prospects for the minerals industry. Miner. Eng. 2007, 20, 1261–1277. [Google Scholar] [CrossRef]
- Tian, X.; Xu, W.; Song, S.; Rao, F.; Xia, L. Effects of curing temperature on the compressive strength and microstructure of copper tailing-based geopolymers. Chemosphere 2020, 253, 126754. [Google Scholar] [CrossRef] [PubMed]
- Xiaolong, Z.; Shiyu, Z.; Hui, L.; Yingliang, Z. Disposal of mine tailings via geopolymerization. J. Clean. Prod. 2021, 284, 124756. [Google Scholar] [CrossRef]
- Castillo, H.; Collado, H.; Droguett, T.; Sánchez, S.; Vesely, M.; Garrido, P.; Palma, S. Methodologies for the Possible Integral Generation of Geopolymers Based on Copper Tailings. Minerals 2021, 11, 1367. [Google Scholar] [CrossRef]
- Rao, F.; Liu, Q. Geopolymerization and Its Potential Application in Mine Tailings Consolidation: A Review. Miner. Process. Extr. Metall. Rev. 2015, 36, 399–409. [Google Scholar] [CrossRef]
- Capasso, I.; Lirer, S.; Flora, A.; Ferone, C.; Cioffi, R.; Caputo, D.; Liguori, B. Reuse of mining waste as aggregates in fly ash-based geopolymers. J. Clean. Prod. 2019, 220, 65–73. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Juilland, P.; Monteiro, P.J.M. Advances in understanding hydration of Portland cement. Cem. Concr. Res. 2015, 78, 38–56. [Google Scholar] [CrossRef]
- Salha, M.S.; Yada, R.Y.; Farrar, D.H.; Chass, G.A.; Tian, K.V.; Bodo, E. Aluminium-catalysed oligomerisation in cement-forming silicate systems. Phys. Chem. Chem. Phys. 2022, 24, 29034–29050. [Google Scholar] [CrossRef]
- Herterich, J.; Richardson, I.G.; Moro, F.; Marchi, M.; Black, L. Microstructure and phase assemblage of low-clinker cements during the early stages of carbonation. Cem. Concr. Res. 2022, 152, 106643. [Google Scholar] [CrossRef]
- Manjarrez, L.; Nikvar-Hassani, A.; Shadnia, R.; Zhang, L. Experimental Study of Geopolymer Binder Synthesized with Copper Mine Tailings and Low-Calcium Copper Slag. J. Mater. Civ. Eng. 2019, 31, 04019156. [Google Scholar] [CrossRef]
- Duan, P.; Yan, C.; Zhou, W.; Ren, D. Fresh properties, compressive strength and microstructure of fly ash geopolymer paste blended with iron ore tailing under thermal cycle. Constr. Build. Mater. 2016, 118, 76–88. [Google Scholar] [CrossRef]
- Fernandez-Jimenez, A.M.; Palomo, A.; Lopez-Hombrados, C. Engineering Properties of Alkali-Activated Fly Ash Concrete. ACI Mater. J. 2006, 103, 106–112. [Google Scholar] [CrossRef]
- Manjarrez, L.; Zhang, L. Utilization of Copper Mine Tailings as Road Base Construction Material through Geopolymerization. J. Mater. Civ. Eng. 2018, 30, 04018201. [Google Scholar] [CrossRef]
- Lazorenko, G.; Kasprzhitskii, A.; Shaikh, F.; Krishna, R.S.; Mishra, J. Utilization potential of mine tailings in geopolymers: Physicochemical and environmental aspects. Process Saf. Environ. Prot. 2021, 147, 559–577. [Google Scholar] [CrossRef]
- ASTM C90-22; Standard Specification for Loadbearing Concrete Masonry Units. ASTM International: West Conshohocken, PA, USA, 2022.
- Temuujin, J.; van Riessen, A. Effect of fly ash preliminary calcination on the properties of geopolymer. J. Hazard. Mater. 2009, 164, 634–639. [Google Scholar] [CrossRef]
- Xu, H.; Van Deventer, J.S.J. The geopolymerisation of alumino-silicate minerals. Int. J. Miner. Process. 2000, 59, 247–266. [Google Scholar] [CrossRef]
- De Weerdt, K.; Haha, M.B.; Le Saout, G.; Kjellsen, K.O.; Justnes, H.; Lothenbach, B. Hydration mechanisms of ternary Portland cements containing limestone powder and fly ash. Cem. Concr. Res. 2011, 41, 279–291. [Google Scholar] [CrossRef]
- Subaer. Influence of Aggregate on the Microstructure of Geopolymer. Ph.D. Thesis, Curtin University, Perth, WA, Australia, 2004. [Online]. Available online: https://espace.curtin.edu.au/handle/20.500.11937/1695 (accessed on 9 November 2024).
- Mavroulidou, M.; Morrison, T.; Unsworth, C.; Gunn, M.J. Properties of concrete made of multicomponent mixes of low-energy demanding binders. Constr. Build. Mater. 2015, 101, 1122–1141. [Google Scholar] [CrossRef]
- Davidovits, J. Waste Solidification and Disposal Method. U.S. Patent US4859367A, 22 August 1989. [En línea]. Available online: https://patents.google.com/patent/US4859367A/en (accessed on 31 October 2024).






| Mix ID | Cu Tailings (kg/m3) | FA (kg/m3) | OPC (kg/m3) | Water (kg/m3) | NaOH (kg/m3) | Sodium Silicate (kg/m3) | L/S | Si/Al | Si/Na |
|---|---|---|---|---|---|---|---|---|---|
| G1 | 1260 | 540 | 0 | 3 | 122 | 500 | 0.28 | 11.4 | 2.9 |
| G2 | 1071 | 459 | 270 | 3 | 122 | 500 | 0.28 | 9.7 | 3.2 |
| Parameter | Cu Tailing | FA | OPC | Method |
|---|---|---|---|---|
| SiO2 (wt.%) | 77.82 | 55.39 | 5.83 | XRF |
| Al2O3 (wt.%) | 5.35 | 13.12 | – | XRF |
| CaO (wt.%) | 0.86 | 9.73 | 13.87 | XRF |
| Fe2O3 (wt.%) | 3.60 | 2.88 | – | XRF |
| Na2O (wt.%) | 0.39 | 0.49 | – | XRF |
| K2O (wt.%) | 0.55 | 0.75 | – | XRF |
| MgO (wt.%) | 0.16 | 0.65 | – | XRF |
| LOI (wt.%) | 5.71 | 11.82 | – | XRF |
| pH | 7.5 | 10.7 | – | Direct measurement |
| Specific gravity | 2.49 | 1.90 | – | Pycnometry |
| SSA (m2/g) | 0.809 | 0.534 | – | Laser Diffraction |
| d90 (µm) | 105.2 | 111.2 | – | Laser Diffraction |
| Amorphous content (wt.%) | - | 64.4 | 2.51 | XRD (Rietveld) |
| Main Crystalline Phases | Quartz (64.56 wt.% [00-046-1045] *) Phillipsite-K (7.77 wt.% [00-046-1427] *) Loveringite (5.05 wt.% [00-042-1368] *) Periclase (4.69 wt.% [00-045-0946] *) Lautite (3.39 wt.% [00-039-0393] *) | Quartz (24.37 wt.% [00-046-1045] *) Mullite (4.18 wt.% [00-015-0776] *) Ettringite (2.48 wt.% [00-041-1451] *) Calcite (1.74 wt.% [00-047-1743] *) | Larnite (19.93 wt.% [00-033-0302] *) Titanite (14.73 wt%) Calcite (13.87 wt.% [00-047-1743] *) Gismondine (12.64 wt.% [00-020-0452] *) | XRD (Rietveld) |
| FESEM Morphology | Irregular, angular particles with coarse texture and voids | Mostly spherical particles, smooth surfaces, some agglomerates | Not characterized in detail | FESEM |
| Parameter | G1 | G2 |
|---|---|---|
| Amorphous content (1.3 months) (wt.%) | 33.25 | 41.81 |
| Porosity (28 days, MIP) (%) | 52.0 | 35.1 |
| Setting time | 24 h | 18 h |
| Compressive strength (28 days → 120 days) (MPa) | 12.4 → 24.2 | 23.1 → 41.2 |
| Main Crystalline Phases (XRD) after 5.7 months ** | Quartz (65.5 wt.% [00-046-1045] *) Muscovite (8.8 wt.% [00-007-0025] *) Clinoclase (6.54 wt.% [00-037-0447] *) Albite (5.1 wt.% [00-041-1480] *) Thenardite (4.6 wt.% [00-037-1465] *) | Quartz (63.2 wt.% [00-046-1045] *) Albite (11.7 wt.% [00-041-1480] *) Clinoclase (5.49 wt.% [00-037-0447]) Thenardite (5.3 wt.% [00-037-1465] *) Muscovite (5.0 wt.% [00-007-0025] *) |
| FESEM Morphology at 5.7 months | Compact matrix with visible voids, partially reacted tailings. | Highly compact, homogeneous structure, minimal voids. |
| Months | Type | Figure | Point | Weight %/Error % | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na | Al | Si | S | K | Ca | Fe | Si/Al | Na/Al | ||||
| 1.3 | G1 | A | 1 | 32.7/7.08 | 0.5/24.26 | 2.8/6.97 | 1.2/9.62 | 0.2/24.70 | 0.2/35.50 | 6.0 | 71.0 | |
| 2 | 19.4/8.11 | 1.5/10.43 | 13.6/5.19 | 9.9/5.41 | 0.5/21.54 | 1.0/13.54 | 1.7/12.81 | 8.9 | 12.7 | |||
| G2 | B | 1 | 1.0/14.87 | 0.6/10.05 | 28.8/3.23 | - | - | 0.2/24.53 | - | 47.3 | 1.6 | |
| 2 | 17.0/7.34 | 4.2/6.46 | 12.2/4.90 | 3.0/5.65 | 0.1/59.47 | 1.6/4.88 | 0.2/26.47 | 2.9 | 4.0 | |||
| 4.8 | G1 | C | 1 | 18.3/6.62 | 2.3/11.58 | 35.8/4.29 | - | - | 3.3/20.88 | - | 15.4 | 7.9 |
| 2 | 2.4/13.24 | 0.8/16.76 | 24.7/4.09 | 2.2/14.55 | 0.7/43.32 | - | 1.8/18.54 | 31.7 | 3.1 | |||
| 3 | 7.7/7.32 | 3.7/7.29 | 26.7/4.10 | - | - | - | - | 7.3 | 2.1 | |||
| G2 | D | 1 | 21.5/5.71 | 0.4/30.82 | 1.7/9.2 | - | - | 1.7/14.60 | - | 4.2 | 53.7 | |
| 2 | 10.1/6.07 | 0.9/9.67 | 6.6/4.53 | - | - | 3.8/9.09 | - | 7.7 | 11.7 | |||
| 5.3 | G1 | E | 1 | 1.3/9.42 | 31.6/3.53 | - | - | - | - | 24.3 | 0.0 | |
| 2 | 11.7/6.61 | 2.6/8.64 | 26.9/4.03 | - | - | 1.3/26.98 | - | 10.5 | 4.6 | |||
| 3 | 8.6/6.84 | 6.4/5.63 | 29.6/3.94 | - | 1.2/19.76 | 1.9/18.52 | - | 4.6 | 1.3 | |||
| G2 | F | 1 | 24.1/5.78 | 3.6/7.44 | 9.0/4.97 | 4.3/8.31 | - | 4.2/10.50 | - | 2.5 | 6.7 | |
| 2 | 7.8/7.31 | 1.1/12.00 | 11.9/4.54 | - | - | 15.3/5.47 | - | 11.2 | 7.3 | |||
| 5.7 | G1 | G | 1 | 12.2/6.66 | 2.4/9.49 | 27.0/4.07 | - | - | 3.5/14.23 | - | 11.2 | 5.1 |
| G2 | H | 1 | 8.9/7.25 | 2.8/8.00 | 14.7/4.43 | 2.0/14.19 | 0.8/28.76 | 19.4/5.39 | - | 5.3 | 3.2 | |
| Leached Concentrations Obtained from the TCLP Test (mg/L) | Determinations by ICP-OES in Raw Material Samples | |||||||
|---|---|---|---|---|---|---|---|---|
| Total Element Concentration | G1 (120 d) | G2 (120 d) | Cu Tailing | FA | Cu Tailing (mg/kg) | FA (mg/kg) | OPC (mg/kg) | MAC (mg/L) |
| As | 1.1 | 1.0 | 4.054 | <0.002 | 3098 | <2.00 | 2.66 | 5.0 |
| Cr | <0.007 | <0.007 | 0.0235 | 0.0593 | 13.8 | 8.13 | 35.50 | 5.0 |
| Hg | <0.002 | <0.002 | <0.002 | <0.002 | n.d * | n.d * | n.d * | 0.2 |
| Pb | <0.051 | <0.051 | <0.051 | <0.051 | 51.10 | <30.00 | <30.00 | 5.0 |
| Se | 0.072 | 0.074 | 0.065 | 0.072 | 2.42 | 16.00 | 6.62 | 1.0 |
| Ba | <1.009 | <1.009 | <1.009 | <1.009 | 4301 | 297.7 | 101.80 | 100.0 |
| Cd | <0.045 | <0.045 | <0.045 | <0.045 | 1.14 | <0.50 | 4.57 | 1.0 |
| Ag | <0.032 | <0.032 | <0.032 | <0.032 | 4.70 | <2.00 | 2.66 | 5.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reyes-Román, A.; Samarina, T.; Castillo-Godoy, D.; Takaluoma, E.; Campo, G.; Araya-Letelier, G.; Silva, Y.F. Use of Abandoned Copper Tailings as a Precursor to the Synthesis of Fly-Ash-Based Alkali Activated Materials. Materials 2025, 18, 3926. https://doi.org/10.3390/ma18173926
Reyes-Román A, Samarina T, Castillo-Godoy D, Takaluoma E, Campo G, Araya-Letelier G, Silva YF. Use of Abandoned Copper Tailings as a Precursor to the Synthesis of Fly-Ash-Based Alkali Activated Materials. Materials. 2025; 18(17):3926. https://doi.org/10.3390/ma18173926
Chicago/Turabian StyleReyes-Román, Arturo, Tatiana Samarina, Daniza Castillo-Godoy, Esther Takaluoma, Giuseppe Campo, Gerardo Araya-Letelier, and Yimmy Fernando Silva. 2025. "Use of Abandoned Copper Tailings as a Precursor to the Synthesis of Fly-Ash-Based Alkali Activated Materials" Materials 18, no. 17: 3926. https://doi.org/10.3390/ma18173926
APA StyleReyes-Román, A., Samarina, T., Castillo-Godoy, D., Takaluoma, E., Campo, G., Araya-Letelier, G., & Silva, Y. F. (2025). Use of Abandoned Copper Tailings as a Precursor to the Synthesis of Fly-Ash-Based Alkali Activated Materials. Materials, 18(17), 3926. https://doi.org/10.3390/ma18173926








